
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 09 September 2021
Sec. Breast Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.724543
 Fabian Tollens1
Fabian Tollens1 Pascal A. T. Baltzer2
Pascal A. T. Baltzer2 Matthias Dietzel3
Matthias Dietzel3 Moritz L. Schnitzer4
Moritz L. Schnitzer4 Wolfgang G. Kunz4
Wolfgang G. Kunz4 Johann Rink1
Johann Rink1 Johannes Rübenthaler4
Johannes Rübenthaler4 Matthias F. Froelich1
Matthias F. Froelich1 Clemens G. Kaiser1*
Clemens G. Kaiser1*Objectives: To evaluate the cost-effectiveness of MR-mammography (MRM) vs. x-ray based mammography (XM) in two-yearly screening women of intermediate risk for breast cancer in the light of recent literature.
Methods: Decision analysis and Markov modelling were used to compare cumulative costs (in US-$) and outcomes (in QALYs) of MRM vs. XM over the model runtime of 20 years. The perspective of the U.S. healthcare system was selected. Incremental cost-effectiveness ratios (ICER) were calculated and related to a willingness to pay-threshold of $ 100,000 per QALY in order to evaluate the cost-effectiveness. Deterministic and probabilistic sensitivity analyses were conducted to test the impact of variations of the input parameters. In particular, variations of the rate of false positive findings beyond the first screening round and their impact on cost-effectiveness were assessed.
Results: Breast cancer screening with MRM resulted in increased costs and superior effectiveness. Cumulative average costs of $ 6,081 per woman and cumulative effects of 15.12 QALYs were determined for MRM, whereas screening with XM resulted in costs of $ 5,810 and 15.10 QALYs, resulting in an ICER of $ 13,493 per QALY gained. When the specificity of MRM in the second and subsequent screening rounds was varied from 92% to 99%, the ICER resulted in a range from $ 38,849 to $ 5,062 per QALY.
Conclusions: Based on most recent data on the diagnostic performance beyond the first screening round, MRM may remain the economically preferable alternative in screening women of intermediate risk for breast cancer due to their dense breast tissue.
In line with current recommendations, MR-mammography (MRM) has been clinically accepted for various indications such as screening women at high risk for breast cancer, diagnostic evaluation in cancer of unknown primary, and as a problem solver in special cases (1, 2).
Recent data has hinted towards a role of MRM in an extended set of indications, such as screening women at intermediate risk of breast cancer due to their elevated density of breast tissue. The superior diagnostic performance of MRM compared to x-ray based techniques has been demonstrated in several prospective multicenter trials (3–5). Data on the first screening round of the DENSE trial indicated an incremental cost-effectiveness ratio (ICER) of two-yearly MRM screening below $ 10,000 per QALY gained as previously published in our model-based cost-effectiveness analysis (6).
Based on current data, the Dutch parliament has lately decided to introduce MRM as a screening tool for women of intermediate risk due to their breast density as the first country to recommend so (7).
Cost-effectiveness analyses have become a widely accepted methodology in order to direct healthcare resource allocation (8). In particular, expensive innovative medical procedures, diagnostic tests and screening programs are inherently afflicted with ongoing claims not only to provide proof of favorable downstream effects but also superior economic value (9). Therefore, modern concepts of economic studies are increasingly being applied to evaluate diagnostic strategies in screening.
The economic potential of MR-based techniques in breast cancer screening has been demonstrated in various collectives - all with a favorable economic outcome compared to x-ray based techniques (6, 10–13). However, the definition of valid input parameters for these model-based analyses depends on representative real-world data. Long-term data on screening women with intermediate risk for breast cancer with MRM due to their elevated breast density has been unavailable up to this point.
Most recently, Veenhuizen et al. closed this gap by delivering unprecedented data on the diagnostic performance of MRM in breast cancer screening beyond the first screening round (5). A reduced incremental cancer detection rate of 5.8 per 1000 screening examinations in the second screening round (respective 16.5 in the first screening interval) as well as an increased specificity of 97% for the second screening round (respective 92% in the first screening round) were observed in the second screening round of the DENSE trial. All breast cancer cases were detected in an early stage (stage 0-1) and were node negative (5), underlining the diagnostic and prognostic potential of MRM, and hinting towards its economic value beyond the first screening interval.
As outlined in our previous cost-effectiveness analyses, an optimal specificity as well as a minimal rate of false positive findings is required to economically justify MRM as a screening tool in patients of intermediate risk for breast cancer.
Therefore, this study seeks to evaluate the cost-effectiveness of MRM in comparison to x-ray mammography (XM) in screening women of intermediate risk for breast cancer due to their elevated breast density, considering the changes in specificity and false positives in a follow-up situation.
To compare the diagnostic strategies XM versus MRM in a screening setting, a decision model was designed that included the diagnostic outcomes true positive, true negative, false positive and false negative (Figure 1A), based on previously published cost-effectiveness analyses (6, 12, 13).
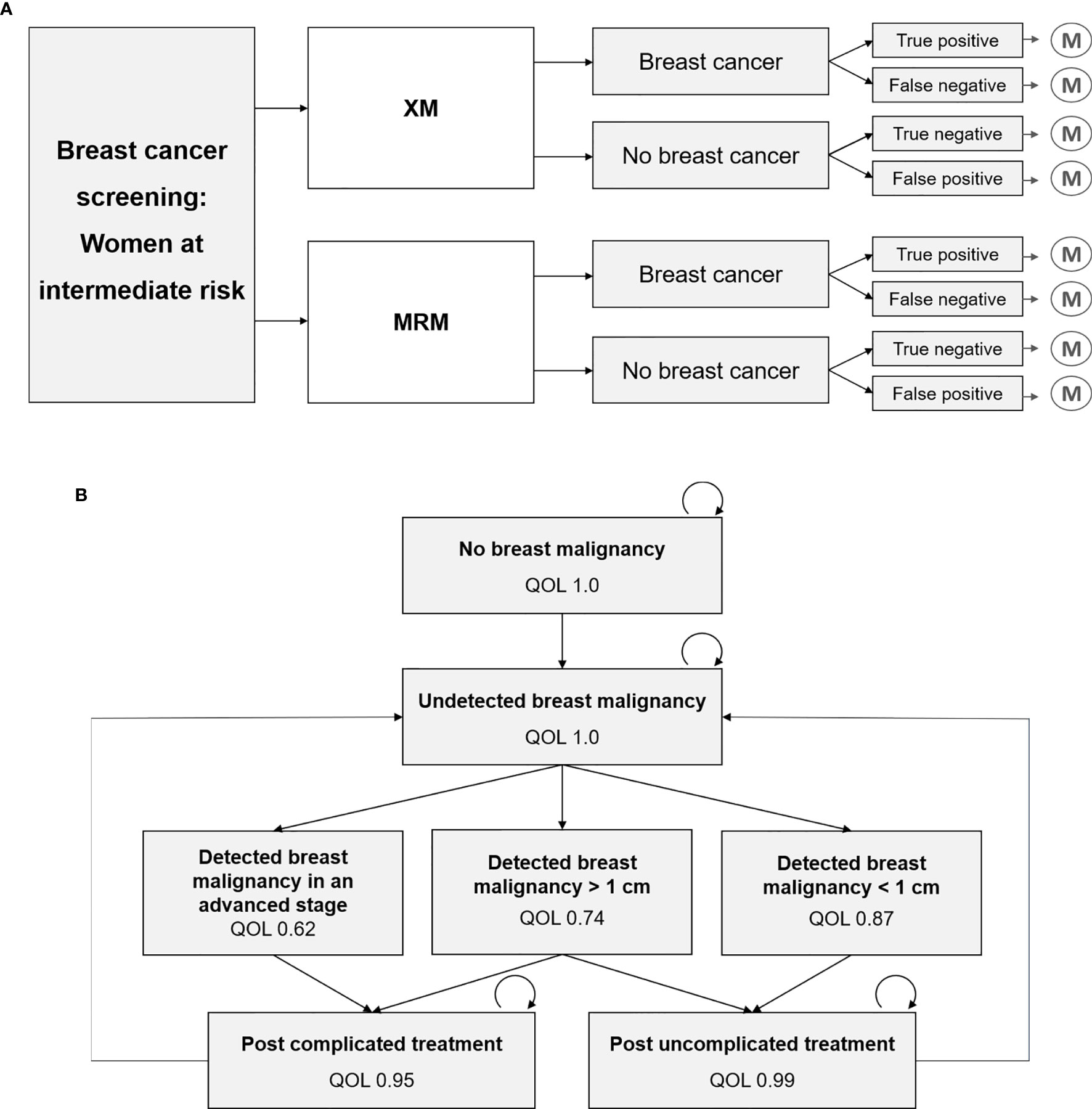
Figure 1 Decision tree and Markov model, which have recently been developed and refined for this study (6, 12, 13). (A) Decision model that represents the diagnostic strategies x-ray based mammography (XM) versus MR-mammography (MRM), and the respective outcomes true positive, false negative, true negative and false positive, that each result in a Markov model simulation. (B) Markov model with various health states and their associated quality of life (QOL). Transition to death is not depicted.
A recently developed Markov Model for breast cancer screening was adapted for the two-yearly screening of women at intermediate risk of breast cancer (Figure 1B). Intermediate risk was defined by elevated breast tissue density, which is further specified in 2.2.1. Long-term costs and outcomes were simulated using a cycle-length of one year and a total model runtime of 20 years. Quality of life in each Markov state was used to calculate cumulative quality-adjusted life years (QALYs) for each diagnostic strategy. Correspondingly, annual costs were assigned to each Markov state and summed up over the total duration of 20 years. In line with current recommendations (1, 2, 14), a screening interval of two years was assumed.
Input parameters were extracted from recent literature (Table 1) closely following international standards on the conduct and methodological practice of cost-effectiveness analyses (16, 31).
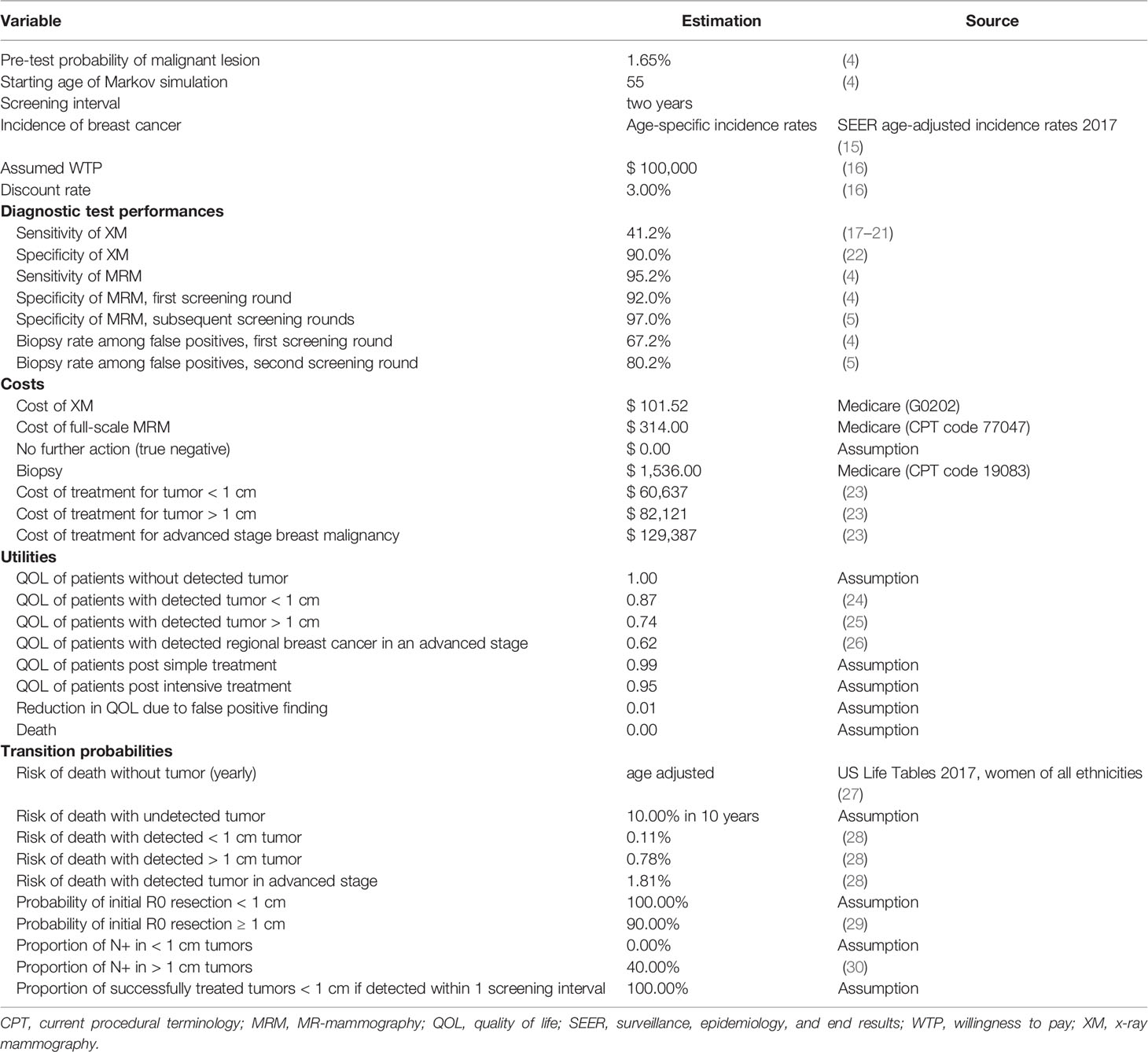
Table 1 Model input parameters for the economic modelling, that have recently been published and adapted for this analysis (6, 12, 13).
Women at intermediate risk of breast cancer due to their elevated breast density (ACR BI-RADS category 4 or D) represented the study collective, with a mean age of 55 years at the beginning of the screening program as reported by Bakker et al. (4).
The diagnostic accuracy of the modalities were extracted from literature (4, 5, 17–22). Importantly, the specificity of MRM has been demonstrated to rise from 92% in the first screening round to 97% in the second round, which was assumed to stay constant in subsequent screening rounds.
Quality of life was estimated for each Markov state based on published literature (24–26). Size of the tumor at the time of detection, stage of disease and the respective therapy affected the patients’ quality of life.
Both short- and long-term costs in US-$ were included (Table 1) in order to calculate cumulative total costs of each diagnostic strategy based on Medicare Current Procedural Terminology (CPT) codes and literature (23). The perspective of the U.S. healthcare system was selected to estimate costs due to standardization and comparability of the data. Costs for false positive results were simulated throughout the entire time span. False positive findings of XM resulted in a biopsy, whereas MRM resulted either in a follow-up examination or a biopsy (Table 1).
Age-adjusted incidence rates were collected from the Surveillance, Epidemiology, and End Results (SEER) Program (15). Disease-specific risk of death was estimated based on the NHS Predict model (28). General age-adjusted death rates were extracted from the U.S. Life Tables (27). Resection status and nodal status were estimated depending on the stage of disease (29, 30).
The U.S. healthcare system was chosen as the study perspective. Outcomes were modelled by calculating the average cumulative QALYs for each diagnostic strategy, costs were measured in US-$. Dedicated software for economic modelling and decision analysis was used to carry out Markov modelling and cost-effectiveness analyses (TreeAge Pro 2020, TreeAge Software, Williamstown, MA). According to recommendations on the conduct of cost-effectiveness analyses, an annual discount rate of 3% was applied both for costs and outcomes (16). The willingness to pay (WTP)-threshold was set to $ 100,00 per QALY gained (32, 33), so that an incremental cost-effectiveness ratio (ICER) below this value indicated favorable cost-effectiveness.
The diagnostic performance and the cost of the screening modalities were varied in a deterministic sensitivity analysis and the resulting ICER was simulated in order to examine the impact of variations in the input parameters (Figure 2). In the next step, the relationship between varying costs of MRM and the specificity of MRM in the second and subsequent screening rounds and their impact on the resulting cost-effectiveness were modelled (Figure 3).
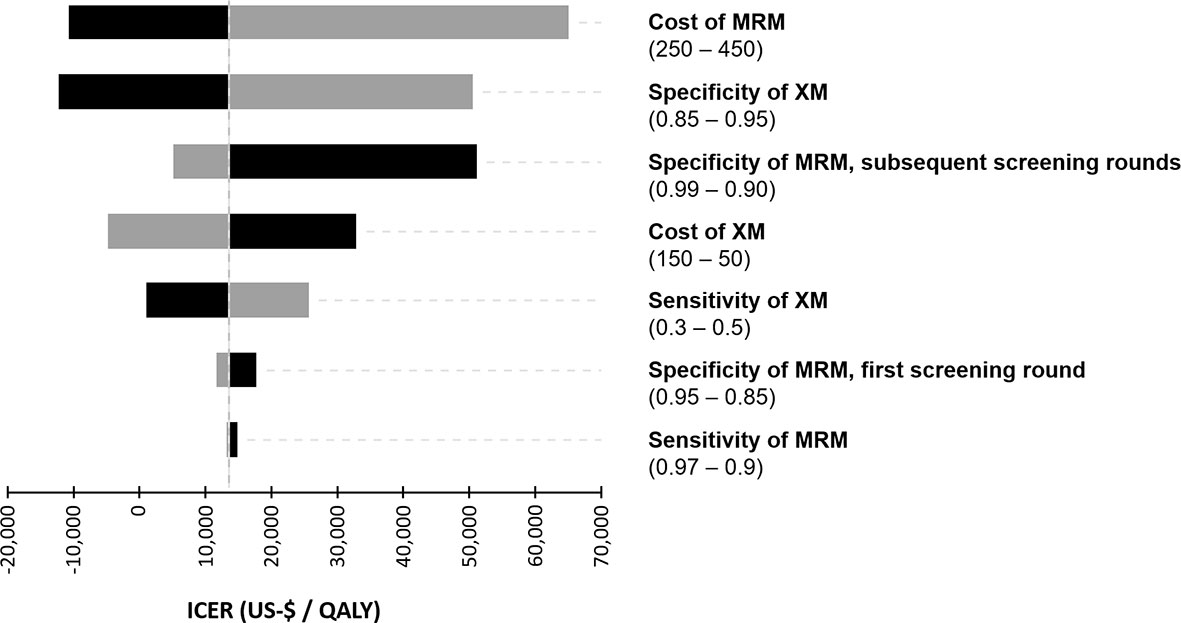
Figure 2 Tornado diagram of the deterministic sensitivity analysis. Costs of the diagnostic procedures (US-$) and the diagnostic performance were varied within a reasonable range to illustrate their impact on the incremental cost-effectiveness ratio (ICER).
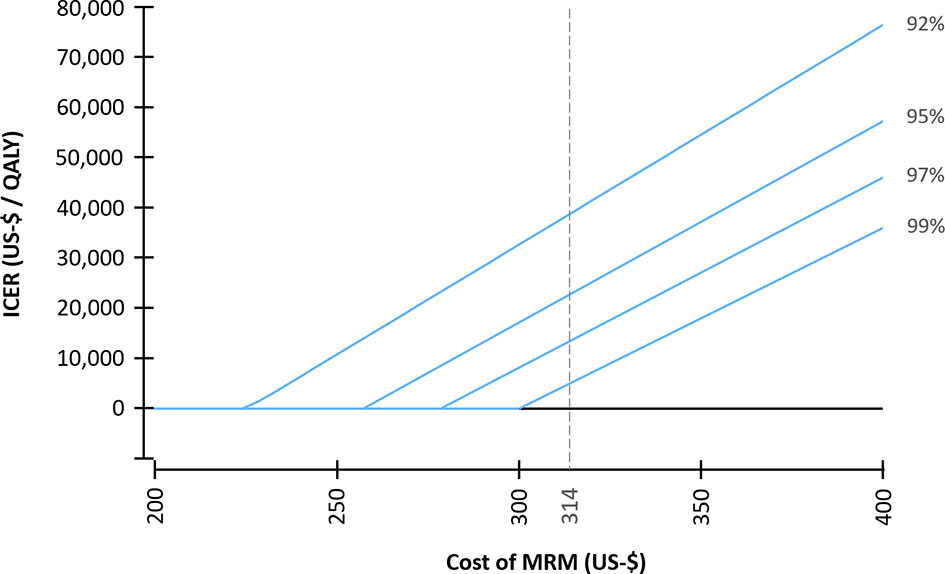
Figure 3 Incremental cost-effectiveness ratio (ICER) for varying costs of MR-mammography (MRM). A specificity of MRM of 92% was selected for the first screening round. For the subsequent screening rounds, varying specificities (92% - 99%) were assumed. An average cost per examination of $ 314 was assumed for MRM in the base case scenario.
A probabilistic sensitivity analysis with 30,000 Monte Carlo iterations was conducted to reflect the uncertainty of the input parameters and a cost-effectiveness acceptability curve was calculated (Figure 4).
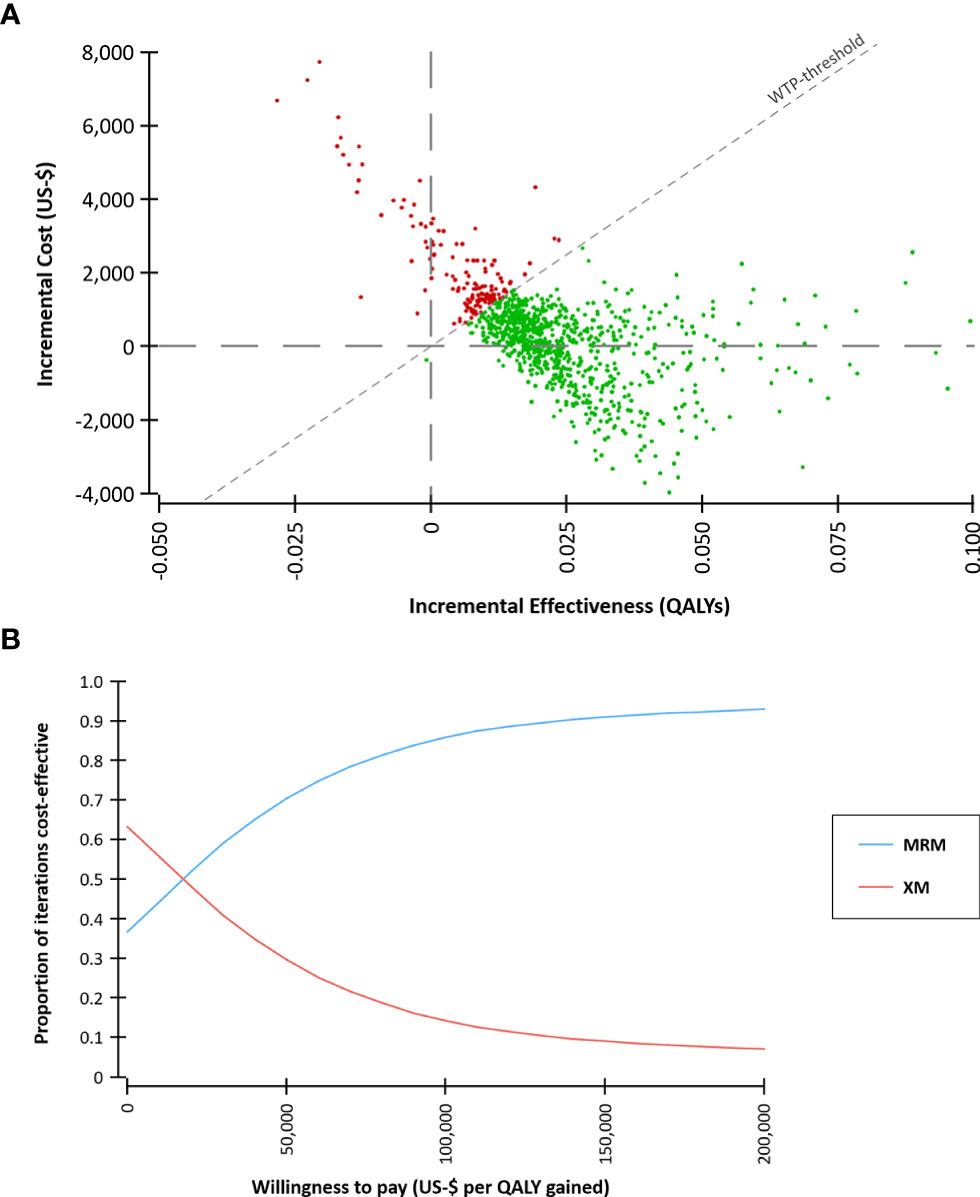
Figure 4 Probabilistic sensitivity analysis based on 30,000 Monte Carlo simulations. (A) Incremental costs and effects comparing MR-mammography (MRM) versus x-ray mammography (XM). A willingness-to-pay (WTP) threshold of $ 100,000 per quality-adjusted life year (QALY) gained was assumed. (B) Cost-effectiveness acceptability curve. At a WTP of $ 100,000 per QALY gained, 86% of the iterations were cost-effective.
In the base case scenario, applying MRM in the screening of women with intermediate risk for breast cancer resulted in more QALYs, i.e. favorable effects, than XM-based screening, but was associated with higher costs. Over the time frame of 20 years, the strategy MRM resulted in average cumulative costs of $ 6,081 per woman and average cumulative effects of 15.12 QALYs, whereas screening with XM resulted in costs of $ 5,810 and 15.10 QALYs (Table 2). The resulting ICER was $ 13,493 per QALY gained.
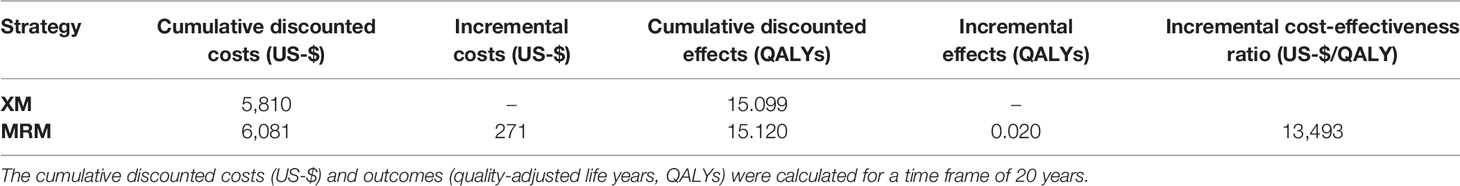
Table 2 Cost-effectiveness analysis of the base-case scenario comparing MR-mammography (MRM) to x-ray mammography (XM).
The specificity of MRM was set to 92% in the first screening round. When varying the specificity for the subsequent screening rounds from 92% to 99%, the ICER resulted in a range from $ 38,849 to $ 5,062 per QALY gained (Table 3).
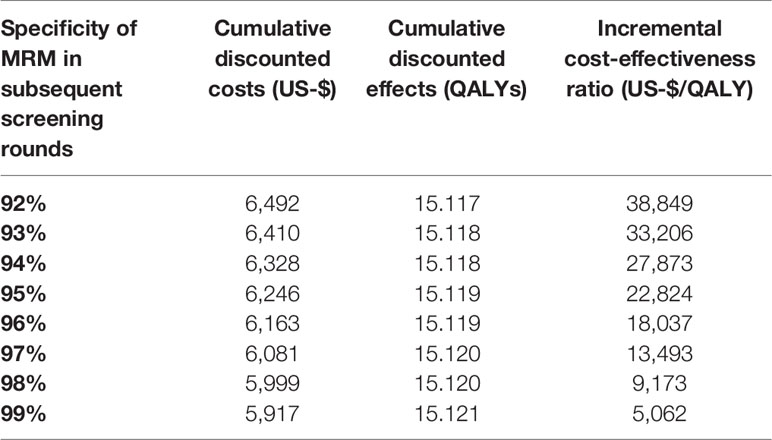
Table 3 Cost-effectiveness of MR-mammography (MRM) for varying specificities of MRM in the second and the following screening rounds, with cumulative costs and effects and the incremental cost-effectiveness ratio compared to x-ray mammography.
In a one-way sensitivity analysis, which is illustrated in a tornado chart (Figure 2), the cost of MRM was identified as the most important driver of cost-effectiveness. The specificity of XM and MRM were also key determinants of the ICER. Interestingly, the sensitivity of MRM did not show a significant impact on cost-effectiveness in the chosen model.
Therefore, the impact of the cost of MRM and the specificity of MRM in subsequent screening rounds on the ICER was further investigated in a deterministic sensitivity analysis (Figure 3). Assuming a specificity in subsequent screening rounds of 99%, a cost per examination of MRM of $ 296 resulted in equal costs of the MRM- and XM-strategy (ICER = 0); whereas for a specificity of 92%, a cost of MRM of $ 224 resulted in equal costs of both strategies.
In the Monte Carlo simulation, the majority of iterations were below a willingness-to-pay threshold of $ 100,000 per QALY gained (Figure 4). 37% of the iterations were characterized by lower costs of the MRM-strategy compared to the XM-strategy. This indicates that screening with MRM might be less costly than with XM in a part of the screening collective. The cost-effectiveness acceptability curve demonstrates the stability of the findings across a range of WTP-thresholds. At a WTP of $ 100,000 per QALY gained, 86% of the iterations were cost-effective.
The new data by Veenhuizen et al. for the first time allow for a cost-effectiveness analysis addressing the diagnostic shifts between screening rounds (5).
In our study, we were able to reconfirm the cost-effectiveness of MRM as a screening tool for patients of intermediate risk for breast cancer, considering the shift in diagnostic performance over the course of two screening rounds.
In the second screening round of the DENSE trial (incidence round), a decreased cancer detection rate was observed due to filtering effects of the first round (prevalence round) while all newly detected breast cancer cases were in an early stage (T1). Thus, the balance of the costs of screening vs. the number of detected cases of breast cancer could be shown to be impaired in the second screening round and the cost per detected case of breast cancer was higher than in the first screening round. On the contrary, improvements in specificity, reduced false positive findings and hence smaller follow-up costs counteract these effects. An improved specificity as well as reduced rate of false positive findings apparently outweighed the decreased cancer detection rate in follow-up examinations. Our results identified ICER levels of around $ 13,493 per QALY gained (respective ICER $ 38,849 per QALY in the first screening round).
Naturally, modelling the cumulative average costs and effects as conducted in this economic evaluation does not allow for a differentiation of these effects.
The reasons for the increased specificity may present a matter of scientific discussion. However, Veenhuizen et al. stressed the role of prior imaging from preceding screening rounds and their possible comparison in order to reduce false positive findings.
Decreased false positive findings in subsequent screening rounds offer the potential to reduce adverse effects of screening, e.g. unnecessary biopsies, and to further improve the cost-effectiveness of screening of women with intermediate risk of breast cancer.
The results of our study are generally in line with recent cost-effectiveness evaluations examining breast cancer screening with various imaging techniques. We have previously indicated an economical benefit of MRM in screening women at intermediate risk in westernized countries with their respective WTP levels, i.e. their accepted monetary thresholds for gains in quality-adjusted life years (6, 13). Compared to DBT, abbreviated breast MRI was cost-effective in screening women at intermediate risk, with an ICER below $ 20,807 per QALY gained (13).
Markov models allow for a simplified simulation of successive disease states and associated costs, but can never accurately reflect any manifestation of clinical reality. For reasons of transparency and comparability of the findings, the U.S. healthcare system perspective was selected for this analysis, which enables comparisons to prior studies but may not be transferable to different contexts.
ICER-levels may be impacted slightly by the evolution of our Markov models over time, leading to some distortion of the effects. However, the authors believe that the development of the model contributes to a more realistic simulation of economic outcomes.
So far, only data on the first two screening rounds have been published from the prospective multi-centre trials on MR-based screening (3–5). Data on the long-term diagnostic performance, interval cancer rates and effects on mortality have been unavailable so far. Therefore, diagnostic performance parameters of the second screening round were extrapolated to subsequent screening rounds in our study.
In conclusion, updating our economic modelling by recently reported data on diagnostic performance beyond the first screening round, the superior cost-effectiveness of MRM in screening women with dense breast tissue for breast cancer could be reconfirmed. Improved specificity and reduced false positive findings in subsequent screening rounds appear to have more impact on cost-effectiveness than reduced cancer detection rates.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
FT, PB, MF, and CK contributed to conception and design of the study. FT and CK performed the economic modelling. MF, MS, WK, and JRu contributed to input data collection. FT and CK wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sardanelli F, Boetes C, Borisch B, Decker T, Federico M, Gilbert FJ. Magnetic Resonance Imaging of the Breast: Recommendations From the EUSOMA Working Group. Eur J Cancer Oxf Engl (2010) 46(8):1296–316. doi: 10.1016/j.ejca.2010.02.015
2. Sardanelli F, Aase HS, Álvarez M, Azavedo E, Baarslag HJ, Balleyguier C. Position Paper on Screening for Breast Cancer by the European Society of Breast Imaging (EUSOBI) and 30 National Breast Radiology Bodies From Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Israel, Lithuania, Moldova, the Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Spain, Sweden, Switzerland and Turkey. Eur Radiol (2017) 27(7):2737–43. doi: 10.1007/s00330-016-4612-z
3. Comstock CE, Gatsonis C, Newstead GM, Snyder BS, Gareen IF, Bergin JT. Comparison of Abbreviated Breast MRI vs Digital Breast Tomosynthesis for Breast Cancer Detection Among Women With Dense Breasts Undergoing Screening. JAMA (2020) 323(8):746. doi: 10.1001/jama.2020.0572
4. Bakker MF, de Lange SV, Pijnappel RM, Mann RM, Peeters PHM, Monninkhof EM. Supplemental MRI Screening for Women With Extremely Dense Breast Tissue. N Engl J Med (2019) 381(22):2091–102. doi: 10.1056/NEJMoa1903986
5. Veenhuizen SGA, de Lange SV, Bakker MF, Pijnappel RM, Mann RM, Monninkhof EM. Supplemental Breast MRI for Women With Extremely Dense Breasts: Results of the Second Screening Round of the DENSE Trial. Radiology (2021) 299(2):276–86. doi: 10.1148/radiol.2021203633
6. Kaiser CG, Dietzel M, Vag T, Froelich MF. Cost-Effectiveness of MR-Mammography vs. Conventional Mammography in Screening Patients at Intermediate Risk of Breast Cancer - a Model-Based Economic Evaluation. Eur J Radiol (2020) 136:109355. doi: 10.1016/j.ejrad.2020.109355
7. Dutch Parliament Supports MRI Screening for Dense Breasts [Internet] . Available at: https://www.auntminnieeurope.com/index.aspx?sec=ser&sub=def&pag=dis&ItemID=619806.
8. Kadom N, Itri JN, Trofimova A, Otero HJ, Horný M. Cost-Effectiveness Analysis: An Overview of Key Concepts, Recommendations, Controversies, and Pitfalls. Acad Radiol (2019) 26(4):534–41. doi: 10.1016/j.acra.2018.10.014
9. Iragorri N, Spackman E. Assessing the Value of Screening Tools: Reviewing the Challenges and Opportunities of Cost-Effectiveness Analysis. Public Health Rev (2018) 39:17. doi: 10.1186/s40985-018-0093-8
10. Plevritis SK, Kurian AW, Sigal BM, Daniel BL, Ikeda DM, Stockdale FE. Cost-Effectiveness of Screening BRCA1/2 Mutation Carriers With Breast Magnetic Resonance Imaging. JAMA (2006) 295(20):2374–84. doi: 10.1001/jama.295.20.2374
11. Taneja C, Edelsberg J, Weycker D, Guo A, Oster G, Weinreb J. Cost Effectiveness of Breast Cancer Screening With Contrast-Enhanced MRI in High-Risk Women. J Am Coll Radiol (2009) 6(3):171–9. doi: 10.1016/j.jacr.2008.10.003
12. Kaiser CG, Dietzel M, Vag T, Rübenthaler J, Froelich MF, Tollens F. Impact of Specificity on Cost-Effectiveness of Screening Women at High Risk of Breast Cancer With Magnetic Resonance Imaging, Mammography and Ultrasound. Eur J Radiol (2021) 137:109576. doi: 10.1016/j.ejrad.2021.109576
13. Tollens F, Baltzer PAT, Dietzel M, Rübenthaler J, Froelich MF, Kaiser CG. Cost-Effectiveness of Digital Breast Tomosynthesis vs. Abbreviated Breast MRI for Screening Women With Intermediate Risk of Breast Cancer-How Low-Cost Must MRI be? Cancers (2021) 13(6):1241. doi: 10.3390/cancers13061241
14. Lee CH, Dershaw DD, Kopans D, Evans P, Monsees B, Monticciolo D. Breast Cancer Screening With Imaging: Recommendations From the Society of Breast Imaging and the ACR on the Use of Mammography, Breast MRI, Breast Ultrasound, and Other Technologies for the Detection of Clinically Occult Breast Cancer. J Am Coll Radiol (2010) 7(1):18–27. doi: 10.1016/j.jacr.2009.09.022
15. Surveillance, Epidemiology, and End Results (SEER) Program. Seer*Stat Database: Incidence - Seer Research Data. Registries, Nov 2020 Sub (1975-2018) - Linked To County Attributes - Time Dependent (1990-2018) Income/Rurality, 1969-2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2021, based on the November 2020 submission.
16. Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-Effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA (2016) 316(10):1093–103. doi: 10.1001/jama.2016.12195
17. Sardanelli F, Podo F, Santoro F, Manoukian S, Bergonzi S, Trecate G. Multicenter Surveillance of Women at High Genetic Breast Cancer Risk Using Mammography, Ultrasonography, and Contrast-Enhanced Magnetic Resonance Imaging (the High Breast Cancer Risk Italian 1 Study): Final Results. Invest Radiol (2011) 46(2):94–105. doi: 10.1097/RLI.0b013e3181f3fcdf
18. Leach MO, Boggis CRM, Dixon AK, Easton DF, Eeles RA, Evans DGR. Screening With Magnetic Resonance Imaging and Mammography of a UK Population at High Familial Risk of Breast Cancer: A Prospective Multicentre Cohort Study (MARIBS). Lancet Lond Engl (2005) 365(9473):1769–78. doi: 10.1016/S0140-6736(05)66481-1
19. Kuhl C, Weigel S, Schrading S, Arand B, Bieling H, König R. Prospective Multicenter Cohort Study to Refine Management Recommendations for Women at Elevated Familial Risk of Breast Cancer: The EVA Trial. J Clin Oncol (2010) 28(9):1450–7. doi: 10.1200/JCO.2009.23.0839
20. Lehman CD, Lee JM, DeMartini WB, Hippe DS, Rendi MH, Kalish G. Screening MRI in Women With a Personal History of Breast Cancer. J Natl Cancer Inst (2016) 108(3):djv349. doi: 10.1093/jnci/djv349
21. Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R. Mammography, Breast Ultrasound, and Magnetic Resonance Imaging for Surveillance of Women at High Familial Risk for Breast Cancer. J Clin Oncol Off J Am Soc Clin Oncol (2005) 23(33):8469–76. doi: 10.1200/JCO.2004.00.4960
22. Pisano ED, Hendrick RE, Yaffe MJ, Baum JK, Acharyya S, Cormack JB. Diagnostic Accuracy of Digital Versus Film Mammography: Exploratory Analysis of Selected Population Subgroups in DMIST. Radiology (2008) 246(2):376–83. doi: 10.1148/radiol.2461070200
23. Blumen H, Fitch K, Polkus V. Comparison of Treatment Costs for Breast Cancer, by Tumor Stage and Type of Service. Am Health Drug Benefits. Februar (2016) 9(1):23–32.
24. Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR. Reliability and Validity of the Functional Assessment of Cancer Therapy-Breast Quality-of-Life Instrument. J Clin Oncol Off J Am Soc Clin Oncol (1997) 15(3):974–86. doi: 10.1200/JCO.1997.15.3.974
25. Ahern CH, Shih Y-CT, Dong W, Parmigiani G, Shen Y. Cost-Effectiveness of Alternative Strategies for Integrating MRI Into Breast Cancer Screening for Women at High Risk. Br J Cancer (2014) 111(8):1542–51. doi: 10.1038/bjc.2014.458
26. Polsky D, Mandelblatt JS, Weeks JC, Venditti L, Hwang Y-T, Glick HA. Economic Evaluation of Breast Cancer Treatment: Considering the Value of Patient Choice. J Clin Oncol (2003) 21(6):1139–46. doi: 10.1200/JCO.2003.03.126
27. Arias E. United States Life Tables, 2017. Natl Vital Stat Rep Cent Dis Control Prev Natl Cent Health Stat Natl Vital Stat Syst (2019) 68(7):1–66.
28. Wishart GC, Azzato EM, Greenberg DC, Rashbass J, Kearins O, Lawrence G. PREDICT: A New UK Prognostic Model That Predicts Survival Following Surgery for Invasive Breast Cancer. Breast Cancer Res BCR (2010) 12(1):R1. doi: 10.1186/bcr2464
29. Lombardi A, Pastore E, Maggi S, Stanzani G, Vitale V, Romano C. Positive Margins (R1) Risk Factors in Breast Cancer Conservative Surgery. Breast Cancer Targets Ther (2019) 11:243–8. doi: 10.2147/BCTT.S210788
30. Heil J, Rauch G, Szabo AZ, Garcia-Etienne CA, Golatta M, Domschke C. Breast Cancer Mastectomy Trends Between 2006 and 2010: Association With Magnetic Resonance Imaging, Immediate Breast Reconstruction, and Hospital Volume. Ann Surg Oncol (2013) 20(12):3839–46. doi: 10.1245/s10434-013-3097-0
31. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement. Value Health J Int Soc Pharmacoeconomics Outcomes Res (2013) 16(2):e1–5. doi: 10.1016/j.jval.2013.02.010
32. Woods B, Revill P, Sculpher M, Claxton K. Country-Level Cost-Effectiveness Thresholds: Initial Estimates and the Need for Further Research. Value Health (2016) 19(8):929–35. doi: 10.1016/j.jval.2016.02.017
Keywords: breast MRI, MR-mammography, breast cancer, intermediate-risk screening, cost-effectiveness analyses, cost-effectiveness threshold
Citation: Tollens F, Baltzer PAT, Dietzel M, Schnitzer ML, Kunz WG, Rink J, Rübenthaler J, Froelich MF and Kaiser CG (2021) Cost-Effectiveness of MR-Mammography in Breast Cancer Screening of Women With Extremely Dense Breasts After Two Rounds of Screening. Front. Oncol. 11:724543. doi: 10.3389/fonc.2021.724543
Received: 13 June 2021; Accepted: 13 August 2021;
Published: 09 September 2021.
Edited by:
Michael Gnant, Medical University of Vienna, AustriaReviewed by:
Michael Fuchsjaeger, Medical University of Graz, AustriaCopyright © 2021 Tollens, Baltzer, Dietzel, Schnitzer, Kunz, Rink, Rübenthaler, Froelich and Kaiser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clemens G. Kaiser, Y2xlbWVucy5rYWlzZXJAdW1tLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.