
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Oncol., 08 November 2021
Sec. Hematologic Malignancies
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.720704
This article is part of the Research TopicInsights in Hematologic Malignancies: 2021View all 28 articles
 Susan M. O’Brien1*
Susan M. O’Brien1* Jennifer R. Brown2
Jennifer R. Brown2 John C. Byrd3
John C. Byrd3 Richard R. Furman4
Richard R. Furman4 Paolo Ghia5
Paolo Ghia5 Jeff P. Sharman6
Jeff P. Sharman6 William G. Wierda7
William G. Wierda7Bruton tyrosine kinase (BTK) inhibitors represent an important therapeutic advancement for B cell malignancies. Ibrutinib, the first-in-class BTK inhibitor, is approved by the US FDA to treat patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), and mantle cell lymphoma (MCL; after ≥1 prior therapy); and by the European Medicines Agency (EMA) for adult patients with relapsed/refractory (R/R) MCL and patients with CLL. Ibrutinib treatment can be limited by adverse events (AEs) including atrial fibrillation, arthralgias, rash, diarrhea, and bleeding events, leading to drug discontinuation in 4%–26% of patients. Acalabrutinib, a second-generation BTK inhibitor, is approved by the FDA to treat adult patients with CLL/SLL or MCL (relapsed after 1 prior therapy); and by the EMA to treat adult patients with CLL or R/R MCL. The most common AE associated with acalabrutinib is headache of limited duration, which occurs in 22%–51% of patients, and is mainly grade 1–2 in severity, with only 1% of patients experiencing grade ≥3 headache. Furthermore, acalabrutinib is associated with a low incidence of atrial fibrillation. Zanubrutinib, a selective next-generation covalent BTK inhibitor, is approved by the FDA to treat adult patients with MCL who have received ≥1 prior therapy, and is under investigation for the treatment of patients with CLL. In the phase 3 SEQUOIA trial in patients with CLL, the most common grade ≥3 AEs were neutropenia/neutrophil count decreased and infections. This review provides an overview of BTK inhibitor-related AEs in patients with CLL, and strategies for their management.
Ibrutinib, the first-in-class Bruton tyrosine kinase (BTK) inhibitor, is approved by the US Food and Drug Administration (FDA) for the treatment of patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL) after at least 1 prior therapy, Waldenstrom’s macroglobulinemia, marginal zone lymphoma in patients who have received at least 1 prior anti-CD20-based therapy, and chronic graft-versus-host disease after failure of at least 1 systemic therapy (1); and by the European Medicines Agency (EMA) for adult patients with relapsed or refractory (R/R) MCL, and patients with CLL (2). Ibrutinib consistently demonstrated benefit in clinical trials, with improved progression-free survival (PFS) and overall survival (OS) in patients with CLL/SLL and R/R MCL, compared to outcomes with conventional therapies (3, 4). However, ibrutinib treatment can be limited by adverse events (AEs) including atrial fibrillation, arthralgias, rash, diarrhea, and bleeding events (3, 5, 6) leading to drug discontinuation in 4% to 26% of patients (3, 6).
Acalabrutinib is a second-generation BTK inhibitor approved by the US FDA for the treatment of patients with CLL/SLL, and patients with MCL who have received at least 1 prior therapy (7), and by the EMA for patients with CLL (8). Zanubrutinib is a selective next-generation covalent BTK inhibitor approved by the FDA for the treatment of adult patients with MCL who have received at least 1 prior therapy (9); it is under investigation for the treatment of patients with CLL (10, 11). This review will discuss the mechanisms of BTK inhibition, review AEs associated with ibrutinib therapy, together with their possible underlying molecular basis, and AEs reported with acalabrutinib therapy in patients with CLL, including recommendations for the management of acalabrutinib-related AEs. Acalabrutinib-associated AEs will be described using data from the phase 3 ASCEND (12) and ELEVATE-TN (13) trials, long-term safety data from the phase 2 ACE-CL-001 study in patients with CLL who were either treatment naïve or who became R/R (14, 15), and from reports of the most common acalabrutinib-related AEs observed in clinical practice. AEs associated with the use of zanubrutinib in the treatment of patients with treatment-naïve or R/R CLL will also be presented (10, 11). In addition, we will discuss AE management strategies based on the authors’ experiences.
Targeting key signaling proteins responsible for driving cancer cell growth and differentiation has provided a revolution in cancer drug development. The B cell receptor (BCR)-signaling pathway is fundamental to CLL cell growth and survival; hence, antagonists have proven highly effective for the treatment of patients with CLL (16). In normal B cells, BCR ligation first induces activation, proliferation, and expansion. Certain B cells generate plasma cells, while others become anergic or undergo apoptosis (17, 18). Following antigen stimulation and activation, certain B cells become memory B cells, and stop proliferation and differentiation (18). When these memory B cells subsequently re-encounter the same antigen, they are activated and proliferate and differentiate into plasma cells (18). In CLL, there is chronic stimulation through the BCR-signaling pathway leading to proliferation, propagation, and increased prosurvival signals, contributing to expansion and prolonged survival of clonal B cells (19). BTK is an important downstream protein in the BCR-signaling pathway, and plays a major role in immune regulation, as indicated by the severe immunodeficiency occurring in patients with X-linked agammaglobulinemia (XLA) who do not express BTK due to the presence of a mutation in this gene (20–22). BTK is uniformly overexpressed at the transcriptional level and constitutively phosphorylated in patients with CLL (19).
BTK is not only a signaling component downstream of the BCR-signaling pathway but it is also involved in additional signaling pathways (i.e., chemokine receptor [e.g., CXCR4], Toll-like receptor [TLR], and activating Fcγ receptor signaling [e.g., FcγRI]) (23). Upon chemokine binding to the extracellular domain of G-protein-coupled chemokine receptors, a conformation change leads to dissociation of the Gα and Gβγ subunits, which independently activate phosphoinositide 3-kinase (PI3K), leading to activation of BTK, protein kinase B (AKT), and mitogen-activated protein kinase (MAPK)-dependent pathways (23). Following ligand recognition, TLRs recruit several proteins (e.g., myeloid differentiation primary response 88 [MYD88], interleukin-1-receptor associated kinase 1 [IRAK1], and TIR-domain-containing adaptor protein [TIRAP]/MyD88 adapter-like [MAL]) (23). These interact with BTK to induce nuclear factor-ĸB (NFĸB) activation, leading to activation, proliferation, antibody secretion, and proinflammatory cytokine production in B cells (23). Following FcγRI cross-linking, Src-kinases, SYK, PI3K-γ, and BTK are activated, while inhibitory Fc-receptors (e.g., FcγRIIB) recruit phosphatases and reduce BTK activation (23). However, it is not clear if BTK inhibition interferes with the signaling of these various receptors.
All covalently binding BTK inhibitors currently in clinical use irreversibly bind to the cysteine at position 481 (CYS-481), blocking the ATP binding pocket of BTK, preventing autophosphorylation at tyrosine residue 223 and full BTK activation (24). Ibrutinib is a highly potent, irreversible BTK inhibitor, which is rapidly absorbed following oral administration, with a pharmacodynamic profile that is maintained over a 24-hour period (25). Ibrutinib also irreversibly binds other kinases possessing an analogous cysteine with varying affinity (interleukin-2-inducible T-cell kinase [ITK] and tyrosine kinase expressed in hepatocellular carcinoma [TEC]-family kinases), thereby potentially disrupting normal T-cell, macrophage, and platelet function (15, 24, 26, 27). These off-target effects may influence the AE profile associated with ibrutinib therapy (26) (Figure 1); bleeding is attributed to effects on BTK and TEC; rash and diarrhea are possibly related to effects on epidermal growth factor receptor (EGFR), while a molecular target leading to the development of atrial fibrillation has been shown to be the C-terminal Src kinase (CSK) (28) (Figure 1).
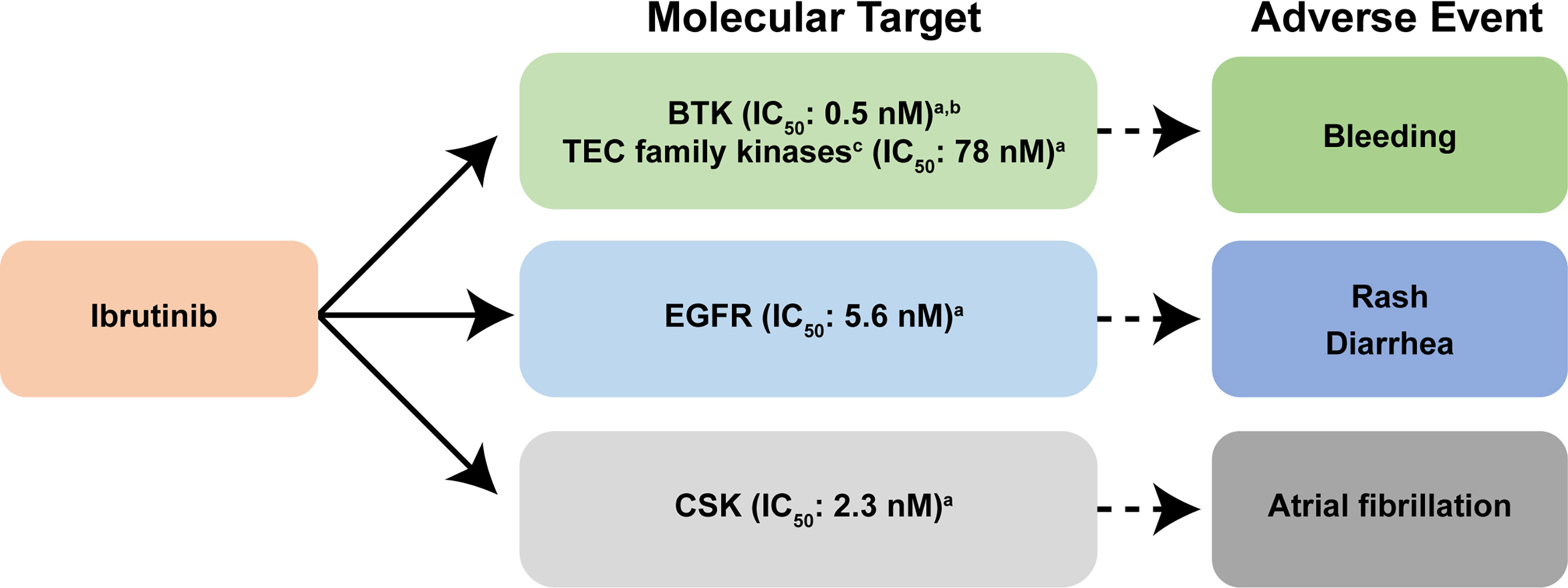
Figure 1 Reported molecular targets of ibrutinib and their associated adverse events. In addition to covalently binding to BTK, ibrutinib also targets other cellular processes regulated by other kinases including EGFR, and TEC family kinases, thereby disrupting normal T-lymphocyte, macrophage, and platelet function (15, 24, 26). Ibrutinib also inhibits other enzymes that contain cysteine residues that are homologous to CYS-481 present within BTK (27). Collectively, these additional off-target effects on cellular process are thought to influence the adverse event profile associated with ibrutinib therapy (26). Bleeding is attributed to effects on BTK and TEC family proteins; rash and diarrhea are related to effects on EGFR; and the molecular target leading to the development of atrial fibrillation is CSK (28). aData are from reference (29). bData are from reference (30). cKinases that contain a cysteine residue that aligns with CYS-481 are present in BTK (29). BTK, Bruton tyrosine kinase; CSK, C-terminal Src kinase; EGFR, epidermal growth factor receptor; IC50, the half maximal inhibitory concentration; TEC, tyrosine kinase expressed in hepatocellular carcinoma.
Based on the toxicity profile observed with ibrutinib, more selective second-generation BTK inhibitors were developed for the treatment of hematological malignancies (7, 24). Acalabrutinib is a highly selective, potent, second-generation BTK inhibitor, with reduced off-target activity (12, 15), rapid absorption, and a short pharmacokinetic half-life (15). An advantage of a short half-life is that there is no lasting impact upon noncovalently bound enzymes. Furthermore, acalabrutinib has an extended pharmacodynamic response (15), such that in patients with B cell malignancies, a 100-mg dose of acalabrutinib every 12 hours led to a mean steady-state BTK occupancy of ≥95% in peripheral blood cells, which was maintained over the 12-hour period, thereby ensuring BTK inhibition during the entire dosing period (7). Acalabrutinib, and its major active metabolite, ACP-5862, form a covalent bond with the CYS-481 residue in the active site of BTK, thereby irreversibly inhibiting BTK enzymatic activity (7, 31). In preclinical studies, acalabrutinib inhibited BTK-mediated activation of downstream signaling proteins; and in mouse xenograft models, acalabrutinib inhibited malignant B cell proliferation and tumor growth (7). Further, acalabrutinib does not inhibit Src-family kinases, and demonstrated less inhibition of TEC kinases compared with that observed with ibrutinib, and displayed no in vitro activity against EGFR or ERBB2 (27) (Table 1), consistent with a low frequency of skin rash and diarrhea (27). However, exposure of acalabrutinib is decreased in the presence of proton-pump inhibitors (PPIs), with a 43% reduction in the area under the concentration-time curve (AUC) observed with coadministration of 40 mg omeprazole for 5 days (7). Consequently, patients are advised to avoid coadministration of acalabrutinib with PPIs (7).
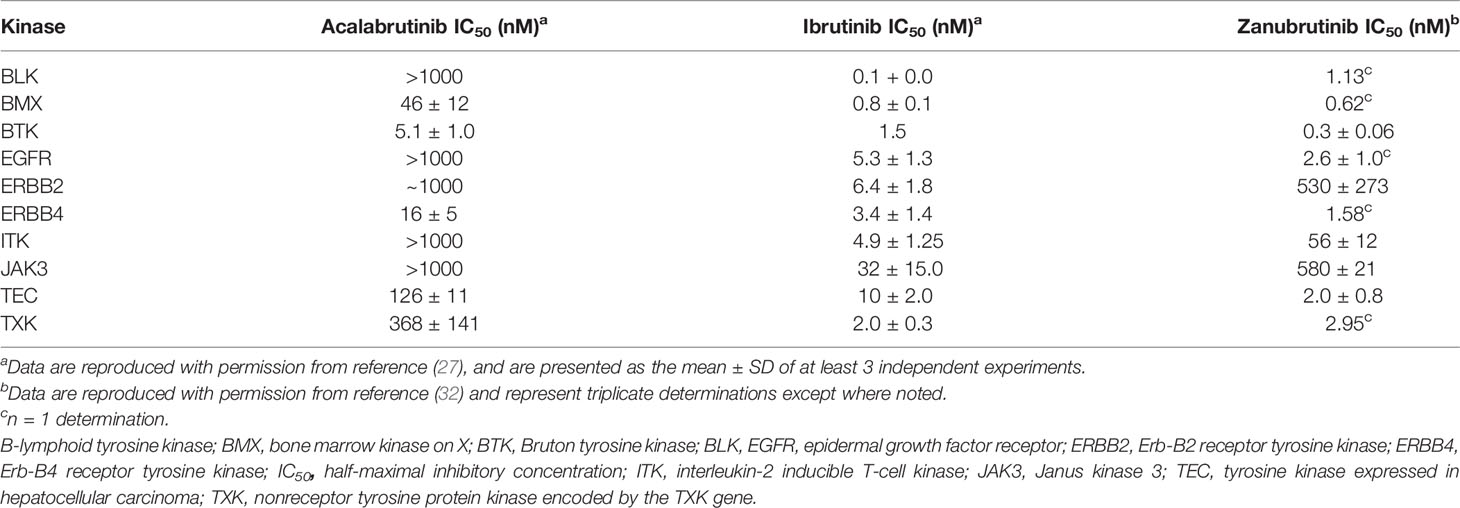
Table 1 A comparison of half maximal inhibitory concentrations of BTK and members of the TEC protein kinase family by acalabrutinib, ibrutinib, and zanubrutinib.
The mean steady-state BTK occupancy by zanubrutinib in peripheral blood was maintained at 100% over 24 hours at a dose of 320 mg once daily in patients with B cell malignancies (9). In vitro half-maximal inhibitory concentration (IC50) values for zanubrutinib for off-target kinases and BTK are presented in Table 1 (32). The median Tmax of zanubrutinib is 2 hours, and mean half-life is 2–4 hours following a single oral dose of 160 mg or 320 mg. No clinically significant differences in the pharmacokinetics of zanubrutinib were observed when coadministered with PPIs (9).
The efficacy and safety of ibrutinib was evaluated in 3 pivotal phase 3 trials: RESONATE (5), RESONATE-2 (3), and ILLUMINATE (33).
RESONATE (PCYC-1112), a multicenter, open-label, phase 3 study in patients with R/R CLL, compared the efficacy and safety of ibrutinib versus the anti-CD20 antibody, ofatumumab (5). The median age of patients was 67 years (range, 30–86) (5). At a median follow-up point of 9.4 months, the most common AEs of any grade occurring in >20% of patients following ibrutinib monotherapy were diarrhea (48%), fatigue (28%), nausea (26%), pyrexia (24%), anemia (23%), and neutropenia (22%) (5). The most common grade ≥3 AEs occurring in ≥5% of patients were neutropenia (16%), pneumonia (7%), thrombocytopenia (6%), and anemia (5%) (5). In addition, atrial fibrillation (any grade) was noted in 5% of ibrutinib-treated patients; and grade ≥3 atrial fibrillation occurred in 3% of ibrutinib-treated patients (5). A subdural hematoma was noted in 1 patient as an AE of interest following ibrutinib monotherapy (5).
RESONATE-2 (PCYC-1115-CA) was a multicenter, open-label, randomized, phase 3 study to evaluate the efficacy and safety of ibrutinib compared with chlorambucil in treatment-naïve patients with CLL who were ≥65 years of age (3). The median age of patients in the ibrutinib-treated group was 73 years (range, 65–89) (3). At a median follow-up point of 18.4 months, the most common AEs of any grade were consistent with those observed in RESONATE (diarrhea, 42%; fatigue, 30%; cough, 22%; and nausea, 22%) (3). Grade ≥3 AEs were neutropenia (10%), anemia (6%), hypertension (4%), pneumonia (4%), and diarrhea (4%) (3). Atrial fibrillation was noted in 6% of patients in the ibrutinib-treated group (grade 2, n = 6; grade 3, n = 2) (3).
ILLUMINATE, a multicenter, randomized, open-label, phase 3 study, evaluated the efficacy and safety of ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in treatment-naïve patients with CLL/SLL, aged either ≥65 or <65 years (33). Overall median age was 71 years (range, 66–76), and 81% of the patients in the ibrutinib plus obinutuzumab group were ≥65 years of age (33). After a median follow-up duration of 31.3 months, the most common grade ≥3 treatment-emergent AEs occurring in ≥5% of patients in the ibrutinib plus obinutuzumab group were neutropenia (36%), thrombocytopenia (19%), pneumonia (7%), and atrial fibrillation (5%) (33). Serious ibrutinib-related AEs occurred in 27% of patients in the ibrutinib plus obinutuzumab group; these AEs were pneumonia (n=5), atrial fibrillation (n=5), and febrile neutropenia (n=4) (33). An ibrutinib treatment-related death was reported in 1 patient in the ibrutinib plus obinutuzumab group (33).
The efficacy and safety of acalabrutinib in patients with CLL were investigated in 2 pivotal phase 3 studies: ELEVATE-TN (13) and ASCEND (12).
ELEVATE-TN was a randomized, multicenter, open-label, controlled study that compared the efficacy and safety of acalabrutinib monotherapy, acalabrutinib plus obinutuzumab, and obinutuzumab plus chlorambucil (1:1:1) in treatment-naïve patients with CLL (13). The median age across treatment groups was 70 years (range, 66–75); 84% of patients were aged ≥65 years (13). After a median follow-up of 28.3 months, the most common AEs of any grade observed for acalabrutinib monotherapy included headache (37%), diarrhea (35%), fatigue (18%), cough (18%), upper respiratory tract infection (18%), arthralgia (16%), and contusion (15%) (13). AEs of grade ≥3 severity that occurred with acalabrutinib monotherapy included neutropenia (10%), anemia (7%), thrombocytopenia (3%), urinary tract infection (2%), pneumonia (2%), dyspnea (2%), headache (1%), fatigue (1%), and back pain (1%) (13). In patients who received acalabrutinib monotherapy, predefined events of clinical interest included atrial fibrillation (4%), any-grade hypertension (5%), grade ≥3 hypertension (2%), and bleeding events (any grade: 39%; grade 1–2: 37%); the most common of which were contusion (15%) and petechiae (9%) (13). Grade ≥3 bleeding events occurred in 2% of patients (13). In total, 9% (16/179) of patients discontinued acalabrutinib treatment because of AEs (e.g., acute myocardial infarction, brain injury, cardiac failure, and fatigue, n=1 each) (13).
The incidence of grade ≥3 AEs in patients receiving acalabrutinib plus obinutuzumab was 70% compared with 50% for patients receiving acalabrutinib monotherapy (13). Grade ≥3 neutropenia was 3 times more frequent in patients treated with acalabrutinib plus obinutuzumab (30%) compared with patients treated with acalabrutinib monotherapy (10%) (13). Grade 4–5 neutropenia occurred in 20% of patients receiving acalabrutinib plus obinutuzumab compared with 6% of patients receiving acalabrutinib monotherapy (13). In addition, grade ≥3 thrombocytopenia occurred in 8% of patients receiving acalabrutinib plus obinutuzumab compared with 3% of those receiving acalabrutinib monotherapy; and grade ≥3 pneumonia occurred in 6% of patients receiving acalabrutinib plus obinutuzumab compared with 2% in patients who received acalabrutinib monotherapy (13). Predefined events of clinical interest in the acalabrutinib plus obinutuzumab group included atrial fibrillation and grade ≥3 hypertension (3% each) (13).
ASCEND, a randomized phase 3 trial, compared the efficacy and safety of acalabrutinib monotherapy versus intestigator’s choice of idelalisib plus rituximab or bendamustine plus rituximab in patients with R/R CLL (12). The median age across treatment groups was 67 years (range, 32–90); 22% of patients in the acalabrutinib group were aged ≥75 years (12). In patients who received acalabrutinib monotherapy, the most common AEs of any grade were headache (22%), neutropenia (19%), diarrhea (18%), cough (15%), upper respiratory tract infection and anemia (14% each), after a median follow-up of 16.1 months (12). In total, 82% of patients had ≥1 treatment-emergent AE of clinical interest, which included infection (57%), atrial fibrillation (5%), hepatotoxicity (5%), hypertension (3%), and major bleeding (2%) (12). Additionally, 19% (30/155) of patients discontinued treatment, 11% (17/155) were ascribed to an AE (e.g., alanine aminotransferase level increased, headache, respiratory tract infection, n=1 each) (12).
Recently, mature results from the phase 2 ACE-CL-001 study provided the longest duration safety follow-up data for acalabrutinib in treatment-naïve and R/R patients with CLL with a median follow-up of 53 months and 41 months, respectively (14, 15). The median age of the patients with treatment-naïve CLL in this study was 64 years (range, 33–85) (14); and 66 years (range, 42–85) for patients with R/R CLL (20% of whom were aged ≥75 years) (15). In treatment-naïve patients, the most common AEs of any grade were diarrhea (51%), headache (45%), upper respiratory tract infection (44%), arthralgia (42%), and contusion (42%) (14). Any-grade and grade ≥3 severity AEs of clinical interest included infection (84% and 15%, respectively), bleeding event (66%/3%), and hypertension (22%/11%) (14). Atrial fibrillation (all grades) occurred in 5% of treatment-naïve patients (14). Serious AEs were reported in 38% of treatment-naïve patients, including pneumonia (n=4) and sepsis (n=3) (14). In total, 6% of patients discontinued due to an AE (14). No new long-term safety issues were reported in treatment-naïve patients (14). In patients with R/R CLL, most AEs were mild or moderate and included: diarrhea (all grades: 52%; grade 1: 31%; grade 2: 16%; grade 3: 5%), headache (all grades: 51%; grade 1: 44%; grade 2; 7%), upper respiratory tract infection (all grades: 37%; grade 1: 10%; grade 2: 26%; grade 3: 1%), and fatigue (all grades: 31%; grade 1: 17%; grade 2: 13%; grade 3: 3%) (15). Treatment discontinuation occurred in 11% of patients due to AEs, including infections that occurred early during treatment (15). No patients with R/R CLL discontinued acalabrutinib treatment because of atrial fibrillation, hypertension, or bleeding events (15).
The phase 3 head-to-head comparison to evaluate the efficacy and safety of acalabrutinib versus ibrutinib in previously treated patients with high-risk CLL was recently completed (NCT02477696; ELEVATE-RR) (34). Patient median age was 66 years (range, 28–89) (34). The results demonstrated that acalabrutinib met the primary efficacy endpoint with noninferior PFS compared to ibrutinib in previously treated patients with high-risk CLL after a median follow-up period of 40.9 months (range, 0.0–59.1) (34). The key secondary endpoint for safety was also met, with a statistically significant lower incidence of atrial fibrillation with acalabrutinib compared to that seen with ibrutinib (9.4% vs 16.0%, P = 0.02) (34). Acalabrutinib treatment was also associated with a lower incidence of any-grade hypertension compared with ibrutinib (8.6% vs 22.8%), as well as incidences of arthralgia (15.8% vs 22.8%) and diarrhea (34.6% vs 46.0%), but there was a higher incidence of headache (34.6% vs 20.2%) and cough (28.9% vs 21.3%) (34). Overall, AEs leading to discontinuation were numerically lower in acalabrutinib-treated patients compared with ibrutinib-treated patients (14.7% vs 21.3%) (34).
In a phase 2 single-arm study, zanubrutinib was generally well tolerated by Chinese patients with R/R CLL/SLL (median age, 61 years [range, 35–87]), after a median follow-up period of 15.1 months (range, 0.8 to 21.1) (11). The most common grade ≥3 AEs were neutropenia (44%), thrombocytopenia (15.4%), lung infection/pneumonia (13.2%), upper respiratory infection (9.9%), and anemia (8.8%) (11). Eight (9%) patients discontinued zanubrutinib treatment due to AEs, and 7 (8%) patients required ≥1 dose reduction (11).
The SEQUOIA trial (NCT03336333), an open-label, global, multicenter, phase 3 study, included a nonrandomized cohort of treatment-naïve patients with del(17p) CLL/SLL who were treated with zanubrutinib 160 mg twice daily (10). Patient median age was 70 years (range, 42–86) (10). After a median follow-up period of 18.2 months (range, 5.0–26.3 months), AEs (of any grade; reported in ≥10% of treated patients) were contusion (20.2%), upper respiratory tract infection (19.3%), neutropenia/neutrophil count decreased (17.4%), diarrhea (16.5%), nausea (14.7%), constipation (13.8%), rash (13.8%), back pain (12.8%), cough (11.9%), arthralgia (11.0%), and fatigue (10.1%) (10). Grade ≥3 AEs occurring in ≥2% of patients were neutropenia/neutrophil count decreased (12.8%) and pneumonia (3.7%) (10). AEs of interest (any grade) reported in ≥10% of patients were infections (64.2%; grade ≥3, 13.8%), minor bleeding (26.6%), bruising (24.8%), neutropenia (18.3%; grade ≥3, 13.8%), diarrhea (15.6%; grade ≥3, 0.9%), nausea (13.8%), arthralgia (11.0%), and fatigue (10.1%; grade ≥3, 0.9%) (10). Dermatological malignancies were reported in 9.2% of patients, and nonskin second malignancies were reported in 4.6% of patients (10). Atrial fibrillation was reported in 3 (2.8%) patients, 2 events of which occurred in the setting of sepsis (10). Four (3.7%) patients discontinued zanubrutinib due to AEs (i.e., pneumonia, sepsis secondary to pseudomonas, melanoma, and acute renal failure [associated with disease progression]), and 2 of these patients died (10). No sudden deaths or deaths of unknown cause were reported (10).
The ALPINE trial was an open-label, global, randomized, phase 3 study that compared zanubrutinib versus ibrutinib treatment in patients with R/R CLL/SLL (NCT03734016) (35, 36). Data from a pre planned interim analysis for the first 12 months after randomization of the first 415 patients was recently reported (35, 36). Patients ≥65 years of age comprised 62.3% of the zanubrutinib-treated group and 61.5% of the ibrutinib-treated group (35). With a median follow-up period of 15 months, grade ≥3 AEs occurred in 55.9% of patients in the zanubrutinib group compared with 51.2% of patients in the ibrutinib group (37). Atrial fibrillation/flutter, a prespecified safety endpoint, was observed at lower rates in patients in the zanubrutinib group versus the ibrutinib group (2.5% vs 10.1%, respectively, P = 0.0014) (35). Rates of other AEs that were lower in the zanubrutinib group versus the ibrutinib group included major bleeding (2.9% vs 3.9%), cardiac disorders of any grade (13.7% vs 25.1%) or grade ≥3 (2.5% vs 6.8%), and AEs leading to discontinuation (7.8% vs 13.0%) or death (3.9% vs 5.8%), respectively (35, 37). The rate of neutropenia was higher with zanubrutinib versus ibrutinib treatment (28.4% vs 21.7%, respectively); however, grade ≥3 infections were lower with zanubrutinib than with ibrutinib (12.7% vs 17.9%, respectively) (35). Because this is an interim analysis, additional data are needed before these results can be fully evaluated.
Headache, occurring in 22% to 51% of patients, is the most common AE experienced by patients receiving acalabrutinib therapy (12, 13, 15). Acalabrutinib treatment-related headaches usually occur early in the course of treatment, are mild, and of limited duration (38). These headaches typically occur within 30 minutes of dosing and in many cases do not need medical intervention, or can be effectively managed with acetaminophen or caffeine, while avoiding the use of nonsteroidal anti-inflammatory drugs (NSAIDs), if possible (Table 2) (39). Only 1% of headaches lead to treatment discontinuation (12). In clinical practice, patient education prior to initiation of therapy (i.e., advising that acalabrutinib-induced headaches are easily managed and should abate over a period of up to 4 weeks) helps to reassure the patient that it is not a long-term consequence. The mechanism(s) for these headaches is unclear, but could include calcitonin gene-related peptide (CGRP) agonism, which is of interest given the new class of migraine medications designed to work by antagonizing CGRP (42).
Atrial fibrillation has been reported in 6%–10% of untreated patients with CLL (43–45). Furthermore, the prevalence of atrial fibrillation increases with age (46). In clinical trials, the incidence rate of atrial fibrillation with ibrutinib treatment, after a 9-month follow-up, was 3% (5), and 6% after a median follow-up of 18 months (3); the 2-year incidence rate—based on randomized and observational studies—was estimated at between 10%–16% (44, 47). With a follow-up ranging from 14 to 28 months in clinical trials, the incidence rate of atrial fibrillation with acalabrutinib was approximately 0%–5% (12, 13, 48), with a time to onset of atrial fibrillation in the range of 23 days to >1–3 years after treatment initiation (15). The slope is slightly higher in the first 6 months, but is then stable, perhaps suggesting minimal risk associated with drug initiation. Monitoring for atrial fibrillation while receiving acalabrutinib therapy is indicated (7). Appropriate management of atrial fibrillation, such as the administration of direct oral anticoagulants, is recommended without necessarily withholding acalabrutinib, and discontinuation should be considered if the atrial fibrillation is not medically controllable (Table 2) (39).
Grade ≥3 hypertension was reported in 38% of patients treated with ibrutinib, including 18% in patients who did not have previous hypertension at baseline (49). In contrast, grade ≥3 hypertension was reported in 2%–7% of patients treated with acalabrutinib, with a median follow-up ranging from 14 to 28 months (12, 13, 48). Further, the updated results from the long-term follow-up (41 months) of the ACE-CL-001 phase 2 study, reported that 18% of patients experienced hypertension (all grades), 10% of which were grade 1–2, and 7% were grade ≥3 (15). In patients treated with ibrutinib, hypertension (all grades) was observed in 14% of patients; 4% of which were grade 3, after a median follow-up of 18 months (3). Patients should be monitored for treatment-emergent hypertension, which should be managed with antihypertensive medication (Table 2). It should be noted that antihypertensive medication dosages may need to be adjusted once any BTK inhibitor therapy is discontinued.
Although a moderate incidence of diarrhea was reported with acalabrutinib treatment in clinical trials and in a long-term follow-up study (18%–52%) (12, 13, 15), it appeared not to be drug-related. Diarrhea usually resolves quickly without the need for further intervention. If intervention is required, patients can be simply treated with an antidiarrheal medication (e.g., loperamide) (Table 2) (40).
Thrombocytopenia is frequently observed in patients with unfavorable biological risk factors for CLL, and is commonly caused by splenomegaly, bone marrow failure secondary to tumor infiltration, recent chemotherapy, or megakaryocyte dysplasia (50). In patients with CLL who were treated with acalabrutinib monotherapy, thrombocytopenia of any grade and grade ≥3 were reported in 7%–11% and 3%–4% of patients, respectively (12, 13). In patients with R/R CLL who were treated with zanubrutinib, all-grade and grade ≥3 thrombocytopenia was reported in 42% and 15% of patients, respectively (11). Thrombocytopenia (all grades) was reported in 6% of patients with treatment-naïve CLL who were treated with zanubrutinib, with 1% of cases of grade ≥3 severity (10). Dose interruptions are recommended for the first to third occurrences of grade 3 or 4 thrombocytopenia, and dose discontinuation is recommended for the fourth occurrence, unless thrombocytopenia is related to CLL infiltration of the marrow (Table 2) (7, 9).
Neutropenia is also commonly observed in patients treated with BTK inhibitors due to an on-target toxicity effect (11). Between 10%–16% of patients treated with acalabrutinib monotherapy developed grade ≥3 neutropenia, although this number can be higher with combination therapies (e.g., 30% of patients experienced grade ≥3 neutropenia when treated with acalabrutinib plus obinutuzumab) (12, 13, 26). When treated with ibrutinib, 10% of patients experienced grade ≥3 neutropenia (3); and 44% of patients treated with zanubrutinib experienced grade ≥3 neutropenia (11). As with thrombocytopenia, dose interruptions are recommended for the first to third occurrences of grade 3 or 4 neutropenia, and dose discontinuation is recommended after a fourth occurrence (Table 2) (7, 9).
Infections were common in patients with CLL who were treated with zanubrutinib; 39% of patients with R/R CLL reported ≥1 grade ≥3 infection (11), and 64% of patients with treatment-naive CLL reported an infection of any grade, 13.8% of which were grade ≥3 (10). Most were respiratory tract infections and were effectively managed without the need for a dose reduction or treatment discontinuation (11). Grade ≥3 infections occurred at similar rates in patients receiving acalabrutinib monotherapy (14%) (13). However, 17.9% of ibrutinib-treated patients developed grade ≥3 infections (35). Prophylactic treatment should be considered for patients at a higher risk of developing opportunistic infections, and all patients should be monitored for signs and symptoms of infection and treated promptly (Table 2) (1, 7, 9).
Monitoring for signs of bleeding is important in patients receiving acalabrutinib therapy (7). Data suggest that BTK inhibitors increase the risk of bleeding by impairing collagen-induced platelet activation, akin to the effects of aspirin (51, 52). Inhibition of Src-kinases is suggested to be associated with bleeding (52). Acalabrutinib has less inhibitory potential with respect to Src-family kinases compared with that seen with ibrutinib (Table 1) (27). Preliminary studies suggest that BTK and TEC kinases have overlapping roles in platelets, which would explain why patients with XLA do not demonstrate a bleeding phenotype (53, 54). In ibrutinib-treated and acalabrutinib-treated patients, BTK and TEC kinases are both irreversibly inhibited (52). The lower affinity of acalabrutinib for TEC kinase (53) may preserve some platelet activity. Further, differences in bleeding events may be related to a greater selectivity of acalabrutinib for BTK over TEC compared to ibrutinib (54). Minor bleeding tends to be less evident, and patients treated with acalabrutinib report fewer minor bleeding occurrences. There is also evidence that major bleeding events are rare in patients treated with acalabrutinib (54). However, in our experience, the risk for a major bleeding event is equal for both acalabrutinib and ibrutinib. The risk of bleeding in acalabrutinib-treated patients can be mitigated as for those treated with ibrutinib. Further, in patients with treatment-naive CLL, grade 1/2 minor bleeding was observed in 27% of patients, and grade ≥3 major bleeding was observed in 5% of patients—2 of whom experienced a grade 3 major bleeding event following a surgical procedure for which there was no per protocol dose hold (10). Minor bleeding events were relatively common in patients with R/R CLL treated with zanubrutinib: 1% of patients experienced grade 1-2 major bleeding, and 1% of patients experienced grade ≥3 major bleeding (gastrointestinal hemorrhage following diagnosis of colon cancer, and posttraumatic right-thalamic hemorrhage) (11). Patients treated with warfarin were excluded early from the ibrutinib studies after complications, and, as such, coadministration of warfarin and acalabrutinib has not been studied. Jones and colleagues assessed the use of anticoagulant or antiplatelet agents and bleeding events in patients with CLL who were treated with ibrutinib monotherapy in 2 multicenter studies (55). In the PCYC-1102 study (median follow-up of 22 months), 9% of patients who received ibrutinib treatment plus an anti-coagulant agent and 4% who received ibrutinib plus an antiplatelet agent reported a major bleeding event (55). In the RESONATE study with a median follow-up of 10 months, 2% and 1% of patients, respectively, reported a major bleeding event (55). Major bleeding events in these patients were typically observed in conjunction with other factors (i.e., coexisting medical conditions, concurrent medications) (55). Therefore, the risk-versus-benefit profile for coadministration of a BTK inhibitor with antiplatelet or anticoagulant therapy should be carefully considered, and the patient should be monitored for signs of bleeding (Table 2) (7, 39). If anticoagulation therapy is required, we generally prefer to use direct oral anticoagulants (Table 2). In addition, we recommend withholding acalabrutinib administration for 3 to 7 days before and after surgery, depending upon the type of surgery, and the potential risk of a bleeding event (Table 2) (7).
Although musculoskeletal pain, including myalgias and arthralgias, is a less serious AE reported with BTK inhibitor therapy, it can be troublesome for the patient and lead to treatment discontinuation (11, 41). Musculoskeletal pain is one of the most common AEs (i.e., occurring in ≥30% of patients) observed with ibrutinib treatment (1). A retrospective analysis of patients with CLL who received ibrutinib treatment reported that 36% of patients developed new or worsening arthralgias/myalgias, with a median time to occurrence of 34.5 months (41). Musculoskeletal pain, of any grade, was reported in 14% of patients treated with zanubrutinib, with grade ≥3 AEs observed in 3.4% (9), and myalgia was also observed in acalabrutinib-treated patients (all grades, 21%; grade ≥3, 0.8%) (7). Interrupting ibrutinib therapy, as recommended, alleviated symptoms in 14% of patients, while a dose reduction alleviated symptoms in 60% of patients (41). Although avoiding the use of NSAIDs has been recommended because this class of drugs may exacerbate the risk of bleeding, 50% of patients who used NSAIDs reported improvements in arthralgia/myalgia symptoms (41). Additionally, 54% of patients who developed arthralgias/myalgias had spontaneous resolution of their symptoms with no changes to ibrutinib treatment, although most patients had grade 1 arthralgias/myalgias (41). Ultimately 22% of patients with arthralgias/myalgias discontinued ibrutinib treatment; however, this increased to 63% in patients with grade 3 events (41).
Treatment options for patients with CLL have evolved considerably over recent years (39). Small-molecule inhibitor-based therapies have significantly improved PFS outcomes in patients with CLL compared with chemoimmunotherapy outcomes, especially in patients with high-risk disease characteristics (e.g., del(17p) or TP53 mutations) (39, 56, 57). Current National Comprehensive Cancer Network and the European Society for Medical Oncology practice guidelines recommend that first-line treatment for patients with CLL should be based on the presence or absence of del(17p) or mutated TP53, regardless of patient age and comorbidities (39, 58), and preference should be given to small molecules. The shift from chemoimmunotherapy to oral targeted therapy provides patients with CLL/SLL convenient options with fewer toxicities. However, since oral therapy can be administered away from the clinic, there must be vigilant monitoring of safety considerations and thorough adverse event management.
Acalabrutinib is well-tolerated and associated with low rates of treatment discontinuation due to AEs [8, 9, 18], which may provide some advantages in routine practice. However, patients taking proton-pump inhibitors might have impaired absorption of acalabrutinib (7).
Long-term data from the phase 2 ACE-CL-001 study reaffirmed that acalabrutinib monotherapy for patients with treatment-naïve and R/R CLL provided durable responses with a favorable safety profile, with no new safety concerns reported (14, 15). Several clinically relevant AEs considered to be associated with BTK inhibition in the clinical setting appear to be present at a low frequency in patients treated with acalabrutinib. The most common AE associated with the use of acalabrutinib is a headache (12, 13, 26). Headaches are mild, easily managed, and of limited duration, lasting only a few days (39, 59).
Zanubrutinib is associated with an overall favorable safety profile, with a low incidence of major bleeding or arrythmias observed in patients with CLL (60). The National Comprehensive Cancer Network guidelines recommend the use of zanubrutinib as a first-line or second-line therapy for patients with CLL/SLL with del(17p)/TP53 mutations who have a contraindication to other BTK inhibitors, and as second-line and subsequent therapy for patients without del(17p)/TP53 mutations who are intolerant of, or have a contraindication to, other BTK inhibitors (61). However, zanubrutinib is currently approved by the FDA only for the treatment of MCL in patients who have received ≥1 prior therapy (9).
In summary, BTK inhibitors are highly effective options for the treatment of patients with CLL, and selection is driven by patient and physician personal choice, as well as available efficacy and tolerability data from clinical trials and clinician experience. Acalabrutinib is safe and effective and provides an additional FDA-approved option for the treatment of patients with CLL.
All authors contributed to the discussion of the content, reviewed each draft, and approved the final version for submission.
SO has served as a consultant for Amgen, Astellas, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences Inc, Vaniam Group LLC, AbbVie, and Alexion; has received research support from Kite Pharma, Regeneron, and Acerta Pharma; and has been a consultant and received research support from Gilead, Pharmacyclics, TG Therapeutics, Pfizer, and Sunesis. JBr has served as a consultant for AbbVie, Acerta, AstraZeneca, BeiGene, Catapult, Dynamo Therapeutics, Eli Lilly, Juno/Celgene, Kite, MEI Pharma, Nextcea, Novartis, Octapharma, Pfizer, Rigel, Sunesis, TG Therapeutics, and Verastem; received honoraria from Janssen; received research funding from Gilead, Loxo, Sun, and Verastem; and served on data safety monitoring committees (DSMC) for Invectys. JBy reports personal fees from Acerta Pharma (a member of the AstraZeneca Group), Genentech, Janssen, and Pharmacyclics. RF reports consulting fees from AbbVie, Acerta, AstraZeneca, BeiGene, Genentech, Janssen, Loxo Oncology, Morphosys, OncoTarget, Pharmacyclics, Sanofi, Sunesis, TG Therapeutics, and Verastem; DSMC: Incyte; and speaker fees from Janssen. PG reports consulting or advisory fees from AbbVie, BeiGene, Janssen Oncology, Gilead Sciences, Juno Therapeutics, Sunesis Pharmaceuticals, ArQule, Adaptive Biotechnologies, MEI Pharma, and Acerta Pharma/AstraZeneca; and research funding from: AbbVie, Janssen Oncology, Gilead Sciences, and Novartis. JS reports personal fees from AbbVie, Acerta Pharma (a member of the AstraZeneca Group), AstraZeneca, Genentech, Pharmacyclics, Sunesis, and TG Therapeutics. WW has received research funding from AbbVie, Acerta Pharma, Genentech, Gilead, GlaxoSmithKline/Novartis, Janssen, Juno, Kite, and Pharmacyclics.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Medical writing and editorial support, conducted in accordance with Good Publication Practice 3 (GPP3) and the International Committee of Medical Journal Editors (ICMJE) guidelines, were provided by Marie-Louise Ricketts, PhD, of Oxford PharmaGenesis Inc., Newtown, PA, and funded by AstraZeneca, Gaithersburg, MD.
2. Imbruvica® [Summary of Product Characteristics]. Beerse, Belgium: Janssen-Cilag International NV (2021).
3. Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as Initial Therapy for Patients With Chronic Lymphocytic Leukemia. N Engl J Med (2015) 373:2425–37. doi: 10.1056/NEJMoa1509388
4. Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, et al. Ibrutinib Versus Temsirolimus in Patients With Relapsed or Refractory Mantle-Cell Lymphoma: An International, Randomised, Open-Label, Phase 3 Study. Lancet (2016) 387:770–8. doi: 10.1016/S0140-6736(15)00667-4
5. Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib Versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. N Engl J Med (2014) 371:213–23. doi: 10.1056/NEJMoa1400376
6. Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum K, et al. Ibrutinib Treatment for First-Line and Relapsed/Refractory Chronic Lymphocytic Leukemia: Final Analysis of the Pivotal Phase Ib/II PCYC-1102 Study. Clin Cancer Res (2020) 26:3918–27. doi: 10.1158/1078-0432.CCR-19-2856
7. Calquence® (Acalabrutinib) [Prescribing Information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP (2019).
8. AstraZeneca. Calquence Recommended for Approval in the EU by CHMP for Chronic Lymphocytic Leukaemia (2020). Available at: https://www.astrazeneca.com/media-centre/press-releases/2020/calquence-recommended-for-approval-in-the-eu-by-chmp-for-chronic-lymphocytic-leukaemia.html (Accessed May 12, 2021).
9. BrukinsaTM (Zanubrutinib) [Full Prescribing Information]. San Mateo, CA: BeiGene USA, Inc (2019).
10. Tam CS, Robak T, Ghia P, Kahl BS, Walker P, Janowski W, et al. Zanubrutinib Monotherapy for Patients With Treatment Naive Chronic Lymphocytic Leukemia and 17p Deletion. Haematologica (2020) 106:2354–63. doi: 10.3324/haematol.2020.259432
11. Xu W, Yang S, Zhou K, Pan L, Li Z, Zhou J, et al. Treatment of Relapsed/Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma With the BTK Inhibitor Zanubrutinib: Phase 2, Single-Arm, Multicenter Study. J Hematol Oncol (2020) 13:48. doi: 10.1186/s13045-020-00884-4
12. Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, et al. ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. J Clin Oncol (2020) 38:2849–461. doi: 10.1200/JCO.19.03355
13. Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib With or Without Obinutuzumab Versus Chlorambucil and Obinutuzmab for Treatment-Naive Chronic Lymphocytic Leukaemia (ELEVATE TN): A Randomised, Controlled, Phase 3 Trial. Lancet (2020) 395:1278–91. doi: 10.1016/S0140-6736(20)30262-2
14. Byrd JC, Woyach JA, Furman RR, Martin P, O’Brien S, Brown JR, et al. Acalabrutinib in Treatment-Naive Chronic Lymphocytic Leukemia. Blood (2021) 137:3327–38. doi: 10.1182/blood.2020009617
15. Byrd JC, Wierda WG, Schuh A, Devereux S, Chaves JM, Brown JR, et al. Acalabrutinib Monotherapy in Patients With Relapsed/Refractory Chronic Lymphocytic Leukemia: Updated Phase 2 Results. Blood (2020) 135:1204–13. doi: 10.1182/blood.2018884940
16. Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK With Ibrutinib in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med (2013) 369:32–42. doi: 10.1056/NEJMoa1215637
17. Tan C, Hiwa R, Mueller JL, Vykunta V, Hibiya K, Noviski M, et al. NR4A Nuclear Receptors Restrain B Cell Responses to Antigen When Second Signals Are Absent or Limiting. Nat Immunol (2020) 21:1267–79. doi: 10.1038/s41590-020-0765-7
18. Wen Y, Jing Y, Yang L, Kang D, Jiang P, Li N, et al. The Regulators of BCR Signalling During B Cell Activation. Blood Sci (2019) 1:119–29. doi: 10.1097/BS9.0000000000000026
19. Woyach JA, Bojnik E, Ruppert AS, Stefanovski MR, Goettl VM, Smucker KA, et al. Bruton’s Tyrosine Kinase (BTK) Function is Important to the Development and Expansion of Chronic Lymphocytic Leukemia (CLL). Blood (2014) 123:1207–13. doi: 10.1182/blood-2013-07-515361
20. Vetrie D, Vořechovský I, Sideras P, Holland J, Davies A, Flinter F, et al. The Gene Involved in X-Linked Agammaglobulinaemia is a Member of the Src Family of Protein-Tyrosine Kinases. Nature (1993) 361:226–33. doi: 10.1038/361226a0
21. Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, et al. Deficient Expression of a B Cell Cytoplasmic Tyrosine Kinase in Human X-Linked Agammaglobulinemia. Cell (1993) 72:279–90. doi: 10.1016/0092-8674(93)90667-f
22. Ponader S, Burger JA. Bruton’s Tyrosine Kinase: From X-Linked Agammaglobulinemia Toward Targeted Therapy for B-Cell Malignancies. J Clin Oncol (2014) 32:1830–9. doi: 10.1200/JCO.2013.53.1046
23. Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton’s Tyrosine Kinase in B Cells and Malignancies. Mol Cancer (2018) 17:57. doi: 10.1186/s12943-018-0779-z
24. Wu J, Liu C, Tsui ST, Liu D. Second-Generation Inhibitors of Bruton Tyrosine Kinase. J Hematol Oncol (2016) 9:80. doi: 10.1186/s13045-016-0313-y
25. Parmar S, Patel K, Pinilla-Ibarz J. Ibrutinib (Imbruvica): A Novel Targeted Therapy for Chronic Lymphocytic Leukemia. Pharm Ther (2014) 39:483–519.
26. Awan FT, Schuh A, Brown JR, Furman RR, Pagel JM, Hillmen P, et al. Acalabrutinib Monotherapy in Patients With Chronic Lymphocytic Leukemia Who Are Intolerant to Ibrutinib. Blood Adv (2019) 3:1553–62. doi: 10.1182/bloodadvances.2018030007
27. Barf T, Covey T, Izumi R, van de Kar B, Gulrajani M, van Lith B, et al. Acalabrutinib (ACP-196): A Covalent Bruton Tyrosine Kinase Inhibitor With a Differentiated Selectivity and In Vivo Potency Profile. J Pharmacol Exp Ther (2017) 363:240–52. doi: 10.1124/jpet.117.242909
28. Xiao L, Salem JE, Clauss S, Hanley A, Bapat A, Hulsmans M, et al. Ibrutinib-Mediated Atrial Fibrillation Due to Inhibition of CSK. Circulation (2020) 142:2443–55. doi: 10.1161/CIRCULATIONAHA.120.049210
29. Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, et al. The Bruton Tyrosine Kinase Inhibitor PCI-32765 Blocks B-Cell Activation and Is Efficacious in Models of Autoimmune Disease and B-Cell Malignancy. Proc Natl Acad Sci U.S.A. (2010) 107:13075–80. doi: 10.1073/pnas.1004594107
30. Akinleye A, Chen Y, Mukhi N, Song Y, Liu D. Ibrutinib and Novel BTK Inhibitors in Clinical Development. J Hematol Oncol (2013) 6:59. doi: 10.1186/1756-8722-6-59
31. Herman SEM, Montraveta A, Niemann CU, Mora-Jensen H, Gulrajani M, Krantz F, et al. The Bruton Tyrosine Kinase (BTK) Inhibitor Acalabrutinib Demonstrates Potent on-Target Effects and Efficacy in Two Mouse Models of Chronic Lymphocytic Leukemia. Clin Cancer Res (2017) 23:2831–41. doi: 10.1158/1078-0432.CCR-16-0463
32. Guo Y, Liu Y, Hu N, Yu D, Zhou C, Shi G, et al. Discovery of Zanubrutinib (BGB-3111), A Novel, Potent, and Selective Covalent Inhibitor of Bruton’s Tyrosine Kinase. J Med Chem (2019) 62:7923–40. doi: 10.1021/acs.jmedchem.9b00687
33. Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, et al. Ibrutinib Plus Obinutuzumab Versus Chlorambucil Plus Obinutuzumab in First-Line Treatment of Chronic Lymphocytic Leukaemia (iLLUMINATE): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2019) 20:43–56. doi: 10.1016/S1470-2045(18)30788-5
34. Byrd JC, Hillmen P, Ghia P, Kater AP, Chanan-Khan A, Furman RR, et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J Clin Oncol (2021), JCO2101210. doi: 10.1200/JCO.21.01210
35. Hillmen P, Eichhorst B, Brown JR, Lamanna N, O’Brien SM, Tam CS, et al. First Interim Analysis of ALPINE Study: Results of a Phase 3 Randomized Study of Zanubrutinib vs Ibrutinib in Patients With Relapsed/Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Eur Hematol Assoc (2021).
36. Hillmen P, Brown JR, Eichhorst BF, Lamanna N, O’Brien SM, Qiu L, et al. ALPINE: Zanubrutinib Versus Ibrutinib in Relapsed/Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Future Oncol (2020) 16:517–23. doi: 10.2217/fon-2019-0844
37. Helwick C. Zanubrutinib vs Ibrutinib in Relasped/Refractory CLL/SLL: ALPINE Trial (2021). Available at: https://ascopost.com/news/june-2021/zanubrutinib-vs-ibrutinib-in-relapsedrefractory-cllsll-alpine-trial/ (Accessed August 13, 2021).
38. Dolan S, Christofides A, Doucette S, Shafey M. Highlights From ASCO 2020: Updates on the Treatment of Chronic Lymphocytic Leukemia. Curr Oncol (2020) 27:e420–e32. doi: 10.3747/co.27.7009
39. Wierda WG, Byrd JC, Abramson JS, Bilgrami SF, Bociek G, Brander D, et al. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Version 4.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2020) 18:185–217. doi: 10.6004/jnccn.2020.0006
40. Badillo M, Nava D, Rosa M, Chen W, Guerrero M, Wang M. Acalabrutinib: Managing Adverse Events and Improving Adherence in Patients With Mantle Cell Lymphoma. Clin J Oncol Nurs (2020) 24:392–8. doi: 10.1188/20.CJON.392-398
41. Rhodes JM, LoRe VA 3rd, Mato AR, Chong EA, Barrientos JC, Gerson JN, et al. Ibrutinib-Associated Arthralgias/Myalgias in Patients With Chronic Lymphocytic Leukemia: Incidence and Impact on Clinical Outcomes. Clin Lymphoma Myeloma Leuk (2020) 20:438–44 e1. doi: 10.1016/j.clml.2020.02.001
42. Carmine Belin A, Ran C, Edvinsson L. Calcitonin Gene-Related Peptide (CGRP) and Cluster Headache. Brain Sci (2020) 10:30. doi: 10.3390/brainsci10010030
43. Shanafelt TD, Parikh SA, Noseworthy PA, Goede V, Chaffee KG, Bahlo J, et al. Atrial Fibrillation in Patients With Chronic Lymphocytic Leukemia (CLL). Leuk Lymphoma (2017) 58:1630–9. doi: 10.1080/10428194.2016.1257795
44. Baptiste F, Cautela J, Ancedy Y, Resseguier N, Aurran T, Farnault L, et al. High Incidence of Atrial Fibrillation in Patients Treated With Ibrutinib. Open Heart (2019) 6:e001049. doi: 10.1136/openhrt-2019-001049
45. Larsson K, Mattsson M, Ebrahim F, Glimelius I, Hoglund M. High Prevalence and Incidence of Cardiovascular Disease in Chronic Lymphocytic Leukaemia: A Nationwide Population-Based Study. Br J Haematol (2020) 190:e245–e8. doi: 10.1111/bjh.16859
46. Aronow WS, Banach M. Atrial Fibrillation: The New Epidemic of the Ageing World. J Atr Fibrillation (2009) 1:154. doi: 10.4022/jafib.154
47. Ganatra S, Sharma A, Shah S, Chaudhry GM, Martin DT, Neilan TG, et al. Ibrutinib-Associated Atrial Fibrillation. JACC Clin Electrophysiol (2018) 4:1491–500. doi: 10.1016/j.jacep.2018.06.004
48. Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med (2016) 374:323–32. doi: 10.1056/NEJMoa1509981
49. Dickerson T, Wiczer T, Waller A, Philippon J, Porter K, Haddad D, et al. Hypertension and Incident Cardiovascular Events Following Ibrutinib Initiation. Blood (2019) 134:1919–28. doi: 10.1182/blood.2019000840
50. Visco C, Barcellini W, Maura F, Neri A, Cortelezzi A, Rodeghiero F. Autoimmune Cytopenias in Chronic Lymphocytic Leukemia. Am J Hematol (2014) 89:1055–62. doi: 10.1002/ajh.23785
51. Goldmann L, Duan R, Kragh T, Wittmann G, Weber C, Lorenz R, et al. Oral Bruton Tyrosine Kinase Inhibitors Block Activation of the Platelet Fc Receptor CD32a (FcgammaRIIA): A New Option in HIT? Blood Adv (2019) 3:4021–33. doi: 10.1182/bloodadvances.2019000617
52. Series J, Garcia C, Levade M, Viaud J, Sié P, Ysebaert L, et al. Differences and Similarities in the Effects of Ibrutinib and Acalabrutinib on Platelet Functions. Haematologica (2019) 104:2292–9. doi: 10.3324/haematol.2018.207183
53. Chen J, Kinoshita T, Gururaja T, Sukbuntherng J, James D, Lu D, et al. The Effect of Bruton’s Tyrosine Kinase (BTK) Inhibitors on Collagen-Induced Platelet Aggregation, BTK, and Tyrosine Kinase Expressed in Hepatocellular Carcinoma (TEC). Eur J Haematol (2018) 101:604–12. doi: 10.1111/ejh.13148
54. Nicolson PLR, Hughes CE, Watson S, Nock SH, Hardy AT, Watson CN, et al. Inhibition of Btk by Btk-Specific Concentrations of Ibrutinib and Acalabrutinib Delays But Does Not Block Platelet Aggregation Mediated by Glycoprotein VI. Haematologica (2018) 103:2097–108. doi: 10.3324/haematol.2018.193391
55. Jones JA, Hillmen P, Coutre S, Tam C, Furman RR, Barr PM, et al. Use of Anticoagulants and Antiplatelet in Patients With Chronic Lymphocytic Leukaemia Treated With Single-Agent Ibrutinib. Br J Haematol (2017) 178:286–91. doi: 10.1111/bjh.14660
56. Shanafelt TD, Wang V, Kay NE, Hanson CA, O’Brien SM, Barrientos JC, et al. A Randomized Phase III Study of Ibrutinib (PCI-32765)-Based Therapy vs. Standard Fludarabine, Cyclophosphamide, and Rituximab (FCR) Chemoimmunotherapy in Untreated Younger Patients With Chronic Lymphocytic Leukemia (CLL): A Trial of the ECOG-ACRIN Cancer Research Group (E1912) [Abstract]. Blood (2018) 132(Suppl 1):LBA–4. doi: 10.1182/blood-2018-120779
57. Woyach J, Ruppert AS, Perez G, Booth AM, Feldman D, Dib EG, et al. Alliance A041702: A Randomized Phase III Study of Ibrutinib Plus Obinutuzumab Versus Ibrutinib Plus Venetoclax and Obinutuzumab in Untreated Older Patients (≥ 70 Years of Age) With Chronic Lymphocytic Leukemia (CLL) [Abstract]. Blood (2019) 134(Suppl 1):1751. doi: 10.1182/blood-2019-127102
58. Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, et al. Chronic Lymphocytic Leukemia: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2021) 31:23–33. doi: 10.1016/j.annonc.2020.09.019
59. Seymour C. Acalabrutinib Demonstrates Activity in Frontline CLL (2019). Available at: https://www.targetedonc.com/view/acalabrutinib-demonstrates-activity-in-frontline-cll (Accessed May 12, 2021).
60. Rhodes JM, Mato AR. Zanubrutinib (BGB-3111), a Second-Generation Selective Covalent Inhibitor of Bruton’s Tyrosine Kinase and Its Utility in Treating Chronic Lymphocytic Leukemia. Drug Des Devel Ther (2021) 15:919–26. doi: 10.2147/DDDT.S250823
Keywords: acalabrutinib, adverse events, Bruton tyrosine kinase inhibitor, chronic lymphocytic leukemia, ibrutinib
Citation: O’Brien SM, Brown JR, Byrd JC, Furman RR, Ghia P, Sharman JP and Wierda WG (2021) Monitoring and Managing BTK Inhibitor Treatment-Related Adverse Events in Clinical Practice. Front. Oncol. 11:720704. doi: 10.3389/fonc.2021.720704
Received: 04 June 2021; Accepted: 18 October 2021;
Published: 08 November 2021.
Edited by:
Rakesh Verma, Consultant, San Francisco, CA, United StatesReviewed by:
Yi Miao, Nanjing Medical University, ChinaCopyright © 2021 O’Brien, Brown, Byrd, Furman, Ghia, Sharman and Wierda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susan M. O’Brien, b2JyaWVuQHVjaS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.