
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 05 October 2021
Sec. Genitourinary Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.719941
This article is part of the Research Topic Panel of Tumor Markers: Towards Precision Medicine in Genitourinary Cancers View all 27 articles
Background: The pretreatment prognostic nutritional index (PNI) is correlated with poor prognosis in several malignancies. However, the prognostic role of PNI in patients with renal cell carcinoma (RCC) remains unclear. Therefore, we performed a meta-analysis to investigate the prognostic significance of PNI in patients with RCC.
Methods: We searched the PubMed, Web of Science, Embase, Scopus, and Cochrane Library databases up to February 2021. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were used to estimate correlation between PNI and survival endpoints in RCC.
Results: Ten studies with 4,908 patients were included in the meta-analysis. The pooled results indicated that a low PNI associated with poor overall survival (HR = 2.10, 95% CI = 1.67–2.64, p<0.001), shorter progression-free survival, disease-free survival, recurrence-free survival (HR = 1.99, 95% CI = 1.67–2.36, p<0.001), and poor cancer-specific survival (HR = 2.95, 95% CI = 1.61–5.39, p<0.001). Additionally, the prognostic ability of PNI was not affected by subgroup analysis factors.
Conclusion: The meta-analysis indicated that low PNI associated with shorter survival outcomes in patients with RCC. Therefore, PNI could be used as an effective prognostic indicator in RCC.
Renal cell carcinoma (RCC) is the most prevalent form of kidney tumor, accounting for 85% of cases (1). RCC is the most lethal urological malignancy and is responsible for approximately 2%–3% of all adult malignancies (2). Surgical resection, including partial and radical nephrectomy, is a treatment with curative intent in patients with localized RCC (3). A majority of the patients with RCC are diagnosed at the localized stage, but 1/3 patients present with locally advanced or metastatic status (4). Moreover, >25% of patients with localized disease show metastatic progression after the initial treatment. Furthermore, the prognosis of patients with advanced disease is dismal, with a 5-year survival rate of 11% (5). Thus, prognostic scores and parameters would be helpful in determining the survival of patients with RCC (6).
Patients with cancer usually experience malnutrition and changes in immune responses during disease development (7). The prognostic nutritional index (PNI) is evaluated according to serum albumin levels and lymphocyte count in the peripheral blood (8). PNI reflects the nutritional and immunologic status of patients with cancer and is a prognostic factor in several solid tumors (9). Low PNI is associated with poor prognosis in some cancers, such as pancreatic (10), lung (11), esophageal cancer (EC) (12), and ovarian (13) cancers, and nasopharyngeal carcinoma (14). Many studies have also explored the prognostic significance of PNI in patients with RCC; however, the results have been inconsistent (15–24). For example, some studies identified low PNI as a significant prognostic factor for RCC (20, 22), whereas others failed to detect the prognostic role of PNI in RCC (17). Therefore, in this study, we performed a meta-analysis to quantitatively evaluate association between PNI and prognosis in patients with RCC.
The meta-analysis was performed under the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (25). We searched PubMed, Web of Science, Embase, Scopus, and Cochrane Library databases up to February 2021. The search was performed using following terms: “prognostic nutritional index,” “PNI,” “kidney cancer,” “renal cell carcinoma,” “renal tumor,” and “renal neoplasms.” Only studies published in English were considered. Additionally, literature references were manually screened to identify potentially relevant studies. No ethical approval or informed consent was required as all data were based on previously published articles.
Studies fulfilling following features were eligible for the meta-analysis: 1) studies using pathological methods to confirm RCC; 2) studies reporting hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for estimating associations between PNI and survival outcomes or had sufficient data to calculate these statistics; 3) identified a cutoff value to stratify low and high PNI; and 4) were full-text articles. The exclusion criteria were as follows: 1) reviews, case reports, meeting abstracts, letters, and comments; 2) studies with no data of interest for this meta-analysis; 3) animal studies; and 4) non-English studies.
The data were extracted by two independent investigators (C.M. and W.X.), and all disagreements were resolved by discussion with a third researcher (J.Y.). The following information was extracted: name of the first author, country of origin, ethnicity, sample size, age, study duration, metastatic status of disease, Fuhrman grade, histological type, treatment methods, cutoff value of PNI, cutoff value determination methods, survival endpoints, survival analysis types, and HRs with 95% CIs. The primary study outcome was overall survival (OS), and secondary study outcomes were progression-free survival (PFS), disease-free survival (DFS), recurrence-free survival (RFS), and cancer-specific survival (CSS). PFS/DFS/RFS were combined because survival was calculated based on the duration of event-free survival. Moreover, this combination of PFS/DFS/RFS was based on previous studies on PNI (26, 27).
Two researchers (W.M. and C.W.) independently evaluated the methodological quality of eligible studies according to the Newcastle-Ottawa Scale (NOS) (28). The NOS was used to assess the quality based on three aspects: selection of subjects (four stars), comparability of study groups (two stars), and outcome measurement (three stars). The NOS ranged from 0 to 9, and studies with NOS ≥6 were considered high-quality.
Pooled HRs and 95% CIs were used to estimate correlations between PNI and survival endpoints. The heterogeneity among studies was evaluated using Cochran’s Q and I2 statistics. I2>50% and P for heterogeneity <0.10 indicated significant heterogeneity; and a random-effects model (REM) was applied. However, a fixed-effects model (FEM) was adopted otherwise. Subgroup analysis was performed to further investigate the prognostic role of PNI in various patient groups. Sensitivity analysis was performed to explore the impact of each study on the overall pooled results of the meta-analysis. Publication bias was estimated by visual inspection of the Begg’s funnel plot. All statistical analyses were performed using Stata software version 15.0 (STATA Corporation, College Station, TX, USA). A p < 0.05 was considered to be statistically significant.
The process of literature selection and screening is shown in Figure 1. The initial literature search identified 394 records. After removing 84 duplicate records, 310 articles were screened. After reviewing the titles, abstracts, and full texts, 10 studies (15–24) were included in the meta-analysis. The basic characteristics of the included studies are listed in Table 1. The studies were published between 2015 and 2020, and were conducted in five countries, including China (n = 4) (16, 17, 22, 24), Korea (n = 3) (18–20), the USA (n = 1) (15), Austria (n = 1) (21), and Turkey (n = 1) (23). The total sample size was 4,908, ranging from 125 to 1,360 and with a median value of 413. Five studies enrolled patients with non-metastatic disease (15, 18, 19, 21, 24), three studies recruited patients with metastatic disease (16, 20, 23), and two studies enrolled patients with mixed disease stages (17, 22). All studies had a retrospective study design. Nine studies recruited patients with clear cell renal cell carcinoma (ccRCC) and non-clear cell renal cell carcinoma (nccRCC) (15–20, 22–24), and one study included patients with ccRCC (21). Regarding treatment methods, six studies applied partial or radical nephrectomy (15, 17, 19, 21, 22, 24), three studies administered tyrosine kinase inhibitors (TKIs) (16, 20, 23), and one study applied radical nephrectomy (18). The cutoff values for PNI ranged from 38.5 to 51.62, with a median value of 46.31. The NOS of all studies was >6, indicating that all eligible studies were of high quality. The detailed items for the NOS scores are shown in Table 2.
Eight studies (15–18, 20, 22–24) with 4,019 patients investigated the association between low PNI and OS in patients with RCC. The heterogeneity was significant (I2 = 56.9%, P=0.023); therefore, REM was applied. The pooled results had HR = 2.10, 95% CI = 1.67–2.64, p<0.001 (Figure 2 and Table 3). Subgroup analyses were performed according to ethnicity, cutoff value, cutoff value determination method, treatment, and survival analysis type. The REM and FEM were selected according to the heterogeneity in each subgroup. As shown in Figure 2; Supplementary Figure 1 and Table 3, a low PNI was a significant prognostic factor in all subgroups (p<0.05). The results indicated that reduced PNI correlated with poor OS, and that the prognostic role was not influenced by subgroup factors.
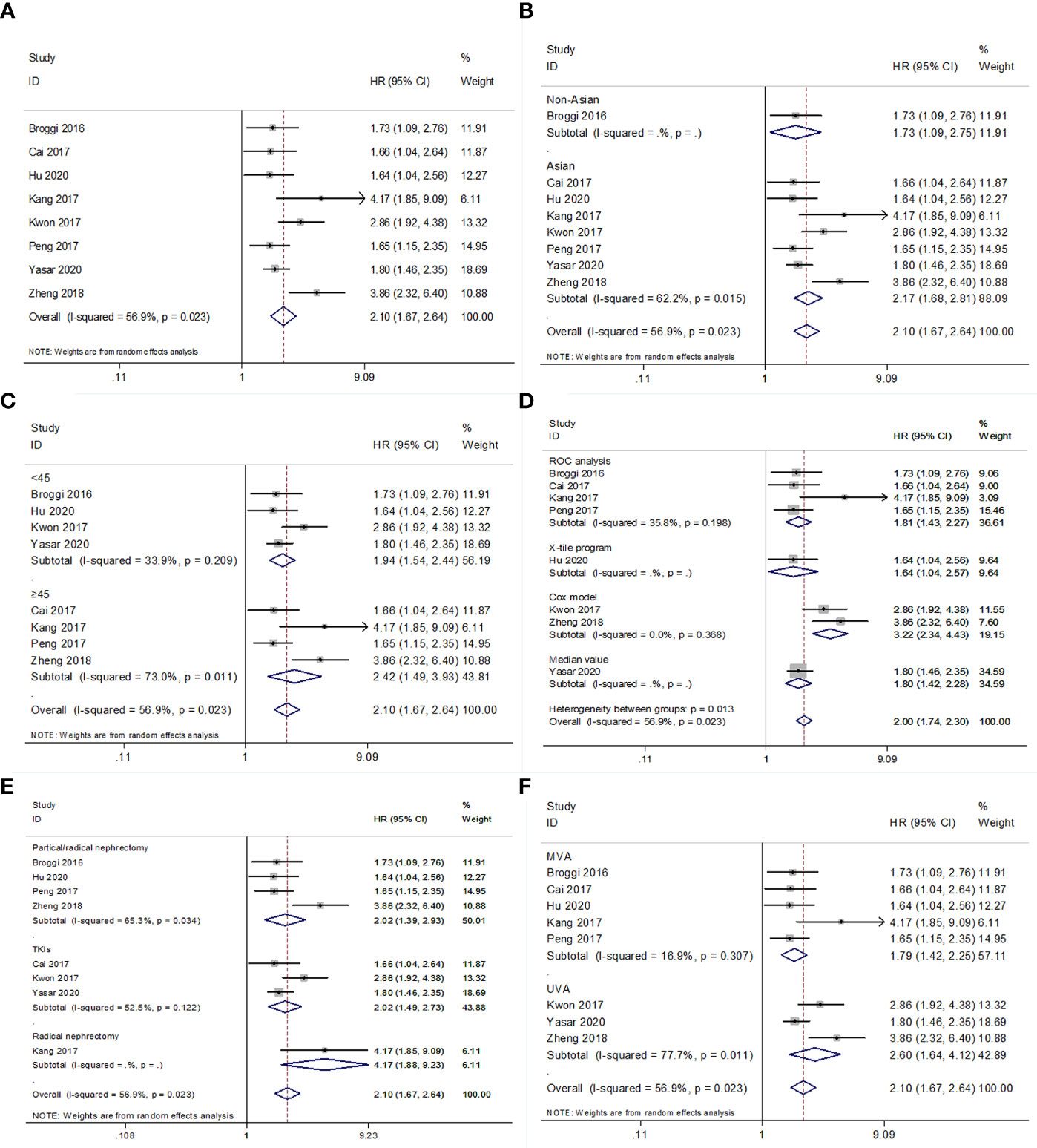
Figure 2 Forest plot examining the association between PNI and OS in patients with RCC. (A) overall patient population; (B) subgroup analysis by various ethnicities; (C) subgroup analysis by various cut-off values of PNI; (D) subgroup analysis by various cut-off value determination methods; (E) subgroup analysis by various treatment methods; (F) subgroup analysis by various survival analysis types.
For PFS/DFS/RFS, the data from seven studies with 3,553 patients were combined (15–17, 19–22). The combined data had HR = 1.99, 95% CI = 1.67–2.36, p<0.001; and an FEM was applied due to non-significant heterogeneity (I2 = 0, P=0.563) (Figure 3 and Table 4). Subgroup analyses were also performed, and the results indicated that low PNI associated with worse PFS/DFS/RFS irrespective of ethnicity, cutoff value, cutoff determination method, treatment, metastatic status, histology, and survival analysis (Figure 3; Supplementary Figure 2 and Table 4).
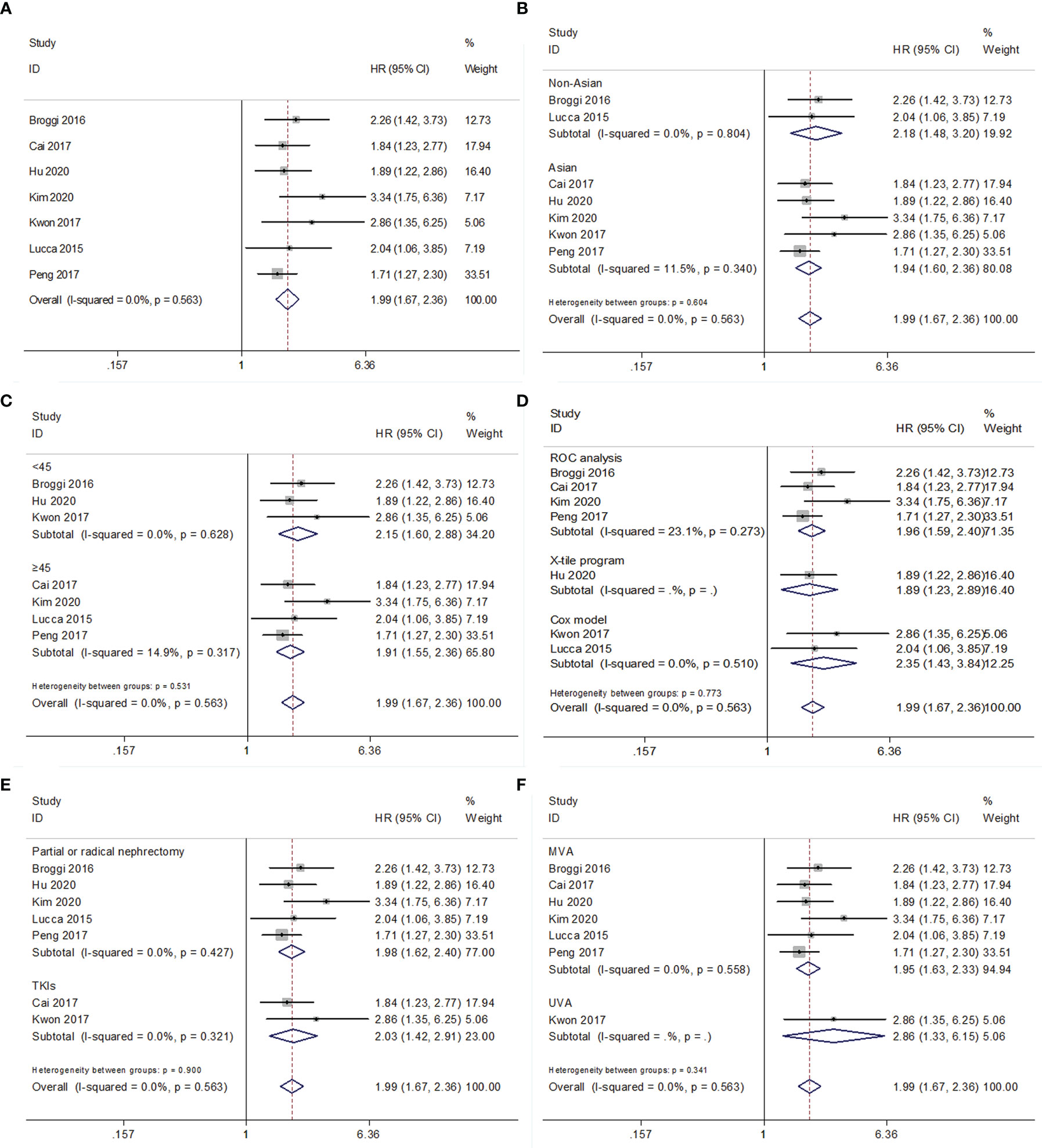
Figure 3 Forest plot examining the association between PNI and PFS/DFS/RFS in patients with RCC. (A) overall patient population; (B) subgroup analysis by various ethnicities; (C) subgroup analysis by various cut-off values of PNI; (D) subgroup analysis by various cut-off value determination methods; (E) subgroup analysis by various treatment methods; (F) subgroup analysis by various survival analysis.
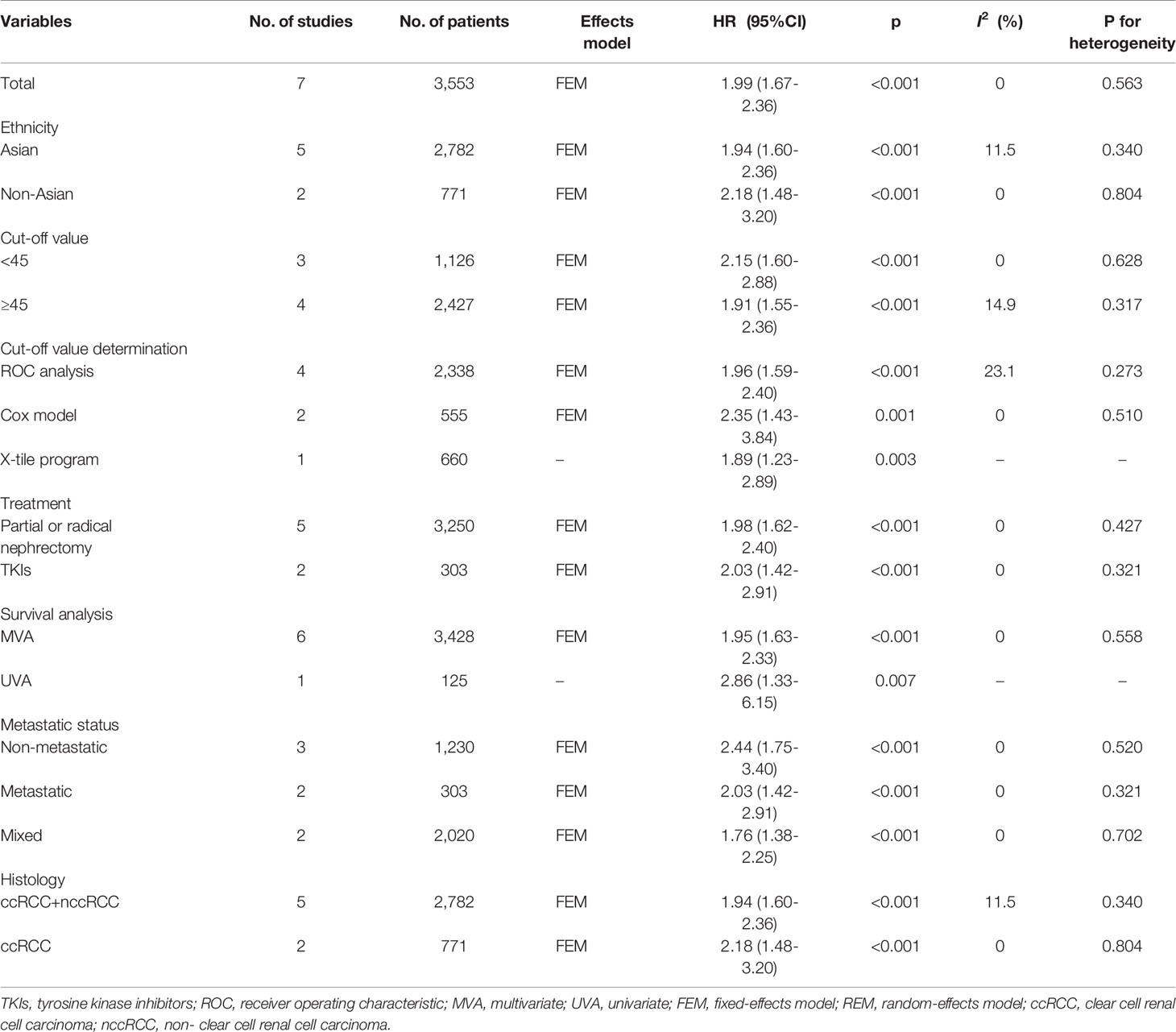
Table 4 Results of subgroup meta-analysis for progression-free survival/disease-free survival/recurrence-free survival.
The association between PNI and CSS was analyzed based on data from four studies comprising 2,078 patients (17–19, 24). The overall results had HR = 2.95, 95% CI = 1.61–5.39, p<0.001 in REM (Figure 4 and Table 5). Subgroup analysis showed that low PNI was a significant prognostic factor for poor CSS when the cutoff value was ≥45, but the prognostic value was invalid with a cutoff value <45 (Figure 4 and Table 5).
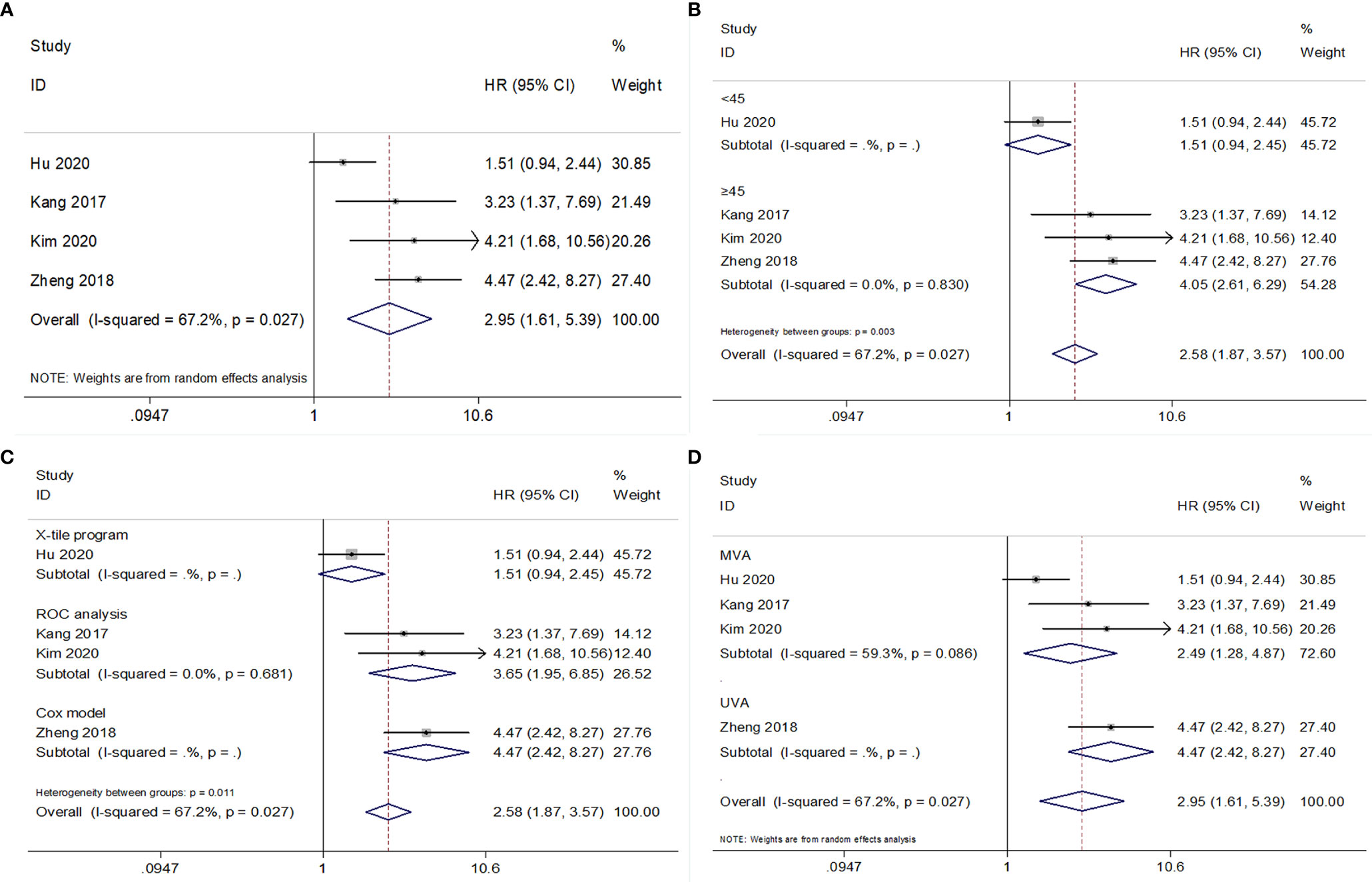
Figure 4 Forest plot examining the association between PNI and CSS in patients with RCC. (A) overall patient population; (B) subgroup analysis by various cut-off values of PNI; (C) subgroup analysis by various cut-off value determination methods; (D) subgroup analysis by various survival analysis types.
We examined the stability and reliability of pooled HRs and 95% CIs for OS, PFS/DFS/RFS, and CSS based on sensitivity. As shown in Figure 5, the conclusions were reliable as the combined data remained substantially unchanged by the removal of any individual study.
Publication bias was tested using the Begg’s test and funnel plots. The Begg’s p values for OS, PFS/DFS/RFS, and CSS were p = 0.063, p = 0.327, and p = 0.734, respectively. Visual inspection of the funnel plots was symmetrical (Figure 6), suggesting that there was no significant publication bias in the meta-analysis.
The pooled analysis of survival data from 10 studies with 4,908 patients showed that reduced PNI associated with poor OS, PFS/DFS/RFS, and CSS in patients with RCC. The results in subgroups stratified by ethnicity, cutoff value, cutoff value determination method, treatment, and survival analysis type were consistent with the overall trend. Sensitivity analysis and publication bias tests confirmed the robustness of the results. Thus, the meta-analysis showed that a low PNI is a significant and reliable prognostic parameter in patients with RCC. PNI could be applied as a promising indicator for survival prediction in RCC.
The PNI is calculated as follows: serum albumin (g/L) + 0.005 × lymphocyte count (per mm3) (29). PNI is a parameter that combines the nutritional and inflammatory statuses of patients. The mechanisms of association between low PNI and poor survival outcomes can be explained as follows: First, a low serum albumin level reflects malnutrition in patients with cancer. Malnutrition accounts for 20% of all cancer-related deaths (30). The presence of cancer cachexia is frequently observed, and reduced albumin levels can directly reflect the severity of malnutrition (31). Low pretreatment serum albumin levels are correlated with inferior survival in patients with urothelial carcinoma (32). Moreover, lymphocyte counts reflect antitumor activity in the host. Lymphocytes play a critical role in T cell-related antitumor responses (33). Tumor-infiltrating lymphocytes (TILs) can induce cytotoxic cell death and suppress tumor cell proliferation and migration (34). Based on this evidence, patients with low PNI may suffer from a weakened antitumor response, and therefore, poor survival.
Several recent meta-analyses have also focused on the prognostic ability of PNI in patients with solid tumors (10, 11, 35). Liao et al. reported that lower PNI correlated with unfavorable prognostic factors and poor prognosis in patients with EC, based on a meta-analysis of 3,118 patients (35). Li et al. showed that a low PNI associated with shorter OS in patients with pancreatic cancer (10). Additionally, prognostic significance in their study was not affected by subgroup variables (10). Another meta-analysis demonstrated that a low PNI could predict short- and long-term survival outcomes in patients with nasopharyngeal carcinoma (36). A recent meta-analysis of nine studies indicated that a low PNI status closely correlated with decreased OS in patients with small cell lung cancer. The findings of the present meta-analysis are consistent with those of previous meta-analyses of PNI in other cancer types. As PNI is cost-effective and easily obtained from laboratory tests, PNI can be helpful for clinicians in the management of patients.
In recent years, several studies have found that lymphopenia is a prognostic factor for poor survival in patients with RCC (37, 38). A low PNI represents poor nutritional status and is associated with worse survival in patients with RCC (17, 19, 23, 24). Moreover, the studies included in the present meta-analysis (15–24) indicated inconsistent prognostic value of PNI in RCC, which led to heterogeneity between studies. There are several factors contributing to this. First, the cutoff values of PNI were not uniform in the studies, ranging from 38.5 to 51.62. Therefore, stratification of patients in the low/high PNI groups varied in the included studies. Second, patients received partial or radical nephrectomy and TKIs in different groups, which might have led to selection bias. Patients receiving nephrectomy usually have a good physical condition and non-metastatic disease. However, patients frequently receive TKIs as an adjuvant treatment and have metastatic disease. Malnutrition is less common in patients with non-metastatic RCC than in those with metastatic RCC. Selection bias may exist across studies. Third, all included studies were retrospective in nature. The inherent nature of retrospective studies may lead to heterogeneity, and therefore, the inconsistent results in the included studies. The subgroup analyses by cutoff values of PNI, treatment methods, and metastatic status confirmed the prognostic role of PNI in these subgroups; but the source of heterogeneity should also be acknowledged.
This meta-analysis had some limitations. First, a majority of the studies included were from Asia, lacking data from other regions. Therefore, the prognostic value of PNI in patients with RCC from non-Asian countries should be further confirmed. Second, the methods for determining cutoff and the cutoff values were not uniform in the included studies. Thus, a standard cutoff value for PNI is needed in the clinical settings. Third, the heterogeneity of OS analysis was significant, and selection bias might have been introduced; although sensitivity analysis and publication bias indicated reliability of the results.
In conclusion, this meta-analysis demonstrated that low PNI associates with shorter survival outcomes in patients with RCC. The prognostic role of PNI is consistent in various patient populations. Furthermore, large-scale studies with standard assessment methods should be conducted to confirm the study findings.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
CM and WX designed the study. CM, WX, and JY performed the literature search, literature selection, and data extraction. WM and CW checked the data extraction. ZG and JY statistically analyzed the obtained data. CM, WX, and JY wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Scientific Research Projects of Jiading District Health Committee (General) (Grant No. 2020-KY-05), Research Projects of Agricultural and Social Undertakings in Jiading District (Grant No. JDKW-2020-0020), and Shanghai Key Specialty Project of Clinical Pharmacy (Grant No. YXZDZK-01). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.719941/full#supplementary-material
Supplementary Figure 1 | Forest plot examining the association between PNI and OS in patients with RCC. (A) subgroup analysis by various metastatic status of disease; (B) subgroup analysis by histological types of RCC.
Supplementary Figure 2 | Forest plot examining the association between PNI and PFS/DFS/RFS in patients with RCC. (A) subgroup analysis by various metastatic status of disease; (B) subgroup analysis by histological types of RCC.
PNI, prognostic nutritional index; RCC, renal cell carcinoma; HR, hazard ratio; CI, confidence interval; OS, overall survival; PFS, progression-free survival; DFS, disease-free survival; RFS, recurrence-free survival; CSS, cancer-specific survival; EC, esophageal cancer; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; NOS, Newcastle-Ottawa Scale; REM, random-effects model; FEM, fixed-effects model; ccRCC, clear cell renal cell carcinoma; nccRCC, non- clear cell renal cell carcinoma; TKIs, tyrosine kinase inhibitors; NR, not reported; ROC, receiver operating characteristic; MVA, multivariate; UVA, univariate.
1. Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International Variations and Trends in Renal Cell Carcinoma Incidence and Mortality. Eur Urol (2015) 67(3):519–30. doi: 10.1016/j.eururo.2014.10.002
2. MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TB, Hilvano-Cabungcal AM, et al. Systematic Review of Oncological Outcomes Following Surgical Management of Localised Renal Cancer. Eur Urol (2012) 61(5):972–93. doi: 10.1016/j.eururo.2012.02.039
3. Sun M, Choueiri TK. Kidney Cancer: Recurrence in Renal Cell Carcinoma: The Work Is Not Done. Nat Rev Urol (2016) 13(5):246–7. doi: 10.1038/nrurol.2016.57
4. Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, et al. Epidemiology of Renal Cell Carcinoma. Eur Urol (2019) 75(1):74–84. doi: 10.1016/j.eururo.2018.08.036
5. Fisher R, Gore M, Larkin J. Current and Future Systemic Treatments for Renal Cell Carcinoma. Semin Cancer Biol (2013) 23(1):38–45. doi: 10.1016/j.semcancer.2012.06.004
6. Klatte T, Rossi SH, Stewart GD. Prognostic Factors and Prognostic Models for Renal Cell Carcinoma: A Literature Review. World J Urol (2018) 36(12):1943–52. doi: 10.1007/s00345-018-2309-4
7. Zitvogel L, Pietrocola F, Kroemer G. Nutrition, Inflammation and Cancer. Nat Immunol (2017) 18(8):843–50. doi: 10.1038/ni.3754
8. Abe A, Hayashi H, Ishihama T, Furuta H. Prognostic Impact of the Prognostic Nutritional Index in Cases of Resected Oral Squamous Cell Carcinoma: A Retrospective Study. BMC Oral Health (2021) 21(1):40. doi: 10.1186/s12903-021-01394-6
9. Sun KY, Chen SL, Xu JB, Li GH, He YL. The Prognostic Significance of the Prognostic Nutritional Index in Cancer: A Systematic Review and Meta-Analysis. J Cancer Res Clin Oncol (2014) 140(9):1537–49. doi: 10.1007/s00432-014-1714-3
10. Li S, Tian G, Chen Z, Zhuang Y, Li G. Prognostic Role of the Prognostic Nutritional Index in Pancreatic Cancer: A Meta-Analysis. Nutr Cancer (2019) 71(2):207–13. doi: 10.1080/01635581.2018.1559930
11. Wang Z, Wang Y, Zhang X, Zhang T. Pretreatment Prognostic Nutritional Index as a Prognostic Factor in Lung Cancer: Review and Meta-Analysis. Clin Chim Acta (2018) 486:303–10. doi: 10.1016/j.cca.2018.08.030
12. Hao J, Chen C, Wan F, Zhu Y, Jin H, Zhou J, et al. Prognostic Value of Pre-Treatment Prognostic Nutritional Index in Esophageal Cancer: A Systematic Review and Meta-Analysis. Front Oncol (2020) 10:797. doi: 10.3389/fonc.2020.00797
13. Dai Y, Liu M, Lei L, Lu S. Prognostic Significance of Preoperative Prognostic Nutritional Index in Ovarian Cancer: A Systematic Review and Meta-Analysis. Med (Baltimore) (2020) 99(38):e21840. doi: 10.1097/md.0000000000021840
14. Gao QL, Shi JG, Huang YD. Prognostic Significance of Pretreatment Prognostic Nutritional Index (PNI) in Patients With Nasopharyngeal Carcinoma: A Meta-Analysis. Nutr Cancer (2021) 73(9):1657–67. doi: 10.1080/01635581.2020.1810715
15. Broggi MS, Patil D, Baum Y, Nieh PT, Alemozaffar M, Pattaras JG, et al. Onodera’s Prognostic Nutritional Index as an Independent Prognostic Factor in Clear Cell Renal Cell Carcinoma. Urology (2016) 96:99–105. doi: 10.1016/j.urology.2016.05.064
16. Cai W, Zhong H, Kong W, Dong B, Chen Y, Zhou L, et al. Significance of Preoperative Prognostic Nutrition Index as Prognostic Predictors in Patients With Metastatic Renal Cell Carcinoma With Tyrosine Kinase Inhibitors as First-Line Target Therapy. Int Urol Nephrol (2017) 49(11):1955–63. doi: 10.1007/s11255-017-1693-9
17. Hu X, Wang YH, Lia T, Yang ZQ, Shao YX, Yang WX, et al. Prognostic Value of Preoperative Prognostic Nutritional Index in Patients With Renal Cell Carcinoma After Nephrectomy. Clin Chim Acta (2020) 509:210–6. doi: 10.1016/j.cca.2020.06.025
18. Kang M, Chang CT, Sung HH, Jeon HG, Jeong BC, Seo SI, et al. Prognostic Significance of Pre- to Postoperative Dynamics of the Prognostic Nutritional Index for Patients With Renal Cell Carcinoma Who Underwent Radical Nephrectomy. Ann Surg Oncol (2017) 24(13):4067–75. doi: 10.1245/s10434-017-6065-2
19. Kim SJ, Kim SI, Cho DS. Prognostic Significance of Preoperative Prognostic Nutritional Index in Patients Undergoing Nephrectomy for Nonmetastatic Renal Cell Carcinoma. Am J Clin Oncol (2020) 43(6):388–92. doi: 10.1097/coc.0000000000000680
20. Kwon WA, Kim S, Kim SH, Joung JY, Seo HK, Lee KH, et al. Pretreatment Prognostic Nutritional Index Is an Independent Predictor of Survival in Patients With Metastatic Renal Cell Carcinoma Treated With Targeted Therapy. Clin Genitour Cancer (2017) 15(1):100–11. doi: 10.1016/j.clgc.2016.07.025
21. Lucca I, de Martino M, Hofbauer SL, Zamani N, Shariat SF, Klatte T. Comparison of the Prognostic Value of Pretreatment Measurements of Systemic Inflammatory Response in Patients Undergoing Curative Resection of Clear Cell Renal Cell Carcinoma. World J Urol (2015) 33(12):2045–52. doi: 10.1007/s00345-015-1559-7
22. Peng D, He ZS, Li XS, Tang Q, Zhang L, Yang KW, et al. Prognostic Value of Inflammatory and Nutritional Scores in Renal Cell Carcinoma After Nephrectomy. Clin Genitour Cancer (2017) 15(5):582–90. doi: 10.1016/j.clgc.2017.04.001
23. Yasar HA, Bir Yucel K, Arslan C, Ucar G, Karakaya S, Bilgin B, et al. The Relationship Between Prognostic Nutritional Index and Treatment Response in Patients With Metastatic Renal Cell Cancer. J Oncol Pharm Pract (2020) 26(5):1110–6. doi: 10.1177/1078155219883004
24. Zheng Y, Bao L, Wang W, Wang Q, Pan Y, Gao X. Prognostic Impact of the Controlling Nutritional Status Score Following Curative Nephrectomy for Patients With Renal Cell Carcinoma. Med (Baltimore) (2018) 97(49):e13409. doi: 10.1097/md.0000000000013409
25. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol (2009) 62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005
26. Qi F, Zhou X, Wang Y, Wang Y, Wang Y, Zhang Q, et al. Pre-Treatment Prognostic Nutritional Index may Serve as a Potential Biomarker in Urinary Cancers: A Systematic Review and Meta-Analysis. Cancer Cell Int (2018) 18:207. doi: 10.1186/s12935-018-0708-7
27. Zhou W. Zhang GL. C-Reactive Protein to Albumin Ratio Predicts the Outcome in Renal Cell Carcinoma: A Meta-Analysis. PloS One (2019) 14(10):e0224266. doi: 10.1371/journal.pone.0224266
28. Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
29. Wang X, Wang Y. The Prognostic Nutritional Index Is Prognostic Factor of Gynecological Cancer: A Systematic Review and Meta-Analysis. Int J Surg (London England) (2019) 67:79–86. doi: 10.1016/j.ijsu.2019.05.018
30. Ottery FD. Cancer Cachexia: Prevention, Early Diagnosis, and Management. Cancer Pract (1994) 2(2):123–31.
31. Esper DH, Harb WA. The Cancer Cachexia Syndrome: A Review of Metabolic and Clinical Manifestations. Nutr Clin Pract (2005) 20(4):369–76. doi: 10.1177/0115426505020004369
32. Liu J, Wang F, Li SH, Huang WH, Jia YJ, Wei CJ. The Prognostic Significance of Preoperative Serum Albumin in Urothelial Carcinoma: A Systematic Review and Meta-Analysis. Biosc Rep (2018) 38:13. doi: 10.1042/bsr20180214
33. Germano G, Allavena P, Mantovani A. Cytokines as a Key Component of Cancer-Related Inflammation. Cytokine (2008) 43(3):374–9. doi: 10.1016/j.cyto.2008.07.014
34. Man YG, Stojadinovic A, Mason J, Avital I, Bilchik A, Bruecher B, et al. Tumor-Infiltrating Immune Cells Promoting Tumor Invasion and Metastasis: Existing Theories. J Cancer (2013) 4(1):84–95. doi: 10.7150/jca.5482
35. Liao G, Zhao Z, Yang H, Chen M, Li X. Can Prognostic Nutritional Index be a Prediction Factor in Esophageal Cancer?: A Meta-Analysis. Nutr Cancer (2020) 72(2):187–93. doi: 10.1080/01635581.2019.1631859
36. Tu X, Ren J, Zhao Y. Prognostic Value of Prognostic Nutritional Index in Nasopharyngeal Carcinoma: A Meta-Analysis Containing 4511 Patients. Oral Oncol (2020) 110:104991. doi: 10.1016/j.oraloncology.2020.104991
37. Saroha S, Uzzo RG, Plimack ER, Ruth K, Al-Saleem T. Lymphopenia Is an Independent Predictor of Inferior Outcome in Clear Cell Renal Carcinoma. J Urol (2013) 189(2):454–61. doi: 10.1016/j.juro.2012.09.166
Keywords: PNI, renal cell carcinoma, meta-analysis, prognosis, immune responses
Citation: Mao C, Xu W, Ma W, Wang C, Guo Z and Yan J (2021) Prognostic Value of Pretreatment Prognostic Nutritional Index in Patients With Renal Cell Carcinoma: A Meta-Analysis. Front. Oncol. 11:719941. doi: 10.3389/fonc.2021.719941
Received: 03 June 2021; Accepted: 10 September 2021;
Published: 05 October 2021.
Edited by:
Matteo Ferro, European Institute of Oncology (IEO), ItalyCopyright © 2021 Mao, Xu, Ma, Wang, Guo and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Yan, eWFuanVuMTA0MEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.