
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 23 September 2021
Sec. Radiation Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.705303
 Qingsong Li1,2
Qingsong Li1,2 Na Liang1,2
Na Liang1,2 Xia Zhang1,3
Xia Zhang1,3 Yi Zhang1,2
Yi Zhang1,2 Weiwei Ouyang1,2
Weiwei Ouyang1,2 Shengfa Su1,2
Shengfa Su1,2 Zhu Ma1,2
Zhu Ma1,2 Yinxiang Hu1,2
Yinxiang Hu1,2 Yichao Geng1,2
Yichao Geng1,2 Xiaxia Chen1,2
Xiaxia Chen1,2 Bing Lu1,2*
Bing Lu1,2*Purpose: The aim of this study was to investigate the reasonable timing of radiotherapy for stage IV non-small-cell lung cancer (NSCLC) with EGFR-positive mutations during targeted therapy based on tumour volume change (TVC).
Patients and Methods: Simulation Computed Tomography Scan (SCTS) measurements were taken to test TVC in patients with stage IV NSCLC during targeted therapy at intervals of 10 days. The SCTS measurement was terminated when the tumour volume shrinkage rate in the latter simulation compared with the previous simulation was ≤5% or when the time after treatment was 90 days. Then, primary tumour radiotherapy was performed. Related parameters of the radiotherapy plan were compared between the implementation and simulation plans.
Results: Twenty-seven patients were enrolled in the analysis. After treatment, shrinkage of the primary tumour was observed in all patients, but the rate and speed were inconsistent. The average tumour volume decreased obviously within 40 days and was significantly different every 10 days (P ≤ 0.001). The average volume decreased slowly and tended to be stable (P>0.05) after 40 days. After the termination of SCTSs, 21 patients accepted primary tumour radiotherapy. No patients experienced grade 3+ acute radiation toxicity. The implementation radiotherapy plan was significantly better than that before treatment (all P<0.05) but not better than that on the 40th day after treatment (all P>0.05).
Conclusions: To obtain a high radiation dose and control radiation toxicity, the 40th day after targeted therapy may be a reasonable time to start radiotherapy for stage IV NSCLC with EGFR-positive mutations.
Clinical Trial Registration: https://www.clinicaltrials.gov/ct2/show/NCT03258671, identifier, NCT03258671.
First-line treatment of stage IV non-small-cell lung cancer (NSCLC) has evolved from chemotherapy alone to chemotherapy, targeted therapy, and immunotherapy (1), and targeted therapy for patients with positive mutations in driver Genes, such as human epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK)/C-ros oncogene 1 receptor tyrosine kinase (ROS1) and T790M, has been shown to significantly prolong progression-free survival (PFS) (2–5). Higginson DS (6) et al. analysed stage III/IV NSCLC patients who received only systemic chemotherapy and found that the state of the primary tumour (large central tumour, pulmonary symptoms, and bronchial or vascular compression) was associated with poor OS. More importantly, recent studies have shown that targeted therapy, chemotherapy, and immunotherapy combined with three-dimensional radiotherapy of primary tumours and metastatic lesions can significantly improve overall survival (OS) (7–9) and significantly reduce the treatment failure rate of primary tumours from 80%-90% to less than 30% (10). A meta-analysis suggested that primary tumour radiotherapy, especially with radical doses, might further prolong survival (11). The local failure (12) was 82% for stage IV NSCLC treated with only EGFR-TKI. Previous studies (13, 14) showed that targeted therapy can increase the sensitivity of radiotherapy, and the combination therapy has the best inhibitory effect on cancer cell proliferation compared with radiotherapy alone or targeted therapy alone. OS benefits may be derived from the synergistic combination of radiotherapy and targeted therapy (15–18). However, the tumour burden of stage IV NSCLC is relatively large, with T3-4 accounting for 60%-70% and N2-3 accounting for 70%-80%, and the median volume of the primary tumour is approximately 134 cm3 (7, 19). The large tumour volume results in a low local control rate (LCR) due to the low radiation dose to reduce the rates of severe radiation toxicities and can also lead to an increase in radiation-induced toxicities due to an increased radiation dose. Therefore, we designed a prospective clinical trial to reduce the tumour to a certain size and maintain a relatively stable state by using EGFR-tyrosine kinase inhibitors (EGFR-TKIs), which have an objective response rate (ORR) of more than 70%, to realize the reasonable timing of radiotherapy to reduce normal tissue toxicity and increase the radiation dose, and to provide a reference for further randomized controlled studies on the reasonable timing of radiotherapy.
The inclusion criteria were as follows: (1) pathologically confirmed, positive for sensitive driver Gene mutations, primary stage IV NSCLC (Union for International Cancer Control,UICC version 8), (2) no previous history of tumour treatment; (3) Karnofsky performance status (KPS)≥70; (4) aged from 18 to 80 years; (5) no contraindications to targeted therapy and radiotherapy; (6) signed informed consent; (7) clear consciousness when the metastatic sites were brain; (8) no influence on pulmonary function when the metastatic sites were lung; and (9) Normal bone marrow and organ function as defined below(absolute neutrophil count ≥ 1,500/mcl, Platelets ≥ 100000/mcl, Haemoglobin ≥ 9.0 g/Dl, Total bilirubin ≤ 2.0 x IULN(institution’s upper limit of normal), SGOT (serum glutamic-oxaloacetic transaminase)/SGPT (serum glutamic-pyruvic transaminase) ≤ 3.0 x IULN; if liver metastases, number ≤ 5.0, Serum creatinine ≤ 1.5 x IULN; LVEF (left ventricular ejection fraction) ≥ 50% performed no more than 4 weeks prior to enrolment; FEV1 (forced expiratory volume in the first second)>50%, mild-moderate pulmonary function dysfunction).
Tumour volume measurement process: (1) A Simulation Computed Tomography Scan (SCTS) was planned within 1 week before targeted therapy and every 10 days after the first day of treatment that patients underwent one simulation scan in sequence for a maximum of 90 days; (2) the SCTS within 1 week before targeted therapy was defined as C0; after the start of treatment, the SCTSs every 10 days were defined as C10-C90; the primary tumour volume before treatment (VP), volume of metastatic lymph nodes in the drainage area (VN) and gross tumour volume (GTV) were defined as VP0, VN0 and GTV0, respectively; and the volumes measured on the SCTSs were VP10-VP90, VN10-VN90 and GTV10-GTV90, respectively; (3) termination criteria for the SCTS were a tumour volume shrinkage (TVS) rate ≤5% in the latter simulation compared with the previous simulation or when the time after treatment was 90 days.
Delineation and calculation of tumour volume: Intensity-modulated radiotherapy (IMRT) was given via 6 MV X-ray. The patient was positioned in the supine position with thermoplastic film fixation, and the 5-mm-thick enhanced Computed Tomography (CT) scans were transferred to the Pinnacle3 planning system. VP was outlined with a lung window (W: 1,600, L: -300), and VN was outlined with a mediastinal window (W: 400, L: 800). Tumour volume was calculated, and the GTV compromised VP and VN. The GTV was outlined on the last simulation CT image. The clinical target volume (CTV) was defined as the GTV plus a margin of 0.6 cm, and the planning target volume (PTV) was defined as the CTV plus another margin of 0.5 to 1.0 cm. The TVS rate of CN was calculated as follows: TVS rate = (pre-treatment volume - simulation volume of CN)/pre-treatment volume × 100%.
Implementation radiotherapy plans and simulation plans: (1) IMRT was given via 6 MV X-ray. The implementation radiotherapy plans were created with the last simulation CT image. The radiotherapy dose was given to patients according to the tolerability of normal tissue and was maintained at ≤76 Gy. For all individual treatment plans, the percentage of the total lung volume receiving ≥20 Gy (V20) was maintained at ≤32% (≤25% in crizotinib-treated patients), V5 at ≤ 70%, mean lung dose (MLD) at ≤20 Gy, mean heart dose (MHD) at ≤26 Gy and maximum point dose to the spinal cord (SC-MPD) at ≤50 Gy. Radiotherapy plans were evaluated as 100% of the prescription dose line including 100% of the GTV and 95% of the prescription dose including 95% of the PTV or 90% of the prescription dose including 98% of the PTV. Patients received late-course accelerated hyperfractionated radiotherapy (LCAHRT) (20–23) to the primary tumour. The first course of radiotherapy was given in 1.8-Gy fractions, 5 days per week, to a total dose of 36-40 Gy/18-20 f. LCAHRT was then delivered in twice-daily fractions of 1.5 Gy each, separated by 6 to 8 hours per day, to a total dose of 21-30 Gy/14-20 f.
Simulation plans were created with the pre-treatment simulation (C0) and 40 days post-treatment (C40) simulation images. Implementation radiotherapy plans were adjusted according to the same dose or the same radiation toxicity control criteria for each patient, and the dose-volume histogram (DVH) was recorded.
Gefitinib (250 mg, qd), erlotinib (150 mg, qd), icotinib (125 mg, tid) or crizotinib (250 mg, bid) was given according to the status of driver Gene-positive mutations. None of the patients received systemic chemotherapy.
For oligometastatic NSCLC, all metastatic lesions were treated with radiotherapy. For non-oligometastatic NSCLC, radiotherapy to metastatic lesions was determined by clinical necessity, such as, brain metastasis, bone metastasis with cancer pain or risk of fracture.
Study endpoints and statistical methods: The primary endpoints were the change patterns of the VP, VN and GTV before and during treatment, and the secondary endpoints were acute radiation pneumonitis (RP) (within 3 months after the end of radiotherapy), oesophagitis (RE) (NCICTC 3.0 criteria) and DVH parameters. Statistical analysis was performed using SPSS software (version 23.0). Measurement data are expressed as the mean ± standard deviation (SD) and were analysed with t-tests or Mann-Whitney U-tests. P<0.05 was considered a statistically significant difference.
Thirty patients met the inclusion criteria, and 27 patients were eligible for analysis (refusal in 1 patient and SCTS not as planned in 2 patients). The ratio of males to females was 1.25, and the median patient age was 60 years. The most common site of metastatic disease at diagnosis was the bone, brain and lung (Table 1). The VP0, VN0 and GTV0 were 6.23~470.00, 0~362.97 and 28.86~470.00 cm3, respectively. The median and average GTV0 were 149.42 cm3 and 189.23 ± 127.03 cm3, respectively (the rest are shown in Table 1). Twenty-seven patients completed the SCTS and volumetric measurements according to the termination criteria. Twenty-three patients harboured EGFR-positive mutations: an exon 19 deletion mutation (19del) was observed in 14 patients, and an exon 21 deletion mutation (L858R) was observed in 9 patients. Four patients harboured an ALK rearrangement. Targeted therapy involved gefitinib in 16 patients, icotinib in 7 patients and crizotinib in 4 patients (Figure 1).
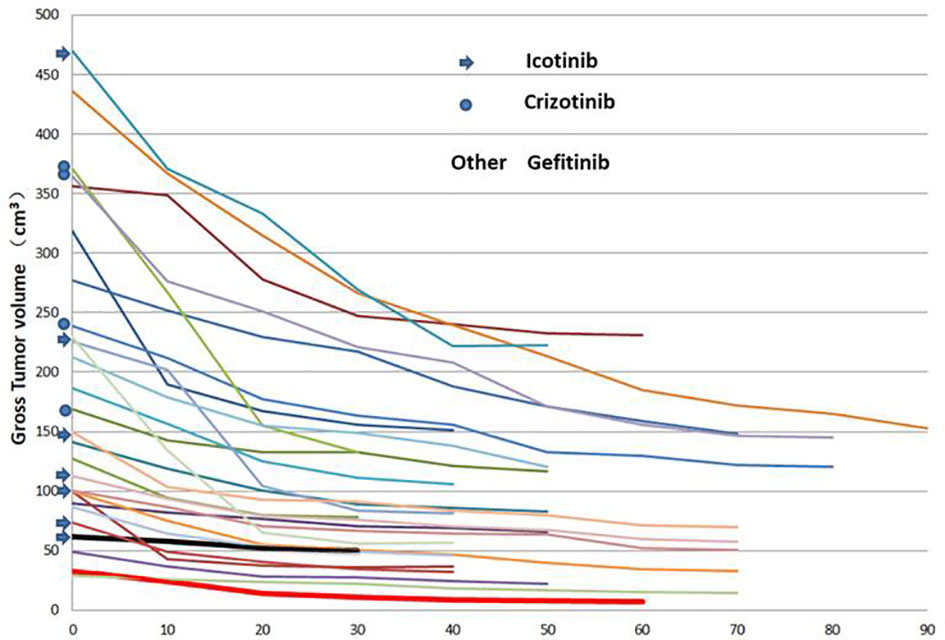
Figure 1 Changes in the primary tumour volume at different times after targeted therapy in 27 patients.
SCTSs from C0 to C30 were performed on 27 patients, and C40, C50, C60, C70, C80 and C90 were performed on 24, 17, 11, 9, 3, and 1 patients, respectively. The GTV of all patients had different degrees of change from C0 to the last SCTS and showed a trend of gradual shrinkage, in which the largest volume shrinkage rate was 78.1% (gefitinib, thick red solid line) and the smallest was 18.8% (icotinib, thick black solid line) (Figure 1). According to the graph of mean GTV, VP, and VN (C0-70) values, tumour volume decreased gradually and significantly within the 40th day after treatment and then tended to stabilize (3 patients in C80 and 1 patient in C90, not analysed). The mean tumour volume continued to shrink or tended to stabilize after slightly increasing at 50 days (Figure 2).
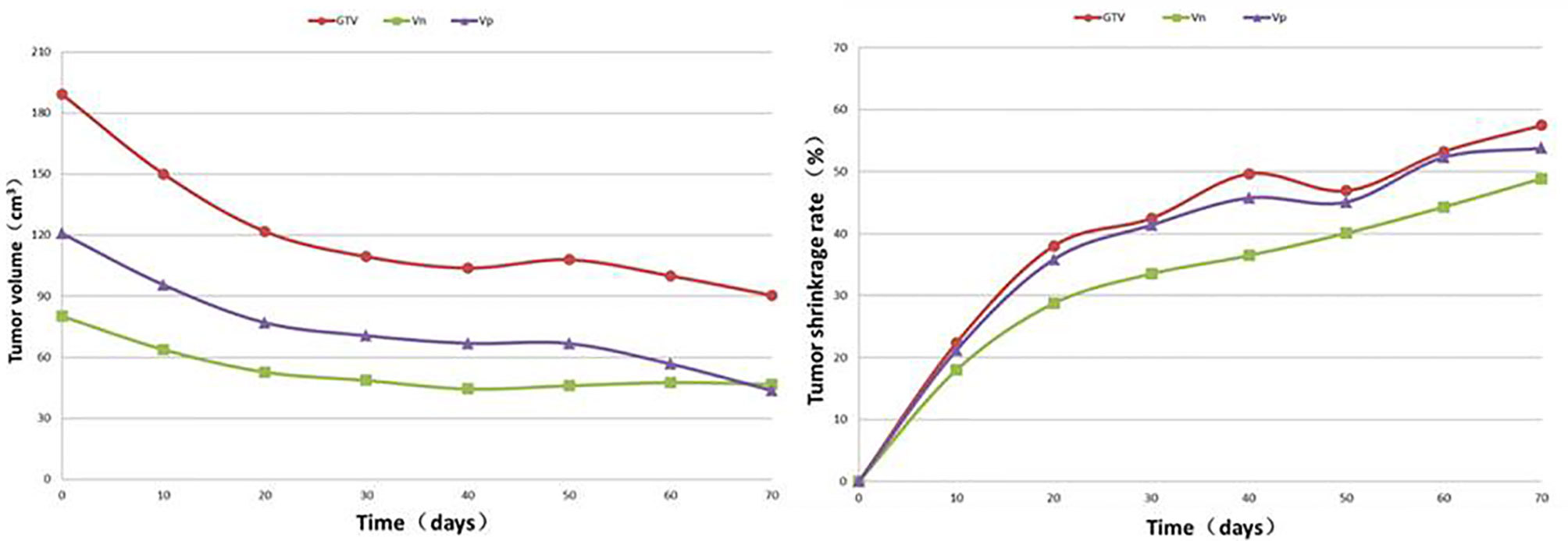
Figure 2 Regularity of the average value and shrinkage rate for the VP, VN and GTV at different times after targeted therapy in 27 patients.
GTV changes in two adjacent SCTSs showed that the tumour volume shrinkage rate was inconsistent before the C40 SCTS every 10-day interval, and the tumour volume shrinkage rate was <5% on the C40 and C50 SCTSs and every 10-day interval thereafter (Table 2).

Table 2 Comparison of the gross tumour volume (cm3) every 10 days after targeted therapy in 27 patients.
The change patterns in VP and VN were similar to that of the GTV after treatment, with the most significant shrinkage rate in the first 10 days (C10). The shrinkage rates of the GTV10-40 were 22.21%, 14.64%, 5.54% and 4.37%, respectively. In every interval from C40 to C90, 3, 7, 6, 2, 6 and 2 patients met the termination criteria due to having a shrinkage rate (adjacent comparison) <5%. The average shrinkage rate from C40 to C70 was 2.67%. Only 1 patient continued to have a >5% shrinkage rate at C90 (Table 3).

Table 3 Changes in the gross tumour volume (GTV), primary tumour volume (VP), and metastatic lymph nodes in drainage areas (VN) at different times after targeted therapy in 29 patients (mean ± SD).
Twenty-one patients (6 of whom refused radiotherapy after the termination of simulation) were treated with primary tumour radiotherapy according to the last CT simulation image and were followed up until 90 days after the end of radiotherapy. There were 5 (23.8%), 2 (9.5%), 1 (4.8%) and 5 (23.8%), 3 (14.3%), 0 (0%) cases of grade I, II and III acute RP and RE, respectively.
The comparison between the implementation plan and the corresponding simulation plan with the same primary tumour dose revealed the following. The lung V20, MLD, and MHD of the C0 plan were significantly higher than those of the implementation plan and the C40 plan. The lung V5, SC-MPD and oesophageal V50 also tended to increase. The C40 plan was similar to the implementation plan (Table 4). The comparison between the implementation plan and the simulation plan with the same radiation damage control criteria revealed the following. The C40 plan increased the radiotherapy dose from 63 ± 7 Gy at C0 to 66 ± 7 Gy (P<0.001), and the implementation plan increased the radiotherapy dose to 68 ± 7 Gy (P<0.001). The radiotherapy dose of the C40 plan was similar to that of the implementation plan (P=0.110).
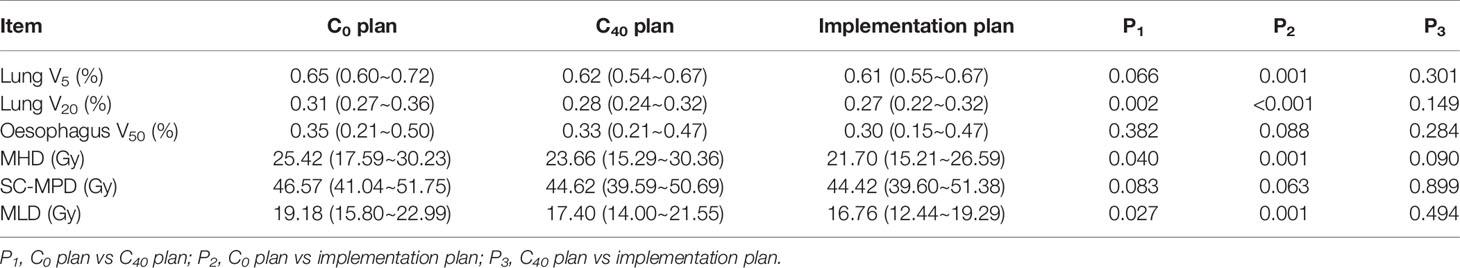
Table 4 Comparison of dose-volume histogram parameters in the pre-treatment localization (C0) and 40 days post-treatment (C40) simulation plans and implementation plans in 21 patients (mean and range).
The median survival time (MST) of patients with stage IV NSCLC who received three-dimensional radiotherapy to the primary tumour combined with chemotherapy was prolonged to 16 months, and radiotherapy may play a very important role in prolonging OS based on the benefits of systemic therapy (21). Stereotactic ablative radiotherapy and stereotactic body radiotherapy to the primary tumour or metastases combined with EGFR-TKIs or first-line chemotherapy (for patients without EGFR mutations) significantly prolonged PFS and OS in patients with oligometastatic NSCLC (16, 24–28). Increasing the radiotherapy dose to the primary tumour was strongly associated with improved OS, and a radical radiation dose may be more beneficial for OS, especially in patients with oligometastases (10, 21, 24, 26). Radiotherapy has become an important treatment for prolonging OS by reducing the failure rate of the primary tumour in patients with stage IV NSCLC (21). However, it is well known that the radical radiation dose can improve the LCR. However, the volume of the irradiated target area is an important factor that affects an increase in the tumour dose and controls radiation injury to normal tissues (27). The large irradiated volume leads to the phenomenon that radiation injury is aggravated by high doses to improve the LCR, or the LCR is reduced by low doses for fear of radiation injury. Therefore, reducing the volume of the irradiated tumour is the key to both increasing the dose and LCR and decreasing the incidence of radiation injury. However, the primary tumour is large in volume and scattered, and patients mainly have T3-4/N2-3 disease according to research data (11, 21, 24). In some patients, when radiotherapy and EGFR-TKIs are started simultaneously, the purposes of both increasing the dose to the primary tumour and protecting normal tissues from radiation injury cannot be achieved because of the primary tumour volume. Therefore, this study was designed to take advantage of the ORR of EGFR-TKI treatment (>70%), disease control rate (>90%), and PFS (9-11 months) (29–31) based on the dosimetric property that a ≥15% shrinkage rate in the primary tumour volume can significantly reduce the low-dose volume to the whole lung and reduce radiation injury (27). Patients underwent SCTSs before EGFR-TKI treatment and every 10 days after treatment. The SCTS measurement was terminated, and then primary tumour radiotherapy began when the TVS in the latter simulation compared with the previous simulation was ≤5% or when the time after treatment was 90 days. The aim was to investigate the timing of administering radiotherapy to the primary tumour to both increase the dose and LCR and reduce the probability of radiation injury.
This study showed that although each patient had positive mutations in driver Genes, the rate and degree of tumour volume shrinkage after EGFR-TKI treatment were not consistent. Until the last SCTS, the maximum and minimum shrinkage rates were 78.1% and 18.8%, respectively. The most significant change in the average volume was within 40 days after the start of treatment. Thereafter, the average volume shrinkage rate slowed and was relatively stable at every 10-day interval. The total and average shrinkage rates from C40 to C70 were 8% and 2.67%, respectively. On day 50, the shrinkage rate increased slightly (by 3%) and continued to decrease thereafter. The regularity of TVC after EGFR-TKI treatment is that the volume shrinkage gradually slows the volume continues to shrink after increasing in some cases, and tumour shrinkage varies due to the different sensitivities of EGFR-TKI treatment in different patients (32). Therefore, it may be most beneficial to start radiotherapy at the time when the tumour volume continues to shrink to a low level after treatment and stabilizes without waiting until the disease progresses. In this study, the primary tumour volume was measured and compared separately at each 10-day interval. The results showed that the tumour volume shrinkage rates were significant and different within 40 days after the start of treatment. The tumour volumes from days C40 to C70 were similar and slow, and the tumour volume increased slightly on day 50 in 1 patient, which suggests that the speed of tumour volume shrinkage is different in each individual. For patients who receive EGFR-TKI treatment, a certain regularity of tumour volume shrinkage may be deduced, or 60 days may be the time to carry out radiotherapy by means of mathematical modelling (33), but an individualized analysis was not performed, and the actual pattern of TVC was not examined. Therefore, the current study shows that TVS was significant within 40 days after EGFR-TKI treatment and entered the stable phase after 40 days in most patients. The 40th day after EGFR-TKI treatment may be a reasonable time to administer radiotherapy to reach the goals of controlling tumours and reducing injury.
The simulated radiotherapy plan and its parameters represent the dose likely to control the primary tumour and the threshold to protect normal tissues from radiation injury (34, 35), while the implementation radiotherapy plan and its parameters validate and summarize the efficacy for each individual tumour and the probability of radiation injury for normal tissues after a given dose of radiotherapy (36). Grade 2 and 3 acute RP and RE were observed in only a small number of patients treated with radiotherapy after the termination of SCTSs in this study, which suggests that the safety and efficacy of radiotherapy are acceptable under the premise of injury control criteria. The simulation radiotherapy plans for the primary tumour at C0 and C40 were designed at the same dose as the implementation plans of the corresponding patients. The DVH parameters of the 3 plans were compared. The results showed that compared to the C0 simulation plan, the implementation plan and the C40 plan significantly reduced the lung V20, MLD, and MHD. There was a trend of a significant reduction in the lung V5 and SC-MPD. The reduction in the lung V20 may significantly reduce the occurrence of RP (28). There was a trend of a significant reduction in the oesophageal DVH of the implementation plans that may reduce the incidence of RE. Under the premise of the same control criteria of radiation injury, the radiation doses were compared among the implementation plan and the C0 and C40 simulation plans. The results showed that the implementation plan and C40 simulation plan could significantly increase the tumour dose compared with the C0 simulation plan and achieved a radical dose of more than 60 Gy, which not only improved the LCR but also did not increase radiation injury (34–37). The implementation plan was similar to the C40 simulation plan in both the tumour dose and DVH parameter regarding radiation injury protection. Therefore, it was further validated that it is a reasonable time to start primary tumour radiotherapy at 40 days after EGFR-TKI treatment in patients with EGFR-positive mutations.
In summary, the tumour volume shrinkage rate after EGFR-TKI treatment in patients with stage IV NSCLC with driver Gene-positive mutations gradually slowed over time and varied in each individual. The shrinkage rate was significant within 40 days after treatment and then entered the stable stage, and it may be the best time to start radiotherapy after 40 days of the initial treatment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Guizhou Cancer Hospital, GuiYang, China. The patients/participants provided their written informed consent to participate in this study.
QL, NL, XZ, YZ, WO, SS, ZM, YH, YG, and XC collected the data. BL conceived the study and participated in its design and coordination. BL and QL performed the statistical analysis and drafted the manuscript. All authors contributed to the article and approved the submitted version.
Guizhou Science and Technology Plan Support Project [Qiankehe support (2019) 2795].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. David SE, Douglas EW, Dara LA, Wallace A, Jessica RB, Ankit B, et al. NCCN Clinical Practice Guidelines in Oncology-Non-Small Cell Lung Cancer. National Comprehensive Cancer Network (2020). Available at: https://www.scienceopen.com/document.
2. Okamoto I, Mitsudomi T, Nakagawa K, Fukuoka M. The Emerging Role of Epidermal Growth Factor Receptor (EGFR) Inhibitors in First-Line Treatment for Patients With Advanced Non-Small Cell Lung Cancer Positive for EGFR Mutations. Ther Adv Med Oncol (2010) 2(5):301–7. doi: 10.1177/1758834010370698
3. Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, et al. Effect of Crizotinib on Overall Survival in Patients With Advanced Non-Small-Cell Lung Cancer Harbouring ALK Gene Rearrangement: A Retrospective Analysis. Lancet Oncol (2011) 12(11):1004–12. doi: 10.1016/S1470-2045(11)70232-7
4. Mok TS, W Y-L, A M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med (2017) 376(7):629–40. doi: 10.1056/NEJMoa1612674
5. Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al. Alectinib Versus Crizotinib in Patients With ALK-Positive Non-Small-Cell Lung Cancer (J-ALEX): An Open-Label, Randomised Phase 3 Trial. Lancet (2017) 390(10089):29–39. doi: 10.1016/S0140-6736(17)30565-2
6. Higginson DS, Chen RC, Tracton G, Morris DE, Halle J, Rosenman JG, et al. The Impact of Local and Regional Disease Extent on Overall Survival in Patients With Advanced Stage IIIB/IV Non-Small Cell Lung Carcinoma. Int J Radiat Oncol Biol Phys (2012) 84(3):e385–92. doi: 10.1016/j.ijrobp.2012.04.045
7. Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-Rearranged Non-Small-Cell Lung Cancer. N Engl J Med (2014) 371(21):1963–71. doi: 10.1056/NEJMoa1406766
8. Su SF, Hu YX, Ouyang WW, Lu B, Ma Z, Li QS, et al. Overall Survival and Toxicities Regarding Thoracic Three-Dimensional Radiotherapy With Concurrent Chemotherapy for Stage IV Non-Small Cell Lung Cancer: Results of a Prospective Single-Center Study. BMC Cancer (2013) 13(1-9):474. doi: 10.1186/1471-2407-13-474
9. Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Three-Year Overall Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-Update From PACIFIC. J Thorac Oncol (2020) 15(2):288–93. doi: 10.1016/j.jtho.2019.10.002
10. Wang C, Lu X, Lyu Z, Bi N, Wang L. Comparison of Up-Front Radiotherapy and TKI With TKI Alone for NSCLC With Brain Metastases and EGFR Mutation: A Meta-Analysis. Lung Cancer (2018) 122:94–9. doi: 10.1016/j.lungcan.2018.05.014
11. Lopez Guerra JL, Gomez D, Zhuang Y, Hong DS, Heymach JV, Swisher SG, et al. Prognostic Impact of Radiation Therapy to the Primary Tumor in Patients With non-Small Cell Lung Cancer and Oligometastasis at Diagnosis. Int J Radiat Oncol Biol Phys (2012) 84(1):e61–7. doi: 10.1016/j.ijrobp.2012.02.054
12. Patel SH, Rimner A, Foster A, Zhang Z, Woo KM, Yu HA, et al. Patterns of Initial and Intracranial Failure in Metastatic EGFR-Mutant Non-Small Cell Lung Cancer Treated With Erlotinib. Lung Cancer (2017) 108:109–14. doi: 10.1016/j.lungcan.2017.03.010
13. Chinnaiyan P, Huang S, Vallabhaneni G, Armstrong E, Varambally S, Tomlins SA, et al. Mechanisms of Enhanced Radiation Response Following Epidermal Growth Factor Receptor Signaling Inhibition by Erlotinib (Tarceva). Cancer Res (2005) 65(8):3328–35. doi: 10.1158/0008-5472.CAN-04-3547
14. Das AK, Chen BP, Story MD, Sato M, Minna JD, Chen DJ, et al. Somatic Mutations in the Tyrosine Kinase Domain of Epidermal Growth Factor Receptor (EGFR) Abrogate EGFR-Mediated Radioprotection in Non-Small Cell Lung Carcinoma. Cancer Res (2007) 67(11):5267–74. doi: 10.1158/0008-5472.CAN-07-0242
15. Zheng L, Wang Y, Xu Z, Yang Q, Zhu G, Liao XY, et al. Concurrent EGFR-TKI and Thoracic Radiotherapy as First-Line Treatment for Stage IV Non-Small Cell Lung Cancer Harboring EGFR Active Mutations. Oncologist (2019). doi: 10.1634/theoncologist.2019-0285
16. Gomez DR, Blumenschein GR, Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local Consolidative Therapy Versus Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer Without Progression After First-Line Systemic Therapy: A Multicentre, Randomised, Controlled, Phase 2 Study. Lancet Oncol (2016) 17(12):1672–82. doi: 10.1016/S1470-2045(16)30532-0
17. Hsu KH, Huang JW, Tseng JS, Chen KW, Weng YC, Yu SL, et al. Primary Tumor Radiotherapy During EGFR-TKI Disease Control Improves Survival of Treatment Naïve Advanced EGFR-Mutant Lung Adenocarcinoma Patients. Onco Targets Ther (2021) 14:2139–48. doi: 10.2147/OTT.S300267
18. Zhang Y, Wang W, Xu X, Li Y, Zhang H, Li J, et al. Impact of Radiotherapy Pattern on the Prognosis of Stage IV Lung Adenocarcinomas Harboring EGFR Mutations. Cancer Manag Res (2021) 13:3293–301. doi: 10.2147/CMAR.S299563
19. Ma JT, Zheng JH, Han CB, Guo QY. Meta-Analysis Comparing Higher and Lower Dose Radiotherapy for Palliation in Locally Advanced Lung Cancer. Cancer Sci (2014) 105(8):1015–22. doi: 10.1111/cas.12466
20. Withers HR, Taylor JM, Maciejewski B. The Hazard of Accelerated Tumor Clonogen Repopulation During Radiotherapy. Acta Oncol (1988) 27(2):131–46. doi: 10.3109/02841868809090333
21. Su S, Li T, Lu B, Wang X, Li J, Chen M, et al. Three-Dimensional Radiation Therapy to the Primary Tumor With Concurrent Chemotherapy in Patients With Stage IV Non-Small Cell Lung Cancer: Results of a Multicenter Phase 2 Study From PPRA-RTOG, China. Int J Radiat Oncol Biol Phys (2015) 93(4):769–77. doi: 10.1016/j.ijrobp.2015.08.012
22. Wang D, Li B, Wang Z, Zhu J, Sun H, Zhang J, et al. Functional Dose-Volume Histograms for Predicting Radiation Pneumonitis in Locally Advanced Non-Small Cell Lung Cancer Treated With Late-Course Accelerated Hyperfractionated Radiotherapy. Exp Ther Med (2011) 2(5):1017–22. doi: 10.3892/etm.2011.301
23. Chen M, Chen YY, Bao Y, Xian CG, Liu GZ, Zhang L, et al. Neoadjuvant Chemotherapy Followed by Late-Course Accelerated Hyperfractionated Radiation Therapy for Locally Advanced Non-Small-Cell Lung Cancer: Long-Term Results of a Phase I/II Clinical Trial. Clin Lung Cancer (2005) 6(5):304–9. doi: 10.3816/CLC.2005.n.010
24. Ouyang WW, Su SF, Hu YX, Lu B, Ma Z, Li QS, et al. Radiation Dose and Survival of Patients With Stage IV Non-Small Cell Lung Cancer Undergoing Concurrent Chemotherapy and Thoracic Three-Dimensional Radiotherapy: Reanalysis of the Findings of a Single-Center Prospective Study. BMC Cancer (2014) 14:491(1–8). doi: 10.1186/1471-2407-14-491
25. Uhlig J, Case MD, Blasberg JD, Boffa DJ, Chiang A, Gettinger SN, et al. Comparison of Survival Rates After a Combination of Local Treatment and Systemic Therapy vs Systemic Therapy Alone for Treatment of Stage IV Non-Small Cell Lung Cancer. JAMA Netw Open (2019) 2(8):e199702. doi: 10.1001/jamanetworkopen.2019.9702
26. Koshy M, Malik R, Mahmood U, Rusthoven CG, Sher DJ. Comparative Effectiveness of Aggressive Thoracic Radiation Therapy and Concurrent Chemoradiation Therapy in Metastatic Lung Cancer. Pract Radiat Oncol (2015) 5(6):374–82. doi: 10.1016/j.prro.2015.07.009
27. Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J Clin Oncol (2018) 36(22):2244–50. doi: 10.1200/JCO.2018.78.7994
28. Gomez DR, Tang C, Zhang J, Blumenschein GR, Hernandez M, Lee JJ, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol (2019) 37(18):1558–65. doi: 10.1200/JCO.19.00201
29. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med (2009) 361(10):947–57. doi: 10.1056/NEJMoa0810699
30. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib Versus Standard Chemotherapy as First-Line Treatment for European Patients With Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol (2012) 13(3):239–46. doi: 10.1016/S1470-2045(11)70393-X
31. Oya Y, Yoshida T, Kuroda H, Shimizu J, Horio Y, Sakao H, et al. Association Between EGFR T790M Status and Progression Patterns During Initial EGFR-TKI Treatment in Patients Harboring EGFR Mutation. Clin Lung Cancer (2017) 18(6):698–705.e2. doi: 10.1016/j.cllc.2017.05.004
32. Park DI, Kim SY, Kim JO, Jung SS, Park HS, Moon JY, et al. The Prognostic Value of the Tumor Shrinkage Rate for Progression-Free Survival in Patients With Non-Small Cell Lung Cancer Receiving Gefitinib. Tuberc Respir Dis (Seoul) (2015) 78(4):315–20. doi: 10.4046/trd.2015.78.4.315
33. Tang Y, Xia B, Xie R, Zhang M, Wu K, Wang B, et al. Timing in Combination With Radiotherapy and Patterns of Disease Progression in Non-Small Cell Lung Cancer Treated With EGFR-TKI. Lung Cancer (2020) 140:65–70. doi: 10.1016/j.lungcan.2019.12.009
34. Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, et al. Predicting Radiation Pneumonitis After Chemoradiation Therapy for Lung Cancer: An International Individual Patient Data Meta-Analysis. Int J Radiat Oncol Biol Phys (2013) 85(2):444–50. doi: 10.1016/j.ijrobp.2012.04.043
35. Hu YX, Lu B, Zhou HY, Gan JY, Hong W. Changes of Lung Dose Volume and its Clinical Significance in Three-Dimensional Conformal Late Course Accelerated Hyperfractionation Radiotherapy for Non-Small Cell Lung Cancer [J]. Chin J Radiat Oncol (2009) 18(1):57–60. doi: 10.3760/cma.j.issn.1004-4221.2009.01.057
36. Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of Normal Tissue Complication Probability Models in the Clinic. Int J Radiat Oncol Biol Phys (2010) 76(3 Suppl):S10–9. doi: 10.1016/j.ijrobp.2009.07.1754
37. Liang J, Bi N, Wu S, Chen M, Lv C, Zhao L, et al. Etoposide and Cisplatin Versus Paclitaxel and Carboplatin With Concurrent Thoracic Radiotherapy in Unresectable Stage III Non-Small Cell Lung Cancer: A Multicenter Randomized Phase III Trial. Ann Oncol (2017) 28(4):777–83. doi: 10.1093/annonc/mdx009
Keywords: non-small-cell lung cancer, targeted therapy, tumour volume change, radiotherapy, reasonable timing
Citation: Li Q, Liang N, Zhang X, Zhang Y, Ouyang W, Su S, Ma Z, Hu Y, Geng Y, Chen X and Lu B (2021) Reasonable Timing of Radiotherapy for Stage IV Non-Small-Cell Lung Cancer During Targeted Therapy Based on Tumour Volume Change. Front. Oncol. 11:705303. doi: 10.3389/fonc.2021.705303
Received: 05 May 2021; Accepted: 06 September 2021;
Published: 23 September 2021.
Edited by:
Jaroslaw T. Hepel, Rhode Island Hospital, United StatesReviewed by:
Pasquale Pisapia, University of Naples Federico II, ItalyCopyright © 2021 Li, Liang, Zhang, Zhang, Ouyang, Su, Ma, Hu, Geng, Chen and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Lu, bGJneW1hYWFhQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.