
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 29 June 2021
Sec. Thoracic Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.703893
This article is part of the Research Topic Treatment for Non Small Cell Lung Cancer in Distinct Patient Populations View all 32 articles
 Lea Daniello1,2
Lea Daniello1,2 Mariam Elshiaty1,2
Mariam Elshiaty1,2 Farastuk Bozorgmehr1,2
Farastuk Bozorgmehr1,2 Jonas Kuon1,2
Jonas Kuon1,2 Daniel Kazdal2,3
Daniel Kazdal2,3 Hannah Schindler1,2
Hannah Schindler1,2 Rajiv Shah1,2
Rajiv Shah1,2 Anna-Lena Volckmar3
Anna-Lena Volckmar3 Fabienne Lusky1,2
Fabienne Lusky1,2 Leonore Diekmann4
Leonore Diekmann4 Stephan Liersch5
Stephan Liersch5 Martin Faehling6
Martin Faehling6 Thomas Muley2,7
Thomas Muley2,7 Mark Kriegsmann2,3
Mark Kriegsmann2,3 Karolina Benesova4
Karolina Benesova4 Albrecht Stenzinger2,3
Albrecht Stenzinger2,3 Michael Thomas1,2
Michael Thomas1,2 Petros Christopoulos1,2*
Petros Christopoulos1,2*Introduction: PD-(L)1 inhibitors have improved prognosis of non-small-cell lung cancer (NSCLC), but can also cause immune-related adverse events (irAEs) that complicate management.
Methods: We analyzed NSCLC patients receiving PD-(L)1 inhibitors from 2012 to 2020 in a German academic center.
Results: IrAE showed comparable frequencies in stage IV (198/894 or 22%) vs. III (14/45 or 31%, p = 0.15), after anti-PD-(L)1 monotherapy vs. chemoimmunotherapy (139/483 vs. 58/213, p = 0.75), and across treatment lines. In stage IV, irAE occurred after 3.1 months in median, affected multiple organs (median 2) in 27/894 patients and were associated with PD-L1 positivity (25 vs. 14%, p = 0.003), lower neutrophil-to-lymphocyte ratios (29 vs. 17%, p < 0.001 for NLR dichotomized at 5), better ECOG status (26 vs. 18% for 0 vs. 1, p = 0.004), but not related to age, sex, smoking and palliative radiotherapy. Two hundred thirty two irAEs occurred mostly in endocrine glands (4.9%), lungs (4.4%), the musculoskeletal system (4.2%), colon (4.1%), liver (3.7%), and skin (2.6%), while pneumonitis was most frequent with durvalumab following definitive chemoradiation (16% or 7/45, p < 0.01). IrAE severity was grade 1 in 11%, 2 in 41%, 3 in 36%, and 4 in 11% events, while two were lethal (<1%, myocarditis and pneumonitis). Therapy was suspended in 72%, while steroids were initiated in 66% and complemented by other immunosuppressants in 6%, with longest treatment duration for rheumatic events (mean >3 months), and average cumulative prednisone doses >700 mg for all organs, except for skin. Patients developing irAE had longer progression-free (PFS) and overall survival (OS) in multivariable 12/14-week landmark analyses including ECOG status, treatment line, treatment type, PD-L1 TPS, and NLR (median PFS 17 vs. 10 months, HR = 0.68, p = 0.009; median OS 37 vs. 15 months, HR = 0.40, p < 0.001), regardless of grade. OS was longest with skin (95% at 2 years) and shortest with pneumonitis, hepatitis, neurologic, and cardiologic irAE (38, 37, 28, and 0% at 2 years, p < 0.001).
Conclusions: Approximately one-fourth of immunotherapy-treated NSCLC patients develop irAEs, most of which necessitate treatment suspension and steroids. Despite more frequent occurrence with PD-L1 positive tumors, lower NLR, and better ECOG PS, irAEs are independently associated with longer survival, especially when affecting the skin. Lethality is below 1%.
Inhibitors of immune checkpoints (ICIs), such as the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein-1 (PD-1), and its ligand programmed death-ligand 1 (PD-L1), are increasingly used for the treatment of metastatic cancers (1). These drugs block inhibitory effects of neoplastic on immune cells, to potentiate immunologic tumor control (2). Nivolumab, pembrolizumab, ipilimumab, and atezolizumab have improved progression-free (PFS) and overall survival (OS) of patients with metastatic non-small-cell lung cancer (NSCLC) in randomized phase 3 trials and currently represent the standard first-line treatment alone or in combination with chemotherapy for most cases (3, 4).
Besides the high antitumor efficacy of ICIs, as exemplified by an unprecedented 5-year survival rate of 32% for stage IV patients with PD-L1 high-expressing NSCLC receiving first-line pembrolizumab monotherapy (5), these drugs can also alter the physiology of immune responses, leading to toxicity collectively described as “immune-related adverse events” (irAEs), which can affect diverse organs and complicate patient management (6). The severity grading for irAEs relies on the National Cancer Institute common toxicity criteria for adverse events (NCI CTCAE) version 5 (7). Grade ≥3 toxicities, especially, can be even life threatening and require special monitoring and therapeutic maneuvers, including dose reductions, treatment interruption, and/or high-dose steroids (8).
With increasing use of ICIs as treatment for NSCLC, precise characterization of predisposing factors, manifestations, management, outcome, and impact on overall prognosis becomes more important for irAEs, because this knowledge could become a valuable aid for decision-making in daily clinical practice. This retrospective study utilizes a large, single-institution cohort to address these issues under real-world conditions.
This study was approved by the ethics committee of Heidelberg University (S-296/2016) and included all advanced NSCLC patients treated with PD-(L)1 inhibitors in the Thoraxklinik Heidelberg between October 2012 and June 2020. Patients that received other immunotherapies, in particular CTLA-4 inhibitors, were excluded from this analysis.
Diagnosis of NSCLC was performed in the Institute of Pathology Heidelberg using tissue specimens according to the criteria of the current WHO classification (2015) for lung cancer, as described previously (9, 10). Clinical data and laboratory results were collected by a systematic review of patient records. The following clinical data were extracted: demographic, baseline clinical and tumor characteristics, including ECOG performance status (PS), smoking status, PD-L1 tumor proportion score (TPS), laboratory results, systemic and local anticancer treatments, date of progression, date of the last follow-up, and date of death. The neutrophile-to-lymphocyte ratio (NLR) was dichotomized at the bibliographical cut-off of 5, which corresponds to the median value for untreated patients (11, 12). PD-L1 TPS was assessed using the clone SP263 (Ventana/Roche, Mannheim, Germany) and trichotomized for analysis as <1, 1–49, and ≥50%. For calculation of PFS, the progression date under immunotherapy was verified by the investigators with review of radiologic images, i.e. chest/abdomen CT and brain MRI-based restaging every 6–12 weeks, without formal RECIST reevaluation, as several studies have demonstrated very good agreement between real-world and RECIST-based assessments (13, 14). Patients with irAEs were diagnosed based on clinicolaboratory criteria and treated according to the current guidelines (7, 15). Diagnosis of pneumonitis was based on high-resolution CT (HRCT), considering that bioptic confirmation is generally not required for subsequent patient management (15). For patients with irAE additional data were collected about severity, management, outcome, and impact of irAEs on anticancer treatment. The subset of stage III patients who received durvalumab as consolidation after chemoradiation was analyzed separately (Figure 1).
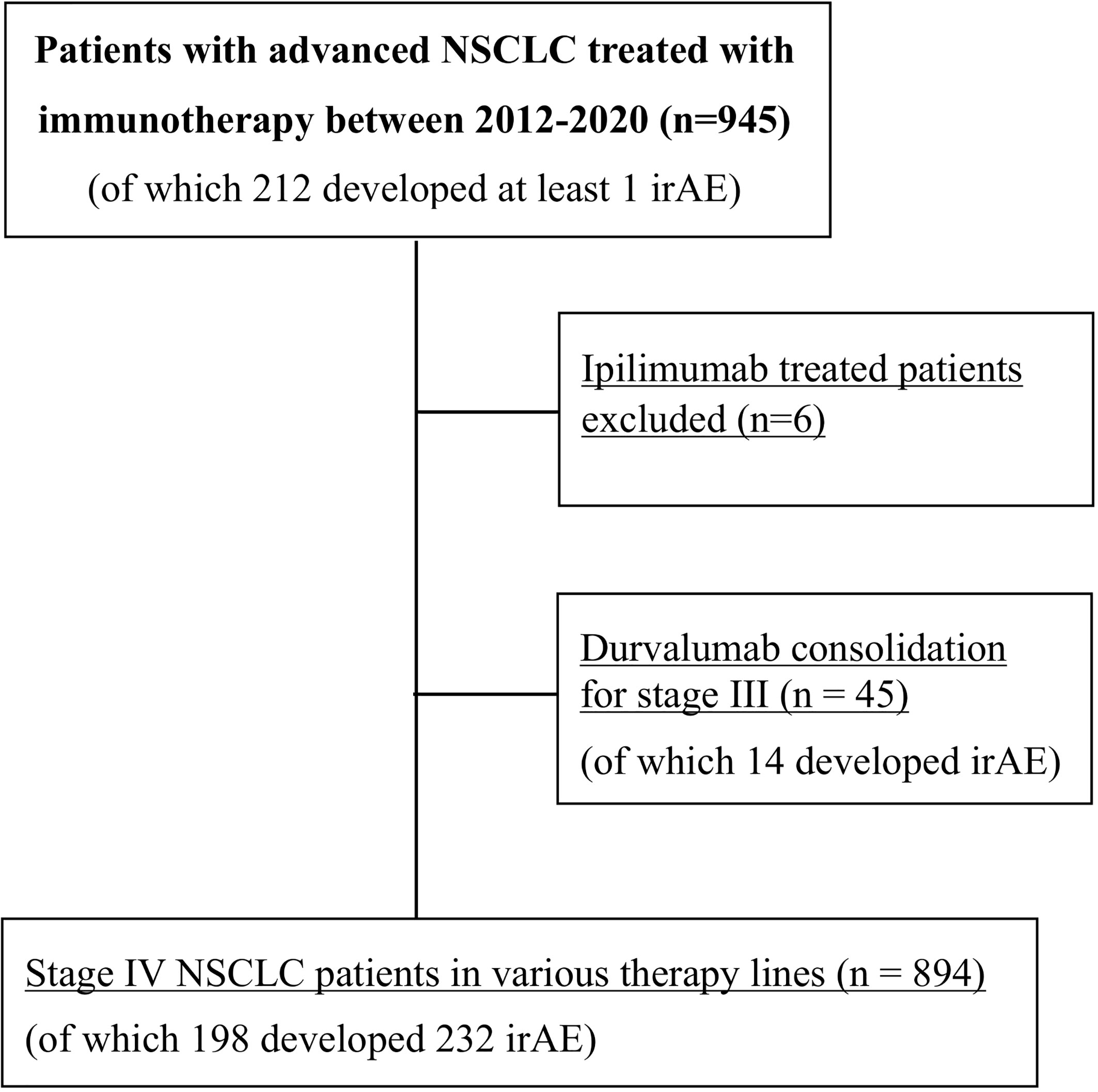
Figure 1 Flowchart of study patients. NSCLC, non-small-cell lung cancer; irAE, immune-related adverse events.
Patients who developed irAEs were analyzed further in more detail regarding affected organs and severity grade, time of onset, treatment with steroids—including start date, dose, duration, whether anticancer therapy had to be interrupted or terminated—or other immunosupressants, and if radiotherapy had been given in the past. Rheumatic irAEs were diagnosed in consultation with an experienced rheumatologist (KB) in order to differentiate them from non-autoimmune joint disease (e.g. osteoarthritis) (16, 17).
Categorical data were analyzed using the chi-square test, including “goodness-of-fit” tests for the observed frequencies against the even distribution, when applicable. Numerical data were compared across two groups using an unpaired t-test and across three or more groups using one-way ANOVA with the Dunnett’s post-hoc test with correction for multiple testing. Survival data were analyzed according to Kaplan–Meier and compared between groups with the logrank test. The association of irAE and other variables with survival was analyzed using Cox regression. Immortal time bias was controlled through two landmark analyses, at 12 and 14 weeks, which included only cases surviving beyond the respective landmark, as well as by a time-dependent Cox regression, in which the occurrence of irAEs was considered as time-dependent covariate. Statistical calculations were performed with SPSS version 27 (IBM Corp., Armonk, NY, USA), and plots were generated with SPSS and Microsoft Excel 365 (Redmond, WA, USA). P-values <0.05 were considered as statistically significant.
Overall, 939 consecutive patients were included in the study, of which 894 were treated with ICI monotherapy (70%) or chemoimmunotherapy (30%) for metastatic disease as shown in Table 1, while 45 patients received durvalumab after chemoradiotherapy of locally advanced tumors (Figure 1 and Supplementary Table 1). Mean age was 65 years, with a predominance of male (60%) and smokers (92%) showing mostly an ECOG PS of 0–1 (98%). IrAEs showed comparable overall frequencies in stage IV vs. III (22 vs. 31%, p = 0.15), after ICI monotherapy vs. chemoimmunotherapy (22 vs. 21%, p = 0.75), across treatment lines (21–26% in the first vs. 20–33% in subsequent lines, p = 0.08–0.68), and across different ICIs (p = 0.16–0.74), with a trend for lower frequency for PD-L1 compared to PD-1 inhibitor monotherapy (13 vs. 23%, p = 0.053, Table 1). Among stage IV patients, 232 irAEs were documented, with involvement of multiple organs (two in median) in 14% (27/198) of patients. Most frequently affected were the endocrine glands (in 4.9% of patients, or 44/894), lungs (4.4%), musculoskeletal system (4.2%), colon (4.1%), and liver (3.7%), followed by the skin (2.6%), nervous system (0.7%), heart (0.4%), kidney (0.3%), pancreas (0.3%), and blood (0.1%, p < 0.001 across organs, Figure 2A and Table 2). CTCAE severity was grade 1 in 11% (25/232), grade 2 in 41%, grade 3 in 36%, and grade 4 in 11% (p < 0.001 across grades, Figure 2B), while two events were lethal (2/939 = 0.2%, one instance of myocarditis, and one instance of pneumonitis). The percentages of patients with at least one grade 3–4 irAE was comparable between stage IV (11% or 97/894) and stage III (18% or 8/45, p = 0.14, Supplementary Table 2) patients. In stage IV, the severity distribution was skewed for several organs, with predominance of grade 2 irAE for the skin (p = 0.0075), endocrine (p < 0.001), and musculoskeletal systems (p < 0.001), while grade 3 was more frequent for pneumonitis (p < 0.001), colitis (p = 0.02), and hepatitis (p < 0.001, Figure 2C). Besides, in stage III patients after definitive chemoradiation, grade ≥3 pneumonitis predominated (6/14, Supplementary Table 2).
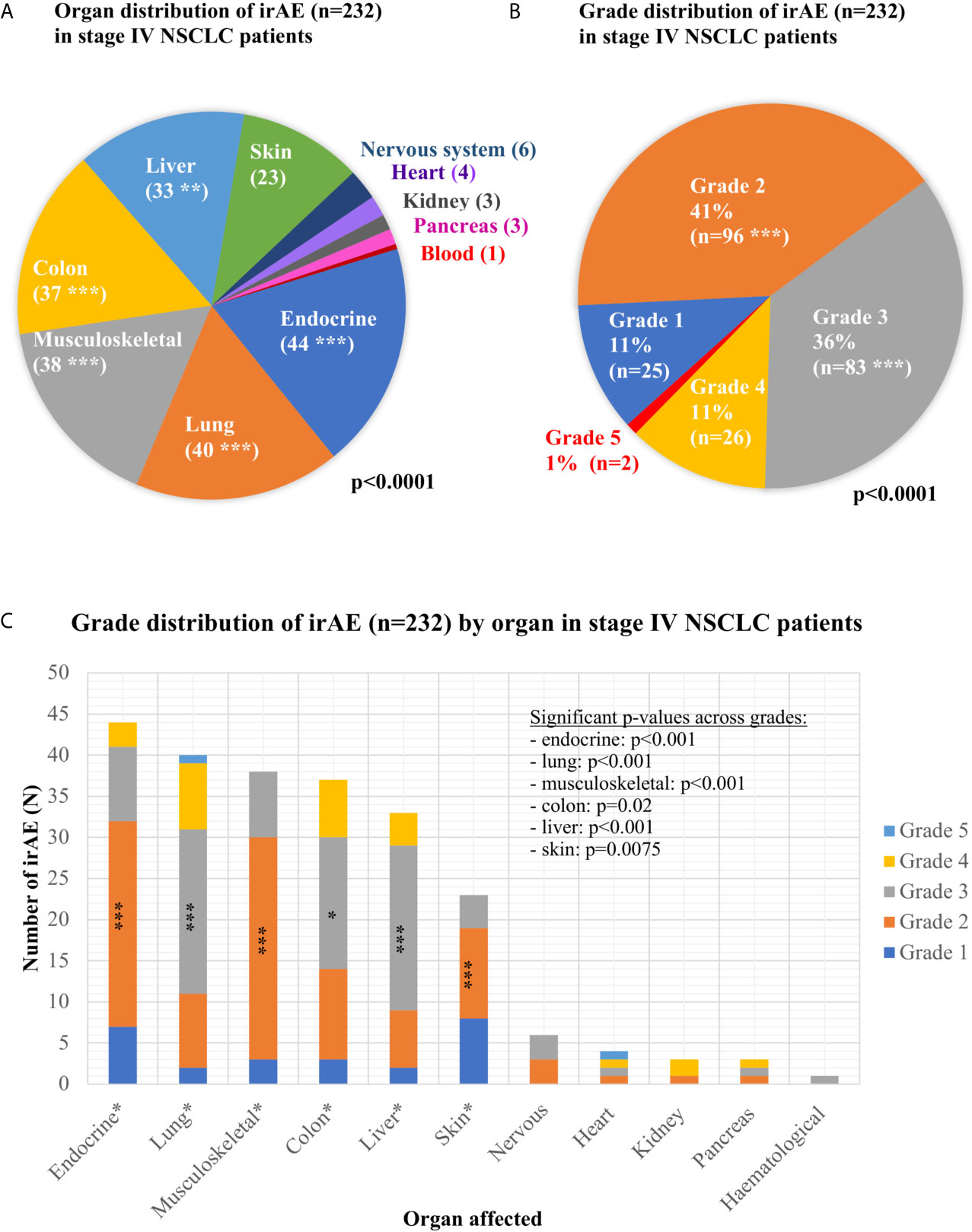
Figure 2 Organ and grade distribution of immune-related adverse events in immunotherapy-treated non-small-cell lung cancer patients. (A) Organ distribution of the 232 immune-related adverse events (irAEs) observed in stage IV non-small-cell lung cancer (NSCLC) patients (p < 0.0001 with a chi-square test across the various affected organs; detailed results are shown in Table 2; ***p < 0.001, **p < 0.01, *p < 0.05). (B) Grade distribution of the 232 irAEs observed in stage IV NSCLC patients (p < 0.0001 with a chi-square test across grades, grades with significantly increased frequency are marked with asterisks). (C) Grade distribution of the irAEs observed in each organ for stage IV NSCLC patients. For each organ, the p-value was calculated with a chi-square test of the observed frequencies for each grade against the even distribution (endocrinological: 44 irAEs overall, grade 1:7, grade 2:25, grade 3:9, grade 4:3, p < 0.001; lungs: 40 irAEs overall, grade 1:2, grade 2:9, grade 3:20, grade 4:8, grade 5:1, p < 0.001; musculoskeletal system: 38 irAEs overall, grade 1:3, grade 2:27, grade 3:8, grade 4:0, p < 0.001; colon: 37 irAE overall, grade 1:3, grade 2:11, grade 3:16, grade 4:7, p = 0.02; hepatitis: 33 irAEs overall, grade 1:2, grade 2:7, grade 3:20, grade 4:4, p < 0.001; skin: 23 irAEs overall, grade 1:8, grade 2:11, grade 3:4, grade 4:0, p = 0.0075; nervous system: six irAEs overall, grade 1:0, grade 2:3, grade 3:3, grade 4:0, p = 0.06; heart: four irAEs overall, grade 1:0, grade 2:1, grade 3:1, grade 4:1, grade 5:1, p = 0.26; kidneys: three irAEs overall, grade 1:0, grade 2:1, grade 3:0, grade 4:2, p = 0.30; pancreas: three irAEs overall, grade 1:0, grade 2:1, grade 3:1, grade 4:0, p = 0.80; hematological: one irAE overall, grade 3).

Table 2 Severity, onset, and management of immune-related adverse events in stage IV non-small-cell lung cancer patients.
Most patient characteristics, like age, sex, and smoking status, were balanced between patients with or without irAE (Table 1). At the same time, irAE occurrence was significantly associated with PD-L1 positivity, i.e. TPS ≥1% (25 vs. 14%, p = 0.003; mean PD-L1 TPS 43 vs. 34% for patients with vs. without irAE, p = 0.008), a lower baseline NLR <5 (29 vs. 17% for patients with NLR ≥5, p < 0.001; mean NLR 7.0 vs. 9.0, p = 0.005), and a better ECOG PS (26 vs. 18% for PS 0 vs. 1, p = 0.004). In stage IV patients, there was no significant relationship between administration of palliative radiotherapy to any organ and development of any irAE (p = 0.37), or between prior palliative thoracic radiotherapy and development of pneumonitis p = 0.68, Table 1). However, the frequency of pneumonitis was significantly higher in stage III patients receiving durvalumab after curative-intent radiotherapy compared to stage IV patients (7/45 = 16% vs. 40/894 = 4%, respectively, p = 0.0009). Similarly, the relative frequency of pneumonitis among the observed irAE was significantly higher for stage III compared to stage IV patients (7/14 = 50% vs. 40/232 = 17%, respectively, p = 0.0025, Table 2 and Supplementary Table 2). Median time-to-onset of irAE from ICI start was 3.1 months (92 days), with significantly later onset for musculoskeletal (246 days, p = 0.046) and renal events (669 days, p < 0.001, Table 2).
The majority of irAE interfered with further administration of ICI therapy, leading to suspension in 72% (168/232), and termination in 55% (128/232) of cases, respectively (Table 2). This affected practically all patients with grade 3–4 events (98 and 100%, respectively), but ICI therapy was also permanently discontinued for 21% of patients with grade 1 (n = 4), and 51% of patients with grade 2 events (n = 35). There were considerable differences depending on the affected organ, with significantly more pneumonitis (95%), colitis (84%), and hepatitis (94%) irAEs leading to suspension (p < 0.001, Table 2). For irAEs of other vital organs, i.e. nervous system, heart, kidneys, and blood, the suspension rate was also very high, approaching 100%, but did not reach statistical significance due to the rarity of these events (Table 2).
In the majority of cases (66% or 155/232), steroid treatment was required, with increasing frequency and dose for more severe events (Figure 3A): no patient with steroid treatment for grade 1 irAE, 51% with grade 2 (mean initial daily dose 20 mg), and >90% with grades 3–4 (mean initial dose 95 mg). The only grade 3–4 cases without steroid therapy were four patients with hypophysitis, who received only hydrocortisone replacement. Overall, hydrocortisone replacement therapy was required for most patients with endocrinologic irAE (24/44 or 55%, namely 22 with hypophysitis, one with thyroiditis, and one with polyendocrinopathy. Utilization of steroid therapy also showed considerable heterogeneity across affected organs and was more frequent for pneumonitis (93%), hepatitis (82%), colitis (81%), and musculoskeletal events (74%, p < 0.001, Table 2). Steroid therapy was longest for musculoskeletal irAEs, which were the only type of events with average steroid duration exceeding 3 months (128 days, Table 2 and Figure 3B). Several patients received steroids for >1 year, either higher prednisone doses >10 mg (six patients, all with musculoskeletal irAEs), or low-dose maintenance therapy with ≤10 mg prednisone daily, which was necessary for approximately one-third (14/38 or 37%) of patients with musculoskeletal irAE. On the other hand, the duration of treatment was shortest for skin irAE (average 23 days) and the single case with a hematologic event (20 days, Table 2). The cumulative steroid dose was highest for patients with renal (3,330 mg), followed by liver (1,622 mg), and lung irAEs (1,519 mg), but exceeded 700 mg also for all other organs, except for the skin (average 237 mg, Figure 3B). A need for additional immunosuppressive therapy was documented in 13/232 events (6%), or 13/198 cases (7%), which corresponded to 13/154 (8.4%) steroid-treated patients: namely 3/28 steroid-treated cases of musculoskeletal events (n = 2 arthritis grade 2, n = 1 arthritis grade 3), 6/30 steroid-treated cases of colitis (n = 1 grade 2, n = 3 grade 3, and n = 2 grade 4), 1/2 steroid-treated cases of myocarditis (grade 4), 2/27 steroid-treated cases of hepatitis (grade 3 and grade 4), and 1/8 steroid-treated cases of dermatitis (grade 3 exacerbated psoriasis). Immunosuppressants administered were mycophenolate mofetil for hepatitis (2×) and myocarditis (1×), mesalamine for colitis (3×), tacrolimus for colitis (1×), infliximab for colitis (2×) and polyarthritis (1×), methotrexate for psoriasis (1×), polyarthritis (1×) and adalimumab as well as leflunomide for arthritis (2× and 1× respectively). Three cases required more than one additional immunosuppressant (n = 2 arthritis, n = 1 colitis). For stage III, steroid therapy was required for most patients with irAEs (79% or 11/14), particularly in case of grade 3–4 events (100% use vs. 25% for grade 2, Supplementary Table 2).
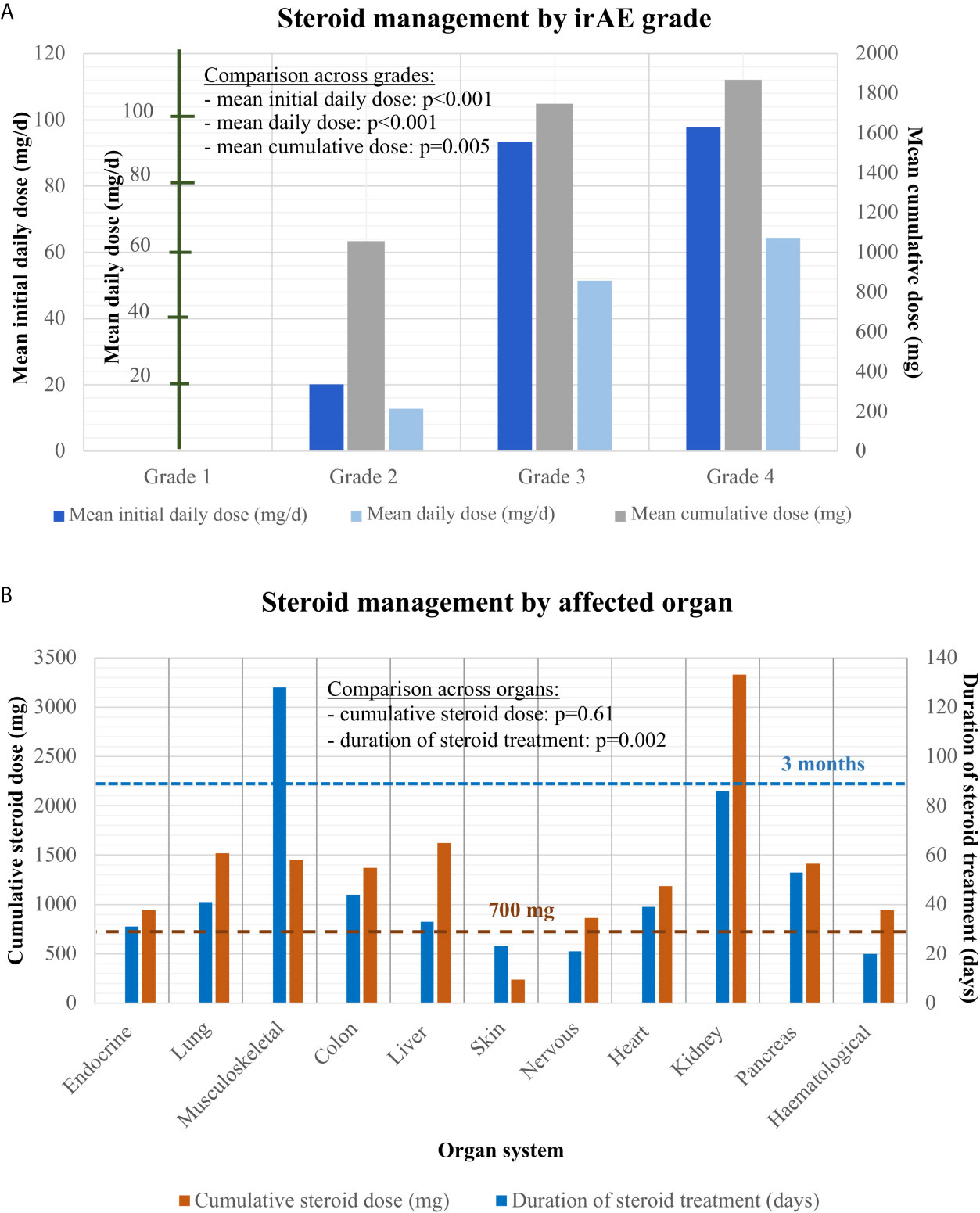
Figure 3 Steroid management of grades 1–4 irAEs. (A) Mean initial daily dose, mean average daily dose, and mean cumulative dose for steroid treatment in patients with grade 2–4 irAEs analyzed by one-way ANOVA. While no grade 1 irAE received steroid treatment, mean initial daily, mean average daily and cumulative steroid dose increased steadily from grades 2–4: for grade 2 irAE mean initial dose: 20.2 mg [standard error (SE): 2.9], cumulative dose: 1056.4 mg (SE: 300.3), mean daily dose: 12.9 mg (SE: 2.0); for grade 3 irAE: mean initial dose: 93.4 mg (SE: 9.7), cumulative dose: 1747.5 mg (SE: 186.3), mean daily dose: 51.5 mg (SE: 5.3); for grade 4 irAE mean initial dose: 96.4 mg (SE: 10.9), cumulative dose: 1823.5 mg (SE: 348.4), mean daily dose: 62.8 mg (SE: 8.0). ANOVA with post-hoc test for trend across grades: p < 0.001 (mean initial daily dose), p < 0.001 (mean average daily dose), p = 0.005 (cumulative dose). (B) Cumulative steroid dose and total duration of steroid treatment by affected organ: endocrine: 942 mg (SE: 536) over 31 days (SE: 16); lungs: 1,519 mg (SE: 248) over 41 days (SE: 6)); musculoskeletal: mean cumulative dose 1455 mg (SE: 442) over 128 days (SE: 33); colon: 1,371 mg (SE: 227) over 44 days (SE: 7); liver: 1,622 mg (SE: 339) over 33 days (SE: 5); skin: 237 mg (SE: 123) over 23 days (SE: 11); nervous system: 863 mg (SE: 427) over 21 days (SE: 9); cardiologic: 1,183 mg (SE: 684) over 39 days (SE: 31); kidney: 3,330 (SE: 2,394) over 86 days (SE: 9); pancreas: 1,413 mg (SE: 1035) over 53 days (SE: 5); blood: 940 mg (SE: na) over 20 days (SE: na). One-way ANOVA p = 0.002 for the cumulative dose across affected organs (with statistical significance in post-hoc testing for musculoskeletal irAE, please see Table 2), and p = 0.61 for the treatment duration. Abbreviations: irAEs, immune related adverse events; SE, standard error of the mean; n/a, not applicable.
Patients who developed irAEs had a longer PFS [15 vs. 9 months, hazard ratio (HR) 0.68 with p = 0.008 in a 12-week landmark analysis, Supplementary Figure 1A, Supplementary Tables 3, 4; 17 vs. 10 months, HR = 0.65 with p = 0.005 in a 14-week landmark analysis, Figure 4A, Tables 3, 4], which was significant in multivariable testing along with PD-L1 TPS (p < 0.01), NLR (p > 0.05), treatment line (p > 0.05), type of treatment (chemoimmunotherapy vs. IO-monotherapy, p > 0.05), and ECOG PS (p > 0.05, Table 4 and Supplementary Table 4). OS from start of IO treatment was also longer for patients developing irAEs (37 vs. 14 months, HR = 0.4 with p < 0.001 in a 12-week landmark analysis, Supplementary Figure 1B, Supplementary Tables 3, 4; 37 vs. 15 months, HR = 0.38 with p < 0.001 in a 14-week landmark analysis, Figure 4B, Tables 3, 4), which was significant in multivariable testing along with PD-L1 TPS (p < 0.01), NLR (p < 0.01) treatment line (p > 0.05), type of treatment (p > 0.05), and ECOG PS (p < 0.05, Table 4, Supplementary Table 4). The independent prognostic value of irAE alongside NLR, PD-L1 TPS, ECOG PS, treatment line, and treatment type was also confirmed in separate multivariable OS analysis using the occurrence of irAE as a time-dependent covariate (Supplementary Table 5). OS of patients with irAE varied widely between irAE affecting different organs, being longest for skin (2-year OS 95%), and shortest for pulmonary, hepatic, nervous system, and cardiologic irAE (2-year OS 38, 37, 28, and 0% respectively, p = 0.007, Figure 5B) but did not differ significantly by irAE grade (p = 0.71, Figure 5A).
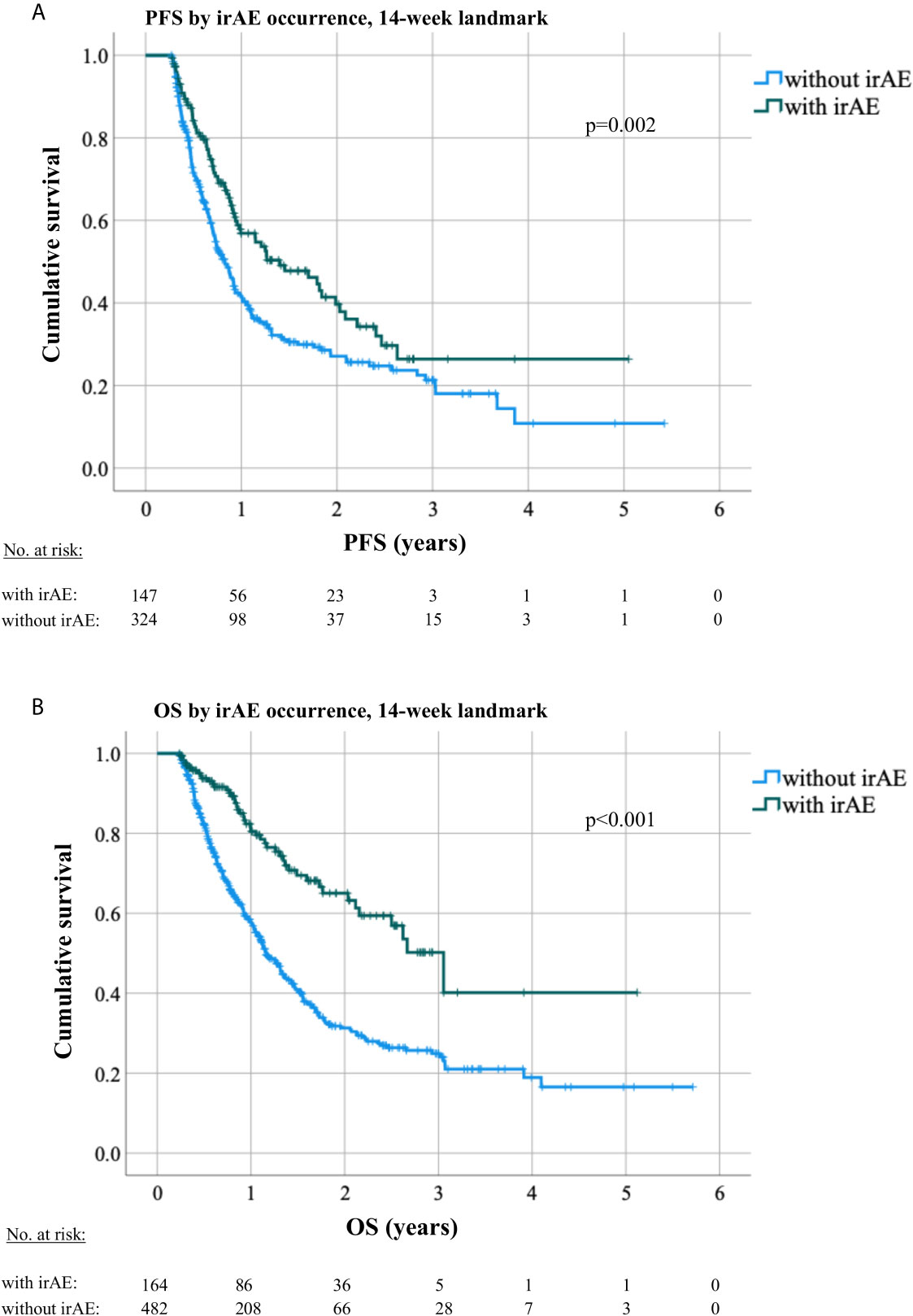
Figure 4 Progression-free and overall survival by occurrence of irAEs in a 14-week landmark analysis. (A) The median PFS under immunotherapy was 10 months (8.7–11.4) for patients without irAE vs. 17 months (10.3–23.6, logrank p = 0.003) for patients with irAEs in a 14-week landmark analysis. (B) The median OS was 15 months (13.5–16.6) for patients without irAE vs. 37 months (28.7–44.6, logrank p < 0.001) for patients with irAE in a 14-week landmark analysis.
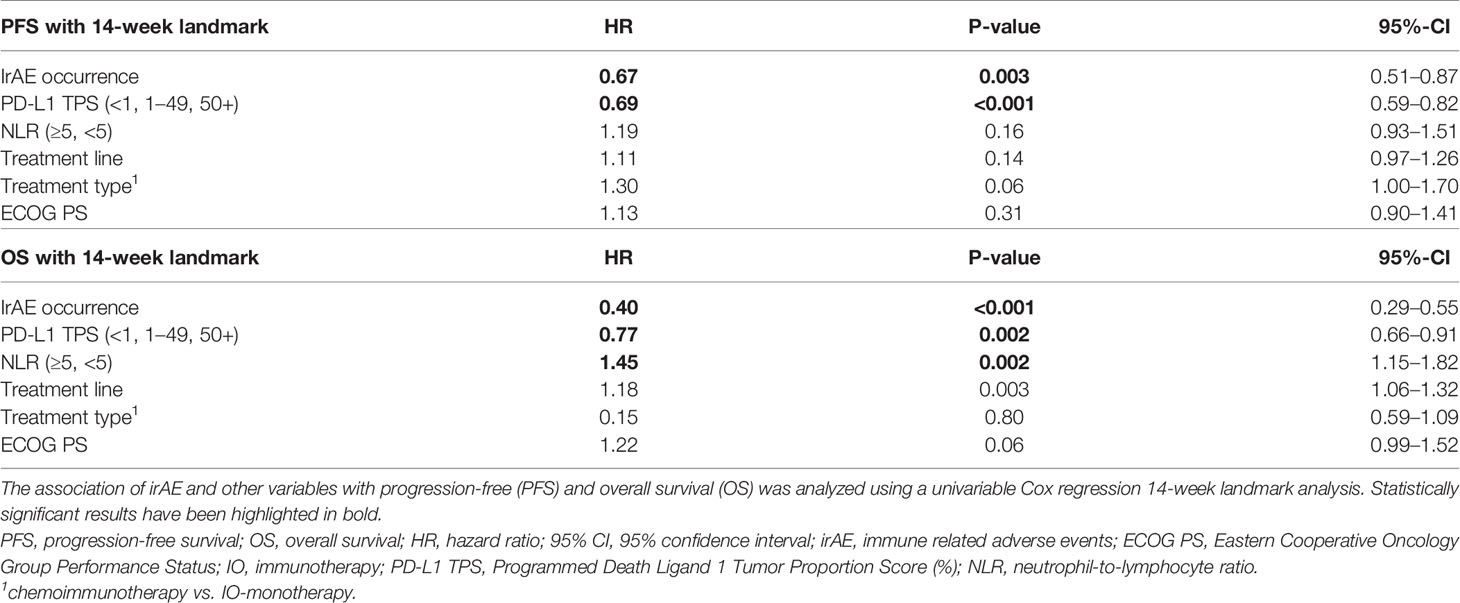
Table 3 Univariable analysis of progression-free and overall survival according to occurrence of irAE in NSCLC.
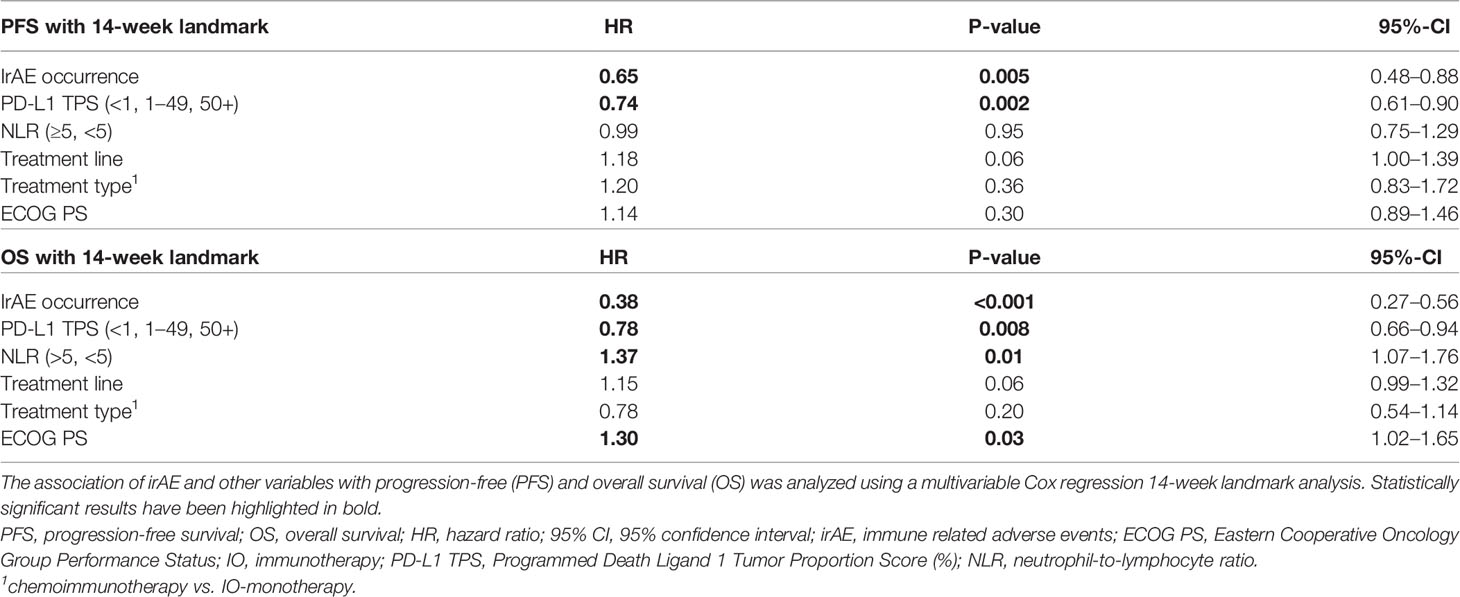
Table 4 Multivariable analysis of progression-free and overall survival according to occurrence of irAE in NSCLC.
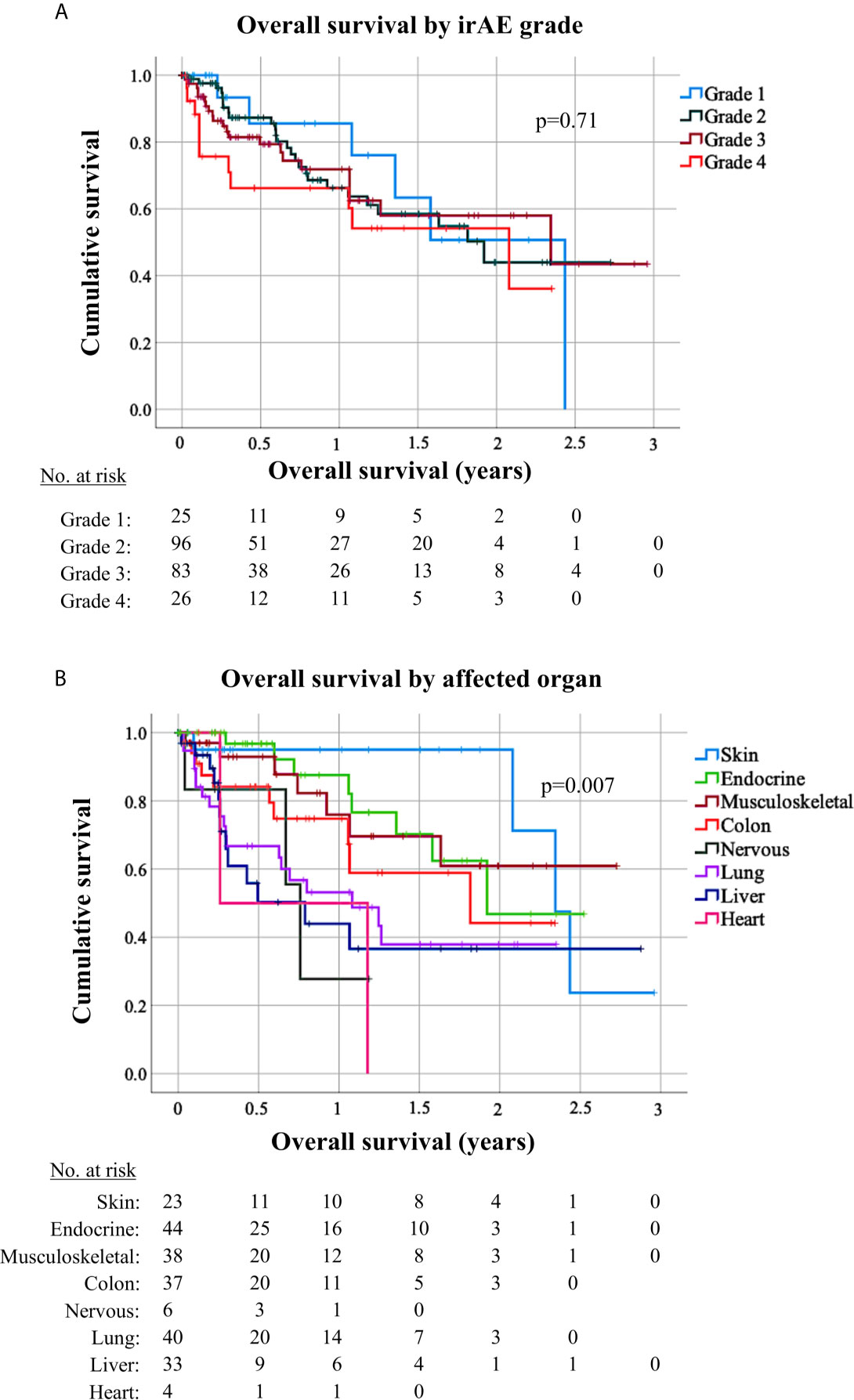
Figure 5 Survival of patients with grade 1–4 immune-related adverse events by affected organ. (A) Overall survival (OS) from start of immunotherapy for non-small-cell lung cancer (NSCLC) patients developing immune-related adverse events (irAEs) did not differ significantly by irAE grade (logrank p = 0.71). Median OS was 29 months [95% confidence interval (CI) n/a] in case of grade 1 irAE, 23 months (13.0–31.2) in case of grade 1 irAE, 28 months (3.7–52.6) in case of grade 3 irAE, and 25 months (8.5–41.4) in case of grade 4 irAE. (B) OS for NSCLC patients developing irAE showed significant differences according to the irAE type (logrank p = 0.007). Median OS was 28.1 months (CI 23.9–32.3) for patients with dermatologic irAE, with 2-year OS rate 95% (CI 85–100); 23 months (CI n/a) for patients with endocrinologic irAE, with 2-year OS rate 47% (CI 15–79); not reached for patients with musculoskeletal irAE, with a 2-year OS rate 61% (36–85); 22 months (3.1–40.6) for patients with colitis, with a 2-year OS rate 44% (14–75); 13 months (4.2–21.8) for patients with pneumonitis, with a 2-year OS rate 38% (19–57); and 9.5 months (1.4–17.6) for patients with hepatitis, with a 2-year OS rate 37% (14–59); 9.1 months (7.3–10.9) with a 2-year OS rate of 27.8% (CI 0–73) for patients with neurological irAE; and 3.1 months (CI na) for patients with cardiologic irAE with a 2-year OS rate 0%. Only irAE with >3 occurrences in our patients were included in this analysis.
As survival of NSCLC under immunotherapy is improving, with long-term, 5-year OS rates of 20–30% for stage IV disease currently (18, 19), and the use of PD-(L)1 inhibitors is expanding in locally-advanced and early stages (20), the interest for thorough analysis of irAEs is growing, because they pose important practical challenges for oncologists and a major limitation for patient outcome.
The irAE frequency in our study was 22% (189/894) overall, 10.7% (96/894) for grade 3–4 events, and similar between ICI-monotherapy and chemoimmunotherapy, which agrees well with the overall frequency of 20–30% for any grade, and 9–10% for grade 3–4 irAEs reported in the Keynote-24 and Keynote-189 clinical trials (21, 22). The spectrum of involved organs, mainly endocrine glands, lungs, musculoskeletal system, colon, and liver (Figure 2A), and median time to onset of 3.1 months were typical and also very similar to that reported by clinical trials and retrospective NSCLC series (21–24). Patient characteristics associated with development of irAEs were PD-L1 positivity (p = 0.003), a lower NLR (p < 0.001), and a better ECOG PS (p = 0.004, Table 1). Of note, each of these three parameters is also a predictor of better antitumor efficacy for immunotherapy in NSCLC, both in our patients (Table 3) and according to several previous studies (25–29). Therefore, it appears that the efficacy and potential for toxicity are interconnected in case of ICIs. Along the same lines, other studies have linked increase of cytokines, like CXCL9, CXCL10, CXCL11, and CXCL19, under ICIs as a sign of enhanced general immune reactivity with both subsequent tumor responses and development of irAE in NSCLC (30–32). A similar close relationship between efficacy and toxicity is also known to exist in another form of immunotherapy, namely between the graft-versus-host and graft-versus leukemia effects of allogeneic hematopoietic cell transplantation (33, 34). Besides systemic immunologic parameters, organ-specific factors probably also play a role in the development of specific irAE; for example the frequency of ICI pneumonitis was higher in cases of stage III NSCLC with invariable administration of full-dose thoracic radiotherapy compared to stage IV in our cohort. At the same time, however, it should be noted that palliative radiotherapy was not associated with detectable increase in risk (Table 1), which echoes the findings of other investigators and is an important consideration for everyday practice (35, 36). Other examples of organ-related factors that modulate the risk of specific irAE are preexisting interstitial lung disease, which is a strict ICI contraindication due to the very high risk of pneumonitis (37, 38), as well as an increased baseline TSH, which is associated with subsequent development of thyroiditis (39, 40). However, no reliable predictive scheme has been devised yet.
Another clinically important and controversial issue is the relationship between irAE and patient survival (41). Earlier studies in melanoma and NSCLC had shown conflicting results, namely favorable (41–45) or indifferent outcome for patients developing irAEs (46–48), which was in part due to different handling of the “immortal-time bias” (ITB, aka “guaranteed-time bias”) by the various investigators (49). In a recent meta-analysis, both the confounding effect of the ITB and the real, positive association between irAE and patient survival that remains significant after control for ITB could be shown (50). Nevertheless, the relationship between irAE and other predictors for longer PFS and OS, such as PD-L1, NLR, and ECOG PS, evident in our patients (Table 1), demonstrates an additional dimension of the question about the potential prognostic utility of irAE, namely whether irAEs have any independent value beyond that of already validated parameters. To our knowledge, the present study is the first to demonstrate this by combining rigorous ITB control using landmark (Tables 3, 4) and time-dependent analyses (Supplementary Table 5), with multivariable testing that includes all currently established survival predictors, both laboratory (PD-L1 TPS, NLR) and clinical (treatment type, treatment line, ECOG PS, Table 4 and Supplementary Table 5). Furthermore, particularly relevant for the contemporary practice is the inclusion of a large chemoimmunotherapy subcohort (n > 250, Table 1) in this analysis, which is the predominant therapeutic strategy for most NSCLC patients currently (3, 4), in contrast to previous studies who have analyzed IO-monotherapy (41, 50) or small chemoimmunotherapy series with less than 100 patients (51). Our results show that the relationship between occurrence of irAEs and ICI efficacy is very strong (HR = 0.4, Table 4), stronger than that of PD-L1 TPS or NLR, and that it persists regardless of concurrent or previous chemotherapy. An additional indication for the potency of this interaction is the lack of negative association between irAE grade and patient survival (Figure 5A), which has also been noted by others (52), as well as the recent finding that NSCLC patients with multiple irAEs have an even longer survival (53).
On the other hand, irAEs are increasingly also recognized as a considerable source of patient morbidity and financial burden for the health system (54), with important differences between the real-world and clinical trial setting (55); however systematic studies about routine irAE management are scarce. Of particular interest in this regard are details about the utilization of corticosteroids, which are used much more frequently than other immunosuppressants and have considerable toxicity potential (56). Our results show that the majority or irAEs (67%) will necessitate treatment with steroids, the average cumulative dose of which will exceed 1 g even for grade 2 events (Figure 3A). This is important, because cumulative corticosteroid doses >700 mg are known to result in clinically overt impairment of immune function, i.e. increased infections (57), which is well in line with the compromised antitumor efficacy of ICIs reported for patients suffering irAEs (58–60). In addition, several other adverse effects, like myopathy, lipodystrophy, memory and mood changes, already commence within the first 1–2 months if the daily dose exceeds 10 mg (61–64), which is the case in the majority of irAEs occurring in NSCLC patients (Figure 2A). In contrast, chronic side-effects, like bone density loss, which commences at 3 months (65), and cataracts, the risk of which becomes relevant after 1 year (66), are mainly relevant for patients with musculoskeletal irAEs, of which the average duration of steroid treatment uniquely exceeded 3 months (Figure 3B), and about one-third required long-term steroid therapy exceeding 12 months in our study. Indeed, immune checkpoint inhibitor-induced inflammatory arthritis has been described to persist after immunotherapy cessation and necessitates long-term therapy to prevent late relapses, for example with lower-dosed (≤10 mg/day) steroids in combination with disease modifying antirheumatic drugs (67). The multifaceted toxicity of corticosteroids is presumably one main reason, why irAEs that usually present with grade ≥3 and require higher steroid doses, like pneumonitis, colitis and hepatitis (Table 2), are associated with shorter OS than irAE affecting other organs (Figure 5B). In keeping with this, patients with skin irAEs, who require steroids least frequently (Table 2) and have the lowest cumulative dose, uniquely below 700 mg on average (Figure 3B), showed the longest OS relative to other irAE types (Figure 5B). An association between higher steroid doses and shorter OS in NSCLC patients with irAEs has also been noted by other investigators (68). IrAE fatality in our study was approximately 0.2%, similar to the 0.36–0.38% reported by a global meta-analysis for PD-(L)1 inhibitors across cancer types (69).
The main limitations of our study stem from its retrospective nature, which cannot exclude potential confounders, and is also not as accurate regarding estimation of PFS and other parameters as prospective clinical trials. In order to control this, we annotated our cases extensively and performed multivariable analysis including all factors known to be associated with patient survival. In addition, we accounted for ITB by 12- and 14-week landmark, as well as time-dependent analyses. It should also be noted that our study only enrolled patients with NSCLC from Germany, which limits generalizability of the results to other cancer types and/or other countries with potentially different patterns of clinical practice. Also, for some less frequently affected organs, like the nervous system, heart, and blood, the number of available cases was small and precluded in-depth study.
In conclusion, our study demonstrates that irAEs affect approximately 20–25% of ICI-treated NSCLC patients regardless of additional previous chemotherapy, most necessitating treatment suspension and initiation of steroids. Despite more frequent occurrence with PD-L1 positive tumors, lower NLR, and better ECOG PS, irAEs, particularly those affecting the skin, are independently associated with longer survival. Lethality is below 1%.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
The studies involving human participants were reviewed and approved by the ethics committee of Heidelberg University (S-296/2016). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
LDa: conceptualization, methodology, investigation; data curation, formal analysis, visualization, writing—original draft. ME: investigation, data curation, validation, writing—review & editing. FB: investigation, data curation, validation, writing—review & editing. JK: investigation, data curation, validation, writing—review & editing. DK: investigation; data curation, validation, writing—original draft. HS: investigation, data curation, validation, writing—review & editing. RS: investigation, data curation, validation, writing—review & editing. A-LV: investigation; data curation, validation, writing—review & editing. FL: investigation, data curation, validation, writing—review & editing. LDi: investigation, data curation, validation, writing—review & editing. SL: investigation, data curation, validation, writing—review & editing. MF: investigation, data curation, validation, writing—review & editing. TM: investigation, data curation, validation, writing—review & editing. MK: investigation; data curation, writing—review & editing. BK: validation, supervision, project administration, writing—review & editing. AS: validation, supervision, project administration, writing—review & editing. MT: conceptualization, methodology, validation, supervision, funding acquisition, writing—review & editing. PC: conceptualization, methodology, investigation; data curation, formal analysis, visualization, supervision, project administration; writing—original draft, writing—review & editing. All authors contributed to the article and approved the submitted version.
This work was supported by the German Center for Lung Research (DZL).
FB: research funding from BMS and travel grants from BMS and MSD. JK: research funding from AstraZeneca and Celgene. DK: advisory board and speaker’s honoraria from AstraZeneca, BMS, Pfizer. RS: research funding from BMS. KB: research funding from Novartis and Abbvie, speaker’s honoraria/advisory board/travel grants from Abbvie, BMS, Gilead/Galapagos, Janssen, Lilly, Medac, MSD, Mundipharma, Novartis, Pfizer, Roche, UCB. AS: advisory board honoraria from BMS, AstraZeneca, ThermoFisher, Novartis, speaker’s honoraria from BMS, Illumina, AstraZeneca, Novartis, ThermoFisher, MSD, Roche, and research funding from Chugai. MT: advisory board honoraria from Novartis, Lilly, BMS, MSD, Roche, Celgene, Takeda, AbbVie, Boehringer, speaker’s honoraria from Lilly, MSD, Takeda, research funding from AstraZeneca, BMS, Celgene, Novartis, Roche and travel grants from BMS, MSD, Novartis, Boehringer. PC: research funding from AstraZeneca, Novartis, Roche, Takeda, and advisory board/lecture fees from AstraZeneca, Boehringer Ingelheim, Chugai, Novartis, Pfizer, Roche, Takeda.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Simone Kuder for help with the data collection.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.703893/full#supplementary-material
1. Robert C. A Decade of Immune-Checkpoint Inhibitors in Cancer Therapy. Nat Commun (2020) 11:3801. doi: 10.1038/s41467-020-17670-y
2. Gaissmaier L, Christopoulos P. Immune Modulation in Lung Cancer: Current Concepts and Future Strategies. Respiration (2020) 99:903–29. doi: 10.1159/000510385
3. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-Small Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2018) 29:iv192–237. doi: 10.1093/annonc/mdy275
4. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Esmo Clinical Practice Living Guidelines - Metastatic non-Small-Cell Lung Cancer (2021). Available at: https://www.esmo.org/guidelines/lung-and-chest-tumours/clinical-practice-living-guidelines-metastatic-non-small-cell-lung-cancer.
5. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50%. J Clin Oncol (2021), JCO.21.00174. doi: 10.1200/JCO.21.00174
6. Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, et al. Association Between Immune-Related Adverse Events and Efficacy of Immune Checkpoint Inhibitors in Non–small-cell Lung Cancer. Clin Lung Cancer (2019) 20:201–7. doi: 10.1016/j.cllc.2018.10.002
7. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer institute. Common Terminology Criteria for Adverse Events (Ctcae) Version 5.0. (2017).
8. Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated With Immune Checkpoint Blockade. N Engl J Med (2018) 378:158–68. doi: 10.1056/NEJMra1703481
9. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thor Oncol (2015) 10:1243–60. doi: 10.1097/JTO.0000000000000630
10. Volckmar A-L, Leichsenring J, Kirchner M, Christopoulos P, Neumann O, Budczies J, et al. Combined Targeted DNA and RNA Sequencing of Advanced NSCLC in Routine Molecular Diagnostics: Analysis of the First 3,000 Heidelberg Cases. Int J Cancer (2019) 145:649–61. doi: 10.1002/ijc.32133
11. Valero C, Lee M, Hoen D, Weiss K, Kelly DW, Adusumilli PS, et al. Pretreatment Neutrophil-to-Lymphocyte Ratio and Mutational Burden as Biomarkers of Tumor Response to Immune Checkpoint Inhibitors. Nat Commun (2021) 12:729. doi: 10.1038/s41467-021-20935-9
12. Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim TM, et al. Post-Treatment Neutrophil-to-Lymphocyte Ratio at Week 6 Is Prognostic in Patients With Advanced Non-Small Cell Lung Cancers Treated With anti-PD-1 Antibody. Cancer Immunol Immunother (2018) 67:459–70. doi: 10.1007/s00262-017-2092-x
13. Bartlett CH, Mardekian J, Cotter M, Huang X, Zhang Z, Parrinello CM, et al. Concordance of Real World Progression Free Survival (PFS) on Endocrine Therapy as First Line Treatment for Metastatic Breast Cancer Using Electronic Health Record With Proper Quality Control Versus Conventional PFS From a Phase 3 Trial. Cancer Res (2018) 78:P3–17-03. doi: 10.1158/1538-7445.Sabcs17-p3-17-03
14. Ma X, Nussbaum NC, Magee K, Bourla AB, Tucker M, Bellomo L, et al. Comparison of Real-World Response Rate (rwRR) to RECIST-based Response Rate in Patients With Advanced Non-Small Cell Lung Cancer (Ansclc). Ann Oncol (2019) 30:1581P. doi: 10.1093/annonc/mdz260.103
15. Haanen JB, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of Toxicities From Immunotherapy: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2017) 28:iv119–42. doi: 10.1093/annonc/mdx225
16. Benesova K, Lorenz H-M, Leipe J, Jordan K. How I Treat Cancer: Treatment of Rheumatological Side Effects of Immunotherapy. ESMO Open (2019) 4:e000529. doi: 10.1136/esmoopen-2019-000529
17. Buder-Bakhaya K, Benesova K, Schulz C, Anwar H, Dimitrakopoulou-Strauss A, Weber TF, et al. Characterization of Arthralgia Induced by PD-1 Antibody Treatment in Patients With Metastasized Cutaneous Malignancies. Cancer Immunol Immunother (2018) 67:175–82. doi: 10.1007/s00262-017-2069-9
18. Brahmer JR, Rodriguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Lba51 KEYNOTE-024 5-Year OS Update: First-Line (1L) Pembrolizumab (Pembro) vs Platinum-Based Chemotherapy (Chemo) in Patients (Pts) With Metastatic NSCLC and PD-L1 Tumour Proportion Score (TPS) ≥50%. Ann Oncol (2020) 31:S1181–2. doi: 10.1016/j.annonc.2020.08.2284
19. Gettinger S, Borghaei H, Brahmer J, Chow L, Burgio M, de Castro Carpeno J, et al. Oa14.04 Five-Year Outcomes From the Randomized, Phase 3 Trials CheckMate 017/057: Nivolumab vs Docetaxel in Previously Treated Nsclc. J Thor Oncol (2019) 14:S244–5. doi: 10.1016/j.jtho.2019.08.486
20. Heigener DF, Reck M. Immune Checkpoint Inhibition in Non-metastatic non-Small Cell Lung Cancer: Chance for Cure? Drugs (2019) 79:1937–45. doi: 10.1007/s40265-019-01222-w
21. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, de Angelis F, et al. Pembrolizumab Plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
22. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
23. Remon J, Mezquita L, Corral J, Vilariño N, Reguart N. Immune-Related Adverse Events With Immune Checkpoint Inhibitors in Thoracic Malignancies: Focusing on Non-Small Cell Lung Cancer Patients. J Thor Dis (2018) 10:S1516–33. doi: 10.21037/jtd.2017.12.52
24. Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the Anti-PD-1 and Anti-PD-L1 Immune Checkpoint Antibodies. Ann Oncol (2015) 26:2375–91. doi: 10.1093/annonc/mdv383
25. Xu Y, Wan B, Chen X, Zhan P, Zhao Y, Zhang T, et al. The Association of PD-L1 Expression With the Efficacy of Anti- PD-1/PD-L1 Immunotherapy and Survival of Non-Small Cell Lung Cancer Patients: A Meta-Analysis of Randomized Controlled Trials. Transl Lung Canc Res (2019) 8:413–28. doi: 10.21037/tlcr.2019.08.09
26. Li Y, Zhang Z, Hu Y, Yan X, Song Q, Wang G, et al. Pretreatment Neutrophil-to-Lymphocyte Ratio (Nlr) May Predict the Outcomes of Advanced Non-Small-Cell Lung Cancer (Nsclc) Patients Treated With Immune Checkpoint Inhibitors (Icis). Front Oncol (2020) 10:654. doi: 10.3389/fonc.2020.00654
27. Dall’Olio FG, Maggio I, Massucci M, Mollica V, Fragomeno B, Ardizzoni A. ECOG Performance Status ≥2 as a Prognostic Factor in Patients With Advanced Non Small Cell Lung Cancer Treated With Immune Checkpoint Inhibitors-a Systematic Review and Meta-Analysis of Real World Data. Lung Cancer (2020) 145:95–104. doi: 10.1016/j.lungcan.2020.04.027
28. Addeo A, Metro G, Signorelli D, Economopoulou P, Roila F, Banna GL, et al. Poor Performance Status and Front-Line Pembrolizumab in Advanced Non-Small-Cell Lung Cancer (NSCLC) Patients With PD-L1>50%. J Clin Oncol (2020) 38:e21651–1. doi: 10.1200/JCO.2020.38.15_suppl.e21651
29. Rheinheimer S, Heussel C-P, Mayer P, Gaissmaier L, Bozorgmehr F, Winter H, et al. Oligoprogressive Non-Small-Cell Lung Cancer Under Treatment With PD-(L)1 Inhibitors. Cancers (Basel) (2020) 12:1046. doi: 10.3390/cancers12041046
30. Wang Y, Chen H, Zhang T, Yang X, Zhong J, Wang Y, et al. Plasma Cytokines interleukin-18 and C-X-C Motif Chemokine Ligand 10 Are Indicative of the Anti-Programmed Cell Death Protein-1 Treatment Response in Lung Cancer Patients. Annal Transl Med (2021) 9:33. doi: 10.21037/atm-20-1513
31. Khan S, Khan SA, Luo X, Fattah FJ, Saltarski J, Gloria-McCutchen Y, et al. Immune Dysregulation in Cancer Patients Developing Immune-Related Adverse Events. Br J Cancer (2019) 120:63–8. doi: 10.1038/s41416-018-0155-1
32. Hommes JW, Verheijden RJ, Suijkerbuijk KP, Hamann D. Biomarkers of Checkpoint Inhibitor Induced Immune-Related Adverse Events-a Comprehensive Review. Front Oncol (2020) 10:585311. doi: 10.3389/fonc.2020.585311
33. Negrin RS. Graft-Versus-Host Disease Versus Graft-Versus-Leukemia. Hematol Am Soc Hematol Educ Program (2015) 2015:225–30. doi: 10.1182/asheducation-2015.1.225
34. Pasquini MC. Impact of Graft-Versus-Host Disease on Survival. Best Pract Res Clin Haematol (2008) 21:193–204. doi: 10.1016/j.beha.2008.02.011
35. Lesueur P, Escande A, Thariat J, Vauléon E, Monnet I, Cortot A, et al. Safety of Combined PD-1 Pathway Inhibition and Radiation Therapy for Non-Small-Cell Lung Cancer: A Multicentric Retrospective Study From the GFPC. Cancer Med (2018) 7:5505–13. doi: 10.1002/cam4.1825
36. Voong KR, Hazell SZ, Fu W, Hu C, Lin CT, Ding K, et al. Relationship Between Prior Radiotherapy and Checkpoint-Inhibitor Pneumonitis in Patients With Advanced Non-Small-Cell Lung Cancer. Clin Lung Cancer (2019) 20:e470–9. doi: 10.1016/j.cllc.2019.02.018
37. Gemmill JA L, Sher A. Anti-Pd-1-Related Exacerbation of Interstitial Lung Disease in a Patient With Non-Small Cell Lung Cancer: A Case Presentation and Review of the Literature. Cancer Invest (2020) 38:365–71. doi: 10.1080/07357907.2020.1783677
38. Shimoji K, Masuda T, Nakanishi Y, Yamaguchi K, Sakamoto S, Horimasu Y, et al. Pre-Existing Interstitial Lung Abnormalities Are Risk Factors for Immune Checkpoint Inhibitor-Induced Interstitial Lung Disease in Non-NSCLC Cancers. J Clin Oncol (2020) 38:e15171–1. doi: 10.1200/JCO.2020.38.15_suppl.e15171
39. Brilli L, Danielli R, Campanile M, Secchi C, Ciuoli C, Calabrò L, et al. Baseline Serum TSH Levels Predict the Absence of Thyroid Dysfunction in Cancer Patients Treated With Immunotherapy. J Endocrinol Invest (2020). doi: 10.1007/s40618-020-01480-6. [Epub ahead of print].
40. Kimbara S, Fujiwara Y, Iwama S, Ohashi K, Kuchiba A, Arima H, et al. Association of Antithyroglobulin Antibodies With the Development of Thyroid Dysfunction Induced by Nivolumab. Cancer Sci (2018) 109:3583–90. doi: 10.1111/cas.13800
41. Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are Immune-Related Adverse Events Associated With the Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer? A Systematic Review and Meta-Analysis. BMC Med (2020) 18:87. doi: 10.1186/s12916-020-01549-2
42. Indini A, Di Guardo L, Cimminiello C, Prisciandaro M, Randon G, de Braud F, et al. Immune-Related Adverse Events Correlate With Improved Survival in Patients Undergoing Anti-PD1 Immunotherapy for Metastatic Melanoma. J Cancer Res Clin Oncol (2019) 145:511–21. doi: 10.1007/s00432-018-2819-x
43. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol (2018) 4:374–8. doi: 10.1001/jamaoncol.2017.2925
44. Kim H, Kim M, Lee S-H, Park SY, Kim YN, Kim H, et al. Development of Thyroid Dysfunction Is Associated With Clinical Response to PD-1 Blockade Treatment in Patients With Advanced Non-Small Cell Lung Cancer. Oncoimmunology (2017) 7:e1375642. doi: 10.1080/2162402X.2017.1375642
45. Judd J, Zibelman M, Handorf E, O’Neill J, Ramamurthy C, Bentota S, et al. Immune-Related Adverse Events as a Biomarker in Non-Melanoma Patients Treated With Programmed Cell Death 1 Inhibitors. Onoclogist (2017) 22:1232–7. doi: 10.1634/theoncologist.2017-0133
46. Horvat TZ, Adel NG, Dang T-O, Momtaz P, Postow MA, Callahan MK, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol (2015) 33:3193–8. doi: 10.1200/JCO.2015.60.8448
47. Bjørnhart B, Hansen KH, Jørgensen TL, Herrstedt J, Schytte T. Efficacy and Safety of Immune Checkpoint Inhibitors in a Danish Real Life non-Small Cell Lung Cancer Population: A Retrospective Cohort Study. Acta Oncol (2019) 58:953–61. doi: 10.1080/0284186X.2019.1615636
48. Chen X, Nie J, Dai L, Hu W, Zhang J, Han J, et al. Immune-Related Adverse Events and Their Association With the Effectiveness of PD-1/PD-L1 Inhibitors in Non-Small Cell Lung Cancer: A Real-World Study From China. Front Oncol (2021) 11:607531. doi: 10.3389/fonc.2021.607531
49. Dall’Olio FG, Di Nunno V, Massari F. Immortal Time Bias Question in the Association Between Toxicity and Outcome of Immune Checkpoint Inhibitors. J Clin Oncol (2019) 38:105–6. doi: 10.1200/JCO.19.01728
50. Dall’Olio FG, Rizzo A, Mollica V, Massucci M, Maggio I, Massari F. Immortal Time Bias in the Association Between Toxicity and Response for Immune Checkpoint Inhibitors: A Meta-Analysis. Immunotherapy (2021) 13:257–70. doi: 10.2217/imt-2020-0179
51. Morimoto K, Yamada T, Takumi C, Ogura Y, Takeda T, Onoi K, et al. Immune-Related Adverse Events Are Associated With Clinical Benefit in Patients With Non-Small-Cell Lung Cancer Treated With Immunotherapy Plus Chemotherapy: A Retrospective Study. Front Oncol (2021) 11:630136. doi: 10.3389/fonc.2021.630136
52. Cortellini A, Chiari R, Ricciuti B, Metro G, Perrone F, Tiseo M, et al. Correlations Between the Immune-Related Adverse Events Spectrum and Efficacy of Anti-PD1 Immunotherapy in NSCLC Patients. Clin Lung Cancer (2019) 20:237–247.e1. doi: 10.1016/j.cllc.2019.02.006
53. Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, et al. Multisystem Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors for Treatment of Non–Small Cell Lung Cancer. JAMA Oncol (2020) 6:1952. doi: 10.1001/jamaoncol.2020.5012
54. Olsson-Brown AC, Baxter M, Dobeson C, Feeney L, Lee R, Maynard A, et al. Real-World Outcomes of Immune-Related Adverse Events in 2,125 Patients Managed With Immunotherapy: A United Kingdom Multicenter Series. J Clin Oncol (2020) 38:7065. doi: 10.1200/JCO.2020.38.15_suppl.7065
55. Raschi E, Gatti M, Gelsomino F, Ardizzoni A, Poluzzi E, De Ponti F. Lessons to be Learnt From Real-World Studies on Immune-Related Adverse Events With Checkpoint Inhibitors: A Clinical Perspective From Pharmacovigilance. Targeted Oncol (2020) 15:449–66. doi: 10.1007/s11523-020-00738-6
56. Aulakh R, Singh S. Strategies for Minimizing Corticosteroid Toxicity: A Review. Indian J Pediatr (2008) 75:1067–73. doi: 10.1007/s12098-008-0211-6
57. Stuck AE, Minder CE, Frey FJ. Risk of Infectious Complications in Patients Taking Glucocorticosteroids. Rev Infect Dis (1989) 11:954–63. doi: 10.1093/clinids/11.6.954
58. Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune Checkpoint Inhibitor Outcomes for Patients With Non-Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J Clin Oncol (2019) 37:1927–34. doi: 10.1200/JCO.19.00189
59. Drakaki A, Luhn P, Wakelee H, Dhillon PK, Kent M, Shim J, et al. Association of Systemic Corticosteroids With Overall Survival in Patients Receiving Cancer Immunotherapy for Advanced Melanoma, non-Small Cell Lung Cancer or Urothelial Cancer in Routine Clinical Practice. Ann Oncol (2019) 30:xi16–7. doi: 10.1093/annonc/mdz449.001
60. de Giglio A, Mezquita L, Auclin E, Blanc-Durand F, El-Amarti L, Caramella C, et al. Impact of Early Introduction of Steroid on Immune-Checkpoint Inhibitors (ICI) in Patients With Advanced non-Small Cell Lung Cancer Treated. Ann Oncol (2019) 30:xi16. doi: 10.1093/annonc/mdz449
61. Moghadam-Kia S, Werth VP. Prevention and Treatment of Systemic Glucocorticoid Side Effects. Int J Dermatol (2010) 49:239–48. doi: 10.1111/j.1365-4632.2009.04322.x
62. Fardet L, Cabane J, Lebbé C, Morel P, Flahault A. Incidence and Risk Factors for Corticosteroid-Induced Lipodystrophy: A Prospective Study. J Am Acad Dermatol (2007) 57:604–9. doi: 10.1016/j.jaad.2007.04.018
63. Brown ES. Effects of Glucocorticoids on Mood, Memory, and the Hippocampus. Treatment and Preventive Therapy. Ann N Y Acad Sci (2009) 1179:41–55. doi: 10.1111/j.1749-6632.2009.04981.x
64. Warrington TP, Bostwick JM. Psychiatric Adverse Effects of Corticosteroids. Mayo Clin Proc (2006) 81:1361–7. doi: 10.4065/81.10.1361
65. van Staa TP, Leufkens HG, Cooper C. The Epidemiology of Corticosteroid-Induced Osteoporosis: A Meta-Analysis. Osteoporosis Int (2002) 13:777–87. doi: 10.1007/s001980200108
66. Urban RC, Cotlier E. Corticosteroid-Induced Cataracts. Surv Ophthalmol (1986) 31:102–10. doi: 10.1016/0039-6257(86)90077-9
67. Braaten TJ, Brahmer JR, Forde PM, Le D, Lipson EJ, Naidoo J, et al. Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis Persists After Immunotherapy Cessation. Ann Rheum Dis (2020) 79:332–8. doi: 10.1136/annrheumdis-2019-216109
68. Riudavets M, Mosquera J, Garcia-Campelo R, Serra J, Anguera G, Gallardo P, et al. Immune-Related Adverse Events and Corticosteroid Use for Cancer-Related Symptoms Are Associated With Efficacy in Patients With Non-small Cell Lung Cancer Receiving Anti-Pd-(L)1 Blockade Agents. Front Oncol (2020) 10:1677. doi: 10.3389/fonc.2020.01677
Keywords: immune-related adverse events, immunotherapy, immune-checkpoint inhibitors, treatment interruption, prognosis, lethality
Citation: Daniello L, Elshiaty M, Bozorgmehr F, Kuon J, Kazdal D, Schindler H, Shah R, Volckmar A-L, Lusky F, Diekmann L, Liersch S, Faehling M, Muley T, Kriegsmann M, Benesova K, Stenzinger A, Thomas M and Christopoulos P (2021) Therapeutic and Prognostic Implications of Immune-Related Adverse Events in Advanced Non-Small-Cell Lung Cancer. Front. Oncol. 11:703893. doi: 10.3389/fonc.2021.703893
Received: 30 April 2021; Accepted: 09 June 2021;
Published: 29 June 2021.
Edited by:
Junji Uchino, Kyoto Prefectural University of Medicine, JapanReviewed by:
Joanna Julia Domagala-Kulawik, Medical University of Warsaw, PolandCopyright © 2021 Daniello, Elshiaty, Bozorgmehr, Kuon, Kazdal, Schindler, Shah, Volckmar, Lusky, Diekmann, Liersch, Faehling, Muley, Kriegsmann, Benesova, Stenzinger, Thomas and Christopoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petros Christopoulos, cGV0cm9zLmNocmlzdG9wb3Vsb3NAbWVkLnVuaS1oZWlkZWxiZXJnLmRl orcid.org/0000-0002-7966-8980
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.