
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 25 June 2021
Sec. Cancer Imaging and Image-directed Interventions
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.693640
 Hao Wang1†
Hao Wang1† Wenwei Zhu1†
Wenwei Zhu1† Shuhua Ren2
Shuhua Ren2 Yanyan Kong2
Yanyan Kong2 Qi Huang2
Qi Huang2 Jun Zhao3
Jun Zhao3 Yihui Guan2
Yihui Guan2 Huliang Jia1
Huliang Jia1 Jinhong Chen1
Jinhong Chen1 Lu Lu1*
Lu Lu1* Fang Xie2*
Fang Xie2* Lunxiu Qin1*
Lunxiu Qin1*Background: Fibroblast activation protein (FAP) is commonly expressed in activated stromal fibroblasts in various epithelial tumours. Recently, 68Ga-FAPI-04 has been used for tumour imaging in positron emission tomography/computed tomography (PET/CT). This study aimed to compare the diagnostic performances of 68Ga-FAPI-04 PET/CT and 18F-FDG PET/CT in hepatocellular carcinoma (HCC), and to assess factors associated with 68Ga-FAPI-04 uptake in HCC.
Materials and Methods: Twenty-nine patients with suspiciously HCC who received both 18F-FDG and 68Ga-FAPI-04 PET/CT were included in this retrospective study. The results were interpreted by two experienced nuclear medicine physicians independently. The maximum and mean standardized uptake values (SUVmax and SUVmean) were measured in the lesions and liver background, respectively. The tumour-to-background ratio (TBR) was then calculated as lesion’s SUVmax divided by background SUVmean.
Results: A total of 35 intrahepatic lesions in 25 patients with HCC were finally involved in the statistical analysis. 68Ga-FAPI-04 PET/CT showed a higher sensitivity than 18F-FDG PET/CT in detecting intrahepatic HCC lesions (85.7% vs. 57.1%, P = 0.002), including in small (≤ 2 cm in diameter; 68.8% vs. 18.8%, P = 0.008) and well- or moderately-differentiated (83.3% vs. 33.3%, P = 0.031) tumors. SUVmax was comparable between 68Ga-FAPI-04 and 18F-FDG (6.96 ± 5.01 vs. 5.89 ± 3.38, P > 0.05), but the TBR was significantly higher in the 68Ga-FAPI-04 group compared with the 18F-FDG group (11.90 ± 8.35 vs. 3.14 ± 1.59, P < 0.001). SUVmax and the TBR in 68Ga-FAPI-04 positive lesions were associated with tumour size (both P < 0.05), but not the remaining clinical and pathological features (all P > 0.05).
Conclusions: 68Ga-FAPI-04 PET/CT is more sensitive than 18F-FDG PET/CT in detecting HCC lesions, and 68Ga-FAPI-04 uptake is correlated mainly with tumour size.
Hepatocellular carcinoma (HCC), the most frequent primary liver cancer, is the fourth most common cause of cancer-related death worldwide (1, 2). The majority of HCCs occur in patients with underlying liver disease, mostly as a result of hepatitis B or C virus (HBV or HCV) infection or alcohol abuse (3). Unlike many other malignant tumours, HCC can be diagnosed by imaging based on non-invasive criteria without confirmatory pathology (4). Therefore, imaging plays a critical role in the detection and diagnosis of HCC. Conventional imaging modalities, including computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound, are mainly utilized for anatomical evaluation, with limited value in the assessment of morphologically atypical lesions (5). In contrast, positron emission tomography/computed tomography (PET/CT) as one of the functional imaging approaches has the potential to provide relevant biological information in HCC and to improve response assessment (6). Furthermore, a subset of HCCs cannot be diagnosed non-invasively either because the patients do not have cirrhosis or the lesions do not follow established enhancement patterns on contrast-enhanced CT or MRI (1, 3). Therefore, molecular imaging with PET/CT offers potential additional advantages to non-invasively confirm a diagnosis of HCC. However, the most widely available clinical PET tracer, 18F-FDG, shows poor sensitivity for the detection of HCC ranging from 40% to 68%, mainly because of the relatively high glucose-6-phosphatase activity found in low-grade HCC (6). In addition, it is not useful for the detection of small HCC lesions (7, 8). Therefore, several new tracers have been developed and applied for HCC detection, including 11C-acetate, 11C-choline, 68Ga-PSMA (9–12).
Cancer-associated fibroblasts (CAFs) are among the most crucial components of the tumour microenvironment that creates a favourable microenvironment for tumour growth, invasion and metastasis (13, 14). Fibroblast activation protein (FAP), a cell surface glycoprotein belonging to the serine protease family, is commonly expressed in activated stromal fibroblasts in various epithelial tumours (15, 16). Recent investigations indicated that 68Ga-labelled FAP inhibitor (FAPI) shows an equal or even improved tumour imaging with lower background uptake in the liver and the brain in comparison to 18F-FDG in various cancers (17, 18). 68Ga-FAPI-04 PET/CT was also revealed to have high sensitivity in detecting hepatic malignancies (19–21). Therefore, 68Ga-FAPI-04 may be a potential tracer for visualizing HCC by targeting CAFs that are abundant in the tumour microenvironment. This study aimed to comparatively assess the diagnostic performances of 68Ga-FAPI-04 PET/CT and 18F-FDG PET/CT in HCC and to assess factors associated with the uptake of 68Ga-FAPI-04 in HCC.
This is a post-hoc analysis of a prior prospective study conducted at the Huashan Hospital of Fudan University. Twenty-nine patients with suspiciously incipient or recurrent HCC determined by clinical manifestations and conventional imaging techniques (CT, MRI and ultrasound) were included in this retrospective study. They underwent both 18F-FDG and 68Ga-FAPI-04 PET/CT examinations with an interval of one day before surgical treatment. In patients who underwent surgery or biopsy, the definitive diagnosis was confirmed by pathology. In patients who underwent transarterial chemoembolization (TACE), HCC diagnosis was based on a specific imaging pattern of hyperenhancement in the arterial phase and washout in the venous or delayed phase, on contrast-enhanced CT or MRI in the setting of liver cirrhosis (1, 3). This study was approved by the institutional ethics committee, and written informed consent was obtained from all patients.
Whole-body static FDG PET/CT scans were obtained as a routine procedure on a dedicated PET/CT scanner (Biograph mCT Flow scanner, Siemens, Germany). Whole-body 68Ga-FAPI-04 PET/CT scans were obtained on another PET/CT scanner (μMI510, Union imaging, Shanghai, China) within 60 min after intravenous injection of ~ 185 MBq (~5 mCi) of 68Ga-FAPI-04. Low-dose CT scans were obtained for attenuation correction and image fusion. PET images were acquired in the 3D mode, and reconstructed by the ordered subset expectation maximization 3D (OSEM 3D) method.
Because two different PETs were applied in this study, SUVs were normalized after data collection for PET/CT system performance harmonization. NEMA IEC body phantom (Data Spectrum Corporation, Durham, NC, USA) with 6 simulated lesion spheres (diameters of 10 mm, 13 mm, 17 mm, 22 mm, 28 mm and 37 mm, respectively) and 2, 4, 8, and 16 times of background activity (2 kBq/mL of background activity concentration) based on routine scan protocols was applied for SUV normalization.
18F-FDG and 68Ga-FAPI-04 PET/CT images were interpreted independently by two experienced nuclear medicine physicians blinded to other imaging and pathology results. The maximum standardized uptake value (SUVmax) was measured by delineating a spherical region of interest (ROI) for each lesion. The mean standardized uptake value (SUVmean) of the liver background was measured by drawing a spherical ROI with 2 cm diameter in the non-tumour hepatic parenchyma of the right lobe in each patient. The tumour-to-background ratio (TBR) was calculated by dividing the lesion’s SUVmax with the background SUVmean. A lesion was considered to be positive on the basis of the visual judgment of elevated uptake in the tumour tissue by 2 experienced nuclear medicine physicians independently, supported by the calculation of the TBRs of 18F-FDG and 68Ga-FAPI-04, respectively. Any difference of opinion between these two physicians was settled by mutual agreement.
All statistical analyses were conducted with the STATA statistical analysis software (StataCorp LLC, version 15.1). Categorical variables were presented as frequency and percentage, and continuous variables as mean ± standard deviation (SD). The McNemar’s test and Fisher exact test were performed to compare categorical variables. Non-parametric tests were carried out for the comparison of continuous variables with non-normal distribution. The Spearman rank correlation coefficient was determined to assess the correlation between continuous variables with non-normal distribution. Two-tailed P < 0.05 was considered statistically significant.
Twenty-nine patients were included in the current study, including 23 treated by hepatic surgery, 5 administered TACE, and one that underwent biopsy only. Except for 3 patients who were diagnosed with benign hepatic nodules, the remaining 26 patients were diagnosed with HCC. One recurrent HCC case who underwent surgical resection had extensive peritoneal dissemination but no intrahepatic lesions. Therefore, 25 HCC patients with 35 intrahepatic lesions were finally involved in the statistical analysis. The study flowchart is presented in Figure 1. According to microvascular invasion (MVI) number and distribution, 2, 8 and 10 patients were categorized into the M0 (no MVI), M1 (≤ 5 MVI in adjacent liver tissue ≤ 1 cm away from the HCC), and M2 (> 5 MVI or MVI in adjacent liver tissue > 1 cm away from the HCC) groups, respectively. According to the American Joint Committee on Cancer (AJCC) cancer staging system (8th Edition), 5, 10, 4 and 1 patients were categorized into stage I, II;, III and IV, respectively. The general characteristics of the 25 HCC patients are summarized in Table 1.
The results of the patient-based analysis of 18F-FDG and 68Ga-FAPI-04 PET/CT are summarized in Table 2. Of the 35 intrahepatic HCCs, 20 were tested positive by both 18F-FDG and 68Ga-FAPI-04 PET/CT, 10 were tested positive by 68Ga-FAPI-04 PET/CT alone, and 5 were not tested positive by either method. 68Ga-FAPI-04 PET/CT showed a better sensitivity in detecting intrahepatic lesions compared with 18F-FDG PET/CT (85.7% vs. 57.1%, P = 0.002). In subgroup analysis, 68Ga-FAPI-04 PET/CT was more sensitive than 18F-FDG PET/CT in the detection of intrahepatic lesions in patients with cirrhosis, low α-fetoprotein (AFP), multiple tumours, and non-serious MVI (M0 and M1) (all P < 0.05). Moreover, 68Ga-FAPI-04 PET/CT detected 4 of the 5 lesions in patients with stage I disease, whereas 18F-FDG PET/CT did not reveal any abnormal finding in these patients (Figure 2). The sensitivity of 18F-FDG PET/CT was associated with AJCC TNM stage (P = 0.016), while that of 68Ga-FAPI-04 PET/CT was correlated with serum AFP levels (P = 0.045). These findings suggested that 68Ga-FAPI-04 PET/CT was more sensitive than 18F-FDG PET/CT in the detection of intrahepatic lesions, particularly in patients with cirrhosis, low AFP, multiple HCCs, and non-serious MVI.

Figure 2 PET/CT images in a 53-year-old male patient with moderately-differentiated HCC. (A) 68Ga-FAPI-04 PET/CT revealed a strongly FAPI-avid lesion (black and white arrows, SUVmax = 7.36, TBR = 6.03) in the right lobe of the liver. (B) No positive finding was observed within liver in 18F-FDG PET/CT images (SUVmax = 2.36, TBR = 1.31).
The results of lesion-based analysis of 18F-FDG and 68Ga-FAPI-04 PET/CT are summarized in Table 3. 68Ga-FAPI-04 PET/CT was more sensitive than 18F-FDG PET/CT in detecting small HCCs (≤ 2 cm in diameter) (P = 0.008) and well- or moderately-differentiated HCCs (P = 0.031), but there were no significant sensitivity differences between the 2 tracers in the detection of HCCs > 2 cm in diameter (both P > 0.05) and poorly-differentiated or undifferentiated HCCs (P > 0.05). The sensitivities of 18F-FDG and 68Ga-FAPI-04 PET/CT were significantly related to the size of intrahepatic lesions (both P < 0.05). These findings indicated that 68Ga-FAPI-04 PET/CT was more sensitive than 18F-FDG PET/CT in the detection of small and well- or moderately-differentiated HCCs.
Among the 25 HCC patients with 35 intrahepatic lesions, uptake of 18F-FDG and 68Ga-FAPI-04 in positive lesions was assessed, respectively (Table 4). Although the lesion uptake (SUVmax) of 68Ga-FAPI-04 was similar to that of 18F-FDG (P > 0.05), its TBR was significantly higher than that of 18F-FDG (P < 0.001) (Figure 3A). Particularly, the background uptake (SUVmean) of 68Ga-FAPI-04 was much lower than that of 18F-FDG in each patient. The SUVmean of 68Ga-FAPI-04 in patients with cirrhosis was significantly higher than that of patients without cirrhosis (0.76 ± 0.39 vs. 0.40 ± 0.07, P < 0.001); however, no significant difference was obtained in SUVmean of 18F-FDG between these two groups (1.82 ± 0.39 vs. 1.97 ± 0.44, P > 0.05) (Figure 3B).
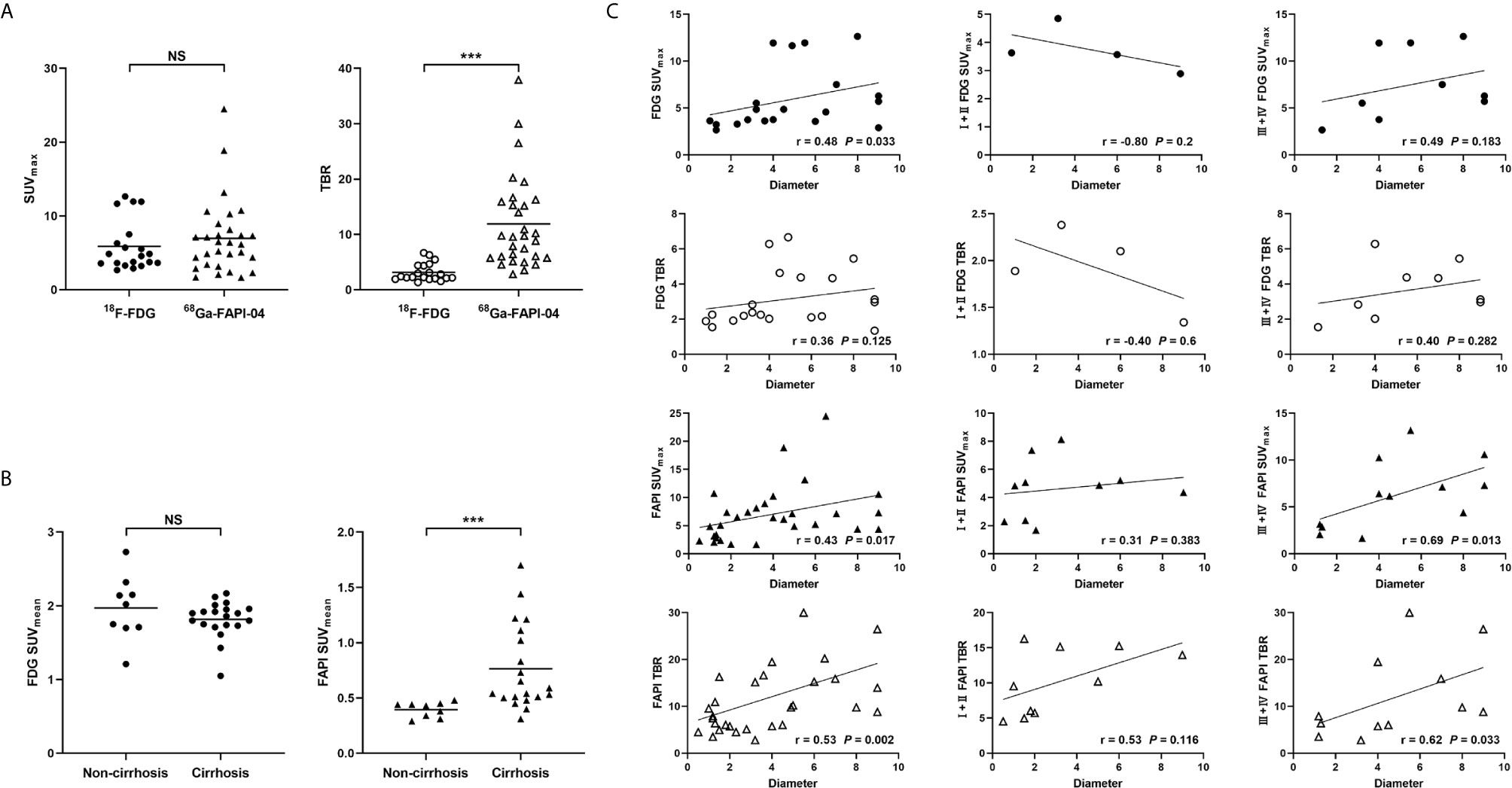
Figure 3 (A) Comparison of uptake intensity (SUVmax and TBR) between 18F-FDG and 68Ga-FAPI-04. (B) Comparison of background uptake (SUVmean) between patients with cirrhosis or those without cirrhosis. (C) Spearman rank correlation analysis of uptake intensity (SUVmax and TBR) and tumour diameter in 18F-FDG and 68Ga-FAPI-04. NS, not statistically significant; ***, P < 0.001.
The SUVmax of 18F-FDG in positive lesions was associated with the degree of MVI (P = 0.048) and tumour differentiation (P = 0.045), while the TBR was only associated with tumour differentiation (P = 0.045). In contrast, the SUVmax and TBR of 68Ga-FAPI-04 in positive lesions were associated only with tumour size (both P < 0.05), but not with other clinical and pathological features (all P > 0.05). In Spearman correlation analysis (Figure 3C), the SUVmax and TBR of 68Ga-FAPI-04 in positive lesions were correlated with tumour size (rSUVmax = 0.43, rTBR = 0.53, both P < 0.05). Further subgroup analysis revealed these correlations in poorly-differentiated or undifferentiated HCCs (rSUVmax = 0.69, rTBR = 0.62, both P < 0.05), rather than well- or moderately-differentiated ones (both P > 0.05). For 18F-FDG, SUVmax, but not TBR, was correlated with tumour size (rSUVmax = 0.48, P = 0.033). However, neither SUVmax nor TBR exhibited a correlation with tumour size in subgroup analysis (all P > 0.05).
Lymph node metastasis in one patient with poorly-differentiated HCC showed strong uptake of 68Ga-FAPI-04, but undetectable uptake of 18F-FDG (Figure 4). 68Ga-FAPI-04 PET/CT detected a small metastatic lesion that was not revealed by 18F-FDG PET/CT in another HCC patient with extensive peritoneal dissemination (Figure 5).
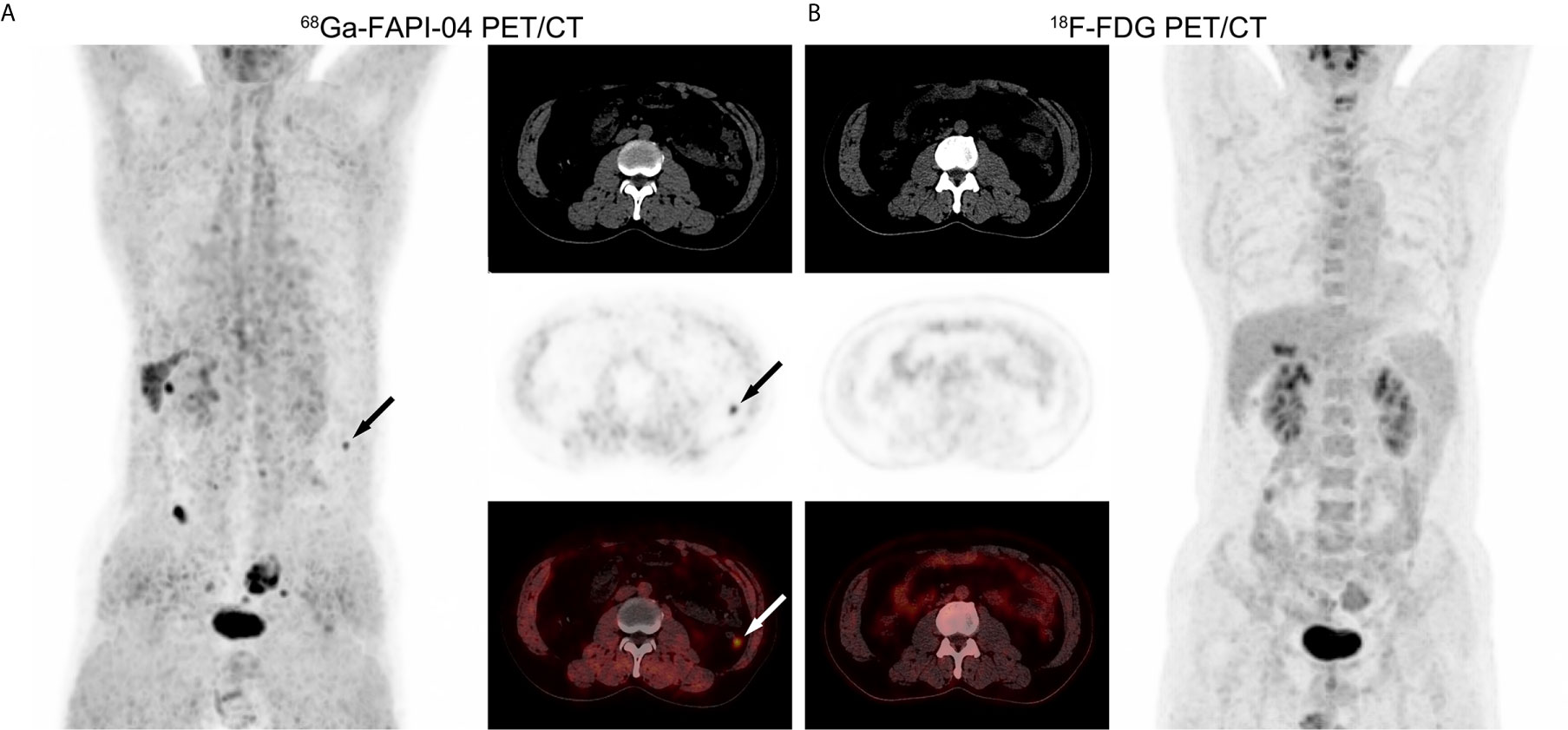
Figure 4 PET/CT images in a 51-year-old male patient with poorly-differentiated HCC and regional lymph node metastasis. (A) 68Ga-FAPI-04 PET/CT revealed a strongly FAPI-avid lesion (black and white arrows, SUVmax = 6.67, TBR = 15.2) that was pathologically confirmed as lymph node metastasis of the porta hepatis. (B) 18F-FDG PET/CT showed no elevated uptake of this extrahepatic metastasis (SUVmax = 2.84, TBR = 1.04).
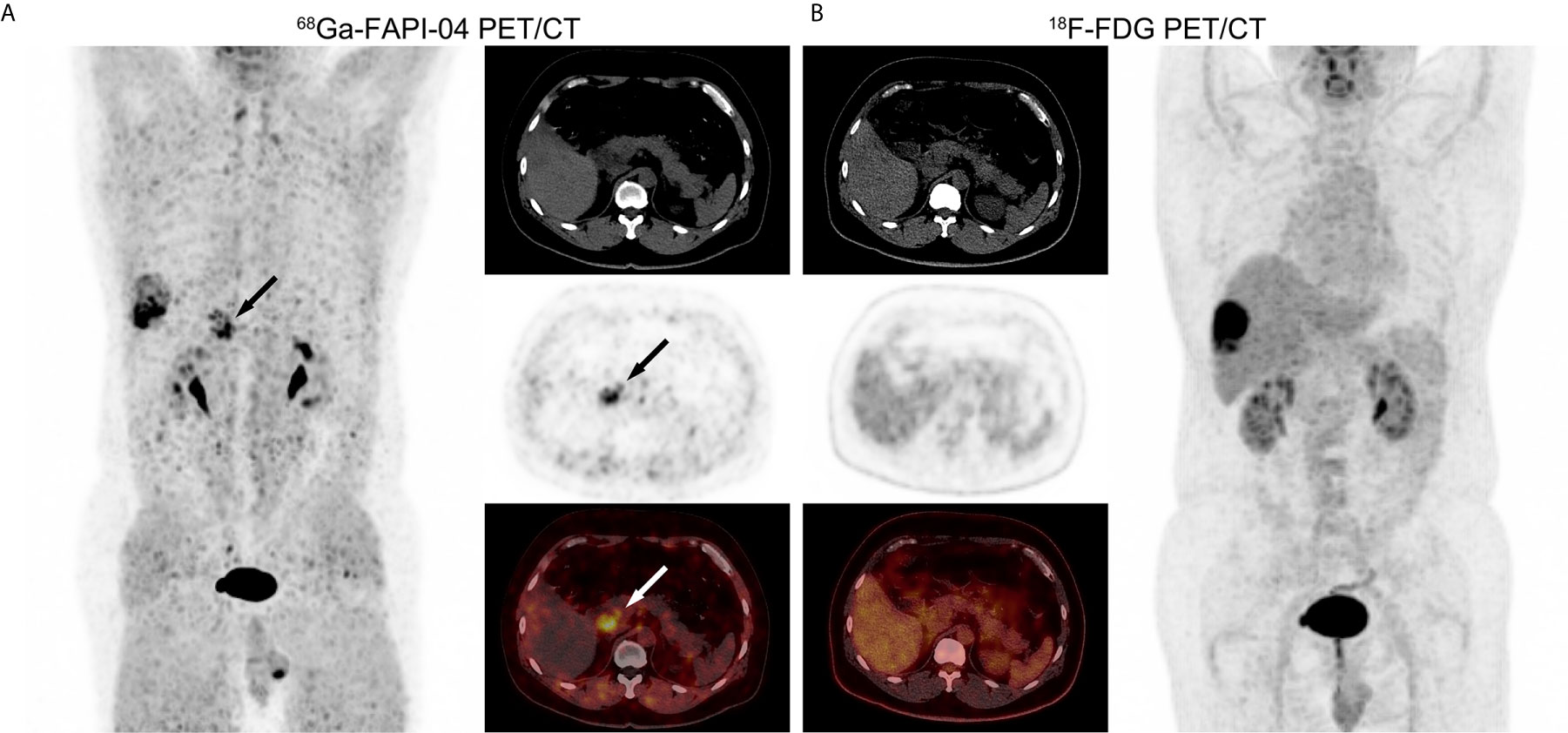
Figure 5 PET/CT images in a 53-year-old male patient with recurrent HCC and extensive peritoneal dissemination. (A) In 68Ga-FAPI-04 PET/CT, a small metastatic lesion confirmed by pathology showed elevated uptake (black and white arrows, SUVmax = 4.72, TBR = 11). (B) 18F-FDG PET/CT did not detect this metastatic lesion (SUVmax = 1.27, TBR = 0.63).
Of the benign lesions, angiomyolipoma (AML) presented strong uptake of 68Ga-FAPI-04 (SUVmax = 8.34 and TBR = 21.92) and mildly increased uptake of 18F-FDG (SUVmax = 2.70 and TBR = 1.54), whereas focal nodular hyperplasia (FNH) presented elevated uptake of 68Ga-FAPI-04 (SUVmax = 2.20 and TBR = 5.77) and negative uptake of 18F-FDG (SUVmax = 1.51 and TBR = 0.86). In two cases of inflammatory nodules in the liver, one showed positive uptake of 68Ga-FAPI-04 (SUVmax = 1.56 and TBR = 3.08) and negative uptake of 18F-FDG (SUVmax = 2.14 and TBR = 1.19), while the other was neither 68Ga-FAPI-04 avid (SUVmax = 0.53 and TBR = 1.20) nor 18F-FDG avid (SUVmax = 2.92 and TBR = 1.36).
Nowadays, 18F-FDG as the most widely available clinical PET tracer has been increasingly utilized for detecting extrahepatic metastases (22), TNM staging (23), selecting patients for liver transplantation (23), and predicting tumour progression or recurrence after treatments (24, 25). However, 18F-FDG has limited value in the early diagnosis of HCC because of its low sensitivity (6, 26). Therefore, there remains an urgent need for highly sensitive tracers in the early diagnosis of HCC by PET/CT. Recently, 68Ga-labelled FAPI was shown to be a novel tracer in PET/CT imaging of various cancers due to its high tumour-to-background contrast (17, 27). Furthermore, 68Ga-FAPI-04 PET/CT has high sensitivity in detecting hepatic malignancies, including HCC and ICC (19, 21).
In line with previous studies reporting a range from 40% to 68%, the sensitivity of 18F-FDG PET/CT in the detection of HCC was 57.1% in the present study (6). 68Ga-FAPI-04 PET/CT had a better sensitivity (85.7%) than 18F-FDG PET/CT in the detection of intrahepatic lesions in HCC patients. Of note, 68Ga-FAPI-04 PET/CT was capable of detecting more than half of small HCC lesions (11 of 16, ≤ 2 cm in diameter) in the present cohort, whereas 18F-FDG PET/CT detected only three of the 16 lesions, which is consistent with previous studies that consider 18F-FDG an inappropriate tracer for visualizing small HCCs (7, 8). Furthermore, 68Ga-FAPI-04 PET/CT exhibited a relatively higher sensitivity in the detection of well- or moderately-differentiated HCCs (10 of 12, histologic grade I or II) compared with 18F-FDG PET/CT (4 of 12). The poor sensitivity of 18F-FDG PET/CT in detecting low-grade HCC is probably related to enhanced glucose-6-phosphatase activity causing the dephosphorylation of 18F-FDG-6-PO4, which is therefore not trapped in HCC cells, resulting in false-negative results (6, 28, 29).
In contrast, a CAF-targeting tracer can circumvent highly heterogeneous avidity exhibited by some tracers that target the tumour per se, because CAFs are among the most abundant stromal components in the tumour microenvironment of many solid tumours, and are found even at the early stages of tumorigenesis (30, 31). The above correlation analysis of 68Ga-FAPI-04 revealed that neither positive incidence nor uptake intensity was associated with tumour differentiation. Moreover, 68Ga-FAPI-04 showed a high lesion-to-background contrast in the liver, which may partially increase sensitivity that is affected by the partial volume effect of PET/CT. As such, 68Ga-FAPI-04 PET/CT can make up for the deficiencies of 18F-FDG PET/CT in the detection of low-grade HCC. Additionally, a higher detection rate was observed with 68Ga-FAPI-04 PET/CT in high-grade HCC in comparison with 18F-FDG PET/CT, although this difference was not statistically significant. Therefore, 68Ga-FAPI-04 PET/CT appears to be a promising new approach for the detection of intrahepatic HCC lesions with higher sensitivity compared with 18F-FDG PET/CT.
68Ga-FAPI-04 PET/CT could not detect 5 intrahepatic HCC lesions with the diameter within 2 cm in this cohort. These negative results may be due to the similar uptake intensity of 68Ga-FAPI-04 between small HCC lesions and the liver background of cirrhosis. Hypoxia may be a reasonable explanation for the positive correlation between the uptake intensity of 68Ga-FAPI-04 and tumour size. It has been reported that the degree of hypoxia correlates positively with tumour size (32) and that hypoxia is a potent factor inducing the expression of FAP in CAFs (33). Therefore, the degree of hypoxia is mild in small HCCs, leading to the low uptake of 68GA-FAPI-04 in these lesions. In the cirrhotic liver, FAP is strongly expressed by activated hepatic stellate cells (34, 35). In line with a previous study (19), this work also found that patients with cirrhosis presented elevated uptake of 68Ga-FAPI-04 in the hepatic parenchyma compared with those without cirrhosis. Therefore, small lesions have relatively lower uptake of 68Ga-FAPI-04 in comparison with large ones, which makes them susceptible to being masked by the background of cirrhosis.
Although 18F-FDG PET/CT has a potential value in detecting extrahepatic metastases in HCC patients (7, 8, 36, 37), heterogeneous uptake of 18F-FDG in metastatic nodules remains a major reason limiting its wide application for tumour staging. CAFs play a critical role in constructing a microenvironment that favours tumour progression at the primary site, and are, moreover, responsible for creating a pre-metastatic niche in distal organs and triggering the subsequent metastatic events (30, 31, 38). Therefore, CAF-targeting tracers seem to be optimal candidates for PET/CT in the evaluation of extrahepatic metastases. In the present study, lymph node metastasis at the porta hepatis in one patient with poorly-differentiated HCC presented a clear visualization in 68Ga-FAPI-04 PET/CT, but an obscure image in 18F-FDG PET/CT (Figure 4). Separately, one small metastatic lesion in another recurrent HCC case with extensive peritoneal dissemination was only clearly visualised by 68Ga-FAPI-04 PET/CT (Figure 5). It appears that 68Ga-FAPI-04 PET/CT may outperform 18F-FDG PET/CT in detecting extrahepatic lesions in patients with advanced HCC. Nevertheless, the comparison of 68Ga-FAPI-04 with 18F-FDG in terms of applicability to the detection of extrahepatic metastasis of HCC needs to be clarified in future studies.
Despite the high sensitivity of 68Ga-FAPI-04 PET/CT in the detection of malignancies, some benign lesions confirmed by pathological examinations in the current cohort presented positive results as well. Especially, AML, FNH, and one of two inflammatory nodules presented elevated uptake of 68Ga-FAPI-04 in contrast to the hepatic background, perhaps because of the enhanced fibrosis around or within lesions. Moreover, increasing uptake of 68Ga-FAPI-04 was observed in the postoperative area of the liver in one patient with recurrent HCC, which is consistent with a previous study that considered 68Ga-FAPI-04 an inappropriate tracer for the discrimination between abnormal malignant progression and normal postoperative reaction (39). Nevertheless, negligible 68Ga-FAPI-04 uptake has been observed in some other benign hepatic lesions such as adenoma (20, 39), dysplastic nodule (20), granuloma (21), and haemangioma (21). Collectively, great caution should be exercised when regarding intrahepatic lesions with elevated uptake of 68Ga-FAPI-04 as malignancy.
There were several limitations in the present study. First, it is unethical to biopsy all lesions as part of a research study, although that is perhaps not practical or needed. As a result, the lack of pathological data in 5 HCC patients whose confirmative diagnosis was based on non-invasive criteria may yield latent bias. Another limitation is that the present cohort only included a small number of patients with suspicious HCC who were willing to receive both 68Ga-FAPI-04 and 18F-FDG PET/CT examinations. Therefore, selection bias was inevitable. Finally, different scanners were used in this study for 68Ga-FAPI-04 and 18F-FDG imaging because of the different production places of PET tracers. For this reason, SUV normalization was applied after data collection for PET/CT system performance harmonization.
68Ga-FAPI-04 PET/CT is more sensitive than 18F-FDG PET/CT in detecting intrahepatic HCCs. This outperformance is more prominent in the detection of small and well- or moderately-differentiated HCCs. The uptake of 68Ga-FAPI-04 was correlated mainly with tumour size in this study; therefore, 68Ga-FAPI-04 PET/CT can be considered a very promising imaging modality in HCC diagnosis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by ethics committee at Huashan Hospital of Fudan University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LQ, FX, LL, WZ, and HW designed the study, interpreted the data and led the writing and review of the manuscript. HW, HJ, JC, WZ, and LL enrolled patients and collected clinical data. YG, JZ, QH, YK, and SR performed examinations. All authors contributed to the article and approved the submitted version.
This study was funded by the Key Program of National Natural Science Foundation of China (81930074), the Major Program of National Natural Science Foundation of China (91959203), the Shanghai Sailing Program (18YF1403200), the Start-up fund of Huashan Hospital, Fudan University (2017QD081), and the Shanghai Municipal Key Clinical Specialty (shslczdzk03402).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors appreciate the excellent technical assistance of the staff at the PET Centre and the Department of General Surgery, Huashan Hospital, Fudan University.
1. Forner A, Reig M, Bruix J. Hepatocellular Carcinoma. Lancet (2018) 391(10127):1301–14. doi: 10.1016/s0140-6736(18)30010-2
2. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management. Nat Rev Gastroenterol Hepatol (2019) 16(10):589–604. doi: 10.1038/s41575-019-0186-y
3. Villanueva A. Hepatocellular Carcinoma. N Engl J Med (2019) 380(15):1450–62. doi: 10.1056/NEJMra1713263
4. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology (2018) 68(2):723–50. doi: 10.1002/hep.29913
5. Oliva MR, Saini S. Liver Cancer Imaging: Role of CT, MRI, US and PET. Cancer Imaging (2004) 4 Spec No A(Spec No A):S42–6. doi: 10.1102/1470-7330.2004.0011
6. Goh V, Sarker D, Osmany S, Cook GJ. Functional Imaging Techniques in Hepatocellular Carcinoma. Eur J Nucl Med Mol Imaging (2012) 39(6):1070–9. doi: 10.1007/s00259-012-2096-x
7. Trojan J, Schroeder O, Raedle J, Baum RP, Herrmann G, Jacobi V, et al. Fluorine-18 FDG Positron Emission Tomography for Imaging of Hepatocellular Carcinoma. Am J Gastroenterol (1999) 94(11):3314–9. doi: 10.1111/j.1572-0241.1999.01544.x
8. Park JW, Kim JH, Kim SK, Kang KW, Park KW, Choi JI, et al. A Prospective Evaluation of 18F-FDG and 11C-Acetate PET/CT for Detection of Primary and Metastatic Hepatocellular Carcinoma. J Nucl Med (2008) 49(12):1912–21. doi: 10.2967/jnumed.108.055087
9. Ho CL, Chen S, Yeung DW, Cheng TK. Dual-Tracer PET/CT Imaging in Evaluation of Metastatic Hepatocellular Carcinoma. J Nucl Med (2007) 48(6):902–9. doi: 10.2967/jnumed.106.036673
10. Castilla-Lièvre MA, Franco D, Gervais P, Kuhnast B, Agostini H, Marthey L, et al. Diagnostic Value of Combining ¹¹C-Choline and 18F-FDG PET/CT in Hepatocellular Carcinoma. Eur J Nucl Med Mol Imaging (2016) 43(5):852–9. doi: 10.1007/s00259-015-3241-0
11. Kesler M, Levine C, Hershkovitz D, Mishani E, Menachem Y, Lerman H, et al. (68)Ga-PSMA is a Novel PET-CT Tracer for Imaging of Hepatocellular Carcinoma: A Prospective Pilot Study. J Nucl Med (2019) 60(2):185–91. doi: 10.2967/jnumed.118.214833
12. Lopci E, Torzilli G, Poretti D, de Neto LJ, Donadon M, Rimassa L, et al. Diagnostic Accuracy of ¹¹C-Choline PET/CT in Comparison With CT and/or MRI in Patients With Hepatocellular Carcinoma. Eur J Nucl Med Mol Imaging (2015) 42(9):1399–407. doi: 10.1007/s00259-015-3079-5
13. Kubo N, Araki K, Kuwano H, Shirabe K. Cancer-Associated Fibroblasts in Hepatocellular Carcinoma. World J Gastroenterol (2016) 22(30):6841–50. doi: 10.3748/wjg.v22.i30.6841
14. Baglieri J, Brenner DA, Kisseleva T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma. Int J Mol Sci (2019) 20(7):1723. doi: 10.3390/ijms20071723
15. Garin-Chesa P, Old LJ, Rettig WJ. Cell Surface Glycoprotein of Reactive Stromal Fibroblasts as a Potential Antibody Target in Human Epithelial Cancers. Proc Natl Acad Sci USA (1990) 87(18):7235–9. doi: 10.1073/pnas.87.18.7235
16. Scanlan MJ, Raj BK, Calvo B, Garin-Chesa P, Sanz-Moncasi MP, Healey JH, et al. Molecular Cloning of Fibroblast Activation Protein Alpha, a Member of the Serine Protease Family Selectively Expressed in Stromal Fibroblasts of Epithelial Cancers. Proc Natl Acad Sci USA (1994) 91(12):5657–61. doi: 10.1073/pnas.91.12.5657
17. Giesel FL, Kratochwil C, Lindner T, Marschalek MM, Loktev A, Lehnert W, et al. (68)Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients With Various Cancers. J Nucl Med (2019) 60(3):386–92. doi: 10.2967/jnumed.118.215913
18. Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F] FDG PET/CT for the Diagnosis of Primary and Metastatic Lesions in Patients With Various Types of Cancer. Eur J Nucl Med Mol Imaging (2020) 47(8):1820–32. doi: 10.1007/s00259-020-04769-z
19. Shi X, Xing H, Yang X, Li F, Yao S, Zhang H, et al. Fibroblast Imaging of Hepatic Carcinoma With (68)Ga-FAPI-04 PET/CT: A Pilot Study in Patients With Suspected Hepatic Nodules. Eur J Nucl Med Mol Imaging (2020) 48(1):196–203. doi: 10.1007/s00259-020-04882-z
20. Guo W, Pang Y, Yao L, Zhao L, Fan C, Ke J, et al. Imaging Fibroblast Activation Protein in Liver Cancer: A Single-Center Post HOC Retrospective Analysis to Compare [(68)Ga]Ga-FAPI-04 PET/CT Versus MRI and [(18)F]-FDG PET/CT. Eur J Nucl Med Mol Imaging (2021) 48(5):1604–17. doi: 10.1007/s00259-020-05095-0
21. Shi X, Xing H, Yang X, Li F, Yao S, Congwei J, et al. Comparison of PET Imaging of Activated Fibroblasts and (18)F-FDG for Diagnosis of Primary Hepatic Tumours: A Prospective Pilot Study. Eur J Nucl Med Mol Imaging (2020) 48(5):1593–603. doi: 10.1007/s00259-020-05070-9
22. Lin CY, Chen JH, Liang JA, Lin CC, Jeng LB, Kao CH. 18F-FDG PET or PET/CT for Detecting Extrahepatic Metastases or Recurrent Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Eur J Radiol (2012) 81(9):2417–22. doi: 10.1016/j.ejrad.2011.08.004
23. Cheung TT, Ho CL, Lo CM, Chen S, Chan SC, Chok KS, et al. 11C-Acetate and 18F-FDG PET/CT for Clinical Staging and Selection of Patients With Hepatocellular Carcinoma for Liver Transplantation on the Basis of Milan Criteria: Surgeon’s Perspective. J Nucl Med (2013) 54(2):192–200. doi: 10.2967/jnumed.112.107516
24. Hong G, Suh KS, Suh SW, Yoo T, Kim H, Park MS, et al. Alpha-Fetoprotein and (18)F-FDG Positron Emission Tomography Predict Tumor Recurrence Better Than Milan Criteria in Living Donor Liver Transplantation. J Hepatol (2016) 64(4):852–9. doi: 10.1016/j.jhep.2015.11.033
25. Song MJ, Bae SH, Lee SW, Song DS, Kim HY, Yoo Ie R, et al. 18F-Fluorodeoxyglucose PET/CT Predicts Tumour Progression After Transarterial Chemoembolization in Hepatocellular Carcinoma. Eur J Nucl Med Mol Imaging (2013) 40(6):865–73. doi: 10.1007/s00259-013-2366-2
26. Ayuso C, Rimola J, Vilana R, Burrel M, Darnell A, García-Criado Á, et al. Diagnosis and Staging of Hepatocellular Carcinoma (HCC): Current Guidelines. Eur J Radiol (2018) 101:72–81. doi: 10.1016/j.ejrad.2018.01.025
27. Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J Nucl Med (2019) 60(6):801–5. doi: 10.2967/jnumed.119.227967
28. Torizuka T, Tamaki N, Inokuma T, Magata Y, Sasayama S, Yonekura Y, et al. In Vivo Assessment of Glucose Metabolism in Hepatocellular Carcinoma With FDG-PET. J Nucl Med (1995) 36(10):1811–7.
29. Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, Wolf AP. Metabolic Trapping as a Principle of Oradiopharmaceutical Design: Some Factors Resposible for the Biodistribution of [18F] 2-Deoxy-2-Fluoro-D-Glucose. J Nucl Med (1978) 19(10):1154–61.
30. Kalluri R. The Biology and Function of Fibroblasts in Cancer. Nat Rev Cancer (2016) 16(9):582–98. doi: 10.1038/nrc.2016.73
31. Chen X, Song E. Turning Foes to Friends: Targeting Cancer-Associated Fibroblasts. Nat Rev Drug Discovery (2019) 18(2):99–115. doi: 10.1038/s41573-018-0004-1
32. Carmona-Bozo JC, Manavaki R, Woitek R, Torheim T, Baxter GC, Caracò C, et al. Hypoxia and Perfusion in Breast Cancer: Simultaneous Assessment Using PET/MR Imaging. Eur Radiol (2021) 31(1):333–44. doi: 10.1007/s00330-020-07067-2
33. Ammirante M, Shalapour S, Kang Y, Jamieson CA, Karin M. Tissue Injury and Hypoxia Promote Malignant Progression of Prostate Cancer by Inducing CXCL13 Expression in Tumor Myofibroblasts. Proc Natl Acad Sci USA (2014) 111(41):14776–81. doi: 10.1073/pnas.1416498111
34. Levy MT, McCaughan GW, Abbott CA, Park JE, Cunningham AM, Müller E, et al. Fibroblast Activation Protein: A Cell Surface Dipeptidyl Peptidase and Gelatinase Expressed by Stellate Cells at the Tissue Remodelling Interface in Human Cirrhosis. Hepatology (1999) 29(6):1768–78. doi: 10.1002/hep.510290631
35. Wang XM, Yu DM, McCaughan GW, Gorrell MD. Fibroblast Activation Protein Increases Apoptosis, Cell Adhesion, and Migration by the LX-2 Human Stellate Cell Line. Hepatology (2005) 42(4):935–45. doi: 10.1002/hep.20853
36. Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, et al. Positron Emission Tomography Scanning in the Evaluation of Hepatocellular Carcinoma. J Hepatol (2000) 32(5):792–7. doi: 10.1016/s0168-8278(00)80248-2
37. Sugiyama M, Sakahara H, Torizuka T, Kanno T, Nakamura F, Futatsubashi M, et al. 18F-FDG PET in the Detection of Extrahepatic Metastases From Hepatocellular Carcinoma. J Gastroenterol (2004) 39(10):961–8. doi: 10.1007/s00535-004-1427-5
38. Liu Y, Cao X. Characteristics and Significance of the Pre-Metastatic Niche. Cancer Cell (2016) 30(5):668–81. doi: 10.1016/j.ccell.2016.09.011
Keywords: 68Ga-FAPI-04, 18F-FDG, hepatocellular carcinoma (HCC), cancer-associated fibroblast (CAF), fibroblast activating protein (FAP)
Citation: Wang H, Zhu W, Ren S, Kong Y, Huang Q, Zhao J, Guan Y, Jia H, Chen J, Lu L, Xie F and Qin L (2021) 68Ga-FAPI-04 Versus 18F-FDG PET/CT in the Detection of Hepatocellular Carcinoma. Front. Oncol. 11:693640. doi: 10.3389/fonc.2021.693640
Received: 11 April 2021; Accepted: 14 June 2021;
Published: 25 June 2021.
Edited by:
Freimut Dankwart Juengling, University of Alberta, CanadaReviewed by:
Niklaus Schaefer, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandCopyright © 2021 Wang, Zhu, Ren, Kong, Huang, Zhao, Guan, Jia, Chen, Lu, Xie and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lunxiu Qin, cWlubHhAZnVkYW4uZWR1LmNu; Fang Xie, ZmFuZ3hpZUBmdWRhbi5lZHUuY24=; Lu Lu, bHVsdUBodWFzaGFuLm9yZy5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.