
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 29 September 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.690662
This article is part of the Research Topic Chemotherapy in Esophageal Cancer View all 10 articles
 Qing Feng1
Qing Feng1 Du Long1
Du Long1 Ming-shan Du2
Ming-shan Du2 Xiao-song Wang1
Xiao-song Wang1 Zhen-shun Li1
Zhen-shun Li1 Yong-liang Zhao1
Yong-liang Zhao1 Feng Qian1
Feng Qian1 Yan Wen1
Yan Wen1 Pei-wu Yu1
Pei-wu Yu1 Yan Shi1*
Yan Shi1*Background: Laparoscopic gastrectomy (LG) has been increasingly used for the treatment of locally advanced Siewert type II and III adenocarcinoma of the esophagogastric junction (AEG). However, whether LG can achieve the same short-term efficacy in the treatment of patients who receive neoadjuvant chemotherapy (NACT) remains controversial. Thus, the aim of this study was to investigate the clinical outcomes of NACT combined with LG for Siewert type II and III AEG.
Methods: This retrospective study identified patients with locally advanced Siewert type II and III AEG diagnosed between May 2011 and October 2020 using the clinical tumor-node-metastasis (cTNM) staging system. The short-term outcomes were compared between the matched groups using a 1:3 propensity score matching (PSM) method, which was performed to reduce bias in patient selection.
Results: After PSM, 164 patients were selected, including 41 in the NACT group and 123 in the LG group. The baseline characteristics were similar between the two groups. Compared with the LG group, the NACT group exhibit a smaller tumor size and significantly less advanced pathological tumor classification and nodal classification stages. The time to first flatus of the NACT group was significantly shorter, but the hospital stay was significantly longer than that of the LG group. The NACT group showed similar overall (29.3% vs 25.2%, P=0.683), systemic (24.4% vs 21.1%, P=0.663), local (12.2% vs 9.8%, P=0.767), minor (19.5% vs 19.5%, P=1.000) and major (9.8% vs 5.7%, P=0.470) complications as the LG group. Subgroup analyses showed no significant differences in most stratified parameters. Operation time≥ 300 minutes was identified as an independent risk factor for overall complications. Age≥ 60 years was identified as an independent risk factor for major complications.
Conclusion: NACT combined with LG for AEG does not increase the risk of postoperative morbidity and mortality compared with LG.
The incidence of adenocarcinoma of the esophagogastric junction (AEG) is rapidly increasing, especially Siewert II and III AEG (1, 2). Surgery remains the only radical cure for AEG (3). Since laparoscopic gastrectomy (LG) was first introduced by Kitano in 1994 (4), it has been widely used for early gastric cancer and advanced gastric cancer with the advantages of less injury, faster recovery, and lower morbidity of postoperative complications (5–8). For Siewert type II and III AEG, Liao’s meta-analysis (9) revealed that LG can achieve short-term surgical outcomes comparable to open gastrectomy (OG). However, the development of surgical procedures did not improve long-term outcomes (10). In addition, due to the special location of this tumor, most cases are diagnosed at an advanced stage (11), seriously impacting on the prognosis of patients and resulting in a lower overall survival.
Accumulating evidence has revealed that neoadjuvant therapy improves the efficacy of AEG compared with surgery alone (12–14). However, chemotherapy-induced tissue fibrosis and oedema provide new technical challenges for minimally invasive procedures and increase the difficulty of the operation. It remains controversial whether LG is suitable for AEG patients after NACT. Therefore, we conducted a single-centre retrospective, propensity score-matched study to determine whether LG is suitable for AEG patients after NACT.
A total of 256 Siewert type II or III AEG patients who underwent laparoscopic gastrectomy were identified from a prospectively maintained database containing all gastric cancers diagnosed at The First Affiliated Hospital of Army Medical University in China between May 2011 and October 2020. The decision for NACT was discussed in the Department of General Surgery and determined by the patients who were informed of the possible complications of the procedure and the potential benefits and harms of NACT compared with the LG approach. Written informed consent was obtained from all patients before the operation.
The inclusion criteria were as follows: patients aged 18 to 85 years who were diagnosed with Siewert type II/III AEG by computed tomography (CT); patients who received gastroscopy and were pathologically confirmed by postoperative biopsy; patients who adopted a complete trans-abdominal approach; patients with no distant metastasis or invasion to adjacent organs; and patients who underwent D2 radical laparoscopic gastrectomy. The exclusion criteria included non-radical operation, emergent operation previous gastrectomy, endoscopic mucosal resection, or endoscopic submucosal dissection. In total, 41 and 192 patients were included in the NACT and LG groups, respectively. Clinical stage was evaluated for all patients using intravenous contrast-enhanced CT before and after NACT. Before the study was conducted, CT data were evaluated by a professional radiologist who was blinded to the clinical information of the patient. This study was approved by the Ethics Committee of the First Affiliated Hospital of Army Medical University, PLA (Approval number: KY2021059).
Patients received different cycles of NACT preoperatively, and a median of 3 (2, 4) cycles was administered. Among the 41 patients in the NACT group, 37 (90.2%) received the SOX (oxaliplatin + S-1) regimen, 2 (4.9%) received the XELOX (oxaliplatin + capecitabine) regimen, and 2 (4.9%) received the FOLFOX (oxalipatin + fluorouracil + leucovorin) regimen. The toxicity and adverse events of NACT were evaluated according to the World Health Organization (WHO) standard criteria (15). The response to chemotherapy was endoscopically and radiologically evaluated by endoscopy and CT scans. Post-NACT evaluation of the target lesions was divided into four categories: complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD) according to the Response Evaluation Criteria in Solid Tumours (RECIST, version 1.1) (16).
Patients in the NACT group underwent radical gastrectomy after the completion of NACT (3-4 weeks). All patients who underwent laparoscopic gastrectomy with D2 lymphadenectomy were treated by three experienced surgeons according to the Japanese Gastric Cancer Treatment Guidelines (17, 18). Specific surgical gastrectomy procedures, including proximal and total gastrectomy, were selected depending on the location of the primary tumor. Reconstruction of the gastrointestinal tract was performed according to the type of gastrectomy. Postoperative outcomes, including the results of the pathological outcomes, postoperative recovery (i.e., the times to first flatus and length of overall and postoperative hospital stay), and morbidity and mortality rates, were evaluated. Pathologic evaluations and staging were updated according to the 8th American Joint Committee on Cancer (AJCC) TNM staging system (19). Postoperative complications were defined as complications that occurred within 30 days after surgery. One month after the operation, outpatient and telephone follow-ups were conducted to determine the survival and severity of the patients after discharge.
To minimize the bias between the NACT group and the LG group, we performed PSM with the R (x64 3.5.0) MatchIt package. Age, sex, body mass index (BMI) on admission, American Society of Anaesthesiologists (ASA) grade, Siewert classification, cT stage, cN stage, cTNM stage, resection range and tumor differentiation were chosen to perform 1:3 matching using the “nearest” method. Data are presented as proportions for categorical variables and as the mean ± SD for continuous variables. Variables with high skew are presented as the median (IQR). Categorical variables were compared using the χ2 test or Fisher’s exact test, whereas continuous variables were compared using Student’s t-test or the Mann-Whitney U test. Variables with P-values<0.10 in univariate analysis were included in the multivariate analysis. Multivariate analysis was conducted with the binary logistic regression model to identify independent risk factors for postoperative complications. A P-value (two-sided)<0.05 was considered statistically significant. Data analyses were conducted using SPSS (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp).
Flow of patient enrolment is presented in Figure 1. Table 1 summarizes the clinicopathological characteristics of the patients in the two groups. Clinical T stage, clinical N stage and tumor differentiation significantly differed between the NACT and LG groups. On the basis of 1:3 PSM, 164 patients (41 in the NACT group and 123 in the LG group) were selected for analysis. After PSM, no significant differences in age, sex, BMI on admission, ASA, Siewert classification, cT stage, cN stage, cTNM stage, resection range and tumor differentiation were noted between the two groups.
In this study, 27 (65.8%) patients exhibited PR, 12 (29.3%) exhibited SD, and 2 (4.9%) patients exhibited PD according to contrast-enhanced CT before and after NACT (Table 2). The BMI of the NACT group after NACT was significantly greater than that on admission (22.50 vs 21.90 P=0.016). 8 (19.5%) of the 41 treated patients experienced at least grade 3-4 toxicity during NACT treatment. The most common grade 3-4 toxicities were leukopenia/neutropenia (9.8%) and nausea and vomiting (12.2%) (Table 2).
The proximal margin of one patient in the NACT group and four patients in the LG group was found to be positive. R0 resection was performed for 97.8% of patients in the NACT group and 93.5% of patients in the LG group (P = 0.453). The amount of blood loss, transfused patient number, and operation time were comparable between the two groups. During the procedure, 6 patients (14.6%) in the NACT group were converted to open gastrectomy, whereas 12 patients (9.8%) in the LG group showed no significant differences (P=0.395). No statistically significant difference was found between the two groups regarding the length of incision, distal margin or proximal margin (Table 3). After PSM, the median time to first flatus of the NACT group was significantly shorter than that of the LG group (3 vs 4 days, P=0.004). Both the total hospital stay and postoperative hospital stay of the NACT group were significantly longer than those of the LG group.
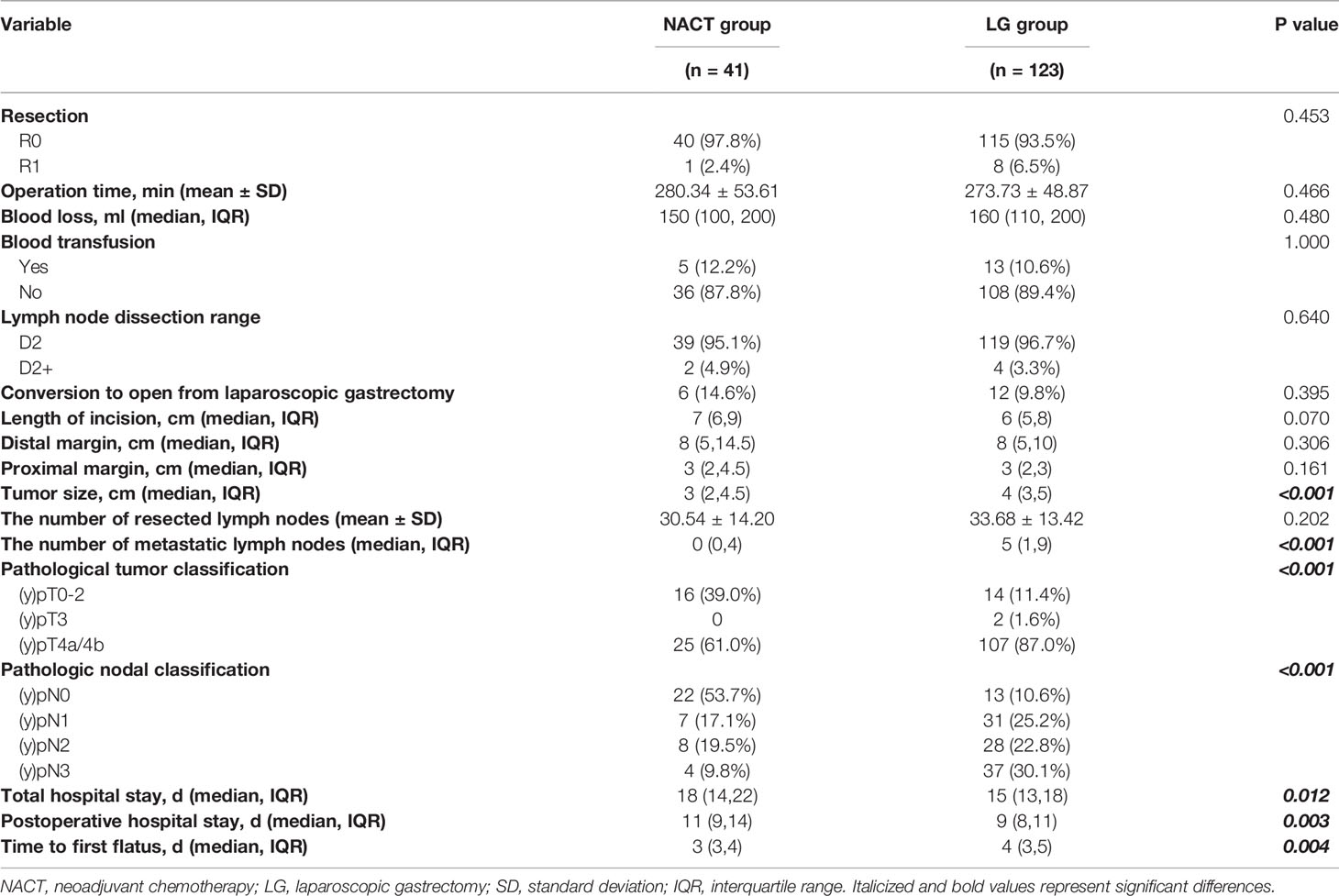
Table 3 Comparison of operative and postoperative parameters between the NACT group and LG group, n (%).
The average number of harvested lymph nodes (LNs) did not significantly differ (P=0.225) in the NACT (30.54) and LG groups (33.51), whereas the number of metastatic LNs was significantly lower in the NACT group (Table 3). The tumor size of the NACT group was smaller than that of the LG group (P<0.001). Following PSM, both the (y)pT and (y)pN stage categories of the NACT group were significantly less advanced than those of the LG group.
The postoperative morbidity and mortality of patients in the PSM cohort are shown in Table 4. Morbidity was comparable between the two groups (29.3% vs 25.2%, P=0.683). No differences in systemic complications (24.4% vs 21.1%, P=0.663) and local complications (12.2% vs 9.8%, P=0.767) were noted between the groups. No significant differences in the comparison of specific complications (all P>0.05) were noted between the groups. More infectious complications were noted in the NACT group compared with the LG group; however, the difference was not significant (24.4% vs 18.7%, P=0.431). No significant differences in complication severity according to the Clavien-Dindo grade were noted (20, 21). Four patients (9.8%) in the NACT group and 7 patients (5.7%) in the LG group experienced grade III or higher complications (P=0.470). One patient in the NACT group and 2 patients in the LG group underwent reoperation due to abdominal bleeding.
Subgroup analyses were performed for overall complications in the PSM cohort. No significant differences in any stratified parameters in terms of overall complications were noted between the two groups (Figure 2).
Univariate analysis showed that BMI ≥25, BMI <18.5, operation time≥ 300 minutes and blood loss ≥200 ml were positively correlated with overall complications (Table 5). Multivariate analysis revealed that operation time≥ 300 minutes (P=0.049) was an independent risk factor for overall complications (Table 5). Regarding major complications, age≥ 60 years, operation time≥ 300 and blood loss ≥200 were correlated with major complications in univariate analysis. In multivariate analysis, age≥ 60 years (P=0.042) was identified as an independent risk factor for major complications.
In our study, NACT did not increase the operation time, blood loss, transfusion during or after surgery or the rate of conversion to open surgery. Although NACT could trigger stomach and metastatic lymph node fibrosis (27) and the tissues of patients with NACT are more likely to bleed (28), the laparoscopic monitoring amplification effect, careful intraoperative procedures and the use of laparoscopic high-resolution imaging help reduce unnecessary damage to prevent accidental bleeding. The wide application of intraoperative ultrasound scalpels can also effectively solve these problems. Therefore, no increase in surgical difficulty was noted after chemotherapy.
Lymph node dissection is a key radical gastrectomy for advanced AEG, and the number of lymph nodes dissected is an important prognostic factor for the surgical treatment of advanced gastric cancer (29). In our study, the average number of lymph nodes dissected in both groups of patients undergoing radical resection was greater than 30, which meets the requirements of current guidelines suggesting that LG is feasible in lymph node dissection (30). No significant difference in the number of dissected lymph nodes was noted between the two groups. The number of metastatic LNs was significantly lower in the NACT group (median 0 vs 5). After NACT, 5% of the total MLNs could achieve complete tumor regression (31), which may explain the difference.
Postoperative complications are the main indicator for evaluating the safety and feasibility of surgery. In our analysis, the incidence of postoperative complications in the NACT group was slightly higher than that in the LG group; however, the difference was not significant (29.3% vs. 25.6%, P= 0.535). Further analysis showed no difference in systemic complications, local complications, minor complications (CD grade<3) or major complications (CD grade≥3). Pulmonary complications obviously accounted for most of the complications in our study, and no difference was noted between the two groups. In their stratified analysis of 92 patients after PSM, Amir et al. (32) found that the NACT group had similar postoperative complications with the surgery alone group. However, in a study of 90 patients, Wei et al. (33) revealed that the NACT group had a higher risk of postoperative infectious complications. Possible explanations for the differences may be that the baselines of the two studies were inconsistent. The cT stage and cN stage in Amir’s study were matched well; however, Wei’s study did not take this factor into consideration. Indeed, a reduction in tumor volume allows less extensive procedures, and nutritional improvement before surgery is helpful to reduce the incidence of complications. Although chemotherapy-induced tissue fibrosis can make surgery more difficult (27) and perhaps increase postoperative complications, LG can provide visual magnification, better exposure, and more detailed organ, blood vessel, and nerve operations, reducing unnecessary intra-operative damage. These problems can be effectively solved by laparoscopy. All patients in this study followed a 3-week rest and nutritional support programme after completing preoperative NACT before surgery. Furthermore, we also performed subgroup analysis to further evaluate complications in different parameters. The results of subgroup analyses showed no significant increase in all types of complications of NACT compared with LG.
Patients with progressive disease and stable disease after neoadjuvant chemotherapy represent a special group of patients, and few studies have been conducted on this group before. However, previous studies (34–37) have shown that approximately 32.1% to 58% of patients inevitably underwent SD or PD after neoadjuvant chemotherapy based on fluorouracil + oxaliplatin, so it is necessary to study the short-term efficacy of this group of patients. Subgroup analysis of complications showed no significant difference in the complications between the SD and PD groups compared with either the PR group or LG group (Appendix, Tables 1, 2). Subgroup analysis of postoperative results revealed no significant differences in operative time, intraoperative blood loss or other results between the SD and PD groups compared with either the PR group or LG group (Appendix, Tables 3, 4). We also noticed a significant increase in the transfer rate of open abdominal surgery and a longer incisional length in the SD and PD groups compared with the direct LG group (Appendix, Table 4). In the SD and PD groups, neoadjuvant chemotherapy was not effective, and some patients experienced tumor progression. Moreover, the oedema of tissues around tumors and metastatic lymph nodes might increase the difficulty of laparoscopic surgery, thus increasing the conversion rate of laparotomy. The increase in the rate of conversion to laparotomy subsequently increased the incision length.
In our analysis, NACT was not an independent risk factor for total complications or for major complications in advanced AEG laparoscopic therapy; thus, the applicability of LG for patients after NACT was further verified. An operation time ≥ 300 minutes was identified as a risk factor for overall complications. A longer operation time always indicates a more complicated situation. In addition, prolonged anaesthesia increases the risk of postoperative complications. According to published studies, old age is a leading risk factor for postoperative complications in gastric cancer surgery (38–40). In our study, old age was an independent risk factor for major complications rather than for overall complications. The reason for this difference may be that LG can effectively reduce the total complications in elderly patients. A previous meta-analysis showed that LG could effectively reduce total complications and minor complications (41). We should also realize that gastrectomy still has higher risks of major complications for elderly patients, and more attention should be given when this procedure is used in elderly patients in clinical practice.
In this study, we also compared the total hospital stay, postoperative hospital stay and time to first flatus between the two procedures. The results showed that the hospital stay was significantly longer in the NACT group. The reason for this finding may be that complications in the NACT group were slightly higher than those in the LG group, and surgeons took a longer time to manage the complications. However, the time to first flatus of the NACT group was significantly shorter than that of the LG group. To investigate whether the difference in the time to first flatus was related to the anastomosis method, we performed a statistical analysis of the anastomosis method between the two groups (Appendix, Tables 5–7). We first conducted statistics on the two groups of anastomosis methods. The results revealed no significant difference between the NACT group and the LG group (Appendix, Table 5). Then, we compared the time to first flatus of the two most common anastomosis methods within the two groups. The results indicated no significant difference in the time to first flatus of end-to-side anastomosis and semi-end-to-end anastomosis in either the NACT group or the LG group (Appendix, Table 6). In addition, in a previous study at our centre, 176 cases of end-to-side esophagojejunostomy and 92 cases of semi-end-to-end esophagojejunostomy were included and compared, and no significant difference in the first time to flatus was noted between the two groups (P = 0.957) (42). Finally, we performed an intergroup comparison between the NACT group and the LG group. The results revealed that the time to first flatus of end-to-side anastomosis in the NACT group was significantly shorter than that in the LG group, and the time to first flatus of semi-end-to-end anastomosis in the NACT group was also significantly shorter than that in the LG group (Appendix, Table 7). Therefore, we hypothesized that the difference in the time to first flatus between the NACT group and the LG group was caused by neoadjuvant chemotherapy rather than the difference in anastomosis. In our study, the BMI of the NACT group before surgery was significantly greater than that on admission. AEG is often accompanied by symptoms of obstruction, leading to poor preoperative nutritional status. NACT can effectively improve the obstruction state preoperatively supplemented with enteral nutrition preparation and prove the preoperative nutritional status.
Nevertheless, there are several limitations in the current study. First, as a retrospective analysis conducted at a single centre, this study is subject to possible selection bias despite the use of PSM to reduce bias, which was intended to mimic randomized controlled trials. Second, the regimens and indications for NACT were not standardized; therefore, the effects of different NACT regimens were not analysed.
In conclusion, the findings of this study suggest that NACT combined with LG is safe and feasible in treating locally advanced Siewert type II and III AEG in terms of morbidity and short-term surgical outcomes. Multicentre, prospective, clinical trials with large sample sizes are still warranted to verify our findings.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of Southwest Hospital (Chongqing, China). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
YS and P-wY contributed to the conception and design of the study. M-sD, FQ, Y-Lz, and YW organized the database. DL and QF performed the statistical analysis. QF wrote the first draft of the manuscript. X-sW and Z-sL wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the Special Science and Technology Innovation Foundation of Social Programs and Livelihood Insurance of Chongqing: Research and application of precise minimally invasive therapy for gastrointestinal malignant tumors (cstc2017shmsA10003-ztzx10001-4).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Xiao-qing Zhan for language editing.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.690662/full#supplementary-material
1. Imamura Y, Watanabe M, Toihata T, Takamatsu M, Kawachi H, Haraguchi I, et al. Recent Incidence Trend of Surgically Resected Esophagogastric Junction Adenocarcinoma and Microsatellite Instability Status in Japanese Patients. Digestion (2019) 99(1):6–13. doi: 10.1159/000494406
2. Buas MF, Vaughan TL. Epidemiology and Risk Factors for Gastroesophageal Junction Tumors: Understanding the Rising Incidence of This Disease. Semin Radiat Oncol (2013) 23(1):3–9. doi: 10.1016/j.semradonc.2012.09.008
3. Saka M, Morita S, Fukagawa T, Katai H. Present and Future Status of Gastric Cancer Surgery. Jpn J Clin Oncol (2011) 41(3):307–13. doi: 10.1093/jjco/hyq240
4. Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-Assisted Billroth I Gastrectomy. Surg Laparosc Endosc (1994) 4(2):146–8.
5. Kinoshita T. Minimally Invasive Approaches for Early Gastric Cancer in East Asia: Current Status and Future Perspective. Transl Gastroenterol Hepatol (2020) 5:20. doi: 10.21037/tgh.2019.10.08
6. Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-Term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg (2016) 263(1):28–35. doi: 10.1097/SLA.0000000000001346
7. Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol (2016) 34(12):1350–7. doi: 10.1200/JCO.2015.63.7215
8. Park YK, Yoon HM, Kim YW, Park JY, Ryu KW, Lee YJ, et al. Laparoscopy-Assisted Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: Results From a Randomized Phase II Multicenter Clinical Trial (COACT 1001). Ann Surg (2018) 267(4):638–45. doi: 10.1097/SLA.0000000000002168
9. Liao C, Feng Q, Xie S, Chen J, Shi Y. Laparoscopic Versus Open Gastrectomy for Siewert Type II/III Adenocarcinoma of the Esophagogastric Junction: A Meta-Analysis. Surg Endosc (2021) 35(2):860–71. doi: 10.1007/s00464-020-07458-y
10. Zhang P, Zhang X, Xue H. Long-Term Results of Hand-Assisted Laparoscopic Gastrectomy for Advanced Siewert Type II and Type III Esophagogastric Junction Adenocarcinoma. Int J Surg (2018) 53:201–5. doi: 10.1016/j.ijsu.2018.03.004
11. Barbour AP, Rizk NP, Gonen M, Tang L, Bains MS, Rusch VW, et al. Adenocarcinoma of the Gastroesophageal Junction: Influence of Esophageal Resection Margin and Operative Approach on Outcome. Ann Surg (2007) 246(1):1–8. doi: 10.1097/01.sla.0000255563.65157.d2
12. Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, et al. Perioperative Chemotherapy Compared With Surgery Alone for Resectable Gastroesophageal Adenocarcinoma: An FNCLCC and FFCD Multicenter Phase III Trial. J Clin Oncol (2011) 29(13):1715–21. doi: 10.1200/JCO.2010.33.0597
13. Coccolini F, Nardi M, Montori G, Ceresoli M, Celotti A, Cascinu S, et al. Neoadjuvant Chemotherapy in Advanced Gastric and Esophago-Gastric Cancer. Meta-Analysis of Randomized Trials. Int J Surg (2018) 51:120–7. doi: 10.1016/j.ijsu.2018.01.008
14. Miao ZF, Liu XY, Wang ZN, Zhao TT, Xu YY, Song YX, et al. Effect of Neoadjuvant Chemotherapy in Patients With Gastric Cancer: A PRISMA-Compliant Systematic Review and Meta-Analysis. BMC Cancer (2018) 18(1):118. doi: 10.1186/s12885-018-4027-0
15. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting Results of Cancer Treatment. Cancer Am Cancer Soc (1981) 47(1):207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6
16. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
17. Nakajima T. Gastric Cancer Treatment Guidelines in Japan. Gastric Cancer (2002) 5(1):1–5. doi: 10.1007/s101200200000
18. Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2010 (Ver. 3). Gastric Cancer (2011) 14(2):113–23. doi: 10.1007/s10120-011-0042-4
19. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge From a Population-Based to a More “Personalized” Approach to Cancer Staging. CA Cancer J Clin (2017) 67(2):93–9. doi: 10.3322/caac.21388
20. Clavien PA, Sanabria JR, Strasberg SM. Proposed Classification of Complications of Surgery With Examples of Utility in Cholecystectomy. Surgery (1992) 111(5):518–26.
21. Dindo D, Demartines N, Clavien PA. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
22. Siewert JR, Stein HJ. Classification of Adenocarcinoma of the Oesophagogastric Junction. Br J Surg (1998) 85(11):1457–9. doi: 10.1046/j.1365-2168.1998.00940.x
23. Sugita S, Kinoshita T, Kaito A, Watanabe M, Sunagawa H. Short-Term Outcomes After Laparoscopic Versus Open Transhiatal Resection of Siewert Type II Adenocarcinoma of the Esophagogastric Junction. Surg Endosc (2018) 32(1):383–90. doi: 10.1007/s00464-017-5687-6
24. Huang CM, Lv CB, Lin JX, Chen QY, Zheng CH, Li P, et al. Laparoscopic-Assisted Versus Open Total Gastrectomy for Siewert Type II and III Esophagogastric Junction Carcinoma: A Propensity Score-Matched Case-Control Study. Surg Endosc (2017) 31(9):3495–503. doi: 10.1007/s00464-016-5375-y
25. Shi Y, Li L, Xiao H, Guo S, Wang G, Tao K, et al. Feasibility of Laparoscopic Gastrectomy for Patients With Siewert-Type II/III Adenocarcinoma of the Esophagogastric Junction: A Propensity Score Matching Analysis. PLoS One (2018) 13(9):e0203125. doi: 10.1371/journal.pone.0203125
26. Zhang YC, Wu QB, Yang XY, Yang TH, Wang ZQ, Wang ZQ, et al. Laparoscopic-Assisted Transhiatal Esophagogastrectomy Without Thoracic or Cervical Access: A Series of One Hundred Three Consecutive Cases. J Laparoendosc Adv Surg Tech A (2018) 28(7):845–52. doi: 10.1089/lap.2017.0692
27. An JY, Kim KM, Kim YM, Cheong JH, Hyung WJ, Noh SH. Surgical Complications in Gastric Cancer Patients Preoperatively Treated With Chemotherapy: Their Risk Factors and Clinical Relevance. Ann Surg Oncol (2012) 19(8):2452–8. doi: 10.1245/s10434-012-2267-9
28. Wu L, Ge L, Qin Y, Huang M, Chen J, Yang Y, et al. Postoperative Morbidity and Mortality After Neoadjuvant Chemotherapy Versus Upfront Surgery for Locally Advanced Gastric Cancer: A Propensity Score Matching Analysis. Cancer Manag Res (2019) 11:6011–8. doi: 10.2147/CMAR.S203880
29. Koh YW, Park YS, Ryu MH, Ryoo BY, Park HJ, Yook JH, et al. Postoperative Nodal Status and Diffuse-Type Histology are Independent Prognostic Factors in Resectable Advanced Gastric Carcinomas After Preoperative Chemotherapy. Am J Surg Pathol (2013) 37(7):1022–9. doi: 10.1097/PAS.0b013e31828778fd
30. Lu J, Wang W, Zheng CH, Fang C, Li P, Xie JW, et al. Influence of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: An Analysis From a Two-Institution Database in China. Ann Surg Oncol (2017) 24(2):486–93. doi: 10.1245/s10434-016-5494-7
31. Kinoshita O, Ichikawa D, Ichijo Y, Komatsu S, Okamoto K, Kishimoto M, et al. Histological Evaluation for Chemotherapeutic Responses of Metastatic Lymph Nodes in Gastric Cancer. World J Gastroenterol (2015) 21(48):13500–6. doi: 10.3748/wjg.v21.i48.13500
32. Charruf AZ, Ramos M, Pereira MA, Dias AR, de Castria TB, Zilberstein B, et al. Impact of Neoadjuvant Chemotherapy on Surgical and Pathological Results of Gastric Cancer Patients: A Case-Control Study. J Surg Oncol (2020) 121(5):833–9. doi: 10.1002/jso.25839
33. Wei Z, Tan B, Cao S, Liu S, Tan X, Yao Z, et al. The Influence of Neoadjuvant Chemotherapy on Gastric Cancer Patients’ Postoperative Infectious Complications: What Is the Negative Role Played by the Intestinal Barrier Dysfunction? Oncotarget (2017) 8(26):43376–88. doi: 10.18632/oncotarget.14758
34. Zhao Q, Lian C, Huo Z, Li M, Liu Y, Fan L, et al. The Efficacy and Safety of Neoadjuvant Chemotherapy on Patients With Advanced Gastric Cancer: A Multicenter Randomized Clinical Trial. Cancer Med (2020) 9(16):5731–45. doi: 10.1002/cam4.3224
35. Zhang X, Huang H, Wei Z, Zhu Z, Yang D, Fu H, et al. Comparison of Docetaxel + Oxaliplatin + S-1 vs Oxalipatin + S-1 as Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Propensity Score Matched Analysis. Cancer Manag Res (2020) 12:6641–53. doi: 10.2147/CMAR.S258360
36. Sah BK, Zhang B, Zhang H, Li J, Yuan F, Ma T, et al. Neoadjuvant FLOT Versus SOX Phase II Randomized Clinical Trial for Patients With Locally Advanced Gastric Cancer. Nat Commun (2020) 11(1):6093. doi: 10.1038/s41467-020-19965-6
37. Zhao Q, Li Y, Huang J, Fan L, Tan B, Tian Y, et al. Short-Term Curative Effect of S-1 Plus Oxaliplatin as Perioperative Chemotherapy for Locally Advanced Gastric Cancer: A Prospective Comparison Study. Pharmazie (2017) 72(4):236–40. doi: 10.1691/ph.2017.6865
38. Zhou J, Yu P, Shi Y, Tang B, Hao Y, Zhao Y, et al. Evaluation of Clavien-Dindo Classification in Patients Undergoing Total Gastrectomy for Gastric Cancer. Med Oncol (2015) 32(4):120. doi: 10.1007/s12032-015-0573-3
39. Li Z, Bai B, Zhao Y, Yu D, Lian B, Liu Y, et al. Severity of Complications and Long-Term Survival After Laparoscopic Total Gastrectomy With D2 Lymph Node Dissection for Advanced Gastric Cancer: A Propensity Score-Matched, Case-Control Study. Int J Surg (2018) 54(Pt A):62–9. doi: 10.1016/j.ijsu.2018.04.034
40. Hamakawa T, Kurokawa Y, Mikami J, Miyazaki Y, Takahashi T, Yamasaki M, et al. Risk Factors for Postoperative Complications After Gastrectomy in Gastric Cancer Patients With Comorbidities. Surg Today (2016) 46(2):224–8. doi: 10.1007/s00595-015-1175-6
41. Chen X, Feng X, Wang M, Yao X. Laparoscopic Versus Open Distal Gastrectomy for Advanced Gastric Cancer: A Meta-Analysis of Randomized Controlled Trials and High-Quality Nonrandomized Comparative Studies. Eur J Surg Oncol (2020) 46(11):1998–2010. doi: 10.1016/j.ejso.2020.06.046
Keywords: esophagogastric junction, neoadjuvant chemotherapy, laparoscopic, postoperative complication, Siewert II and III
Citation: Feng Q, Long D, Du M-s, Wang X-s, Li Z-s, Zhao Y-l, Qian F, Wen Y, Yu P-w and Shi Y (2021) Short-Term Clinical Efficacy of Neoadjuvant Chemotherapy Combined With Laparoscopic Gastrectomy for Locally Advanced Siewert Type II and III Adenocarcinoma of the Esophagogastric Junction: A Retrospective, Propensity Score-Matched Study. Front. Oncol. 11:690662. doi: 10.3389/fonc.2021.690662
Received: 03 April 2021; Accepted: 24 August 2021;
Published: 29 September 2021.
Edited by:
Hao Liu, Southern Medical University, ChinaReviewed by:
Lu Zang, Shanghai Jiao Tong University, ChinaCopyright © 2021 Feng, Long, Du, Wang, Li, Zhao, Qian, Wen, Yu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Shi, c2hpeWFuZG9jdG9yQHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.