- 1Department of Oncology, Xiangya Hospital, Central South University, Changsha, China
- 2Department of Pathology, Xiangya Hospital, Central South University, Changsha, China
- 3Department of Pancreatic Surgery, Xiangya Hospital, Central South University, Changsha, China
- 4Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
Sarcoma or sarcomatoid malignancies are a set of mesenchymal-origin malignancies with vast heterogeneity in clinical and molecular characteristics. Anaplastic lymphoma kinase (ALK) is a tyrosine kinase oncoprotein expressed by several tumors, including sarcomas. Crizotinib is an effective ALK inhibitor. In this review paper, we summarized findings from the literature regarding the use of crizotinib for the treatment of sarcoma and sarcomatoid malignancies harboring ALK fusions with definitive partners (with the given gene(s) name) from the years 2010 to 2021.One hundred and four articles were retrieved and after exclusion, 28 studies containing 33 patients were finally selected. All 33 patients were treated with crizotinib. Among the 33 cases, 19 were adult patients, 11 were pediatric patients, and 3 cases did not have data on age and/or gender. Most cases had a primary abdominal lesion (16/30), followed by thoracic (10/30), trunk (3/30), retroperitoneal (1/30), and one case of right medial thigh (case 7). Stage IV disease was reported in 76.7% (23/30) of patients. The objective response rate and disease control rate was 86.7% (26/30) and 96.7% (29/30), respectively, which were assessed on average of 8 weeks after crizotinib initiation. Rapid improvement of symptoms was observed within one to two weeks in some cases including patients with extensive diseases or poor performance. There was no difference in crizotinib response between pediatrics and adult cases. Crizotinib is effective; however, surgery remains the mainstay of therapy, with newer evidence showing concurrent crizotinib with surgery conferring long-term overall survival. However, we should still be cognizant of the heterogeneous landscape of crizotinib efficacy and its associated fatal adverse events.
Introduction
Anaplastic lymphoma kinase (ALK), a single-chain transmembrane receptor tyrosine kinase, was first described as a fusion partner in the t (2;5) chromosomal translocation in anaplastic large cell lymphoma (ALCL) in 1994. The C-terminal 563 amino acids (aa 1058-1620) that constitute the cytoplasmic portion of ALK, including the kinase domain, are encoded by exons 20-29 at the 3’ end of the ALK gene. The resulting fusion oncoproteins are chimeric self-associating polypeptides with a variety of N-terminal domains and a common, constitutively active C-terminal tyrosine kinase domain, in the vast majority of cases including all 563 cytoplasmic amino acids from ALK. Novel ALK fusion protein partners are increasingly uncovered in sarcoma or sarcomatoid carcinoma (1–3). Endoplasmic reticulum ribosomal-binding protein 1 (RRBP 1), Glypican1 (GPC1), leucine-rich repeat flightless-1-interacting protein 1 (LRRFIP1), Fibronectin 1 (FN1), or Tensins1 (TNS1), and Nuclear Mitotic Apparatus proteins (NUMA1), were correlated with carcinogenesis and cancer progression via multifaceted methods such as reducing ER stress, as modulators of growth factor signaling, promoting epithelial mesenchymal transition (EMT), participating in cellular adhesion and migration processes, or be involved in deoxyribonucleic acid (DNA) damage repair and homologous recombination et al. So different ALK fusion proteins exhibit divergent stability, cellular distribution and sensitivity to ALK tyrosine kinase inhibitors(TKIs) due to distinct characteristics of the partners which were fused to ALK gene.
Crizotinib is a kinase inhibitor approved by Food and Drug Administration (FDA) for the treatment of ALK or c-ros oncogene 1 receptor kinase (ROS1) -positive patients with metastatic non-small cell lung cancer (NSCLC). It was gradually off-label in the treatment of inoperable sarcomas that are carried with ALK fusions since the first case of inflammatory myofibroblastic sarcoma (IMT) with the ALK rearrangement showed a favorable response to crizotinib (4). However, several questions remain unanswered, including if single-agent crizotinib is optimal, or if a partner of ALK fusion kinase variants influences tumor response to crizotinib (5), and if next-generation ALK inhibitors are effective post-crizotinib resistance.
Owing to the low incidence of ALK-fusion sarcoma and sarcomatoid malignancies, most of the available data are case reports with detailed molecular profiles. Therefore, we aim to provide a systematic review of the available data on the therapeutic effects of crizotinib in ALK-positive sarcoma and sarcomatoid malignancies.
Materials And Methods
Medical Subject Heading Terms (MESH)
An electronic literature search in the PUBMED database was performed on August 22nd, 2021. The search period was from 2010 to 2021. MESH terms used for the search were “ ALK*[AB] AND [CRIZOTINIB*(AB) OR ALK inhibitor (TI) OR kinase inhibitor (TI)] AND [SARCOMA*(AB) OR SARCOMATOID(TI) OR INFLAMMATORY MYOFIBROBLASTIC TUMOR (TI)]”. All articles included in this review were identified according to the inclusion criteria. Conference abstracts were excluded.
Eligibility Criteria
All articles were screened as per inclusion criteria: All patients with sarcoma or sarcomatous malignancies, presence of ALK fusion with definitive partner(s) and use of crizotinib. The exclusion criteria include non-human models, non-English language publications, and reviews that do not contain original case reports.
Data Collection
All included articles were reviewed, and the following information was retrieved: Year of publication, partners for ALK fusions, tumor histomorphology, patient age and gender, sequence of crizotinib use and its combination, treatment response, survival time, and adverse events (AEs).
Statistical Methods for Pooled Analyses
Pooled analyses were performed using SPSS software, in which normally distributed data were represented as average (95%CI, lower and upper limit). Data that were not normally distributed were presented as medians (95% CI, lower and upper limit). Chi-square test was used to compare the response of crizotinib between the different subgroups. Survival was analyzed using the Kaplan-Meier curves. P value less than 0.05 was considered statistically significant.
Results
Study Selection
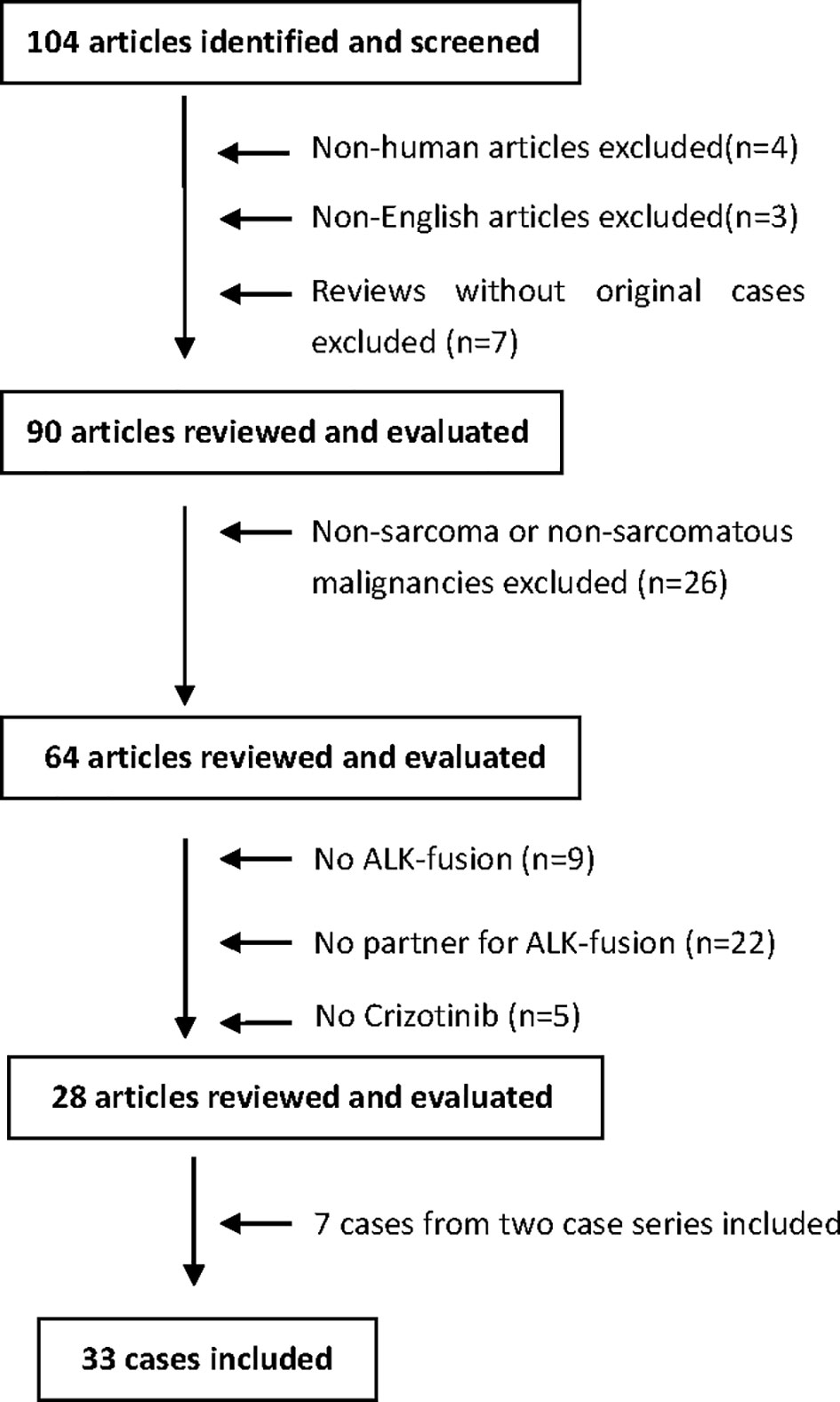
As shown in Figure 1, a total of 104 search results were found. After screening, the following articles were excluded: non-human data (n=4), non-English language articles (n=3), reviews without original cases (n=7), non-sarcoma/non-sarcomatous malignancies (n=26), lack of ALK-fusion (n=9), lack of ALK-fusion partners (n=22), and lack of crizotinib-based drug regimens (n=5). In addition, 7 cases from two case series (n=7) were presented individually and included to generate a total of 33 cases (26 + 7) that were analyzed in this review.
Patients and Tumors Characteristics
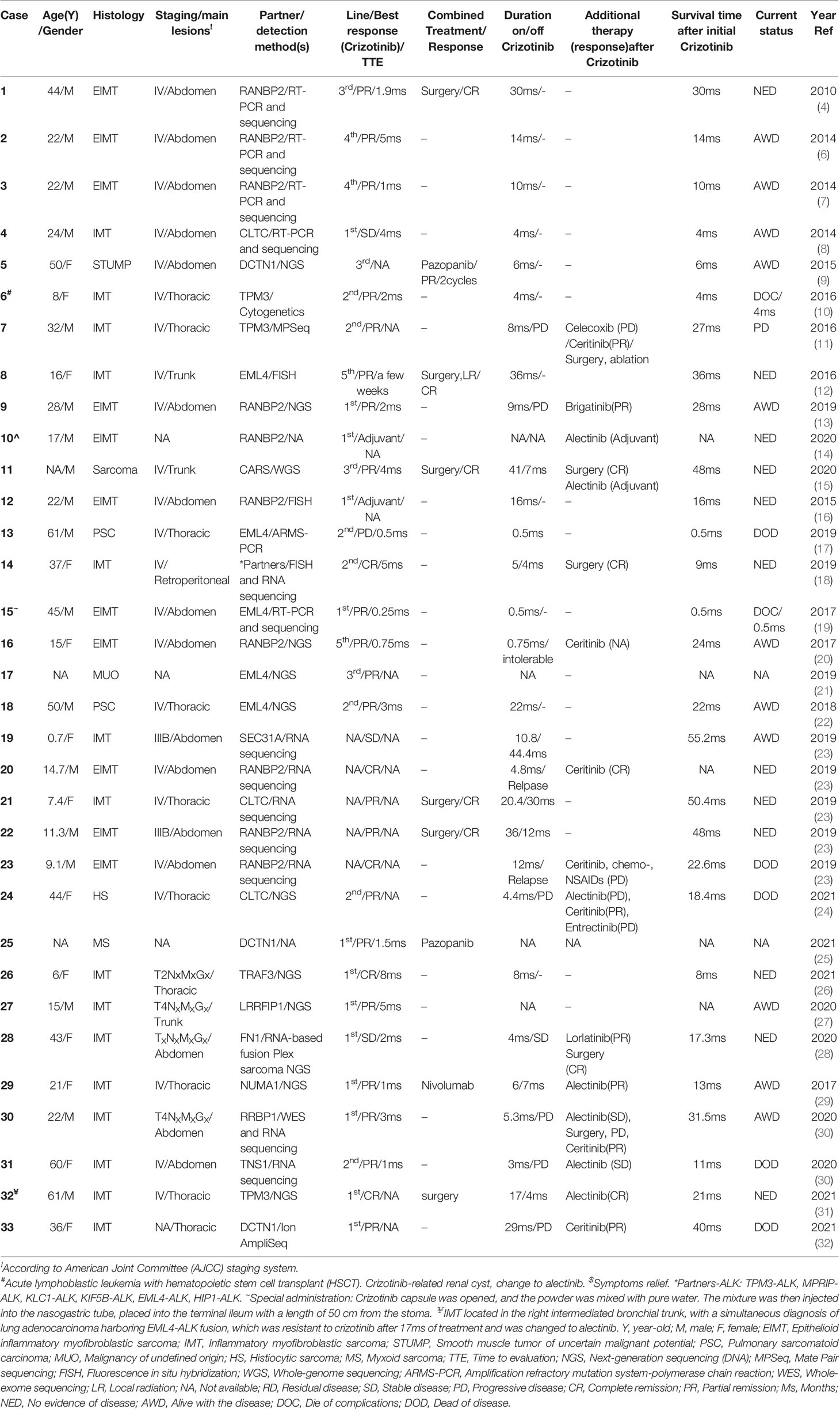
Among the 33 cases in Table 1, 19 were adult patients, 11 were pediatric patients, and 3 cases did not have data on age and/or gender. Most cases had a primary abdominal lesion (16/30), followed by thoracic (10/30), trunk (3/30), retroperitoneal (1/30), and one case of right medial thigh (case 7). Stage IV disease was reported in 76.7% (23/30) of patients.
The histomorphology included 11 cases of epithelioid inflammatory myofibroblastic sarcoma (EIMT), 15 cases of IMT, 2 cases of pulmonary sarcomatoid carcinoma (PSC), 1 case of low-grade sarcoma, 1 case of smooth muscle tumor of uncertain malignant potential (STUMP), 1 malignancy with unknown origin, 1 histiocytic sarcoma (HS), and 1 myxoid sarcoma (MS).
Generally, most EIMTs harbor RANBP2-ALK (10/11) rearrangement. Using newer Ribonucleic Acid (RNA) sequencing technologies, the 15 cases of IMT were found to harbor various fusion partners of ALK, including CLTC, TPM3, EML4, SEC31A, TRAF3, LRRFIP1, FN1, NUMA1, RRBP1, TNS1, and DCTN1. Both cases of PSC harbored EML4-ALK. Similar to IMT, HS and MS harbored partners of CLTC and DCTN1. Intriguingly, one case report by Ogata et al. showed multiple fusion partners, including TPM3 (tropomyosin 3), MPRIP (myosin phosphatase Rho interacting protein), KLC1 (kinesin light chain 1), KIF5B (kinesin family member 5B), EML4 (echinoderm microtubule-associated protein-like 4), and HIP1 (huntingtin interacting protein 1).
Response to Crizotinib Treatment
As shown in Table 1, the objective response rate (ORR) and disease control rate (DCR) were 86.7% (26/30) and 96.7% (29/30), respectively, which were assessed on average of 8 weeks after crizotinib initiation. One case with concurrent KRAS exon 2 mutation deteriorated clinically and died (17). Rapid improvement of symptoms was observed within one to two weeks in some cases (case 3, 15, 29). Patients with extensive diseases or poor performance status (PS) (case 6, 15, 29, 30) still had good responses to crizotinib. Intriguingly, EIMT had an excellent ORR of 100% to crizotinib (9/9). One IMT case with FN1 partner mutation had no response to crizotinib, but the tumor dramatically shrunk following lorlatinib neoadjuvant treatment followed by radical resection (28). Another IMT case with a TNS1 partner progressed shortly after the crizotinib response and died upon switching to alectinib (30). Three pediatric cases (cases 21, 22, 19) were off crizotinib upon achieving no evidence of disease (NED) or stable disease (SD), with recurrent lesions surgically removed, hence achieving long-term survival (23). There was no difference in crizotinib response between pediatric and adult cases (P=0.809) (Table 2).
Improved Outcomes in Patients Treated With Surgery and Crizotinib
The survival time for cases included in our analysis ranged from 0.5 to 55.2 months. Patients treated with both surgery and crizotinib had an improved clinical outcome compared to cases treated with crizotinib alone as indicated by complete remission (CR) rates of 80% (8/10) and 5.3% (1/19), respectively (P<0.05) (Table 3). The median overall survival (mOS) time in the combination group was significantly longer compared to crizotinib treatment alone (31.5ms, 95% CI, 17.3-48.0ms; versus 14ms, 95% CI, 8.0-22.0ms, respectively, P<0.05) (Figure 2). Gaudichon et al. reported a metastatic IMT case that achieved CR following multiple primary lesion resection and a metastasectomy, all while on crizotinib maintenance therapy (12).

Table 3 Response to crizotinib therapy with or without surgery in patients with sarcomatous malignancies.
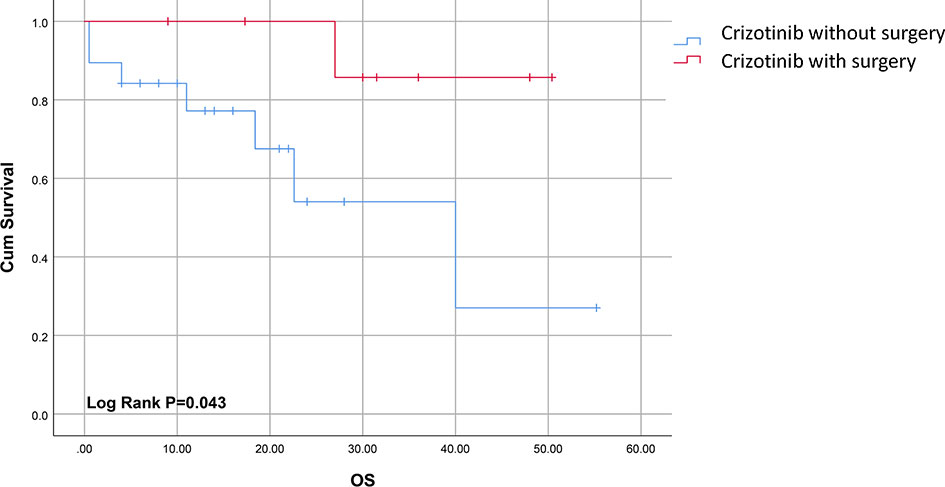
Figure 2 Survival curves of ALK-positive sarcomatous malignancies for patients treated with crizotinib with or without surgery.
AEs of Crizotinib
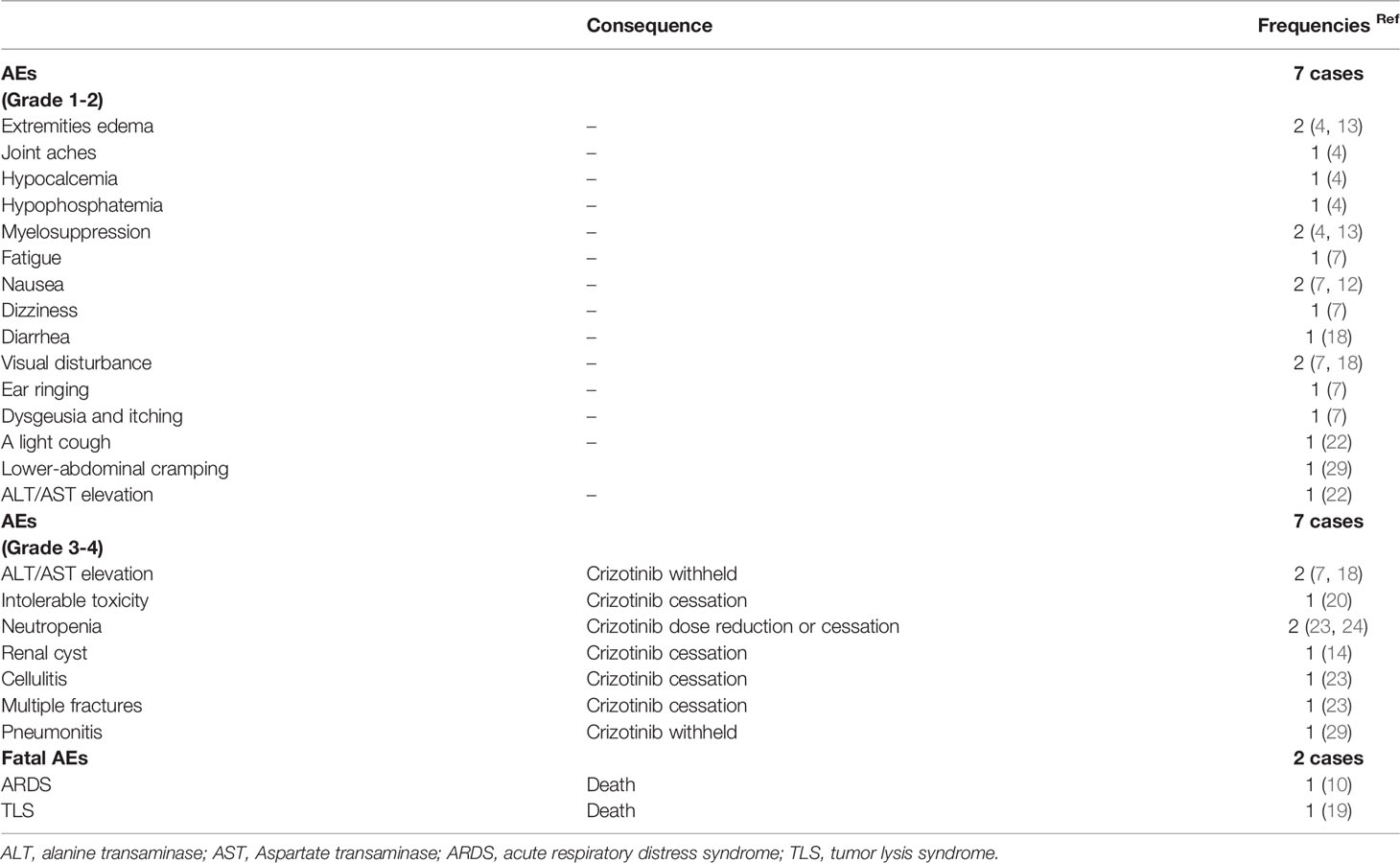
As shown in Table 4, crizotinib-related AEs were observed in 16 cases, with some cases having multiple AEs (4, 7, 13, 18, 22). Serious AEs, including transaminitis, neutropenia, renal cyst, cellulitis, and multiple fractures, were seen in 7 cases prompting crizotinib cessation or dose reduction (1, 7, 18, 20, 23, 29, 30). Fatal AEs in 2 cases led to death, which occurred in 0.5 and 4ms, respectively, after crizotinib initiation. One case was an undiagnosed tumor lysis syndrome (TLS), in which a heavy tumor burden caused a rapid fatal response to crizotinib, while the other was due to criztoinib-related acute respiratory distress syndrome (ARDS) (10, 19). Notably, one case achieved partial remission (PR) following combination therapy with crizotinib and nivolumab due to high PD-L1 expression in tumor tissues but later presented with recurrent pneumonitis resulting from both agents prompting a switch to alectinib (29).
Post-Crizotinib Treatment Options and Outcomes
Ten cases had no response or progressed on crizotinib treatment (11, 13, 15, 23, 24, 28, 30, 32). Nine of them received next-generation ALK inhibitors (ceritinib, brigatinib, alectinib, or lorlatinib) with 77.8% (7/9) achieving ORR (11, 13, 23, 24, 28, 30, 32), and the remaining two cases resistant to ceritinib or alectinib died within one year after their progression on crizotinib (23, 30). One case with radical surgery followed by alectinib adjuvant treatment was still alive without evidence of disease (15). Two cases showed no response to alectinib but achieved tumor shrinkage following subsequent ceritinib treatment (24, 30).
Discussion
ALK protein is normally expressed in the central nervous system and encodes a novel receptor, tyrosine kinase (33). ALK rearrangement leads to its dysregulation under a new promoter from its fusion partner genes, resulting in ligand-independent tyrosine kinase activation. ALK fusions were identified in about 50% of IMT cases, 3.6% of sarcomatous malignancies, and extremely rare in rhabdomyosarcomas and leiomyosarcoma (34).
ALK-positive sarcoma is predominantly seen in pediatric or middle-aged patients, with only 2 patients older than 60-years in our cohort of 33 cases. IMT and its variant EIMTs composed the majority of our cases, accounting for 78.8% (26/33). Crizotinib is an effective Adenosine triphosphate (ATP)-competitive ALK inhibitor. It achieved 74% ORR in ALK-fusion positive NSCLC (35). But the efficacy of crizotinib in sarcoma or sarcomatous malignancies is largely unknown. Although limited by sample size, our pooled analysis showed up to 86.7% efficacy of crizotinib in ALK-positive sarcoma or sarcomatoid malignancies with equal effectiveness in both adult and pediatric patients.
The partners of ALK fusion kinase variants impact cellular phenotypes and response to ALK inhibitors (5). As shown in case 28, FN1 is ubiquitously and abundantly expressed in human cells; therefore, high-level expression of ALK under the control of a strong FN1 promoter, as revealed by immunohistochemistry, leads to reduced crizotinib efficacy (36). TNS1-ALK fusion in case 31 also represents a distinct variant of crizotinib resistance but was later sensitive to brigatinib, which controlled disease for at least 9 months (37). Similar to previous reports, most EIMTs have RANBP2-ALK mutation. RANBP2 is a perinuclear protein closely related to mitochondrial stress, a feature that likely explains distinct staining patterns around the perinuclear and nuclear membrane and the aggressive nature of EIMTs (38). However, RANBP2 does not affect crizotinib response; as shown in Table 1, all EIMT cases responded to crizotinib.
Surgical resection remains the mainstay of treatment for localized ALK-positive sarcoma and sarcomatous malignancies. Crizotinib combined with surgery improved long-term survival, with mOS of up to 31.5ms, compared to crizotinib alone(mOS 14ms). Resection of resistant or recurrent lesions such as oligo-residual or oligo-progression is optimal for treating sarcomas (39). A main example of this approach is the role of surgery in prolonging the progression-free survival (PFS) after imatinib resistance in gastrointestinal stromal tumors (GIST), mainly by resection of resistant tumor lesions (40).
Mutations within the ALK fusion gene attribute to about 30% of cases of crizotinib resistance. Second generation of ALK TKIs, including ceritinib, brigatinib, alectinib, were usually effective in later lines (13, 32), similar to their current indications in NSCLC. Interestingly, 2 cases that progressed on alectinib following crizotinib resistance showed response to subsequent ceritinib (24, 30). Different ALK inhibitors bind slightly differently within the ATP-binding pocket of the ALK kinase domain, and thus coax different resistance mutation patterns. As part of the underlying resistance mechanism to ALK inhibitors, a complex chromoplectic residue forms involving two focal regions on the q-arms of chromosomes 1, 9, and 20 containing the ALK locus following ceritinib treatment, indicating a multifaceted resistance mechanism (11).
Crizotinib is predominantly metabolized by CYP3A4/5 existed in the liver. Hepatotoxicity, as well as interstitial lung disease/pneumonitis, QT interval prolongation, bradycardia, and severe visual loss were previously observed in crizotinib-treated NSCLC cases. Here, the rare incidence of serious or fatal complications such as cellulitis, multiple bone fractures, ARDS or TLS require prompt attention from the treating physicians, albeit with CR of the disease (10, 19).
This study is not without limitations. Due to the low incidence and high heterogeneity of sarcoma, most of the available data are case reports or subgroup analyses from small size clinical trials. So, the pooled data should be carefully interpreted. Multiple center cooperation for large prospective studies is warranted. A brief clinical routine for treating ALK-positive sarcomatous malignancies was presented in Figure 3 as a reference for clinical decision-making.
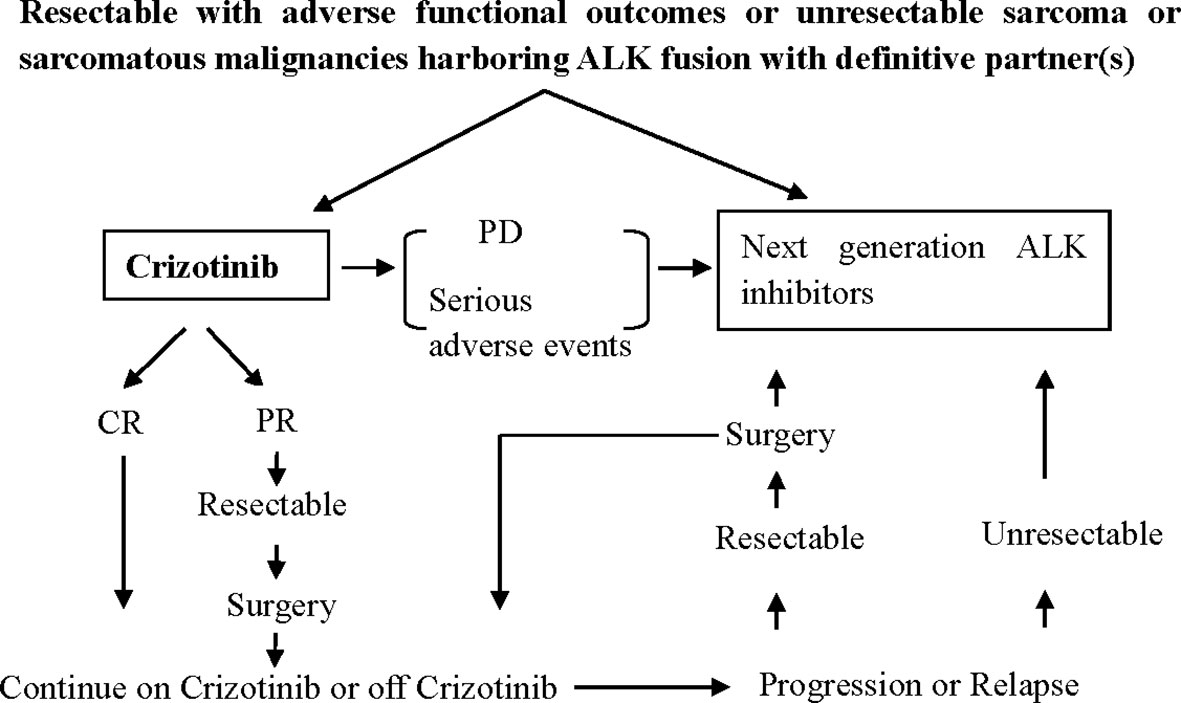
Figure 3 Proposed clinical routine for the treatment of metastatic ALK-positive sarcomatous malignancies. PD, progressive disease; CR, complete remission; PR, partial remission.
Conclusion
Findings from this analysis indicated that crizotinib-based palliative treatment regimens improved outcomes in patients with ALK-positive sarcoma and sarcomatoid malignancies. Nevertheless, further investigations are necessary to understand the efficacy of crizotinib treatment over the different types of sarcomas and sarcomatoid malignancies. The potential AEs should be carefully evaluated if crizotinib-based regimens are considered for treatment.
Author Contributions
Contribution to data retrieval and evaluation: BL and JW collected and entered data. SX, GH and OA reviewed the data. YH and SX, who are sarcoma pathologists, evaluated the outcomes of the information. Contribution to manuscript: BL designed the article. JW drafted the manuscript. YH and GH contributed to the literature search and discussion. OA and SX helped to revise the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by a grant from the Province Natural Science Foundation of Hunan (No. 2021JJ31069).
Conflict of Interest
The authors declare that the research was conducted in the absence any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Shan Shan Feng from the Department of Public Health of Guangzhou Medical University for conducting all the statistical analyses included in this study.
References
1. Lee JC, Li CF, Huang HY, Zhu MJ, Marino-Enriquez A, Lee CT, et al. ALK Oncoproteins in Atypical Inflammatory Myofibroblastic Tumours: Novel RRBP1-ALK Fusions in Epithelioid Inflammatory Myofibroblastic Sarcoma. J Pathol (2017) 241:316–23. doi: 10.1002/path.4836
2. Davis LE, Nusser KD, Przybyl J, Pittsenbarger J, Hofmann NE, Varma S, et al. Discovery and Characterization of Recurrent, Targetable ALK Fusions in Leiomyosarcoma. Mol Cancer Res (2019) 17:676–85. doi: 10.1158/1541-7786.MCR-18-1075
3. Xiong L, Li X, Chen D, Li S, Luo L. GPC1-ALK: A Novel ALK Fusion in a Patient With Pulmonary Sarcomatoid Carcinoma. Lung Cancer (2021) 151:104–5. doi: 10.1016/j.lungcan.2020.11.021
4. Butrynski JE, D’adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, et al. Crizotinib in ALK-Rearranged Inflammatory Myofibroblastic Tumor. N Engl J Med (2010) 363:1727–33. doi: 10.1056/NEJMoa1007056
5. Childress MA, Himmelberg SM, Chen H, Deng W, Davies MA, Lovly CM. ALK Fusion Partners Impact Response to ALK Inhibition: Differential Effects on Sensitivity, Cellular Phenotypes, and Biochemical Properties. Mol Cancer Res (2018) 16:1724–36. doi: 10.1158/1541-7786.MCR-18-0171
6. Kurihara-Hosokawa K, Kawasaki I, Tamai A, Yoshida Y, Yakushiji Y, Ueno H, et al. Epithelioid Inflammatory Myofibroblastic Sarcoma Responsive to Surgery and an ALK Inhibitor in a Patient With Panhypopituitarism. Intern Med (2014) 53:2211–4. doi: 10.2169/internalmedicine.53.2546
7. Kimbara S, Takeda K, Fukushima H, Inoue T, Okada H, Shibata Y, et al. A Case Report of Epithelioid Inflammatory Myofibroblastic Sarcoma With RANBP2-ALK Fusion Gene Treated With the ALK Inhibitor, Crizotinib. Jpn J Clin Oncol (2014) 44:868–71. doi: 10.1093/jjco/hyu069
8. Lorenzi L, Cigognetti M, Medicina D, Pellegrini V, Balzarini P, Cestari R, et al. ALK-Positive Inflammatory Myofibroblastic Tumor of the Abdomen With Widespread Microscopic Multifocality. Int J Surg Pathol (2014) 22:640–4. doi: 10.1177/1066896914525232
9. Subbiah V, Mcmahon C, Patel S, Zinner R, Silva EG, Elvin JA, et al. STUMP Un“Stumped”: Anti-Tumor Response to Anaplastic Lymphoma Kinase (ALK) Inhibitor Based Targeted Therapy in Uterine Inflammatory Myofibroblastic Tumor With Myxoid Features Harboring DCTN1-ALK Fusion. J Hematol Oncol (2015) 8:66. doi: 10.1186/s13045-015-0160-2
10. Shash H, Stefanovici C, Phillips S, Cuvelier GD. Aggressive Metastatic Inflammatory Myofibroblastic Tumor After Allogeneic Stem Cell Transplant With Fatal Pulmonary Toxicity From Crizotinib. J Pediatr Hematol Oncol (2016) 38:642–45. doi: 10.1097/MPH.0000000000000594
11. Mansfield AS, Murphy SJ, Harris FR, Robinson SI, Marks RS, Johnson SH, et al. Chromoplectic TPM3-ALK Rearrangement in a Patient With Inflammatory Myofibroblastic Tumor Who Responded to Ceritinib After Progression on Crizotinib. Ann Oncol (2016) 27:2111–17. doi: 10.1093/annonc/mdw405
12. Gaudichon J, Jeanne-Pasquier C, Deparis M, Veyssiere A, Heyndrickx M, Minckes O, et al. Complete and Repeated Response of a Metastatic ALK-Rearranged Inflammatory Myofibroblastic Tumor to Crizotinib in a Teenage Girl. J Pediatr Hematol Oncol (2016) 38:308–11. doi: 10.1097/MPH.0000000000000498
13. Xu X, Li H, Peng K, Yu Y, Chen L, Fang Y, et al. ALK-G1269A Mutation in Epithelioid Inflammatory Myofibroblastic Sarcoma After Progression on Crizotinib: A Case Report. Oncol Lett (2019) 17:2370–76. doi: 10.3892/ol.2018.9865
14. Kopelevich A, Holzman SA, La J, Lai HA, Torno L, Stephany HA. Crizotinib-Associated Renal Cyst Formation in a Pediatric Patient With ALK+ Epithelioid Inflammatory Myofibroblastic Sarcoma. Urology (2021) 149:222–24. doi: 10.1016/j.urology.2020.08.032
15. Jiao XD, Liu K, Xu M, Yu G, Liu D, Huang T, et al. Metastatic Low-Grade Sarcoma With CARS-ALK Fusion Dramatically Responded to Multiple ALK Tyrosine Kinase Inhibitors: A Case Report With Comprehensive Genomic Analysis. Oncologist (2021) 26:e524–e29. doi: 10.1002/onco.13543
16. Liu Q, Kan Y, Zhao Y, He H, Kong L. Epithelioid Inflammatory Myofibroblastic Sarcoma Treated With ALK Inhibitor: A Case Report and Review of Literature. Int J Clin Exp Pathol (2015) 8:15328–32.
17. Chen F, Gu Q, Hu C, Cai X, Lei S. Poor Prognosis of Pulmonary Sarcomatoid Carcinoma With KRAS Mutation and ALK Fusion. Onco Targets Ther (2019) 12:3321–25. doi: 10.2147/OTT.S196751
18. Ogata M, Hatachi Y, Ogata T, Satake H, Imai Y, Yasui H. Effectiveness of Crizotinib for Inflammatory Myofibroblastic Tumor With ALK Mutation. Intern Med (2019) 58:1029–32. doi: 10.2169/internalmedicine.1640-18
19. Jiang Q, Tong HX, Hou YY, Zhang Y, Li JL, Zhou YH, et al. Identification of EML4-ALK as an Alternative Fusion Gene in Epithelioid Inflammatory Myofibroblastic Sarcoma. Orphanet J Rare Dis (2017) 12:97. doi: 10.1186/s13023-017-0647-8
20. Fang H, Langstraat CL, Visscher DW, Folpe AL, Schoolmeester JK. Epithelioid Inflammatory Myofibroblastic Sarcoma of the Ovary With RANB2-ALK Fusion: Report of a Case. Int J Gynecol Pathol (2018) 37:468–72. doi: 10.1097/PGP.0000000000000431
21. Seol YM, Kwon CH, Lee SJ, Lee SJ, Choi Y, Choi YJ, et al. A Pilot Prospective Study of Refractory Solid Tumor Patients for NGS-Based Targeted Anticancer Therapy. Transl Oncol (2019) 12:301–07. doi: 10.1016/j.tranon.2018.10.011
22. Valter A, Roosipuu R, Tamm H, Padrik P. An Anaplastic Lymphoma Kinase (ALK) Fusion Oncogene Positive Metastatic Sarcomatoid Carcinoma of the Lung With Good Response to Crizotinib. AME Case Rep (2018) 2:2. doi: 10.21037/acr.2018.01.01
23. Trahair T, Gifford AJ, Fordham A, Mayoh C, Fadia M, Lukeis R, et al. Crizotinib and Surgery for Long-Term Disease Control in Children and Adolescents With ALK-Positive Inflammatory Myofibroblastic Tumors. JCO Precis Oncol (2019) 3:PO.18.00297. doi: 10.1200/PO.18.00297
24. Takeyasu Y, Okuma HS, Kojima Y, Nishikawa T, Tanioka M, Sudo K, et al. Impact of ALK Inhibitors in Patients With ALK-Rearranged Nonlung Solid Tumors. JCO Precis Oncol (2021) 5:756–66. doi: 10.1200/PO.20.00383
25. Piha-Paul SA, Dumbrava EE, Nair BC, Xiong W, Xu L, Mostorino R, et al. A Phase I Trial of the MET/ALK/ROS1 Inhibitor Crizotinib Combined With the VEGF Inhibitor Pazopanib in Patients With Advanced Solid Malignancies. Onco Targets Ther (2021) 14:3037–49. doi: 10.2147/OTT.S291801
26. Di Ruscio V, Mastronuzzi A, Russo I, Neri M, Stracuzzi A, Giovannoni I, et al. Inflammatory Myofibroblastic Tumor of the Upper Airways Harboring a New TRAF3-ALK Fusion Transcript. Children (Basel) (2021) 8:505. doi: 10.3390/children8060505
27. Liu W, Duan Q, Gong L, Yang Y, Huang Z, Guo H, et al. A Novel LRRFIP1-ALK Fusion in Inflammatory Myofibroblastic Tumor of Hip and Response to Crizotinib. Invest New Drugs (2021) 39:278–82. doi: 10.1007/s10637-020-00984-5
28. Reinhart S, Trachsel Y, Fritz C, Wagner U, Bode-Lesniewska B, John H, et al. Inflammatory Myofibroblastic Tumor of the Bladder With FN1-ALK Gene Fusion: Different Response to ALK Inhibition. Urology (2020) 146:32–5. doi: 10.1016/j.urology.2020.09.026
29. Rao N, Iwenofu H, Tang B, Woyach J, Liebner DA. Inflammatory Myofibroblastic Tumor Driven by Novel NUMA1-ALK Fusion Responds to ALK Inhibition. J Natl Compr Canc Netw (2018) 16:115–21. doi: 10.6004/jnccn.2017.7031
30. Zhang C, Wang Z, Zhuang R, Guo X, Feng Y, Shen F, et al. Efficacy and Resistance of ALK Inhibitors in Two Inflammatory Myofibroblastic Tumor Patients With ALK Fusions Assessed by Whole Exome and RNA Sequencing. Onco Targets Ther (2020) 13:10335–42. doi: 10.2147/OTT.S270481
31. Zhao S, Liu W, Li S, Shi T, Chen Q, Li Q, et al. A Case of Simultaneously Diagnosed Lung Adenocarcinoma and Endobronchial Inflammatory Myofibroblastic Tumor With Two Distinct Types of ALK Translocation. Cancer Res Treat (2021) 53:601–6. doi: 10.4143/crt.2020.952
32. Michels SYF, Scheel AH, Wundisch T, Heuckmann JM, Menon R, Puesken M, et al. ALK(G1269A) Mutation as a Potential Mechanism of Acquired Resistance to Crizotinib in an ALK-Rearranged Inflammatory Myofibroblastic Tumor. NPJ Precis Oncol (2017) 1:4. doi: 10.1038/s41698-017-0004-3
33. Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui X, et al. ALK, the Chromosome 2 Gene Locus Altered by the T (2,5) in Non-Hodgkin’s Lymphoma, Encodes a Novel Neural Receptor Tyrosine Kinase That Is Highly Related to Leukocyte Tyrosine Kinase (LTK). Oncogene (1997) 14:2175–88. doi: 10.1038/sj.onc.1201062
34. Theilen TM, Soerensen J, Bochennek K, Becker M, Schwabe D, Rolle U, et al. Crizotinib in ALK(+) Inflammatory Myofibroblastic Tumors-Current Experience and Future Perspectives. Pediatr Blood Cancer (2018) 65:e26920. doi: 10.1002/pbc.26920
35. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-Line Crizotinib Versus Chemotherapy in ALK-Positive Lung Cancer. N Engl J Med (2014) 371:2167–77. doi: 10.1056/NEJMoa1408440
36. Ouchi K, Miyachi M, Tsuma Y, Tsuchiya K, Iehara T, Konishi E, et al. FN1: A Novel Fusion Partner of ALK in an Inflammatory Myofibroblastic Tumor. Pediatr Blood Cancer (2015) 62:909–11. doi: 10.1002/pbc.25424
37. Lee J, Singh A, Ali SM, Lin DI, Klempner SJ. TNS1-ALK Fusion in a Recurrent, Metastatic Uterine Mesenchymal Tumor Originally Diagnosed as Leiomyosarcoma. Acta Med Acad (2019) 48:116–20. doi: 10.5644/ama2006-124.248
38. Cho KI, Yi H, Yeh A, Tserentsoodol N, Cuadrado L, Searle K, et al. Haploinsufficiency of RanBP2 Is Neuroprotective Against Light-Elicited and Age-Dependent Degeneration of Photoreceptor Neurons. Cell Death Differ (2009) 16:287–97. doi: 10.1038/cdd.2008.153
39. Grilley-Olson JE, Webber NP, Demos DS, Christensen JD, Kirsch DG. Multidisciplinary Management of Oligometastatic Soft Tissue Sarcoma. Am Soc Clin Oncol Educ Book (2018) 38:939–48. doi: 10.1200/EDBK_200573
Keywords: crizotinib, sarcoma, sarcomatoid malignancies, ALK fusions, definitive partner(s), adverse events
Citation: Wu J, Hu Y, Abdihamid O, Huang G, Xiao S and Li B (2021) Crizotinib in Sarcomatous Malignancies Harboring ALK Fusion With a Definitive Partner(s): Response and Efficacy. Front. Oncol. 11:684865. doi: 10.3389/fonc.2021.684865
Received: 09 July 2021; Accepted: 27 September 2021;
Published: 14 October 2021.
Edited by:
Nehad M. Ayoub, Jordan University of Science and Technology, JordanReviewed by:
Teodora Alexa-Stratulat, Grigore T. Popa University of Medicine and Pharmacy, RomaniaOsama Alshogran, Jordan University of Science and Technology, Jordan
Copyright © 2021 Wu, Hu, Abdihamid, Huang, Xiao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Li, YmluY3N1eHlAY3N1LmVkdS5jbg==
 Jinchun Wu
Jinchun Wu Yongbin Hu
Yongbin Hu Omar Abdihamid1
Omar Abdihamid1 Gengwen Huang
Gengwen Huang Sheng Xiao
Sheng Xiao Bin Li
Bin Li