- 1State Key Laboratory of Oncogenes and Related Genes, Department of Urology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 2Department of Nuclear Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Positron emission tomography/computed tomography (PET/CT) is widely used in prostate cancer to evaluate the localized tumor burden and detect symptomatic metastatic lesions early. 18F-FDG is the most used tracer for oncologic imaging, but it has limitations in detecting early-stage prostate cancer. 68Ga-PSMA is a new tracer that has high specificity and sensibility in detecting local and metastatic tumors. But with the progression of prostate cancer, the enhancement of glucose metabolism in progressive prostate cancer provides a chance for 18F-FDG. This review focuses on PET/CT in the detection and prognosis of prostate cancer, summarizing the literature on 18F-FDG and 68Ga-PSMA in prostate cancer and highlighting that 18F-FDG has advantages in detecting local recurrence, visceral and lymph node metastases compared to 68Ga-PSMA in partial progressive prostate cancer and castration-resistant prostate cancer patients. We emphasize 18F-FDG PET/CT can compensate for the weakness of 68Ga-PSMA PET/CT in progressive prostate cancer.
Introduction
The incidence and mortality of prostate cancer rank first among all cancers, accounting for 1/10 of all cancer deaths in the United States (1). After receiving effective treatment such as surgery and radiotherapy, 2/5 of patients with prostate cancer experience a detectable rise in the serum prostate-specific antigen (PSA) level in the next 10 years from the first treatment, which includes local recurrence and metastatic disease (2, 3). With the increased management of hormone therapy, nearly all patients eventually develop castration-resistant prostate cancer (CRPC), which is the main cause of disease-related death (4, 5). Some CRPC patients would progress into a rare pathological subtype called neuroendocrine prostate cancer (NEPC). The resistance to treatment is caused by following the mechanisms as TP53 mutation, AR amplification, or mutations or RB1 loss. For the difficulty in early diagnosis and prediction of prognosis, PSA monitoring along with radiologic evaluations is standard for tumor burden assessment. Multiple imaging techniques are used for diagnosis or staging, assessment of treatment, and prognosis prediction including Magnetic Resonance Imaging (MRI), Computed Tomography (CT), ultrasound, Single-Photon Emission Computed Tomography (SPECT), and PET/CT. Guidelines recommend MRI or CT for staging, detecting lymph node metastases, and local recurrence. Besides traditional anatomic imaging, nuclear imaging-based PET/CT is a rapidly developing field for compensating the limitation of whole-body evaluation and precise prognosis prediction. The most commonly used tracer is 18F-fluorodeoxyglucose (FDG), and the newer tracer 68Ga-prostate-specific membrane antigen (PSMA) also provides good detection. 18F-FDG PET/CT is shown to be a useful prognostic tool in selected patients with advanced disease (6–8). Intraprostatic uptake of 18F-FDG imaged by PET/CT suggests that aggressive behavior and castration resistance with increased glucose uptake (9, 10). In addition, 18F-FDG PET/CT has played a significant role in many kinds of cancer for a long time. 18F-FDG PET/CT is widely used in lymphoma, breast cancer, lung cancer, esophageal cancer, colorectal cancer, and many other cancers (11); it performs well in diagnosis, staging, prediction of recurrence, and prognosis prediction of the above diseases.
This article mainly summarizes the former research of the mechanism and usage of 18F-FDG and 68Ga-PSMA PET/CT in prostate cancer. We compare the pros and cons of 18F-FDG and 68Ga-PSMA in localized prostate cancer. We then focus on exploring the potential of 18F-FDG PET/CT in aggressive and progressive prostate cancer. In addition, in order to maintain a comprehensive review, we also briefly summarized the clinical use of choline PET in prostate cancer.
The Basic Mechanism of Detecting Prostate Cancer
PET/CT is a non-invasive examination for diagnosis, and many radiotracers are widely used in different malignant tumors. PET-based radiotracers, 18F-FDG, is the most commonly used radiotracer for oncologic imaging and is based on the increased glucose metabolism in malignant tumors (12, 13). FDG, a glucose analog, is transported into the cell through GLUTs and then phosphorylated by hexokinases to FDG-6-phosphate which then is stored within the cells. Malignant cancers convert to an increased rate of glycolysis at an advanced stage named Warburg effects in the transformation process (14, 15). Androgens enhance glucose metabolism by modulating glycolytic-related genes (16). Furthermore, Glucose uptake and metabolism rely on the transporter GLUT family and hexokinase which are involved in tumor progression and overall survival (17–20). Compared with normal epithelial cells, cancer cells enhancee glycolysis, showing higher SUVmax in PET/CT. The expression of GLUT1 correlated with disease progression, and tumor cells enhance glycolysis with the elevation of GLUT1 (19). A significant association was found between GLUT1 expression levels and SUVmax level (p = 0.005), lymph node status (p = 0.05), volume of cancer (p = 0.01), CRPC disease progression (p = 0.02), and metastases development (p = 0.04). Prostate cancer upgrades the expression of hexokinase2 when progressing to CRPC. Several studies have proven that Pten/p53 deficient mice elevated levels of hexokinase2 and its binding to mitochondrial enhanced enzyme activities (21, 22). 18F-FDG is used as an auxiliary method to analyze glucose metabolism in prostate cancer cells and find new targets and methods for the diagnosis and treatment of prostate cancer.
The new tracer PSMA in prostate cancer is a transmembrane protein with a 707-amino-acid extracellular portion located in the apical prostate cell surrounding ducts. In the physiological statue, PSMA shows accumulation in the salivary gland, the liver, the spleen, the small bowel, and the urinary tract. In normal prostate, PSMA localizes in the cytoplasm and apical side of the epithelium surrounding prostatic ducts. Dysplastic or neoplastic transformation transfers PSMA from the apical membrane to the luminal surface of the ducts, and this has been detected by PET/CT (23). PSMA has become the standard method for diagnosing and staging prostate cancer. Several meta-analyses concluded 68Ga-PSMA PET/CT improved detection of localized prostate cancer and metastases (24, 25).
Localized Prostate Cancer
18F-FDG PET/CT acts as a detection tool to detect metastases during the observation period but has low sensitivity in localized prostate cancer. At present, many viewpoints believed that for localized prostate cancer, compared with observation, active radical prostatectomy or external-beam radiotherapy didn’t have much effect on the mortality of patients but it could control the progression of some prostate cancer (26, 27). Although the current data showed that the detection of 18F-FDG PET/CT had some limitations, there were still some studies suggesting the value of 18F-FDG PET/CT. Serendipitous high focal 18F-FDG uptake in the prostate gland in several case reports suggested that this imaging tool was useful for specific types of tumors (5, 28–30). In an investigation among 47,109 men who underwent 18F-FDG PET in a 10-year period, 1,335 (2.83%) showed incidental prostatic 18F-FDG uptake, and 99 of these men underwent prostate biopsy (31). Prostate cancer occurred in 1 of 26 patients (3.8%) with serum PSA<2.5 ng/mL, compared with 40 of 67 patients (59.7%) with serum PSA≥2.5 ng/mL. It revealed that patients with high 18F-FDG uptake in the prostate should be further evaluated by the measurement of serum PSA and prostate biopsy. Despite the low sensitivity, 18F-FDG could predict therapy effects for patients who exhibiting high absorption of 18F-FDG in tumor lesions. We looked at a patient who was primarily diagnosed with prostate cancer with oligometastatic lesions, and he was sensitive to androgen deprivation therapy (Figure 1). With the continuous decrease of PSA level, the FDG accumulation decreased in the prostate and right ischium. New tracers, 68Ga-PSMA and 11C-choline, have higher sensitivities in localized prostate cancer. In primary prostate cancer, the sensitivity of PSMA was 40–95%, which correlated with the levels of serum PSA. When PSA was over 2ng/mL, the sensitivity elevated to 95% (24). A meta-analysis including nine studies and a total of 547 patients with primary prostate cancer found the sensitivity of PSMA ranging from 67–97% (32). This study pointed out that PSMA had higher sensitivity and specificity in detecting primary prostate cancer compared with conventional imaging examinations. Similarly, the sensitivities of 11C- and 18F-choline in the diagnosis of primary prostate cancer were 66–86.5%, and the specificities were 43–81%. However, some studies showed that choline PET could not distinguish between benign and malignant tumors, or between inflammatory and malignant tissue in microcarcinomas and small tumors (33–41). Besides, choline PET/CT has a limited role in staging and is only beneficial in the detection of distant metastases such as bone metastases (33).
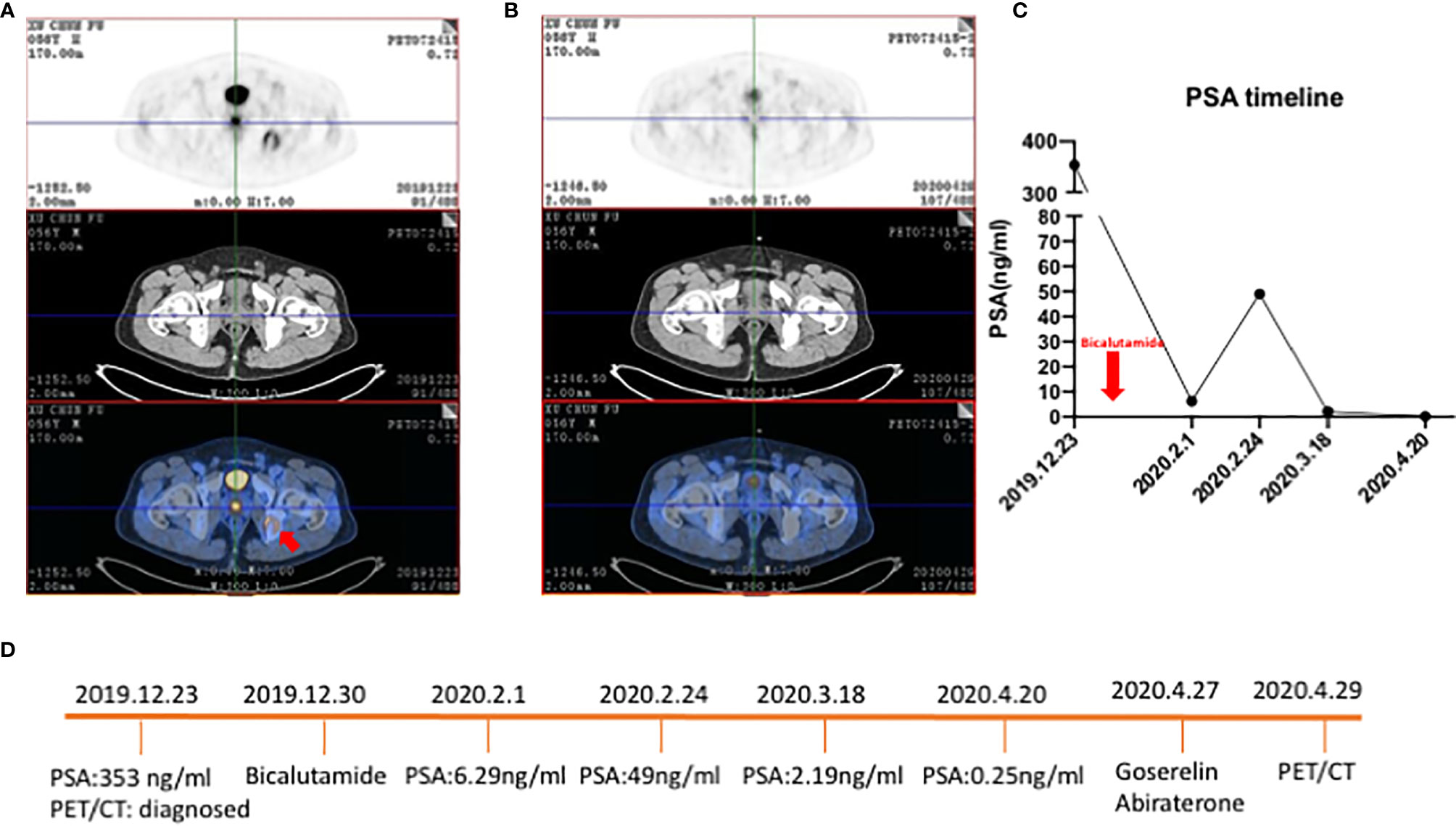
Figure 1 18F-FDG PET/CT of a 56-year-old patient with primary prostate cancer who was sensitive to ADT. (A) Patient was diagnosed with primary prostate cancer and an oligometastatic lesion in the right ischium (red arrow) with an osteogenic bone lesion. SUVmax of primary prostate cancer was 7.2. (B) After ADT tumor burden was significantly decreased and the oligometastatic lesion regressed. (C) Line graph of PSA level. The red arrow was the timepoint patient taking bicalutamide. (D) Timeline of patient underwent ADT and time for PET/CT.
There is the consensus that CT/MRI is preferred for detecting primary prostate cancer. PET/CT is not particularly satisfactory because of the lower or similar sensitivity and being less convenient and commercial in clinical practice. 18F-FDG, 68Ga-PSMA, and 11C-choline have relatively low sensitivity when PSA is lower than 2ng/mL and increase the sensitivity when PSA is over 2ng/mL. Patients whose PSA is over 2ng/mL are often required for biopsy to diagnose. For primary prostate cancer, PET/CT may act as an auxiliary method to evaluate the tumor burden and metastases.
Progressive and Aggressive Prostate Cancer
After initial treatment as radical prostatectomy or androgen deprivation therapy (ADT), the disease progresses into metastasis, recurrence, or resistance to treatment. 18F-FDG PET/CT has been used as a commonly used means to observe the effects of treatment and surveillance together with CT, MRI, PSMA, and bone scan (42).
Metastasis and Recurrence
PET/CT is a promising tool to identify lymph node metastases. Both 18F-FDG and 68Ga-PSMA PET/CT have high specificity in detecting metastases with direct therapy. Yi, C. et al. evaluated the efficacy of 18F-FDG in the detection of advanced prostate cancer and its metastases (43). In the detection of 26 patients with high-risk prostate cancer (Gleason score 8–10 or PSA> 20 ng/mL or clinical tumor extension ≥ T2c), the sensitivity and specificity for the diagnosis of lymph node metastases were 75% and 44.4%, respectively. This shows that 18F-FDG plays a role in asymptomatic people with high PSA and lymph node metastases. Moreover, 18F-FDG guides stage and restage of prostate cancer by detection of tumor burden and metastases. 18F-FDG has a high positive predictive value for untreated metastases in viscera but no lymph nodes (44). Beauregard et al. performed 18F-FDG PET/CT on 44 patients with known Gleason≥8 for the staging workup (9). High 18F-FDG uptake was found in the prostate gland, lymph nodes, and bone in 44%, 13%, and 6% of the patients, respectively. The absence or presence of intraprostatic 18F-FDG uptake was associated with a median cancer-free survival probability of 70.2% and 26.9% (P = 0.0097), respectively. 18F-FDG PET/CT can detect local and distant metastases with relatively high accuracy. Compared with 18F-FDG, 11C-choline PET/CT appears to have a better value for biochemical recurrence and restaging (45), but neither of them was satisfactory. Researchers focused on the potential utilization of 68Ga-PSMA to detect metastases and restage tumor burden accurately. Lars et al. assessed the diagnostic accuracy of 68Ga-PSMA before radical prostatectomy (46). Their work revealed the specificity of PSMA was 100%, but the sensitivity was only 33.3%. The author concluded the accuracy of PSMA based on the size of enlarged lymph. Another retrospective research analyzed the PSMA-positive metastasis with different nomograms (47). The sensitivity ranged from 22.3% to 40.5%. Some researchers compared 68Ga-PSMA with another new tracer. Ali. et al. compared 68Ga-PSMA to 11C-choline in 78 lesions from 32 patients and found 68Ga-PSMA could detect all lesions that were positive by 11C-choline; meanwhile, 68Ga-PSMA had a clearer tumor to background ratio (48). In summary, PET/CT is recommended for localizing metastases and identifying diagnoses to assist in surgery planning. Among these tracers, 68Ga-PSMA has proved high specificity in detecting lymph nodules lesions while 11C-choline has been recommended for bone metastasis in NCCN guidelines.
Recurrence means the failure of treatment and poor prognosis. 18F-FDG PET/CT can detect the recurrence of the disease earlier than traditional imaging methods, which may be more meaningful for the remedial treatment and prolonging the survival time of patients with prostate cancer. Jadvar, H. et al. reported a prospective investigation to assess the association of 18F-FDG PET/CT with time to hormonal treatment failure (THTF) in 76 men with metastatic castration-sensitive prostate cancer (8). The conclusion is that sum of SUVmax and the number of lesions derived from 18F-FDG PET/CT provided independent prognostic information on THTF in men with metastatic castration-sensitive prostate cancer. In addition, FDG accumulation in the prostate decreased in all patients 1–5 months after the initiation of hormone therapy (49). FDG had a 31% sensitivity in detecting biochemical recurrence after radical prostatectomy when the PSA level exceeded 1.9ng/mL and the sensitivity increased to 40% (50). These studies indicate that the sensitivity of detection for non-castrated resistant prostate cancer was relatively low. Judging from the listed data, many studies have pointed out the limited value of 18F-FDG in the detection of primary prostate cancer. For 11C-choline PET/CT, a study reported that the overall detection rate of biochemical recurrence was 45%. High PSA levels and advanced pathological stage were significantly associated with an increased risk of positive PET/CT findings (51). These features were similar to FDG PET imaging data, but the relationship with the Gleason score was not clear (52). 68Ga-PSMA has advantages in detecting recurrence compared to other tracers. A retrospective analysis included 294 patients and showed the sensitivity and specificity were both around 70%, and the SUVmax impacted therapeutic management (53). A meta-analysis showed PSMA-positive rate in patients was 68% and it was correlated with early biochemical recurrence (54). Several prospective studies report management changes after PSMA with biochemical recurrence of prostate cancer (55, 56). 68Ga-PSMA could clarify recurrence which improved management in patients with biochemical recurrence.
In summary, 18F-FDG and 11C-choline PET/CT have certain similarities in the detection of primary and recurrent tumors. Meanwhile, 68Ga-PSMA PET/CT performs better in the detection of lymph nodule metastases and recurrence than others. And it has the potential to assist therapeutic management in patients with biochemical recurrence.
CRPC and NEPC
After ADT or castration, treatment response varies from months to years. The insensitivity to the castration treatment is inevitable and is termed CRPC defined as three consecutive rises in PSA with two rises of 50% above or progression of bone lesions or progression of soft tissue lesions (57). During the development of CRPC, the rise of serum PSA was termed as biochemical recurrence (BCR) and several researchers reported 18F-FDG PET/CT as a predictor of time to BCR.
High uptake of 18F-FDG in prostate cancer meant a shorter time to happen biochemical recurrence. Lavallee, E. et al. analyzed 18F-FDG PET/CT before prostatectomy in 148 prostate cancer patients with Gleason≥8 (10). In multivariate analysis, SUVmax≥4.6 in the prostate was associated with double the risk of biochemical recurrence 1 year after operation. The median biochemical recurrence-free survival (BFS) was 11.3 months while that of patients with lower SUVmax was 49.5 months. In addition, the high FDG uptake in the prostate was related to the shorter time of castration resistance after radical prostatectomy. The authors concluded that preoperative intraprostatic FDG uptake can predict BFS and castration resistance following radical prostatectomy in patients. It revealed that increased utilization of 18F-FDG identified invasive prostate cancers.
Castration resistance prostate cancer cells have higher 18F-FDG uptake and glucose metabolism than primary tumors, which means they are more malignant. Prognosis of patients with CRPC correlated inversely with SUVmax (58). Fox et al. reported FDG along with flurodihydrostosterone (FDHT) PET/CT to distinguish patients for sensitivity to androgen receptor signaling inhibitors (59). The results showed that FDHT positive or FDG positive had an independent negative effect. FDHT-negative with FDG positive was most insensitive to ADT (hazard ratio [HR], 1.11; 95% CI, 1.05-1.16; P < .001), followed by FDHT-positive with FDG positive (HR, 1.05; 95% CI, 1.03-1.06; P < .001).
CRPC would inevitably develop into metastatic castration-resistant prostate cancer (mCRPC). Both PSMA and FDG were good predictions on prognosis and treatment response in mCRPC. In a prospective investigation, parameters derived from baseline 18F-FDG PET/CT were associated with overall survival (OS) in Eighty-seven castrate-resistant metastatic prostate cancer patients (7). The authors observed that the fourth-quartile range of the sum of SUVmax was related to the shortest OS. In another study, the authors used the CAPRA-S prognostic tool to conclude that the absence and presence of FDG uptake in the prostate were associated with a median 5-year cancer-free survival rate of 70.2% and 26.9% (P=0.0097), respectively (9). The expression of PSMA portended a poor prognosis in patients with mCRPC. High radiographic PSMA uptake had a shorter OS than low PSMA uptake (15.8 to 22.7 months) (60). 68Ga-PSMA had a sensitive response to therapy in mCPRC patients. Compared to the serum PSA level, 52.6% of patients showed partial remission on PSMA while 23.7% of serum PSA. Median OS stratified to PSA/PSMA response was 25.6/25.6 months (61).
Several researchers focused on the rare pathologic type, neuroendocrine prostate cancer, as more aggressive prostate cancer with castration resistance and rise of NE markers. Bakht, M. K. et al. evaluated the association between neuroendocrine gene signature and 18F-FDG uptake-associated genes including GLUTs and hexokinases, with the goal of providing a genomic signature to explain the reported 18F-FDG avidity of PSMA-suppressed tumors (20). Their work demonstrated that a neuroendocrine gene signature is associated with differential expression of genes encoding GLUT and hexokinase proteins. The authors concluded that alteration of 18F-FDG uptake-associated genes correlated positively with higher glucose uptake in AR- and PSMA-suppressed tumors. This suggested that when the detection of prostate cancer by PSMA was not ideal and 18F-FDG PET/CT played a more important role. Spratt et al. reported high SUVmax in NEPC bone lesions and soft tissue lesions was associated with a shorter survival time (62). Stratified by the median survival from NEPC diagnosis, patients who survived below 2.2 years had more PET avid bone (8 vs. 2, P = 0.06) and soft tissue lesions (7 vs. 1, P = 0.01), higher average SUVmax of bone (5.49 vs. 3.40, P = 0.04), and soft tissue lesions (8.02 vs. 3.90, P = 0.0002).
18F-FDG Versus68Ga-PSMA
It is widely accepted that 68Ga-PSMA PET/CT has more considerable accuracy in diagnosis and staging in primary prostate cancer than 18F-FDG (63). In a study focusing on the sensitivity of 68Ga-PSMA in evaluating biochemical recurrence, the total positive predictive values of the prostate, pelvic lymph nodes, extra-pelvic lymph nodes, bones, and distant organs were 28%, 38%, 13%, 22%, and 5%, respectively (64). Multiple studies have revealed that PSMA needs to improve sensitivity but has high specificity for detection of nodal metastases in intermediate-to-high-risk prostate cancer.
Plenty of studies have shown 68Ga-PSMA PET/CT had advantages in evaluating local recurrence or distance metastases but in CRPC PSMA had some limitations. A prospective single-arm clinical trial focused on accuracy in localizing recurrent prostate cancer. The data showed 75% of recurrent prostate cancer were positive and detection rates increased with PSA: 38% for <0.5 ng/mL, 57% for 0.5 to <1.0 ng/mL, 84% for 1.0 to <2.0 ng/mL, 86% for 2.0 to <5.0 ng/mL (n = 158), and 97% for ≥5.0 ng/mL (n = 173, P <.001) (65). This study demonstrated PSMA had high sensitivity in recurrent prostate cancer while it also showed PSMA might neglect tumor lesions for patients with low PSA. There are still some missed lesions because of the negative PSMA. A 61-year-old mCPRC patient had widespread metastases (Figure 2). 68Ga-PSMA showed negative in suspicious lesions in the liver while 18F-FDG exhibited high enhancement. This case demonstrated for some mCRPC patients FDG still had a role in risk stratification and recognizing metastatic lesions. A prospective trial included 37 patients which underwent both FDG and PSMA PET/CT (66). Of all 114 lesions, 81 were PSMA+FDG+, and 33 were PSMA-FDG+. PSMA-FDG+ lesions had a poor prognosis and resistance to castration. PSMA was a more sensitive and specific agent in prostate cancer, but in castration-resistant lesions, the sensitivity would reduce and mean more malignant lesions (67–69). Several case reports observed heterogeneity results that relied on pathological and clinical grading of prostate cancer. Some castration-resistant cancers showed PSMA negative and neuroendocrine tumors showed FDG and DOTATATE positive (68–70).
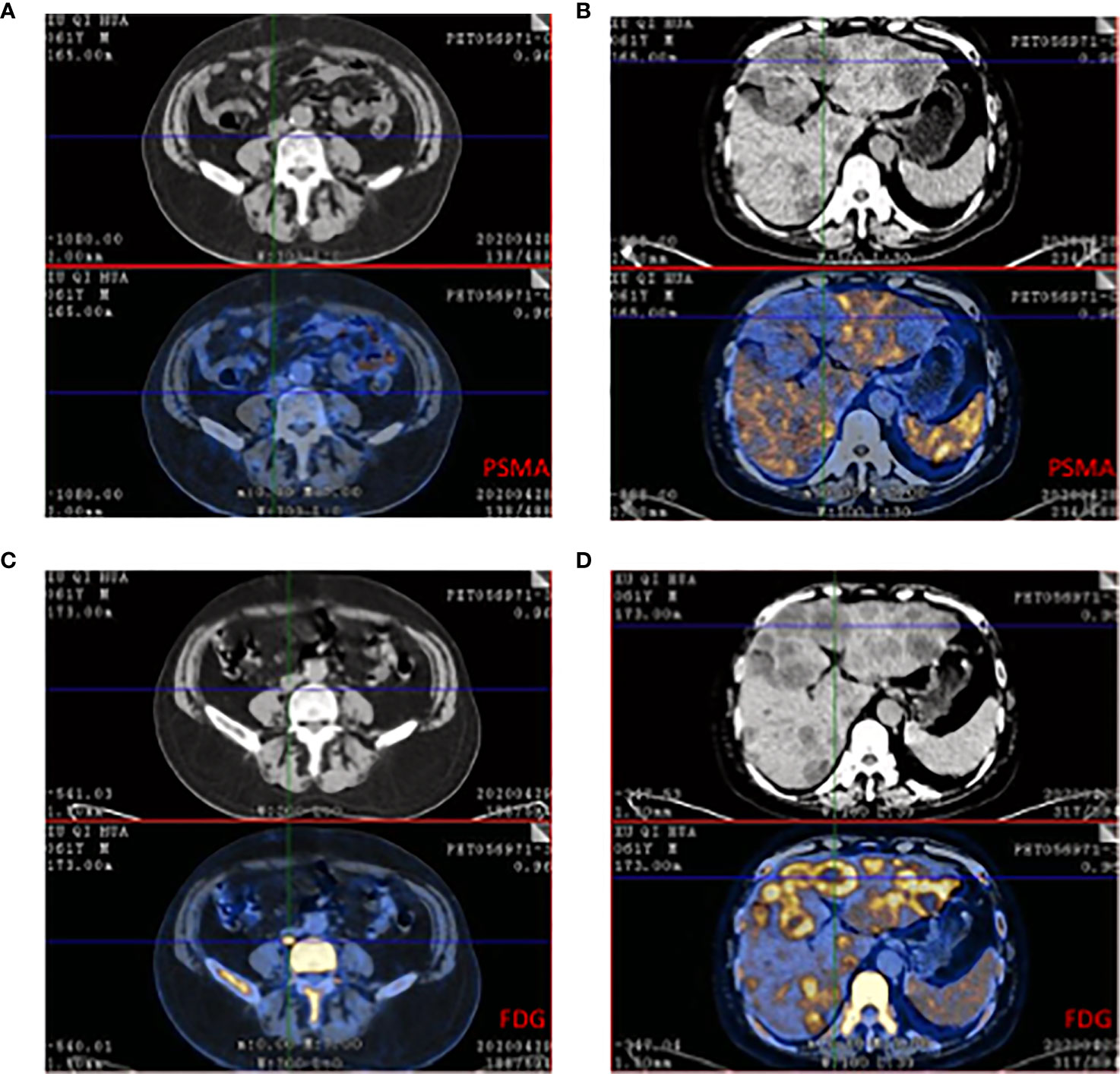
Figure 2 68Ga-PSMA and 18F-FDG PET/CT of a 61-year-old patient who was resistant to ADT and had widespread metastases in lymph node and liver. (A) CT showed the enlarged lymph node while PSMA expression was low. (B) Multiple low-intensity lesions were in the whole liver and the largest one was in the left hepatic lobe. The PSMA PET/CT showed low absorption in the suspicious lesion. (C) The same plane compared to A, FDG showed high absorption beside the vertebra. The size was 25*18mm and Sova=14.1. (D) The same plane compared to B, FDG showed heterogeneous enhanced in liver and SUVmax=4.8-11.0.
In the detection of primary and metastatic prostate cancer, the overall sensitivity and specificity of 68Ga-PSMA PET/CT are significantly higher than 18F-FDG PET/CT, but this does not mean that PSMA PET/CT has an excellent performance in all types of prostate cancer and all clinical environments, on the contrary, 18F-FDG plays a crucial role in CRPC and NEPC. For patients with negative 68Ga-PSMA, 18F-FDG has a diagnostic value correlated with PSA and the Gleason score (71). With the continuous improvement of the status of 68Ga-PSMA in the detection of prostate cancer, the auxiliary role of 18F-FDG should not be underestimated. The combination of 68Ga-PSMA and 18F-FDG may be more beneficial to the treatment and prognosis of patients.
Conclusion
PET/CT has an important role in initial diagnosis, staging, and recurrence surveillance in a variety of cancers. The conventional tracer 18F-FDG was efficient for detecting lesions that maintained high glucose metabolism both in the primary tumor and metastases. Several clinical guidelines, like myeloma and lymphoma, recommended 18F-FDG PET/CT in diagnosis (72, 73). In solid tumors, 18F-FDG showed its high sensitivity for detecting metastases (74).
Prostate cancer is characterized by slow development and low glucose metabolism. In the 2020 prostate cancer NCCN guideline, CT/MRI is the priority when it comes to identifying primary lesions and evaluating the tumor volume. Conventional molecular imaging as 18F-FDG PET/CT should not be used routinely for staging in primary prostate cancer. In guidelines, new tracers such as 11C-choline can be used to detect small volume disease in soft tissues and bone. 68Ga-PSMA PET/CT is under several clinical trials and shows its advantages in both specificity and sensitivity in detecting lesions, but it requires more prospective clinical trials (75). Recent research focused on a group with prostate cancer, which was suitable for 68Ga-PSMA, and data prompts its appliance in primary prostate cancer to generally evaluate tumor burden and metastases.
Aggressive prostate cancer, however, transforms into higher glucose metabolism and makes it possible for 18F-FDG PET/CT to detect tumors. In high-risk prostate cancer, 18F-FDG still has limitations on low sensitivity while 18F-FDG is competitive to 68Ga-PSMA in recurrence or CRPC. Both 18F-FDG and 68Ga-PSMA are predictors of prognosis and therapeutic effect. 68Ga-PSMA PET/CT is the mainstream of nuclear imaging in CRPC and mCRPC. The high sensitivity and specificity make it promising in treatment management. While partial patients are PSMA negative, which increases the difficulty in disease surveillance. Some characters may be found such as low serum PSA level, special pathological subtype, or different molecular mechanisms. Several clinical trials revealed 18F-FDG could effectively detect metastases after ADT. Tumors in CRPC and mCRPC generally elevate glucose metabolism, and FDG can localize the recurrence and metastases, which are negative in PSMA. Although 68Ga-PSMA is effective in evaluating recurrence, and 18F-FDG PET/CT can compensate for the shortage and effectively verify the tumor lesions in CRPC.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
KS and BL wrote the first manuscript. KS and BL contributed equally as first authors. YJ, LC, and XZ read, reviewed, edited, and wrote sections related to their areas of expertise. WX and QW read, reviewed, edited throughout the whole writing process, and signed off on the final paper. All authors contributed to the article and approved the submitted version.
Funding
We are grateful for funding provided by the National Natural Science Foundation of China (No 81702542, 81972578, 82072847, and 81772742); Science and Technology Commission of Shanghai Municipality (19XD1402300); Shanghai Municipal Health Commission (2019LJ11); Shanghai Jiao Tong University Medical Engineering Cross Fund (YG2019GD02).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA-Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Kessler B, Albertsen P. The Natural History of Prostate Cancer. Urol Clin North Am (2003) 30(2):219–26. doi: 10.1016/S0094-0143(02)00182-9
3. Carroll P. Rising PSA After a Radical Treatment. Eur Urol (2001) 40 Suppl 2:9–16. doi: 10.1159/000049879
4. Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and End Points of Clinical Trials for Patients With Progressive Prostate Cancer and Castrate Levels of Testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol (2008) 26(7):1148–59. doi: 10.1200/JCO.2007.12.4487
5. Jadvar H. Imaging Evaluation of Prostate Cancer With 18F-Fluorodeoxyglucose PET/CT: Utility and Limitations. Eur J Nucl Med Mol Imaging (2013) 40 Suppl 1:S5–10. doi: 10.1007/s00259-013-2361-7
6. Chang CH, Wu HC, Tsai JJ, Shen YY, Changlai SP, Kao A. Detecting Metastatic Pelvic Lymph Nodes by 18F-2-Deoxyglucose Positron Emission Tomography in Patients With Prostate-Specific Antigen Relapse After Treatment for Localized Prostate Cancer. Urol Int (2003) 70(4):311–5. doi: 10.1159/000070141
7. Jadvar H, Desai B, Ji L, Conti PS, Dorff TB, Groshen SG, et al. Baseline 18f-FDG PET/CT Parameters as Imaging Biomarkers of Overall Survival in Castrate-Resistant Metastatic Prostate Cancer. J Nucl Med (2013) 54(8):1195–201. doi: 10.2967/jnumed.112.114116
8. Jadvar H, Velez EM, Desai B, Ji L, Colletti PM, Quinn DI. Prediction of Time to Hormonal Treatment Failure in Metastatic Castration-Sensitive Prostate Cancer With (18)F-FDG PET/Ct. J Nucl Med: Off Publication Soc Nucl Med (2019) 60(11):1524–30. doi: 10.2967/jnumed.118.223263
9. Beauregard JM, Blouin AC, Fradet V, Caron A, Fradet Y, Lemay C, et al. FDG-PET/CT for Pre-Operative Staging and Prognostic Stratification of Patients With High-Grade Prostate Cancer at Biopsy. Cancer Imaging (2015) 15:2. doi: 10.1186/s40644-015-0038-0
10. Lavallee E, Bergeron M, Buteau FA, Blouin AC, Duchesnay N, Dujardin T, et al. Increased Prostate Cancer Glucose Metabolism Detected by (18)F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Localised Gleason 8-10 Prostate Cancers Identifies Very High-Risk Patients for Early Recurrence and Resistance to Castration. Eur Urol Focus (2019) 5(6):998–1006. doi: 10.1016/j.euf.2018.03.008
11. Ben-Haim S. Ell P. F-18-FDG PET and PET/CT in the Evaluation of Cancer Treatment Response. J Nucl Med (2009) 50(1):88–99. doi: 10.2967/jnumed.108.054205
12. Jadvar H. Molecular Imaging of Prostate Cancer: PET Radiotracers. AJR Am J Roentgenol (2012) 199(2):278–91. doi: 10.2214/AJR.12.8816
13. Jadvar H. Positron Emission Tomography in Prostate Cancer: Summary of Systematic Reviews and Meta-Analysis. Tomography (2015) 1(1):18–22. doi: 10.18383/j.tom.2015.00130
14. Cutruzzola F, Giardina G, Marani M, Macone A, Paiardini A, Rinaldo S, et al. Glucose Metabolism in the Progression of Prostate Cancer. Front Physiol (2017) 8:97. doi: 10.3389/fphys.2017.00097
15. Gonzalez-Menendez P, Hevia D, Mayo JC, Sainz RM. The Dark Side of Glucose Transporters in Prostate Cancer: Are They a New Feature to Characterize Carcinomas? Int J Cancer (2018) 142(12):2414–24. doi: 10.1002/ijc.31165
16. Vaz CV, Marques R, Alves MG, Oliveira PF, Cavaco JE, Maia CJ, et al. Androgens Enhance the Glycolytic Metabolism and Lactate Export in Prostate Cancer Cells by Modulating the Expression of GLUT1, GLUT3, PFK, LDH and MCT4 Genes. J Cancer Res Clin Oncol (2016) 142(1):5–16. doi: 10.1007/s00432-015-1992-4
17. Yu M, Yongzhi H, Chen S, Luo X, Lin Y, Zhou Y, et al. The Prognostic Value of GLUT1 in Cancers: A Systematic Review and Meta-Analysis. Oncotarget (2017) 8(26):43356–67. doi: 10.18632/oncotarget.17445
18. Wang J, Ye C, Chen C, Xiong H, Xie B, Zhou J, et al. Glucose Transporter GLUT1 Expression and Clinical Outcome in Solid Tumors: A Systematic Review and Meta-Analysis. Oncotarget (2017) 8(10):16875–86. doi: 10.18632/oncotarget.15171
19. Meziou S, Ringuette Goulet C, Hovington H, Lefebvre V, Lavallee E, Bergeron M, et al. GLUT1 Expression in High-Risk Prostate Cancer: Correlation With (18)F-FDG-PET/CT and Clinical Outcome. Prostate Cancer Prostatic Dis (2020) 23(3):441–8. doi: 10.1038/s41391-020-0202-x
20. Bakht MK, Lovnicki JM, Tubman J, Stringer KF, Chiaramonte J, Reynolds MR, et al. Differential Expression of Glucose Transporters and Hexokinases in Prostate Cancer With a Neuroendocrine Gene Signature: A Mechanistic Perspective for (18)F-FDG Imaging of PSMA-Suppressed Tumors. J Nucl Med: Off Publication Soc Nucl Med (2020) 61(6):904–10. doi: 10.2967/jnumed.119.231068
21. Wang L, Xiong H, Wu F, Zhang Y, Wang J, Zhao L, et al. Hexokinase 2-Mediated Warburg Effect Is Required for PTEN- and P53-Deficiency-Driven Prostate Cancer Growth. Cell Rep (2014) 8(5):1461–74. doi: 10.1016/j.celrep.2014.07.053
22. Martin PL, Yin JJ, Seng V, Casey O, Corey E, Morrissey C, et al. Androgen Deprivation Leads to Increased Carbohydrate Metabolism and Hexokinase 2-Mediated Survival in Pten/Tp53-Deficient Prostate Cancer. Oncogene (2017) 36(4):525–33. doi: 10.1038/onc.2016.223
23. DeMarzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and Molecular Aspects of Prostate Cancer. Lancet (London England) (2003) 361(9361):955–64. doi: 10.1016/S0140-6736(03)12779-1
24. Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, Specificity, and Predictors of Positive (68)Ga-Prostate-Specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-Analysis. Eur Urol (2016) 70(6):926–37. doi: 10.1016/j.eururo.2016.06.021
25. Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 Prostate-Specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-Specific Membrane Antigen-Avid Lesions: A Systematic Review and Meta-Analysis. Eur Urol (2020) 77(4):403–17. doi: 10.1016/j.eururo.2019.01.049
26. Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical Prostatectomy Versus Observation for Localized Prostate Cancer. N Engl J Med (2012) 367(3):203–13. doi: 10.1056/NEJMoa1113162
27. Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year Outcomes After Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med (2016) 375(15):1415–24. doi: 10.1056/NEJMoa1606220
28. de Carvalho Flamini R, Yamaga L, Mello ME, Wagner J, Livorsi da Cunha M, Osawa A, et al. F-18 FDG PET/CT Imaging in Small Cell Prostate Cancer. Clin Nucl Med (2010) 35(6):452–3. doi: 10.1097/RLU.0b013e3181db4ce9
29. Minamimoto R, Uemura H, Sano F, Terao H, Nagashima Y, Yamanaka S, et al. The Potential of FDG-PET/CT for Detecting Prostate Cancer in Patients With an Elevated Serum PSA Level. Ann Nucl Med (2011) 25(1):21–7. doi: 10.1007/s12149-010-0424-4
30. Bailly M, Besse H, Kerdraon R, Metrard G, Gauvain S. 18f-FDG PET/CT Superscan in Prostate Cancer. Clin Nucl Med (2014) 39(10):912–4. doi: 10.1097/RLU.0000000000000376
31. Kwon T, Jeong IG, You D, Hong JH, Ahn H, Kim CS. Prevalence and Clinical Significance of Incidental (18)F-Fluoro-2-Deoxyglucose Uptake in Prostate. Korean J Urol (2015) 56(4):288–94. doi: 10.4111/kju.2015.56.4.288
32. Hu X, Wu Y, Yang P, Wang J, Wang P, Cai J. Performance of 68Ga-Labeled Prostate-Specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography in the Diagnosis of Primary Prostate Cancer: A Systematic Review and Meta-Analysis. Int Braz J Urol: Off J Braz Soc Urol (2021) 47. doi: 10.1590/S1677-5538.IBJU.2020.0986
33. Nitsch S, Hakenberg OW, Heuschkel M, Drager D, Hildebrandt G, Krause BJ, et al. Evaluation of Prostate Cancer With C-11- and F-18-Choline PET/CT: Diagnosis and Initial Staging. J Nucl Med (2016) 57:38S–42S. doi: 10.2967/jnumed.115.169748
34. Farsad M, Schiavina R, Castellucci P, Nanni C, Corti B, Martorana G, et al. Detection and Localization of Prostate Cancer: Correlation of (11)C-Choline PET/CT With Histopathologic Step-Section Analysis. J Nucl Med (2005) 46(10):1642–9.
35. Giovacchini G, Picchio M, Coradeschi E, Scattoni V, Bettinardi V, Cozzarini C, et al. [(11)C]choline Uptake With PET/CT for the Initial Diagnosis of Prostate Cancer: Relation to PSA Levels, Tumour Stage and Anti-Androgenic Therapy. Eur J Nucl Med Mol Imaging (2008) 35(6):1065–73. doi: 10.1007/s00259-008-0716-2
36. Martorana G, Schiavina R, Corti B, Farsad M, Salizzoni E, Brunocilla E, et al. 11C-Choline Positron Emission Tomography/Computerized Tomography for Tumor Localization of Primary Prostate Cancer in Comparison With 12-Core Biopsy. J Urol (2006) 176(3):954–60; discussion 60. doi: 10.1016/j.juro.2006.04.015
37. Scher B, Seitz M, Albinger W, Tiling R, Scherr M, Becker HC, et al. Value of 11C-Choline PET and PET/CT in Patients With Suspected Prostate Cancer. Eur J Nucl Med Mol Imaging (2007) 34(1):45–53. doi: 10.1007/s00259-006-0190-7
38. Souvatzoglou M, Weirich G, Schwarzenboeck S, Maurer T, Schuster T, Bundschuh RA, et al. The Sensitivity of [11C]Choline PET/CT to Localize Prostate Cancer Depends on the Tumor Configuration. Clin Cancer Res (2011) 17(11):3751–9. doi: 10.1158/1078-0432.CCR-10-2093
39. Beheshti M, Imamovic L, Broinger G, Vali R, Waldenberger P, Stoiber F, et al. 18F Choline PET/CT in the Preoperative Staging of Prostate Cancer in Patients With Intermediate or High Risk of Extracapsular Disease: A Prospective Study of 130 Patients. Radiology (2010) 254(3):925–33. doi: 10.1148/radiol.09090413
40. Bundschuh RA, Wendl CM, Weirich G, Eiber M, Souvatzoglou M, Treiber U, et al. Tumour Volume Delineation in Prostate Cancer Assessed by [11C]Choline PET/CT: Validation With Surgical Specimens. Eur J Nucl Med Mol Imaging (2013) 40(6):824–31. doi: 10.1007/s00259-013-2345-7
41. Grosu AL, Weirich G, Wendl C, Prokic V, Kirste S, Geinitz H, et al. 11c-Choline PET/pathology Image Coregistration in Primary Localized Prostate Cancer. Eur J Nucl Med Mol Imaging (2014) 41(12):2242–8. doi: 10.1007/s00259-014-2861-0
42. Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. Lu-177 -PSMA-617 Radionuclide Treatment in Patients With Metastatic Castration-Resistant Prostate Cancer (LuPSMA Trial): A Single-Centre, Single-Arm, Phase 2 Study. Lancet Oncol (2018) 19(6):825–33. doi: 10.1016/S1470-2045(18)30198-0
43. Yi C, Yu D, Shi X, Zhang X, Luo G, He Q, et al. The Combination of 13N-Ammonia and 18F-FDG Whole-Body PET/CT on the Same Day for Diagnosis of Advanced Prostate Cancer. Nucl Med Commun (2016) 37(3):239–46. doi: 10.1097/MNM.0000000000000444
44. Takahashi N, Inoue T, Lee J, Yamaguchi T, Shizukuishi K. The Roles of PET and PET/CT in the Diagnosis and Management of Prostate Cancer. Oncology (2007) 72(3-4):226–33. doi: 10.1159/000112946
45. Picchio M, Messa C, Landoni C, Gianolli L, Sironi S, Brioschi M, et al. Value of [11C]Choline-Positron Emission Tomography for Re-Staging Prostate Cancer: A Comparison With [18F]Fluorodeoxyglucose-Positron Emission Tomography. J Urol (2003) 169(4):1337–40. doi: 10.1097/01.ju.0000056901.95996.43
46. Budäus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial Experience of (68)Ga-PSMA PET/CT Imaging in High-Risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur Urol (2016) 69(3):393–6. doi: 10.1016/j.eururo.2015.06.010
47. Onal C, Ozyigit G, Oymak E, Guler OC, Hurmuz P, Tilki B, et al. Clinical Parameters and Nomograms for Predicting Lymph Node Metastasis Detected With (68) Ga-PSMA-PET/CT in Prostate Cancer Patients Candidate to Definitive Radiotherapy. Prostate (2021) 81(10):648–56. doi: 10.1002/pros.24142
48. Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET Imaging With a (68)Ga-Labelled PSMA Ligand and (18)F-Choline-Based PET/CT for the Diagnosis of Recurrent Prostate Cancer. Eur J Nucl Med Mol Imaging (2014) 41(1):11–20. doi: 10.1007/s00259-013-2525-5
49. Oyama N, Akino H, Suzuki Y, Kanamaru H, Ishida H, Tanase K, et al. FDG PET for Evaluating the Change of Glucose Metabolism in Prostate Cancer After Androgen Ablation. Nucl Med Commun (2001) 22(9):963–9. doi: 10.1097/00006231-200109000-00004
50. Richter JA, Rodriguez M, Rioja J, Penuelas I, Marti-Climent J, Garrastachu P, et al. Dual Tracer C-11-Choline and FDG-PET in the Diagnosis of Biochemical Prostate Cancer Relapse After Radical Treatment. Mol Imaging Biol (2010) 12(2):210–7. doi: 10.1007/s11307-009-0243-y
51. Giovacchini G, Picchio M, Coradeschi E, Bettinardi V, Gianolli L, Scattoni V, et al. Predictive Factors of [(11)C]choline PET/CT in Patients With Biochemical Failure After Radical Prostatectomy. Eur J Nucl Med Mol Imaging (2010) 37(2):301–9. doi: 10.1007/s00259-009-1253-3
52. Giovacchini G, Giovannini E, Leoncini R, Riondato M, Ciarmiello A. PET and PET/CT With Radiolabeled Choline in Prostate Cancer: A Critical Reappraisal of 20 Years of Clinical Studies. Eur J Nucl Med Mol Imaging (2017) 44(10):1751–76. doi: 10.1007/s00259-017-3700-x
53. Fourquet A, Lahmi L, Rusu T, Belkacemi Y, Créhange G, de la Taille A, et al. Restaging the Biochemical Recurrence of Prostate Cancer With [(68)Ga]Ga-PSMA-11 PET/CT: Diagnostic Performance and Impact on Patient Disease Management. Cancers (2021) 13(7). doi: 10.3390/cancers13071594
54. Diao W, Cao Y, Su D, Jia Z. Impact of (68) Gallium Prostate-Specific Membrane Antigen Tracers on the Management of Patients With Prostate Cancer Who Experience Biochemical Recurrence. BJU Int (2021) 127(2):153–63. doi: 10.1111/bju.15257
55. Fendler WP, Ferdinandus J, Czernin J, Eiber M, Flavell RR, Behr SC, et al. Impact of (68)Ga-PSMA-11 PET on the Management of Recurrent Prostate Cancer in a Prospective Single-Arm Clinical Trial. J Nucl Med: Off Publication Soc Nucl Med (2020) 61(12):1793–9. doi: 10.1055/s-0040-1708125
56. Deandreis D, Guarneri A, Ceci F, Lillaz B, Bartoncini S, Oderda M, et al. (68)Ga-PSMA-11 PET/CT in Recurrent Hormone-Sensitive Prostate Cancer (HSPC): A Prospective Single-Centre Study in Patients Eligible for Salvage Therapy. Eur J Nucl Med Mol Imaging (2020) 47(12):2804–15. doi: 10.1007/s00259-020-04809-8
57. Carles J, Castellano D, Climent MA, Maroto P, Medina R, Alcaraz A. Castration-Resistant Metastatic Prostate Cancer: Current Status and Treatment Possibilities. Clin Transl Oncol (2012) 14(3):169–76. doi: 10.1007/s12094-012-0780-8
58. Meirelles GS, Schoder H, Ravizzini GC, Gonen M, Fox JJ, Humm J, et al. Prognostic Value of Baseline [18F] Fluorodeoxyglucose Positron Emission Tomography and 99mtc-MDP Bone Scan in Progressing Metastatic Prostate Cancer. Clin Cancer Res: Off J Am Assoc Cancer Res (2010) 16(24):6093–9. doi: 10.1158/1078-0432.CCR-10-1357
59. Fox JJ, Gavane SC, Blanc-Autran E, Nehmeh S, Gönen M, Beattie B, et al. Positron Emission Tomography/Computed Tomography-Based Assessments of Androgen Receptor Expression and Glycolytic Activity as a Prognostic Biomarker for Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol (2018) 4(2):217–24. doi: 10.1001/jamaoncol.2017.3588
60. Vlachostergios PJ, Niaz MJ, Sun M, Mosallaie SA, Thomas C, Christos PJ, et al. Prostate-Specific Membrane Antigen Uptake and Survival in Metastatic Castration-Resistant Prostate Cancer. Front Oncol (2021) 11:630589. doi: 10.3389/fonc.2021.630589
61. Prasad V, Huang K, Prasad S, Makowski MR, Czech N, Brenner W. In Comparison to PSA, Interim Ga-68-PSMA PET/CT Response Evaluation Based on Modified RECIST 1.1 After 2(Nd) Cycle Is Better Predictor of Overall Survival of Prostate Cancer Patients Treated With (177)Lu-PSMA. Front Oncol (2021) 11:578093. doi: 10.3389/fonc.2021.578093
62. Spratt DE, Gavane S, Tarlinton L, Fareedy SB, Doran MG, Zelefsky MJ, et al. Utility of FDG-PET in Clinical Neuroendocrine Prostate Cancer. Prostate (2014) 74(11):1153–9. doi: 10.1002/pros.22831
63. Tu X, Zhang C, Liu Z, Shen G, Wu X, Nie L, et al. The Role of(68)Ga-PSMA Positron Emission Tomography/Computerized Tomography for Preoperative Lymph Node Staging in Intermediate/High Risk Patients With Prostate Cancer: A Diagnostic Meta-Analysis. Front Oncol (2020) 10. doi: 10.3389/fonc.2020.01365
64. Alipour R, Azad A, Hofman MS. Guiding Management of Therapy in Prostate Cancer: Time to Switch From Conventional Imaging to PSMA PET? Ther Adv Med Oncol (2019) 11:14. doi: 10.1177/1758835919876828
65. Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol (2019) 5(6):856–63. doi: 10.1001/jamaoncol.2019.0096
66. Wang B, Liu C, Wei Y, Meng J, Zhang Y, Gan H, et al. A Prospective Trial of (68)Ga-PSMA and (18)F-FDG PET/CT in Nonmetastatic Prostate Cancer Patients With an Early PSA Progression During Castration. Clin Cancer Res (2020) 26(17):4551–8. doi: 10.1158/1078-0432.CCR-20-0587
67. Pianou NK, Stavrou PZ, Vlontzou E, Rondogianni P, Exarhos DN, Datseris IE. More Advantages in Detecting Bone and Soft Tissue Metastases From Prostate Cancer Using (18)F-PSMA PET/Ct. Hellenic J Nucl Med (2019) 22(1):6–9. doi: 10.1967/s002449910952
68. Khreish F, Rosar F, Kratochwil C, Giesel FL, Haberkorn U, Ezziddin S. Positive FAPI-PET/CT in a Metastatic Castration-Resistant Prostate Cancer Patient With PSMA-Negative/FDG-Positive Disease. Eur J Nucl Med Mol Imaging (2020) 47(8):2040–1. doi: 10.1007/s00259-019-04623-x
69. Perez PM, Hope TA, Behr SC, van Zante A, Small EJ, Flavell RR. Intertumoral Heterogeneity of 18F-FDG and 68Ga-PSMA Uptake in Prostate Cancer Pulmonary Metastases. Clin Nucl Med (2019) 44(1):e28–32. doi: 10.1097/RLU.0000000000002367
70. Acar E, Kaya G. 18f-FDG, 68ga-DOTATATE and 68Ga-PSMA Positive Metastatic Large Cell Neuroendocrine Prostate Tumor. Clin Nucl Med (2019) 44(1):53–4. doi: 10.1097/RLU.0000000000002322
71. Chen R, Wang Y, Shi Y, Zhu Y, Xu L, Huang G, et al. Diagnostic Value of (18)F-FDG PET/CT in Patients With Biochemical Recurrent Prostate Cancer and Negative (68)Ga-PSMA PET/Ct. Eur J Nucl Med Mol Imaging (2021) 48(9):2970–7. doi: 10.1007/s00259-021-05221-6
72. Younes A, Hilden P, Coiffier B, Hagenbeek A, Salles G, Wilson W, et al. International Working Group Consensus Response Evaluation Criteria in Lymphoma (RECIL 2017). Ann Oncol: Off J Eur Soc Med Oncol (2017) 28(7):1436–47. doi: 10.1093/annonc/mdx097
73. Cavo M, Terpos E, Nanni C, Moreau P, Lentzsch S, Zweegman S, et al. Role of (18)F-FDG PET/CT in the Diagnosis and Management of Multiple Myeloma and Other Plasma Cell Disorders: A Consensus Statement by the International Myeloma Working Group. Lancet Oncol (2017) 18(4):e206–e17. doi: 10.1016/S1470-2045(17)30189-4
74. Na F, Wang J, Li C, Deng L, Xue J, Lu Y. Primary Tumor Standardized Uptake Value Measured on F18-Fluorodeoxyglucose Positron Emission Tomography Is of Prediction Value for Survival and Local Control in non-Small-Cell Lung Cancer Receiving Radiotherapy: Meta-Analysis. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer (2014) 9(6):834–42. doi: 10.1097/JTO.0000000000000185
75. Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-Specific Membrane Antigen PET-CT in Patients With High-Risk Prostate Cancer Before Curative-Intent Surgery or Radiotherapy (proPSMA): A Prospective, Randomised, Multicentre Study. Lancet (London England) (2020) 395(10231):1208–16. doi: 10.1016/S0140-6736(20)30314-7
Keywords: 18F-FDG, PET/CT, 68Ga-PSMA, glucose metabolism, prostate cancer
Citation: Shen K, Liu B, Zhou X, Ji Y, Chen L, Wang Q and Xue W (2021) The Evolving Role of 18F-FDG PET/CT in Diagnosis and Prognosis Prediction in Progressive Prostate Cancer. Front. Oncol. 11:683793. doi: 10.3389/fonc.2021.683793
Received: 07 April 2021; Accepted: 08 June 2021;
Published: 29 July 2021.
Edited by:
Bradley T. Scroggins, National Cancer Institute (NCI), United StatesReviewed by:
Partha Choudhury, Rajiv Gandhi Cancer Institute and Research Centre, IndiaOrazio Schillaci, University of Rome Tor Vergata, Italy
Copyright © 2021 Shen, Liu, Zhou, Ji, Chen, Wang and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Wang, d3FpQHNqdHUuZWR1LmNu; Wei Xue, dXJveHVld2VpQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Kai Shen
Kai Shen Bo Liu
Bo Liu Xiang Zhou
Xiang Zhou Yiyi Ji
Yiyi Ji Lei Chen1
Lei Chen1 Qi Wang
Qi Wang