- 1Department of Radiation Oncology, University Hospital Schleswig-Holstein, Kiel, Germany
- 2Department of Otorhinolaryngology, Head and Neck Surgery, University Hospital Schleswig-Holstein, Kiel, Germany
- 3University Library Kiel, Christian-Albrechts-University Kiel, Kiel, Germany
Incurable head and neck cancer has a poor prognosis and impairs a patient’s health-related quality of life. Palliative radiotherapy may improve or stabilize health-related quality of life and symptoms, best measured by patient-reported outcomes. There is no systematic analysis if palliative radiotherapy for head and neck cancer improves or stabilizes health-related quality of life or symptoms as validly measured by patient-reported outcomes. Therefore, the primary objective of this systematic review (PROSPERO-ID: CRD42020166434) was to assess the effect of palliative radiotherapy for head and neck cancer on patient-reported outcomes. The secondary objective was to assess the rate and quality of use of patient-reported outcomes in relevant studies claiming a “palliative effect” of radiotherapy. The databases MEDLINE/PubMed, EMBASE, Cochrane CENTRAL, “ClinicalTrials.gov” were searched. Concerning the primary objective, four studies were eligible to assess the effectiveness of palliative radiotherapy as measured by patient-reported outcomes. A narrative synthesis suggests a favorable impact of palliative radiotherapy on health-related quality of life and symptom burden. The risk of bias, however, is considerable and the overall quality of evidence low. Concerning the secondary objective, over 90% of studies claiming a “palliative effect” of palliative radiotherapy did either not use patient-reported outcomes or did so by limited quality. In conclusion, implementation of patient-reported outcomes in studies assessing palliative radiotherapy for head and neck cancer should be fostered. Palliative radiotherapy remains an option for head and neck cancer patients, although more studies focusing on patient-reported outcomes are needed.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42020166434
Introduction
Patients suffering from incurable head and neck cancer face a poor prognosis (1). A recent prospective clinical cohort study reported a 1-year overall survival of only 32% for head and neck cancer patients treated in “non-curative” intent (2). Even in the era of immune checkpoint-inhibitors the median overall survival is still roughly one year, for example in recurrent or metastatic cases (3, 4). In addition, severe symptoms may have a marked negative impact on a patient’s health-related quality of life (5). Palliative radiotherapy can be an option to improve or stabilize health-related quality of life and symptoms. Although palliative radiotherapy is routinely used for cancer patients in general (6), there may be a more restrictive use in head and neck cancer patients. A Canadian study suggests that compared to the average palliative cancer patient, head and neck cancer patients are more frequently referred to radiation oncology consultations, although these consultations result less frequently in actual palliative radiation (7). While the high referral rates underline the need for symptom control, low actual treatments by palliative radiotherapy indicate some degree of skepticism or other obstacles for palliative radiotherapy in head and neck cancer patients. In fact, the effectiveness of palliative radiotherapy for head and neck cancer has been questioned (8).
The main goal of any palliative treatment should be an improvement and/or stabilization of health-related quality of life and/or symptoms (9). Patient-reported outcomes are essential to measure health-related quality of life and relevant symptoms (10). Specific patient-reported outcome measures have been developed also for head and neck cancer patients, for example in the form of multi-item questionnaires (11). In order to contribute robust evidence in clinical studies, these instruments should ideally be applied in a validated, longitudinal, and prospective setting. Yet recent reviews of palliative radiotherapy for head and neck cancer synthesized any concept of palliative response; including tumor response rates or unvalidated assessments of symptom control in retrospective studies (12, 13). Hence, to date there is little guidance, if palliative radiotherapy meets the main goal of improving or stabilizing health-related quality of life or symptoms in head and neck cancer patients as validly measured by patient-reported outcomes.
Therefore, we conducted a systematic review of patient-reported outcomes of head and neck cancer patients treated with palliative radiotherapy. The primary objective was to assess the impact of palliative radiotherapy on health-related quality of life and symptoms based on patient-reported outcomes in studies using a sound methodology. The secondary objective was to evaluate the rate and quality of use of patient-reported outcomes in studies claiming a “palliative effect”.
Materials and Methods
This is a systematic review with a narrative synthesis (14, 15). A protocol was developed on the basis of the Cochrane methodology and PRISMA statement with support of a statistician (SS) (16, 17). The protocol was registered on PROSPERO (ID: CRD42020166434) (Supplementary Data Sheet 1). Amendments were documented and explained on PROSPERO as far as possible at the time of publication (Supplementary Data Sheet 2).
Objectives and Eligibility
The primary objective was to assess the effect of palliative radiotherapy for head and neck cancer on patient-reported outcomes before and after treatment in eligible studies. Inclusion criteria were: head and neck squamous cell carcinoma, use of palliative radiotherapy (as defined by the respective study author), prospective trial design or case series, and English language. Exclusion criteria were: radical radiotherapy, cutaneous primary, case report, no use of patient-reported outcomes, and poor quality of patient-reported outcome measurement. Poor quality of patient-reported outcome measurement was defined on the basis of a prior publication as presence of at least one of the following (18): no evidence of validity or responsiveness, not self-reported, no longitudinal assessment, no compliance data available, and time point of assessment not indicated.
The secondary objective was to assess the rate and quality of use of patient-reported outcomes in relevant studies claiming a “palliative effect” over time. The secondary objective was a post-hoc analysis decided at abstract screening stage without impact on the initial search strategy. All clinical studies of palliative radiotherapy for head and neck squamous cell carcinoma reporting a “palliative effect” were included to full text screening. The semantic term of “palliative effect” was assessed concisely. Terms like “effective palliation”, “significant palliative effect”, or “meaningful palliation” were counted; studies solely stating terms like “improvement of symptoms” were not counted.
Search Strategy
The PICO-elements included head and neck cancer patients in a palliative setting (population), palliative radiotherapy (intervention), and health-related quality of life or common symptoms (outcome). An extensive search strategy was realized by a librarian (OW) in February 2020 and updated in November 2020 without time restriction (Supplementary Table 1). The databases MEDLINE/PubMed, EMBASE, Cochrane Center Register of Controlled Trials (CENTRAL), and “ClinicalTrials.gov” were searched. In addition, cross references of eligible studies were screened for relevant further studies. The online tool Covidence was used for record management. Title and abstract screening as well as full text screening for eligibility were performed independently by two authors (AF, JDo). In case of disagreement, mutual consent was found by discussion.
Data Extraction and Data Synthesis
Concerning the primary objective, data extraction of eligible studies was done independently by two authors (AF, JDo) via predefined data extraction forms. The conduct of a meta-analysis was deemed infeasible due to the paucity of available data and due to mostly categorical outcome reporting without clear sample sizes. Instead, a narrative synthesis was performed following the framework proposed by Popay and colleagues (15). This framework for narrative syntheses consists of four iterative stages. The first stage is to develop a theory of how, why, and for whom an intervention may work. As radiotherapy is a standard treatment for many cancer patients, we limited this stage to the description provided in the second paragraph of the introduction. The second stage, conducting a preliminary synthesis of findings of included studies, was performed via tabulation in the findings section (Table 3) (19). The table is ordered by the first author’s name of the study. The groups and domains of patient-reported outcomes displayed in Table 3 represent all validly reported patient-reported outcomes of the eligible studies. One study reported two additional domains that are not displayed in Table 3 due to a lack of comparability with the other included studies for these domains (20). Results of patient-reported outcomes are categorically displayed in Table 3 as reported by the respective study. Vote counting based on the direction of effects in patient-reported outcomes was performed in order to avoid subjective synthesis rules. The reported data of included studies did not allow for other synthesis methods or standardized metrics (19). The binary distinction between harm and benefit in the vote counting process was defined as benefit if more than 50% of the patients in an individual study reported “improved” or “unchanged” results for a respective patient-reported outcome domain. The third stage is to explore relationships within the findings. A textual exploration is provided in the findings section. The exploration was performed in collaboration by three authors (AF, JDo, DK). Due to the paucity of eligible studies and available data resulting in adequate tabular display, we did not provide a graphical overall synthesis. The fourth stage is to assess the robustness of the synthesis. For this purpose, the overall quality of evidence for patient-reported outcomes was assessed independently by two authors (AF, JDo) per GRADE criteria (21). The ROBINS-I tool was used independently by two authors (AF, JDo) to evaluate the risk of bias of included individual non-randomized studies (22). A formal risk of bias assessment across studies was not performed, as this mostly relies on quantitative summary data (23). Finally, Popay and colleagues suggest to critically reflect on the synthesis process and to provide conclusions and recommendations as end of the synthesis (15). Both steps are provided in the discussion section.
Concerning the secondary objective, data extraction was performed independently by two authors (AF, JDo). Clinical studies stating a “palliative effect” were counted and assessed for use of patient-reported outcomes. If patient-reported outcomes were used, their measurement was assessed for validity, compliance data, availability of baseline assessment, and indication for time of assessment.
Any disagreements in the data extraction and synthesis processes were resolved by discussion among the authors (AF, JDo, DK).
Results
Screening Results
The database search and assessment of cross references resulted in 4863 records to screen for title and abstract as shown by the PRISMA flow diagram (Figure 1) (17). Ninety-three records were included to full text screening. Four studies were eligible for the primary objective and included in the narrative synthesis (20, 24–26). The most prevalent reasons for exclusion were unvalidated patient-reported outcomes, no use of patient-reported outcomes, and no prospective study design (see Supplementary Tables 2 and 3).
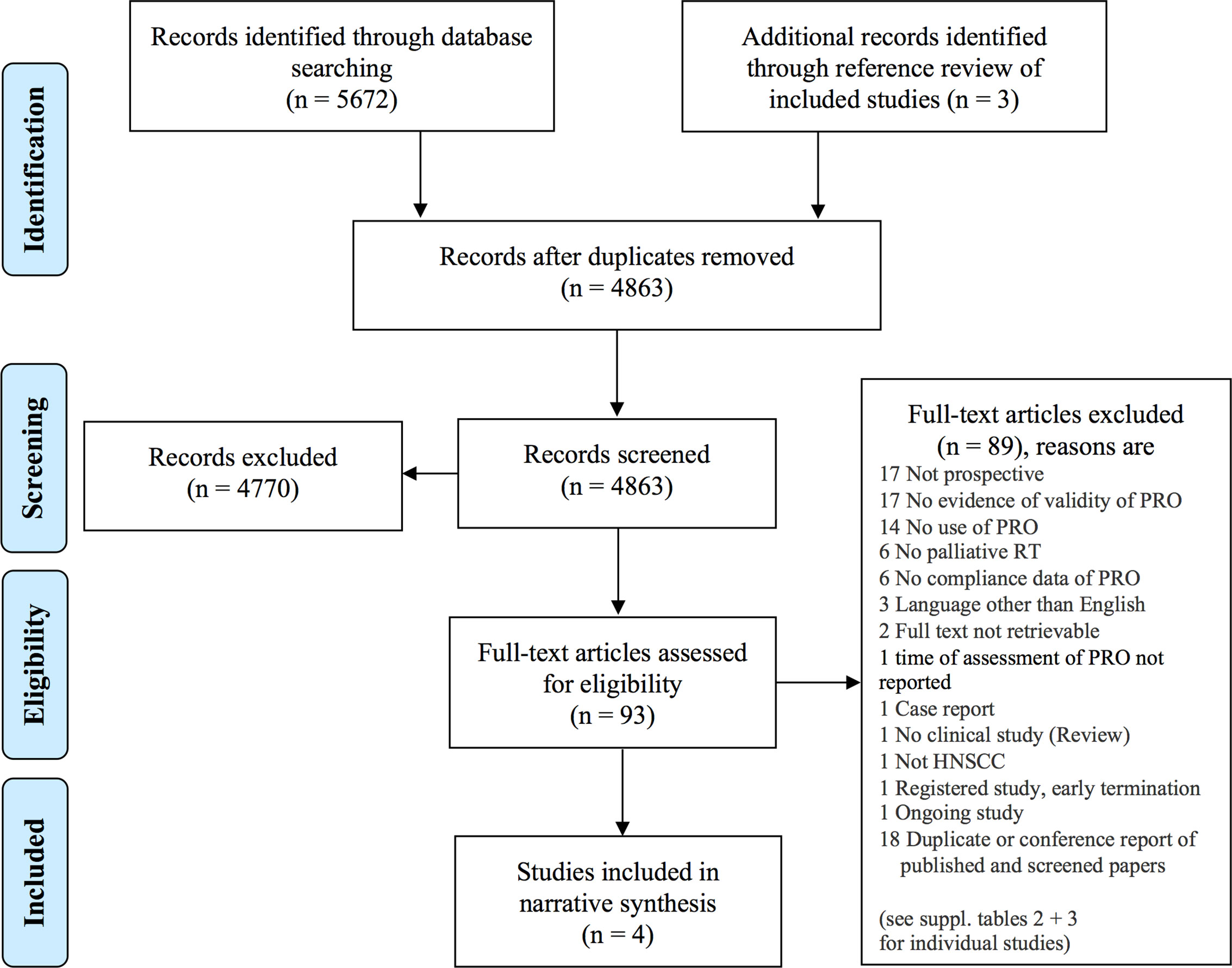
Figure 1 PRISMA flow diagram (17). The databases MEDLINE/Pubmed, EMBASE, Cochrane CENTRAL, and “ClinicalTrials.gov” were searched. Screening was performed independently by two authors. HNSCC, head and neck squamous cell carcinoma; PRO, patient-reported outcome; RT, radiotherapy.
Use of Patient-Reported Outcomes in Studies Reporting a “Palliative Effect”
Concerning the secondary objective, 37 clinical studies reported a “palliative effect” of palliative radiotherapy for head and neck cancer patients (Table 1). Of these studies, 12 (32%) did not use patient-reported outcomes. Twenty-two studies (60%) applied patient-reported outcome measurement with limited quality; “no evidence of validity” and “compliance not reported” being the most common reason. The remaining three studies (8%) are three out of the four eligible studies mentioned above (20, 24, 26). From 1979 to 2020, the number of clinical studies reporting a “palliative effect” of palliative radiotherapy for head and neck cancer patients increased per decade (Figure 2). From 2010 to 2020, 22% of these studies did still not report patient-reported outcomes.
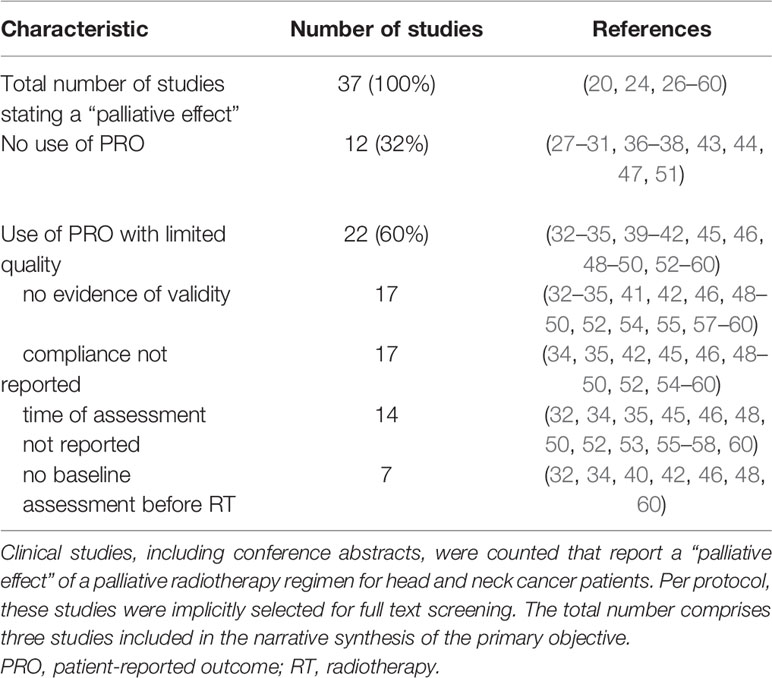
Table 1 Use of patient-reported outcomes and reasons for limited quality of their assessment in studies reporting a “palliative effect”.
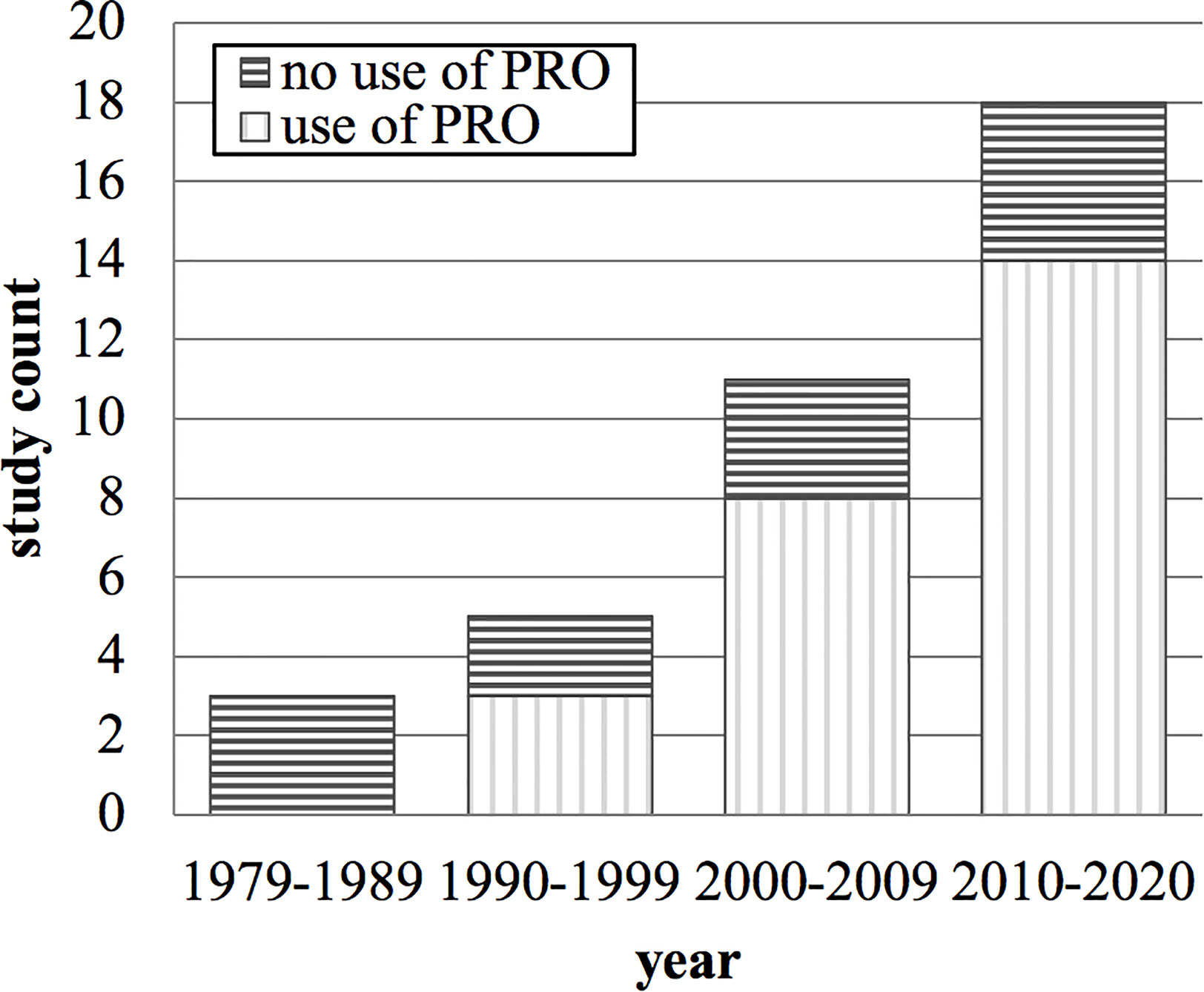
Figure 2 Count of clinical studies stating a “palliative effect” of palliative radiotherapy for head and neck cancer and their use of patient-reported outcomes over time in years. PRO, patient-reported outcome.
Characteristics and Findings of Eligible Studies for the Primary Objective
Preliminary Synthesis
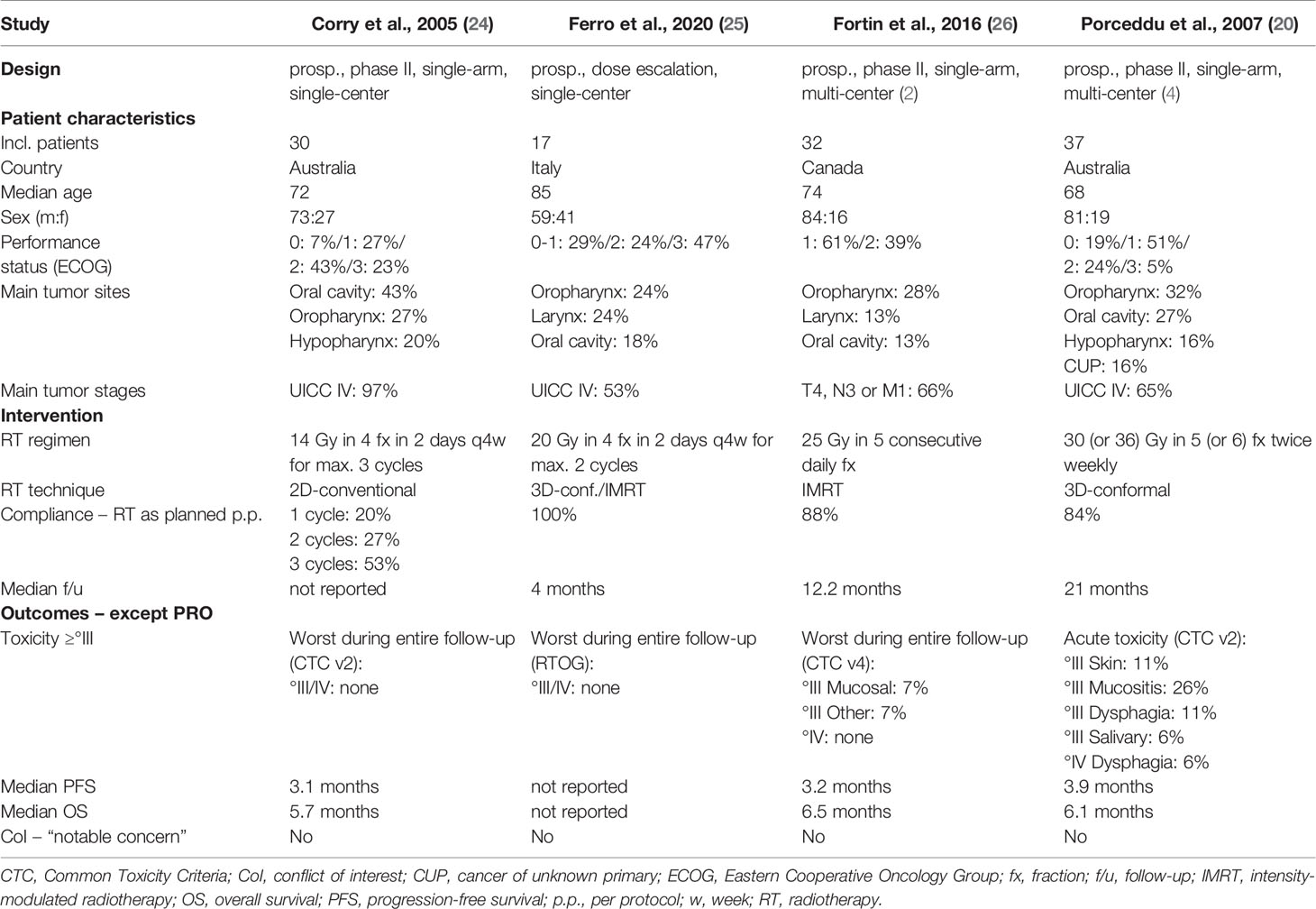
The characteristics of all four eligible studies for the primary objective are shown in Table 2. Three were prospective, non-randomized phase II studies (20, 24, 26). One study was a prospective radiation dose-escalation study (25). Quality of life was the primary endpoint in only one study (26). All studies were conducted in high-income countries. No patient had prior radiotherapy. There was no concurrent systemic therapy per protocol in any study. All trials studied hypofractionated external beam radiotherapy. One study assessed a cyclical regimen known as “Quad Shot” with four fractions in two consecutive days repeated every four weeks for up to three cycles (24). Another study assessed a similar protocol at higher radiation doses (25). The remaining two studies employed once daily consecutive or intermittent fractions (20, 26). Toxicity of grade three or higher was absent in two studies (24, 25), moderate (e.g. mucositis 7%) in one study (26), and more pronounced (e.g. mucositis 26%) in the remaining study (20). The progression-free survival ranged from 3.1 to 3.9 and the overall survival from 5.7 to 6.5 months, respectively.
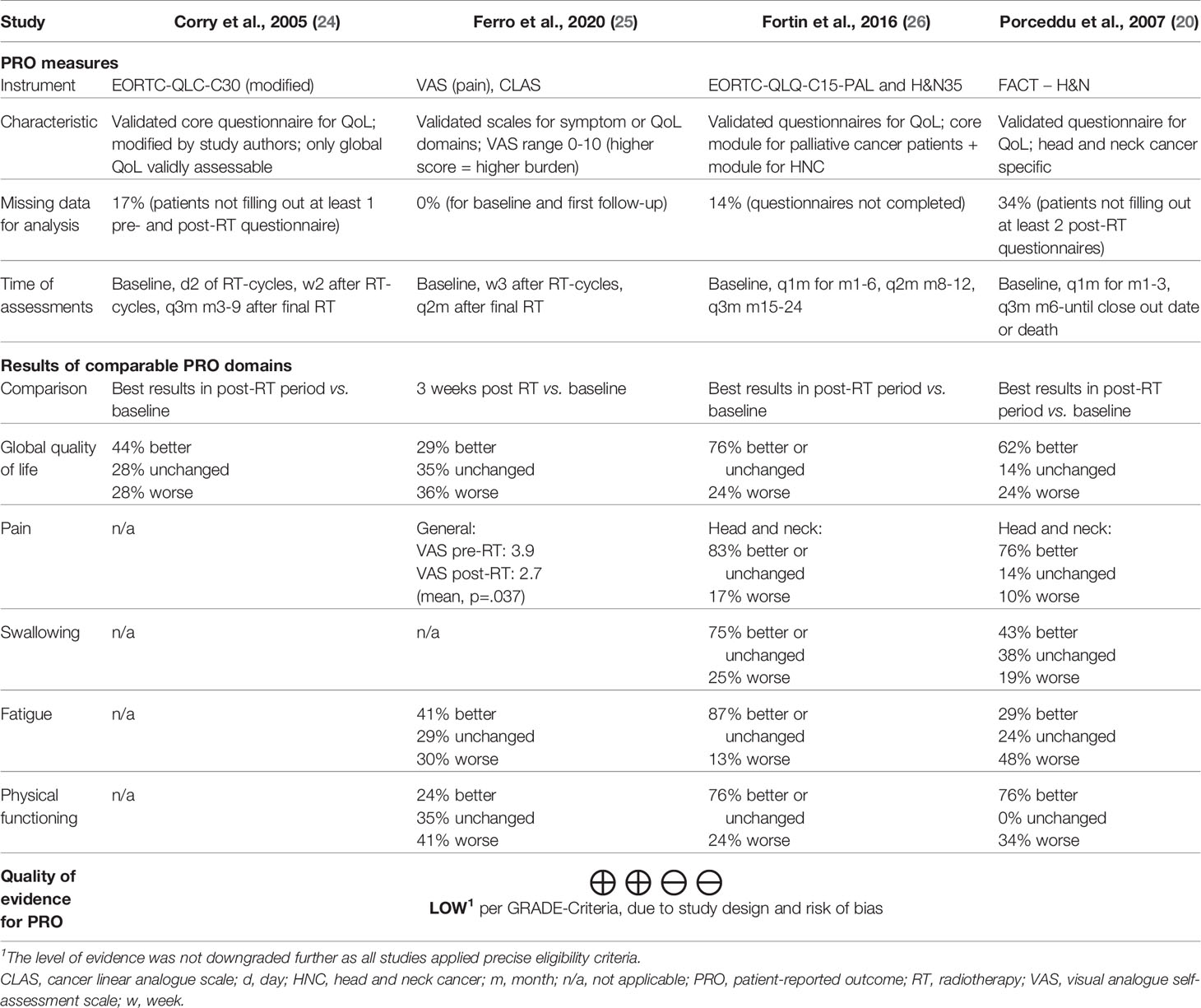
Table 3 displays the summary of findings for patient-reported outcomes of all four eligible studies. The studies employed different patient-reported outcome measures. One study used linear analogue scales for three domains and the visual analogue scale for the domain “pain” (25). The other studies used multi-item questionnaires (20, 24, 26). Of note, one study modified the questionnaire extensively resulting in only one validly measured outcome domain (“global quality of life”) (24). All studies scheduled multiple assessments after the completion of radiotherapy for comparison to baseline. One study reported only outcomes for the first follow-up (25). Two studies reported only the best results at any follow-up (20, 24). The remaining study provided multiple outcomes for a follow-up period of six months (26). In order to allow for better comparison between studies, we have chosen to display the best results after the completion of radiotherapy for the latter study. Results of comparable patient-reported outcome domains are juxtaposed in Table 3, as the instruments used share comparable constructs for (head and neck) cancer patients. The specific domain definition may, however, differ for some outcomes per instrument used. For example, the domain “physical functioning” refers to “ability to perform daily activities” (25), “physical functioning” (26), or “ability to work” (20). Per vote counting, as defined in the methods section, palliative radiotherapy resulted in a benefit for all patient-reported outcome domains across the studies (Table 3). This is also the case for all time points in the study that reported multiple assessments in a six month follow-up period (data not displayed in this review) (26).
Relationships Within the Findings
Tables 2 and 3 offer insights into relationships within the findings with regard to patient-reported outcomes. Yet if differences in patient-reported outcomes reflect relations to study characteristics, should only cautiously be explored on a hypothesis-generating basis. This is due to small sample sizes, reporting of categorical rather than absolute changes, and heterogeneity in the reported comparison to baseline of patient-reported outcomes. Nevertheless, three relating aspects may be identified.
First, differences in effect sizes of specific outcome domains could in part relate to different patient-reported outcome measures used in the studies. The domain fatigue is, for example, a composite score in the EORTC-QLQ-C15-PAL (26), whereas it refers to a single question on “lack of energy” in the FACT-H&N questionnaire (20). Second, the results of patient-reported outcomes tend to be inferior in one study (25) compared to the other studies (20, 24, 26). This might relate to the patient’s significantly higher age as well as to the comparison to baseline (25); only the first follow-up was reported instead of the best result within multiple assessments. Third, a relevant fraction of patients in all studies reported worsened health-related quality of life scores despite limited treatment-related toxicity. This aspect may be related to advanced disease stages, the fact that all radiotherapy protocols treated only the symptomatic gross disease in order to limit toxicity, and short progression-free survival. Competing tumor growth may therefore be related to worsened health-related quality of life scores in some patients.
Robustness of the Synthesis
The overall quality of evidence for a positive impact of palliative radiotherapy for head and neck cancer on health-related quality of life or symptoms as measured by patient-reported outcomes was “low” per GRADE-criteria (Table 3). The risk of bias assessment of included, individual studies showed “serious” overall risk of bias in three studies (24–26) and “critical” overall risk of bias in one study (20) per ROBINS-I tool (22, 61) (Figure 3).
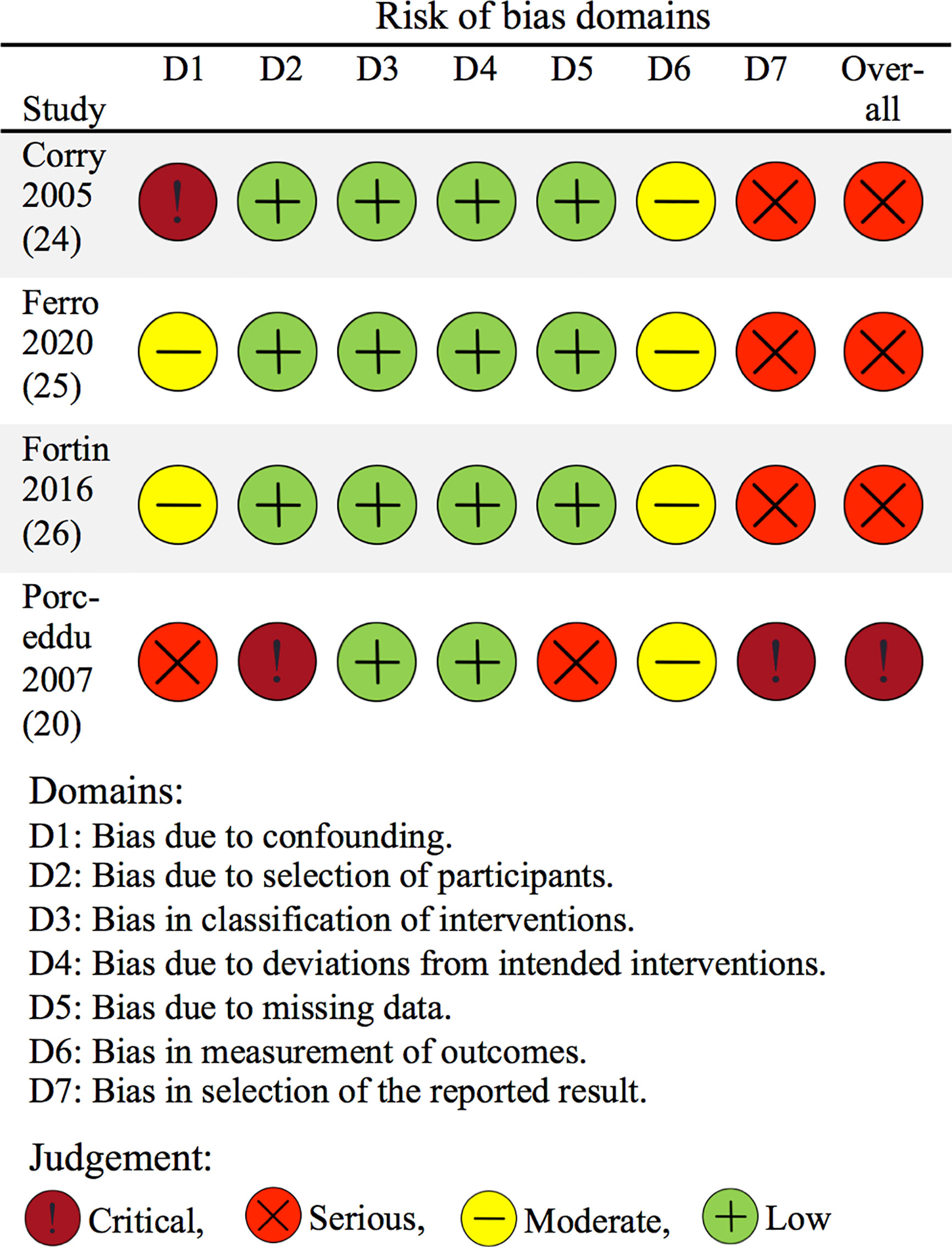
Figure 3 Risk of bias of included studies concerning patient-reported outcomes. The risk of bias of included studies was assessed using the ROBINS-I tool for non-randomized studies (22). The figure is based on the “robvis” tool (61).
Registered Studies
Relevant studies that are registered on “ClinicalTrials.gov”, but were not reported in November 2020, are displayed in Table 4. One study was terminated early due to poor recruitment. One ongoing prospective study uses the patient-reported outcome measure “EORTC-QLQ-C15-PAL” and awaits primary completion in November 2022.
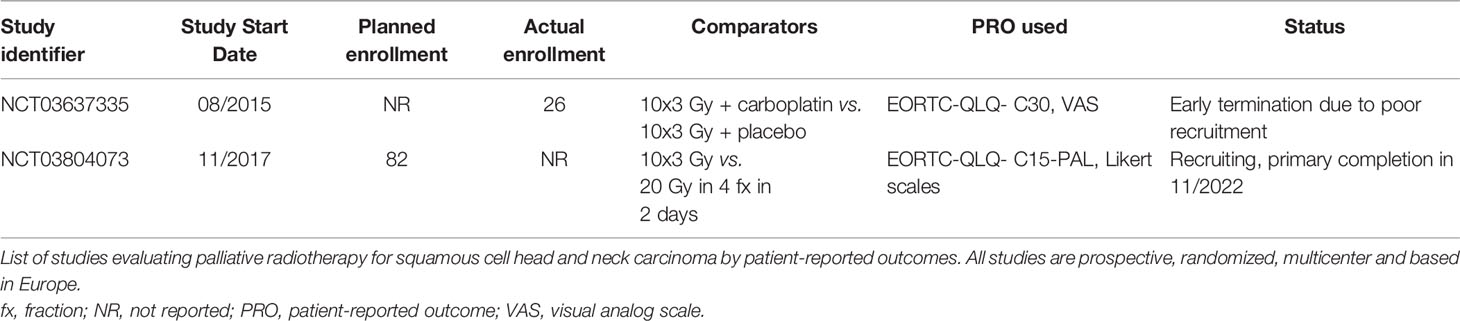
Table 4 Studies registered on “CinicalTrials.gov” and not yet reported in November 2020.
Discussion
This systematic review of patient-reported outcomes in patients treated with palliative radiotherapy for head and neck cancer shows that over 30% of studies claiming a “palliative effect” did not use patient-reported outcomes. Sixty percent of the studies applied patient-reported outcomes with limited quality, for example owing to unvalidated instruments or unreported compliance data. Four studies were eligible to analyze the primary objective: the effectiveness of palliative radiotherapy as validly measured by patient-reported outcomes. While a planned meta-analysis or calculation of summary effect sizes was not feasible, all studies suggested a positive impact of palliative radiotherapy on global health-related quality of life. In addition, relevant symptoms seemed to be alleviated or stabilized. The risk of bias, however, was high and the overall quality of the evidence concerning patient-reported outcomes remains low per GRADE-criteria.
The low rate of patient-reported outcomes and standard of their use in studies of palliative radiotherapy for head and neck cancer is in part also reflected in other entities. In fact, a systematic review of palliative radiotherapy for rectal cancer showed that none of 27 eligible studies assessed patient-reported outcomes (62). Furthermore, a systematic review of palliative radiotherapy for prostate cancer could neither identify any study applying patient-reported outcomes (63). Another systematic review evaluated patient-reported outcomes in palliative radiotherapy for esophageal cancer (18), a patient population sharing risk factors with head and neck cancer patients. Only six studies conducted patient-reported outcome assessment of sufficient standard as defined by the review authors. Next to these overall relatively low rates of patient-reported outcome evaluation, a recent systematic review on patient-reported outcome measures in palliative radiotherapy studies across entities confirmed quality issues, for instance in patient-reported outcome reporting (64). Hence, our finding is reflective of the current literature and might be rooted in a long-recognized difficulty in defining the right outcome measure for palliation (65). Patient-reported outcomes, however, are generally accepted to measure health-related quality of life and symptom burden, also in a palliative setting (66).
Even though patient-reported outcome measures are the cornerstone to assess health-related quality of life and symptom burden, their implementation is challenging. A major concern is missing data. In fact, it has long been recognized that missing patient-reported outcome data represent not only random effects but also important information (67). For example, missing data could result from a missed patient visit that was cancelled due to a deterioration of the patient’s health. In order to allow for a meaningful interpretation of patient-reported outcomes, the rate of missing data should be as low as possible. The rate of missing patient-reported outcome data for analysis was 34% in one of the eligible studies for our primary objective (Table 3) (20). This might raise the question if patient-reported outcome measures are an appropriate tool to assess health-related quality of life and symptom burden in patients with incurable head and neck cancer. In fact, missing data in trials of palliative interventions is an issue for most endpoints. A systematic review and meta-analysis has reported a rate of 23% of missing data for all primary endpoints in randomized trials of palliative interventions (68). Health-related quality of life was even associated with significantly higher rates of missing data in the meta-analysis. In this light, one of the eligible studies for the primary endpoint of our review reported only 14% of missing patient-reported outcome data for all scheduled questionnaire assessments (26). This demonstrates that the rate of missing patient-reported outcome data can be lower than average in head and neck cancer patients undergoing palliative radiotherapy. In our view, the use of patient-reported outcome measures should therefore not be discouraged in this setting.
Despite limited evidence in terms of validly measured patient-reported outcomes regarding the effectiveness of palliative radiotherapy for head and neck cancer, recent data demonstrate the frequent use of radiotherapy in this context. A British prospective cohort study of head and neck cancer patients treated at 76 UK centers reported that over 50% of those treated in “non-curative” intent received radiotherapy (2). This goes in line with guidelines suggesting palliative local therapy such as radiotherapy for head and neck cancer patients when indicated (69, 70). Nevertheless, the burden of radiotherapy-related toxicity should be carefully considered. Most included studies for our primary objective reported remarkably low toxicity rates (24–26), which may have less negative impact on health-related quality of life compared to more intensive radiotherapy regimens. In light of limited prognosis, toxicity is crucial, as patients with head and neck cancer and palliative patients in general show the highest rates of radiotherapy-associated hospital admissions (71). Keeping in mind that health-related quality of life should be a main outcome, a review of palliative radiotherapy for head and neck cancer also judges patient-reported outcomes essential to optimize decisions on therapy (13). The eligibility criteria for the primary objective of our study were designed in order to ensure an appropriate quality of studies reporting patient-reported outcomes, the latter being a crucial aspect as stated by the Cochrane foundation (72). All four eligible studies reported a positive impact of palliative radiotherapy on health-related quality of life and relevant symptoms. Another systematic review on palliative radiotherapy for head and neck cancer used less stringent eligibility criteria. This review included for example retrospective studies assessing local tumor control rates or unvalidated patient-reported outcome measures (12). Nevertheless, the studies presented there are as well in favor of palliative radiotherapy. The question remains, however, how to define the patient population that may benefit from palliative radiotherapy for symptomatic head and neck cancer instead of radical radiotherapy or also omission of radiotherapy. Put simply as a framework, this question relates to four key aspects: i.) comorbidity and/or performance status, ii.) tumor extension, iii.) prognosis as judged clinically, and iv.) an individual patient’s preferences. These aspects need to be merged and weighed individually in a shared decision-making process. Even in a similar clinical situation, this process may result in divergent decisions reaching from a more radical to a palliative radiotherapy approach or also omission of radiotherapy.
Our systematic review has limitations. The number of eligible studies for the primary objective was small. This precluded a meta-analysis and reduces the confidence in the evidence. The narrative synthesis conducted instead followed a methodological framework (15) but inflicted in part personnel judgement of the authors. This is for example reflected in the choice of findings to be explored regarding their potential relationships. Furthermore, two studies only reported the best results of patient-reported outcomes after completion of radiotherapy (20, 24). This approach favors the display of a positive effect of radiotherapy. One study, however, reported multiple assessments after radiotherapy that all showed a benefit of palliative radiotherapy per vote counting (26). Next, the sample sizes of all eligible studies are small. As head and neck cancer is a heterogeneous and relatively rare disease in Western countries, large sample sizes are difficult to achieve. This is also shown by one European study closed early due to poor recruitment (73), while only one ongoing study is listed on “ClinicalTrials.gov” (Table 4). Moreover, most head and neck cancer cases are located in low- and middle-income countries in Asia and on the Indian subcontinent (74). Palliative radiotherapy for head and neck cancer is of major importance in this setting. Yet no local study met our eligibility criteria, in part due to linguistically unvalidated patient-reported outcome measures. On the other hand, one of the strengths of our systematic review is the emphasis on health-related quality of life and symptom burden. This is essential in the palliative setting and of greater relevance than surrogate measures such as radiographic response rates. Furthermore, we applied strict eligibility criteria for studies reporting patient-reported outcomes. In contrast to earlier reviews (12, 13), this approach allows for a meaningful minimum standard in the critical appraisal of the evidence. Finally, to our knowledge, we are the first to use certain tools of high standard for risk of bias assessment [ROBINS-I (22)] and overall evidence assessment [GRADE (21)] in our study setting.
In conclusion, the overall quality of evidence concerning the effectiveness of palliative radiotherapy for head and neck cancer is low as measured by patient-reported outcomes. Nevertheless, existing evidence suggests a positive impact on health-related quality of life and symptom burden. Although further validation by studies including patient-reported outcomes is urgently needed, palliative radiotherapy should be considered in appropriate cases.
Data Availability Statement
The original contributions presented in the study are included in part in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
The protocol was developed by AF, JDu, OW, and DK. The search strategy was realized and conducted by OW. Screening, data extraction, and data synthesis were performed by AF, JDo, and DK. Figures, tables, and manuscript were compiled by AF and JDo and substantially revised and adapted by JDu, MH, CS, and DK. All authors contributed to the article and approved the submitted version.
Funding
We acknowledge financial support by Land Schleswig-Holstein within the funding program “Open Access Publikationsfonds”.
Conflict of Interest
DK has received honoraria from Merck Sharp & Dome.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Silke Szymczak, University of Lübeck—Institute of Medical Biometry and Statistics, for statistical and methodological counselling.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.683042/full#supplementary-material
References
1. Ledeboer QCP, van der Schroeff MP, Pruyn JFA, de Boer MF, Baatenburg de Jong RJ, van der Velden L-A. Survival of Patients With Palliative Head and Neck Cancer. Head Neck (2011) 33:1021–6. doi: 10.1002/hed.21572
2. Mayland CR, Ingarfield K, Rogers SN, Dey P, Thomas S, Waylen A, et al. Disease Trajectories, Place and Mode of Death in People With Head and Neck Cancer: Findings From the “Head and Neck 5000” Population-Based Prospective Clinical Cohort Study. Palliat Med (2020) 34:639–50. doi: 10.1177/0269216320904313
3. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de CG, et al. Pembrolizumab Alone or With Chemotherapy Versus Cetuximab With Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet (2019) 394:1915–28. doi: 10.1016/S0140-6736(19)32591-7
4. Szturz P, Vermorken JB. Management of Recurrent and Metastatic Oral Cavity Cancer: Raising the Bar a Step Higher. Oral Oncol (2020) 101:104492. doi: 10.1016/j.oraloncology.2019.104492
5. Mayland CR, Ho QM, Doughty HC, Rogers SN, Peddinti P, Chada P, et al. The Palliative Care Needs and Experiences of People With Advanced Head and Neck Cancer: A Scoping Review. Palliat Med (2020) 35(1):27–44. doi: 10.1177/0269216320963892
6. Lutz ST, Jones J, Chow E. Role of Radiation Therapy in Palliative Care of the Patient With Cancer. J Clin Oncol (2014) 32:2913–9. doi: 10.1200/JCO.2014.55.1143
7. Lavergne MR, Johnston GM, Gao J, Dummer TJ, Rheaume DE. Variation in the Use of Palliative Radiotherapy At End of Life: Examining Demographic, Clinical, Health Service, and Geographic Factors in a Population-Based Study. Palliat Med (2011) 25:101–10. doi: 10.1177/0269216310384900
8. Nilsen ML, Johnson JT. Potential for Low-Value Palliative Care of Patients With Recurrent Head and Neck Cancer. Lancet Oncol (2017) 18:e284–9. doi: 10.1016/S1470-2045(17)30260-7
9. Kelley AS, Morrison RS. Palliative Care for the Seriously Ill. N Engl J Med (2015) 373:747–55. doi: 10.1056/NEJMra1404684
10. LeBlanc TW, Abernethy AP. Patient-Reported Outcomes in Cancer Care - Hearing the Patient Voice At Greater Volume. Nat Rev Clin Oncol (2017) 14:763–72. doi: 10.1038/nrclinonc.2017.153
11. Rogers SN, Barber B. Using Proms to Guide Patients and Practitioners Through the Head and Neck Cancer Journey. Patient Relat Outcome Meas (2017) 8:133–42. doi: 10.2147/PROM.S129012
12. Shahid Iqbal M, Kelly C, Kovarik J, Goranov B, Shaikh G, Morgan D, et al. Palliative Radiotherapy for Locally Advanced Non-Metastatic Head and Neck Cancer: A Systematic Review. Radiother Oncol (2018) 126:558–67. doi: 10.1016/j.radonc.2017.12.011
13. Grewal AS, Jones J, Lin A. Palliative Radiation Therapy for Head and Neck Cancers. Int J Radiat Oncol Biol Phys (2019) 105:254–66. doi: 10.1016/j.ijrobp.2019.05.024
14. Grant MJ, Booth A. A Typology of Reviews: An Analysis of 14 Review Types and Associated Methodologies. Health Info Libr J (2009) 26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x
15. Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product From the ESRC Methods Programme. ESRC Methods Programme (2006). doi: 10.13140/2.1.1018.4643
16. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd. Chichester (UK: John Wiley & Sons (2019).
17. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ (2009) 339:b2535. doi: 10.1136/bmj.b2535
18. Amdal CD, Jacobsen A-B, Guren MG, Bjordal K. Patient-Reported Outcomes Evaluating Palliative Radiotherapy and Chemotherapy in Patients With Oesophageal Cancer: A Systematic Review. Acta Oncol (2013) 52:679–90. doi: 10.3109/0284186X.2012.731521
19. McKenzie JE, Brennan SE. “Chapter 12: Synthesizing and Presenting Findings Using Other Methods. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0,” in (Cochrane). John Wiley & Sons (2019).
20. Porceddu SV, Rosser B, Burmeister BH, Jones M, Hickey B, Baumann K, et al. Hypofractionated Radiotherapy for the Palliation of Advanced Head and Neck Cancer in Patients Unsuitable for Curative Treatment–”Hypo Trial”. Radiother Oncol (2007) 85:456–62. doi: 10.1016/j.radonc.2007.10.020
21. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE Guidelines: 1. Introduction-GRADE Evidence Profiles and Summary of Findings Tables. J Clin Epidemiol (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
22. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ (2016) 355:i4919. doi: 10.1136/bmj.i4919
23. Page MJ, Higgins JPT, Sterne JAC. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, Editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0,” in (Cochrane). John Wiley & Sons (2019).
24. Corry J, Peters LJ, Costa ID, Milner AD, Fawns H, Rischin D, et al. The ‘QUAD SHOT’–A Phase II Study of Palliative Radiotherapy for Incurable Head and Neck Cancer. Radiother Oncol (2005) 77:137–42. doi: 10.1016/j.radonc.2005.10.008
25. Ferro M, Macchia G, Re A, Buwenge M, Ferro M, Boccardi M, et al. Advanced Head and Neck Cancer in Older Adults: Results of a Short Course Accelerated Radiotherapy Trial. J Geriatr Oncol (2020) 12(3):441–5. doi: 10.1016/j.jgo.2020.10.006
26. Fortin B, Khaouam N, Filion E, Nguyen-Tan PF, Bujold A, Lambert L. Palliative Radiation Therapy for Advanced Head and Neck Carcinomas: A Phase 2 Study. Int J Radiat Oncol Biol Phys (2016) 95:647–53. doi: 10.1016/j.ijrobp.2016.01.039
27. Pilepich MV, Munzenrider JE, Rene JB. Unorthodox Fractionation in the Treatment of Head and Neck Tumors. Int J Radiat Oncol Biol Phys (1979) 5:249–52. doi: 10.1016/0360-3016(79)90727-2
28. Vikram B, Hilaris BS, Anderson L, Strong EW. Permanent Iodine-125 Implants in Head and Neck Cancer. Cancer (1983) 51:1310–4. doi: 10.1002/1097-0142(19830401)51:7<1310::aid-cncr2820510722>3.0.co;2-i
29. Weissberg JB, Pillsbury H, Sasaki CT, Son YH, Fischer JJ. High Fractional Dose Irradiation of Advanced Head and Neck Cancer. Implications for Combined Radiotherapy and Surgery. Arch Otolaryngol (1983) 109:98–102. doi: 10.1001/archotol.1983.00800160032008
30. Haraf DJ, Vokes EE, Panje WR, Weichselbaum RR. Survival and Analysis of Failure Following Hydroxyurea, 5-Fluorouracil and Concomitant Radiation Therapy in Poor Prognosis Head and Neck Cancer. Am J Clin Oncol (1991) 14:419–26. doi: 10.1097/00000421-199110000-00012
31. Gandia D, Wibault P, Guillot T, Bensmaine A, Armand JP, Marandas P, et al. Simultaneous Chemoradiotherapy as Salvage Treatment in Locoregional Recurrences of Squamous Head and Neck Cancer. Head Neck (1993) 15:8–15. doi: 10.1002/hed.2880150103
32. Paris KJ, Spanos WJ Jr, Lindberg RD, Jose B, Albrink F. Phase I-II Study of Multiple Daily Fractions for Palliation of Advanced Head and Neck Malignancies. Int J Radiat Oncol Biol Phys (1993) 25:657–60. doi: 10.1016/0360-3016(93)90012-k
33. Suwinski R, Pilecki B, Skladowski K, Swiatnicka J, Zajusz A, Przeorek W, et al. Sequential Combination of Radio-Chemotherapy (5-Fluorouracil, Cis-Platinum and Irradiation) in the Management of Locally Advanced Head and Neck Cancers. Neoplasma (1996) 43:37–41.
34. Erkal HS, Mendenhall WM, Amdur RJ, Villaret DB, Stringer SP. Squamous Cell Carcinomas Metastatic to Cervical Lymph Nodes From an Unknown Head and Neck Mucosal Site Treated With Radiation Therapy With Palliative Intent. Radiother Oncol (2001) 59:319–21. doi: 10.1016/s0167-8140(01)00282-1
35. Schleicher UM, Andreopoulos D, Ammon J. Palliative Radiotherapy in Recurrent Head-and-Neck Tumors by a Percutaneous Superfractionated Treatment Schedule. Int J Radiat Oncol Biol Phys (2001) 50:65–8. doi: 10.1016/s0360-3016(00)01567-4
36. Barrett WL, Gleich L, Wilson K, Gluckman J. Organ Preservation With Interstitial Radiation for Base of Tongue Cancer. Am J Clin Oncol (2002) 25:485–8. doi: 10.1097/00000421-200210000-00013
37. Voynov G, Heron DE, Burton S, Grandis J, Quinn A, Ferris R, et al. Frameless Stereotactic Radiosurgery for Recurrent Head and Neck Carcinoma. Technol Cancer Res Treat (2006) 5:529–35. doi: 10.1177/153303460600500510
38. Kolotas C, Tselis N, Sommerlad M, Roddiger S, Schnabel T, Baltas D, et al. Reirradiation for Recurrent Neck Metastases of Head-and-Neck Tumors Using CT-Guided Interstitial 192Ir HDR Brachytherapy. Strahlenther Onkol (2007) 183:69–75. doi: 10.1007/s00066-007-1632-2
39. Chen AM, Vaughan A, Narayan S, Vijayakumar S. Palliative Radiation Therapy for Head and Neck Cancer: Toward an Optimal Fractionation Scheme. Head Neck (2008) 30:1586–91. doi: 10.1002/hed.20894
40. Al-mamgani A, Tans L, Van rooij PHE, Noever I, Baatenburg de jong RJ, Levendag PC. Hypofractionated Radiotherapy Denoted as the “Christie Scheme”: An Effective Means of Palliating Patients With Head and Neck Cancers Not Suitable for Curative Treatment. Acta Oncol (2009) 48:562–70. doi: 10.1080/02841860902740899
41. Ghoshal S, Chakraborty S, Moudgil N, Kaur M, Patel FD. Quad Shot: A Short But Effective Schedule for Palliative Radiation for Head and Neck Carcinoma. Indian J Palliat Care (2009) 15:137–40. doi: 10.4103/0973-1075.58460
42. Pearson RA, Bannister-Young RH, Ivison D, Kelly CG, Chatterjee S. Split-Course Hypofractionated Palliative Radiotherapy for Patients With Head and Neck Squamous Cell Carcinoma - A Worthwhile Treatment Schedule in the UK? Clin Oncol (2010) 22:890–1. doi: 10.1016/j.clon.2010.06.003
43. Kancherla KN, Oksuz DC, Prestwich RJ, Fosker C, Dyker KE, Coyle CC, et al. The Role of Split-Course Hypofractionated Palliative Radiotherapy in Head and Neck Cancer. Clin Oncol (2011) 23:141–8. doi: 10.1016/j.clon.2010.09.006
44. Tselis N, Ratka M, Vogt HG, Kolotas C, Baghi M, Baltas D, et al. Hypofractionated Accelerated CT-Guided Interstitial 192Ir-HDR-Brachytherapy as Re-Irradiation in Inoperable Recurrent Cervical Lymphadenopathy From Head and Neck Cancer. Radiother Oncol (2011) 98:57–62. doi: 10.1016/j.radonc.2010.10.025
45. Khan L, Tjong M, Raziee H, Lee J, Erler D, Chin L, et al. Role of Stereotactic Body Radiotherapy for Symptom Control in Head and Neck Cancer Patients. Support Care Cancer (2015) 23:1099–103. doi: 10.1007/s00520-014-2421-y
46. Lok BH, Jiang G, Gutiontov S, Lanning RM, Sridhara S, Sherman EJ, et al. Palliative Head and Neck Radiotherapy With the RTOG 8502 Regimen for Incurable Primary or Metastatic Cancers. Oral Oncol (2015) 51:957–62. doi: 10.1016/j.oraloncology.2015.07.011
47. Nguyen NT, Doerwald-Munoz L, Zhang H, Kim DH, Sagar S, Wright JR, et al. 0-7-21 Hypofractionated Palliative Radiotherapy: An Effective Treatment for Advanced Head and Neck Cancers. Br J Radiol (2015) 88:20140646. doi: 10.1259/bjr.20140646
48. van Beek KM, Kaanders JH, Janssens GO, Takes RP, Span PN, Verhoef CG. Effectiveness and Toxicity of Hypofractionated High-Dose Intensity-Modulated Radiotherapy Versus 2- and 3-Dimensional Radiotherapy in Incurable Head and Neck Cancer. Head Neck (2016) 38 Suppl 1:E1264–70. doi: 10.1002/hed.24203
49. Murthy V, Kumar D, Budrukkar A, Gupta T, Ghosh-Laskar S, Agarwal J. Twice-Weekly Palliative Radiotherapy for Locally Very Advanced Head and Neck Cancers. Indian J Cancer (2016) 53:138–41. doi: 10.4103/0019-509X.180847
50. Gamez ME, Agarwal M, Hu KS, Lukens JN, Harrison LB. Hypofractionated Palliative Radiotherapy With Concurrent Radiosensitizing Chemotherapy for Advanced Head and Neck Cancer Using the “QUAD-SHOT Regimen.” Anticancer Res (2017) 37:685–92. doi: 10.21873/anticanres.11364
51. Jakhar SL, Purohit R, Solanki A, Murali P, Kothari T, Sharma N, et al. Accelerated Hypofractionation (OCTA SHOT): Palliative Radiation Schedule in Advanced Head and Neck Carcinoma. J Can Res Ther (2017) 13:943–6. doi: 10.4103/jcrt.JCRT_767_16
52. Choudhary A, Gupta A. Conventional Fractionation Versus Quad Shot in Advanced Head-and-Neck Cancers: A Randomized Controlled Trial. Indian J Palliat Care (2019) 25:527–34. doi: 10.4103/IJPC.IJPC_209_18
53. Kumaravelu AS, Eswaran P. Palliative Hypofractionated Radiotherapy and Chemotherapy in Advanced Head and Neck Squamous Cell Carcinoma (HNSCC). Support Care Cancer (2012) 20:S62. doi: 10.1007/s00520-012-1479-7
54. Singh C, Gupta S. Palliative Radiotherapy in Locally Advanced Head and Neck Cancer: A Quality of Life Study. Support Care Cancer (2014) 22:S187. doi: 10.1007/s00520-014-2222-3
55. Van Beek KM, Kaanders JHAM, Janssens GORJ, Takes RP, Span P, Verhoef CG. Hypofractionated High-Dose IMRT: Effective and Less Toxic Than 2D-RT in Incurable Head and Neck Cancer. Radiother Oncol (2015) 115:S383–4. doi: 10.1016/S0167-8140(15)40763-7
56. Ferro M, Macchia G, Cilla S, Ianiro A, Picardi V, Boccardi M, et al. Short-Course Accelerated Palliative EBRT for Advanced Head and Neck Cancer in Elderly Patients. Radiother Oncol (2019) 133:S884–5. doi: 10.1016/S0167-8140(19)32062-6
57. Basree MM, Mitchell DL, Dibs K, Jhawar SR, Baliga S, Bonomi M, et al. Initial Experience With Palliative “Quad-Shot” Radiotherapy With Concurrent and Adjuvant Pd-1 Inhibitor for Recurrent and/or Metastatic Head and Neck Cancer. Int J Radiat Oncol Biol Phys (2020) 108:e826. doi: 10.1016/j.ijrobp.2020.07.347
58. Zamboglou N, Wurm R, Pape H, Schnabel TH, Kuhn FP, Streffer C, et al. Simultaneous Radiotherapy and Intratumoral Instillation of Mitoxantrone in Locoregional Recurrence of Head and Neck Carcinoma. Reg Cancer Treat (1991) 4:79–84.
59. Das S, Thomas S, Pal SK, Isiah R, John S. Hypofractionated Palliative Radiotherapy in Locally Advanced Inoperable Head and Neck Cancer: CMC Vellore Experience. Indian J Palliat Care (2013) 19:93–8. doi: 10.4103/0973-1075.116709
60. Carrascosa LA, Yashar CM, Paris KJ, Larocca RV, Faught SR, Spanos WJ. Palliation of Pelvic and Head and Neck Cancer With Paclitaxel and a Novel Radiotherapy Regimen. J Palliative Med (2007) 10:877–81. doi: 10.1089/jpm.2006.0192
61. McGuinness LA, Higgins JPT. Risk-of-Bias Visualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res Synth Methods (2020) 12(1):55–61. doi: 10.1002/jrsm.1411
62. Cameron MG, Kersten C, Vistad I, Fosså S, Guren MG. Palliative Pelvic Radiotherapy of Symptomatic Incurable Rectal Cancer - A Systematic Review. Acta Oncol (2014) 53:164–73. doi: 10.3109/0284186X.2013.837582
63. Cameron MG, Kersten C, Guren MG, Fosså SD, Vistad I. Palliative Pelvic Radiotherapy of Symptomatic Incurable Prostate Cancer - A Systematic Review. Radiother Oncol (2014) 110:55–60. doi: 10.1016/j.radonc.2013.08.008
64. Oldenburger E, Oldenburger F, Coolbrandt A, Isebaert S, Neyens I, Sevenants A, et al. The Use of Patient Reported Outcome Measures (Proms) in Palliative Radiotherapy: A Topical Review. Radiother Oncol (2020) 149:94–103. doi: 10.1016/j.radonc.2020.04.045
65. Stephens RJ, Hopwood P, Girling DJ. Defining and Analysing Symptom Palliation in Cancer Clinical Trials: A Deceptively Difficult Exercise. Br J Cancer (1999) 79:538–44. doi: 10.1038/sj.bjc.6690085
66. Bausewein C, Simon ST, Benalia H, Downing J, Mwangi-Powell FN, Daveson BA, et al. PRISMA. Implementing Patient Reported Outcome Measures (Proms) in Palliative Care–Users’ Cry for Help. Health Qual Life Outcomes (2011) 9:27. doi: 10.1186/1477-7525-9-27
67. Bernhard J, Cella DF, Coates AS, Fallowfield L, Ganz PA, Moinpour CM, et al. Missing Quality of Life Data in Cancer Clinical Trials: Serious Problems and Challenges. Stat Med (1998) 17:517–32. doi: 10.1002/(sici)1097-0258(19980315/15)17:5/7<517::aid-sim799>3.0.co;2-s
68. Hussain JA, White IR, Langan D, Johnson MJ, Currow DC, Torgerson DJ, et al. Missing Data in Randomized Controlled Trials Testing Palliative Interventions Pose a Significant Risk of Bias and Loss of Power: A Systematic Review and Meta-Analyses. J Clin Epidemiol (2016) 74:57–65. doi: 10.1016/j.jclinepi.2015.12.003
69. Machiels J-P, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V. Squamous Cell Carcinoma of the Oral Cavity, Larynx, Oropharynx and Hypopharynx: EHNS–ESMO–ESTRO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Annals Oncol (2020) 31:1462–75. doi: 10.1016/j.annonc.2020.07.011
70. Cocks H, Ah-See K, Capel M, Taylor P. Palliative and Supportive Care in Head and Neck Cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol (2016) 130:S198–207. doi: 10.1017/S0022215116000633
71. Waddle MR, Chen RC, Arastu NH, Green RL, Jackson M, Qaqish BF, et al. Unanticipated Hospital Admissions During or Soon After Radiation Therapy: Incidence and Predictive Factors. Pract Radiat Oncol (2015) 5:e245–53. doi: 10.1016/j.prro.2014.08.004
72. Johnston BC, Patrick DL, Devji T, Maxwell LJ, Bingham CO II, Beaton D, et al. “Chapter 18: Patient-Reported Outcomes. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019).,” in (Cochrane). John Wiley & Sons (2019).
73. Al-Mamgani A, Kessels R, Verhoef CG, Navran A, Hamming-Vrieze O, Kaanders JHAM, et al. Randomized Controlled Trial to Identify the Optimal Radiotherapy Scheme for Palliative Treatment of Incurable Head and Neck Squamous Cell Carcinoma. Radiother Oncol (2020) 149:181–8. doi: 10.1016/j.radonc.2020.05.020
Keywords: head and neck neoplasms, squamous cell carcinoma of head and neck, palliative care, radiotherapy, patient reported outcome measures, symptom assessment, quality of life, systematic review
Citation: Fabian A, Domschikowski J, Hoffmann M, Weiner O, Schmalz C, Dunst J and Krug D (2021) Patient-Reported Outcomes Assessing the Impact of Palliative Radiotherapy on Quality of Life and Symptom Burden in Head and Neck Cancer Patients: A Systematic Review. Front. Oncol. 11:683042. doi: 10.3389/fonc.2021.683042
Received: 19 March 2021; Accepted: 28 April 2021;
Published: 04 June 2021.
Edited by:
Markus Brunner, Medical University of Vienna, AustriaReviewed by:
Shilpi Sharma, Narayana Superspeciality Hospital, Gurugram, IndiaChia-Jung Busch, University of Greifswald, Germany
Copyright © 2021 Fabian, Domschikowski, Hoffmann, Weiner, Schmalz, Dunst and Krug. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Fabian, YWxleGFuZGVyLmZhYmlhbkB1a3NoLmRl
 Alexander Fabian
Alexander Fabian Justus Domschikowski
Justus Domschikowski Markus Hoffmann
Markus Hoffmann Oliver Weiner3
Oliver Weiner3 Claudia Schmalz
Claudia Schmalz Jürgen Dunst
Jürgen Dunst