- Department of Pulmonary, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China
Background: Co-mutations was associated with poor response to EGFR-TKIs. First-generation EGFR-TKIs combined with chemotherapy was reported to be more effective than TKIs alone in advanced lung adenocarcinoma patients.
Objective: This retrospective study aimed to explore whether EGFR-mutant patients with co-mutations can benefit from EGFR-TKIs plus chemotherapy.
Patients and Methods: We retrospectively collected data of 137 EGFR-mutant patients with advanced lung adenocarcinoma who underwent next-generation sequencing in our hospital in 2018. Among them, 96 were treated with EGFR–TKIs alone and 41 received EGFR–TKIs plus chemotherapy. We analyzed the progression-free survival (PFS) of patients with co-mutations using different treatments.
Results: Concurrent TP53 mutations, especially exon 4 and 6, were associated with a markedly shorter time to progression on EGFR-TKI monotherapy (11.4 months vs. 16.6 months, P=0.003), while EGFR–TKIs plus chemotherapy would benefit those patients more (with TP53: 11.4 months vs. 19.1 months, P=0.001, HR=0.407; without TP53: 16.6 months vs. 18.9 months, P=0.379, HR=0.706). The incidence of T790M after resistance was equal in patients treated with different treatments (53% vs. 53%, P=0.985).
Conclusions: In our study, concurrent TP53 mutations were found to be risk factors for EGFR-TKI monotherapy, but TKI combined with chemotherapy could eliminate this heterogeneity.
Introduction
In lung adenocarcinoma (LAC), Epidermal growth factor receptor (EGFR) is one of the most common driver genes and can be detected in 40%-50% of Asian patients (1, 2). With the development of targeted therapy, most patients with EGFR mutations can benefit from first-generation EGFR tyrosine kinase inhibitors (EGFR-TKIs) (such as gefitinib, erlotinib, and icotinib, etc.) (3, 4). Several recent prospective studies have shown that EGFR-mutant patients using EGFR-TKIs combined with chemotherapy can have a better prognosis than TKI alone (5–7).
However, there is significant heterogeneity in patients’ objective responses to EGFR-TKI monotherapy, with about 20%-30% of patients failing to respond well or developing drug resistance in the early stage. Previous reports indicated that co-mutations may be associated with poor response to EGFR-TKIs (8–10). Therefore, we tried to explore whether patients with co-mutations can benefit from EGFR-TKIs plus chemotherapy.
In our research, we collected information on patients who used EGFR-TKIs plus chemotherapy and TKIs alone in our hospital. We used the data of next-generation sequencing (NGS) to analyze the most frequent co-mutations, and tried to provide some references for precise treatment.
Materials And Methods
Patients
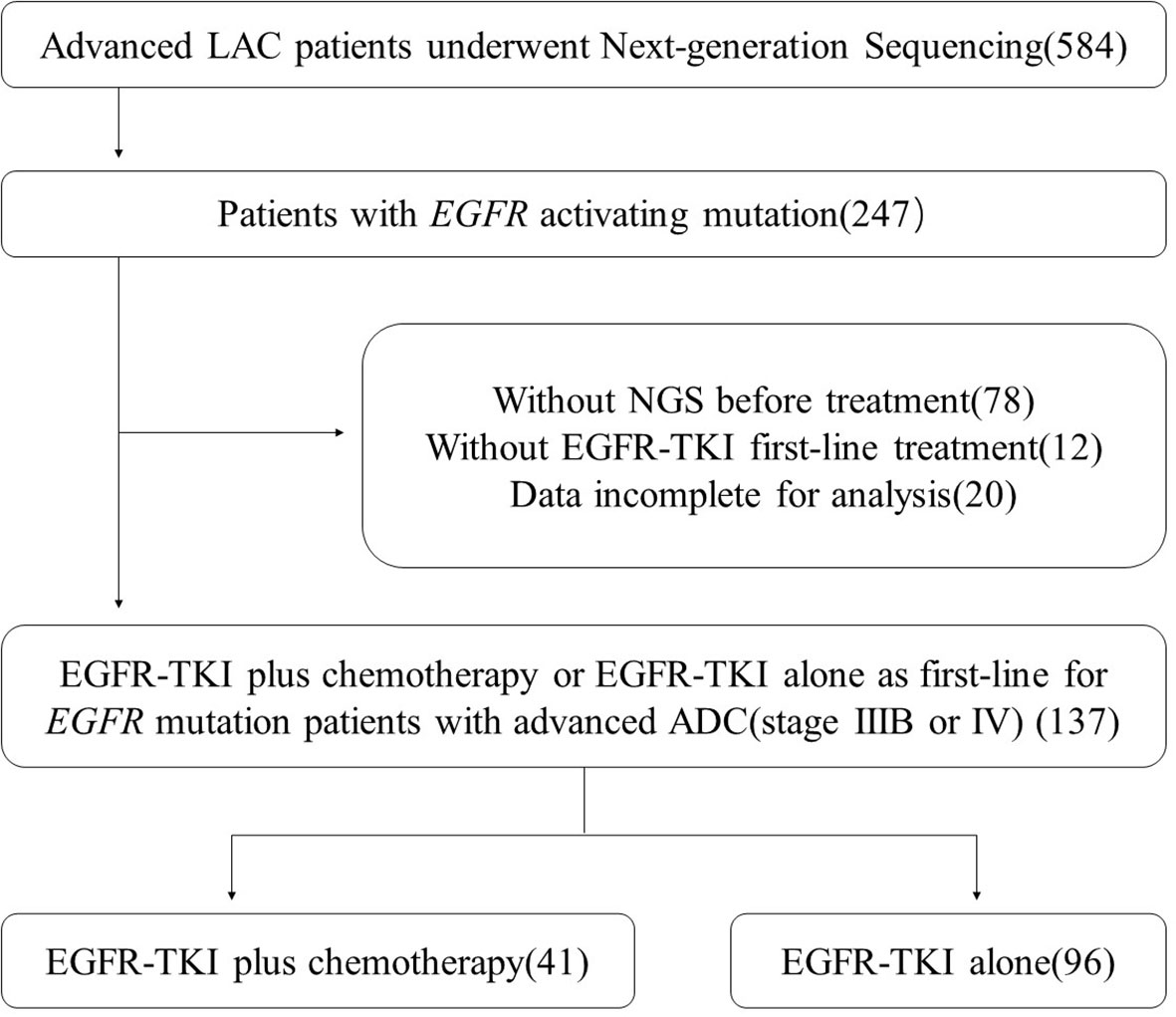
We collected LAC patients who underwent NGS in our hospital in 2018. The specific flow chart for screening patients is shown in Figure 1. We also collected the baseline characteristics of the enrolled patients, including age, gender, smoking status, TNM stage, ECOG-PS score, metastases status, and EGFR subtype.
At last, 137 patients were enrolled in our study and they met all the following screening criteria. First, they were diagnosed with advanced lung cancer (TNM stage IIIB or IV) and detected EGFR sensitive mutations (ex19 deletion or ex21 L858R mutation). Second, they underwent NGS before their first-line treatment and had complete follow-up data in our hospital. All patients gave informed consent before performing operation and treatment.
Next-Generation Sequencing
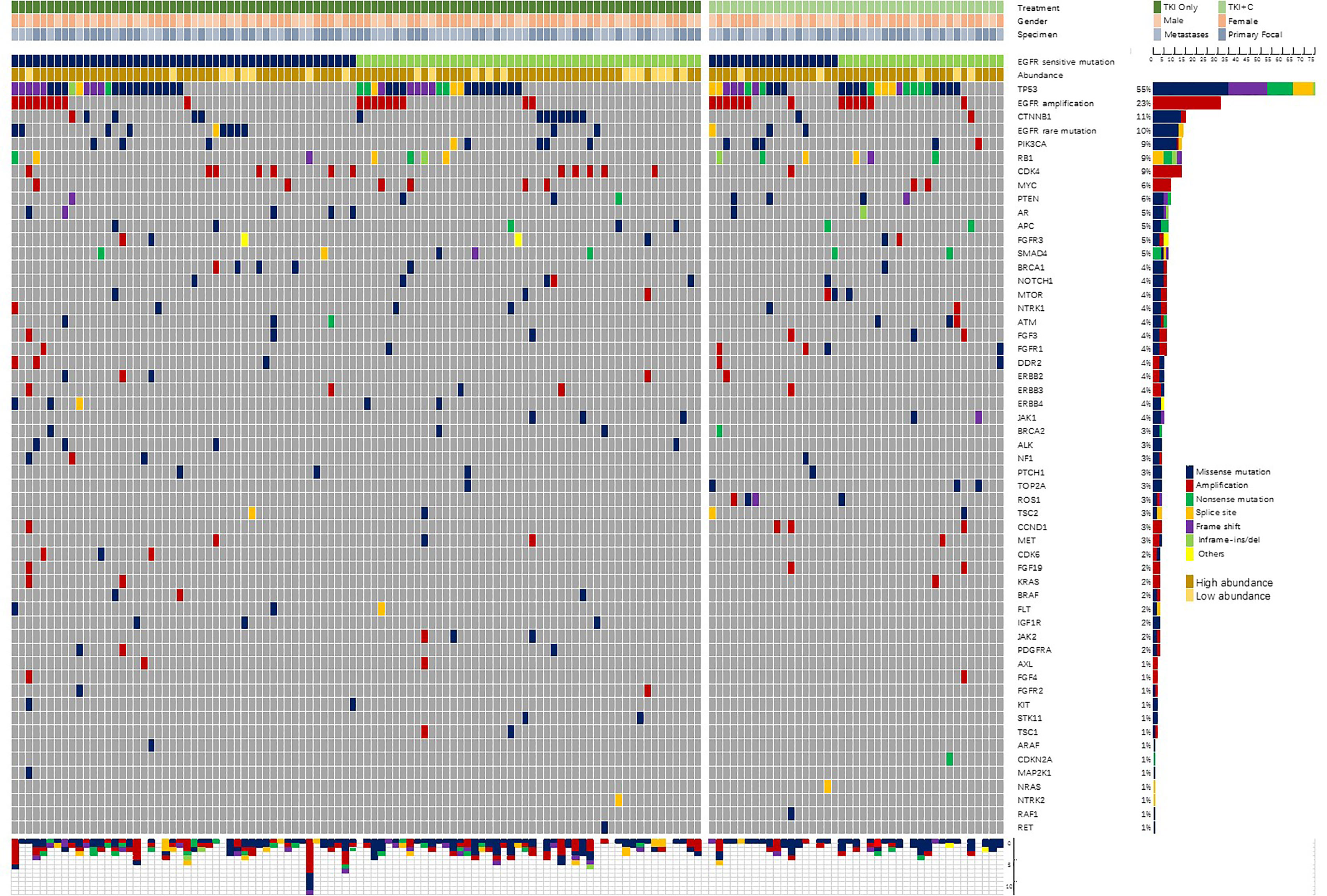
All surgically removed or biopsy tissues were fixed with formalin and embedded in paraffin. Tumor genomic DNA was extracted with the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). As described previously (11), samples were sequenced by Nextseq500 sequencer (Illumina, Inc, San Diego, CA) and evaluated by a panel covering hotspot regions of 68 key cancer-related genes (Supplementary Table 1). The coverage depth of each sample could reach more than 1000×. The genetic profile of samples was shown in Figure 2.
According to previous reports (12), TP53 mutations were divided into 2 groups according to different exon mutation sites.
Treatment and Follow-Up
The monotherapy group was administered first-generation EGFR-TKIs, and the specific dose was gefitinib 250 mg once a day, erlotinib 150 mg once a day, or icotinib 125 mg three times a day. The combination therapy group was given EGFR-TKIs combined with chemotherapy (mainly pemetrexed plus platinum) until the condition worsened or unacceptable toxicity occurred. The mean interval between consecutive chemotherapies was 4 weeks. Bases on the Response Evaluation Criteria in Solid Tumors (RECIST v1.1), patients were clinically evaluated every 4 to 6 weeks. Progression-free survival (PFS) is defined as the time from the initiating EGFR-TKIs to the occurrence of disease progression or the last follow-up (October 10, 2020). The median follow-up time was 24 months.
Statistical Analysis
Chi-square test, Fisher’s exact test and Rank sum test were used to compare categorical variables and continuous variables between groups as appropriate. The Kaplan-Meier method and Log-rank tests were used for PFS analysis to compare the PFS of different groups. A P value of less than 0.05 was considered statistically significant. All analyses were performed on the Statistical Package for Social Science (SPSS, Chicago, IL version 22.0).
Results
Characteristics of Patients
A total of 584 advanced LAC patients underwent next-generation sequencing were included in the preliminary screening. The specific flow chart for screening patients is shown in Figure 1. Finally, 137 patients with advanced LAC (stage IIIB or IV) receiving EGFR-TKI plus chemotherapy or EGFR-TKI alone as first-line were included in our analysis.
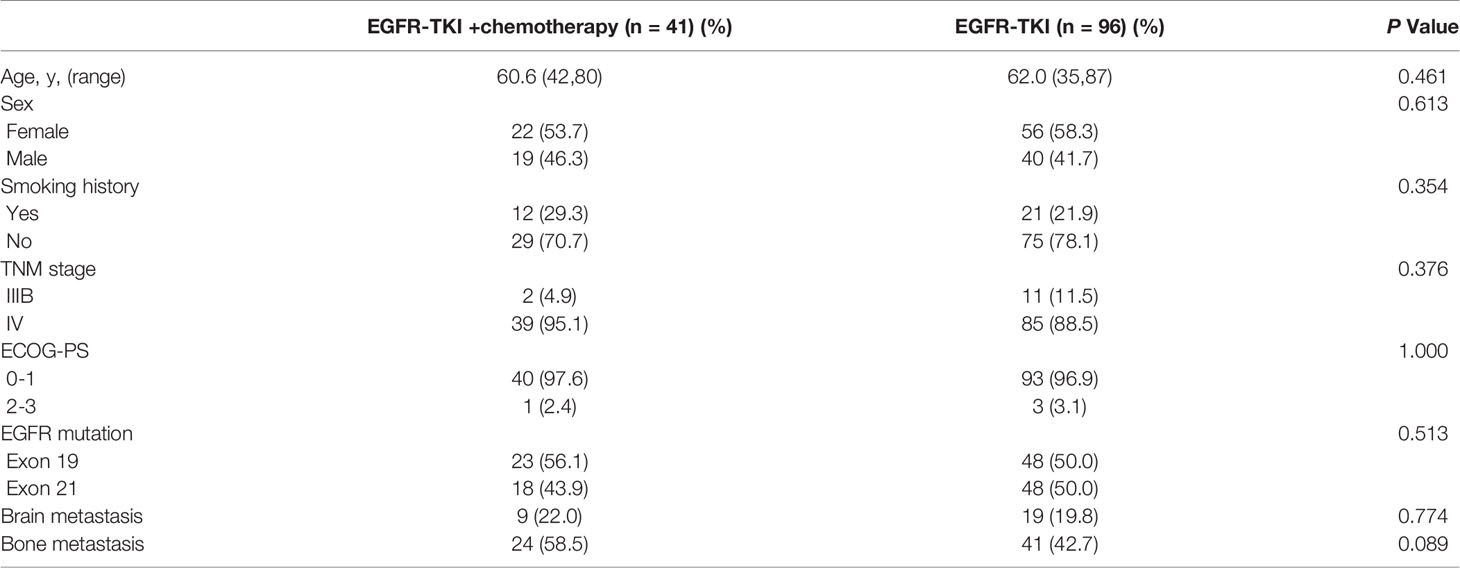
Among the 137 patients, 96 (70%) were treated with EGFR–TKIs alone and 41 (30%) received EGFR–TKIs plus chemotherapy. In the combination therapy group, 39 patients were received pemetrexed plus platinum, and the other 2 patients with gemcitabine plus platinum. The average age in the monotherapy and combination therapy group was 61 years (42 to 80 years) and 62 years (35 to 87 years), respectively. There was no significant difference between the two groups in age, gender, smoking history, ECOG-PS, EGFR subtype, and metastasis status (Table 1). Among the specimens analyzed by NGS, 52% (71/137) were obtained from primary lung sites, while the others were from metastatic lymph node biopsy or pleural effusion embedding.
Baseline Genomic Characteristics
In addition to EGFR sensitive mutations, a total of 364 individual cell mutations and functional mutations were found. At average, a single patient had 2.66 accompanying mutations. Patients with 21L858R mutation tended to have more concomitant mutations than patients with 19del mutation (2.89 vs. 2.44, P=0.183). The majority were missense mutations (47%, 171/364) and amplification (29%, 106/364). Figure 2 showed the frequency and composition of the somatic mutations. TP53 (55%, 75/137) was the most frequent concurrent mutation, followed by EGFR amplification (23%), CTNNB1 (11%), EGFR rare mutations (10%), PIK3CA (9%), RB1 (9%), CDK4 (9%), etc. Twenty-five patients (18%) were identified with low-abundance EGFR mutations, which were detected in samples with a mutation frequency of less than 10%. What’s more, EGFR amplification (84%, 26/31), RB1 (85%, 11/13) and PTEN (75%, 6/8) were often accompanied by TP53 mutations.
In our cohort, TP53 mutation sites were distributed in exons 3-10. Of the 75 patients with TP53 mutations, 1, 6, 24, 14, 15, 9, 5, 1 were located in each exon, respectively.
Outcomes in Monotherapy Group and Combination Therapy Group
After monotherapy or combination therapy, the ORR (the proportion of patients with a confirmed complete or partial response) were 53.1% and 73.2%, respectively(P=0.029). The disease control rate (the proportion of patients with a confirmed complete or partial response or stable disease) were 90.6% and 97.6%, respectively(P=0.284). Of the 28 patients with brain metastases at baseline, excluding 8 patients who received local therapy, the intracranial ORR were 72.7% and 77.7%, respectively (P=1.000).
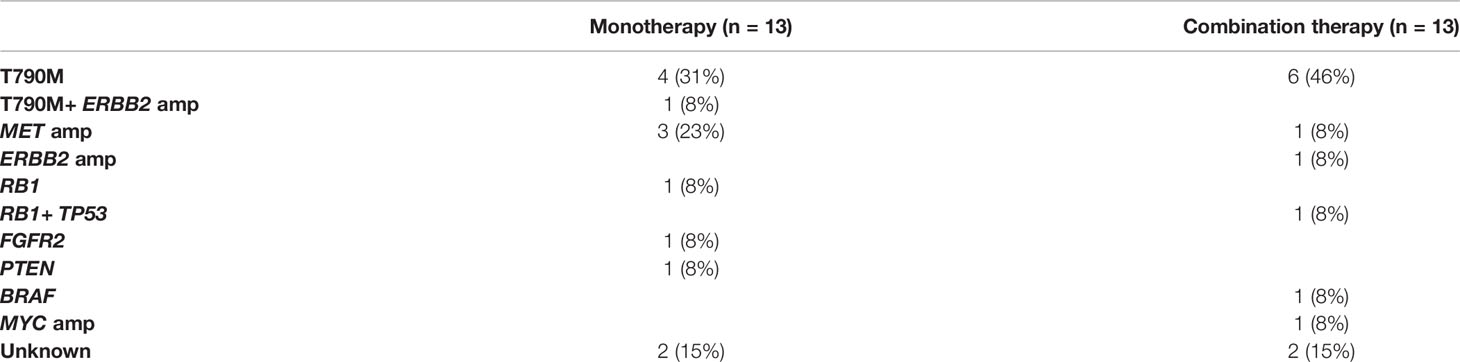
The patients who received combination therapy had significantly longer PFS than those who received monotherapy (Figure 3A; 19.1 months vs. 14.2 months, P=0.018, HR=0.598 95%Cl, 0.391-0.914). Compared with patients with EGFR 19del, patients with EGFR 21L858R tended to have shorter PFS in monotherapy group (Figure 3B; 12.5 months vs. 15.7 months, P=0.133), whereas they benefited more from combination therapy (19del: 19.0 months vs. 15.7 months, P=0.234, HR=0.709; 21L858R: 19.3 months vs. 12.5 months, P=0.046, HR=0.516).
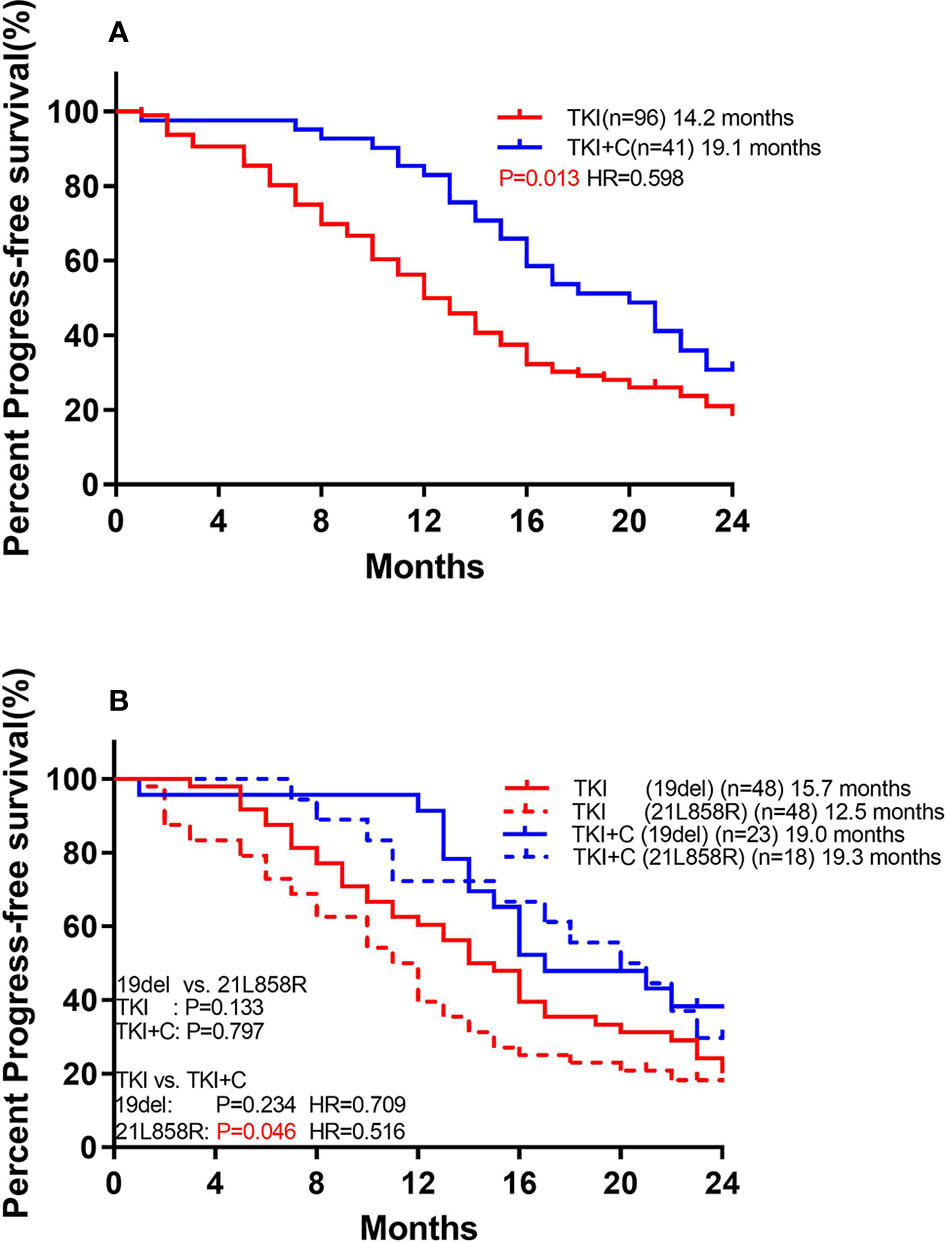
Figure 3 (A) Comparison of monotherapy and combination therapy for PFS in patients with EGFR mutation. (B) Comparison of association of EGFR subtype with PFS to monotherapy and combination therapy in patients with EGFR mutation.
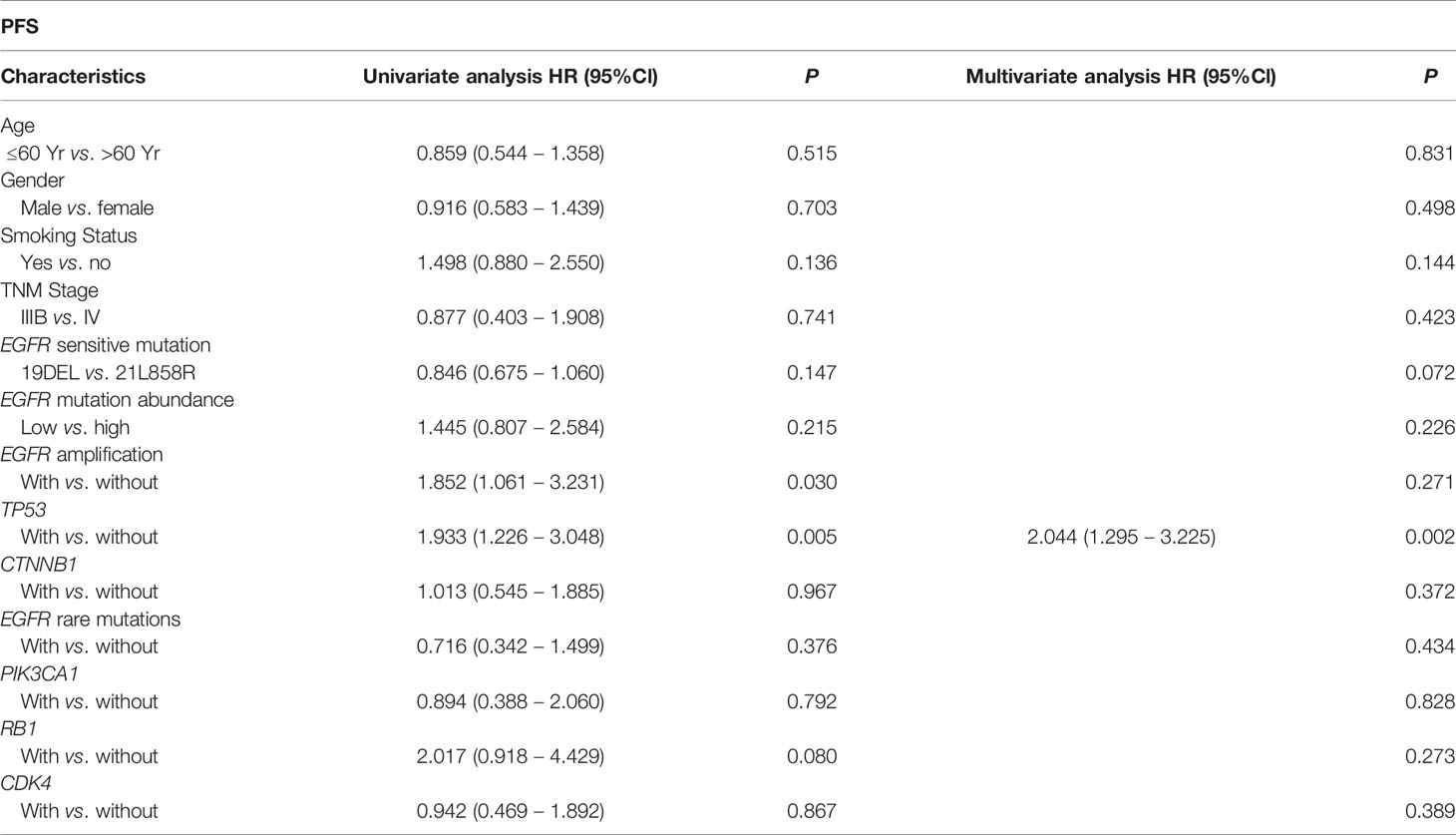
We performed univariate and multivariate analyses of baseline characteristics and high-frequency mutations (including TP53, EGFR amplification, CTNNB1, EGFR rare mutations, PIK3CA, RB1 and CDK4) in patients treated with monotherapy (Table 2). We found that concurrent TP53 mutations (HR: 2.044, 95%Cl, 1.295 to 3.225, P=0.002) had a significant effect on PFS in both analyses, while EGFR amplification only had a negative effect in univariate analysis (HR: 1.852, 95%Cl, 1.061 to 3.231, P=0.030).
We also compared the outcomes of patients with and without TP53 mutations. Of the 96 patients receiving monotherapy, those with concomitant TP53 mutation showed a significantly worse response (Figure 4A; 11.4 months vs. 16.6 months, P=0.003). However, patients with or without TP53 yielded equivalent PFS in combination therapy group (18.9 months vs. 19.1 months, P=0.552). Patients with TP53 benefited more from combination therapy (with TP53: 11.4 months vs. 19.1 months, P=0.001, HR=0.407; without TP53: 16.6 months vs. 18.9 months, P=0.379, HR=0.706).
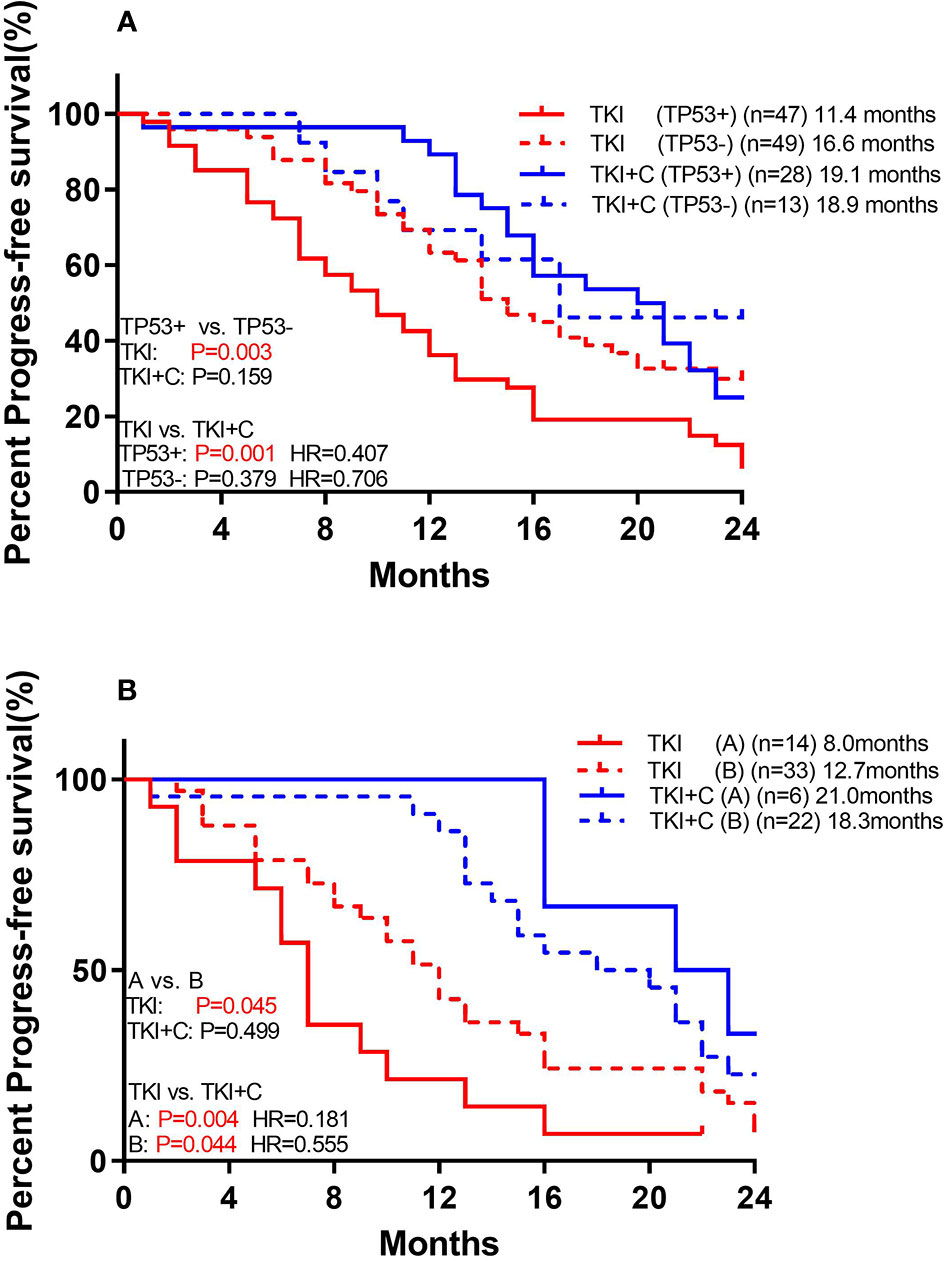
Figure 4 (A) Comparison of association of concurrent TP53 mutation with PFS to monotherapy and combination therapy in patients with EGFR mutation (B) Comparison of association of TP53 subtypes with PFS to monotherapy and combination therapy in patients with EGFR mutation. [Group (A) included exon 4 and 6. Group (B) included the remaining mutant types].
TP53 mutations were further divided into two groups. Group A included exon 4 and 6, and both of these two types of mutations had poor prognosis. Group B included the remaining mutant types which shared a good prognosis. Among monotherapy patients, PFS was significantly lower in group A than in group B (Figure 4B; 8.0 months vs. 12.7 months, P=0.045). Both groups benefited from the combination therapy, but group A benefited more (Group A: 8.0 months vs. 21.0 months, P=0.004, HR=0.181; Group B: 12.7 months vs. 18.3 months, P=0.044, HR=0.555).
Resistance Mechanisms in Monotherapy Group and Combination Therapy Group
Then, we analyzed the baseline and post-resistance samples to explore the resistance mechanisms associated with different treatments. After first-line treatment progression, in patients undergoing EGFR T790M testing, 53% (20/38) in monotherapy group and 53% (11/21) in combination therapy group were positive, which was pretty equivalent (P=0.985). Mechanisms related to resistance in 26 patients who underwent NGS again after progression were summarized in Table 3. Among them, the emergence of MET amplification at PD occurred in 3 of monotherapy group and 1 of combination therapy group. One ERBB2 amplification patient was observed in both groups.
Discussion
In our study, we retrospectively analyzed the concomitant genomic alterations of advanced LAC patients with EGFR mutations and accessed the clinical efficacy of EGFR-TKI plus chemotherapy as first-line treatment.
We found that EGFR mutations were frequently associated with other mutations, with an average of 2.66 accompanying mutations, consistent with previous reports (13). The most common accompanying mutations were TP53 (55%), EGFR amplification (23%), CTNNB1 (11%), EGFR rare mutations (10%), PIK3CA (9%), RB1 (9%), CDK4 (9%), and so on. Previous studies have found that co-mutations may activate the alternative signaling pathway or increase tumor heterogeneity, thereby affecting the efficacy of EGFR-TKIs (9, 14).
We found that TP53 mutations, especially exon 4 and 6, were associated with a markedly shorter time to progression on EGFR-TKI monotherapy, which was consistent with previous reports (8–10, 12). TP53 is a key tumor suppressor gene that can enhance sensitivity to EGFR-TKIs and radiotherapy by inducing cell-cycle arrest, apoptosis, and repair of DNA damage (15). The complete loss of TP53 function, mainly manifested as single-base substitution and loss of alleles, can catalyze the transformation potential of oncogene drivers in lung cancer and inhibit tumor response to chemotherapy, radiotherapy and EGFR-TKIs (15, 16). However, in the combination therapy group, patients with TP53 also showed a good response, and there was no significant difference in PFS compared with patients without TP53. This means that the combination of EGFR-TKI and chemotherapy will benefit patients with concurrent TP53 mutations more.
Several previous studies reported that EGFR amplification in EGFR-mutant patients was associated with a longer PFS in TKI treatment (17, 18). In our study, EGFR amplification was a risk factor for PFS in univariate analysis but not in multivariate analysis, possibly because it was mainly accompanied by TP53.
In addition, many studies have shown that patients with EGFR 21L858R mutation do not respond as well to EGFR-TKI as patients with EGFR 19del mutation (19, 20). This may be attributed to the different intrinsic sensitivity of the two mutations to EGFR-TKIs (21). This trend was also observed in our study, in which patients with 21L858R had shorter PFS than those with 19del, but they benefited more from combination therapy.
EGFR-TKI combined with chemotherapy has been reported in a large number of prospective studies to delay resistance (5–7). This combination therapy was found to induce cell apoptosis and inhibit Akt and extracellular signal-regulated kinase phosphorylation (22), and EGFR-TKIs could reduce the level of thymidine synthase to improve the efficacy of pemetrexate (23). What’s more, the proportions of patients with T790M positive after progression were similar in the combination therapy and monotherapy group, which meant that the majority of patients with the first-line combination therapy could be successfully treated with the sequential therapy of osimertinib. However, in addition to excellent effect, clinically relevant grade ≥ 3 toxicity in the combination therapy group were doubled (5, 6). There may be more patients over the age of 75 with a high ECOG-PS score in the clinical course, so we need to identify patients who would benefit more from the combination therapy. In our study, we found that patients with 21L858R or coexisting TP53 mutations did not respond well to monotherapy, but benefited more from combination therapy. In addition, FLAURA (NCT02296125) showed osimertinib as first-line treatment yielded more benefits than first-generation EGFR-TKIs, providing an alternative option for patients with EGFR mutations (24, 25).
Our study has the following limitations. First, as a retrospective study, we failed to compare the adverse effects of different treatments due to incomplete records. Second, although there was no significant difference in baseline characteristics among patients receiving different treatments, we recognized the existence of selection bias that patients with comorbidities were more likely to be recommended for monotherapy. Third, the mechanism by which combination therapy benefits patients with TP53 mutations remains unclear and needs further study.
In summary, we retrospectively analyzed genomic changes in patients with advanced lung adenocarcinoma with sensitizing EGFR mutations and found that TP53 was the most frequent concurrent mutations. Grouped by next-generation sequencing results, we compared the efficacy of monotherapy versus combination therapy. We found that patients with 21L858R mutation or concurrent TP53 mutations did not respond well to EGFR-TKIs alone, but benefited more from EGFR-TKIs plus chemotherapy. In the future clinical treatment process, we should consider to stratify patients according to their EGFR subtype and concurrent mutations, and develop more targeted treatment programs.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by The institutional review board of Shanghai Chest Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZY, YC, and YW both have substantial contributions to the conception or design of the work, the collection and analysis of data, the writing and edit of the article. The rest authors have given substantial contributions to the work by providing editing and writing assistance. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the foundation of Shanghai Chest Hospital (Project No. YJXT20190102); the program of system biomedicine innovation center from Shanghai Jiao Tong University (Project No. 15ZH4009); Shanghai Jiao Tong University School of Medicine (Project No. 15ZH1008) and the foundation of Chinese society of clinical oncology (Project No. Y2019AZZD-0355).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all of the patients and their families for their contributions to this study. The institutional review board of Shanghai Chest Hospital approved our study, and all patients provided written consent before surgery and clinical treatment.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.681429/full#supplementary-material
Supplementary Table 1 | Sixty-eight cancer-related Genes sequenced in our panel.
References
1. Mitsudomi T, Kosaka T, Endoh H, Horio Y, Hida T, Mori S, et al. Mutations of the Epidermal Growth Factor Receptor Gene Predict Prolonged Survival After Gefitinib Treatment in Patients With Non-Small-Cell Lung Cancer With Postoperative Recurrence. J Clin Oncol (2005) 23(11):2513–20. doi: 10.1200/JCO.2005.00.992
2. Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for Epidermal Growth Factor Receptor Mutations in Lung Cancer. N Engl J Med (2009) 361(10):958–67. doi: 10.1056/NEJMoa0904554
3. Gao G, Ren S, Li A, Xu J, Xu Q, Su C, et al. Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor Therapy is Effective as First-Line Treatment of Advanced Non-Small-Cell Lung Cancer With Mutated EGFR: A Meta-Analysis From Six Phase III Randomized Controlled Trials. Int J Cancer (2012) 131(5):E822–9. doi: 10.1002/ijc.27396
4. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib Versus Cisplatin Plus Docetaxel in Patients With non-Small-Cell Lung Cancer Harbouring Mutations of the Epidermal Growth Factor Receptor (WJTOG3405): An Open Label, Randomised Phase 3 Trial. Lancet Oncol (2010) 11(2):121–8. doi: 10.1016/s1470-2045(09)70364-x
5. Hosomi Y, Morita S, Sugawara S, Kato T, Fukuhara T, Gemma A, et al. Gefitinib Alone Versus Gefitinib Plus Chemotherapy for Non-Small-Cell Lung Cancer With Mutated Epidermal Growth Factor Receptor: NEJ009 Study. J Clin Oncol (2020) 38(2):115–23. doi: 10.1200/JCO.19.01488
6. Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, et al. Gefitinib Versus Gefitinib Plus Pemetrexed and Carboplatin Chemotherapy in EGFR-Mutated Lung Cancer. J Clin Oncol (2020) 38(2):124–36. doi: 10.1200/JCO.19.01154
7. Yang JC, Cheng Y, Murakami H, Yang PC, He J, Nakagawa K, et al. A Randomized Phase 2 Study of Gefitinib With or Without Pemetrexed as First-Line Treatment in Nonsquamous NSCLC With EGFR Mutation: Final Overall Survival and Biomarker Analysis. J Thorac Oncol (2020) 15(1):91–100. doi: 10.1016/j.jtho.2019.09.008
8. Labbe C, Cabanero M, Korpanty GJ, Tomasini P, Doherty MK, Mascaux C, et al. Prognostic and Predictive Effects of TP53 Co-Mutation in Patients With EGFR-Mutated Non-Small Cell Lung Cancer (NSCLC). Lung Cancer (2017) 111:23–9. doi: 10.1016/j.lungcan.2017.06.014
9. VanderLaan PA, Rangachari D, Mockus SM, Spotlow V, Reddi HV, Malcolm J, et al. Mutations in TP53, PIK3CA, PTEN and Other Genes in EGFR Mutated Lung Cancers: Correlation With Clinical Outcomes. Lung Cancer (2017) 106:17–21. doi: 10.1016/j.lungcan.2017.01.011
10. Cheng Y, Ma L, Liu Y, Zhu J, Xin Y, Liu X, et al. Comprehensive Characterization and Clinical Impact of Concomitant Genomic Alterations in EGFR-Mutant NSCLCs Treated With EGFR Kinase Inhibitors. Lung Cancer (2020) 145:63–70. doi: 10.1016/j.lungcan.2020.04.004
11. Zhao Y, Dong Y, Zhao R, Zhang B, Wang S, Zhang L, et al. Expression Profiling of Driver Genes in Female Never-Smokers With Non-Adenocarcinoma Non-Small-Cell Lung Cancer in China. Clin Lung Cancer (2020) 21(5):e355–e62. doi: 10.1016/j.cllc.2020.02.005
12. Jiao X-D, Qin B-D, You P, Cai J, Zang Y-S. The Prognostic Value of TP53 and its Correlation With EGFR Mutation in Advanced Non-Small Cell Lung Cancer, an Analysis Based on Cbioportal Data Base. Lung Cancer (2018) 123:70–5. doi: 10.1016/j.lungcan.2018.07.003
13. Yu HA, Suzawa K, Jordan E, Zehir A, Ni A, Kim R, et al. Concurrent Alterations in EGFR-Mutant Lung Cancers Associated With Resistance to EGFR Kinase Inhibitors and Characterization of mTOR as a Mediator of Resistance. Clin Cancer Res (2018) 24(13):3108–18. doi: 10.1158/1078-0432.CCR-17-2961
14. Chen M, Xu Y, Zhao J, Zhong W, Zhang L, Bi Y, et al. Concurrent Driver Gene Mutations as Negative Predictive Factors in Epidermal Growth Factor Receptor-Positive Non-Small Cell Lung Cancer. EBioMedicine (2019) 42:304–10. doi: 10.1016/j.ebiom.2019.03.023
15. Huang S, Benavente S, Armstrong EA, Li C, Wheeler DL, Harari PM. P53 Modulates Acquired Resistance to EGFR Inhibitors and Radiation. Cancer Res (2011) 71(22):7071–9. doi: 10.1158/0008-5472.CAN-11-0128
16. Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T, et al. A Murine Lung Cancer Co-Clinical Trial Identifies Genetic Modifiers of Therapeutic Response. Nature (2012) 483(7391):613–7. doi: 10.1038/nature10937
17. Shan L, Wang Z, Guo L, Sun H, Qiu T, Ling Y, et al. Concurrence of EGFR Amplification and Sensitizing Mutations Indicate a Better Survival Benefit From EGFR-TKI Therapy in Lung Adenocarcinoma Patients. Lung Cancer (2015) 89(3):337–42. doi: 10.1016/j.lungcan.2015.06.008
18. Ruiz-Patino A, Castro CD, Ricaurte LM, Cardona AF, Rojas L, Zatarain-Barron ZL, et al. EGFR Amplification and Sensitizing Mutations Correlate With Survival in Lung Adenocarcinoma Patients Treated With Erlotinib (MutP-CLICaP). Target Oncol (2018) 13(5):621–9. doi: 10.1007/s11523-018-0594-x
19. Wang H, Huang J, Yu X, Han S, Yan X, Sun S, et al. Different Efficacy of EGFR Tyrosine Kinase Inhibitors and Prognosis in Patients With Subtypes of EGFR-Mutated Advanced Non-Small Cell Lung Cancer: A Meta-Analysis. J Cancer Res Clin Oncol (2014) 140(11):1901–9. doi: 10.1007/s00432-014-1709-0
20. Jackman DM, Yeap BY, Sequist LV, Lindeman N, Holmes AJ, Joshi VA, et al. Exon 19 Deletion Mutations of Epidermal Growth Factor Receptor Are Associated With Prolonged Survival in Non-Small Cell Lung Cancer Patients Treated With Gefitinib or Erlotinib. Clin Cancer Res (2006) 12(13):3908–14. doi: 10.1158/1078-0432.CCR-06-0462
21. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR Mutations in Lung Cancer: Correlation With Clinical Response to Gefitinib Therapy. Science (2004) 304(5676):1497–500. doi: 10.1126/science.1099314
22. Yang Z, Tam KY. Combination Strategies Using EGFR-TKi in NSCLC Therapy: Learning From the Gap Between Pre-Clinical Results and Clinical Outcomes. Int J Biol Sci (2018) 14(2):204–16. doi: 10.7150/ijbs.22955
23. Galvani E, Peters GJ, Giovannetti E. Thymidylate Synthase Inhibitors for non-Small Cell Lung Cancer. Expert Opin Investig Drugs (2011) 20(10):1343–56. doi: 10.1517/13543784.2011.617742
24. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378(2):113–25. doi: 10.1056/NEJMoa1713137
Keywords: epidermal growth factor receptor, tyrosine kinase inhibitors, next-generation sequencing, TP53, co-mutations
Citation: Yang Z, Chen Y, Wang Y, Wang S, Hu M, Zhang B and Han B (2021) Efficacy of EGFR-TKI Plus Chemotherapy or Monotherapy as First-Line Treatment for Advanced EGFR-Mutant Lung Adenocarcinoma Patients With Co-Mutations. Front. Oncol. 11:681429. doi: 10.3389/fonc.2021.681429
Received: 16 March 2021; Accepted: 27 July 2021;
Published: 16 August 2021.
Edited by:
Hideharu Kimura, Kanazawa University, JapanReviewed by:
Sagun Parakh, University of Melbourne, AustraliaShuhang Wang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2021 Yang, Chen, Wang, Wang, Hu, Zhang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baohui Han, 18930858216@163.com; Bo Zhang, zb1063253078@163.com
†These authors have contributed equally to this work
 Zhengyu Yang†
Zhengyu Yang†