
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 03 June 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.672222
This article is part of the Research Topic Chemo-resistance in Gastrointestinal Cancers View all 12 articles
 Xiaojie Liu1,2,3
Xiaojie Liu1,2,3 Mingjing He1,2,3
Mingjing He1,2,3 Linlin Li1,2,3
Linlin Li1,2,3 Xiya Wang1,2,3
Xiya Wang1,2,3 Shuhua Han1,2,3
Shuhua Han1,2,3 Jinzhu Zhao1,2,3
Jinzhu Zhao1,2,3 Yalong Dong1,2,3
Yalong Dong1,2,3 Mushtaq Ahmad1,2,3
Mushtaq Ahmad1,2,3 Leilei Li1,2,3
Leilei Li1,2,3 Xueyan Zhang1,2,3
Xueyan Zhang1,2,3 Junfeng Huo1,2,3
Junfeng Huo1,2,3 Yunfan Liu1
Yunfan Liu1 Chengxue Pan1,3*
Chengxue Pan1,3* Cong Wang1,2,3*
Cong Wang1,2,3*Drug resistance often occurs after chemotherapy in esophageal cancer patients, leading to cancer metastasis and recurrence. However, the relationship among cancer cell migration, recurrence and drug resistance in esophageal cancer drug-resistant cells has not been clearly explained. In this study, we constructed paclitaxel (PTX)-resistant esophageal cancer cells to explore the causes of drug resistance and poor prognosis after chemotherapy in esophageal cancer. Colony formation assay was used to evaluate the difference of colony formation between parental cells and drug resistance cells. Microsphere formation assay was used to examine the phenotype of stem cells. Wound healing and Transwell assays were used to detect the migration ability of drug-resistant cells. Western blotting and immunofluorescence assays were used to explore the mechanisms. Finally, we used nude mouse xenograft model to explore the tumor characteristics and the expression of relative proteins to verify our findings in vivo. Our study demonstrated that the cancer cell stemness characteristics exist in drug-resistant esophageal cancer cells, that expressed the biomarkers of stem cells and were prone to epithelial-mesenchymal transition (EMT). Our results suggested that the expression of EMT biomarkers and stemness-related proteins increased in esophageal cancer cells after continuously using chemotherapeutic drugs for a period of time. This study indicated that simultaneously targeting EMT and stemness could be a better strategy for the treatment of esophageal cancer drug resistance.
Esophageal cancer is one of the most common human digestive tract cancers and ranked as the sixth leading cause of cancer-related death worldwide (1). The 5-year overall survival rate of patients with advanced esophageal cancer is less than 10% (2). Esophageal squamous cell carcinoma (ESCC), as the predominant histologic type in China, seriously endangering people’s health (3). Surgical treatment, radiation therapy, chemotherapy, targeted therapy, and immunotherapy are accepted treatment options for patients. Among them, chemotherapy plays a dominant role. Chemotherapeutic agents, such as paclitaxel (PTX), are used widely for the treatment of advanced human cancers. However, long-term application of chemotherapeutic agents often leads to drug resistance even they are effective initially (4). In particular, tumor cells are more prone to invasion, metastasis, and recurrence after cancers develop chemotherapeutic resistance, leading to poor prognosis.
Cancer stem cells (CSCs) refer to a group of tumor cells with self-renewal capacity and differentiation potential, which can re-initiate tumor formation (5, 6). Studies have shown that the increase of recognizable stemness-related biomarkers in tumor cells is associated with driving the proliferation of tumor cells, the resistance of treatment and the recurrence of cancers (7–10). Previous studies have shown that CSCs exist in esophageal cancer (11). Although there is less consensus on biomarkers of CSCs in esophageal cancer so far, it has been determined that the poor prognosis of esophageal cancer patients is closely related to CSCs (12, 13).
CSCs also showed the tendency to invade and metastasize (14, 15). Epithelial-mesenchymal transition (EMT) plays a significant role in cancer metastasis and invasion (16). EMT, which transforms polarized epithelial cells to a motile mesenchymal phenotype, plays a primary role in morphologic changes in various physiological processes (17). A number of studies have shown that EMT contributes to the early spread of cancer cells, which is often activated during tumor invasion and metastasis (18). EMT is closely associated with poor prognosis in multiple types of cancers, including prostate cancer (19), breast cancer (20), lung cancer (21), hepatocellular carcinoma (22), and other cancers.
In this study, we investigate the association of cancer cell stemness along with EMT characteristics in drug-resistant esophageal cancer cells.
Paclitaxel was purchased from Hainan Quanxing Pharmaceutical Co., Ltd. (Hainan, China). MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide) was purchased from Solarbio (Beijing, China). DMSO (Dimethyl sulfoxide) was acquired from Solarbio (Shanghai, China). Hoechst 33258 was purchased from Beyotime Biotechnology (Shanghai, China). Primary antibodies against ZO-1, Claudin-1, E-cadherin, β-catenin, Vimentin, N-cadherin, NANOG, SOX2, and OCT4 were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Human esophageal cancer cell lines TE-1 was purchased from the Chinese Academy of Sciences (Shanghai, China), EC109 was purchased from the Institute of Basic Medical Sciences Chinese Academy of Medical Sciences & School of Basic Medicine Peking Union Medical College (Beijing, China). Esophageal cancer cell lines resistant to paclitaxel (TE-1/PTX and EC109/PTX) were established in our team according to the method of LIU-BIN GUO (23). All the cell lines were cultured in Roswell Park Memorial Institute (RPMI) 1640 (Biological Industries, Kibbutz Beit HaEmek, Israe) with 10% fetal bovine serum (FBS, purchased from Biological Industries, Kibbutz Beit HaEmek, Israel). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Cell sensitivity to PTX was examined by MTT assay. Briefly, cells were seeded in the 96-well plate at a density of 4.5 × 103 cells per well and incubated overnight. After that, the medium was changed to fresh medium containing various concentrations of PTX. After 72 h, 20 μl MTT was added to each well and cells were incubated for 4 h with MTT at the concentration of 500 mg/ml. Then, the precipitate was dissolved completely by 150 μl DMSO and the light absorbance was measured at 570 nm using the Multiskan Spectrum spectrophotometer (BioTek Instruments, Inc. Vermont, USA).
Cells were seeded in six-well plate overnight and a sterile 100 μl pipette tip was used to scraped off a part of cells in each well. After that, plates were washed twice with PBS buffer solution to remove the detached cells. Subsequently, cells were incubated in culture medium with 2% FBS. The wound gaps were acquired by a microscope connected to a digital camera. After 24 and 48 h incubation, the wound gaps were acquired again, and the migration rates were evaluated.
Transwell assays were performed by Transwell chamber (Corning Life Sciences, NY, USA) in a 24-well plate. About 1 × 104 EC109 and EC109/PTX cells and 1 × 103 TE-1 and TE-1/PTX cells were added in the upper chamber in 300 μl culture medium with 0% FBS and 600 μl culture medium with 20% FBS was placed in the lower chamber. The cells were incubated at 37°C with 5% CO2 for 24 h. At the end, the cells were stained by 1% crystal violet solution and imaged under a Nikon microscope.
1 × 103 cells were seeded per well in 6-wells plate with culture medium with 10% FBS. The cells were incubated for 10 days. After that, the cells were washed three times with PBS and fixed in methanol for 30 min. Cells were stained with 1% crystal violet for 30 min. Then, the cells were washed with PBS at least five times.
200 cells of EC109, EC109/PTX, and TE-1, TE-1/PTX were cultured in serum-free medium DMEM/F12 containing supplement 2% B27 (Gibco, USA) and 0.1% growth factor EGF (Gibco, USA) in the ultra-low adsorption 96-well plate. Take photos every 24 h for a week under the microscope. The sphere numbers (diameter ≥50 μm) were calculated.
Limit dilution assay (LDA) was used to select the tight cloning of cells. 30 to 50 cells were seeded in the 6-well plates. After 2 to 3 weeks, the cells of tight cloning were selected under microscope. Cells were suspended, counted, and cultured in the medium of cancer stem cells (98 ml DMEM/F12 + 2 μg EGF + 2 μg bFGF + 275 2 μl insulin + 2 ml B27) in the ultra-low adsorption 24-well plate. 10 days later, take photos under the microscope.
Cells were lysed on ice by RIPA lysis buffer containing phosphatase inhibitor cocktail and 1% protease for 30 min. Then, the lysates of the cells were centrifuged at 12,000 rpm for 10 min at 4°C and supernatant liquid were collected. The lysates were measured for protein concentration with a BCA protein assay kit. Total protein was resolved in 10% polyacrylamide gel by SDS-PAGE and transferred to polyvinylidene difluoride membranes. The nonspecific binding was blocked by 5% non-fat milk. The membranes were incubated with primary antibodies overnight at 4°C and then incubated with the secondary antibodies for 2 h at room temperature. The resolved protein bands were visualized by enhanced chemiluminescence (ECL) detection system. The densitometric analysis were performed using Image J software.
The cells were seeded in 24-well plates. After incubating overnight, the cells were fixed with methanol, permeabilized 10 min with 0.1% Triton X-100, blocked 1 h with 5% BSA and incubated with the primary antibodies (SOX2 1: 200, NANOG 1: 200, OCT4 1: 200) at 4°C overnight. After that, the cells were washed with PBS and incubated 1 h with Alexa Fluor 561 goat anti-rabbit IgG and Alexa Fluor 488 goat anti- rabbit IgG at room temperature. The nuclei of the cells were counterstained 20 min with 10 μg/ml of Hoechst 33258 in the dark. Images were acquired by laser scanning confocal microscopy (Nikon, A1, Tokyo, Japan). Target protein staining was presented in red (TE-1 and TE-1/PTX) and green (EC109 and EC109/PTX), and the nuclei were stained with Hoechst 33258 (blue).
Animal experiments were approved by the Institutional Animal Care and Use Committee of the Zhengzhou University. Approximately 5-week-old female nude mice were purchased from Hunan SJA Animal Laboratory Co. Ltd. The xenograft tumor model of nude mice was established. Briefly, 3 × 106 EC109 cells and EC109/PTX cells were subcutaneously implanted into the right scapula of the mice. The volume of the tumors and the weight of the mice were measured every 2 days. Tumor size were calculated using the formula (A×B×B)/2 (A was the longest diameter and B was the shortest diameter of the tumor). The mice were sacrificed after 20 days. Then, tumors were dissected and weighed. Besides, the whole blood and the serum were obtained before the mice were sacrificed.
Tumor tissues were embedded in paraffin, and the paraffin-embedded tissue was cut into 4- to 5-μm slices with a microtome. The slices were pasted on clean slides with ultrapure water, heated slightly with an alcohol lamp to make them flat. After that the paraffin slices were soaked in xylene for 20 min, they were soaked in gradient alcohol, soaked in ultrapure water for 5 min, then, soaked in PBS 20 min twice to carry out dewaxing and hydration. The slices were stained in hematoxylin staining solution for 2 min, rinsed with tap water and put into 1% alcoholic hydrochloric acid to decolorize and 1% ammonia solution to turn blue, and the nuclear staining was observed under a microscope, followed by putting into eosin staining solution for 1 min, tap water rinse and the staining was observed under the microscope. Before covering the slices, slides were dehydrated in gradient alcohol and cleared in xylene. They were sealed with neutral resin. The slices were dried and photographed using the Leica DM 3000 microscope at 200× magnification.
All the experiments were independently repeated three times. Data were shown as mean ± SD. Statistical analysis was performed using one-way ANOVA, using the GraphPad Prism 8.0. All comparisons were made relative to controls and significance of difference was indicated as * P < 0.05 and **P < 0.01.
EC109 and TE-1 were treated with different concentrations of PTX for 72 h, and MTT assay was applied to evaluate the sensitivity of cells to PTX. The results showed that the IC50 value is about 8.69 nmol and 93.88 nmol in the EC109 and its drug-resistant cells (EC109/PTX), respectively. The PTX-resistant index is 10.80. The IC50 value is about 5.47 nmol and 56.84 nmol in the TE-1 and its drug-resistant cells (TE-1/PTX), respectively. The PTX-resistant index is 10.38 (Figure 1A and Table 1). This result indicated that EC109/PTX and TE-1/PTX exhibited moderate drug resistance. Besides, we applied Cis-platinum and 5-Fluorouracil as the other drug sensitivity results upon PTX-resistant esophageal cancer cells. As a result, the Cis-platinum-resistant index is 2.36 (TE-1/PTX) and 1.54 (EC109/PTX), showed as mild drug resistance. The 5-Fluorouracil-resistant index is 3.10 (TE-1/PTX) and 9.93 (EC109/PTX), showed as mild drug resistance (TE-1/PTX) and moderate drug resistance (EC109/PTX). In other words, the resistant cells we stimulated were not only more resistant to the inducible drug PTX than the other therapeutic drugs but also showed different degrees of resistance to other therapeutic drugs (Figure 1B and Table 2). Morphologically, EC109 and TE-1 had regular form and uniform size, in contrast, EC109/PTX and TE-1/PTX lost their original shape and became elongated (Figure 1C), indicating that the cells changed after they gained drug resistance. The colony formation ability of the parental cells was significantly higher than that of the drug-resistant cancer cells in the colony formation assay. While when the parental cells and resistant cells were treated with PTX solvent as control, the colony formation ability of the parental cells was significantly lower than that of the drug-resistant cancer cells. This result further suggested that the drug-resistant cells were more resistant to paclitaxel than the parental cells (Figures 1D, E).
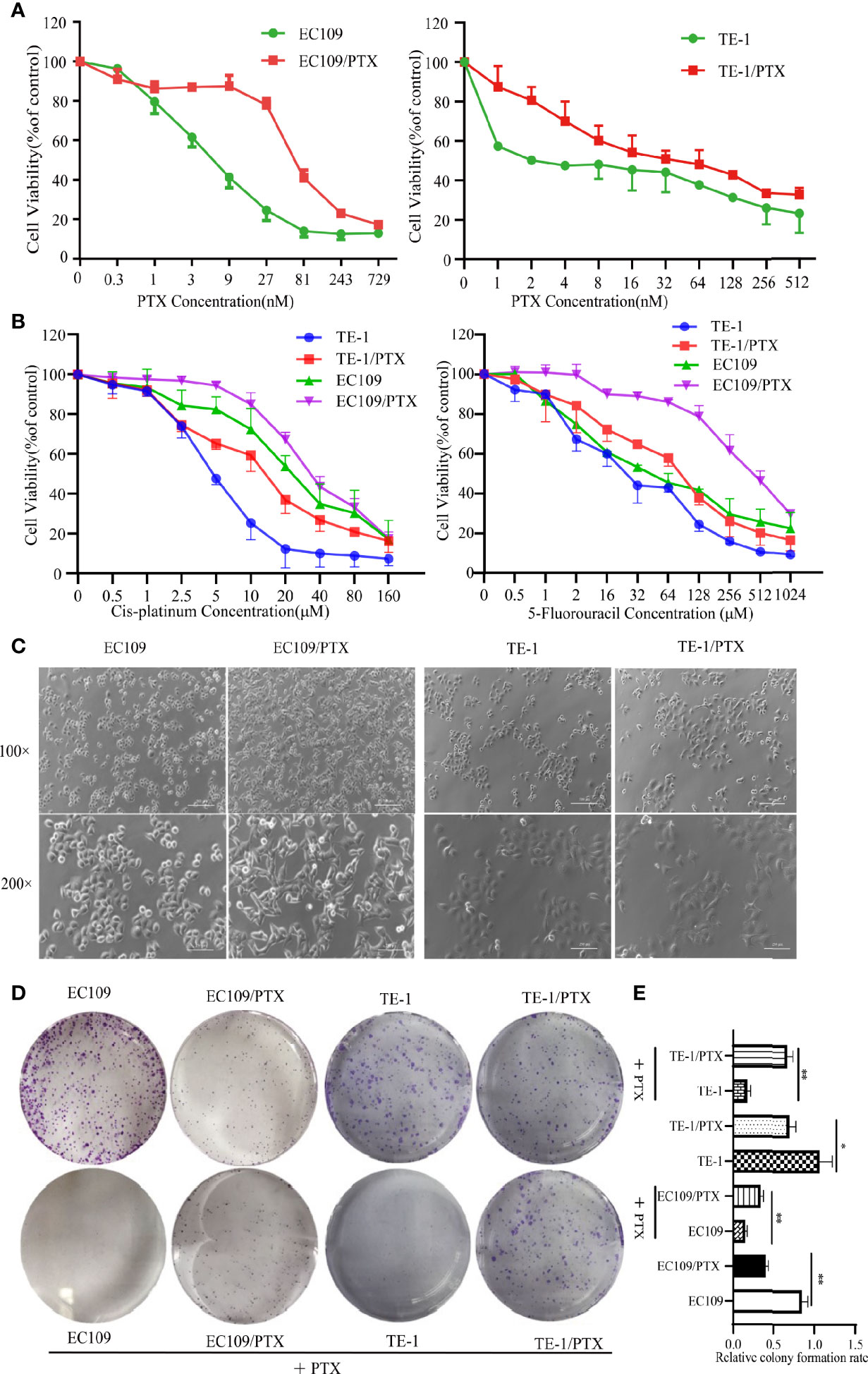
Figure 1 PTX-resistant EC109 and TE-1cells. (A, B) MTT assay on the survival of parental cells and PTX-resistant cells under the treatment with PTX, Cis-platinum and 5-Fluorouracil. (C) Cell morphology photographed under an inverted microscope. Scale bar: 500 µm (100×), 250 µm (200×). (D) Colony formation assay on the two groups of cells and the corresponding PTX solvent as control. (E) The corresponding statistical results of colony formation. *P < 0.05 indicates statistically significance vs. parental cells. **P < 0.01 indicates highly statistically significant vs. parental cells.
The microsphere formation assay was used to test the property of cancer cell stemness, and the results showed that the microsphere formation rate in EC109/PTX and TE-1/PTX is 1.63-fold and 3.35-fold greater than their parental cells respectively (Figures 2A, B). Besides, we used LDA to repeat sphere formation. The results showed that the microsphere formation rate in EC109/PTX and TE-1/PTX was greater than their parental cells respectively. Also, in general, the microsphere in EC109/PTX and TE-1/PTX was bigger than that in their parental cells, respectively (Figures 2C, D). These results indicated that the self-renewal ability of drug-resistant cells is stronger than the parental cells. Western blotting assay was used to examine the expression of related proteins, and the result showed that the expression of embryonic stem cell transcription factors NANOG, OCT4, and SOX2 enhanced in the drug-resistant cell lines (TE-1/PTX and EC109/PTX) (Figures 2E, F). The immunofluorescence analysis showed similar results as the Western blotting (Figures 2G, H).
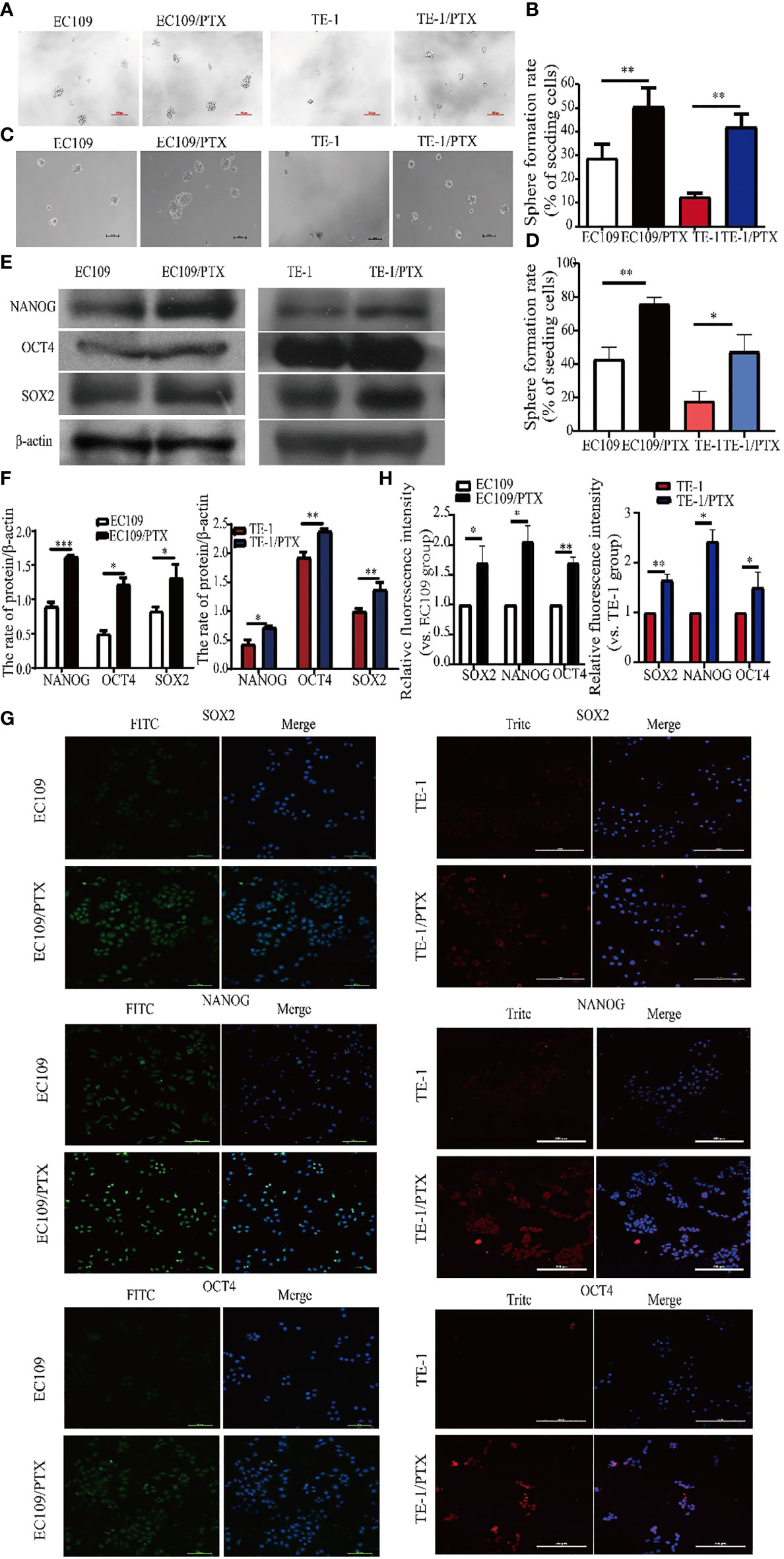
Figure 2 Enhanced cancer cell stemness in the drug-resistant cells. (A, B) Sphere formation assay on the two groups of cells and the corresponding statistical results. Scale bar: 100 µm. (C, D) LDA-Sphere formation assay on the two groups of cells and the corresponding statistical results. Scale bar: 100 µm. (E, F) Western blotting on the stem cell protein biomarkers and the corresponding statistical results. (G, H) Immunofluorescence analysis detected the expression of stem cell protein biomarkers NANOG, OCT4 and SOX2 in the two groups of cells. Scale bar: 100 µm (TE-1, TE-1/PTX), 200 µm (EC109, EC109/PTX). *P < 0.05 indicates statistically significance vs. parental cells. **P < 0.01 indicates highly statistically significant vs. parental cells.
Wound healing and Transwell assays were used to investigate the difference of cell migration ability in the drug sensitive and -resistant cells. The results of the wound healing assay indicated that after culturing the cells for 24 and 48 h, the size of the healed space in EC109 and TE-1 cells is 1.875-fold and 1.64-fold greater than that of EC109/PTX and TE-1/PTX cells (Figures 3A, B). The results of the Transwell assay indicated that the number of the migrated EC109/PTX and TE-1/PTX cells was 4.2-fold and 2.3-fold of that of EC109 and TE-1 cells (Figures 3C, D). These results suggested that once the cells gained the drug-resistant property, they have stronger ability of migration and invasion than their parental cells. Western blotting assay was used to explore the expression of EMT-related proteins. The results showed that the expression of the epithelial protein biomarkers Claudin-1, ZO-1, and E-cadherin were decreased in the drug-resistant cells, while the expression of the mesenchymal protein biomarkers Vimentin, N-cadherin, and the transcription factor β-catenin were increased (Figures 3E–G).
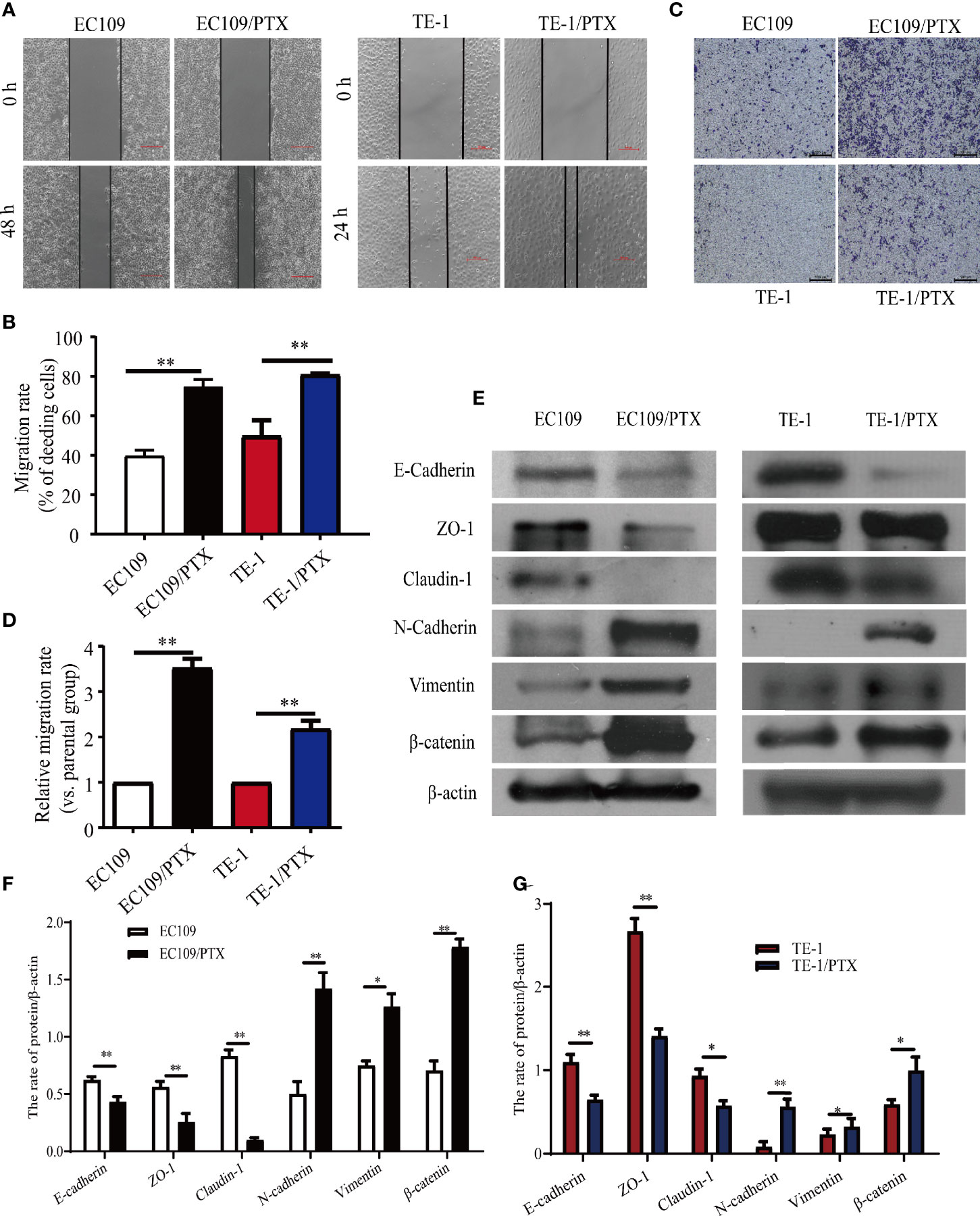
Figure 3 The migratory ability enhanced and the expression of EMT biomarkers changed in the PTX-treated cells. (A, B) Wound healing assay was used for the migration ability of the two groups of the cells and the corresponding statistical results. Scale bar: 500 µm. (C, D) Transwell assay on the two groups of the cells and the corresponding statistical results. Scale bar: 500 µm. (E–G) Western blotting evaluated the representative proteins of EMT and the corresponding statistical results. *P < 0.05 indicates statistically significance vs. parental cells. **P < 0.01 indicates highly statistically significant vs. parental cells.
To further explore the EMT and stemness in the drug-resistant esophageal cancer, we applied the xenograft tumor model in nude mice. It was found that, there is hardly any difference in the Blood Biochemical Index and Serum Biochemical Indices(ALT, AST and creatinine), except the uric acid and white blood cells. The level of Uric acid in the drug-resistant cells group is lower than that in the parental cells group, but both of them are in the normal range. It was also found that there was no significant difference in liver and kidney function between the mice in the parental cells group and the drug-resistant cells group. The difference in the white blood cells exist in the PTX -resistant cells group, and might due to the bone marrow inhibition, causing decrease of white blood cells (Tables 3, 4). The mice were sacrificed when we finished collecting blood from the eyeball. The average volume and the tumor weight of the in the mice of implanting EC109/PTX were smaller than the parental cells-group while the body weight was minimally affected (Figures 4A–D). The expression of EMT- and stemness-related proteins was analyzed. It was found that except E-cadherin, the expression of epithelial protein biomarkers ZO-1 and Claudin-1 were decreased in the group of EC109/PTX cells, while the expression of mesenchymal protein biomarkers Vimentin, N-cadherin, and the transcription factor TCF-8 and β-catenin were increased. The expression stemness-related proteins SOX2 and NANOG enhanced (Figures 4E, F). Besides, E-cadherin is related to proliferation closely and the volume of tumor tissue in the parent group increased significantly compared with the drug-resistant group in vivo. The expression of E-cadherin enhanced in vivo, which may be caused by the xenograft tumor model. In addition, hematoxylin-eosin staining was performed in tumor tissues (Figure 4G). These in vivo results showed that once the tumor cells became drug resistant, the expression of the CSCs biomarkers and the EMT biomarkers enhanced. All the results were in consistent with the results in vitro.
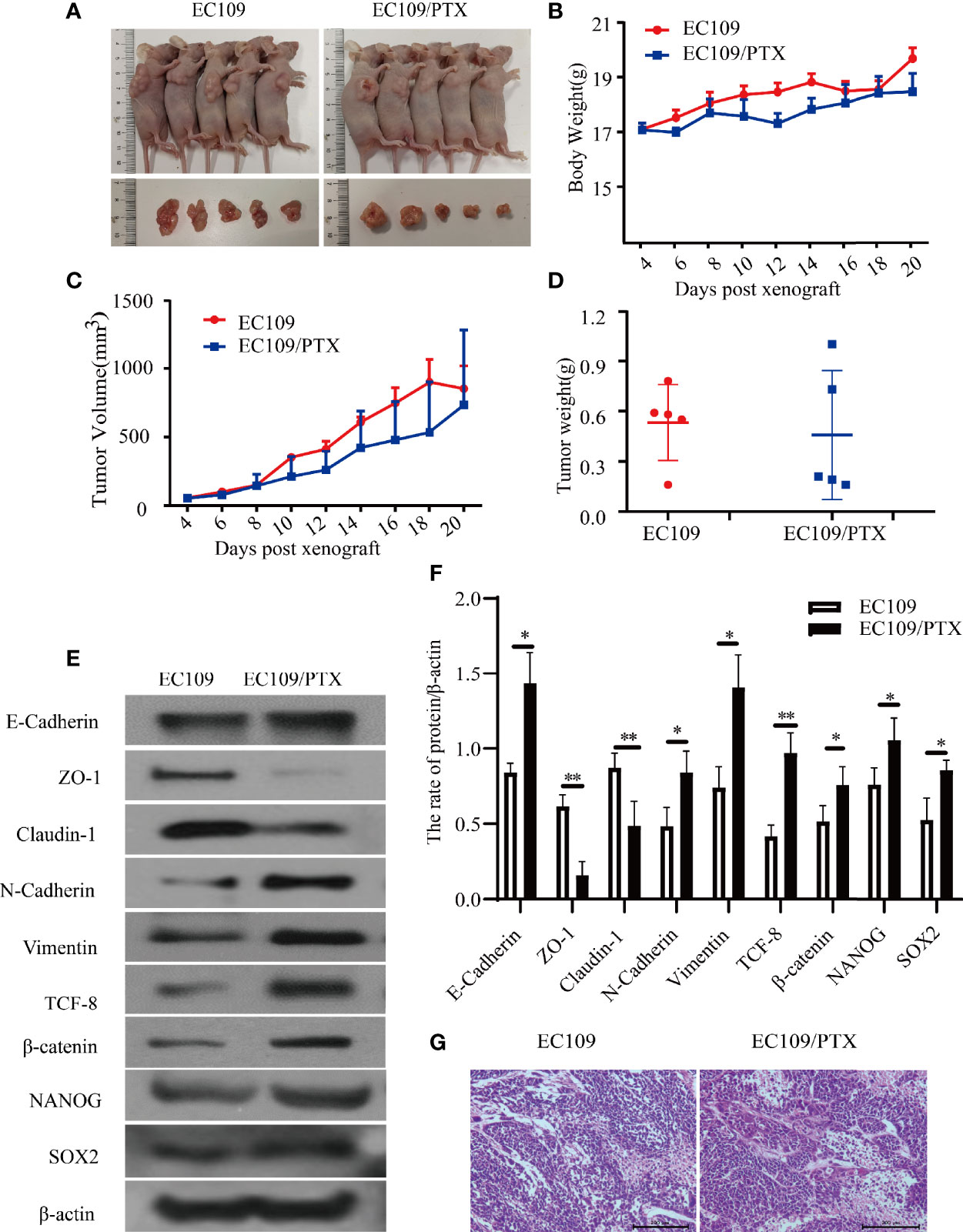
Figure 4 EC109 and EC109/PTX cell growth in vivo and the expression of EMT-related and stemness-related proteins. (A) The mice and tumor tissues of the two groups after implanting tumor cells for 20 days. (B–D) The body weight, tumor volume and tumor weight of the mice and tumor. (E, F) Western blotting for the expression of the EMT-related and stemness-related proteins and the corresponding statistical results. (G) Tumor tissue was stained with HE, the nucleus was stained blue-violet, and the cytoplasm was stained red. Scale bar: 200 µm. *P < 0.05 indicates statistically significance vs. EC109 cells. **P < 0.01 indicates highly statistically significant vs. EC109 cells.
Chemotherapy using PTX as a first-line chemotherapeutic agent is the main treatment for patients with esophageal cancer (24, 25). However, drug resistance occurs in a large proportion of patients after treatment, which affects the efficacy of PTX treatment (26). In this study, we showed that esophageal cancer cells became less sensitive to PTX and exhibited moderate-drug-resistance after treatment with PTX. Moreover, the PTX-resistant cells develop stemness characteristics. We found that the expression of stem cell biomarkers NANOG, OCT4, SOX2 increased in the PTX-resistant esophageal cancer cells. Simultaneously, we found that the expression of epithelial biomarkers decreased and the mesenchymal biomarkers increased in drug-resistant esophageal cancer cells. Also, we verified these findings in vivo (Figure 5). OCT4, SOX2 and NANOG, as the embryonic stem cell transcription factors, transform cancer cells to stem-like cell phenotype in different tumor types (27). Among them, NANOG is an important transcription factor involved in the regulation of cell stemness (28). NANOG plays an important role in self-renewal, undifferentiated state and differentiation ability, high tumorigenicity, and resistance to current standard chemotherapy and radiotherapy (29–31). It can be activated by different transcription factors, such as OCT4 and SOX2 (32, 33). Previous studies have shown that NANOG is closely related to the poor prognosis of cancers (34).
In addition, we also found that after the cells were induced into drug-resistant esophageal cancer cells by PTX, the expression of EMT-related transcription factor β-catenin was up-regulated, the expression of epithelial biomarkers Claudin-1, ZO-1 and E-cadherin was reduced and the mesenchymal biomarkers N-cadherin, Vimentin expression increased. EMT plays an important role in the infiltration and metastasis of tumor cells, the formation of tumor drug resistance and tumor stem cells (35). The results indicated that once esophageal cancer cells become drug resistant, the expression of both cancer stem cell biomarkers and EMT-related biomarkers changed, which indicated clinical recurrence and migration ability (36, 37). Some studies have demonstrated that mesenchymal cells share similar molecular characteristics with CSCs, to some extent (38). Previous studies also revealed that the activation of EMT program led to the acquisition of the characteristics of CSCs in tumor cells (39).
In conclusion, our study suggested that drug resistance and cancer cell stemness develop at the same time during chemotherapy in esophageal cancer. The emergence of stemness explained why recurrence and metastasis occurred after drug resistance and caused poor prognosis. Our results indicated that targeting EMT and stemness at the same time in drug-resistant esophageal cancer could provide a better therapeutic effect.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of the Zhengzhou University.
CW, CP, and XL designed and performed the experiments. XL drafted the manuscript. MH, LinL, XW, SH, JZ, YD, MA, LeiL, XZ, JH, and YL participated in the experiments and revised the manuscript. CW supervised this study. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (no. U1904156) and Zhengzhou University Student Innovation Experiment Project (UIEP).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. CA Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338
3. Liu Y, Xiong Z, Beasley A, D’Amico T, Chen XL. Personalized and Targeted Therapy of Esophageal Squamous Cell Carcinoma: An Update. Ann N Y Acad Sci (2016) 1381:66–73. doi: 10.1111/nyas.13144
4. Yang CS, Chen X, Tu S. Etiology and Prevention of Esophageal Cancer. Gastrointest Tumors (2016) 3:3–16. doi: 10.1159/000443155
5. Lathia JD, Liu H. Overview of Cancer Stem Cells and Stemness for Community Oncologists. Target Oncol (2017) 12:387–99. doi: 10.1007/s11523-017-0508-3
6. Wicha MS, Liu S, Dontu G. Cancer Stem Cells: An Old Idea–a Paradigm Shift. Cancer Res (2006) 66:1883–90. doi: 10.1158/0008-5472. discussion 1895-1886CAN-05-3153.
7. Brown Y, Hua S, Tanwar PS. Extracellular Matrix-Mediated Regulation of Cancer Stem Cells and Chemoresistance. Int J Biochem Cell Biol (2019) 109:90–104. doi: 10.1016/j.biocel.2019.02.002
8. Kolev VN, Tam WF, Wright QG, McDermott SP, Vidal CM, Shapiro IM, et al. Inhibition of FAK Kinase Activity Preferentially Targets Cancer Stem Cells. Oncotarget (2017) 8:51733–47. doi: 10.18632/oncotarget.18517
9. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem Cells, Cancer, and Cancer Stem Cells. Nature (2001) 414:105–11. doi: 10.1038/35102167
10. Liu K, Zhao T, Wang J, Chen Y, Zhang R, Lan X, et al. Etiology, Cancer Stem Cells and Potential Diagnostic Biomarkers for Esophageal Cancer. Cancer Lett (2019) 458:21–8. doi: 10.1016/j.canlet.2019.05.018
11. Wise JGV, Pia DL, Alexander RN, Amila KA, Maha A. Inhibitors of Multidrug Resistance Transporter P-glycoprotein. United States Patent US. 20190216811 (2019).
12. Taniguchi D, Saeki H, Nakashima Y, Kudou K, Nakanishi R, Kubo N, et al. CD44v9 is Associated With Epithelial-Mesenchymal Transition and Poor Outcomes in Esophageal Squamous Cell Carcinoma. Cancer Med (2018) 7:6258–68. doi: 10.1002/cam4.1874
13. Huang D, Gao Q, Guo L, Zhang C, Jiang W, Li H, et al. Isolation and Identification of Cancer Stem-Like Cells in Esophageal Carcinoma Cell Lines. Stem Cells Dev (2009) 18:465–73. doi: 10.1089/scd.2008.0033
14. Zhang F, Wang J, Wang X, Wei N, Liu H, Zhang X. CD146-Mediated Acquisition of Stemness Phenotype Enhances Tumour Invasion and Metastasis After EGFR-TKI Resistance in Lung Cancer. Clin Respir J (2019) 13:23–33. doi: 10.1111/crj.12976
15. Steinbichler TB, Savic D, Dudás J, Kvitsaridze I, Skvortsov S, Riechelmann H. Cancer Stem Cells and Their Unique Role in Metastatic Spread. Semin Cancer Biol (2020) 60:148–56. doi: 10.1016/j.semcancer.2019.09.007
16. Du B, Shim JS. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules (2016) 21:965. doi: 10.3390/molecules21070965
17. Polyak K, Weinberg RA. Transitions Between Epithelial and Mesenchymal States: Acquisition of Malignant and Stem Cell Traits. Nat Rev Cancer (2009) 9:265–73. doi: 10.1038/nrc2620
18. Yeung KT, Yang J. Epithelial-Mesenchymal Transition in Tumor Metastasis. Mol Oncol (2017) 11:28–39. doi: 10.1002/1878-0261.12017
19. Montanari M, Rossetti S, Cavaliere C, D’Aniello C, Malzone MG, Vanacore D. Epithelial-Mesenchymal Transition in Prostate Cancer: An Overview. Oncotarget (2017) 8:35376–89. doi: 10.18632/oncotarget.15686
20. Zeng K, He B, Yang BB, Xu T, Chen X, Xu M. The Pro-Metastasis Effect of circANKS1B in Breast Cancer. Mol Cancer (2018) 17:160. doi: 10.1186/s12943-018-0914-x
21. Che D, Zhang S, Jing Z, Shang L, Jin S, Liu F. Macrophages Induce EMT to Promote Invasion of Lung Cancer Cells Through the IL-6-mediated Cox-2/PGE2/beta-catenin Signalling Pathway. Mol Immunol (2017) 90:197–210. doi: 10.1016/j.molimm.2017.06.018
22. Li X, Zeng X. Shikonin Suppresses Progression and Epithelial Mesenchymal Transition in Hepatocellular Carcinoma (HCC) Cells Via Modulating miR-106b/SMAD7/TGF-beta Signaling Pathway. Cell Biol Int (2020) 44:467–76. doi: 10.1002/cbin.11247
23. Wang C, Guo LB, Ma JY, Li YM, Liu HM. Establishment and Characterization of a Paclitaxelresistant Human Esophageal Carcinoma Cell Line. Int J Oncol (2013) 43:1607–17. doi: 10.3892/ijo.2013.2083
24. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Corvera C, Das P. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Net: JNCCN (2019) 17:855–83. doi: 10.6004/jnccn.2019.0033
25. Feng W, Xiaoyan X, Xuan Y, Xiangke L, Zichang Y, Ran Z. Silencing Stathmin-Modulating Efficiency of Chemotherapy for Esophageal Squamous Cell Cancer With Paclitaxel. Cancer Gene Ther (2015) 22:115–21. doi: 10.1038/cgt.2014.74
26. Zhao WS, Yan WP, Chen DB, Dai L, Yang YB, Kang XZ. Genome-Scale CRISPR Activation Screening Identifies a Role of ELAVL2-CDKN1A Axis in Paclitaxel Resistance in Esophageal Squamous Cell Carcinoma. Am J Cancer Res (2019) 9:1183–200.
27. Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An Embryonic Stem Cell-Like Gene Expression Signature in Poorly Differentiated Aggressive Human Tumors. Nat Genet (2008) 40:499–507. doi: 10.1038/ng.127
28. de Vicente JC, Rodríguez-Santamarta T, Rodrigo JP, Allonca E, Vallina A, Singhania A, et al. The Emerging Role of NANOG as an Early Cancer Risk Biomarker in Patients With Oral Potentially Malignant Disorders. J Clin Med (2019) 8:1376. doi: 10.3390/jcm8091376
29. Siddiqui Z, Srivastava AN, Sankhwar SN, Dalela D, Singh V, Zaidi N, et al. Synergic Effects of Cancer Stem Cells Markers, CD44 and Embryonic Stem Cell Transcription Factor Nanog, on Bladder Cancer Prognosis. Br J BioMed Sci (2020) 77:69–75. doi: 10.1080/09674845.2019.1692761
30. Lin T, Ding YQ, Li JM. Overexpression of Nanog Protein is Associated With Poor Prognosis in Gastric Adenocarcinoma. Med Oncol (2012) 29:878–85. doi: 10.1007/s12032-011-9860-9
31. Meng HM, Zheng P, Wang XY, Liu C, Sui HM, Wu SJ, et al. Over-Expression of Nanog Predicts Tumor Progression and Poor Prognosis in Colorectal Cancer. Cancer Biol Ther (2010) 9:295–302. doi: 10.4161/cbt.9.4.10666
32. Lu Y, Zhu H, Shan H Lu Y, Zhu H, Shan H, Lu J, et al. Knockdown of Oct4 and Nanog Expression Inhibits the Stemness of Pancreatic Cancer Cells. Cancer Lett (2013) 340:113–23. doi: 10.1016/j.canlet.2013.07.009
33. Wang X, Jin J, Wan F, Zhao L, Chu H, Chen C, et al. Ampk Promotes Spop-Mediated NANOG Degradation to Regulate Prostate Cancer Cell Stemness. Dev Cell (2019) 48:345–60.e347. doi: 10.1016/j.devcel.2018.11.033
34. Yang L, Zhang X, Zhang M, Zhang J, Sheng Y, Sun X, et al. Increased Nanog Expression Promotes Tumor Development and Cisplatin Resistance in Human Esophageal Cancer Cells. Cell Physiol Biochem (2012) 30:943–52. doi: 10.1159/000341471
35. Culig Z. Epithelial Mesenchymal Transition and Resistance in Endocrine-Related Cancers. Biochim Biophys Acta Mol Cell Res (2019) 1866:1368–75. doi: 10.1016/j.bbamcr.2019.05.003
36. Martins-Neves SR, Cleton-Jansen AM, Gomes CMF. Therapy-Induced Enrichment of Cancer Stem-Like Cells in Solid Human Tumors: Where do We Stand? Pharmacol Res (2018) 137:193–204. doi: 10.1016/j.phrs.2018.10.011
37. Li J, Qi D, Hsieh TC, Huang JH, Wu JM, Wu E. Trailblazing Perspectives on Targeting Breast Cancer Stem Cells. J Pharmacol Ther (2021) 223:107800. doi: 10.1016/j.pharmthera.2021.107800
38. Li Y, Kong D, Ahmad A, Bao B, Sarkar FH. Pancreatic Cancer Stem Cells: Emerging Target for Designing Novel Therapy. Cancer Lett (2013) 338:94–100. doi: 10.1016/j.canlet.2012.03.018
Keywords: esophageal cancer, drug resistance, paclitaxel, epithelial-mesenchymal transition, stemness
Citation: Liu X, He M, Li L, Wang X, Han S, Zhao J, Dong Y, Ahmad M, Li L, Zhang X, Huo J, Liu Y, Pan C and Wang C (2021) EMT and Cancer Cell Stemness Associated With Chemotherapeutic Resistance in Esophageal Cancer. Front. Oncol. 11:672222. doi: 10.3389/fonc.2021.672222
Received: 25 February 2021; Accepted: 05 May 2021;
Published: 03 June 2021.
Edited by:
Dong-Hua Yang, St. John’s University, United StatesReviewed by:
Zheng Qiu, China Pharmaceutical University, ChinaCopyright © 2021 Liu, He, Li, Wang, Han, Zhao, Dong, Ahmad, Li, Zhang, Huo, Liu, Pan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cong Wang, d2FuZ2NvbmdAenp1LmVkdS5jbg==; Chengxue Pan, cGFuY3hAenp1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.