Corrigendum: LncRNA FGD5-AS1 Facilitates the Radioresistance of Breast Cancer Cells by Enhancing MACC1 Expression Through Competitively Sponging miR-497-5p
- 1Union Hospital, Tongji Medical College, Huazhong University of Science & Technology, Wuhan, China
- 2Department of General Surgery, The Fifth Hospital of Wuhan, Wuhan, China
- 3Department of Rehabilitation Medicine, The 910th Hospital of The People's Liberation Army Joint Logistics Support Unit, Quanzhou, China
- 4Department of Radiology, China Resources & WISCO General Hospital, Wuhan University of Science and Technology, Wuhan, China
Background: LncRNA-FGD5-AS1, as an oncogene, participates in the development and progress of various cancers. However, the exact role and the molecular mechanisms by which FGD5-AS1 regulates radiosensitivity in breast cancer (BC) remains largely unknown.
Methods: We used X-Ray weekly-dose-increase method to establish radiation-resistance cell lines. Bioinformatics tools analyze the expression of FGD5-AS1 in breast cancer tissue and evaluated the relationship between FGD5-AS1 and clinic-pathological features. CCK-8 and colony formation were used to analyze cell proliferation. Western blotting and qPCR were applied to detect protein and gene expression, respectively. RNA interference was used to knock down the endogenous gene expression. Luciferase reporter system and immunoprecipitates were applied to verify the target of FGD5-AS1.
Result: FGD5-AS1 was overexpressed in BC tissues and radiation-resistance cell lines. Higher levels of FGD5-AS1 predicted poorer clinical characteristics and prognosis. Loss-of-function FGD5-AS1 sensitized BC cells to X-ray, meanwhile, the cell gained radiation-resistance when exogenous FGD5-AS1 was expressed. FGD5-AS1 depletion arrested cells at G0/G1 and triggers cell apoptosis. The starBase database (ENCORI), predicted binding site of miR-497-5p in FGD5-AS1 sequence, and luciferase reporter system and immunoprecipitates verified miR-497-5p was the target of FGD5-AS1. Furthermore, MACC1 was predicted and verified as the target of miR-497-5p. Loss-of-function FGD5-AS1 sensitized ionizing radiation was rescued by the up-regulation of MACC1 and the inhibition of miR-497.
Conclusion: FGD5-AS1 displays an oncogene profile in CRC; patients with high expression of FGD5-AS1 should benefit less from radiotherapy and need a more frequent follow-up. Besides, FGD5-AS1 may be a potential therapeutic target for CRC.
Introduction
Breast cancer (BC) accounts for the most common cancer by mobility and the fifth leading cause of mortality in China (1). Despite all efforts and notable advances for improving CRC treatment including surgery, target therapy, radiotherapy, endocrine therapy, and chemotherapy, the average 5-year survival rates of stage II and stage III patients were 75% and 61%, respectively (2). Radiation therapy is an integral part of the multidisciplinary management of breast cancer (3). One of the main concerns around radiotherapy is how to improve biological effect without compromising organ at risk (4, 5); however, promoting the therapeutic effect o is still a challenge for radiation oncologists.
Long non-coding RNAs (lncRNAs) are non-protein coding transcripts with a length of more than 200 nucleotides. Of note, it is clear that lncRNAs are important regulators of biological processes, including chromosome structure modulation, epigenetic regulation, transcription, mRNA splicing, and translation (2, 6). lncRNAs may carry out both gene inhibition and activation via a range of diverse mechanisms to affect the progress of BC such as H19 (7), SRA (8), Zfas1 (9), BCAR (10), and LSINCT5 (11). However, to date, only a few lncRNAs have been characterized in detail for radiotherapy in BC.
FGD5-AS1 has been identified as an oncogene in multiple cancers. Li D et al. reported FGD5-AS1 promoted cell proliferation and metastasis in colorectal cancer and predicted poor survival (12). Similarly, in oral cancer cells, FGD5-AS1 affected cell proliferation, invasion, and migration, and apoptosis as well (13). Furthermore, the tumorigenic potentialities of FGD-AS1 had been revealed in glioblastoma (14), and melanoma (15), as it was related to poor clinic-pathological features and prognosis.
In the current study, we determined the levels of FGD5-AS1 lncRNA in Breast cancer. Furthermore, to understand the possible mechanism of FGD5-AS1 in radiation sensitivity, we established radiation-resistance cell lines. The effects of abnormal expression of FGD5-AS1 on radiation sensitivity were then evaluated by detecting cell viability, apoptosis. Several studies reported that lncRNAs serving as competitive endogenous RNAs (ceRNAs) and sponging miRNAs in many cancers (12–14). For example, FGD5-AS1 lncRNA, as a ceRNA, was proved to target miR-142-3p, miR-153-3p, and miR-129-5p (11).
Metastasis-associated in colon cancer 1 (MACC1), was first identified by Arlt et al. in colon cancer tissues (16). Scholars reported MACC acted as an oncogene involved in cancer progress and metastasis and predicted a poor prognosis in many cancers (17–25). Moreover, many scholars revealed that MACC1 expression has also been associated with treatment response via interrupting Notch pathway (26), β-catenin (27, 28), and HGF/cMet pathway (29). These investigations implicate MACC1 as a multifunctional regulator of cancer progression.
The starBase database (ENCORI), predicted the binding site of miR-497-5p in the FGD5-AS1 sequence, we investigated whether FGD5-AS1 regulates radiation-sensitivity via sponging miR-497-5p. Moreover, a regulatory pattern between miR-497-5p and MACC1 was detected. We, therefore, believe that our discoveries would supply a theoretical basis for overcoming the radiation-resistance of breast cancer.
Materials and Methods
Patients and Sample
A total of 50 BC and paired normal tissues were collected at the China Resources & WISCO General Hospital, Wuhan University of Science and Technology. All samples were confirmed by two pathologists independently. The samples were frozen in liquid nitrogen immediately after surgical resection and then stored at −80°C for later use. Patients who received any neoadjuvant therapies and had a past-tumor-history were excluded from this study. This study was approved by the Ethics Committees of China Resources & WISCO General Hospital, Wuhan University of Science and Technology. The consent was obtained from the study participants before study commencement via signing the Informed Consent Form, and this study was strictly conducted in accordance with the Declaration of Helsinki.
Cell Culture
All the human colorectal cancer cell lines MCF-7 and MDA-MB-231 were cultured in DMEM medium with 10% fetal bovine serum, 100 unit/ml penicillin, and 100 µg/ml streptomycin at 37°C with 5% CO2 under sterile conditions.
Irradiation of Cells and Development of Radio-Resistant Cell Lines
Cells were irradiated using a Faxitron cabinet X-ray system 43855D (Faxitron X-ray Corporation, IL, USA). Radio-resistant cell lines (MCF-7R and MDA-MB-231R) were established on their parental cell lines. Briefly, an initial dose of 2 Gy was followed by weekly incremental doses of 0.5 Gy for 12 weeks for a total radiation dose of 57 Gy. Cells were subsequently maintained with further weekly doses of 5 Gy.
Cell Transfection
shRNA were synthesized and subcloned into pLKO.1-TRC-puro plasmid. We applied the lipofectamine 2000 system to transfect the plasmids. Briefly, 8×105 cells were seeded into a 6-well plate and cultured for 24 h, then mix the lipofectamine 2000 and plasmid (5 μg) with OPTI-MEM, sequentially, incubated the mixture for 30 min at room temperature, then added the mixture into each well, complemented the OPTI-MEM up to 1 ml and cultured for 6 h. After 6 h, added DMED with 20% FBS into each well and up to 2 ml medium, continued to culture 48 h for mRNA extraction and protein collection.
The shRNA sequences are: FGD5-AS1: 5′-GCA AUG AUG CGC CAC UAG AUU G-3′; miRNA-497: 5′-ACAAACCACAGUGUGCUGCUG-3′; Negative control:5′-CCA UCA GUC CCA AAU CCA-3′. Mimics sequences:miR-497 mimic (5ʹ-CAG CAG CAC ACT GTG GTTGT-3ʹ) and negative control (5ʹ-GTC GTC CTC TGT CAC CAGACT-3ʹ). MACC1 shRNA were purchased from Santa Cruz (Cat#: SC-89466). To overexpressing MACC1, the whole coding sequence of MACC1 was subcloned into the pZSG vector.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assays
Total RNA was extracted by using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The first-strand cDNA was synthesized using a reverse transcription kit (Takara, Dalian, China). GAPDH was used as an internal control.
The PCR primers for lncRNA FGD-AS1 and GAPDH were listed the primers: FGD5-AS1 forward, 5′-AGA AGC GGA GGG GTG AAA AT-3′ and reverse, 5′-CCG CCT TAT AGT TGG CCC TC-3′; GAPDH forward, 5′-AAC GGA TTT GGT CGT ATT G-3′ and reverse, 5′-GGA AGA TGG TGA TGG GAT T-3′. miRNA-497: forward, 5′-CAG CCC TGT CCA GTAGC-3′ and reverse, 5′-GCC TGA CTT TAC TGT TGC-3′; MACC1: forward, 5′-TTC TTT TGA TTC CTC CGG TGA-3′ and reverse, 5′-ACT CTG ATG GGC ATG TGC TG-3′. The relative expression of FGD-AS1 was calculated using the 2-ΔΔCt method. All samples were performed in triplicate.
Cell Proliferation Assay
For cell proliferation assay, HCT116 and SW480 cells were seeded onto 96-well plates (2× 103 cells/well) and cultured for 24, 48, and 72 h. The incubation was conducted at 37°C. After adding 10 μl CCK8 (Dojindo, Japan) to each well and incubating at 37°C for 2 h, the absorbance at 480 nm was measured by the Rayto-6000 system (Rayto, China), and normalized to that of DMEM medium as control.
Flow Cytometry Analysis
For cell cycle analysis, the cells were harvested after starving for 6 hours and fixed with cold ethanol overnight and then incubated with propidium iodide and RNAse (BD, USA) in the dark for 15 min; for apoptosis analysis, the cells were harvested after starving for 6 h and washed twice with cold PBS, and stained with FITC-conjugated Annexin V for 20 min and propidium iodide for 15 min in the dark.
The stained cells were assessed by flow cytometry (FACS AriaIII, BD, USA) and analyzed by FlowJo vX.0.7 software (30, 31).
Western Blot Analysis
RIPA lysis buffer (Beyotime Biotechnology) containing protease inhibitors (Roche) was applied to extract the proteins. Quantification of proteins was carried out applying the BCA™ Protein Assay Kit (Pierce, Appleton, WI, USA). After that, proteins (30 μg/sample) were loaded and electrophoresed with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by the transfer to the polyvinylidene difluoride membranes.
The antibodies were prepared in 5% blocking buffer with a dilution of 1:1000, incubated with the membrane at 4°C overnight, washed twice with TBST, and later cultivated with a secondary antibody (1:2000), which was marked by horseradish peroxidase at room temperature for 2 h. Immobilon Western Chemiluminescent HRP Substrate (Millipore) was used to cover the surface of the membrane. Finally, the signals were captured, and the concentration of the bands was quantified using Image Lab™ software (Bio-Rad Laboratories, Hercules, CA, USA).
Luciferase Reporter Assay
FGD5-AS1-WT, FGD5-AS1-Mut were respectively subcloned into pGL3 vectors (Promega, Madison, WI), constructing plasmids for further transfection with indicated mimics or siRNAs for 48 h. the cells were digested with restriction enzymes, the amplified PCR product was cloned into the polyclonal site of the reconstructed pGL3 expression vector. Finally, the luciferase activity was detected using the Dual-Luciferase® Reporter assay kit (Promega, Madison, Wisconsin, USA).
Statistical Analysis
All experiments were performed three times. The results are reported as the mean ± SEM. All statistical analyses were performed using GraphPad Prism version 8.0 (GraphPad Software, La Jolla, CA). The association between FGD5-AS1 expression and clinical characteristics was analyzed by the chi-square tests. Survival was compared by Kaplan-Meier analysis. The difference was calculated by the Student’s t-tests, one-way ANOVA analysis or Mann-Whitney U tests. Correlations were evaluated by Pearson correlation coefficient. A p-value of less than 0.05 was considered statistically.
Results
FGD5-AS1 Is Highly Expressed in Radioresistant BC Tissues and Cell Lines
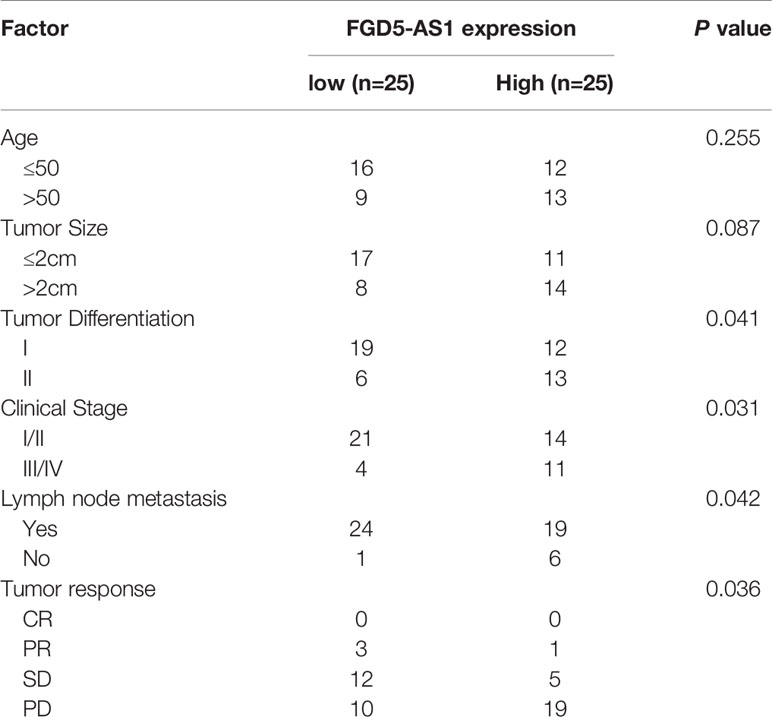
To begin with, we analyzed FGD5-AS1 expression in our patient samples. Quantitative RT-PCR revealed that FGD5-AS1 increased in cancer tissues (Figure 1A). Meanwhile, we search the GENT2 database, not to our surprise, FGD5-AS1 expression significantly elevated in BC tissues (P < 0.001, Figure 1B). Afterward, we divided the sample into 2 groups as a radiation-sensitive group (n=25) and a radiation-resistant group (n=25) by median OS. Of note, QPCR reveals that FGD5-AS1 expression was remarkably increased in the radiation-resistant group (P < 0.005, Figure 1C). As previously described, we successfully established two radiation-resistant BC cell lines MCF-7R and MDA-MB-231R. Continuously, we noted that FGD5-AS1 was up-regulated in the radiation-resistant BC cells (P < 0.005, Figure 1D).
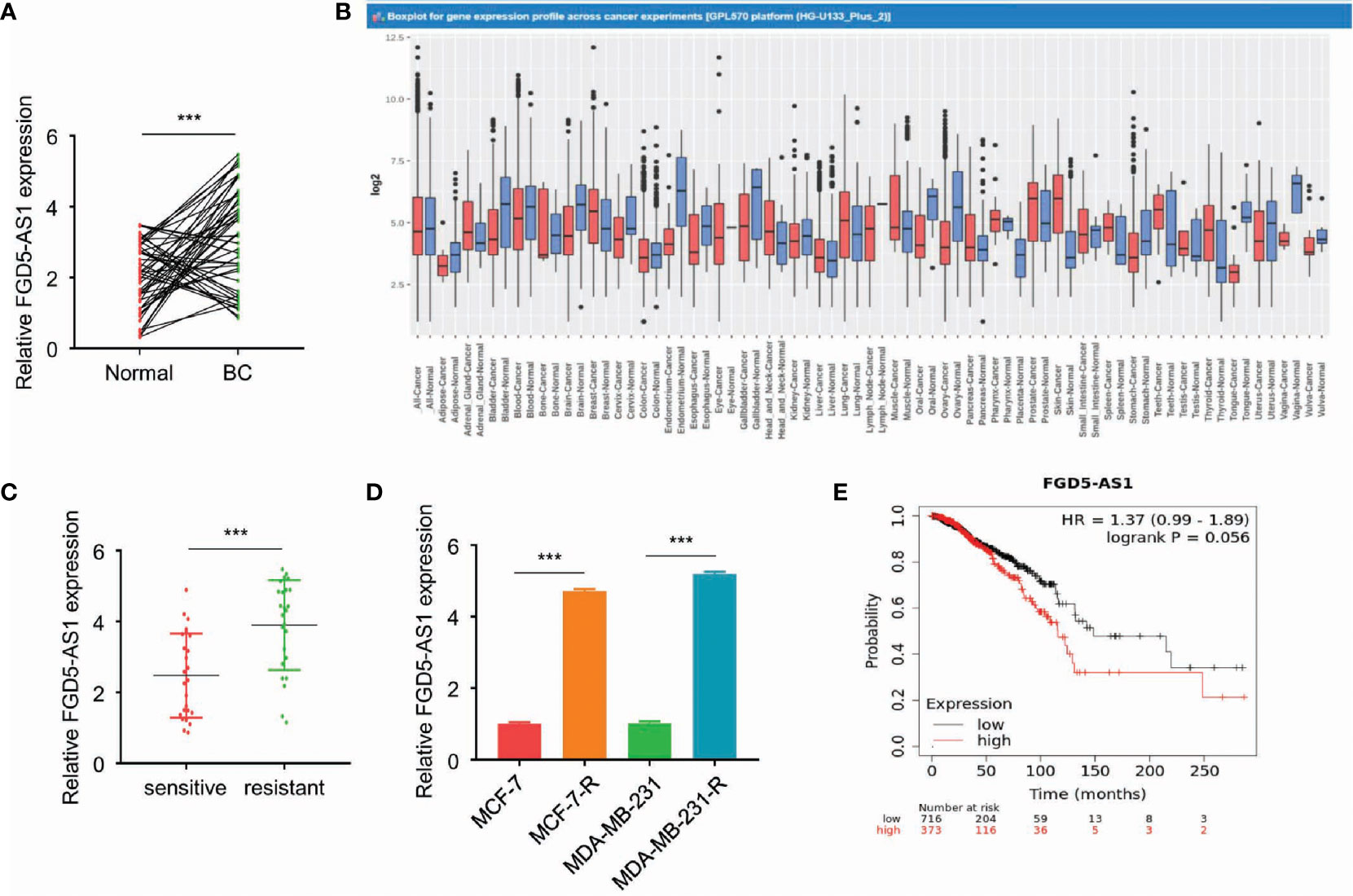
Figure 1 FGD5-AS1 is highly expressed in radioresistant BC tissues and cell lines. (A) The expression of FGD5-AS1 in BC tissues (n=50) and corresponding adjacent normal tissues (n=50) via qRT-PCR assays. (B) GENT2 database showed the significant upregulation of FGD5-AS1 in BC tissues compared with normal tissues (breast-normal) (P < 0.001); (C) Examining the expression of FGD5-AS1 in the radio-sensitive group and radio-resistant via qRT-PCR assays. As indicated, FGD5-AS1 expression was significantly increased in the radio-resistant group than the radio-sensitive group. (D) The expression of FGD5-AS1 in the radio-resistant BC cell lines. As indicated, the expression level of FGD5-AS1 was significantly increased in the radio-resistant BC cells compared to that in their parental cells. (E) Using the KM plotter database, to show that higher FGD5-AS1 expression was correlated with worse survival in BC patients. Intra-group comparison ***p < 0.001.
The mean value of FGD5-AS1 expression was set as the threshold value, and the BC tissue specimens were divided into the highly-expressed group (n=25) and under-expressed group (n=25). As shown in Table 1, higher levels of FGD5-AS1 were positively associated with poor histological grade, advanced tumor stage, lymph node metastasis, and less radiotherapy response. Moreover, the KM plotter database also indicated that higher FGD5-AS1 expression was correlated with worse survival (P < 0.005, Figure 1E).
FGD5-AS1 Strengthens the Radio-Resistance of BC Cell
We successfully transfected FGD5-AS1 siRNA and expression plasmids into MCF-7-R or MDA-MB-231-R cells (P < 0.005, Figures 2A, B). The cells were exposed to different doses of ionizing radiation (0, 3, and 6 Gy), and confirmed that the radiation was conducted as a dose-dependent suppressive effect. CCK-8 and colony formation assays results indicated the deficiency of FGD5-AS1 facilitated cellular radiation-sensitivity and could be rescued by exogenous FGD5-AS1 expression (P < 0.005, Figures 2C, D).
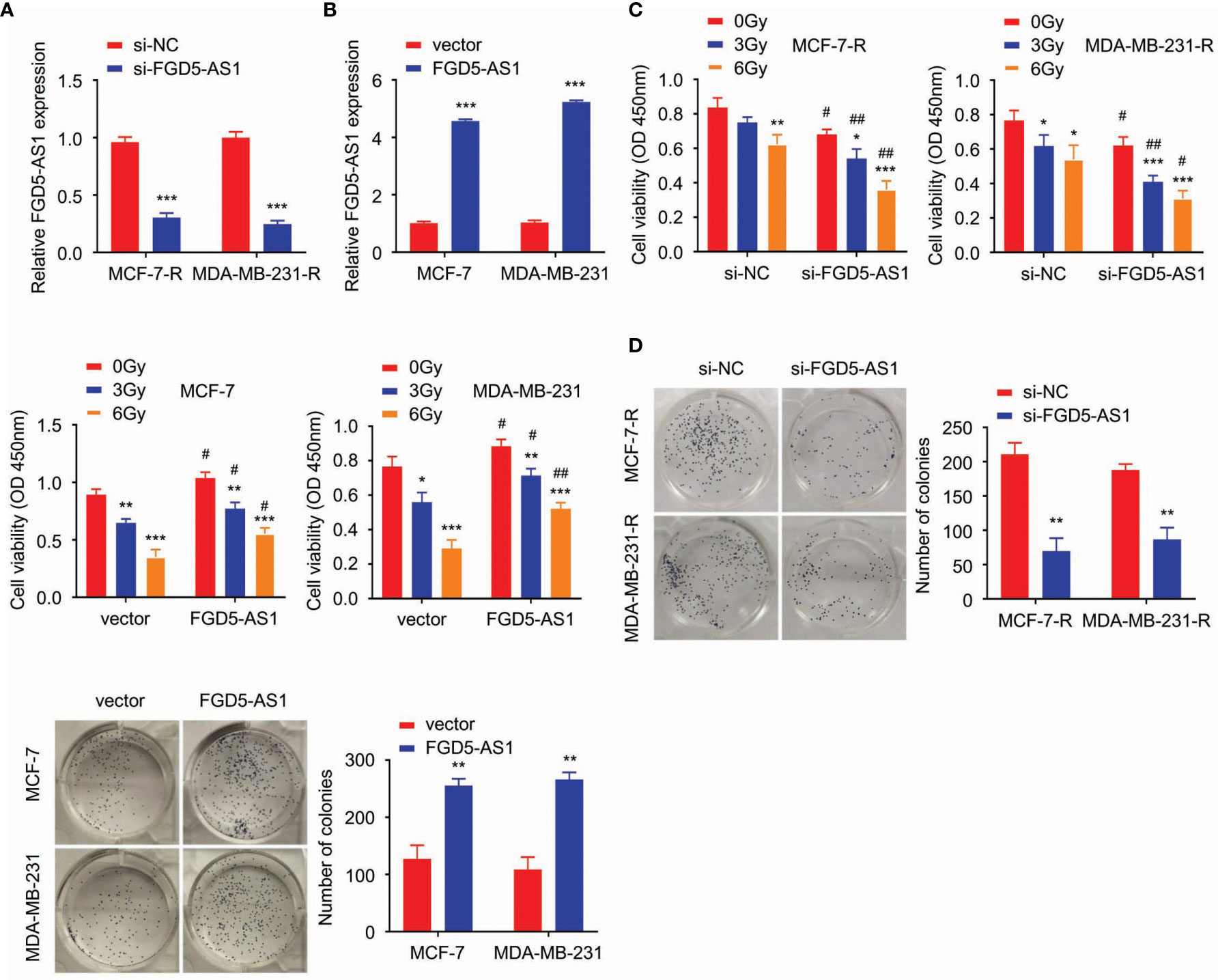
Figure 2 FGD5-AS1 strengthens the radio-resistance of BC cells. (A) MCF-7-R and MDA-MB-231-R were transfected with specific siRNA of human FGD5-AS1. qRT-PCR to confirm the downregulation of FGD5-AS1 in transfected BC cells. (B) As indicated, basing on qPCR, FGD5-AS1 exogenous expression was carried out in MCF-7 and MDA-MB-231 cells. (C) cell viability was detected by CCK-8. Cells were exposed to different doses of ionizing radiation (0, 3, and 6 Gy). FGD5-AS1 deficiency significantly promoted radiation sensitivity of MCF-7-R or MDA-MB-231-R cells; while FGD5-AS1 exogenous expression attenuated the radiation sensitivity of MCF-7 or MDA-MB-231 cells. (D) Colony formation assays, BC cells treated with 2 Gy ionizing radiation. Colony formation assays displayed that FGD5-AS1 deficiency significantly promoted radiation sensitivity of MCF-7-R or MDA-MB-231-R cells; while FGD5-AS1 exogenous expression attenuated the radiation sensitivity of MCF-7 or MDA-MB-231 cells. Intra-group comparison *p < 0.05, **p < 0.01, ***p < 0.001; Inter-group comparison #p < 0.05, ##p < 0.01.
FGD5-AS1 Depletion Triggers Cell Apoptosis in BC Cells
Next, we wondered how FGD5-AS1 performed its biological function in radiation-resistant cells. FACS results revealed that loss-of-function FGD5-AS1 arrested MCF-7-R or MDA-MB-231-R at the G0/G1 phase (Figure 3A) and induced more apoptosis when exposed to 2 Gy X-ray (Figure 3B).
Figure 3 FGD5-AS1 depletion triggers cell apoptosis in BC cells. (A) flow cytometry analysis indicated downregulation of FGD5-AS1 arrested MCF-7-R or MDA-MB-231-R cells at G0/G1and induced much more apoptosis after treated with 2 Gy of ionizing radiation. Meanwhile, overexpression of FGD5-AS1 arrested MCF-7 and MDA-MB-231 at the S phase, and less apoptosis was observed after ionizing radiation. (B) WB showed that knockdown of FGD5-AS1 significantly increased the protein levels of Bax, cleaved caspase-3, and caspase-9 in MCF-7-R or MDA-MB-231-R cells treated with 2 Gy of ionizing radiation. Overexpression of FGD5-AS1 significantly decreased the protein levels of Bax, cleaved caspase-3, and caspase-9 in MCF-7-R or MDA-MB-231-R cells treated ionizing radiation. Intra-group comparison *p < 0.05, ***p < 0.001.
FGD5-AS1 Directly Binds to miR-497-5p and Suppresses Its Expression in BC Cells
Afterward, we analyzed the targets of FGD-AS1 via starBase2. Among the targets discovered, miR-497-5p got a high score and the predicted binding site of FGD5-AS1 and miR-497-5p was listed in Figure 4A. QPCR revealed that miR-497-5p expression was significantly decreased in the radiation-resistant BC tissues and cells (P < 0.005, Figures 4B, C). Also, miR-497-5p was dramatically attenuated in FGD-AS1 deficient cells and rescued by exogenous FGD5-AS1 gene transfected (Figure 4D).
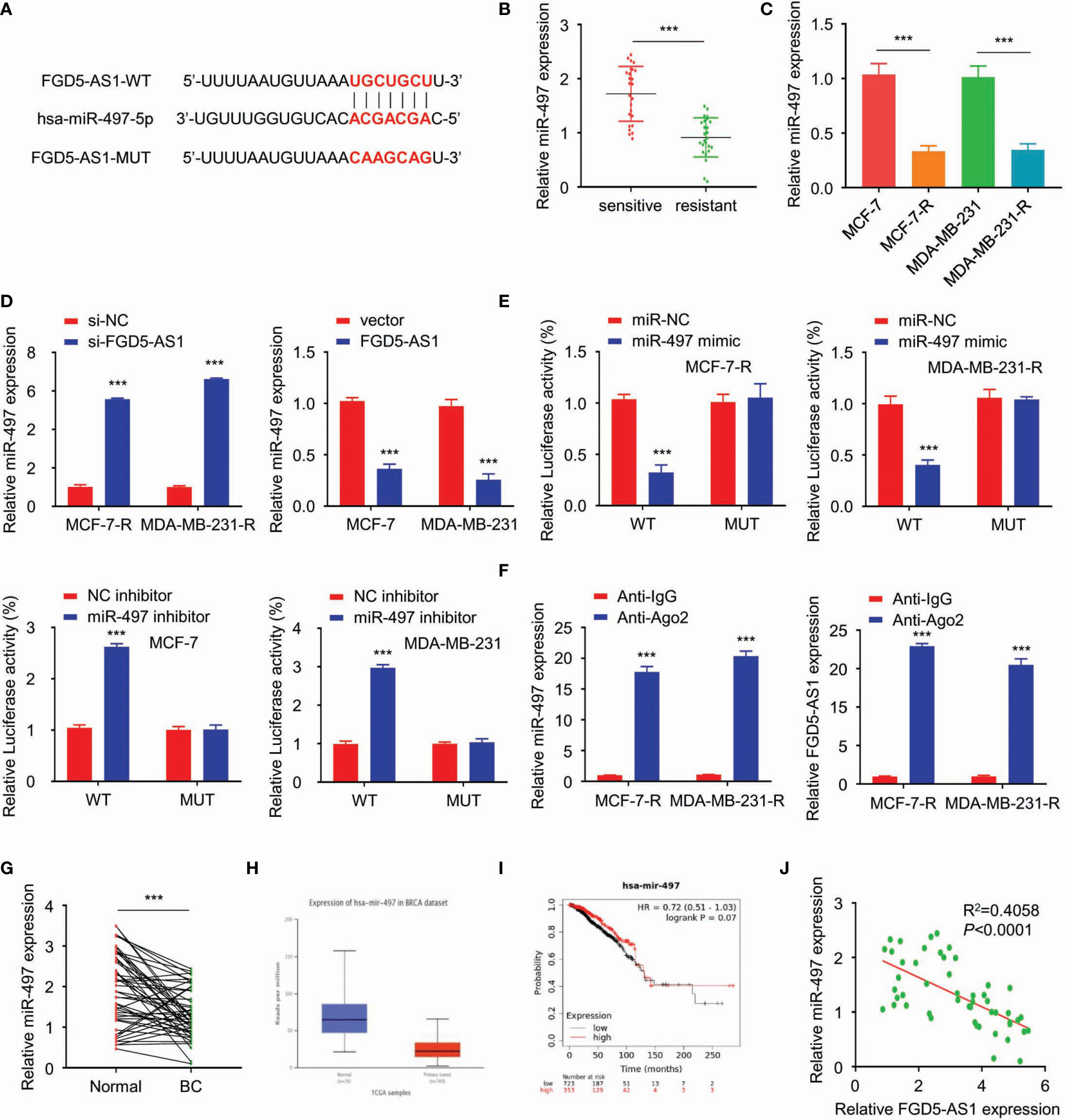
Figure 4 FGD5-AS1 directly binds to miR-497-5p and suppresses its expression in BC cells. (A) Using the starBase database to predict the binding site of miR-497-5p in the FGD5-AS1 sequence. (B) qRT-PCR assays showed that miR-497 expression was significantly decreased in the radio-resistant BC tissues group (n=25). (C) qRT-PCR assays indicated the lower levels of miR-497 in MCF-7-R or MDA-MB-231-R cells than MCF-7 or MDA-MB-231 cells. (D) Using qRT-PCR assays revealed that FGD5-AS1 exogenous expression significantly reduced the expression of miR-497. Meanwhile, FGD5-AS1 silencing significantly upregulated the expression of miR-497. (E) Using the luciferase reporter assays, miR-497 mimic significantly reduced the luciferase activity of the pGL3-FGD5-AS1-WT and FGD5-AS1-MT cell were had not been affected. In contrast, miR-497 Inhibitor significantly promoted the luciferase activity of the pGL3-FGD5-AS1-WT, as the same, FGD5-AS1-MT cell were had not been affected. (F) RNA binding protein immunoprecipitation against Ago2 indicated that FGD5-AS1 or miR-497 were significantly enriched in Ago2 pellets. (G) qRT-PCR assays showed that miR-497 levels were significantly downregulated in BC tissues. (H) UALCAN database indicated the significant downregulation of miR-497 in TCGA BC tissues (P<1E-12). (I) KM plotter showed that lower miR-497 expression was significantly correlated with worse survival in BC patients. (J) PCR showed a negative relationship between FGD5-AS1 and miR-497 expression levels in BC tissues (n=50). Intra-group comparison ***p < 0.001.
We used a pGL3-FGD5-AS1-WT and pGL3-FGD5-AS1-Mut based double luciferase reporter to figure out the target of FGD5-AS1. As a result, the luciferase activity of the pGL3-FGD5-AS1-WT was significantly attenuated when cotransfected with miR-497 mimic. Then, the miR-497 mimic was failed to affect the luciferase activity of the pGL3-FGD5-AS1-MT. Moreover, the luciferase activity of the pGL3-FGD5-AS1-WT was significantly promoted when miR-497 cotransfected, while, miR-497 was unable to affect the luciferase activity of the pGL3-FGD5-AS1-MT (Figure 4E).
RNA binding protein immunoprecipitation experiments were performed on MCF-7-R or MDA-MB-231-R cells extracts by the Ago2 antibody. Not to our surprise, FGD5-AS1 or miR-497 were significantly enriched in Ago2 pellets relative to control IgG immunoprecipitates (Figure 4F). We investigated that miR-497 levels were significantly downregulated in BC tissues (Figures 4G, H) and lower expression of miR-497 predicted an unfavored prognosis (Figure 4I). Also, our samples showed a negative relationship between FGD5-AS1 expression levels and miR-497 expression (Figure 4J). Taken together, FGD5-AS1 may act as an endogenous sponge for miR-497 to suppress its expression in BC cells.
FGD5-AS1 Competitively Sponges miR-497 to Enhance MACC1 Expression in BC Cells
Continuously, we analyzed the targets of miR-497 via starBase2. Among the targets discovered, MACC1 got a high score, and the predicted binding site of miR-497-5p and MACC1 was listed in Figure 5A. Besides, studies have shown that the up-regulation of MACC1 promotes tolerance to radiotherapy. Therefore, this study investigated whether FGD5-AS1 affects the protein stability of MACC1 by regulating the expression of miR-497 in BC cells. To begin with, we found that MACC1 was elevated in BC tissues and radiation-resistant cells (Figures 5B, C) and predicted a poorer prognosis (Figure 5D). Also, luciferase reporter assays indicated the direct binding between miR-497 and MACC1 in MCF-7-R or MDA-MB-231-R cells (Figure 5E). Of note, MACC1 was elevated by exogenous FGD5-AS1 expression and rescued by miR-497 inhibition (Figure 5F). Likewise, we observed a negative relationship between miR-497 and MACC1 expression levels in BC tissues. These findings demonstrated that FGD5-AS1 could induce the expression of MACC1 via acting as a ceRNA to sponge miR-497 in BC cells (Figure 5G).
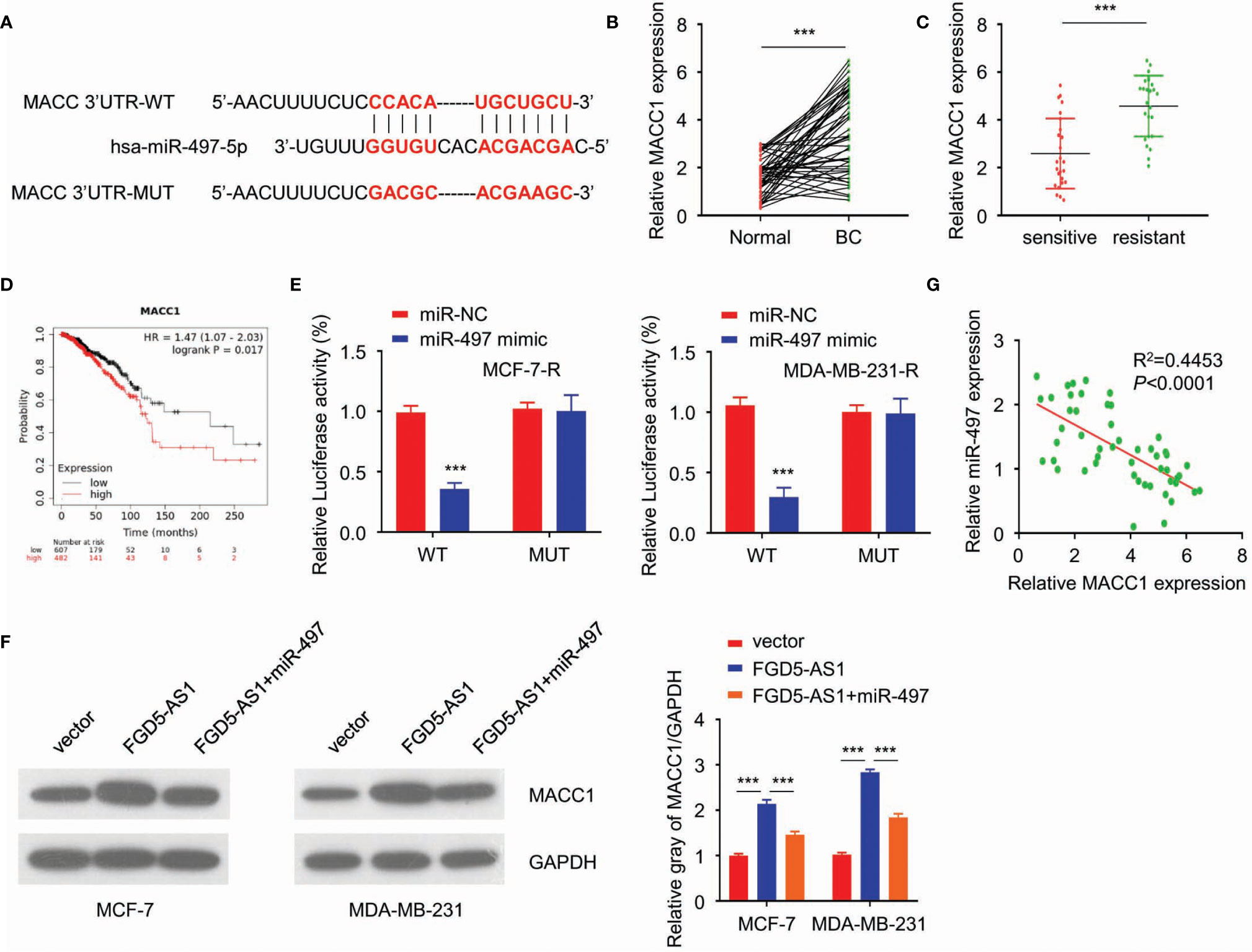
Figure 5 FGD5-AS1 competitively sponges miR-497 to enhance MACC1 expression in BC cells. (A) TargetScan predicted the potential binding between miR-497 and MACC1 sequence. (B) qRT-PCR assays indicated that MACC1 levels were significantly upregulated in BC tissues (n=50). (C) qRT-PCR assays showed that MACC1 expression was significantly increased in the radio-resistant BC tissues group (n=25). (D) KM plotter showed that higher MACC1 expression was significantly correlated with worse survival in BC patients. (E) Luciferase reporter assays indicated the direct binding between miR-497 and MACC1 in MCF-7-R and MDA-MB-231-R cells. (F) WB showed FGD5-AS1 exogenous expression increased MACC1 protein expression and could be reduced by the overexpression of miR-497. (G) qRT-PCR assays indicated the negative relationship between miR-497 expression and MACC1 mRNA expression levels in BC tissues (n=50). Intra-group comparison ***p < 0.001.
FGD5-AS1 Induces MACC1 Expression to Promote the Radio-Resistance of BC Cells Through Competitively Sponging miR-497
Further experiments exhibited MACC1 was significantly upregulated in MCF-7-R and MDA-MB-231-R cells (Figures 6A–C). After we interrupting the function of MACC1, and MCF-7-R and MDA-MB-231-R cells triggered the apoptosis pathway and regained sensitivity to the ionizing radiation (Figures 6D, E). As previously described, FGD5-AS1 deficiency was able to sensitize the cells to radiation. This phenotype could be rescued by exogenous MACC1 expression or miR-497 depletion (Figure 6F). Similarly, we found a positive relationship between miR-497 and MACC1 expression levels in BC tissues. To sum up, lncRNA FGD5-AS1 promoted the radioresistance of BC cells by upregulating MACC1 expression through competitively sponging miR-497.
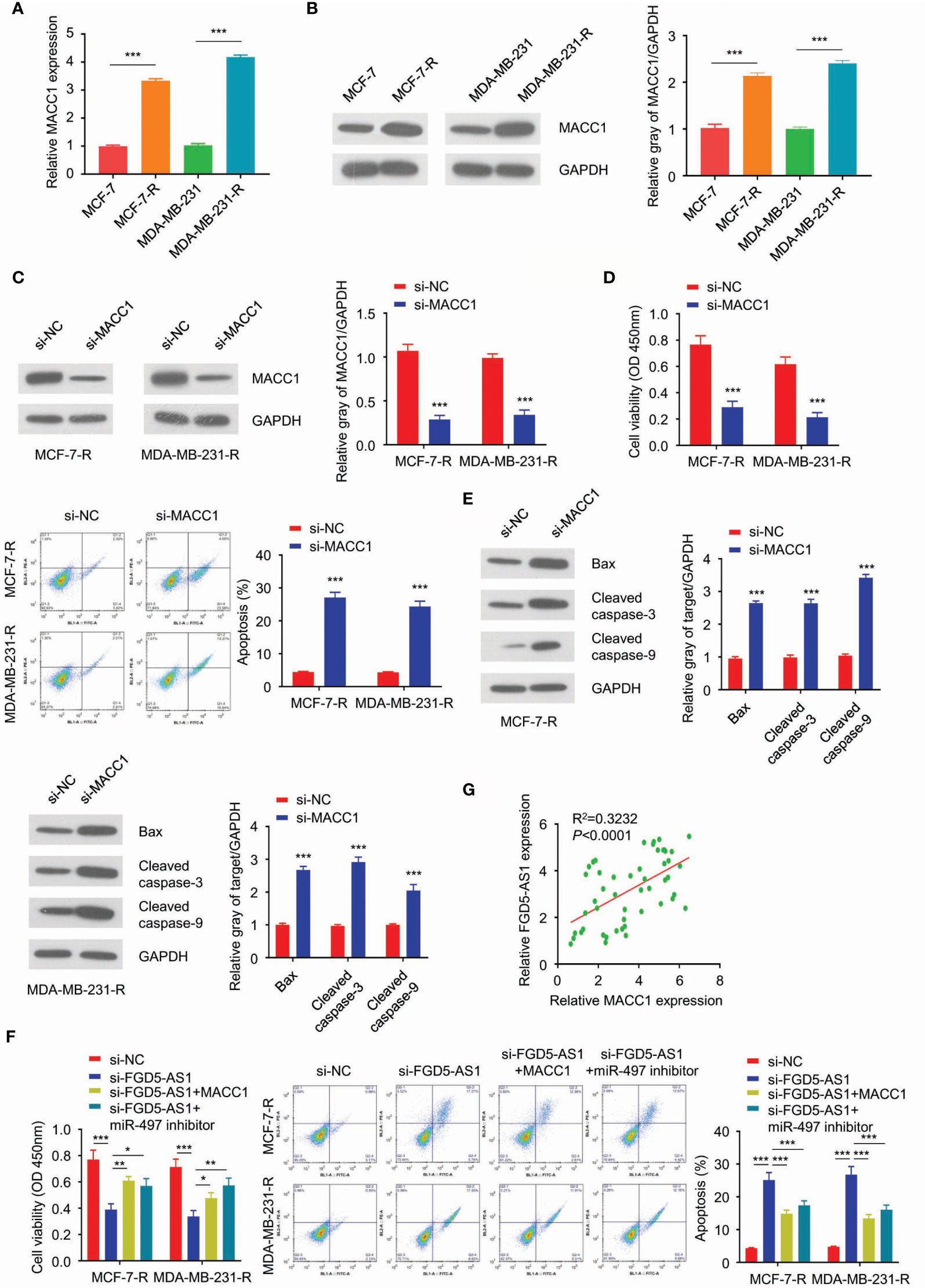
Figure 6 FGD5-AS1 induces MACC1 expression to promote the radio-resistance of BC cells through competitively sponging miR-497. (A) qRT-PCR assays showed that mRNA levels of MACC1 were significantly upregulated in MCF-7-R and MDA-MB-231-R cells. (B) WB analysis showed that the protein level of MACC1 was increased in the radio-resistant BC cells. (C) Transfect MCF-7-R or MDA-MB-231-R cells with human MACC1 siRNA and WB analysis showed the downregulation of MACC1 in transfected cells. (D) CCK-8 assay, colony formation assay, and flow cytometry analysis indicated that MACC1deficiency decreased the survival fraction with ionizing radiation, and led to more apoptosis in BC cells. (E) The western blotting analysis showed MACC1 deficiency significantly increased the protein levels of Bax, cleaved caspase-3, and caspase-9 in MCF-7-R or MDA-MB-231-R cells. (F) CCK-8 assays, clone formation assays, and flow cytometry analysis, to show that knockdown of FGD5-AS1 decreased the survival fraction and induced more apoptosis in BC cells. However, these phenotypes were significantly rescued by MACC1 exogenous expression and the inhibition of miR-497. (G) Spearman’s correlation analysis showed the positive correlation between FGD5-AS1 and MACC1 expression in 50 BC tissues, as measured by qRT-PCR assays. Intra-group comparison *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
Recent studies revealed that lncRNAs acted as a key regulator in the pathogenesis, progression, and phenotype development of breast cancer (32). Growing data delineated LncRNAs dysregulated in breast cancer (33–37). As previously described, lncRNA FGD5-AS1 participated as an essential mediator in various cancer. As we reviewed the published paper, lncRNA FGD5-AS1 might serve as a prognosis factor of colon cancer, oral cancer, and melanoma (38–43). Scholars araised interest of lncRNA FGD5-AS1 from 2018, and reported different molecular mechanism of lncRNA FGD5-AS1 in various cancer, including: regulating miR-140-5p/WEE1 axis (44), miR-153-3p/CITED2 (40), ERK/AKT signaling (45), MCL1 (42), Wnt/β-catenin (28, 46), miR-103a-3p/TPD52 (47). However, the function of lncRNA FGD5-AS1 regarding breast cancer remained rarely studied.
More than 50% of breast cancer patients would undergo radiotherapy during their course of treatment. Radiotherapy, in most cases, through X-ray ionizing radiation, inducing damage of DNA via directly or indirectly attacking the backbone of DNA and triggering a sequence of cellular response (12, 48, 49).
As showing in the schematic diagram (Figure 7), we found FGD5-AS1 over-expression reduced cell apoptosis and induced radioresistance in BC cells. miR-497-5p was identified as a potential target of FGD-AS1. Among the targets discovered, got a high score and the predicted binding site of FGD5-AS1 and miR-497-5p. Further, our data indicated that FGD5-AS1 competitively sponges miR-497 to enhance MACC1 expression in BC cells. After we interrupting the function of MACC1, and breast cancer cells triggered the apoptosis pathway and regained sensitivity to ionizing radiation. This phenotype could be rescued by exogenous MACC1 expression or miR-497 depletion. Similarly, we found a positive relationship between miR-497 and MACC1 expression levels in breast tissues.
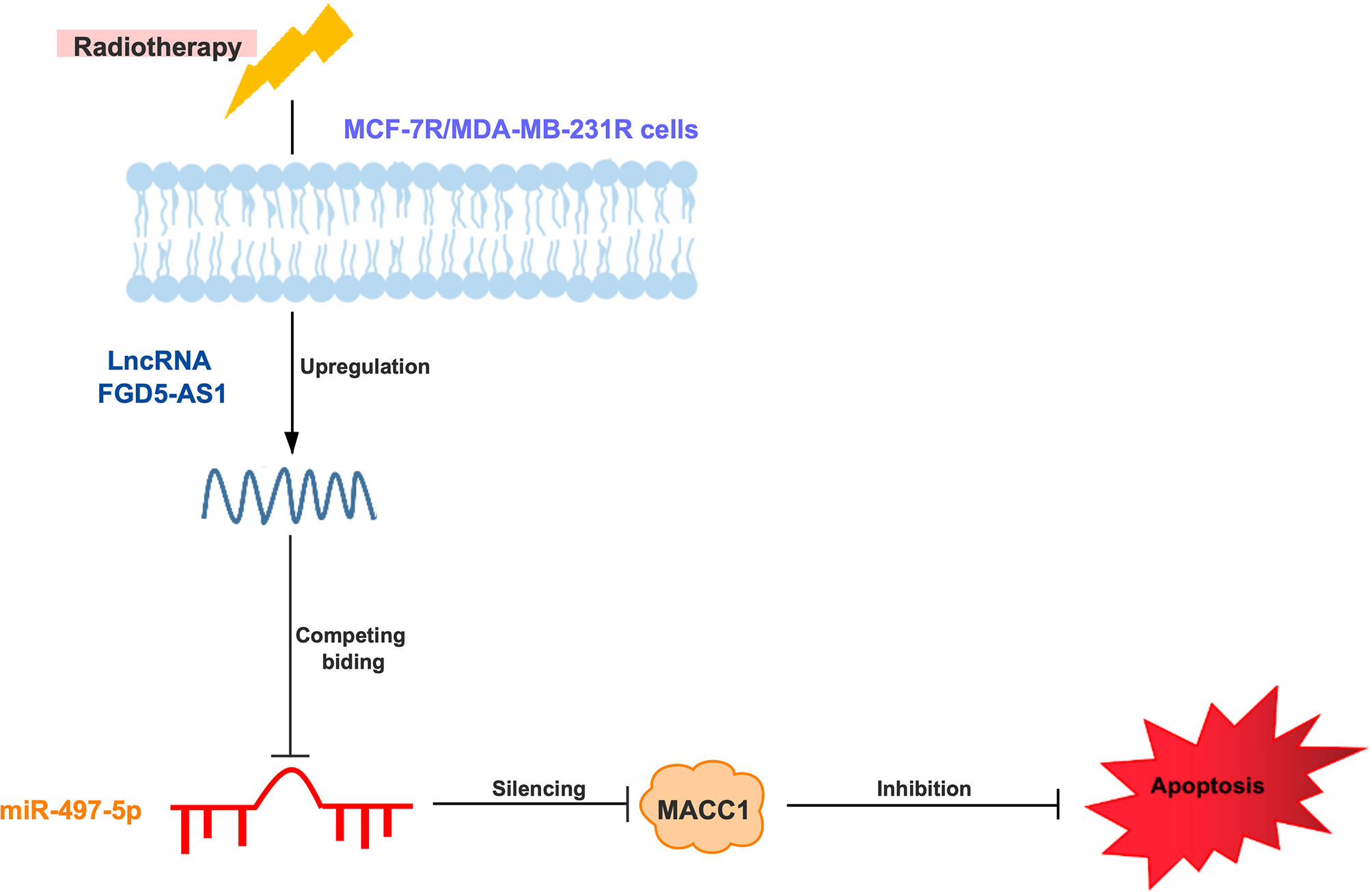
Figure 7 Schematic diagram of lncRNA FGD5-AS1/miR-497-5p/MACC1 axis to regulate MCF-7 or MDA-MB-231 cells radioresistance.
MACC1 has been well known as a key oncogenesis regulator and biomarker of tumor metastasis in a broad panel of cancer entities, associated with poor prognosis (29, 50–54). In 2013, Huang et al. reported serum MACC1 presented a diagnostic and prognostic value in breast cancer (55); Aiko Sueta et al. revealed MACC1 was failed to affect the MET pathway in the breast (56), which differed from colon cancer. Up to 2020, there was 9 articles showed MACC1 might serve as a good biomarker for breast cancer, and our result keeps consistency with previously reported (25, 55–59).
Additionally, we found that MACC1 promoted cell progress and radiosensitivity through antagonizing miR-497. FGD5-AS1 deficiency was able to sensitize the cells to radiation. This phenotype could be rescued by exogenous MACC1 expression or miR-497 depletion, which robustly suggested that they are connected in a loop. Besides, previous studies have reported that MACC1 exerts an inhibitory effect on drug resistance (60). As reported, FGD5-AS1 deficiency can promote chemoresistance via triggering the PI3K pathway, Wnt/β-catenin et al. (41, 42, 45).
The radiation-induced apoptotic mitochondria pathway is regulated by the Bcl-2 family. The Bcl-2 family may act as pro-apoptotic. Bax expression triggers the mitochondrial outer membrane permeabilization and releases cytochrome C to the cytoplasm. As a consequence, activation of the procaspase 9 through with apoptosome leads to cell death (61–63). In our data, we observed that LncRNA FGD5-AS1 deficiency sensitized the cell to X-ray via activating the BAX, Caspase 3, and Caspase 9, which indicate cell apoptosis pathway blockade is the main mechanism of LncRNA FGD5-AS1 induced radioresistance.
However, our study have some limitations. Currently, the expression of FGD5-AS1 was detected in limited number of tissue samples due to the number of available patient participants. Increased number of respondents shall be recruited in future studies. Secondly, the downstream effectors that are essential to FGD5-AS1-regulated radiation-resistance of breast cancer cells need to be thoroughly explored. Finally, we also need to further elucidate the regulatory role of MACC1 in radiation-resistance breast cancer.
In conclusion, the present results suggested that the FGD5-AS1/miR-497/MACC1 axis contributed to radiation resistance in breast cancer. These findings indicated patients with high expression of LncRNA FGD5-AS1 might gain more benefit from radiotherapy.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
This study was approved by the Ethics Committees of China Resources & WISCO General Hospital, Wuhan University of Science and Technology. The consent was obtained from the study participants before study commencement via signing the Informed Consent Form, and this study was strictly conducted in accordance with the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
QZ mainly participated in literature search, study design, writing. JL mainly participated in data collection, data analysis and data interpretation. CL conducted the most of cell experiment. BC maily participated in the critical revision. All authors contributed to the article and approved the submitted version. The authors declare that they don't have any conflict and interests in the study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA: Cancer J Clin (2018) 68:7–30. doi: 10.3322/caac.21442
2. Wang W, Kandimalla R, Huang H, Zhu L, Li Y, Gao F, et al. Molecular Subtyping of Colorectal Cancer: Recent Progress, New Challenges and Emerging Opportunities. Semin Cancer Biol (2019) 55:37–52. doi: 10.1016/j.semcancer.2018.05.002
3. Kunkler I. Radiotherapy for Breast Cancer. N Engl J Med (2002) 346:862–4. doi: 10.1056/NEJM200203143461116
4. Darby SC, Ewertz M, Hall P. Ischemic Heart Disease After Breast Cancer Radiotherapy. N Engl J Med (2013) 368:2527. doi: 10.1056/NEJMc1304601
5. Coles CE, Haviland JS, Kirby AM, Titley J, Wilcox M, Bliss JM, et al. Targeted Radiotherapy for Early Breast Cancer Reply. Lancet (2018) 391:27–8. doi: 10.1016/S0140-6736(17)33317-2
6. Liu SJ, Lim DA. Modulating the Expression of Long Non-Coding RNAs for Functional Studies. EMBO Rep (2018) 19(12):e46955. doi: 10.15252/embr.201846955
7. Luo J, Xu LN, Zhang SJ, Jiang YG, Zhuo DX, Wu LH, et al. Downregulation of LncRNA-RP11-317J10.2 Promotes Cell Proliferation and Invasion and Predicts Poor Prognosis in Colorectal Cancer. Scandinavian J Gastroenterol (2018) 53:38–45. doi: 10.1080/00365521.2017.1392597
8. Peng CL, Zhao XJ, Wei CC, Wu JW. LncRNA HOTAIR Promotes Colon Cancer Development by Down-Regulating miRNA-34a. Eur Rev Med Pharmacol Sci (2019) 23:5752–61. doi: 10.26355/eurrev_201907_18312
9. Yu Y, Nangia-Makker P, Farhana L, Majumdar APN. A Novel Mechanism of lncRNA and miRNA Interaction: CCAT2 Regulates miR-145 Expression by Suppressing Its Maturation Process in Colon Cancer Cells. Mol Cancer (2017) 16:155. doi: 10.1186/s12943-017-0725-5
10. Ouyang S, Zhou X, Chen Z, Wang M, Zheng X, Xie M. Lncrna BCAR4, Targeting to miR-665/STAT3 Signaling, Maintains Cancer Stem Cells Stemness and Promotes Tumorigenicity in Colorectal Cancer. Cancer Cell Int (2019) 19:72. doi: 10.1186/s12935-019-0784-3
11. Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen S, et al. Carcinoma-Associated Fibroblasts Promote the Stemness and Chemoresistance of Colorectal Cancer by Transferring Exosomal lncRNA H19. Theranostics (2018) 8:3932–48. doi: 10.7150/thno.25541
12. Wen J, Wang H, Dong T, Gan P, Fang H, Wu S, et al. STAT3-Induced Upregulation of Lncrna ABHD11-AS1 Promotes Tumour Progression in Papillary Thyroid Carcinoma by Regulating miR-1301-3p/STAT3 Axis and PI3K/AKT Signalling Pathway. Cell Prolif (2019) 52:e12569. doi: 10.1111/cpr.12569
13. Wu DD, Chen X, Sun KX, Wang LL, Chen S, Zhao Y. Role of the Lncrna ABHD11-AS1 in the Tumorigenesis and Progression of Epithelial Ovarian Cancer Through Targeted Regulation of Rhoc. Mol Cancer (2017) 16:138. doi: 10.1186/s12943-017-0709-5
14. Liu Y, Feng W, Liu W, Kong X, Li L, He J, et al. Circulating LncRNA ABHD11-AS1 Serves as a Biomarker for Early Pancreatic Cancer Diagnosis. J Cancer (2019) 10:3746–56. doi: 10.7150/jca.32052
15. Qiao X, Lv SX, Qiao Y, Li QP, Ye B, Wang CC, et al. Long Noncoding RNA Abhd11-AS1 Predicts the Prognosis of Pancreatic Cancer Patients and Serves as a Promoter by Activating the PI3K-AKT Pathway. Eur Rev Med Pharmacol Sci (2018) 22:8630–9. doi: 10.26355/eurrev_201812_16627
16. Arlt F, Stein U. Colon Cancer Metastasis: MACC1 and Met as Metastatic Pacemakers. Int J Biochem Cell Biol (2009) 41:2356–9. doi: 10.1016/j.biocel.2009.08.001
17. Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I, et al. MACC1, a Newly Identified Key Regulator of HGF-MET Signaling, Predicts Colon Cancer Metastasis. Nat Med (2009) 15:59–67. doi: 10.1038/nm.1889
18. Ge SH, Wu XJ, Wang XH, Xing XF, Zhang LH, Zhu YB, et al. Over-Expression of Metastasis-Associated in Colon Cancer-1 (Macc1) Associates With Better Prognosis of Gastric Cancer Patients. Chin J Cancer Res (2011) 23:153–9. doi: 10.1007/s11670-011-0153-9
19. Chen L, Wang J, Fu L, Zhang B, Zhang H, Ye B. Prognostic Significance of Metastasis Associated in Colon Cancer 1 (MACC1) Expression in Patients With Gallbladder Cancer. J Cancer Res Ther (2014) 10:1052–6. doi: 10.4103/0973-1482.137977
20. Wang Y, Hong Q, Wang J, Fang Y, Hu C. Downregulated Expression of Metastasis Associated in Colon Cancer 1 (MACC1) Reduces Gallbladder Cancer Cell Proliferation and Invasion. Tumour Biol (2014) 35:3771–8. doi: 10.1007/s13277-013-1499-z
21. Koelzer VH, Herrmann P, Zlobec I, Karamitopoulou E, Lugli A, Stein U. Heterogeneity Analysis of Metastasis Associated in Colon Cancer 1 (MACC1) for Survival Prognosis of Colorectal Cancer Patients: A Retrospective Cohort Study. BMC Cancer (2015) 15:160. doi: 10.1186/s12885-015-1150-z
22. Ge Y, Meng X, Zhou Y, Zhang J, Ding Y. Positive MACC1 Expression Correlates With Invasive Behaviors and Postoperative Liver Metastasis in Colon Cancer. Int J Clin Exp Med (2015) 8:1094–100.
23. Kopczynska EK. The Potential Therapeutic Applications and Prognostic Significance of Metastasis-Associated in Colon Cancer-1 (MACC1) in Cancers. Contemp Oncol (Pozn) (2016) 20:273–80. doi: 10.5114/wo.2016.61846
24. Zhao Y, Dai C, Wang M, Kang H, Lin S, Yang P, et al. Clinicopathological and Prognostic Significance of Metastasis-Associated in Colon Cancer-1 (MACC1) Overexpression in Colorectal Cancer: A Meta-Analysis. Oncotarget (2016) 7:62966–75. doi: 10.18632/oncotarget.11287
25. Prguda-Mujic J, Milde-Langosch K, Mueller V, Suljagic M, Coric J, Ler D. The Predictive Significance of Metastasis-Associated in Colon Cancer-1 (MACC1) in Primary Breast Cancer. Ann Clin Lab Sci (2018) 48:191–6.
26. Chen Q, Chen XF, Li DG, Yang L, Xu LP, Hu Y, et al. Long Non-Coding RNA Macc1-AS1 Promoted Pancreatic Carcinoma Progression Through Activation of PAX8/NOTCH1 Signaling Pathway. J Exp Clin Canc Res (2019) 38. doi: 10.1186/s13046-019-1332-7
27. Guo L, Ou S, Ma X, Zhang S, Lai Y. MACC1 Silencing Inhibits Cell Proliferation and Induces Cell Apoptosis of Lung Adenocarcinoma Cells Through the Beta-Catenin Pathway. Neoplasma (2018) 65:552–60. doi: 10.4149/neo_2018_170918N595
28. Zhao JB, Xue JF, Zhang WZ, Ren YL, Yan DM. Long Noncoding RNA FGD5-AS1 Promotes Glioma Cell Proliferation, Migration and Invasion by Regulating Wnt/beta-Catenin Pathway. Cancer Manag Res (2020) 12:6187–93. doi: 10.2147/CMAR.S250284
29. Wen J, Xie Y, Zhang Y, Li J, Li J, Zhang Y, et al. Macc1 Contributes to the Development of Osteosarcoma Through Regulation of the HGF/c-Met Pathway and Microtubule Stability. Front Cell Dev Biol (2020) 8:825. doi: 10.3389/fcell.2020.00825
30. Jurisic V, Bogdanovic G, Kojic V, Jakimov D, Srdic T. Effect of TNF-alpha on Raji Cells At Different Cellular Levels Estimated by Various Methods. Ann Hematol (2006) 85:86–94. doi: 10.1007/s00277-005-0010-3
31. Jurisic V, Srdic-Rajic T, Konjevic G, Bogdanovic G, Colic M. TNF-Alpha Induced Apoptosis Is Accompanied With Rapid CD30 and Slower CD45 Shedding From K-562 Cells. J Membr Biol (2011) 239:115–22. doi: 10.1007/s00232-010-9309-7
32. Zare K, Shademan M, Ghahramani Seno MM, Dehghani H. Crispr/Cas9 Knockout Strategies to Ablate CCAT1 Lncrna Gene in Cancer Cells. Biol Proced Online (2018) 20:21. doi: 10.1186/s12575-018-0086-5
33. Wang S, Cheng M, Zheng X, Zheng L, Liu H, Lu J, et al. Interactions Between Lncrna TUG1 and Mir-9-5p Modulate the Resistance of Breast Cancer Cells to Doxorubicin by Regulating Eif5a2. Onco Targets Ther (2020) 13:13159–70. doi: 10.2147/OTT.S255113
34. Chang KC, Diermeier SD, Yu AT, Brine LD, Russo S, Bhatia S, et al. MaTAR25 lncRNA Regulates the Tensin1 Gene to Impact Breast Cancer Progression. Nat Commun (2020) 11:6438. doi: 10.1038/s41467-020-20207-y
35. Shaath H, Vishnubalaji R, Elango R, Khattak S, Alajez NM. Single-Cell Long Noncoding RNA (lncRNA) Transcriptome Implicates MALAT1 in Triple-Negative Breast Cancer (TNBC) Resistance to Neoadjuvant Chemotherapy. Cell Death Discovery (2021) 7:23. doi: 10.1038/s41420-020-00383-y
36. Zhang M, Wang Y, Jiang L, Song X, Zheng A, Gao H, et al. Lncrna CBR3-AS1 Regulates of Breast Cancer Drug Sensitivity as a Competing Endogenous RNA Through the JNK1/MEK4-mediated MAPK Signal Pathway. J Exp Clin Cancer Res (2021) 40:41. doi: 10.1186/s13046-021-01844-7
37. Zhang H, Zhang J, Dong L, Ma R. LncRNA ATXN8OS Enhances Tamoxifen Resistance in Breast Cancer. Open Med (Wars) (2021) 16:68–80. doi: 10.1515/med-2021-0012
38. Li D, Jiang X, Zhang X, Cao G, Wang D, Chen Z. Long Noncoding RNA Fgd5-AS1 Promotes Colorectal Cancer Cell Proliferation, Migration, and Invasion Through Upregulating CDCA7 Via Sponging Mir-302e. In Vitro Cell Dev Biol Anim (2019) 55:577–85. doi: 10.1007/s11626-019-00376-x
39. Gao Y, Zhu H, Mao Q. Expression of LncRNA FGD5-AS1 Correlates With Poor Prognosis in Melanoma Patients. J Gene Med (2020) 22:e3278. doi: 10.1002/jgm.3278
40. Gao Y, Xie M, Guo Y, Yang Q, Hu S, Li Z. Long Non-Coding RNA FGD5-AS1 Regulates Cancer Cell Proliferation and Chemoresistance in Gastric Cancer Through miR-153-3p/CITED2 Axis. Front Genet (2020) 11:715. doi: 10.3389/fgene.2020.00715
41. Chen H, Lan Z, Li Q, Li Y. Abnormal Expression of Long Noncoding RNA Fgd5-AS1 Affects the Development of Periodontitis Through Regulating miR-142-3p/SOCS6/NF-kappaB Pathway. Artif Cells Nanomed Biotechnol (2019) 47:2098–106. doi: 10.1080/21691401.2019.1620256
42. Ge C, Dong J, Chu Y, Cao S, Zhang J, Wei J. Lncrna FGD5-AS1 Promotes Tumor Growth by Regulating MCL1 Via Sponging miR-153-3p in Oral Cancer. Aging (Albany NY) (2020) 12:14355–64. doi: 10.18632/aging.103476
43. Liu L, Zhan Y, Huang Y, Huang L. Lncrna FGD5-AS1 Can be Predicted as Therapeutic Target in Oral Cancer. J Oral Pathol Med (2020) 49:243–52. doi: 10.1111/jop.12989
44. Fu J, Cai H, Wu Y, Fang S, Wang D. Elevation of FGD5-AS1 Contributes to Cell Progression by Improving Cisplatin Resistance Against Non-Small Cell Lung Cancer Cells Through Regulating miR-140-5p/WEE1 Axis. Gene (2020) 755:144886. doi: 10.1016/j.gene.2020.144886
45. Yang Y, Dong MH, Hu HM, Min QH, Xiao L. Lncrna FGD5-AS1/miR-5590-3p Axis Facilitates the Proliferation and Metastasis of Renal Cell Carcinoma Through ERK/AKT Signalling. Eur Rev Med Pharmacol Sci (2020) 24:8756–66. doi: 10.26355/eurrev_202009_22814
46. Wu L, Zhu X, Song Z, Guo M, Liang J, Yan D. Fgd5-AS1 Facilitates Glioblastoma Progression by Activation of Wnt/beta-catenin Signaling Via Regulating miR-129-5p/HNRNPK Axis. Life Sci (2020) 256:117998. doi: 10.1016/j.lfs.2020.117998
47. Su D, Ji Z, Xue P, Guo S, Jia Q, Sun H. Long-Noncoding RNA Fgd5-As1 Enhances the Viability, Migration, and Invasion of Glioblastoma Cells by Regulating the miR-103a-3p/TPD52 Axis. Cancer Manag Res (2020) 12:6317–29. doi: 10.2147/CMAR.S253467
48. Brownmiller T, Juric JA, Ivey AD, Harvey BM, Westemeier ES, Winters MT, et al. Y Chromosome LncRNA Are Involved in Radiation Response of Male Non-Small Cell Lung Cancer Cells. Cancer Res (2020) 80:4046–57. doi: 10.1158/0008-5472.CAN-19-4032
49. Ferrandon S, DeVecchio J, Duraes L, Chouhan H, Karagkounis G, Davenport J, et al. Coa Synthase (Coasy) Mediates Radiation Resistance Via PI3K Signaling in Rectal Cancer. Cancer Res (2020) 80:334–46. doi: 10.1158/0008-5472.CAN-19-1161
50. Wang J, Wang W, Cai H, Du B, Zhang L, Ma W, et al. MACC1 Facilitates Chemoresistance and Cancer Stem Celllike Properties of Colon Cancer Cells Through the PI3K/AKT Signaling Pathway. Mol Med Rep (2017) 16:8747–54. doi: 10.3892/mmr.2017.7721
51. Peng T, Li Z, Li D, Wang S. MACC1 Promotes Angiogenesis in Cholangiocarcinoma by Upregulating VEGFA. Onco Targets Ther (2019) 12:1893–903. doi: 10.2147/OTT.S197319
52. Li Z, Guo T, Fang L, Li N, Wang X, Wang P, et al. MACC1 Overexpression in Carcinomaassociated Fibroblasts Induces the Invasion of Lung Adenocarcinoma Cells Via Paracrine Signaling. Int J Oncol (2019) 54:1367–75. doi: 10.3892/ijo.2019.4702
53. Hohmann T, Hohmann U, Kolbe MR, Dahlmann M, Kobelt D, Stein U, et al. MACC1 Driven Alterations in Cellular Biomechanics Facilitate Cell Motility in Glioblastoma. Cell Commun Signal (2020) 18:85. doi: 10.1186/s12964-020-00566-1
54. Imbastari F, Dahlmann M, Sporbert A, Mattioli CC, Mari T, Scholz F, et al. MACC1 Regulates Clathrin-Mediated Endocytosis and Receptor Recycling of Transferrin Receptor and EGFR in Colorectal Cancer. Cell Mol Life Sci (2021) 78(7):3525–42. doi: 10.1007/s00018-020-03734-1
55. Huang Y, Zhang H, Cai J, Fang L, Wu J, Ye C, et al. Overexpression of MACC1 and Its Significance in Human Breast Cancer Progression. Cell Biosci (2013) 3:16. doi: 10.1186/2045-3701-3-16
56. Sueta A, Yamamoto Y, Yamamoto-Ibusuki M, Hayashi M, Takeshita T, Yamamoto S, et al. Differential Role of MACC1 Expression and Its Regulation of the HGF/cMet Pathway Between Breast and Colorectal Cancer. Int J Oncol (2015) 46:2143–53. doi: 10.3892/ijo.2015.2907
57. Muendlein A, Hubalek M, Geller-Rhomberg S, Gasser K, Winder T, Drexel H, et al. Significant Survival Impact of MACC1 Polymorphisms in HER2 Positive Breast Cancer Patients. Eur J Cancer (2014) 50:2134–41. doi: 10.1016/j.ejca.2014.05.007
58. Tan W, Xie X, Li L, Tang H, Ye X, Chen L, et al. Diagnostic and Prognostic Value of Serum MACC1 in Breast Cancer Patients. Oncotarget (2016) 7:84408–15. doi: 10.18632/oncotarget.12910
59. Soyleyici NA, Aslan F, Avcykurt AS, Akgun GA. Importance of MACC1 Expression in Breast Cancer and its Relationship With Pathological Prognostic Markers. Indian J Pathol Microbiol (2020) 63:19–24. doi: 10.4103/IJPM.IJPM_658_19
60. Li N, Li Y, Gao H, Li J, Ma X, Liu X, et al. Forkhead-Box A3 (FOXA3) Represses Cancer Stemness and Partially Potentiates Chemosensitivity by Targeting Metastasis-Associated in Colon Cancer 1 (MACC1) Signaling Pathway in Colorectal Cancer Cells. Curr Cancer Drug Targets (2020). doi: 10.2174/1568009620666201207150632
61. Bao S, Tibbetts RS, Brumbaugh KM, Fang Y, Richardson DA, Ali A, et al. ATR/ATM-Mediated Phosphorylation of Human Rad17 Is Required for Genotoxic Stress Responses. Nature (2001) 411:969–74. doi: 10.1038/35082110
62. Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J. The ATM-Chk2-Cdc25A Checkpoint Pathway Guards Against Radioresistant DNA Synthesis. Nature (2001) 410:842–7. doi: 10.1038/35071124
Keywords: breast cancer, radiation sensitivity, FGD5-AS1, lncRNA, MACC1
Citation: Li J, Lei C, Chen B and Zhu Q (2021) LncRNA FGD5-AS1 Facilitates the Radioresistance of Breast Cancer Cells by Enhancing MACC1 Expression Through Competitively Sponging miR-497-5p. Front. Oncol. 11:671853. doi: 10.3389/fonc.2021.671853
Received: 24 February 2021; Accepted: 26 April 2021;
Published: 18 June 2021.
Edited by:
Masataka Sawaki, Aichi Cancer Center, JapanReviewed by:
Vladimir Jurisic, University of Kragujevac, SerbiaRakesh Kumar, Rajiv Gandhi Centre for Biotechnology, India
Copyright © 2021 Li, Lei, Chen and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingfang Zhu, cWl1dGszNjlAMTYzLmNvbQ==
†These authors share first authorship
 Ji Li1†
Ji Li1† Qingfang Zhu
Qingfang Zhu