- 1Department of Biotherapy, Cancer Center, West China Hospital of Sichuan University, Chengdu, China
- 2Sichuan Clinical Research Center of Biotherapy, Chengdu, China
Cancer immunotherapy can induce sustained responses in patients with cancers in a broad range of tissues, however, these treatments require the optimized combined therapeutic strategies. Despite immune checkpoint inhibitors (ICIs) have lasting clinical benefit, researchers are trying to combine them with other treatment modalities, and among them the combination with personalized cancer vaccines is attractive. Neoantigens, arising from mutations in cancer cells, can elicit strong immune response without central tolerance and out-target effects, which is a truly personalized method. Growing studies show that the combination can elevate the antitumor efficacy with acceptable safety and minimal additional toxicity compared with single agent vaccine or ICI. Herein, we have searched these preclinical and clinical trials and summarized safety and efficacy of personalized cancer vaccines combined with ICIs in several malignancies. Meanwhile, we discuss the rationale of the combination and future challenges.
Introduction
Neoantigen, an abnormal protein stemming from “non-synonymous mutation”, is specific to tumor cells (1). Neoantigens are non-self-peptides without central tolerance and off-target immune-toxicity, which are the main barriers of previous cancer vaccines and the primary obstacles in the development of personalized cancer therapy (2–4). Therefore, they are “perfect” targets with strong immunogenicity to elicit effective antitumor activity. Currently, a lot of preclinical and clinical trials have proven that neoantigen vaccines are personalized therapy and can activate hosts’ immune systems which then promote redirected T cells to kill tumor cells. However, the single use of neoantigen vaccines has a limited efficacy.
In 2013, immune therapy of cancer was regarded as the most breakthrough in Science. From then, immunotherapy became a major focus in cancer therapy, of which immune checkpoint inhibitors are the most promising and concerned topic (5). Immune checkpoint inhibitors such as anti-PD-1 and anti-CTLA-4 monoclonal antibodies, improve antitumor efficacy and prolong overall survival time in patients with many solid tumors, including lung cancer, melanoma, gastrointestinal cancer and so on (6–9). Though immune checkpoint inhibitors mark the arrival of a new era of cancer immunotherapy, using them alone has limited effect, for many patients encounter primary resistance or initial responses but eventually becoming resistant (10–12). Therefore, there is an urgent need to combine other treatments with ICIs to improve the therapeutic efficacy and prolong overall survival.
Immune checkpoint inhibitors produce antitumor effects through eliminating immune inhibition, recovering or even enhancing hosts’ immunity. The process of immune responses includes capturing, presenting, recognizing targets on tumor cells and finally killing tumor cells. Among so many targets on tumor cells, neoantigens are ideal ones to activate the immune system. Moreover, the neoantigen-specific CD8+ T cell reactivity plays a core role in immunotherapy (13). With observations that the absence of pre-existing immunity or the inhibition of tumor microenvironment may lead to invalidation of both methods (10, 14, 15), there is a strong rationale for combining ICIs with neoantigen vaccines (16). On one hand, adding neoantigen vaccine to ICIs can improve response rates of “hot” tumors through broadening cytotoxic T cell repertoire, as well as turn “cold” tumors to “hot” ones, therefore widening the scope of population who can benefit from immunotherapy (17–26). On the other hand, ICIs can unleash immunity to facilitate the efficacy of neoantigen vaccine. In this review, we focus on the safety and efficacy of personalized cancer vaccines combined with ICIs for the treatment of several malignancies. We highlight the recent development, challenges and possible improvements of personalized cancer vaccines in combination with ICIs, and hope to provide theoretical foundations for the development and application of personalized cancer vaccines in clinical settings.
Rationale for Combination Immunotherapy
To better understand the mechanism of combination immunotherapy, it is necessary to learn the dynamics of anti-tumor immune responses. Researchers propose a concept called ‘‘Cancer-Immunity Cycle’’, which illustrate crucial points during anti-tumor response and consist of seven steps (27). First step, dying tumor cells release tumor antigens such as CRT, HSPs, HMGB1 and ATP. Insufficient tumor antigens release may hamper the proceeding of this cycle. Second step, immature dendritic cells (DC) capture these antigens via signals such as CD92, TLR4 and P2RX7, which can bind to CRT, HMGB1 and ATP, respectively. Then the DCs maturate and migrate to draining lymph nodes. Inadequate activation of DCs may halt the process. Third step, DCs will process the captured tumor antigens and present them to prime and activate effector T cells. In this process, captured antigens with MHC class I and II molecules and DC co-stimulatory signals are required to stimulate T cells. However, some factors may affect T cell priming and activation, including defective expression of MHC molecules in tumors, over-expression of inhibitory signals (CTLA4/CD80, 86, PD-1/PD-L1), limited T cell repertoire (central tolerance), suppressive cells such as regulatory T cells (Tregs). Fourth and fifth steps, the activated effector T cells traffic to and infiltrate into tumors. Sixth and final steps, these cytotoxic T lymphocytes recognize via MHC/peptide complexes on the cell surface and kill their target cells. Many inhibitory mechanisms are active within tumor microenvironment, such as lack of MHC molecules in tumors, increased inhibitory signals (PD-1/PD-L1, Tim-3/phospholipids, BTLA, LAG3, IDO, Arginase), Tregs, myeloid-derived suppressor cells, M2 macrophages and hypoxia. When eventually killing tumor cells, they also release additional tumor antigens to provoke further Cancer-Immunity Cycle. This secondary immunity increasing the repertoire of tumor antigens is recognized as “antigen spreading” with increased breadth and depth of anti-tumor immunity (28). However, tumors are clever, which can take various strategies to attenuate the efficiency of anti-tumor immunity, resulting in incompetent immunity in a process designated as “cancer immune-editing” (29, 30). Therefore, the Cancer-Immunity Cycle is broken. Given the complicated immunity network, it is reasonable to combine appropriate immunotherapeutic strategies to pave the way and promote the Cancer-Immunity Cycle forward. For instance, neoantigen vaccines can bypass the first two steps and directly initiate an immune cycle. Immune checkpoint inhibitors can help overcome immune-suppression in steps 3 and 6. On the basis of cancer immunity cycle and recent studies (25, 31–38), we conclude the main mechanisms for combination immunotherapy are as follows.
Improve Sensitivity and Efficacy of Immune Checkpoint Inhibitors
Many patients with cancer initially do not response to anti-PD-1 inhibitors, possibly because of “cold” tumors, which are with no or few immune cells in tumor tissues and insensitive to ICIs (10, 39–41). In these tumors, tumor antigen cannot effectively prime and activate T cells, and further lead to the cycle halting at step 1 or 2. Vaccination with neoantigens bypassing initial two steps can produce many neoepitope-specific T cells which can traffic to tumor microenvironment and destroy tumor cells expressing these antigens. For instance, neoantigen-specific T cells were found in periphery blood after vaccination (10, 42–44). Furthermore, this strategy produces CD8+ neoantigen-specific T cells and memory T cells, and broadens the TCR repertoire of T cells, intensifying steps 3 and 4, which can further lead to tumor regression (16). Meanwhile, other studies have shown that clonally expanding neoantigen-reactive cells within tumor infiltrating lymphocytes (TILs) expressed PD-1 orPD-L1, suggesting that neoantigen vaccines could create a proper setting for ICIs and lay a foundation for the combination (31, 45). In conclusion, a potent personalized cancer vaccine with strong immunogenicity, can diversify the tumor-specific T cell repertoire, activate immune systems, activate robust effective T cells responses, and enhance the efficacy of ICIs.
Overcome Acquired Resistance of Immune Checkpoint Inhibitors
Effective ICIs result in immune-editing, which will result in subpopulation change, depletion of neoantigens, T cells expansion constraint and finally resistance to ICIs (46–49). Neoantigen repertoire variation can also lead to acquired resistance to ICIs (50). Immune-editing and variation of neoantigen repertoire are involved in many steps of the immunity cycle. In a study, patients with non-small cell lung cancer, who became resistant to ICIs after initial response, experienced the evolution of tumor neoantigens. Neoantigens, which were targets of initial response to ICIs, were eliminated in this process (50). Meanwhile, another study showed that loss of neoantigens could deter the specific T cells expansion (51). However, in the study, two patients with recurrent tumor lesions and resistant to ipilimumab, had tumor regression after injecting neoantigen vaccines (52). Researchers have tried to explore the influence of neoantigen cancer vaccines on neoantigen-specific T cell receptor repertoire, and they found that vaccination with neoantigens could elevate TCR-β clonotypes (52). What’s more, neoantigen vaccines, which can provide strong immunogenicity to activate immune system based on the neoantigen variation spectrum, eventually overcome the acquired resistance (50). Therefore, identifying neoantigens in the tumor evolution and listing them as targets of personalized cancer vaccines can improve antitumor efficacy of ICIs in patient with resistance. Neoantigen vaccines not only speed the proceeding of the immunity cycle, but also augment some crucial points in the activity such as steps 3 and 4.
Immune Checkpoint Inhibitors Overcome Suppressive Microenvironment
The suppression of tumor immune microenvironment is the main reason for the failure of neoantigen vaccine alone to control tumor. Some researchers found that after the neoantigen vaccine was applied, the expression of PD-1 on neoantigen-specific T cells and PD-L1 on tumor cells increased. Meanwhile, compared to CD8+ TILs, neoantigen-specific TILs displayed a more exhausted-like phenotype, which indicated that neoantigen vaccines could contribute to the inhibitory immune microenvironment (1, 13, 53–55). Both conditions weaken the potency of steps 3, 6 and 7. Some researchers tried to investigate the effectiveness of ICIs to overcome the immune-suppression and prove that ICIs could diminish the suppression of immune system and help induce strong T cells targeting at neoantigen epitopes (18). Schumacher and colleagues conducted some studies and indicated that adding ICIs to the treatment of neoantigen vaccine could elevate neoantigen T-cell response (18). In another study, in one out of three melanoma patients who have a relapse and distant metastasis after vaccination with neoantigens, a complete response was observed by subsequent pembrolizumab treatment (53). ICIs can mitigate the impact of inhibitory factors such as PD-1, PD-L1 and CTLA-4 to the Cancer-Immunity Cycle. The general hypothesis is that ICIs may unleash neoantigens with less immunogenicity or reactivate T cells with exhausting phenotypes to enhance antitumor effects (13, 24, 56–59). Increasing studies certify that ICIs can relieve immune inhibition in neoantigen vaccine, and have a promising prospect (50, 60).
Preclinical and Clinical Trials and Recent Development
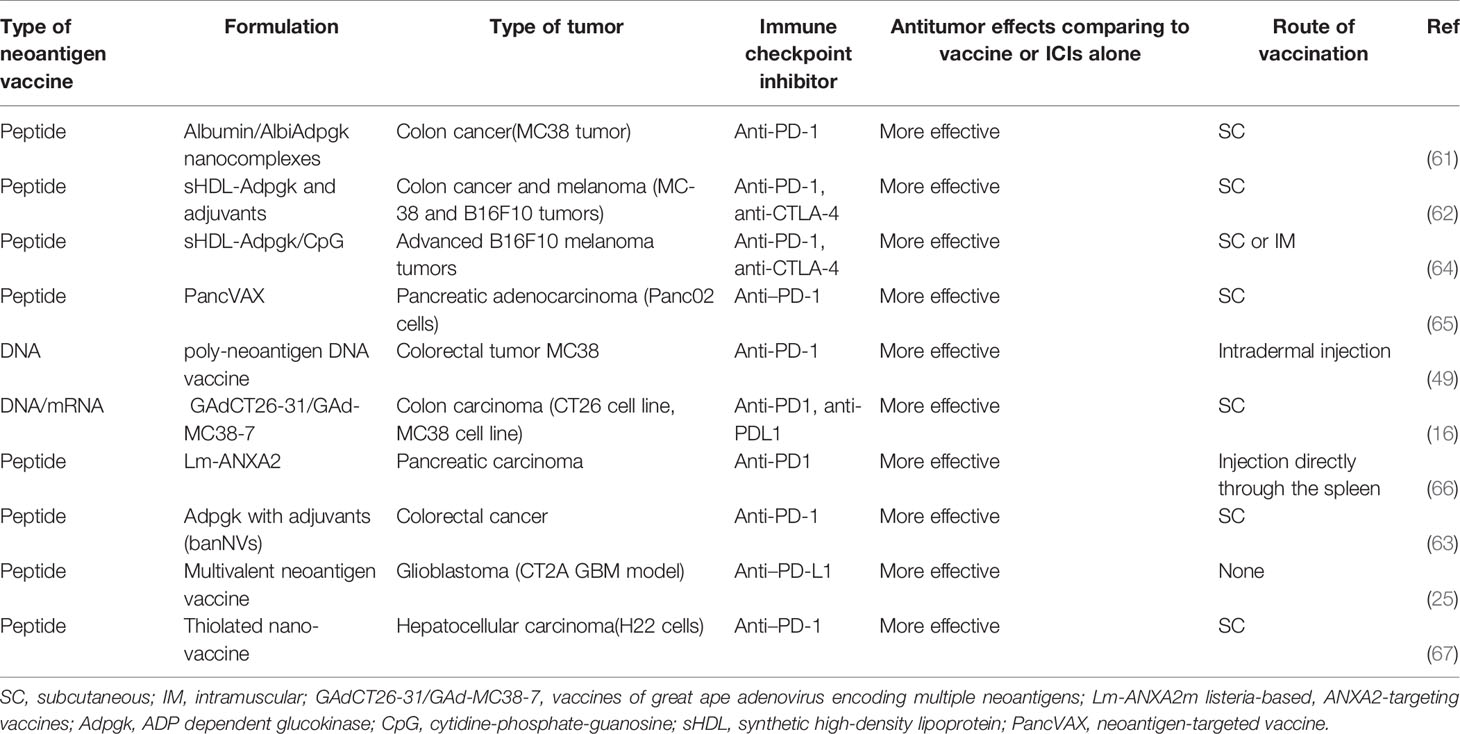
A personalized cancer vaccine, when combined with the ICIs, has shown efficacy in many preclinical trials. Meanwhile, there are many finished and on-going clinical trials trying to further prove the efficacy of the combination in real world.
Recent Preclinical Trials of the Combinatory Modality
In the aggressive glioblastoma CT2A murine model, researchers generated the neoantigen vaccine comprising 27-mer peptides targeting the mutant Plin2G332R, Pomgnt1R497L, and Epb4H471L neoepitopes, as well as poly-ICLC adjuvant. Mice treated either with vaccine or anti-PD-L1 alone exhibited a median overall survival of 17.5 and 25 days, respectively. In contrast, 60% of mice treated with vaccine and anti-PD-L1 blockade demonstrated long-term survival. What’s more, tumor-infiltrating neoepitope-specific CD8 T cells increased in the combinatory condition (25). In five murine colon carcinoma models, researchers investigated the clinical efficacy of the combining a peptide or DNA vaccine with anti-PD-1/L1 and anti-CTLA-4. The neoantigen vaccines combined with immune checkpoint inhibitors can potentiate neoantigen-specific immunity, elicit robust and long-lived T-cell response with a more diversified TCR repertoire and potentially inhibit even eradicate tumors without re-challenge (16, 49, 61–63). Furthermore, three studies have initially proven the central role of CD8+ T cells in the combination regimen by depletion of CD8+ T cells, CD4+ T cells and natural killer 1.1, respectively (16, 49, 63). Besides, vaccines with nanocomplexes markedly improve Ag/adjuvant co-delivery to lymphoid organs and sustain Ag presentation on dendritic cells (61–64). In two highly aggressive and poorly immunogenic murine models of B16F10 melanoma, researchers obtain the similar result that neoantigen vaccine combined with ICIs can initiate potent anti-tumor efficacy (62, 64). Additionally, Kuai et al., also reported vaccines administered via the subcutaneous (SC) or intramuscular (IM) routes were well tolerated in mice without any significant systemic or local toxicity, whereas SC could more efficiently deliver vaccines and intensify neoantigen-specific T cells responses (64). Finally, Panc02 cells models provide proof of concept that triple therapy with PancVAX (a personalized cancer vaccine), anti-PD-1, and agonist OX40 induces vaccine-specific TILs, lower the threshold for T cell activation, and reducing TIL exhaustion markers such as LAG3 and PD-1. In KPC mice (with KRAS and p53 mutations, pancreatic ductal adenocarcinoma), a “cold” tumor, combination treatment can also elicit objective tumor responses and prolonged survival. More importantly, this study shows that sequential combination treatment, neoantigen vaccine prior to anti-PD-1 antibodies significantly increased INF-γ expression and cure rates compared to single regimen or in combination with anti-PD-1 blockadeconcurrently (65, 66). What’s more, in a hepatic cell cancer model, similar results are reported (67). These preclinical trials are displayed in Table 1.
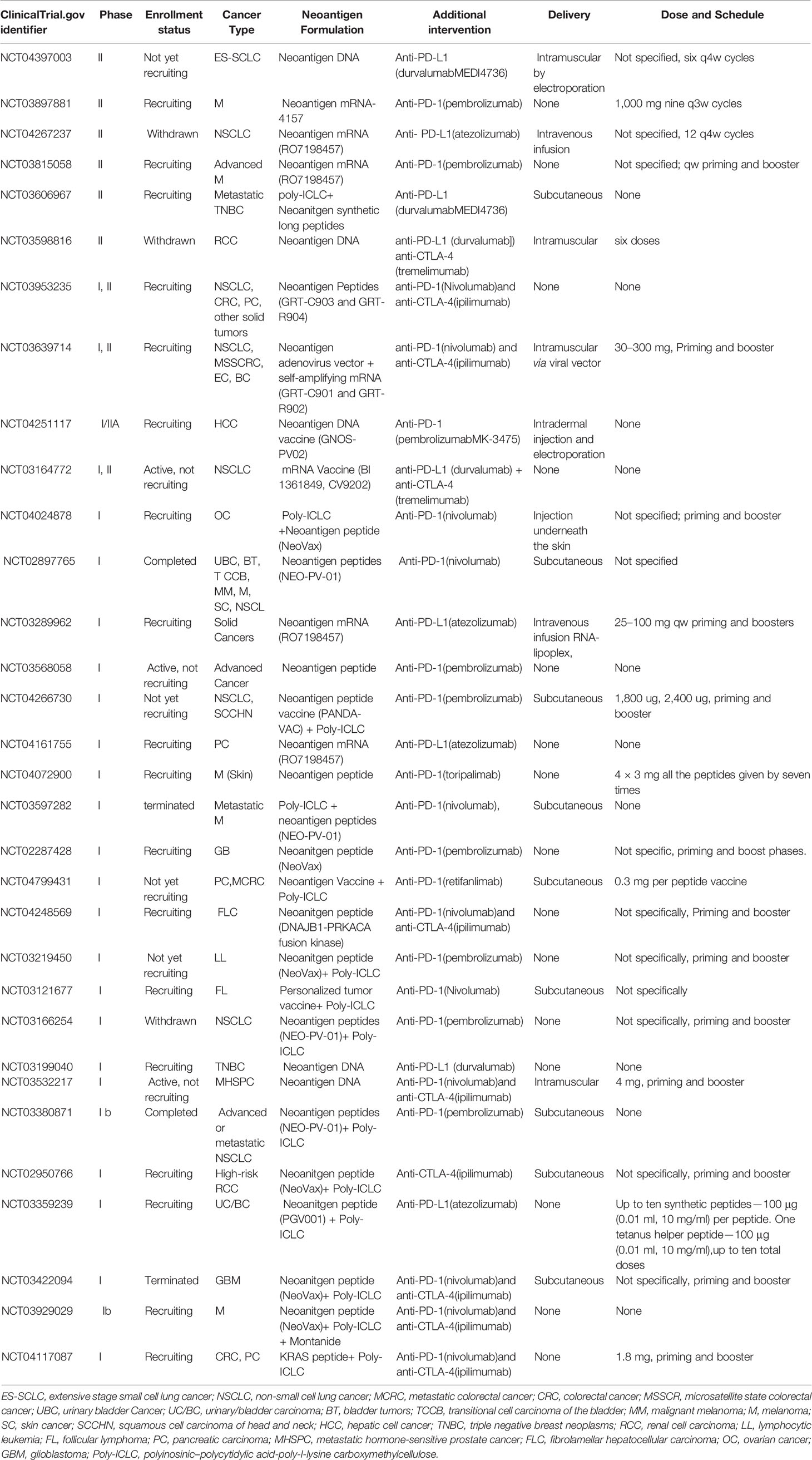
Recent Completed and Ongoing Clinical Trials
The first open-label phase IB clinical trial (NCT02897765) of a personalized neoantigen-based vaccine, NEO-PV-01, in combination with PD-1 blockade, included 82 patients with advanced solid tumors. Analyzing 82 patients, the median progress free survival (PFS) among vaccinated patients was 23.5, 8.5, and 5.8 months in the melanoma, NSCLC, and bladder cancer cohorts, respectively. The median OS for vaccinated patients was not reached in the melanoma and NSCLC cohorts, while for the bladder cancer cohort, the median OS was 20.7 months. The primary objective of the study was to evaluate the safety and tolerability of NEO-PV-01 in combination with nivolumab. The most common adverse events in vaccinated patients were injection-site reactions and influenza-like illness (52 and 35% of the patients, respectively). No treatment-related serious adverse events were observed. These data support the safety and immunity of this regimen in patients with advanced solid tumors (68).
A phase IB study (NCT03289962) evaluated RO7198457, an individualized neoantigen-specific Immunotherapy (iNeST), in combination with atezolizumab in 144 patients with locally advanced or metastatic solid tumors. RO7198457 is a kind of mRNA vaccine including up to 20 neoantigens. In 108 analyzable patients, nine patients responded to the therapy (ORR 8%) including one complete response (CR) and 54 patients (49%) experienced stable disease (SD). The vaccine induced neoantigen-specific T cell response in 77% patients. The combination was well tolerated, and most adverse events were infusion-related reaction, fatigue, cytokine release syndrome, nausea, pyrexia in over 10% patients, classified as grade 1 or 2. No increase in immune-mediated AEs compared with anti-PD-1 alone. Results prove that the combination of RO7198457and atezolizumab is safe and effective, which can induce significant neoantigen-specific immune response (69).
Another phase I multicenter study (NCT03313778) is to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in 13 patients with resected solid tumors and in combination with pembrolizumab in 20 patients with unresectable solid tumors. Indications include melanoma, NSCLC, MSI-high CRC, metastatic cutaneous squamous cell cancer, bladder cancer and so on. mRNA-4157, is a lipid-encapsulated RNA-based neoantigen-based vaccine. Of the 13 patients, 12 patients remain disease free on study with median follow-up of 8 months. While in another 20 patients, researchers observed one CR, two partial responses (PR), five SD, five progressive diseases (PD), two introduced immune unconfirmed progressive disease, and one patient non-evaluable for response. No dose of limited toxicity (DLT) and no drug related SAEs or AEs ≥ grade 3were reported, and treatment related AEs have generally been of low grade and reversible. These results also demonstrate the antitumor efficacy and safety of the combination with pembrolizumab and neoantigen-specific T cells, proceeding mRNA-4157 to phase2 (70, 71).
ADXS-NEO-02 is an ongoing Phase 1 trial (NCT03265080), which preliminarily investigates the safety and efficacy of ADXSNEO alone and in combination with anti-PD-1 antibody therapy in solid tumors. ADXS-NEO is composed of the listeriolysin O(tLLO) and personalized tumor antigens with 20–21mer peptides. This initial result reveals the optimal dose of ADXS-NEO with safety and efficacy. Only mild/moderate, controlled, and reversible events (e.g., chills, fever, tachycardia) were observed at this level. Efficient and rapid priming of substantial CD8+ T cells against most neoantigens and increased secretion of chemokines consistent with T-cell trafficking into tumor microenvironment also existed. Further investigation results about the combination of ADXS-NEO + anti-PD-1 antibody therapy are waiting (72).
Another ongoing phase I/IIA study (NCT03633110), is exploring the tolerability and antitumor activity of GEN-009 combined with anti-PD-1/L1 in multiple advanced tumors. GEN-009 is a personalized neoantigen-based vaccine comprising 4–20 synthetic long peptides formulated with poly-ICLC. The preliminary study of GEN-009 alone in solid tumors displayed that neoantigen provoked sustained peripheral neoantigen-specific CD4+ T cell and CD8+responses in all eight patients. Besides, repeated dosing has been well tolerated with mild local discomfort and no DLT. Further data from this trial about the vaccine in combination with PD-1 inhibitors in patients with advanced tumors, are awaited (71, 73).
Another open-label, phase IB study (NCT03380871) of NEO-PV-01 with pembrolizumab plus chemotherapy in patients with advanced or metastatic non-squamous non-small cell lung cancer is completed, and the results are expected. More clinical trials combining personalized vaccines with immune checkpoint inhibitors are listed in Table 2.
Safety and Toxicity Associated with the Combination Modality
The application of ICI therapy comes with the possibility of occurring immune related adverse events (IRAE). IRAE are regarded as an “over-activation” of the immune system leading to autoimmune inflammatory events affecting virtually any organ, most commonly the skin, gastrointestinal tract, liver, endocrine system and lung (74–76). In this review, we have highlighted the combination of neoantigen vaccine with ICI. Neoantigen vaccine and ICI both effect via provoking the immune system. Consequently, there is a relevant concern that the combination may lead to excessive toxicity. Overall, clinical experience with the combination strategies discussed in this review is limited. However, in a phase I trial (NCT02897765 n = 82) evaluating the safety and tolerability of NEO-PV-01 in combination with nivolumab, injection-site reactions (52%) and influenza-like illness (35%) are most common. Injection site reactions such as warmth and erythema, were often reversible and mild (Common Terminology Criteria for Adverse Events [CTCAE] grade 1), except one patient with a grade 2 injection site erythema. Drug-related events of grade ≥3 severity appeared in two patients. One patient with grade 2 gastritis discontinued the treatment. There is no treatment-related serious adverse events (68). In another phase Ib study (NCT03289962 n=142) to assess RO7198457 in combination with atezolizumab, immune-mediated adverse events (AEs) in atezolizumab alone were similar to those of the combination in >10% of patients include infusion-related reaction (60%), cytokine release syndrome (15%), influenza-like illness (10%), fatigue (30%), nausea (22%), pyrexia (15%), diarrhea (19%), decreased appetite (15%), vomiting (14%), headache (12%), cough (15%), dyspnea (15%), arthralgia (10%), constipation (15%),anemia (12%). Individual signs and symptoms of systemic reaction in more than five patients involve pyrexia, chills, nausea, tachycardia, headache, vomiting, hypertension, myalgia, back pain, fatigue, and hypoxia. 1% patients had grade 3 infusion-related reactions, anemia, fatigue, dyspnea, vomiting, nausea, pyrexia, diarrhea, headache, respectively. Most AEs are grade 1 or 2, and systemic reactions were transient and generally manageable in the outpatient setting. No grade 4 or 5 AE was observed (69). InmRNA-4157 trial, there was also no serious treatment related AEs, with only low grade and reversible reactions. Generally speaking, initial results of studies prove the treatment regimen of neoantigen vaccine combined with ICIs efficient and safe. Whereas, the actual risk for severe adverse events with combinations and potential factors influencing safety such as dose, delivery platforms, ways of administration, adjuvant, personal status, will be required to more and larger randomized studies.
Main Considerations Relating to Therapeutic Combinatory Regimen
Various factors may affect the anti-tumor efficacy in the combinatory modality. For neoantigen vaccine, neoantigen selection is critical, which will determine the production of antigen-specific T cells and eventually influence the anti-tumor response. The pipeline for neoantigen identification includes five main steps, and details have been extensively reviewed in other reviews (77–79). Unfortunately, there is no perfect method to predict appropriate neoantigen without bias and negatively positive. What’s more, the selection of proper delivery platforms is also crucial. Seven kinds of vectors are explored, including synthetic peptides, messenger RNA, DNA plasmids, viral vectors (adenoviral and vaccinia), engineered attenuated bacterial vectors (Salmonella, Listeria), e x vivo antigen-loaded DCs, and nanodiscs. Their advantages and disadvantages have been listed in detail in previous reviews (80, 81). The central problems are the delivery efficacy and manufacturing time, which play an important role in initiating enhanced T cell responses to inhibitor tumors. The sequence of manufacturing time from shorter to long is approximately tumor lysate-pulsed DCs, DNA/RNA, peptide, neoantigen-pulsed DC (71). For both peptide and mRNA vaccine platforms, time less than 4 weeks is expected (80). Additional variables include the route of administration of the vaccine, total number of doses, and induction (priming) and booster (maintenance) intervals. In a preclinical trial, Rui Kuai et al. declared that subcutaneous injection, comparing with intramuscular way, have stronger capability to deliver vaccines and provoke neoantigen-specific T cells responses (64). With the dose increasing, the immune response is intensified and the occurrence of immune related adverse events also elevate. The key point is to find the balance between efficacy and safety (82–87). Owing to lack of more clinical data, further studies about dose and administrating route are urgently required (64). When it comes to the combination, a vital problem emerges, the sequence of administration of neoantigen vaccines and ICIs. Pre-vaccination promote baseline immunity, significantly increase expression of INF-γ, Fms-related tyrosine kinase 3 ligand (FLT3L) and granulocyte–macrophage colony-stimulating factor(GM-CSF), and help “cold” tumor, such as glioblastoma and pancreatic ductal carcinoma, respond to ICIs (65, 66, 71). Additionally, administration of ICIs at vaccination or post-vaccination can boost vaccine-induced immune response. Unfortunately, limited data is presented, so further endeavors are necessary to explore the proper sequence of administration. To monitoring antitumor efficacy, applications of gene sequencing and single-cell sequencing technologies might find why resistance appear and discover the alternative neoantigens.
Challenges and Improvements
Though the combination of neoantigen vaccines and ICIs is promising in personalized treatment, there are still many problems.
Selection of Population Who May Potentially Benefit From the Combination
ICIs and neoantigen vaccines alone can only benefit a fraction of patients, efforts to find out the most proper population to receive the combination therapy are urgently required (88).
Recent studies have shown that somatic mutation, neoantigen burden and neoantigen density associated with long-term benefit from immune checkpoint inhibitors in several solid tumors, which indicated that more mutation-associated neoantigens may enhance the immunogenicity and improve immune responses of ICIs (19, 44, 89). The possible explanation is that higher mutational burden provides a base to generate more immunogenic neoantigens. Furthermore, researchers have found there were neoantigen-specific T cells in the peripheral blood in patients with tumor regression, and this also demonstrated that some neoantigens indeed could activate T cell which had an antitumor effect (19, 44, 88, 90). Therefore, neoantigen burden may be a significant predictor for combinational immunotherapy to distinguishing responders from non-responders.
However, some studies also revealed that responders to ICIs were not restricted to patients with high neoantigen burden, which demonstrated that not only the quantity of neoantigens is significant, rather their “quality” is vital as well (91, 92). For example, in melanoma, an effective antitumor CD8+ T cells response can be produced by a few epitopes, which have affinity to TCRs (26, 43). Another instance, renal cell carcinoma (RCC) has only a moderate to low mutation rate; however, it is universally known that RCC has a good response to immunotherapy (93). Researchers tried to explain this phenomenon and discovered that RCC possess the highest level of insertion and deletion type (indel) mutations, which are regarded to frequently create a new open reading frame and a higher proportion of neoantigens (94). Considering the quality of mutation into the prediction is also significant.
What’s more, lower neoantigen intra-tumor heterogeneity (ITH) is correlated with significantly longer progression-free survival. Neoantigen heterogeneity has a high variable rate with an average of 44% neoantigens found heterogeneously, in a subset of tumor regions (range of 10 to 78%) (55). Researchers have observed that lower hazard ratios when considering both neoantigen burden and ITH compared with the use of neoantigen burden alone to screen the potential population (55). Additionally, tumors with low ITH had an elevated PD-L1 expression (55). Thus, combining heterogeneity selecting a proper threshold with neoantigen burden is critical to find out the population who may benefit from ICIs and neoantigen vaccines.
Effectively controlling tumors with personalized vaccines and ICIs, is associated with neoantigen-specific T cells, which can exist in peripheral blood and show a phenotype different from patients without a response. Further studies to investigate characterization of neoantigen-specific T cells in responder patients, showed that there was relatively high expression of the activation markers CD161, TIGIT, 2B4 and KLRG1,low level of expression of inhibition markers CD27, CD28 and CD127, and a high level of co-inhibitory molecules, including PD-1 (95, 96). These results indicate that identification and characterization of neoantigen-specific T cells may contribute to predict who will response to the combination therapy.
Neoantigens Heterogeneity and Dynamic Variation of Neoantigen Landscape
Neoantigens arise from tumor-specific mutations, and they are variable in different tumors or patients. ITH has a significant impact on the response to immunotherapies. In addition, in patients who initially respond to ICIs, tumors could experience an evolution of epitopes, which will alter the neoantigen landscape and lead to sequential resistance. More importantly, mechanisms of ITH and the dynamic variation of neoantigens landscape are unclear for now (13, 97, 98). We just observed that high heterogeneity may lead to a poorer response to both vaccination and ICIs. For this problem, researchers provide that designing a neoantigen vaccine with multiple targets and based on the variation landscape may help overcome ITH and the dynamic alteration (55).
Identification of Potential Neoantigens
Exact identification of the neoantigens is capable of producing potent epitopes which can be recognized specifically by TCRs and induce strong immune response. However, this is difficult to achieve in current silico predicting systems (49). Some studies showed that in predicted neoantigens, only a fraction (20%) had immunogenicity, demonstrating the low accuracy of current neoantigens prediction algorithms (99, 100). For now, there is no single method providing an accurate and reliable prediction and identification of neoantigens. The possible solutions for this problem are as following. One method is designing multi-epitope vaccines. Using of a platform which can accommodate a large number of neoantigens, may help overcome limits of the prediction algorithms. In a study, combing treatment with the adenoviral vaccine targeting 31 neoantigens increased the number of mice with complete regression (~50%), which may be due to the increasing specific T-cell clones (8). These findings demonstrate that multi-epitope vaccines may be a solution for inaccuracy of the bioinformatics tools for predicting neoantigens (8). Another option is to broaden our analysis of potential neoantigens to include other types of potentially immunogenic alterations, for current predicted neoantigens are mainly resulting from missense mutations (2). For example, chromosomal insertions, inversions, and translocations can lead to fusion transcripts, which exist in certain cancers, such as chronic myelogenous leukemia, lung cancer, bladder cancer, and ovarian cancer (101, 102).
Faster and Cost-Effective Vaccine Production
Currently, although the emergence of NGS, WES and other techniques improve the identification and production of neoantigen vaccines, verifying neoantigens and vaccines generation are still time-consuming and expensive, and usually 3–5 months are required to prepare vaccines from tumor samples (26, 43). Additionally, problems that the identification of neoantigens needs lots of tumor tissues, while the yield of usable epitopes or neoantigens is very low, and it is difficult to solve for technical limits (103). These obstacles have been largely hampering the development of neoantigen vaccines in clinical settings. There is an urgent need to develop better neoantigen prediction algorithms and manufacturing technologies which can decrease the price and shorten the time (4, 104). Efforts for this work are going on, and Anna have established a fast process assembling 60 unique patient mutanome-specific neoantigens and producing personalized adenoviral vaccines within 6 weeks from the time of patient biopsy (16).
Safe and Efficient Delivery System
To generate a potent neoantigen vaccine, a safe and efficient delivery system is needed, which can help induce strong immune response. Effector T cells can be induced by the specific antigens or epitopes within the tumor cells and neoantigen-specific T cells clonal expansion in tumors symbols the effective antitumor response. Neoantigen vaccines based on virus could be the proper candidates to produce potent antitumor immunity. Many studies have proven adenoviruses were powerful genetic vaccine platforms with unique feature encoding for large antigen to activate effective CD8+ and CD4+ T-cell responses safely (105–107). In a preclinical study, a adenoviral vaccine encoding 31 neoantigens, the largest number used so far for neoantigen-based vaccines, selected from the murine CT26 colon carcinoma cell line, produced potent antitumor immune responses, and more than 1,000 antigen-specific IFN-γ secreting lymphocytes/million splenocytes, CD8+ and CD4+ T lymphocytes were generated (16).
Low Efficacy of Neoantigen Vaccines
In preclinical trials, researchers did not detect neoantigen-specific T cells in untreated mice with tumors. The possible reasons are showed below: the inadequate mutated gene expression; antigen can’t be presented effectively due to low affinity to HLA or TCRs on T cells; dynamic variation of neoantigen landscape leading to dominant neoantigens eliminated; T cells in the repertoire which can bind to mutant neoepitopes are in short or undergoing apoptosis (65). Two approaches are capable of enhancing neoantigen-specific T cell responses and improving the antitumor potency.
One is to use a potent adjuvant to stimulate innate immunity. In some preclinical studies, researchers found that adding an agonist OX40 antibody to PancVAX, decreased T cell exhausted markers, such as Lag3 and PD-1, and helpedCD4+ T cell avoid immunosuppressive Treg phenotype (108, 109). What’s more, FoxP3+CD4+T cells decreased and tumor-specific IFN-γ-secreting CD4+ T cells appeared when combining PancVAX with OX40 (65). Furthermore, OX40 is capable of increasing the survival rate of antitumor T cells with low avidity (94). These findings suggest that an effective adjuvant can produce neoantigen-specific TILs, help activate T cells and maintain TILs through survival improvement.
Another strategy to improve the efficacy of neoantigen vaccines is taking MHC class II peptides into vaccination design (88). The clonal expansion of tumor-specific T cells with potent antitumor ability is the core of the success in the personalized cancer immunotherapy (49). Kreiter and colleagues conducted a study suggesting that many personalized cancer vaccines with immunogenic neoantigens were correlated to MHC class II molecules on CD4+ helper T cells (110). In the study, PancVAX played its role to control or shrink tumors primarily through CD8+ T cells, meanwhile, CD4+ T cells may have influence as well. Researchers further investigated the influence of both CD8+ T cells and CD4+ T cells, and noted that antitumor response disappeared when depleting CD8+ T cells while partial responses loss with CD4+ T cells eliminated. The successful example of immunotherapy in melanoma was due to existence of epitopes targeting both CD4+ and CD8+ T cells (16, 20, 26, 65). Therefore, vaccination with neoantigens targeting both CD8+ T cells and CD4+ T cells is vital to induce powerful antitumor immune responses, for the primary immune driver CD8+ T cells can work better with the synergy of CD4+ T cells (111, 112).
Dose and Sequence of ICIs and Vaccines
Though many preclinical and clinical trials investigate the safety and efficacy of the combination of ICIs and neoantigen vaccines, dose and schedule for ICIs and vaccines have been minimally studied. Some researchers found that after the response to interferon secreted by T cells, both PD-1 on activated T cells and PD-L1 on tumors emerged in a short time, which supports that using ICIs first or concomitant with vaccines may be both rational (113). In some studies, after vaccination with neoantigens in patients, the expression of both PD-1 on neoantigen-specific T cells and PD-L1 in tumor tissues increased, and anti-PD-1 or anti-PD-L1 immunotherapy improved the efficacy of vaccines, suggesting that administering neoantigen vaccines before ICIs may have a greater opportunity to achieve the maximal antitumor response (1, 13, 20, 36, 53–55). We have searched up many preclinical and clinical trials, unfortunately, we didn’t find a standard sequence or dose for the combination, which need further exploration.
Conclusion
Neoantigen vaccines alone have a limited efficacy, and ICIs has been limited to a minority of patients with certain cancer types. However, the combination of personalized vaccines and ICIs can significantly improve the antitumor efficacy with minimal additional toxicity compared to either single method by improving sensitivity and efficacy of ICIs, overcoming acquired resistance of ICIs and relieving suppressive microenvironment. This has led to envisaging and developing combined strategies that might augment tumor regression and prolong overall survival for patients with metastatic cancer. However, there still some important aspects for the combination to achieve the maximal efficacy, including optimizing the identification, predication and production of neoantigen vaccines, selecting proper population for the combination therapy, and the optimized dose and sequence of the two agents.
Author Contributions
SZ contributed conception and overall idea of the study. J-YL wrote the first draft of the manuscript. SZ wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank the colleagues in Department of Biotherapy, Cancer Center, West China Hospital.
Abbreviations
CRT, calreticulin; ATP, adenosine triphosphate; HMGB1, High Mobility Group Box 1; HSPs, heat shock proteins; LN, lymph node; DCs, dendritic cells; CTLs, cytotoxic T lymphocytes; IDO, indoleamine 2,3-dioxygenase; mAb, monoclonal antibody; LAG3, Lymphocyte activation gene 3; BTLA, B and T lymphocyte associated gene.
References
1. Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong F, et al. Neoantigen Vaccine: An Emerging Tumor Immunotherapy. Mol Cancer (2019) 18(1):128. doi: 10.1186/s12943-019-1055-6
2. Hacohen N, Fritsch EF, Carter TA, Lander ES, Wu CJ. Getting Personal With Neoantigen-Based Therapeutic Cancer Vaccines. Cancer Immunol Res (2013) 1(1):11–5. doi: 10.1158/2326-6066
3. Tran E, Robbins PF, Rosenberg SA. ‘Final Common Pathway’ of Human Cancer Immunotherapy: Targeting Random Somatic Mutations. Nat Immunol (2017) 18(3):255–62. doi: 10.1038/ni.3682
4. Aldous AR, Dong JZ. Personalized Neoantigen Vaccines: A New Approach to Cancer Immunotherapy. Bioorg Med Chem (2018) 26(10):2842–9. doi: 10.1016/j.bmc.2017.10.021
5. Nakajima H, Nakatsura T. Towards the Era of Immune Checkpoint Inhibitors and Personalized Cancer Immunotherapy. Immunol Med (2020) 9:1–6. doi: 10.1080/25785826.2020.1785654
6. Pardoll DM. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
7. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival With Ipilimumab in Patients With Metastatic Melanoma. N Engl J Med (2010) 363(8):711–23. doi: 10.1056/NEJMoa1003466
8. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, Activity, and Immune Correlates of anti-PD-1 Antibody in Cancer. N Engl J Med (2012) 366(26):2443–54. doi: 10.1056/NEJMoa1200690
9. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab Versus Ipilimumab in Advanced Melanoma. N Engl J Med (2015) 372(26):2521–32. doi: 10.1056/NEJMoa1503093
10. Bassani-Sternberg M, Digklia A, Huber F, Wagner D, Sempoux C, Stevenson BJ, et al. A Phase Ib Study of the Combination of Personalized Autologous Dendritic Cell Vaccine, Aspirin, and Standard of Care Adjuvant Chemotherapy Followed by Nivolumab for Resected Pancreatic Adenocarcinoma-A Proof of Antigen Discovery Feasibility in Three Patients. Front Immunol (2019) 10:1832. doi: 10.3389/fimmu.2019.01832
11. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell (2017) 168(4):707–23. doi: 10.1016/j.cell.2017.01.017
12. Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, et al. Evolution of Neoantigen Landscape During Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Discovery (2017) 7(3):264–76. doi: 10.1158/2159-8290.CD-16-0828
13. Yi M, Qin S, Zhao W, Yu S, Chu Q, Wu K. The Role of Neoantigen in Immune Checkpoint Blockade Therapy. Exp Hematol Oncol (2018) 7:28. doi: 10.1186/s40164-018-0120-y
14. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive Correlates of Response to the anti-PD-L1 Antibody MPDL3280A in Cancer Patients. Nature (2014) 515(7528):563–7. doi: 10.1038/nature14011
15. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature (2014) 515(7528):568–71. doi: 10.1038/nature13954
16. D’Alise AM, Leoni G, Cotugno G, Troise F, Langone F, Fichera I, et al. Adenoviral Vaccine Targeting Multiple Neoantigens as Strategy to Eradicate Large Tumors Combined With Checkpoint Blockade. Nat Commun (2019) 10(1):2688. doi: 10.1038/s41467-019-10594-2
17. Fritsch EF, Hacohen N, Wu CJ. Personal Neoantigen Cancer Vaccines: The Momentum Builds. Oncoimmunology (2014) 3:e29311. doi: 10.4161/onci.29311
18. van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, et al. Tumor Exome Analysis Reveals Neoantigen-Specific T-cell Reactivity in an Ipilimumab-Responsive Melanoma. J Clin Oncol (2013) 31(32):e439–42. doi: 10.1200/JCO.2012.47.7521
19. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N Engl J Med (2014) 371(23):2189–99. doi: 10.1056/NEJMoa1406498
20. Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint Blockade Cancer Immunotherapy Targets Tumour-Specific Mutant Antigens. Nature (2014) 515(7528):577–81. doi: 10.1038/nature13988
21. Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 Combination Blockade Expands Infiltrating T Cells and Reduces Regulatory T and Myeloid Cells Within B16 Melanoma Tumors. Proc Natl Acad Sci USA (2010) 107(9):4275–80. doi: 10.1073/pnas.0915174107
22. Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 Blockade Synergizes With Tumor-Derived Granulocyte-Macrophage Colony-Stimulating Factor for Treatment of an Experimental Mammary Carcinoma. Proc Natl Acad Sci U S A (1998) 95(17):10067–71. doi: 10.1073/pnas.95.17.10067
23. Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual Blockade of PD-1 and CTLA-4 Combined With Tumor Vaccine Effectively Restores T-cell Rejection Function in Tumors. Cancer Res (2013) 73(12):3591–603. doi: 10.1158/0008-5472.CAN-12-4100
24. Lee CH, Yelensky R, Jooss K, Chan TA. Update on Tumor Neoantigens and Their Utility: Why it Is Good to Be Different. Trends Immunol (2018) 39(7):536–48. doi: 10.1016/j.it.2018.04.005
25. Liu CJ, Schaettler M, Blaha DT, Bowman-Kirigin JA, Kobayashi DK, Livingstone AJ, et al. Treatment of an Aggressive Orthotopic Murine Glioblastoma Model With Combination Checkpoint Blockade and a Multivalent Neoantigen Vaccine. Neuro Oncol (2020) 22(9):1276–88. doi: 10.1093/neuonc/noaa050
26. Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An Immunogenic Personal Neoantigen Vaccine for Patients With Melanoma. Nature (2017) 547(7662):217–21. doi: 10.1038/nature22991
27. Chen DS, Mellman I. Oncology Meets Immunology: The Cancerimmunity Cycle. Immunity (2013) 39:1–10. doi: 10.1016/j.immuni.2013.07.012
28. Corbiere V, Chapiro J, Stroobant V, Ma W, Lurquin C, Lethe B, et al. Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res (2011) 71:1253–62. doi: 10.1158/0008-5472.CAN-10-2693
29. Schreiber RD, Old LJ, Smyth MJ. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science (2011) 331:1565–70. doi: 10.1126/science.1203486
30. Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, et al. Cancer Exome Analysis Reveals a T-cell-dependent Mechanism of Cancer Immunoediting. Nature (2012) 482:400–4. doi: 10.1038/nature10755
31. Fu J, Malm I-J, Kadayakkara DK, Levitsky H, Pardoll D, Kim YJ. Preclinical Evidence That PD1 Blockade Cooperates With Cancer Vaccine TEGVAX to Elicit Regression of Established Tumors. Cancer Res (2014) 74:4042–52. doi: 10.1158/0008-5472
32. Sierro SR, Donda A, Perret R, Guillaume P, Yagita H, Levy F, et al. Combination of Lentivector Immunization and Low-Dose Chemotherapy or PD-1/PD-L1 Blocking Primes Self-Reactive T Cells and Induces Anti-Tumor Immunity. Eur J Immunol (2011) 41:2217–28. doi: 10.1002/eji.201041235
33. Morse MA, Lyerly HK. Checkpoint Blockade in Combination With Cancer Vaccines. Vaccine (2015) 33:7377–85. doi: 10.1016/j.vaccine.2015.10.057
34. Draube A, Klein-Gonzalez N, Mattheus S, Brillant C, Hellmich M, Engert A, et al. Dendritic Cell Based Tumor Vaccination in Prostate and Renal Cell Cancer: a Systematic Review and Meta-Analysis. PloS One (2011) 6:e18801. doi: 10.1371/journal.pone.0018801
35. Boudewijns S, Westdorp H, Koornstra RHT, Aarntzen EHJG, Schreibelt G, Creemers JHA, et al. Immune-Related Adverse Events of Dendritic Cell Vaccination Correlate With Immunologic and Clinical Outcome in Stage III and IV Melanoma Patients. J Immunother (2016) 39:241–8. doi: 10.1097/CJI.0000000000000127
36. Nesselhut J, Marx D, Lange H, Regalo G, Cillien N, Chang RY, et al. Systemic Treatment With anti-PD-1 Antibody Nivolumab in Combination With Vaccine Therapy in Advanced Pancreatic Cancer. J Clin Oncol (2016) 34:3092. doi: 10.1200/JCO.2016.34.15_suppl.3092
37. Philip R, Murthy S, Krakover J, Sinnathamby G, Zerfass J, Keller L, et al. Shared Immunoproteome for Ovarian Cancer Diagnostics and Immunotherapy: Potential Theranostic Approach to Cancer. J Proteome Res (2007) 6(7):2509–17. doi: 10.1021/pr0606777
38. Tang Z, Li D, Hou S, Zhu X. The Cancer Exosomes: Clinical Implications, Applications and Challenges. Int J Cancer (2020) 146(11):2946–59. doi: 10.1002/ijc.32762
39. von Bernstorff W, Voss M, Freichel S, Schmid A, Vogel I, Johnk C, et al. Systemic and Local Immunosuppression in Pancreatic Cancer Patients. Clin Cancer Res (2001) 7(3 Suppl.):925s–32s.
40. Schmidt J, Mocevicius P, Werner J, Ryschich E. The Role of the Tumor Endothelium in Leukocyte Recruitment in Pancreatic Cancer. Surgery (2012) 152(3 Suppl 1.):S89–94. doi: 10.1016/j.surg.2012.05.027
41. Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, et al. Immune Cell Infiltration as an Indicator of the Immune Microenvironment of Pancreatic Cancer. Br J Cancer (2013) 108:914–23. doi: 10.1038/bjc.2013.32
42. Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, et al. A Dendritic Cell Vaccine Increases the Breadth and Diversity of Melanoma Neoantigen-specific T Cells. Sci (2015) 348(6236):803–8. doi: 10.1126/science.aaa3828
43. Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, et al. Personalized RNA Mutanome Vaccines Mobilize Poly-Specific Therapeutic Immunity Against Cancer. Nature (2017) 547(7662):222–6. doi: 10.1038/nature23003
44. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer Immunology. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non-small Cell Lung Cancer. Science (2015) 348(6230):124–8. doi: 10.1126/science.aaa1348
45. Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, et al. PD-1 Identifies the Patient-Specific CD8+ Tumor-Reactive Repertoire Infiltrating Human Tumors. J Clin Invest (2014) 124:2246–59. doi: 10.1172/JCI73639
46. Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, et al. Tumor and Microenvironment Evolution During Immunotherapy With Nivolumab. Cell (2017) 171(4):934–49. doi: 10.1016/j.cell.2017.09.028
47. Snyder A, Chan TA. Immunogenic Peptide Discovery in Cancer Genomes. CurrOpin Genet Dev (2015) 30:7–16. doi: 10.1016/j.gde.2014.12.003
48. Alioto TS, Buchhalter I, Derdak S, Hutter B, Eldridge MD, Hovig E, et al. A Comprehensive Assessment of Somatic Mutation Detection in Cancer Using Whole-Genome Sequencing. Nat Commun (2015) 6:10001. doi: 10.1038/ncomms10001
49. Tondini E, Arakelian T, Oosterhuis K, Camps M, van Duikeren S, Han W, et al. A Poly-Neoantigen DNA Vaccine Synergizes With PD-1 Blockade to Induce T Cell-Mediated Tumor Control. Oncoimmunology (2019) 8(11):1652539. doi: 10.1080/2162402X.2019.1652539
50. Kim TM, Laird PW, Park PJ. The Landscape of Microsatellite Instability in Colorectal and Endometrial Cancer Genomes. Cell (2013) 155(4):858–68. doi: 10.1016/j.cell.2013.10.015
51. Dudley ME, Roopenian DC. Loss of a Unique Tumor Antigen by Cytotoxic T Lymphocyte Immunoselection From a 3-Methylcholanthrene-Induced Mouse Sarcoma Reveals Secondary Unique and Shared Antigens. J Exp Med (1996) 184(2):441–7. doi: 10.1084/jem.184.2.441
52. Xu H, DiCarlo J, Satya RV, Peng Q, Wang Y. Comparison of Somatic Mutation Calling Methods in Amplicon and Whole Exome Sequence Data. BMC Genomics (2014) 15:244. doi: 10.1186/1471-2164-15-244
53. Andor N, Graham TA, Jansen M, Xia LC, Aktipis CA, Petritsch C, et al. Pan-Cancer Analysis of the Extent and Consequences of Intratumor Heterogeneity. Nat Med (2016) 22(1):105–13. doi: 10.1038/nm.3984
54. McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal Neoantigens Elicit T Cell Immunoreactivity and Sensitivity to Immune Checkpoint Blockade. Science (2016) 351(6280):1463–9. doi: 10.1126/science.aaf1490
55. Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, et al. Predicting Immunogenic Tumour Mutations by Combining Mass Spectrometry and Exome Sequencing. Nature (2014) 515(7528):572–6. doi: 10.1038/nature14001
56. Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol (2017) 8:561. doi: 10.3389/fphar.2017.00561
57. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab Versus Docetaxel for Previously Treated, PD-L1-positive, Advanced non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
58. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in Patients With Locally Advanced and Metastatic Urothelial Carcinoma Who Have Progressed Following Treatment With Platinum-Based Chemotherapy: A Single-Arm, Multicentre, Phase 2 Trial. Lancet (2016) 387(10031):1909–20. doi: 10.1016/S0140-6736(16)00561-4
59. Tan S, Li D, Zhu X. Cancer Immunotherapy: Pros, Cons and Beyond. BioMed Pharmacother (2020) 124:109821. doi: 10.1016/j.biopha.2020.109821
60. Robbins PF, El-Gamil M, Li YF, Kawakami Y, Loftus D, Appella E, et al. A Mutated Beta-Catenin Gene Encodes a Melanoma-Specific Antigen Recognized by Tumor Infiltrating Lymphocytes. J Exp Med (1996) 183(3):1185–92. doi: 10.1084/jem.183.3.1185
61. Zhu G, Lynn GM, Jacobson O, Chen K, Liu Y, Zhang H, et al. Albumin/Vaccine Nanocomplexes That Assemble In Vivo for Combination Cancer Immunotherapy. Nat Commun (2017) 8(1):1954. doi: 10.1038/s41467-017-02191-y
62. Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer Vaccine Nanodiscs for Personalized Cancer Immunotherapy. Nat Mater (2017) 16(4):489–96. doi: 10.1038/nmat4822
63. Ni Q, Zhang F, Liu Y, Wang Z, Yu G, Liang B, et al. A Bi-Adjuvant Nanovaccine That Potentiates Immunogenicity of Neoantigen for Combination Immunotherapy of Colorectal Cancer. Sci Adv (2020) 6(12):eaaw6071. doi: 10.1126/sciadv.aaw6071
64. Kuai R, Sun X, Yuan W, Xu Y, Schwendeman A, Moon JJ. Subcutaneous Nanodisc Vaccination With Neoantigens for Combination Cancer Immunotherapy. Bioconjug Chem (2018) 29(3):771–5. doi: 10.1021/acs.bioconjchem.7b00761
65. Kinkead HL, Hopkins A, Lutz E, Wu AA, Yarchoan M, Cruz K, et al. Combining STING-based Neoantigen-Targeted Vaccine With Checkpoint Modulators Enhances Antitumor Immunity in Murine Pancreatic Cancer. JCI Insight (2018) 3(20):e122857. doi: 10.1172/jci.insight.122857
66. Kim VM, Blair AB, Lauer P, Foley K, Che X, Soares K, et al. Anti-Pancreatic Tumor Efficacy of a Listeria-based, Annexin A2-targeting Immunotherapy in Combination With anti-PD-1 Antibodies. J Immunother Cancer (2019) 7(1):132. doi: 10.1186/s40425-019-0601-5
67. Zhang D, Lin Z, Wu M, Cai Z, Zheng Y, He L, et al. Cytosolic Delivery of Thiolated Neoantigen Nano-Vaccine Combined With Immune Checkpoint Blockade to Boost Anti-Cancer T Cell Immunity. Adv Sci (Weinh) (2021) 8(6):2003504. doi: 10.1002/advs.202003504
68. Ott PA, Hu-Lieskovan S, Chmielowski B, Govindan R, Naing A, Bhardwaj N, et al. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients With Advanced Melanoma, Non-small Cell Lung Cancer, or Bladder Cancer. Cell (2020) 183(2):347–62. doi: 10.1016/j.cell.2020.08.053
69. Lopez J, Camidge DR, Iafolla M, Rottey S, Schuler M, Hellmann MD, et al. A Phase Ib Study to Evaluate RO7198457, an Individualized Neoantigen-Specific Immunotherapy (Inest). In: Combination With Atezolizumab in Patients With Locally Advanced or Metastatic Solid Tumors. AACR (2020). Available at: https://medically.roche.com/global/en/asset-viewer.6c343ffb-6afa-43f4-ae5d-9c7c0806d4c3.qr.html.
70. Burris HA, Patel MR, Cho DC, Clarke JM, Gutierrez M, Zaks TZ, et al. A Phase I Multicenter Study to Assess the Safety, Tolerability, and Immunogenicity of mRNA-4157 Alone in Patients with Resected Solid Tumors and in Combination with Pembrolizumab in Patients with Unresectable Solid Tumors. J Clin Oncol (2019) 37(Suppl. 15):2523. doi: 10.1200/JCO.2019.37.15_suppl.2523
71. Blass E, Ott PA. Advances in the Development of Personalized Neoantigen-Based Therapeutic Cancer Vaccines. Nat Rev Clin Oncol (2021) 18(4):215–29. doi: 10.1038/s41571-020-00460-2
72. Hecht JR, Goldman JW, Hayes S, Balli D, Princiotta MF, Dennie JG, et al. Safety and Immunogenicity of a Personalized Neoantigen-Listeria Vaccine in Cancer Patients. In: Presented at the American Association for Cancer Research (AACR) Annual Meeting, March 29–April 3 (2019). Available at: https://scholar.lanfanshu.cn/scholar?q=Safety+and+Immunogenicity+of+a+Personalized+Neoantigen-Listeria+Vaccine+in+Cancer+Patients.
73. Cohen RB, Johnson ML, Twardowski P, Stein MN, Vaishampayan UN, Dobson JR, et al. A Phase 1/2a Study of GEN-009, a Neoantigen Vaccine Based on Autologous Peptide Immune Responses. J Clin Oncol (2019) 37(15 Suppl):2611. doi: 10.1200/JCO.2019.37.15_suppl.2611
74. Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture, et al. Toxicities of the anti-PD-1 and anti-PD-L1 Immune Checkpoint Antibodies. Ann Oncol (2015) 26:2375–91. doi: 10.1093/annonc/mdv383
75. Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel, et al. Management of Immune Checkpoint Blockade Dysimmune Toxicities: a Collaborative Position Paper. Ann Oncol (2016) 27:559–74. doi: 10.1093/annonc/mdv623
76. Huemer F, Leisch M, Geisberger R, Melchardt T, Rinnerthaler G, Zaborsky N, et al. Combination Strategies for Immune-Checkpoint Blockade and Response Prediction by Artificial Intelligence. Int J Mol Sci (2020) 21(8):2856. doi: 10.3390/ijms21082856
77. Shibata H, Zhou L, Xu N, Egloff AM, Uppaluri R. Personalized Cancer Vaccination in Head and Neck Cancer. Cancer Sci (2021) 112(3):978–88. doi: 10.1111/cas.14784
78. Roudko V, Greenbaum B, Bhardwaj N. Computational Prediction and Validation of Tumor-Associated Neoantigens. Front Immunol (2020) 11:27. doi: 10.3389/fimmu.2020.00027
79. Holtsträter C, Schrörs B, Bukur T, Löwer M. Bioinformatics for Cancer Immunotherapy. Methods Mol Biol (2020) 2120:1–9. doi: 10.1007/978-1-0716-0327-7_1
80. Sahin U, Türeci Ö. Personalized Vaccines for Cancer Immunotherapy. Science (2018) 359(6382):1355–60. doi: 10.1126/science.aar7112
81. Liu Q, Duo Y, Fu J, Qiu M, Sun Z, Adah D, et al. Nano-immunotherapy: Unique Mechanisms of Nanomaterials in Synergizing Cancer Immunotherapy. Nano Today (2021) 36:101023. doi: 10.1016/j.nantod.2020.101023
82. Shemesh CS, Hsu JC, Hosseini I, Shen BQ, Rotte A, Twomey P, et al. Personalized Cancer Vaccines: Clinical Landscape, Challenges, and Opportunities. Mol Ther (2021) 29(2):555–70. doi: 10.1016/j.ymthe.2020.09.038
83. Mackiewicz J, Mackiewicz A. Design of Clinical Trials for Therapeutic Cancer Vaccines Development. Eur J Pharmacol (2009) 625(1-3):84–9. doi: 10.1016/j.ejphar.2009.09.069
84. Wages NA, Slingluff CL Jr, Bullock TN, Petroni GR. Tailoring Early-Phase Clinical Trial Design to Address Multiple Research Objectives. Cancer Immunol Immunother (2020) 69:95–102. doi: 10.1007/s00262-019-02442-5
85. Zhang L, Wang W, Wang S. Effect of Vaccine Administration Modality on Immunogenicity and Efficacy. Expert Rev Vaccines (2015) 14:1509–23. doi: 10.1586/14760584.2015.1081067
86. Lehmann PV, Zhang W. Unique Strengths of ELISPOT for T Cell Diagnostics. Methods Mol Biol (2012) 792:3–23. doi: 10.1007/978-1-61779-325-7_1
87. Kudrin A. Cancer Vaccines: What do We Need to Measure in Clinical Trials? Hum Vaccin Immunother (2014) 10:3236–40. doi: 10.4161/hv.27586
88. Desrichard A, Snyder A, Chan TA. Cancer Neoantigens and Applications for Immunotherapy. Clin Cancer Res (2016) 22(4):807–12. doi: 10.1158/1078-0432
89. Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic Correlates of Response to CTLA-4 Blockade in Metastatic Melanoma. Science (2015) 350(6257):207–11. doi: 10.1126/science.aad0095
90. Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer Immunotherapy Based on Mutation-Specific CD4+ T Cells in a Patient With Epithelial Cancer. Science (2014) 344(6184):641–5. doi: 10.1126/science.1251102
91. Balachandran VP, Łuksza M, Zhao JN, Makarov V, Moral JA, Remark R, et al. Identification of Unique Neoantigen Qualities in Long-Term Survivors of Pancreatic Cancer. Nature (2017) 551(7681):512–6. doi: 10.1038/nature24462
92. Łuksza M, Riaz N, Makarov V, Balachandran VP, Hellmann MD, Solovyov A, et al. A Neoantigen Fitness Model Predicts Tumour Response to Checkpoint Blockade Immunotherapy. Nature (2017) 551(7681):517–20. doi: 10.1038/nature24473
93. Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, et al. Pd-1/Pd-L1 Blockade Together With Vaccine Therapy Facilitates Effector T-cell Infiltration Into Pancreatic Tumors. J Immunother (2015) 38(1):1–11. doi: 10.1097/CJI.0000000000000062
94. Black CM, Armstrong TD, Jaffee EM. Apoptosis-Regulated Low-Avidity Cancer-Specific CD8(+) T Cells can be Rescued to Eliminate HER2/neu-expressing Tumors by Costimulatory Agonists in Tolerized Mice. Cancer Immunol Res (2014) 2(4):307–19. doi: 10.1158/2326-6066.CIR-13-0145
95. Fehlings M, Jhunjhunwala S, Kowanetz M, O’Gorman WE, Hegde PS, Sumatoh H, et al. Late-Differentiated Effector Neoantigen-Specific CD8+ T Cells are Enriched in Peripheral Blood of non-Small Cell Lung Carcinoma Patients Responding to Atezolizumab Treatment. J Immunother Cancer (2019) 7(1):249. doi: 10.1186/s40425-019-0695-9
96. Xiong H, Mittman S, Rodriguez R, Pacheco-Sanchez P, Moskalenko M, Yang Y, et al. Coexpression of Inhibitory Receptors Enriches for Activated and Functional CD8+ T Cells in Murine Syngeneic Tumor Models. Cancer Immunol Res (2019) 7(6):963–76. doi: 10.1158/2326-6066
97. Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N Engl J Med (2012) 366(10):883–92. doi: 10.1056/NEJMoa1113205
98. Bruin EC, McGranahan N, Mitter R, Salm M, Wedge DC, Yates L, et al. Spatial and Temporal Diversity in Genomic Instability Processes Defines Lung Cancer Evolution. Science (2014) 346(6206):251–6. doi: 10.1126/science.1253462
99. Castle JC, Kreiter S, Diekmann J, Löwer M, van de Roemer N, de Graaf J, et al. Exploitingthe Mutanome for Tumor Vaccination. Cancer Res (2012) 72(5):1081–91. doi: 10.1158/0008-5472.CAN-11-3722
101. Mertens F, Johansson B, Fioretos T, Mitelman F. The Emerging Complexity of Gene Fusions in Cancer. Nat Rev Cancer (2015) 15(6):371–81. doi: 10.1038/nrc3947
102. Francis RW, Thompson-Wicking K, Carter KW, Anderson D, Kees UR, Beesley AH. FusionFinder: A Software Tool to Identify Expressed Gene Fusion Candidates From RNA-Seq Data. PloS One (2012) 7(6):e39987. doi: 10.1371/journal.pone.0039987
103. Caron E, Aebersold R, Banaei-Esfahani A, Chong C, Bassani-Sternberg M. A Case for a Human Immuno-Peptidome Project Consortium. Immunity (2017) 47:203–8. doi: 10.1016/j.immuni.2017.07.010
104. Woroniecka K, Fecci PE. Immuno-Synergy? Neoantigen Vaccines and Checkpoint Blockade in Glioblastoma. Neuro Oncol (2020) 22(9):1233–4. doi: 10.1093/neuonc/noaa170
105. Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, et al. Novel Adenovirus-Based Vaccines Induce Broad and Sustained T Cell Responses to HCV in Man. Sci Transl Med (2012) 4(115):115ra1. doi: 10.1126/scitranslmed.3003155
106. Borthwick N, Ahmed T, Ondondo B, Hayes P, Rose A, Ebrahimsa U, et al. Vaccine-Elicited Human T Cells Recognizing Conserved Protein Regions Inhibit HIV-1. Mol Ther (2014) 22(2):464–75. doi: 10.1038/mt.2013.248
107. Green CA, Scarselli E, Voysey M, Capone S, Vitelli A, Nicosia A, et al. Safety and Immunogenicity of Novel Respiratory Syncytial Virus (RSV) Vaccines Based on the RSV Viral Proteins F, N and M2-1 Encoded by Simian Adenovirus (PanAd3-RSV) and MVA (Mva-RSV); Protocol for an Open-Label, Dose-Escalation, Single-Centre, Phase 1 Clinical Trial in Healthy Adults. BMJ Open (2015) 5(10):e008748. doi: 10.1136/bmjopen-2015-008748
108. Huang RY, Eppolito C, Lele S, Shrikant P, Matsuzaki J, Odunsi K. LAG3 and PD1 Co-Inhibitory Molecules Collaborate to Limit CD8+ T Cell Signaling and Dampen Antitumor Immunity in a Murine Ovarian Cancer Model. Oncotarget (2015) 6(29):27359–77. doi: 10.18632/oncotarget.4751
109. Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-cell Function to Promote Tumoral Immune Escape. Cancer Res (2012) 72(4):917–27. doi: 10.1158/0008-5472
110. Kreiter S, Vormehr M, van de Roemer N, Diken M, Löwer M, Diekmann J, et al. Mutant MHC Class II Epitopes Drive Therapeutic Immune Responses to Cancer. Nature (2015) 520(7549):692–6. doi: 10.1038/nature14426
111. Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T Helper Cell Requirement for Optimal Induction of Cytotoxic T Lymphocytes Against Major Histocompatibility Complex Class II Negative Tumors. J Exp Med (1998) 187(5):693–702. doi: 10.1084/jem.187.5.693
112. Borst J, Ahrends T, Babala N, Melief CJM, Kastenmuller W. Cd4 (+) T Cell Help in Cancer Immunology and Immunotherapy. Nat Rev Immunol (2018) 18(10):635–47. doi: 10.1038/s41577-018-0044-0
Keywords: personalized cancer vaccine, immune checkpoint inhibitor, combination therapy, neoantigen, immunotherapy
Citation: Liao J-Y and Zhang S (2021) Safety and Efficacy of Personalized Cancer Vaccines in Combination With Immune Checkpoint Inhibitors in Cancer Treatment. Front. Oncol. 11:663264. doi: 10.3389/fonc.2021.663264
Received: 02 February 2021; Accepted: 04 May 2021;
Published: 28 May 2021.
Edited by:
Jian-Guo Zhou, University of Erlangen Nuremberg, GermanyReviewed by:
Faezzah Baharom, National Institutes of Health (NIH), United StatesJie Tang, The University of Queensland, Australia
Hidehiro Yamane, National Cancer Institute, National Institutes of Health (NIH), United States
Copyright © 2021 Liao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuang Zhang, c2h1YW5nLnpoYW5nQHNjdS5lZHUuY24=
 Juan-Yan Liao
Juan-Yan Liao Shuang Zhang1,2*
Shuang Zhang1,2*