- 1Special Needs Department of Proton Therapy Center, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
- 2Department of Oncology, Jinan Central Hospital, The First Hospital Affiliated with Shandong First Medical University, Jinan, China
- 3Human Resources Department, Jinan Central Hospital Affiliated to Shandong University, Jinan, China
- 4Jinan Clinical Research Center of Shandong First Medical University, Jinan, China
- 5Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
Background: Understanding the safety and adverse event profiles of PD-1/PD-L1 inhibitors is important in guiding cancer immunotherapy. Consequently, we designed this meta-analysis to evaluate the safety of PD-1/PD-L1 inhibitors in clinical trials involving cancer patients.
Methods: Four safety indicators comprising treatment-related adverse events, death, discontinuation of therapy and grades 3–5 adverse events were evaluated using the random effect model. The quality of enrolled trials was assessed using the Newcastle Ottawa Scale (NOS).
Results: Forty-four clinical trials were included in the final meta-analysis. Compared with chemotherapy, the risk of death due to the use of PD-1/PD-L1 inhibitors was much lower than that experienced in the control group (OR = 0.65, 95%CI: [0.47, 0.91], I2 = 0%, Z = 2.52 (P = 0.01)). Similar observations were apparent regarding the other three indicators of safety and also when the use of PD-1/PD-L1 inhibitors alone is compared with the combined use of PD-1/PD-L1 and CTLA-4. When used together with chemotherapy, PD-1/PD-L1 inhibitors increased the incidence of the adverse events as compared to the use of chemotherapy alone. Increased risks for adverse events were also noticed with the use of PD-1/PD-L1 inhibitors over the use of a placebo.
Conclusion: The use of PD-1/PD-L1 inhibitors alone is associated with a better safety profile compared to either the use of chemotherapy or the use of PD-1/PD-L1 inhibitors with other anticancer regimens.
Introduction
Cancer immunotherapies, including immune checkpoint inhibitors (ICIs) and adoptive cell therapy, have revolutionized the treatment landscape and improved the survival prognosis for most cancer patients (1). Among these, PD-1/PD-L1 inhibitors are the most common type of immunosuppressants used in the treatment of solid tumors (1–4). PD-1/PD-L1 inhibitors can block the interaction between tumor cells and T cells, restore the immune recognition function of T cells, and then eliminate tumor cells (1–4). The unique anti-tumor mechanism of PD-1/PD-L1 inhibitors means that the toxicities caused by these agents are also different from other traditional anti-tumor drugs (1).
Although PD-1/PD-L1 inhibitors have shown remarkable clinical benefits in the treatment of tumors, the spectrum of immune-related adverse events (irAEs) that affect body organs are a major concern with the use of these agents (5, 6). Serious adverse events are a frequent limitation in the use of PD-1/PD-L1 inhibitors among cancer patients (5–9). It, therefore, behooves clinicians to conduct adequate and elaborate systematic assessment of potential recipients of these therapies, to ensure that the benefits outweigh the potential risks in the use of PD-1/PD-L1 inhibitors. In view of the limitations of previous meta-analyses regarding the safety and toxicity of PD-1/PD-L1 inhibitors (10–12), and the availability of recent information from results of clinical trials, we designed this study to reassess the safety of PD-1/PD-L1 inhibitors in cancer chemotherapy.
Method
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (13).
Selection Criteria for Clinical Trials
All randomized, open-label, controlled clinical trials with efficacy and safety data of PD-1/PD-L1 inhibitors were explored. Although Phase III clinical trials were given priority, the phase of clinical trials was not the primary inclusion criterion. Malignancies were limited to solid tumors and, as such, hematological tumors were excluded from the study. The four safety indicators evaluated in the meta-analysis were: a) treatment-related death, b) treatment-related adverse events leading to discontinuation of therapy, c) treatment-related grades 3–5 adverse events and d) any treatment-related adverse events.
Search Strategy
We followed the guidelines of the participants, interventions, comparisons, outcomes (PICOS) as recommended by the Cochrane Collaboration (13). A PubMed search was conducted using the search terms: “neoplasm”, “cancer”, “precancer”, “pre-cancer”, “malignant”, “premalignant”, “tumor”, “PD-1”, “PD-L1”, “nivolumab”, “Opdivo”, “pembrolizumab”, “Keytruda”, “Imfinzi”, “MK-3475”, “atezolizumab”, “Tecentriq”, “MPDL3280A”, “avelumab”, “Bavencio”, “durvalumab”, “camrelizumab”, and “BMS-963558”.
Articles were only included if they were published in English between 09 July 2013 and 19 Sep 2020. Three researchers were designated to independently scrutinize all the data and where there was duplication of clinical trials in selected articles, only one was used for the final analysis.
Assessment of Study Quality and Publication Bias
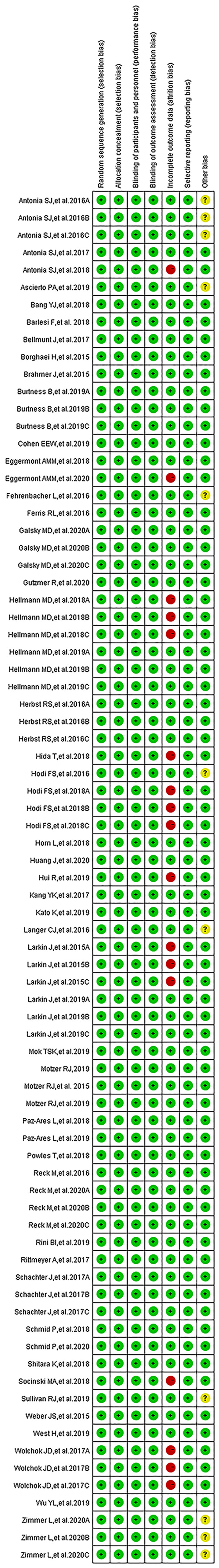
The Cochrane Collaboration tool was used to assess risk of bias in randomized trials (14), while the Funnel plot and Egger’s test were applied to evaluate publication bias (15). Three researchers independently checked the quality of all the included clinical trials. The quality assessment comprised evaluating: a) Selection bias (random sequence generation and allocation concealment), b) Performance bias (blinding of participants and personnel), c) Detection bias (blinding of outcome assessment), d) Attrition bias (incomplete outcome data) and e) Reporting bias (selective outcome reporting).
Outcome and Exposure of Interest
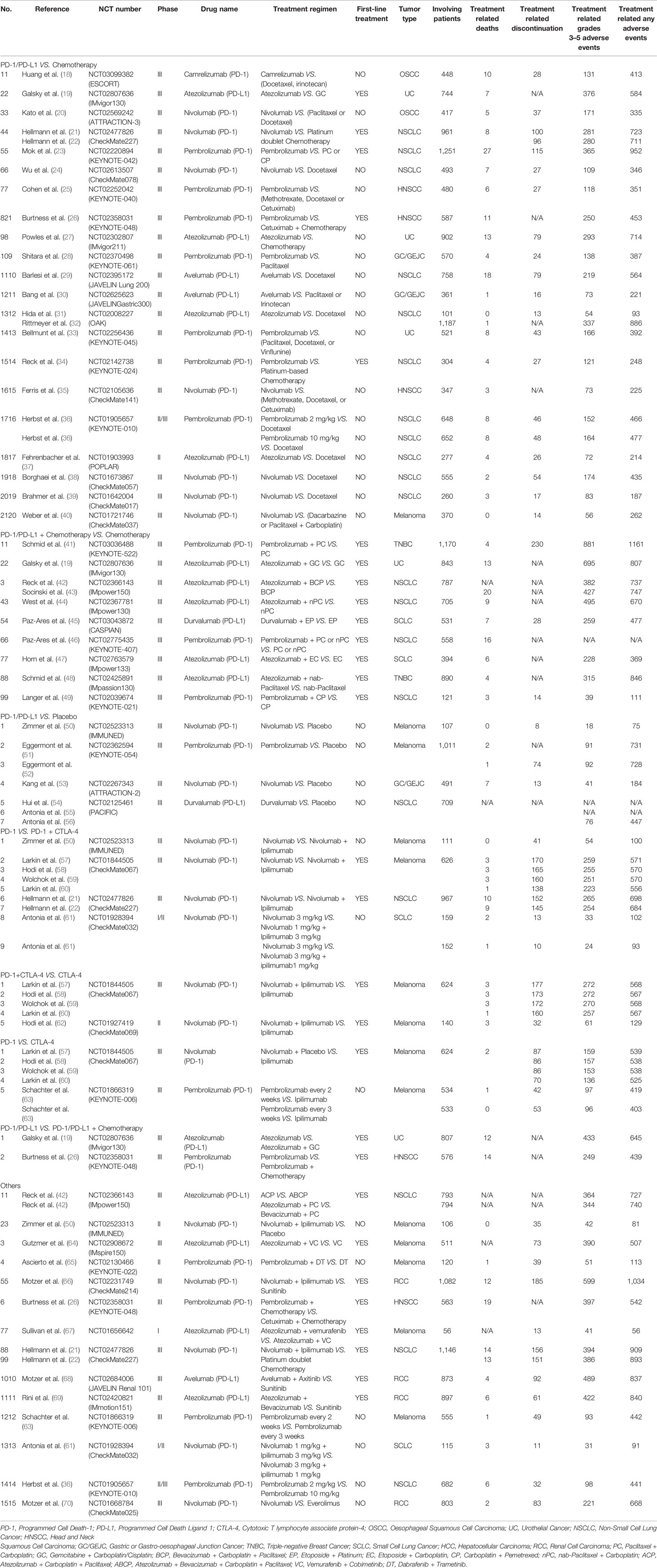
Our primary assessment indicators were the incidence of PD-1/PD-L1 inhibitors-induced “treatment-related death” and “treatment-related adverse events leading to discontinuation”. “Treatment-related grades 3-5 adverse events” and “any treatment-related adverse events” were also recorded. The basic characteristics and information on all the enrolled clinical trials were collected, including the first author’s name, year of publication, trial number, trial title, trial phase, the specific name of the anti-PD-1/PD-L1 agent, treatment regimens, whether treatment was first-line or not, tumor types and the number of participants, treatment-related death and treatment-related discontinuation.
Assessment of Heterogeneity and Statistical Analysis
Heterogeneity of all the eligible trials was evaluated using Cochrane’s Q statistic and the I2 statistic as reported by Higgins and colleagues (13, 16). Publication bias was checked using the Harbord test (16). Using the I2 value, heterogeneity was regarded as low (<25%), moderate (25–50%) or high (>50%). Odds ratio (OR) and the corresponding 95% confidence interval (CI) were calculated using the random effect (RE) (17). Data analysis was conducted using Review Manager 5.3 and all statistical tests were two-sided with a value of P <0.05 considered statistically significant. Subgroup analysis was performed according to the tumor type, treatment regimen and PD-1/PD-L1 inhibitor used.
Results
Literature Search Results
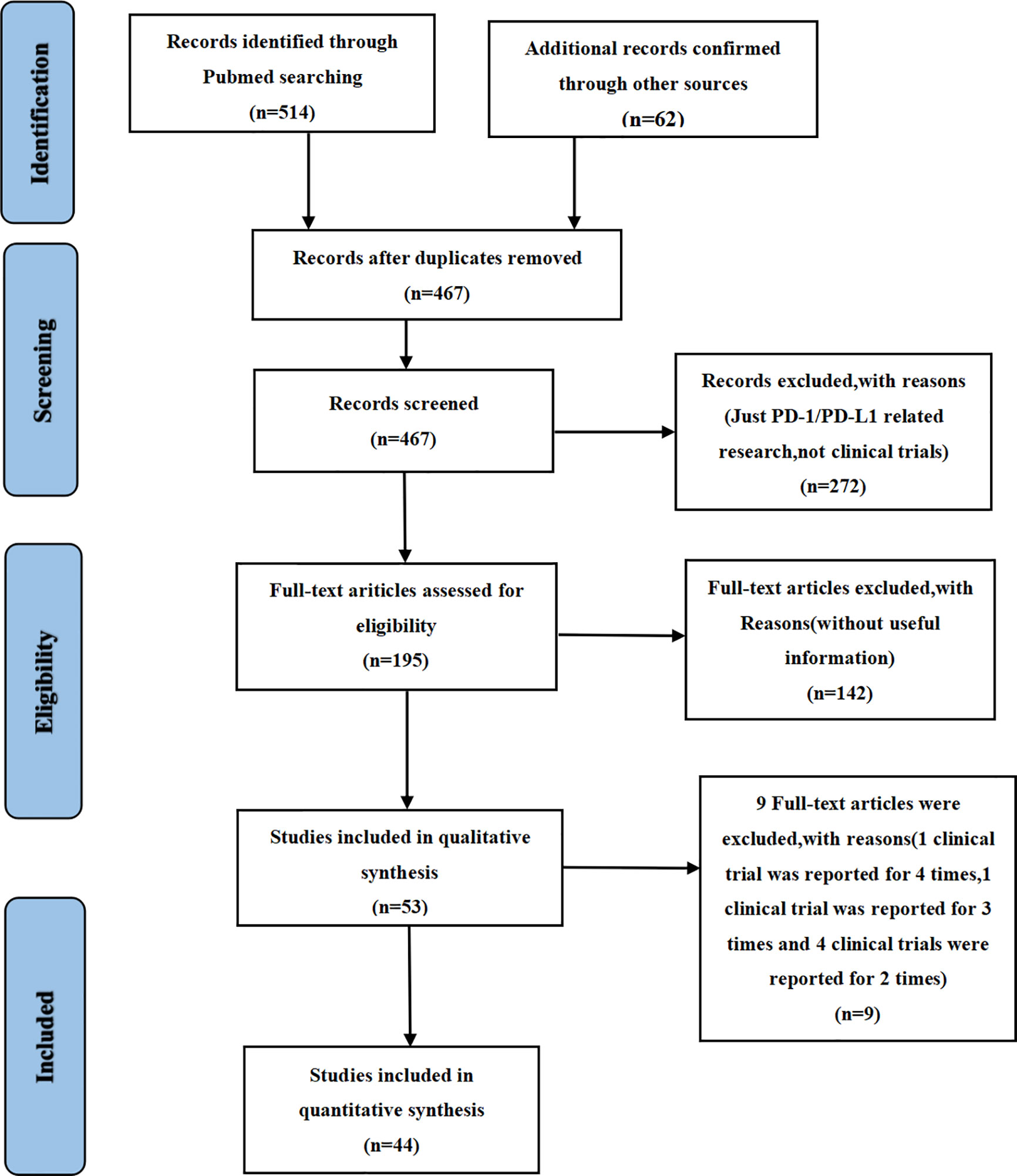
We found 514 clinical trials investigating PD-1/PD-L1 inhibitors after conducting an initial search through the PubMed website. Fifty-three articles were deemed to meet our preliminary selection criteria (18–70), of which 44 articles were selected for the final comprehensive analysis (18–21, 23–30, 32–42, 44–50, 52, 53, 56, 57, 61–70). The results of 6 clinical trials had been reported in multiple platforms: CheckMate 067 (n = 4) (57–60), PACIFIC (n = 3) (54–56), CheckMate 227 (n = 2) (21, 22), OAK (n = 2) (31, 32), KeyNote 054 (n = 2) (51, 52) and IMpower 150 (n = 2) (42, 43). When such duplications were noted, only one was selected for the meta-analysis. The PRISMA flow diagram of the screening process for the clinical trials is shown in Figure 1 while the quality assessment of included studies is shown in Figure 2.
Characteristics of Clinical Trials
The basic characteristics of the 53 eligible articles are summarized in Table 1 (18–70). Most of the articles (45) were about phase III clinical trials (18–35, 38–48, 51–60, 63, 64, 66, 68–70), while five were phase II trials (37, 49, 50, 62, 65). The rest were a phase I trial (67), a phase I/II trial (61), and a phase II/III trial (36). As shown in Table 1, 28 clinical trials (reported in 33 articles) were associated with PD-1 inhibitors (18, 20–26, 28, 33–36, 38–41, 46, 49–53, 57–63, 65, 66, 70), while the other 16 clinical trials (reported in 20 articles) were associated with PD-L1 inhibitors (19, 27, 29–32, 37, 42–45, 47, 48, 54–56, 64, 67–69). Nivolumab (14 clinical trials) (20–22, 24, 35, 38–40, 50, 53, 57–62, 66, 70), Pembrolizumab (13 clinical trials) (23, 25, 26, 28, 33, 34, 36, 41, 46, 49, 51, 52, 63, 65), and atezolizumab (11 clinical trials) (19, 27, 31, 32, 37, 42–44, 47, 48, 64, 67, 69), were the most reported PD-1/PD-L1 inhibitors. Fewer studies involved Camrelizumab (18), Durvalumab (45, 54–56), and Avelumab (29, 30, 68).
There were nine different types of tumors in all the recruited clinical trials. Most of these were non-small cell lung cancer (NSCLC) (15) (21–24, 29, 31, 32, 34, 36–39, 42–44, 46, 49, 54–56), and melanoma (9) (40, 50–52, 57–60, 62–65, 67). The other tumors included renal cell carcinoma (RCC) (4) (66, 68–70), urothelial cancer (UC) (3) (19, 27, 33), head and neck squamous cell carcinoma (HNSCC) (3) (25, 26, 35), and gastric or esophageal junction cancer (GC/GEJC) (3) (28, 30, 53). PD-1/PD-L1 inhibitors were prescribed as the first-line treatment regimen in 20 clinical trials (19, 21–23, 26, 34, 41–49, 57–60, 62, 64, 66–69), while previous anti-cancer treatments were found in 24 clinical trials (18, 20, 24, 25, 27–33, 35–40, 50–56, 61, 63, 65, 70).
The clinical trials were further stratified into seven groups according to the treatment regimen as shown in Table 1. The classes are Group A (PD-1/PD-L1 vs Chemotherapy) (18–40), Group B (PD-1/PD-L1 + Chemotherapy vs Chemotherapy) (19, 41–49), Group C (PD-1/PD-L1 vs Placebo) (50–56), Group D (PD-1 vs PD-1+CTLA-4) (21, 22, 50, 57–61), Group E (PD-1+CTLA-4 vs CTLA-4) (57–60, 62), Group F (PD-1 vs CTLA-4) (57–60, 63), and Group G (PD-1/PD-L1 vs PD-1/PD-L1 + Chemotherapy) (19, 26). The risks for the various types of adverse events within each group were then evaluated.
Risk of Bias
The funnel plots assessing publication bias are as shown in the Supplementary Figures S2–S6. Other types of bias involving the 53 articles are summarized in Figure 2. Six clinical trials were associated with unclear risk of bias while high risk of bias (37, 50, 61, 62, 65, 67), mainly due to incomplete outcome data, hence attrition bias, was found with seven clinical trials (22, 31, 43, 51, 54, 55, 58–60).
Incidence of Treatment-Related Death
Treatment-related death in studies comparing the use of PD-1/PD-L1 and chemotherapy (Group A) was reported in 21 clinical trials (18–40). Less deaths were reported in the PD-1/PD-L1 group as compared to the control chemotherapy group (OR = 0.65, 95%CI: [0.47, 0.91], I2 = 0%, Z = 2.52 (P = 0.01) (Figure 3A) (18–40). This observation was more evident with the NSCLC subgroup (OR = 0.53, 95% CI:[0.34, 0.83], I2 = 0%, Z = 2.75 (P = 0.006) (Figure 3A). In addition to a lack of heterogeneity between the groups (I2 = 0%) the funnel plots revealed that there was no obvious publication bias (Supplementary Figure S3A). Upon subgroup stratification this trend was more obvious with the PD-L1 related subgroup [OR = 0.39, 95% CI:(0.20, 0.74); Supplementary Figures S1A and Figure S2A]. Furthermore, we found that the risk of death in the PD-L1-related subgroup was lower than that in the PD-1-related subgroup [OR (0.39 VS. 0.78); P = 0.07, Supplementary Figure S1A]. Similar trends of treatment-related death were found in Group D (Figure 3D; Supplementary Figure S3D) and Group G (Figure 3G; Supplementary Figure S3G), when PD-1/PD-L1 inhibitors were compared with either PD-1 + CTLA-4 or PD-1/PD-L1 + Chemotherapy (19, 21, 22, 26, 50, 57–61).

Figure 3 Forest plots of treatment-related adverse events leading to death. (A) The odds ratio of treatment-related adverse events leading to death calculated by the random effect (RE) model in Group A (PD-1/PD-L1 vs Chemotherapy). Subgroup analysis was performed based on tumor types. (B) The odds ratio of treatment-related adverse events leading to death calculated by the random effect (RE) model in Group B (PD-1/PD-L1 + Chemotherapy vs Chemotherapy). Subgroup analysis was performed based on tumor types. (C) The odds ratio of treatment-related adverse events leading to death calculated by the random effect (RE) model in Group C (PD-1/PD-L1 vs Placebo). (D) The odds ratio of treatment-related adverse events leading to death calculated by the random effect (RE) model in Group D (PD-1 vs PD-1 + CTLA-4). Subgroup analysis was performed based on tumor types. (E) The odds ratio of treatment-related adverse events leading to death calculated by the random effect (RE) model in Group E (PD-1 + CTLA-4 vs CTLA-4). (F) The odds ratio of treatment-related adverse events leading to death calculated by the random effect (RE) model in Group F (PD-1 vs CTLA-4). (G) The odds ratio of treatment-related adverse events leading to death calculated by the random effect (RE) model in Group G (PD-1/PD-L1 vs PD-1/PD-L1 + Chemotherapy).
When PD-1/PD-L1 inhibitors were prescribed in combination with chemotherapy, the risk of death was increased [OR = 1.76, 95%CI:(1.01, 3.08), I2 = 0%, Z = 1.99 (P = 0.05) (Figure 3B)] (19, 41, 44–49). Similar risk trends, although not statistically significant, were observed for the other Groups: Group C (Figure 3C), Group E (Figure 3E) and Group F (Figure 3F) (50–60, 62, 63). The corresponding funnel plot analyses confirmed that there were no obvious publication bias (Supplementary Figures S3B, C, E, F).
Incidence of Treatment-Related Adverse Events Leading to Discontinuation
The risk of treatment-related adverse events leading to discontinuation of therapy in the use of PD-1/PD-L1 was significantly lower than witnessed with the group that received chemotherapy [OR = 0.55, 95%CI:(0.40, 0.75), I2 = 77%, Z = 3.79 (P = 0.0001); Figure 4A] (18, 20, 22–25, 27–30, 33, 34, 36–40). Subgroup analysis showed that the risk of such adverse events was lower with the PD-L1-related subgroup as compared to the PD-1-related subgroup [OR (0.39 vs. 0.64); P = 0.15, Supplementary Figure S1B] (18, 20, 22–25, 27–30, 33, 34, 36–40). We also found high heterogeneity (I2 = 73%, Figure 4A and Supplementary Figure S1B) but no obvious publication bias (Supplementary Figure S4A). This trend is replicated when the use of PD-1 is compared with combined use of PD-1 plus CTLA-4, in Group D [OR = 0.33, 95%CI: (0.15, 0.72), I2 = 85%, Z = 2.81(P = 0.005); Figure 4D; Supplementary Figure S4D] (21, 50, 57, 61). However, a dissimilar trend was evident when PD-1 combined with CTLA-4 was compared with CTLA-4 alone, in Group E [OR = 4.04, 95%CI:(2.81, 5.80), I2 = 0%, Z = 7.55(P <0.00001); Figure 4E] (57, 62). Additional subgroup analyses did not yield statistically different results (Figures 4B, C, F) (41, 45, 49, 50, 52, 53, 57, 63).
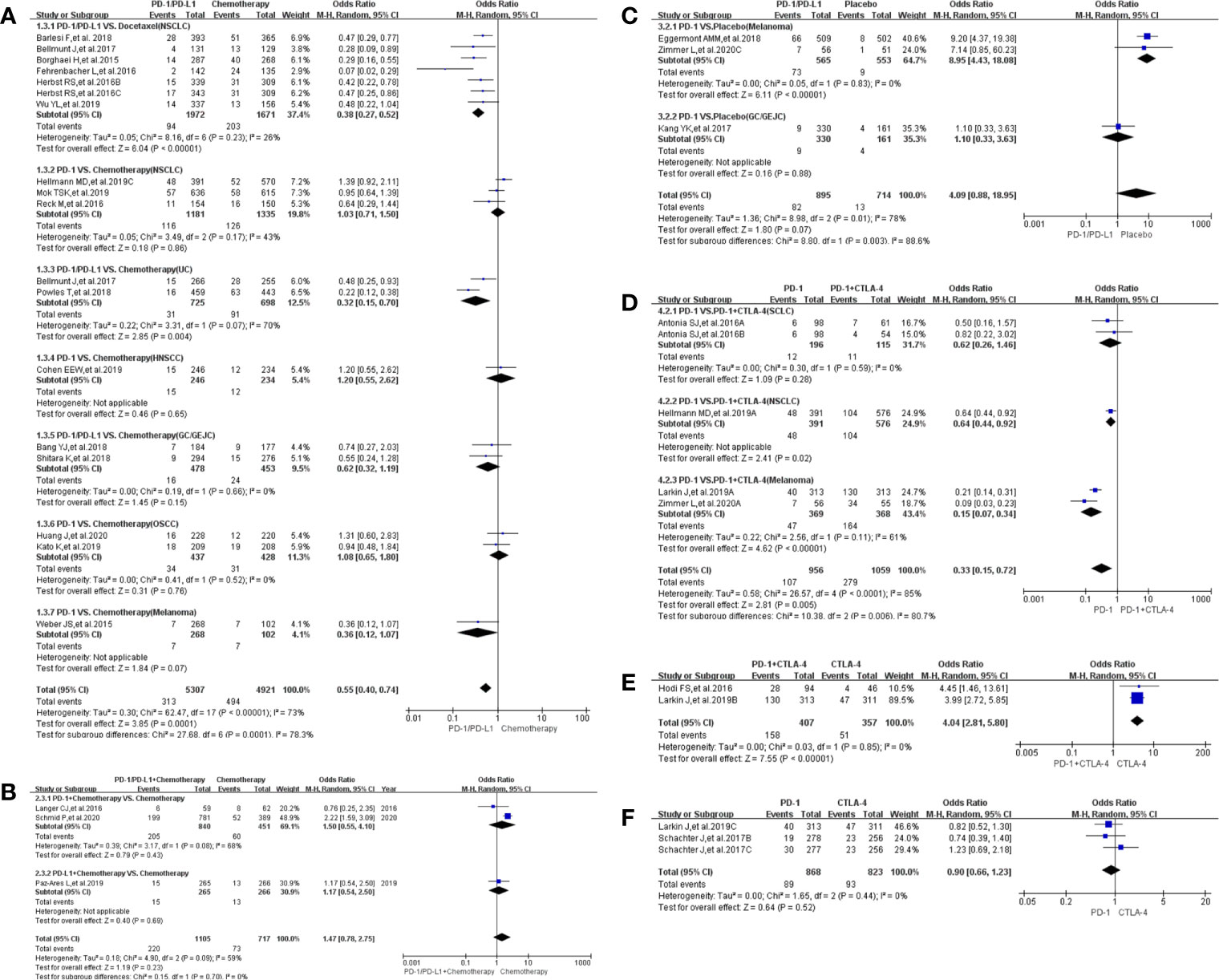
Figure 4 Forest plots of treatment-related adverse events leading to discontinuation of therapy. (A) The odds ratio of treatment-related adverse events leading to discontinuation of therapy calculated by the random effect (RE) model in Group A (PD-1/PD-L1 vs Chemotherapy): subgroup analysis was performed based on tumor types, PD-1/PD-L1 and treatment regimens. (B) The odds ratio of treatment-related adverse events leading to death calculated by the random effect (RE) model in Group B (PD-1/PD-L1 + Chemotherapy vs Chemotherapy). Subgroup analysis was performed based on PD-1/PD-L1. (C) The odds ratio of treatment-related adverse events leading to discontinuation of therapy calculated by the random effect (RE) model in Group C (PD-1/PD-L1 vs Placebo). Subgroup analysis was performed based on tumor types. (D) The odds ratio of treatment-related adverse events leading to discontinuation calculated by the random effect (RE) model in Group D (PD-1 vs PD-1 + CTLA-4). Subgroup analysis was performed based on tumor types. (E) The odds ratio of treatment-related adverse events leading to discontinuation of therapy calculated by the random effect (RE) model in Group E (PD-1 + CTLA-4 vs CTLA-4). (F) The odds ratio of treatment-related adverse events leading to discontinuation of therapy calculated by the random effect (RE) model in Group F (PD-1 vs CTLA-4).
Incidence of Any Treatment-Related Adverse Events
A lower incidence of any treatment-related adverse events was observed in the PD-1/PD-L1 group as compared to the control group, Group A (OR = 0.29, 95%CI:[0.24, 0.36], I2 = 81%, Z = 11.14 (P <0.00001), Figure 5A) (18–40). High heterogeneity, through subgroup analyses, was associated with the OSCC and PD-L1 related UC groups (I2 = 81%; Figure 5A) (18–20, 27). Differences between PD-1 and PD-L1 groups were statistically insignificant (P = 0.19; Supplementary Figure S1C). Converging trends emerged when the use of PD-1 only was compared with the regimen comprising PD-1 in combination with CTLA-4, in Group D (OR = 0.36, 95%CI:[0.23, 0.56], I2 = 54%, Z = 4.56 (P <0.00001); Figure 5D) (21, 50, 57, 61). High heterogeneity (I2 = 54%), attributed to the lung cancer subgroup, was observed (I2 = 59%; Figure 5D) (21, 61), but there were no obvious publication bias (Supplementary Figure S5D).

Figure 5 Forest plots of all-grade treatment-related adverse events. (A) The odds ratio of all-grade treatment-related adverse events calculated by the random effect (RE) model in Group A (PD-1/PD-L1 vs Chemotherapy). Subgroup analysis was performed based on tumor types, PD-1/PD-L1 and treatment regimens. (B) The odds ratio of all-grade treatment-related adverse events calculated by the random effect (RE) model in Group B (PD-1/PD-L1 + Chemotherapy vs Chemotherapy). Subgroup analysis was performed based on PD-1/PD-L1 and tumor types. (C) The odds ratio of all-grade treatment-related adverse events calculated by the random effect (RE) model in Group C (PD-1/PD-L1 vs Placebo). Subgroup analysis was performed based on PD-1/PD-L1 and tumor types. (D) The odds ratio of all-grade treatment-related adverse events calculated by the random effect (RE) model in Group D (PD-1 vs PD-1 + CTLA-4). Subgroup analysis was performed based on tumor types. (E) The odds ratio of all-grade treatment-related adverse events calculated by the random effect (RE) model in Group E (PD-1 + CTLA-4 vs CTLA-4). (F) The odds ratio of all-grade treatment-related adverse events calculated by the random effect (RE) model in Group F (PD-1 vs CTLA-4).
Compared to the placebo in Group C (50, 52, 53, 56), PD-1/PD-L1 increased the incidence risk of any treatment-related adverse events with low heterogeneity being observed mainly due to the melanoma subgroup (OR = 1.94, 95%CI:[1.58, 2.38], I2 = 13%, Z = 6.41 (P <0.00001); Figure 5C) (50, 52). There was neither obvious publication bias (Supplementary Figures S5C, B, E, F) nor statistically significant differences in the subgroup analyses (Figures 5B, E, F).
Incidence of Treatment-Related Grades 3–5 Adverse Events
As observed for any treatment-related adverse events in Group A, the incidence of grades 3–5 adverse events among recipients of PD-1/PD-L1 was significantly lower than for those in the control group [OR = 0.20, 95%CI:(0.16, 0.26), I2 = 88%, Z = 12.05 (P <0.00001); Figure 6A] (18–21, 23–25, 27–30, 32–40). Both OSCC and PD-L1 related UC were determined, through subgroup analysis, to lead to the observed high heterogeneity (I2 = 88%) (Figure 6A) (18–20, 27). No statistically significant differences were apparent in the risk of grades 3–5 adverse events between either the PD-1 and PD-L1 groups (P = 0.19; Supplementary Figure S1D) (18–21, 23–25, 27–30, 32–40) or the use of PD-1 alone or in combination with CTLA-4, in Group D [OR = 0.31, 95%CI:(0.18, 0.53), I2 = 79%, Z = 4.37 (P <0.00001); Figure 6D] (21, 50, 57, 61). The high heterogeneity seen with these groups was inherent to the data and no publication bias was found (Figure 6D; Supplementary Figure S6D) (21, 50, 57, 61). No statistical analysis results was also found in Group F (Figure 6F and Supplementary Figure S6F) (57, 63).

Figure 6 Forest plots of the risk of grades 3–5 treatment-related adverse events (A) The odds ratio of grades 3–5 treatment-related adverse events calculated by the random effect (RE) model in Group A (PD-1/PD-L1 vs Chemotherapy). Subgroup analysis was performed based on tumor types, PD-1/PD-L1 and treatment regimens. (B) The odds ratio of grades 3–5 treatment-related adverse events calculated by the random effect (RE) model in Group B (PD-1/PD-L1 + Chemotherapy vs Chemotherapy). Subgroup analysis was performed based on PD-1/PD-L1 and tumor types. (C) The odds ratio of grades 3–5 treatment-related adverse events calculated by the random effect (RE) model in Group C (PD-1/PD-L1 vs Placebo). Subgroup analysis was performed based on PD-1/PD-L1. (D) The odds ratio of grades 3–5 treatment-related adverse events calculated by the random effect (RE) model in Group D (PD-1 vs PD-1 + CTLA-4). Subgroup analysis was performed based on PD-1/PD-L1 and tumor types. (E) The odds ratio for grades 3–5 of treatment-related adverse events calculated by the random effect (RE) model in Group E (PD-1 + CTLA-4 vs CTLA-4). (F) The odds ratio for grades 3–5 of treatment-related adverse events calculated by the random effect (RE) model in Group F (PD-1 vs CTLA-4).
When combined with chemotherapy, PD-1/PD-L1 increased the risk of treatment-related grades 3–5 adverse events as compared with the use of chemotherapy alone [OR = 1.28, 95%CI:(1.05, 1.57), I2 = 63%, Z = 2.43(P = 0.01); Figure 6B] (19, 41, 43–45, 47–49). The overall high heterogeneity (I2 = 63%) was traced to the NSCLC subgroup (I2 = 47%) (Figure 6B) (43, 44). Similar findings were evident in Group E (OR = 3.99, 95%CI: [2.92, 5.44], I2 = 0%, Z = 8.70 (P <0.00001), (Figure 6E) (57, 58), when PD-1/PD-L1 in combination with CTLA-4 is compared with the sole use of CTLA-4. The corresponding funnel plot are depicted in Supplementary Figure S6E.
Finally, compared to the placebo in Group C (50, 52, 53, 56), PD-1/PD-L1 increased the incidence (37, 50, 61, 62, 65, 67) of treatment-related grades 3–5 adverse events with low heterogeneity which was considered to be mainly caused by the PD-L1 related subgroup (OR = 3.57, 95%CI:[2.40, 5.31], I2 = 16%, Z = 6.28 (P <0.00001); Figure 6C) (56). As with other groups, there was no apparent publication bias (Supplementary Figure S6C) (50, 52, 53, 56) as also witnessed for Group F featuring the comparison between PD-1 and CTLA-4 (Figure 5F and Supplementary Figure S5F) (57, 63).
Discussion
PD-1/PD-L1 inhibitors have been playing an increasingly important role in anti-tumor therapy (1, 5, 6, 8). While these agents have been reported to achieve gratifying clinical anti-tumor efficacy, they are beset by a growing list of diverse treatment-related side effects (18–70). As more clinical trials have been completed in recent years, it is critical that information about the safety and efficacy of PD-1/PD-L1 inhibitors are updated to provide the latest guidance in the administration and use of these therapeutic agents (1, 5, 6, 8, 18–70). The need to provide the most recent information on the safety and adverse effect profiles of PD-1/PD-L1 inhibitors motivated the current meta-analysis.
Following the selection criteria, 44 clinical trials reported by 53 articles were included in the meta-analysis (18–70). High risk of attrition bias was noticeable due to articles with incomplete data (Figure 2) (22, 31, 43, 51, 54, 55, 58–60).
Our meta-analysis found that PD-1/PD-L1 inhibitors were generally distinguished in having a more favorable safety profile as compared to chemotherapy, across the four safety indicators applied to the analysis. Similarly, stratified investigation also revealed that between them, PD-L1 inhibitors were associated with fewer cases of adverse events as compare to PD-1 inhibitors, especially when considering the incidences of treatment-related adverse events leading to discontinuation of therapy or death. This observation is contrary to the conclusion reached in the mirror principle based meta-analysis (71). As there lacked randomized controlled trials between PD-1 and PD-L1 (18–70), the differences in the adverse event profiles between these two groups of agents were controversial as well as inconclusive (71). High heterogeneity was found across three evaluation indicators (Figures 4A; 5A and 6A) and the subgroup analyses suggested the role of the tumor types and the inherent quality of the data in this observation (18–21, 27, 33, 61). Notably, however, there was no obvious publication bias in the articles (Supplementary Figures S3A; S4A; S5A and S6A). In addition, the trend in adverse events was repeated when PD-1/PD-L1 inhibitors were compared with combinational use with CTLA-4 (Figures 3D; 4D; 5D and 6D) (21, 22, 50, 57–61). The combined results from the above analyses led us to the conclusion that PD-1/PD-L1 inhibitors display better safety characteristics than chemotherapy or the combined use of PD-1/PD-L1 with CTLA-4.
Although PD-1/PD-L1 inhibitors, when prescribed in combination with chemotherapy, increased the occurrence of the four classes of adverse events (Figures 3B; 4B; 5B and Figure 6B) (19, 41–49), the increase was only statistically significant regarding grades 3–5 treatment-related adverse events [OR = 1.28, 95%CI:(1.05, 1.57), I2 = 63%, Z = 2.43 (P = 0.01); 6B] (19, 41, 43–45, 47–49). The high heterogeneity (I2 = 63%) was tied to the NSCLC group (I2 = 47%; Figure 6B) (43, 44). The failure to note any meaningful differences with the other groups (Figures 3B; 4B and 5B) might be due to the limitation of data. In order to draw more conclusive statistically significant analysis, more clinical trial results need to be considered.
Similar trends in the profile of adverse events were seen when the use of PD-1/PD-L1 inhibitors is compared to placebo (Figures 3C; 4C; 5C and 6C) (50–56). We, however, had too few clinical trials to enable us to evaluate the comparisons in the differences in the incidence of treatment-related death [OR = 1.47, 95%CI: (0.34, 6.39), I2 = 0%, Z = 0.52 (P = 0.61); Figure 3C] (52, 53).
We experienced similar challenges and limitations in the attempt to carry out subgroup analysis based on the treatment regimen and safety indicators, due to insufficient volumes of data. The observed trends and potential differences within the various subgroups need to be verified by using more clinical trials data.
At the time of conducting this study, results from some randomized controlled clinical trials involving PD-1/PD-L1 combined with targeted therapy had also been reported. However, due to the differences among articles and the results obtained, they could not be included in the current meta-analysis. These references were, nonetheless, listed in Table 1 (21, 22, 26, 36, 42, 50, 61, 63–70).
In summary, our meta-analysis indicates that there is a better safety profile in the use of PD-1/PD-L1 inhibitors as compared to either chemotherapy or the use of combined regimens incorporating PD-1/PD-L1 inhibitors. The PD-1/PD-L1 inhibitors, however, had a worse adverse event profile over placebo. The present study, therefore, suggests caution and awareness of the occurrence of treatment-related adverse events when PD-1/PD-L1 inhibitors are used solely or in combination with other interventions. Clinicians should be aware that should adverse events occur in combinational treatment, withdrawing PD-1/PD-L1 inhibitor may not be the first approach to alleviate severe drug-related toxicities. This meta-analysis provides insights into important considerations to bear in mind when using PD-1/PD-L1 inhibitors and what adverse events to anticipate.
Conclusion
PD-1/PD-L1 inhibitors display better safety profiles than either chemotherapy or combinational treatment regimens involving PD-1/PD-L1 inhibitors.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
YT, AH, YY, QD, and QW collected the data. YT, AH, YY, and QD performed data cleaning and analysis. YT drafted the manuscript. YS and LW reviewed the manuscript for scientific soundness. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Academic Promotion Program of Shandong First Medical University (2019QL025; YS), Natural Science Foundation of Shandong Province (ZR2019MH042; YS), Jinan Science and Technology Program (201805064; YS), and Postdoctoral Innovation Project of Jinan (YT).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.662392/full#supplementary-material
Supplementary Figure 1 | Forest plots for group A (PD-1/PD-L1 vs Chemotherapy) (A) The odds ratio of treatment-related adverse events leading to death calculated by the random effect (RE) model (PD-1/PD-L1 vs Chemotherapy). Subgroup analysis was conducted based on PD-1/PD-L1. (B) The odds ratio of treatment-related adverse events leading to discontinuation calculated by the random effect (RE) model (PD-1/PD-L1 vs Chemotherapy). Subgroup analysis was performed based on PD-1/PD-L1. (C) The odds ratio of all-grade treatment-related adverse events calculated by the random effect (RE) model (PD-1/PD-L1 vs Chemotherapy). Subgroup analysis was carried out based on PD-1/PD-L1. (D) The odds ratio for grade 3-5 of treatment-related any adverse events calculated by the random effect (RE) model (PD-1/PD-L1 vs Chemotherapy). Subgroup analysis was conducted based on PD-1/PD-L1.
Supplementary Figure 2 | Funnel plots for group A (PD-1/PD-L1 vs Chemotherapy) (A) The odds ratio of treatment-related adverse events leading to death calculated by the fixed effect (FE) model (PD-1/PD-L1 vs Chemotherapy). Subgroup analysis was performed based on PD-1/PD-L1. (B) The odds ratio of treatment-related adverse events leading to discontinuation calculated by the fixed effect (FE) model (PD-1/PD-L1 vs Chemotherapy). Subgroup analysis was carried out based on PD-1/PD-L1. (C) The odds ratio of all-grade treatment-related adverse events calculated by the fixed effect (FE) model (PD-1/PD-L1 vs Chemotherapy). Subgroup analysis was conducted based on PD-1/PD-L1. (D) The odds ratio of grade 3-5 treatment-related adverse events calculated by fixed effect (FE) model (PD-1/PD-L1 vs Chemotherapy). Subgroup analysis was performed based on PD-1/PD-L1.
Supplementary Figure 3 | Funnel plots of treatment-related adverse events leading to death (A) The funnel plot of treatment-related adverse events leading to death calculated by the fixed effect (FE) model in Group A (PD-1/PD-L1 vs Chemotherapy). Subgroup analysis was performed based on tumor types. (B) The funnel plot of treatment-related adverse events leading to death calculated by the fixed effect (FE) model in Group B (PD-1/PD-L1+Chemotherapy vs Chemotherapy). Subgroup analysis was performed based on tumor types. (C) The funnel plot of treatment-related adverse events leading to death calculated by the fixed effect (FE) model in Group C (PD-1/PD-L1 vs Placebo). (D) The funnel plot of treatment-related adverse events leading to death calculated by the fixed effect (FE) model in Group D (PD-1 vs PD-1+CTLA-4). Subgroup analysis was performed based on tumor types. (E) The funnel plot of treatment-related adverse events leading to death calculated by the fixed effect (FE) model in Group E (PD-1+CTLA-4 vs CTLA-4). (F) The funnel plot of treatment-related adverse events leading to death calculated by the fixed effect (FE) model in Group F (PD-1 vs CTLA-4). (G) The funnel plot of treatment-related adverse events leading to death calculated by the fixed effect (FE) model in Group G (PD-1/PD-L1 vs PD-1/PD-L1+Chemotherapy).
Supplementary Figure 4 | Funnel plots of the risk of treatment-related adverse events leading to discontinuation (A) The funnel plot of treatment-related adverse events leading to discontinuation calculated by the fixed effect (FE) model in Group A (PD-1/PD-L1 vs Chemotherapy). Subgroup analysis was performed based on tumor types, PD-1/PD-L1 and treatment regimens. (B) The funnel plot of treatment-related adverse events leading to death calculated by the fixed effect (FE) model in Group B (PD-1/PD-L1+Chemotherapy vs Chemotherapy). Subgroup analysis was performed based on PD-1/PD-L1. (C) The funnel plot of treatment-related adverse events leading to discontinuation calculated by the fixed effect (FE) model in Group C (PD-1/PD-L1 vs Placebo). Subgroup analysis was performed based on tumor types. (D) The funnel plot of treatment-related adverse events leading to discontinuation calculated by the fixed effect (FE) model in Group D (PD-1 vs PD-1+CTLA-4). Subgroup analysis was performed based on tumor types. (E) The funnel plot of treatment-related adverse events leading to discontinuation calculated by the fixed effect (FE) model in Group E (PD-1+CTLA-4 vs CTLA-4). (F) The funnel plot of treatment-related adverse events leading to discontinuation calculated by the fixed effect (FE) model in Group F (PD-1 vs CTLA-4).
Supplementary Figure 5 | Funnel plots of all-grade treatment-related adverse events (A) The funnel plot of all-grade treatment-related adverse events calculated by the fixed effect (FE) model in Group A (PD-1/PD-L1 vs Chemotherapy) Subgroup analysis was performed based on tumor types, PD-1/PD-L1 and treatment regimens. (B) The funnel plot of all-grade treatment-related adverse events calculated by the fixed effect (FE) model in Group B (PD-1/PD-L1+Chemotherapy vs Chemotherapy). Subgroup analysis was performed based on PD-1/PD-L1 and tumor types. (C) The funnel plot for all grade treatment-related adverse events calculated by the fixed effect (FE) model in Group C (PD-1/PD-L1 vs Placebo). Subgroup analysis was performed based on PD-1/PD-L1 and tumor types. (D) The funnel plot of all-grade treatment-related adverse events calculated by the fixed effect (FE) model in Group D (PD-1 vs PD-1+CTLA-4). Subgroup analysis was performed based on tumor types. (E) The funnel plot of all-grade treatment-related adverse events calculated by the fixed effect (FE) model in Group E (PD-1+CTLA-4 vs CTLA-4). (F) The funnel plot of all-grade treatment-related adverse events calculated by the fixed effect (FE) model in Group F (PD-1 vs CTLA-4).
Supplementary Figure 6 | Funnel plots of grade 3-5 treatment-related adverse events. (A) The funnel plot of grade 3-5 treatment-related adverse events calculated by the fixed effect (FE) model in Group A (PD-1/PD-L1 vs Chemotherapy). Subgroup analysis was performed based on tumor types, PD-1/PD-L1 and treatment regimens. (B) The funnel plot of grade 3-5 treatment-related adverse events calculated by the fixed effect (FE) model in Group B (PD-1/PD-L1+Chemotherapy vs Chemotherapy).Subgroup analysis was performed based on PD-1/PD-L1 and tumor types. (C) The funnel plot of grade 3-5 treatment-related adverse events calculated by the fixed effect (FE) model in Group C (PD-1/PD-L1 vs Placebo). Subgroup analysis was performed based on PD-1/PD-L1. (D) The funnel plot of grade 3-5 treatment-related adverse events calculated by the fixed effect (FE) model in Group D (PD-1 vs PD-1+CTLA-4). Subgroup analysis was performed based on PD-1/PD-L1 and tumor types. (E) The funnel plot of grade 3-5 treatment-related adverse events calculated by the fixed effect (FE) model in Group E (PD-1+CTLA-4 vs CTLA-4). (F) The funnel plot of grade 3-5 treatment-related adverse events calculated by the fixed effect (FE) model in Group F (PD-1 vs CTLA-4).
Abbreviations
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PICOS, Participants, Interventions, Comparisons, Outcomes, and Study design; PD-1, Programmed Cell Death-1; PD-L1, Programmed Cell Death Ligand 1; CTLA-4, Cytotoxic T lymphocyte associate protein-4; OR, Odds Ratio; CI, Confidence Interval; RE, Random Effect; NSCLC, Non-Small Cell Lung Cancer; SCLC, Small Cell Lung Cancer; OSCC, Oesophageal Squamous Cell Carcinoma; HNSCC, Head and Neck Squamous Cell Carcinoma; UC, Urothelial Cancer; TNBC, Triple-negative Breast Cancer; RCC, Renal Cell Carcinoma; GC/GEJC, Gastric or Gastro-oesophageal Junction Cancer.
References
1. Kennedy LB, Salama AKS. A Review of Cancer Immunotherapy Toxicity. CA Cancer J Clin (2020) 70(2):86–104. doi: 10.3322/caac.21596
2. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med (2012) 366(26):2443–54. doi: 10.1056/NEJMoa1200690
3. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and Activity of Anti-PD-L1 Antibody in Patients With Advanced Cancer. N Engl J Med (2012) 366(26):2455–65. doi: 10.1056/NEJMoa1200694
4. Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med (2016) 375(18):1767–78. doi: 10.1056/NEJMra1514296
5. Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated With Immune Checkpoint Blockade. N Engl J Med (2018) 378(2):158–68. doi: 10.1056/NEJMra1703481
6. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse Effects of Immune-Checkpoint Inhibitors: Epidemiology, Management and Surveillance. Nat Rev Clin Oncol (2019) 16(9):563–80. doi: 10.1038/s41571-019-0218-0
7. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2018) 36(17):1714–68. doi: 10.1200/JCO.2017.77.6385
8. Thompson JA. New NCCN Guidelines: Recognition and Management of Immunotherapy-Related Toxicity. J Natl Compr Canc Netw (2018) 16(5S):594–6. doi: 10.6004/jnccn.2018.0047
9. Castinetti F, Albarel F, Archambeaud F, Bertherat J, Bouillet B, Buffier P, et al. French Endocrine Society Guidance on Endocrine Side Effects of Immunotherapy. Endocr Relat Cancer (2019) 26(2):G1–G18. doi: 10.1530/ERC-18-0320
10. Larkin J, Lao CD, Urba WJ, McDermott DF, Horak C, Jiang J, et al. Efficacy and Safety of Nivolumab in Patients With BRAF V600 Mutant and BRAF Wild-Type Advanced Melanoma: A Pooled Analysis of 4 Clinical Trials. JAMA Oncol (2015) 1(4):433–40. doi: 10.1001/jamaoncol.2015.1184
11. Devji T, Levine O, Neupane B, Beyene J, Xie F. Systemic Therapy for Previously Untreated Advanced Braf-Mutated Melanoma: A Systematic Review and Network Meta-Analysis of Randomized Clinical Trials. JAMA Oncol (2017) 3(3):366–73. doi: 10.1001/jamaoncol.2016.4877
12. Zhou Y, Chen C, Zhang X, Fu S, Xue C, Ma Y, et al. Immune-Checkpoint Inhibitor Plus Chemotherapy Versus Conventional Chemotherapy for First-Line Treatment in Advanced non-Small Cell Lung Carcinoma: A Systematic Review and Meta-Analysis. J Immunother Cancer (2018) 6(1):155. doi: 10.1186/s40425-018-0477-9
13. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med (2009) 151(4):264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135
14. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
15. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
17. DerSimonian R, Laird N. Meta-Analysis in Clinical Trials. Control Clin Trials (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
18. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab Versus Investigator’s Choice of Chemotherapy as Second-Line Therapy for Advanced or Metastatic Oesophageal Squamous Cell Carcinoma (Escort): A Multicentre, Randomised, Open-Label, Phase 3 Study. Lancet Oncol (2020) 21(6):832–42. doi: 10.1016/S1470-2045(20)30110-8
19. Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab With or Without Chemotherapy in Metastatic Urothelial Cancer (Imvigor130): A Multicentre, Randomised, Placebo-Controlled Phase 3 Trial. Lancet (2020) 395(10236):1547–57. doi: 10.1016/S0140-6736(20)30230-0
20. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab Versus Chemotherapy in Patients With Advanced Oesophageal Squamous Cell Carcinoma Refractory or Intolerant to Pnrevious Chemotherapy (Attraction-3): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2019) 20(11):1506–17. doi: 10.1016/S1470-2045(19)30626-6
21. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab Plus Ipilimumab in Advanced non-Small-Cell Lung Cancer. N Engl J Med (2019) 381(21):2020–31. doi: 10.1056/NEJMoa1910231
22. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab Plus Ipilimumab in Lung Cancer With a High Tumor Mutational Burden. N Engl J Med (2018) 378(22):2093–104. doi: 10.1056/NEJMoa1801946
23. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab Versus Chemotherapy for Previously Untreated, PD-L1-expressing, Locally Advanced or Metastatic non-Small-Cell Lung Cancer (Keynote-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
24. Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol (2019) 14(5):867–75. doi: 10.1016/j.jtho.2019.01.006
25. Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab Versus Methotrexate, Docetaxel, or Cetuximab for Recurrent or Metastatic Head-and-Neck Squamous Cell Carcinoma (Keynote-040): A Randomised, Open-Label, Phase 3 Study. Lancet (2019) 393(10167):156–67. doi: 10.1016/S0140-6736(18)31999-8
26. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab Alone or With Chemotherapy Versus Cetuximab With Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (Keynote-048): A Randomised, Open-Label, Phase 3 Study. Lancet (2019) 394(10212):1915–28. doi: 10.1016/S0140-6736(19)32591-7
27. Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab Versus Chemotherapy in Patients With Platinum-Treated Locally Advanced or Metastatic Urothelial Carcinoma (Imvigor211): A Multicentre, Open-Label, Phase 3 Randomised Controlled Trial. Lancet (2018) 391(10122):748–57. doi: 10.1016/S0140-6736(17)33297-X
28. Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, et al. Pembrolizumab Versus Paclitaxel for Previously Treated, Advanced Gastric or Gastro-Oesophageal Junction Cancer (Keynote-061): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet (2018) 392(10142):123–33. doi: 10.1016/S0140-6736(18)31257-1
29. Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab Versus Docetaxel in Patients With Platinum-Treated Advanced Non-Small-Cell Lung Cancer (Javelin Lung 200): An Open-Label, Randomised, Phase 3 Study. Lancet Oncol (2018) 19(11):1468–79. doi: 10.1016/S1470-2045(18)30673-9
30. Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Phase III, Randomised Trial of Avelumab Versus Physician’s Choice of Chemotherapy as Third-Line Treatment of Patients With Advanced Gastric or Gastro-Oesophageal Junction Cancer: Primary Analysis of JAVELIN Gastric 300. Ann Oncol (2018) 29(10):2052–60. doi: 10.1093/annonc/mdy264
31. Hida T, Kaji R, Satouchi M, Ikeda N, Horiike A, Nokihara H, et al. Atezolizumab in Japanese Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer: A Subgroup Analysis of the Phase 3 OAK Study. Clin Lung Cancer (2018) 19(4):e405–15. doi: 10.1016/j.cllc.2018.01.004
32. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab Versus Docetaxel in Patients With Previously Treated non-Small-Cell Lung Cancer (Oak): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet (2017) 389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X
33. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med (2017) 376(11):1015–26. doi: 10.1056/NEJMoa1613683
34. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive non-Small-Cell Lung Cancer. N Engl J Med (2016) Nov 10375(19):1823–33. doi: 10.1056/NEJMoa1606774
35. Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med (2016) 375(19):1856–67. doi: 10.1056/NEJMoa1602252
36. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab Versus Docetaxel for Previously Treated, PD-L1-positive, Advanced non-Small-Cell Lung Cancer (Keynote-010): A Randomised Controlled Trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
37. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab Versus Docetaxel for Patients With Previously Treated non-Small-Cell Lung Cancer (Poplar): A Multicentre, Open-Label, Phase 2 Randomised Controlled Trial. Lancet (2016) 387(10030):1837–46. doi: 10.1016/S0140-6736(16)00587-0
38. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
39. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab Versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
40. Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab Versus Chemotherapy in Patients With Advanced Melanoma Who Progressed After Anti-CTLA-4 Treatment (CheckMate 037): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol (2015) 16(4):375–84. doi: 10.1016/S1470-2045(15)70076-8
41. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med (2020) 382(9):810–21. doi: 10.1056/NEJMoa1910549
42. Reck M, Wehler T, Orlandi F, Nogami N, Barone C, Moro-Sibilot D, et al. Safety and Patient-Reported Outcomes of Atezolizumab Plus Chemotherapy With or Without Bevacizumab Versus Bevacizumab Plus Chemotherapy in Non-Small-Cell Lung Cancer. J Clin Oncol (2020) 38(22):2530–42. doi: 10.1200/JCO.19.03158
43. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Impower150 Study Group. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous Nsclc. N Engl J Med (2018) 378(24):2288–301. doi: 10.1056/NEJMoa1716948
44. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in Combination With Carboplatin Plus Nab-Paclitaxel Chemotherapy Compared With Chemotherapy Alone as First-Line Treatment for Metastatic non-Squamous non-Small-Cell Lung Cancer (Impower130): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2019) 20(7):924–37. doi: 10.1016/S1470-2045(19)30167-6
45. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab Plus Platinum-Etoposide Versus Platinum-Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (Caspian): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet (2019) 394(10212):1929–39. doi: 10.1016/S0140-6736(19)32222-6
46. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab Plus Chemotherapy for Squamous non-Small-Cell Lung Cancer. N Engl J Med (2018) 379(21):2040–51. doi: 10.1056/NEJMoa1810865
47. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab Plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med (2018) 379(23):2220–9. doi: 10.1056/NEJMoa1809064
48. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med (2018) 379(22):2108–21. doi: 10.1056/NEJMoa1809615
49. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and Pemetrexed With or Without Pembrolizumab for Advanced, non-Squamous non-Small-Cell Lung Cancer: A Randomised, Phase 2 Cohort of the Open-Label Keynote-021 Study. Lancet Oncol (2016) 17(11):1497–508. doi: 10.1016/S1470-2045(16)30498-3
50. Zimmer L, Livingstone E, Hassel JC, Fluck M, Eigentler T, Loquai C, et al. Adjuvant Nivolumab Plus Ipilimumab or Nivolumab Monotherapy Versus Placebo in Patients With Resected Stage IV Melanoma With No Evidence of Disease (Immuned): A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet (2020) 395(10236):1558–68. doi: 10.1016/S0140-6736(20)30417-7
51. Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson VG, Dalle S, et al. Longer Follow-Up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results From the EORTC 1325-MG/KEYNOTE-054 Trial. J Clin Oncol (2020) 38(33):3925–36. doi: 10.1200/JCO.20.02110
52. Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant Pembrolizumab Versus Placebo in Resected Stage III Melanoma. N Engl J Med (2018) 378(19):1789–801. doi: 10.1056/NEJMoa1802357
53. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in Patients With Advanced Gastric or Gastro-Oesophageal Junction Cancer Refractory to, or Intolerant of, At Least Two Previous Chemotherapy Regimens (Ono-4538-12, Attraction-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet (2017) 390(10111):2461–71. doi: 10.1016/S0140-6736(17)31827-5
54. Hui R, Özgüroğlu M, Villegas A, Daniel D, Vicente D, Murakami S, et al. Patient-Reported Outcomes With Durvalumab After Chemoradiotherapy in Stage Iii, Unresectable non-Small-Cell Lung Cancer (Pacific): A Randomised, Controlled, Phase 3 Study. Lancet Oncol (2019) 20(12):1670–80. doi: 10.1016/S1470-2045(19)30519-4
55. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Pacific Investigators. Overall Survival With Durvalumab After Chemoradiotherapy in Stage Iii Nsclc. N Engl J Med (2018) 379(24):2342–50. doi: 10.1056/NEJMoa1809697
56. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab After Chemoradiotherapy in Stage Iii Non-Small-Cell Lung Cancer. N Engl J Med (2017) 377(20):1919–29. doi: 10.1056/NEJMoa1709937
57. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-Year Survival With Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2019) 381(16):1535–46. doi: 10.1056/NEJMoa1910836
58. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab Alone in Advanced Melanoma (CheckMate 067): 4-Year Outcomes of a Multicentre, Randomised, Phase 3 Trial. Lancet Oncol (2018) 19(11):1480–92. doi: 10.1016/S1470-2045(18)30700-9
59. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall Survival With Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2017) 377(14):1345–56. doi: 10.1056/NEJMoa1709684
60. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med (2015) 373(1):23–34. doi: 10.1056/NEJMoa1504030
61. Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab Alone and Nivolumab Plus Ipilimumab in Recurrent Small-Cell Lung Cancer (CheckMate 032): A Multicentre, Open-Label, Phase 1/2 Trial. Lancet Oncol (2016) 17(7):883–95. doi: 10.1016/S1470-2045(16)30098-5
62. Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined Nivolumab and Ipilimumab Versus Ipilimumab Alone in Patients With Advanced Melanoma: 2-Year Overall Survival Outcomes in a Multicentre, Randomised, Controlled, Phase 2 Trial. Lancet Oncol (2016) 17(11):1558–68. doi: 10.1016/S1470-2045(16)30366-7
63. Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab Versus Ipilimumab for Advanced Melanoma: Final Overall Survival Results of a Multicentre, Randomised, Open-Label Phase 3 Study (Keynote-006). Lancet (2017) 390(10105):1853–62. doi: 10.1016/S0140-6736(17)31601-X
64. Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Atezolizumab, Vemurafenib, and Cobimetinib as First-Line Treatment for Unresectable Advanced BRAFV600 Mutation-Positive Melanoma (Imspire150): Primary Analysis of the Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet (2020) 395(10240):1835–44. doi: 10.1016/S0140-6736(20)30934-X
65. Ascierto PA, Ferrucci PF, Fisher R, Del Vecchio M, Atkinson V, Schmidt H, et al. Dabrafenib, Trametinib and Pembrolizumab or Placebo in BRAF-mutant Melanoma. Nat Med (2019) 25(6):941–6. doi: 10.1038/s41591-019-0448-9
66. Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab Plus Ipilimumab Versus Sunitinib in First-Line Treatment for Advanced Renal Cell Carcinoma: Extended Follow-Up of Efficacy and Safety Results From a Randomised, Controlled, Phase 3 Trial. Lancet Oncol (2019) 20(10):1370–85. doi: 10.1016/S1470-2045(19)30413-9
67. Sullivan RJ, Hamid O, Gonzalez R, Infante JR, Patel MR, Hodi FS, et al. Atezolizumab Plus Cobimetinib and Vemurafenib in BRAF-mutated Melanoma Patients. Nat Med (2019) 25(6):929–35. doi: 10.1038/s41591-019-0474-7
68. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab Plus Axitinib Versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2019) 380(12):1103–15. doi: 10.1056/NEJMoa1816047
69. Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab Plus Bevacizumab Versus Sunitinib in Patients With Previously Untreated Metastatic Renal Cell Carcinoma (Immotion151): A Multicentre, Open-Label, Phase 3, Randomised Controlled Trial. Lancet (2019) 393(10189):2404–15. doi: 10.1016/S0140-6736(19)30723-8
70. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab Versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med (2015) 373(19):1803–13. doi: 10.1056/NEJMoa1510665
Keywords: PD-1/PD-L1 inhibitors, cancer, meta-analysis, safety assessment, clinical trial
Citation: Tian Y, Huang A, Yang Y, Dang Q, Wen Q, Wang L and Sun Y (2021) Assessment of the Clinical Trials Safety Profile of PD-1/PD-L1 Inhibitors Among Patients With Cancer: An Updated Systematic Review and Meta-Analysis. Front. Oncol. 11:662392. doi: 10.3389/fonc.2021.662392
Received: 01 February 2021; Accepted: 27 April 2021;
Published: 24 May 2021.
Edited by:
Selvarangan Ponnazhagan, University of Alabama at Birmingham, United StatesReviewed by:
Amir Sharabi, Beth Israel Deaconess Medical Center and Harvard Medical School, United StatesSairah Ahmed, University of Texas MD Anderson Cancer Center, United States
Copyright © 2021 Tian, Huang, Yang, Dang, Wen, Wang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linlin Wang, d2FuZ2xpbmxpbmF0am5AMTYzLmNvbQ==; Yuping Sun, MTMzNzA1ODIxODFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yuan Tian
Yuan Tian Alan Huang
Alan Huang Yue Yang3†
Yue Yang3† Qing Wen
Qing Wen Linlin Wang
Linlin Wang Yuping Sun
Yuping Sun