
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 25 March 2021
Sec. Skin Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.659332
This article is part of the Research Topic Multidisciplinary Approach to the Diagnosis and Therapy of Skin Neoplasms View all 19 articles
 Daniela Russo1†
Daniela Russo1† Silvia Varricchio1
Silvia Varricchio1 Gennaro Ilardi1†
Gennaro Ilardi1† Francesco Martino1
Francesco Martino1 Rosa Maria Di Crescenzo1
Rosa Maria Di Crescenzo1 Sara Pignatiello1
Sara Pignatiello1 Massimiliano Scalvenzi2
Massimiliano Scalvenzi2 Claudia Costa2
Claudia Costa2 Massimo Mascolo1
Massimo Mascolo1 Francesco Merolla3*
Francesco Merolla3* Stefania Staibano1*
Stefania Staibano1*Basal cell carcinoma (BCC) is the most common cancer in the white-skinned population accounting for about 15% of all neoplasms. Its incidence is increasing worldwide, at a rate of about 10% per year. BCC, although infrequently metastasizing, very often causes extensive tissue losses, due to the high propensity toward stromal infiltration, particularly in its dedifferentiated forms, with disfiguring and debilitating results. To date, there still is limited availability of therapeutic treatments alternative to surgery. We evaluated the immunohistochemical expression of the carbonic anhydrase IX (CAIX), one of the main markers of tissue hypoxia, in a set of 85 archived FFPE BCC tissues, including the main subtypes, with different clinical outcomes, to demonstrate a possible relationship between hypoxic phenotype and biological aggressiveness of these neoplasms. Our results showed that the expression level of the CAIX protein contributes to the stratification of BCC in the different risk classes for recurrence. We hypothesize for CAIX a potential therapeutic role as a target therapy in the treatment of more aggressive BCCs, thus providing an alternative to surgical and pharmacological therapy with Hedgehog inhibitors, a promising example of target therapy in BCCs.
Basal cell carcinoma (BCC) is defined by the World Health Organization (WHO) as a locally invasive, slow-growing tumor that originates from the basal layer cells of the epidermis, placed peripherally to the hair bulbs, and that rarely hesitates in metastasis. The main risk associated to BCC are multiple relapses, an event more frequently occurring in case of incomplete excision or multiple primitive tumors, Relapsing BCC can produce, over time, serious anatomic, functional and aesthetic damage, with serious problems of co-morbidity, severely affecting the quality of life (1). Accounting for about 15% of all solid tumors, BCC is the most common malignant neoplasm in the world, with more than 2.8 million new cases diagnosed each year in the United States of America (2). In the context of non-melanoma skin cancer (NMSC), BCC accounts for about 80% of the cases (3), with a global incidence increase of 3% to 7%/year over last decades (4), BCC represents a serious public health problem. In Italy, the incidence is approximately 100 cases per 100,000 inhabitants (5). These figures could be underestimated because of the diagnostic-therapeutic management for this neoplasia. BCC treatment, in fact, does not usually include hospitalization, and BCC generally does not cause patients’ death. BCC develops predominantly in the mature-elderly population (>40 years), prevalently males, with average age at diagnosis of 68 years, in regions of the body chronically exposed to the sun (particularly face and neck, 70% to 85% of cases; 25% to 30% being represented by the nose alone, to follow the trunk and less frequently the limbs). Recently, an epidemiological shift has been reported, with increased incidence in young female population, probably due to the varied habits of exposure of the population (not adequately protected) in the Sun (6). BCC recognizes as the main risk factor exposure to sunlight, especially UVA and UVB ultraviolet rays. Different BCC variants have been described, based on clinical behavior, morphology, growth pattern, architecture, and differentiation (7). Hypoxia is a pathological condition determined by a lack of oxygen in the whole organism (generalized hypoxia) or in one tissue (tissue hypoxia). Hypoxia has emerged as an important feature of tumor microenvironment of neoplasms with more aggressive biological behavior. The uncontrolled growth of tumors is, in fact, accompanied by the induction of insufficient vascularization which results in the formation in most of the malignant solid tumors of heterogeneously distributed hypoxia regions (8, 9). Hypoxia generates a passage to the glycolic metabolism that allows the production of energy in low or absent oxygen conditions and is crucial for the survival of hypoxic cancer cells. Among the molecules most expressed in hypoxia condition are HIF-1 α and carbonic anhydrase IX (CAIX). These molecules are responsible for the process of adapting cells to oxygen deficiency with the formation of new blood vessels, a mechanism that is exploited by tumor tissues to grow and metastasize (10). CAIX belongs to the family of Carbonic Anhydrases (CA), a group of metal zinc-containing enzymes that catalyze the reversible hydration of CO2 in HCO3 and H + ions and has recently emerged as the most promising endogenous marker of cellular hypoxia (10, 11). This reaction is fundamental at the level of cells, tissues, and organs in a wide range of biochemical and physiological processes such as acid-base equilibrium, gas exchange, ionic transport, and carbon dioxide fixation. To date, 15 human isoforms of CA have been characterized that differ in catalytic activity, subcellular localization, and tissue distribution (11). Carbonic Anhydrase IX is encoded by a gene located on chromosome 9 and is a transmembrane isoform with a catalytic site in the extracellular portion and has the highest efficiency for the transport of H + between CAs. It consists of a proteoglycan-like domain at the N-terminal end (involved in adhesion and intercellular communication), an extracellular catalytic domain, a trans-membrane hydrophobic portion and a C-terminal cytoplasmic tail (essential for correct localization on the plasma membrane and proper functioning of the enzyme) (12). It is a tumor-associated protein, as it is expressed in limited quantities in normal tissue, such as the stomach or intestine, and the expression is however limited to the basolateral membrane of epithelial cells endowed with increased proliferative activity, while it is hyper expressed in solid tumor cells linked to a hypoxic phenotype (13). The overexpression of CAIX on the cell membrane of many solid tumors is mediated by the HIF-1 transcription factor and is often associated with poor reactivity to classical radio and chemotherapy. In a recent work, a close association between overexpression of CAIX and the markers of staminality CD44 and Nestin, has been demonstrated in several aggressive and metastasizing neoplasms, with relevance in a series of squamous carcinomas of the tongue (14). This indicates that CAIX action in hypoxic tumors goes beyond intra-tumoral PH control. The clear majority of existing data, in fact, indicates that CAIX has multiple functions in solid tumors, in particular, it plays a key role in encouraging the establishment of chemo-and radio-resistance in the most advanced cases and opens new therapeutic perspectives (14). In the present study, we deepened the role of the Carbonic Anhydrase IX as a possible leading actor and marker of hypoxia in BCC, by evaluating the immunohistochemistry expression of the CAIX protein in a series of archived FFPE BCC tissue samples.
Formalin-fixed, paraffin-embedded tissue blocks of 85 BCCs, diagnosed and excised with healthy surgical margins from February 2002 to November 2017, were retrieved from the archives of the Pathology Section of the Department of Advanced Biomedical Sciences, “Federico II” University of Naples. Out of 85 cases, 55 males and 30 females, the age at diagnosis ranged between 38 and 88 years (mean age, 67 years). Table 1 summarizes the histological groups of the study population, together with the associated risk. The clinical data and pathological features of the tumors are reported in Table 2. The study design and procedures involving tissue samples collection and handling were performed according to the Declaration of Helsinki, in agreement with the current Italian law, and to the Institutional Ethical Committee guidelines.
Two pathologists (SS and DR) reviewed the whole routine hematoxylin-eosin (H&E) sections to confirm the original diagnosis and to mark the most representative tumor areas useful for the TMA construction. Tissue cores with a diameter of 3 mm were punched from morphologically representative tissue areas of each “donor” tissue block and brought into one recipient paraffin block using a manual tissue arrayer. The filled recipient blocks were then placed on a metal base mold. The paraffin-embedding was then carried-out, by heating the blocks at 42°C, for 10 min, and flattening their surface by pressing a clean glass slide on them. As a result, four TMAs were built. 4-μm sections were cut from each TMA using an ordinary microtome (15, 16). The first section was stained with H&E to confirm the presence of the tumor and the integrity of tissues. The other section was mounted on a super frost slide (Microm, Walldorf, Germany) for the immunohistochemical evaluation of CAIX. For CAIX IHC assay the sections were deparaffinized routinely in xylene and rehydrated through a series of graded ethanol. CAIX antigen retrieval was performed in EDTA buffer (pH 8) in a hot water bath (94°C) for 20 min and in CITRATE buffer (pH 6) by microwave oven (3 min × 3 times); the backdrop (for blocking non-specific background staining) was removed using the universal blocking serum (Dako Diagnostics, Glostrup, Denmark) for 15 min at room temperature. Endogenous alkaline phosphatase activity was quenched adding Levamisole to buffer AP (Substrate Buffer); the slides were rinsed with TRIS+Tween20 pH 7.4 buffer and incubated in a humidified chamber with the primary rabbit polyclonal antibody anti-CAIX (sc-25599, Santa Cruz Biotechnology, diluted 1:200 overnight at 4°C). Then used a biotinylated secondary antibody and streptavidin conjugated with alkaline phosphatase. The reaction has been highlighted with the chromogen Fast Red, which showed the presence of antigen that we sought in red (Dako REAL Detection System, Alkaline Phosphatase/RED, Rabbit/Mouse). Again, after a weak nuclear counterstain with hematoxylin, the sections were then mounted with a synthetic medium (Entellan, Merck, Darmstadt, Germany). Positivity for CAIX was visualized as red membranous and cytoplasmic staining. The CAIX expression was defined as high or low depending on whether the percentage of neoplastic cells stained was respectively >/= or <5%.
Correlation between CAIX immunohistochemical expression and BCC clinical-pathologic characteristics was asses through contingency analysis with Fisher exact test. Statistical analysis has been performed using SPSS software (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.).
Our case series included 85 tumor samples (Table 2), out of which, 7 (8%) were not evaluable for CAIX tissue expression due to loss of core integrity. The CAIX protein showed LOW expression score in 35 (45%) out of 78 cases, and a HIGH score in the residual 43 (55%) (Table 3). The study population was subdivided, according to the histotype, into two groups: aggressive BCCs (i.e., BCCs with higher risk of recurrence; including basosquamous, morphoeic, infiltrating and micronodular) consisting of 60 cases (60/78, 77%) and the group of ordinary BCCs (i.e., BCCs with lower risk of recurrence; including nodular, superficial, and with adnexal differentiation) consisting of 18 cases (18/78, 23%) (Table 1). Among aggressive BCC, 41 out of 60 evaluable cases (68.3%) showed a HIGH CAIX expression score, while in the group of ordinary BCCs only 2 out of 18 cases (11.1%) showed a high score (Table 4; Figure 1A). Table 5 shows the distribution of CAIX expression scores per histologic subtypes. The follow-up data for recurrence, detailed per tumor variants, are shown in Table 6.
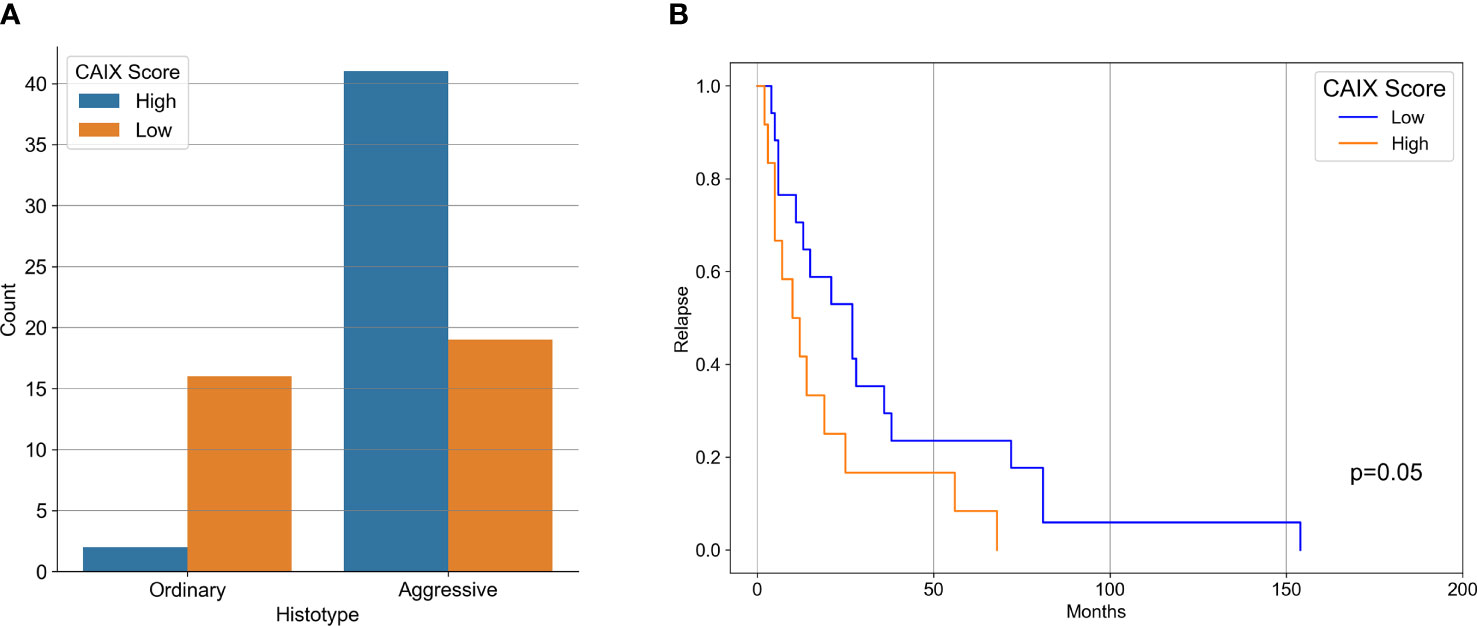
Figure 1 (A) Bar-Graph representation of CAIX immunohistochemical expression in aggressive and ordinary BCC. (B) Kaplan-Meier curves of recurrence survival. Difference between CAIX HIGH and CAIX LOW curved proved statistically significant (p = 0.05) as tested by Log-Rank.
Correlation between CAIX immunohistochemical expression and BCC histotype was assessed through contingency analysis with Fisher exact test, that proved to be statistical significant, with a P value <0.0001. A survival analysis, taking recurrence as endpoint, was carried out and Kaplan-Meier curves are shown in Figure 1B: difference between CAIX HIGH and LOW curves is significant as resulted from Log-Rank test (p = 0.05). Taken together our results show that the higher CAIX expression significantly correlates with BCC aggressive behavior. Representative images of CAIX IHC staining in low-risk BCCs are shown in Figure 2; representative high-risk BCCs immunostained with anti-CAIX antibody are shown in Figure 3.
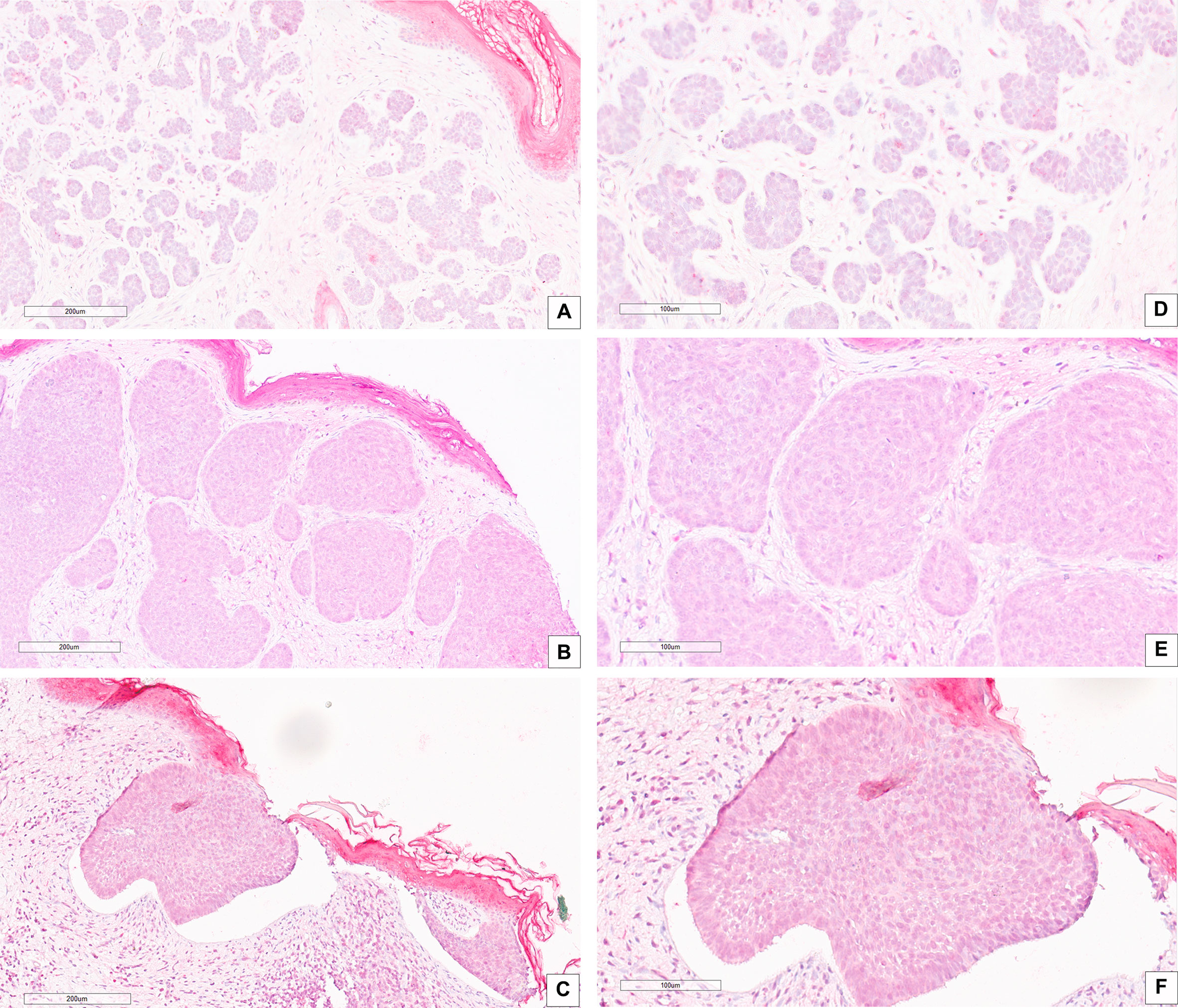
Figure 2 IHC stain with an anti-CAIX antibody in low risk BCC histological variants: (A–D) BCC with adnexal differentiation (magnification, 20× and 40×, respectively); (B–E) Nodular BCC (magnification, 20× and 40×, respectively); (C–F) Superficial BCC (magnification, 20× and 40×, respectively). Scale bars are shown.
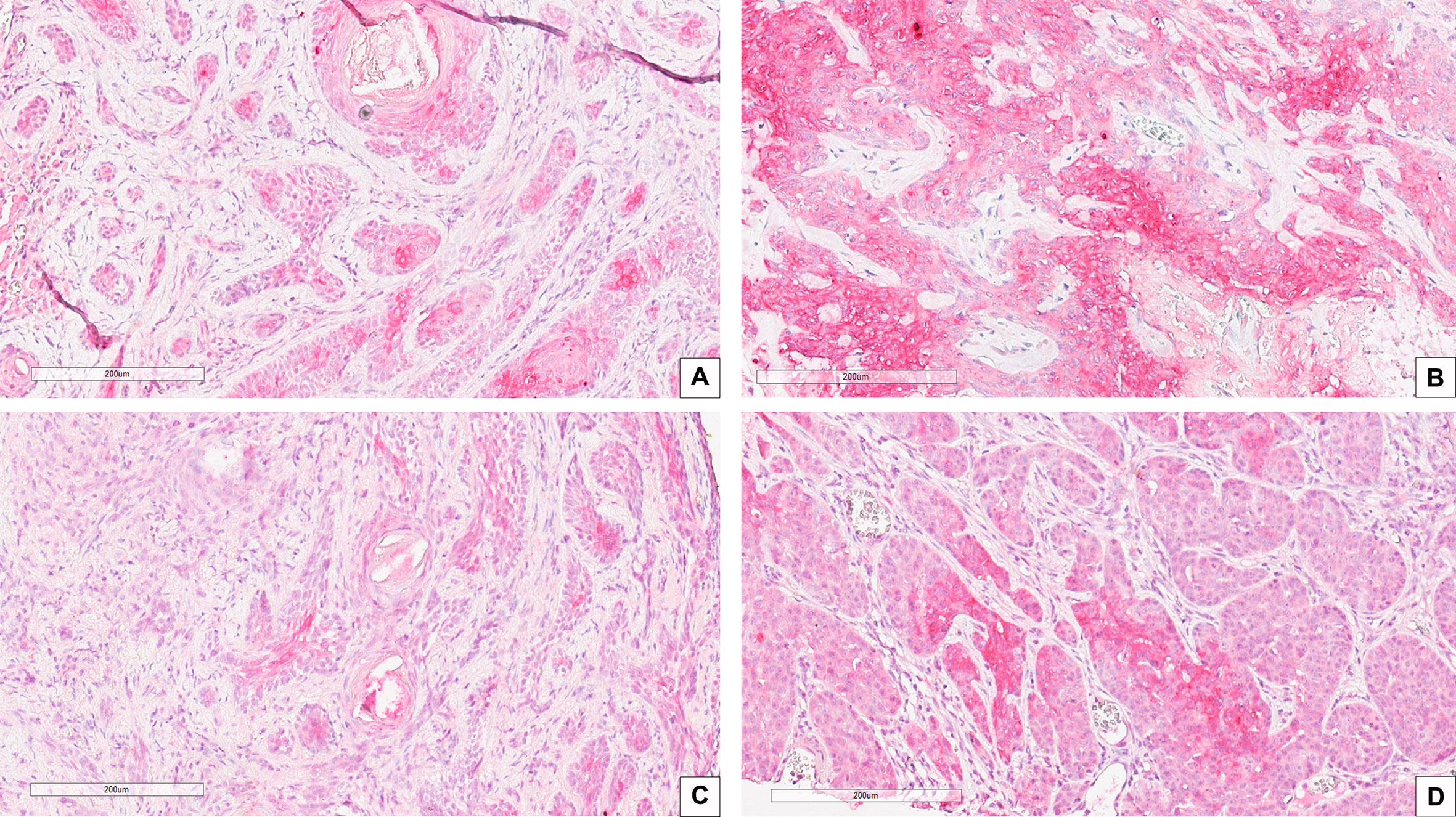
Figure 3 IHC stain with an anti-CAIX antibody in high risk BCC histological variants: (A) Infiltrating BCC; (B) Morpheaform BCC; (C) Micronodular BCC; (D) Basosquamous BCC. Scale bars are shown, magnification is 20×.
Basal carcinoma (BCC) represents 15% of all neoplasms and constitutes a serious public health problem, being the most common cancer in the white-skinned population. Its incidence is increasing worldwide, with an increase of ≥ 10%/year [Lomas et al. (2)]. BCC is a tumor that, despite its low frequency of distant metastasis, frequently causes extensive tissue losses, due to a marked tendency to stromal infiltration, particularly in its dedifferentiated forms, with disfiguring and debilitating results. The maximum expression of this aggressive behavior is the so-called Ulcus Rodens with destructive consequences for the cartilage and bone tissues. To date, there is still limited availability of alternative therapeutic treatments to surgery. Recently, promising results seem to emerge from the early follow-up of patients treated with molecular anti-Sonic Hedgehog therapy, a pathway associated with the BCC carcinogenesis process. It is compelling to unravel the pathogenetic mechanisms underlying the aggressiveness potential of each BCC subtypes, in order to achieve an effective personalized therapy for these tumors. The need of greater understanding of BCC biology appears even more urgent given that this neoplasia preferentially affects the adult and elderly population and that prevention and early diagnosis are still unattained goals, especially in emerging areas of the World and in Western countries peripheral areas. In recent years, a large body of data has highlighted the importance of the interaction of cancer cells with the tumor microenvironment, which provides support for the growth and development of neoplasia. This assumption brought to light new study hypotheses, in order to characterize the heterotypic interaction between the tumor and its microenvironment. Among the alterations of the tumor microenvironment, much attention has been paid, in recent decades, to hypoxia, a pathophysiological feature of locally advanced tumors, resulting from genetic instability, diminished apoptotic potential, and angiogenesis. The hypoxic state also plays an important role in relapsing, metastasis and poor response to treatments, including radiotherapy, chemotherapy and angiogenic treatment. Among the molecules most expressed in hypoxic condition, the carbonic anhydrase IX (CAIX) is considered a marker of hypoxia in vivo (17), whose overexpression has been correlated with increased tumor aggression in different types of cancer (18–22). To date, a series of CAIX inhibitors have been synthesized, both in the form of small inhibitory molecules and as monoclonal antibodies, used as antitumor agents in different models of neoplasms (23–31). In a previous work we tested the expression of CAIX in several human solid tumors, extending the CAIX expression information to the expression of the stem cells markers CD44 and nestin in solid cancers, to explore their relationship with the biological behavior of tumors. We found that CAIX is strongly expressed in advanced tumors, including squamous cell invasive cancer of the tongue (14). The role of CAIX as a prognostic biomarker in oral cancer has been recently reviewed by (32), whose systematic review and meta-analysis showed that that immunohistochemical CAIX assessment is a useful OSCC prognostic biomarker. In the present work, the immunohistochemistry expression of the CAIX protein was evaluated in a selected series of patients with BCC divided into two groups based on the histological subtype. The highest levels of protein were found in the aggressive BCC group consisting mainly of morphoeic BCC and Basosquamous BCC. 68.3% of cases showed a high level of expression, and the remaining 31.7% a low level. CAIX expression frequency distribution has been reported in all the histotypes described in our case series, and the statistics of CAIX expression correlation with BCC subtypes have been carried out grouping BCC samples into two categories, aggressive and ordinary one, according to clinical behavior of each subtype, in order to overcome the relative small number of subjects for some subtypes. In the ordinary group, 92% of cases expressed low levels of CAIX and only 8% show a high score. The Fisher Exact Test confirmed that the difference in CAIX immunostain observed between the two BCC groups was statistically significant. The most significant result was obtained by comparing the averages of expression between the aggressive and ordinary groups, and the difference showed a value of P<0.0001. In conclusion, these results suggest that the expression levels of the CAIX protein can help to stratify BCCs in different risk classes; moreover, our results let envisage a role for CAIX as a therapeutic target to counteract the most aggressive BCC, providing a viable alternative to the surgical approach, and to the inhibitors of Hedgehog Pathway, a promising tool for target therapy in BCCs, often associated with various degrees of toxicity (muscle spasms, alopecia, dysgeusia, weight loss, fatigue, nausea, decreased appetite and diarrhea) that in the most severe forms (hypovolemic shock, myocardial infarction, meningeal disease, Ischemic stroke) determine the interruption of treatment, with no resolution of the pathology in place.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was performed according to the Italian law, and according to the Declaration of Helsinki for studies based only on retrospective analyses on routine archival FFPE-tissue.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication. All authors contributed to the article and approved the submitted version.
The Pathology Section of the University of Naples “Federico II”, Department of Advanced Biomedical Sciences, was funded by a POR Campania FESR 2014-2020 grant; “Technological Platform: eMORFORAD-Campania” grant PG/2017/0623667.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Verkouteren JAC, Ramdas KHR, Wakkee M, Nijsten T. Epidemiology of basal cell carcinoma: scholarly review. Br J Dermatol (2017) 177:359–72. doi: 10.1111/bjd.15321
2. Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol (2012) 166:1069–80. doi: 10.1111/j.1365-2133.2012.10830.x
3. Chinem VP, Miot HA. Epidemiology of basal cell carcinoma. Anais Brasileiros Dermatol (2011) 86:292–305. doi: 10.1590/s0365-05962011000200013
4. Leiter U, Keim U, Garbe C. Epidemiology of skin cancer: Update 2019. Adv Exp Med Biol (2020) 1268:123–39. doi: 10.1007/978-3-030-46227-7_6
5. AIRTUM Working Group. [Italian cancer figures, report 2011: Survival of cancer patients in Italy]. Epidemiol E Prevenzione (2011) 35:1–200.
6. Christenson LJ, Borrowman TA, Vachon CM, Tollefson MM, Otley CC, Weaver AL, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA (2005) 294:681–90. doi: 10.1001/jama.294.6.681
7. Elder DE, Massi D, Scolyer RA, Willemze R. WHO classification of skin tumours. World Health Organization classification of tumours. 4th. Lyon: International Agency for Research on Cancer (2018).
8. Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev (2007) 26:225–39. doi: 10.1007/s10555-007-9055-1
9. Bache M, Kappler M, Said HM, Staab A, Vordermark D. Detection and specific targeting of hypoxic regions within solid tumors: current preclinical and clinical strategies. Curr Med Chem (2008) 15:322–38. doi: 10.2174/092986708783497391
10. Pastorekova S, Gillies RJ. The role of carbonic anhydrase IX in cancer development: links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev (2019) 38:65–77. doi: 10.1007/s10555-019-09799-0
11. Pérez-Sayáns M, Supuran CT, Pastorekova S, Suárez-Peñaranda JM, Pilar GD, Barros-Angueira F, et al. The role of carbonic anhydrase IX in hypoxia control in OSCC. J Oral Pathol Med: Off Publ Int Assoc Oral Pathologists Am Acad Oral Pathol (2013) 42:1–8. doi: 10.1111/j.1600-0714.2012.01144.x
12. De Simone G, Supuran CT. Carbonic anhydrase IX: Biochemical and crystallographic characterization of a novel antitumor target. Biochim Et Biophys Acta (2010) 1804:404–9. doi: 10.1016/j.bbapap.2009.07.027
14. Ilardi G, Zambrano N, Merolla F, Siano M, Varricchio S, Vecchione M, et al. Histopathological determinants of tumor resistance: a special look to the immunohistochemical expression of carbonic anhydrase IX in human cancers. Curr Med Chem (2014) 21:1569–82. doi: 10.2174/09298673113209990227
15. Mascolo M, Ilardi G, Merolla F, Russo D, Vecchione M, de Rosa G, et al. Tissue microarray-based evaluation of chromatin assembly factor-1 (caf-1)/p60 as tumour prognostic marker. Int J Mol Sci (2012) 13:11044–62. doi: 10.3390/ijms130911044
16. Martino F, Varricchio S, Russo D, Merolla F, Ilardi G, Mascolo M, et al. A machine-learning approach for the assessment of the proliferative compartment of solid tumors on hematoxylin-eosin-stained sections. Cancers (2020) 12. doi: 10.3390/cancers12051344
17. Swinson DEB, Jones JL, Richardson D, Wykoff C, Turley H, Pastorek J, et al. Carbonic anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non-small-cell lung cancer. J Clin Oncol: Off J Am Soc Clin Oncol (2003) 21:473–82. doi: 10.1200/JCO.2003.11.132
18. Hussain SA, Ganesan R, Reynolds G, Gross L, Stevens A, Pastorek J, et al. Hypoxia-regulated carbonic anhydrase IX expression is associated with poor survival in patients with invasive breast cancer. Br J Cancer (2007) 96:104–9. doi: 10.1038/sj.bjc.6603530
19. Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Thymoglobulin Induction Study Group. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. New Engl J Med (2006) 355:1967–77. doi: 10.1056/NEJMoa060068
20. Baniak N, Flood TA, Buchanan M, Dal Cin P, Hirsch MS. Carbonic anhydrase IX (CA9) expression in multiple renal epithelial tumour subtypes. Histopathology (2020) 77:659–66. doi: 10.1111/his.14204
21. Jin MS, Lee H, Park IA, Chung YR, Im SA, Lee KH, et al. Overexpression of HIF1α and CAXI predicts poor outcome in early-stage triple negative breast cancer. Virchows Archiv: Int J Pathol (2016) 469:183–90. doi: 10.1007/s00428-016-1953-6
22. Lodewijk L, van Diest P, van der Groep P, Ter Hoeve N, Schepers A, Morreau J, et al. Expression of HIF-1α in medullary thyroid cancer identifies a subgroup with poor prognosis. Oncotarget (2017) 8:28650–9. doi: 10.18632/oncotarget.15622
23. Supuran CT. Carbonic Anhydrase Inhibition and the Management of Hypoxic Tumors. Metabolites (2017) 7. doi: 10.3390/metabo7030048
24. Chafe SC, McDonald PC, Saberi S, Nemirovsky O, Venkateswaran G, Burugu S, et al. Targeting Hypoxia-Induced Carbonic Anhydrase IX Enhances Immune-Checkpoint Blockade Locally and Systemically. Cancer Immunol Res (2019) 7:1064–78. doi: 10.1158/2326-6066.CIR-18-0657
25. Lau J, Lin KS, Bénard F. Past, Present, and Future: Development of Theranostic Agents Targeting Carbonic Anhydrase IX. Theranostics (2017) 7:4322–39. doi: 10.7150/thno.21848
26. Alberti D, Michelotti A, Lanfranco A, Protti N, Altieri S, Deagostino A, et al. In vitro and in vivo BNCT investigations using a carborane containing sulfonamide targeting CAIX epitopes on malignant pleural mesothelioma and breast cancer cells. Sci Rep (2020) 10:19274. doi: 10.1038/s41598-020-76370-1
27. Giuntini G, Monaci S, Cau Y, Mori M, Naldini A, Carraro F. Inhibition of Melanoma Cell Migration and Invasion Targeting the Hypoxic Tumor Associated CAXII. Cancers (2020) 12. doi: 10.3390/cancers12103018
28. Peppicelli S, Andreucci E, Ruzzolini J, Bianchini F, Nediani C, Supuran CT, et al. The Carbonic Anhydrase IX inhibitor SLC-0111 as emerging agent against the mesenchymal stem cell-derived pro-survival effects on melanoma cells. J Enzyme Inhibition Med Chem (2020) 35:1185–93. doi: 10.1080/14756366.2020.1764549
29. Ciccone V, Filippelli A, Angeli A, Supuran CT, Morbidelli L. Pharmacological Inhibition of CA-IX Impairs Tumor Cell Proliferation, Migration and Invasiveness. Int J Mol Sci (2020) 21. doi: 10.3390/ijms21082983
30. Aneja B, Queen A, Khan P, Shamsi F, Hussain A, Hasan P, et al. Design, synthesis & biological evaluation of ferulic acid-based small molecule inhibitors against tumor-associated carbonic anhydrase IX. Bioorg Med Chem (2020) 28:115424. doi: 10.1016/j.bmc.2020.115424
31. Podolski-Renić A, Dinić J, Stanković T, Jovanović M, Ramović A, Pustenko A, et al. Sulfocoumarins, specific carbonic anhydrase IX and XII inhibitors, interact with cancer multidrug resistant phenotype through pH regulation and reverse P-glycoprotein mediated resistance. Eur J Pharm Sci: Off J Eur Fed Pharm Sci (2019) 138:105012. doi: 10.1016/j.ejps.2019.105012
32. Lorenzo-Pouso AI, Gallas-Torreira M, Pérez-Sayáns M, Chamorro-Petronacci CM, Alvarez-Calderon O, Takkouche B, et al. Prognostic value of CAIX expression in oral squamous cell carcinoma: a systematic review and meta-analysis. J Enzyme Inhibition Med Chem (2020) 35:1258–66. doi: 10.1080/14756366.2020.1772250
Keywords: basal cell carcinoma, carbonic anhydrase IX, IHC, skin cancer, prognosis, risk stratification
Citation: Russo D, Varricchio S, Ilardi G, Martino F, Di Crescenzo RM, Pignatiello S, Scalvenzi M, Costa C, Mascolo M, Merolla F and Staibano S (2021) Tissue Expression of Carbonic Anhydrase IX Correlates to More Aggressive Phenotype of Basal Cell Carcinoma. Front. Oncol. 11:659332. doi: 10.3389/fonc.2021.659332
Received: 27 January 2021; Accepted: 05 March 2021;
Published: 25 March 2021.
Edited by:
Renato Franco, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Eugenio Maiorano, University of Bari Aldo Moro, ItalyCopyright © 2021 Russo, Varricchio, Ilardi, Martino, Di Crescenzo, Pignatiello, Scalvenzi, Costa, Mascolo, Merolla and Staibano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Merolla, ZnJhbmNlc2NvLm1lcm9sbGFAdW5pbW9sLml0; Stefania Staibano, c3RhaWJhbm9AdW5pbmEuaXQ=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.