
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 02 June 2021
Sec. Cancer Imaging and Image-directed Interventions
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.659313
This article is part of the Research Topic Breakthrough in Imaging-Guided Precision Medicine in Oncology View all 35 articles
Background: For individuals with cervical cancer, large tumor volume, lymph node metastasis, distant metastasis, and parauterine infiltration are usually associated with a poor prognosis. Individuals with stage 1B1 and 1B2 cervical cancer usually do not have these unfavorable prognostic factors. Once the disease progresses, the prognosis becomes extremely poor. Therefore, investigating the prognostic markers of these cervical cancer patients is necessary for treatment.
Methods: This retrospective study included 95 cervical cancer patients treated with surgery. The patients were divided into progressor and non-progressor groups according to postoperative follow-up results. T-test (or Mann−Whitney U test), chi-squared test (or Fisher’s exact test) and receiver operating characteristic (ROC) curves were used to evaluate imaging, hematology, and clinicopathological index differences between the two groups. Cox analysis was performed to select the independent markers of progression-free survival (PFS) when developing the nomogram. Validation of the nomogram was performed with 1000 bootstrapped samples. The performance of the nomogram was validated with ROC curves, generated calibration curves, and Kaplan-Meier and decision curve analysis (DCA).
Results: Cervical stromal invasion depth, lymphovascular space invasion (LVSI), human papilloma virus (HPV-16), Glut1, D-dimer, SUVmax and SUVpeak showed significant differences between the two groups. Multivariate Cox proportional hazard model showed SUVpeak (p = 0.012), and HPV-16 (p = 0.007) were independent risk factors and were used to develop the nomogram for predicting PFS. The ROC curves, Kaplan-Meier method, calibration curves and DCA indicated satisfactory accuracy, agreement, and clinical usefulness, respectively.
Conclusions: SUVpeak level (≥7.63 g/cm3) and HPV-16 negative status before surgery were associated with worse PFS for patients with cervical cancer. Based on this result, we constructed the nomogram and showed satisfactory performance. Clinically, individualized clinical decision-making can be performed on patients based on this result.
Cervical cancer has the second highest incidence of female malignant tumors (1). The International Federation of Gynecology and Obstetrics (FIGO) cancer staging system is used in the formulation of treatment and prognosis plans for patients with cervical cancer. In the recently updated 2018 FIGO staging, it is noteworthy that, assessment of the abdominopelvic retroperitoneal lymph nodes was included in the FIGO system (2–4). Regardless of parametrial infiltration and tumor size, the presence of nodal metastases now indicates stage IIIC. Radical hysterectomy with lymphadenectomy will be effective for those cervical cancer patients (stage 1B1, 1B2) with the following characteristics: invasive carcinoma confined to the uterine cervix, no more than 5 mm invasion, tumor size < 4 cm in its greatest dimension and no lymph node metastasis. Fortunately, these patients often do not need postoperative adjuvant radiotherapy, since, of course, radiotherapy has serious side effects (5). However, although the prognosis of cervical cancer patients with these features is excellent, if they relapse, then their prognosis is very poor (6). Therefore, identifying those cervical cancer patients who are prone to recurrence after surgery is of great significance, since they will require postoperative adjuvant treatment such as radiotherapy and chemotherapy, as well as closer follow-up, to reduce the chance of recurrence and death.
The following three methods were used in predicting the prognosis of cervical cancer: 1) evaluation of pathological characteristics obtained from postoperative pathological specimens, including aberrant molecular signaling pathway proteins; 2) preoperative imaging; and 3) hematological examination.
It is well known that pathological features such as age, tumor stage and grade, cervical stromal invasion depth, lymphovascular space invasion (LVSI); and preoperative high-risk human papillomavirus (HR-HPV) statue were important factors in the prognosis of cervical cancer. Different molecular factors involving loss of tumor suppressor genes and aberrant molecular signaling pathways, such as TP53-induced glycolysis and apoptosis regulator (TIGAR), cytokine involved primarily in angiogenesis (Vegf-A), mammalian facilitative glucose transporter family (Glut-1), epithelial mesenchymal transition related protein (E-cadherin), immune-linked factor by triggering pro-inflammatory immune-associated reactions (Cox-2), the extracellular matrix molecule (Tenascin-C), have recently been identified in the pathogenesis of cervical cancer (7–11). The impact of these proteins on cervical cancer prognosis needs further investigation.
18F-FDG PET/CT, a functional imaging technique, provides quantified metabolic information (the mean and maximum standardized uptake values (SUVmean and SUVmax), metabolic tumor volume (MTV) and total lesion glycolysis (TLG))and has a well-established role in the management of patients with cervical cancer (12–14). Many studies have shown that hematological parameters such as hemoglobin (Hb), coagulation indexes [D-dimer, fibrinogen (Fg)], tumor marker for squamous cell carcinoma [squamous cell carcinoma antigen (SCCA)], and novel systemic inflammation response index (SIRI) are potential prognostic marker of malignant tumors (15–19).
In this article, our aim was to find independent prediction parameters from these parameters and establish and validate a nomogram to predict the progression-free survival (PFS) of patients with cervical cancer.
Patients deemed to have a high suspicion of cervical cancer with strong evidence from PET/CT (PET/CT examinations were performed within one week before treatment) were retrospectively enrolled from January 1st, 2013, to December 31th, 2015 with the following criteria: (1) The patient underwent surgery (open surgery) and was confirmed as cervical squamous cell carcinoma by postoperative pathological results; (2) According to FIGO2018 pathological staging, the patient was confirmed to be stage IB1 or IB2; (3) Patients who had not undergone other treatments without surgery, and (4) Patients with complete follow-up records.
The Institutional Review Boards at the local institutions endorsed the study. We implemented it in accordance with the ethical standards of the 1964 Helsinki Declaration and subsequent amendments, and gave up informed consent of all participants.
Numerous studies have compared the effect of the Minimally Invasive and Abdominal Radical Hysterectomy for cervical cancer (20–22). The conclusion shows that for early cervical cancer the minimally invasive surgery (laparoscopic or robot-assisted radical hysterectomy) has a higher recurrence rate than open surgery. In recent years, patients of our hospital have also accepted open abdominal surgery as the main surgical method. Therefore, the patients included all received the open abdominal Type-C radical hysterectomy. The incision was located on the lower abdomen. The resection scope included the uterus, parauterine, upper vagina, and partial tissues around the vagina and pelvic lymph nodes. A sufficient length of adjacent connective tissues, including the front vesicocervix ligament (anterior and posterior lobes), the lateral main ligament, the posterior uterosacral ligament and rectovaginal ligament, also should be removed. The ovary was selectively removed according to the patient’s clinical conditions and willingness. The resection of lymph nodes involved the obturator lymph nodes and the internal, common, and external iliac vessel lymph nodes. Para-aortic nodes should be removed only in the following cases: (1) The preoperative PET/CT showed that there were suspicious metastatic lymph nodes in the para-aortic area [SUVmax of lymph nodes is higher than the background metabolism level (23, 24)]; and (2) the possibility of metastasis in the pelvic or para-aortic lymph nodes was suspected intra-operatively.
PFS was defined as the time interval between the date of surgery and the date at which the first recurrence of disease was confirmed. Starting from the completion of the surgery, follow-up examinations (chest CT, abdominal CT and pelvic MR) were performed approximately every 3 months for the first 2 years, and then every 6 months or 1 year for the next 3 years. Recurrent disease was defined as local recurrence, metastasis of pelvic or para-aortic lymph nodes, and metastases in distant organs following surgery. For patients with abnormal follow-up examinations results, PET/CT or biopsy was recommended, and the results used as the standard for recurrence. The end of the follow-up time is December 31th, 2020.
A pathologist with more than 10 years of experience evaluated sections using HE staining and immunohistochemistry (IHC) of CD31 and D2-40. The following results based on postoperative pathology report were obtained: tumor differentiation grade, stage, lymph node metastasis, LVSI, cervical stromal invasion depth and histologic tumor type.
Tumor sections were obtained from our hospital pathology department and we purchased antibodies from Abcam (Shanghai, China). In addition, Cox-2, E-cadherin, Tenascin-C, Glut-1, Tigar, and Vegf-A protein expression were studied based on IHC, which was performed using the Leica BOND-MAX system (Leica Biosystems, Shanghai, China). Thorough mixing was done with the following polyclonal antibodies: Cox-2 at 1:100 dilution; E-cad at 1:700 dilution; Tenascin-C at 1:100 dilution; Glut-1 at 1:500 dilution; Tigar at 1:300 dilution; and Vegf-A at 1:50 dilution. Then the tissue section was sealed after the Imaging Mass Cytometry (IMC) was finished, the section was placed under the lens of a scanner (Pannoramic MIDI, 3D Histech), and moved gradually, imaging while moving and then scanning and imaging all the tissue information on the tissue section to form a file. After image scanning was completed, the DensitoQuant software application in the QuantCenter (QuantCenter is a piece of analysis software for Pannoramic viewer) automatically recognized and set all dark browns on the tissue section as strongly positive, brown-yellow as moderately positive, light yellow as weakly positive, and blue cell nuclei as negative. Furthermore, for each tissue, strongly positive, moderately positive, weakly positive and negative areas, and the percentage of positive areas, were identified. A negative expression was defined by a < 10% positive expression.
For HPV detection we used an AB I2Prism7000 PCR detector, a Hybrid-Max medical nucleic acid molecular hybridizer (AB, USA), and a PCR kit (Qiagen, USA). HPV detection used an AB I2Prism7000 PCR detector, a Hybrid-Max medical nucleic acid molecular hybridizer (AB, USA), and a PCR kit (Qiagen, USA). Fifteen types of the HR-HPV types (HPV16, 18, 31, 33, 34, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68) were detected and diagnosed by a pathologist with more than 10 years of diagnostic experience.
Included in the novel systemic inflammation index were the following: NLR, defined as neutrophil-to-lymphocyte ratio; LMR, defined as mononuclear cell-to-lymphocyte ratio; and SII, defined as ratio of product of platelets and neutrophils to lymphocytes (25, 26). Evaluation was undertaken of the relationship between the novel systemic inflammation index and prognosis based on the ROC curve and calculation of the optimal cut-off value for meaningful indexes. Anemia was defined as a hemoglobin count below 11.0 g/dL. The optimal D-dimer, Fg and SCCA cut-off range for assessing prognosis was 0-0.5 mg/L, 2-4 g/L, and 0-2.5 ng/ml. These cut-off ranges were selected as they have been recognized as standard pathological definitions.
All patients were fasted for more than 6 hours before the 18F-FDG PET/CT examination, blood glucose levels were controlled below 7 mmol/L, and 18F-FDG was injected with the patient in a quiet state from 3.70 to 5.55 MBq/kg. After 45–60 minutes, 18F-FDG PET/CT was performed. The patient was placed in a GE Discovery Elite scanner (GE Healthcare, USA) with the scan ranging from the skull top to the middle of the thigh (120s/bed). The thickness of the CT scan layer was 3.75 mm, the tube voltage was 120–140 keV, and the tube current was 80mA. PET images were reconstructed using an Iterative adaptive algorithm.
Transmitting all 18F-FDG PET/CT images to GE AW4.6 workstation (GE Healthcare, USA), 2 radiologists with more than 5 years of radiodiagnostic experience used PET volume computer-assisted reading (PET VCAR) software to perform imaging analysis. After outline of a lesion region of interest (ROI) at the cross-sectional level of the largest area of the tumor, the fused-PET software automatically calculated SUVmean, SUVmax, MTV and TLG of the entire tumor. MTV was calculated by a 40% SUVmax threshold (27, 28).
The patients were divided into a positive and a negative group according to prognosis. Differences in patient characteristics between the groups were compared with the t-test (Mann-Whitney U test, if not normal distribution) or chi-squared (Fisher’s exact test, if not the number of assumptions necessary). For quantitative variables, receiver operating characteristic (ROC) curves were generated to assess the area under the ROC curve (AUC) for differentiating prognosis. We used the cut-off threshold values for differentiating these meaningful quantitative variables. The Kaplan-Meier method was used to build survival functions and the log-rank test was used to compare survivals for all variables. Prognostic variables were evaluated by univariate and multivariate Cox proportional hazard model (variables with p<0.1 in the univariate Cox proportional hazard model were used in the multivariate analysis). In multiple testing, a correction was performed, based on the Benjamini-Hochberg procedure. Finally, these final prognostic variables were incorporated to construct the nomogram. The nomogram adopted the 1-, 2-, and 5-year PFS as primary endpoints. Validation of the nomogram was performed with 1000 bootstrapped samples, that is, we adopted the method of bootstrapped samples to set up a control group, randomly selected 1000 times from the existing samples, arbitrarily once, and then a final time to get the original sample number (95). The predictive capability was evaluated by ROC analysis. Generated calibration curves were used to visualize the difference between the predicted and actual 1-, 2-, and 5-year PFS. Decision curve analysis (DCA) was introduced to evaluate clinical utility of the nomogram (29). Based on the median of the total scores, a risk stratification system was developed, and the cervical cancer patients were divided into two risk subgroups, including high-risk and low-risk groups, and the Kaplan-Meier method and log rank test were used to compare the differences between the two subgroups. The statistical analysis was conducted by MedCalc (version 15.2.2), SPSS (version 22.0), R (version 3.5.3), and p<0.05 indicated a significant statistical difference (if not specified).
The median follow-up was 62.8 months (range, 2-96). A total of 95 patients were enrolled: 24 (25.3%) patients manifested progressive disease and 71 (74.7%) patients presented no evidence of disease (Figure 1).
The clinicopathological characteristics of the patients are summarized in Table 1A. Cervical stromal invasion depth (chi-squared test, p=0.046; log-rank test, p=0.032), LVSI (chi-squared test, p=0.032; log-rank test, p=0.008) and HPV-16 (chi-squared test, p=0.001; log-rank test, p<0.001) showed differences between patients with and without disease progression. The remaining indicators had no significant correlation.
Chi-squared test and Kaplan-Meier method showed that patients with Glut1-negative had a statistically significant better clinical outcome than patients with Glut-1-positive (chi-squared test, p=0.039; log-rank test, p=0.039) (Table 1B). The remaining protein indicators were not statistically significant.
Chi-squared test and Kaplan-Meier method showed only D-dimer was related to disease progression and PFS (Table 1C). The ROC curve showed that no novel systemic inflammation index was statistically significant for disease progression.
SUVmax (U test, p=0.014) and SUVpeak (U test, p=0.002) showed significant between-group differences (Table 2). ROC analysis showed that SUVmax (AUC 0.668, p=0.006), SUVpeak (AUC 0.716, p<0.001) had a positive effect on predicting disease progression (Figure 2). The optimal cut-off threshold values for SUVmax, SUVpeak were 9.12 g/cm3 (sensitivity 91.67, specificity 39.44) and 7.63 g/cm3 (sensitivity 91.67, specificity 47.89), respectively. Kaplan-Meier method showed the DFS rates of patients exhibiting high SUVmax and SUVpeak of the primary tumor were significantly lower than those of patients exhibiting low SUVmax and SUVpeak of the primary tumor (p=0.006 and p=0.001, respectively) (Figure 2).
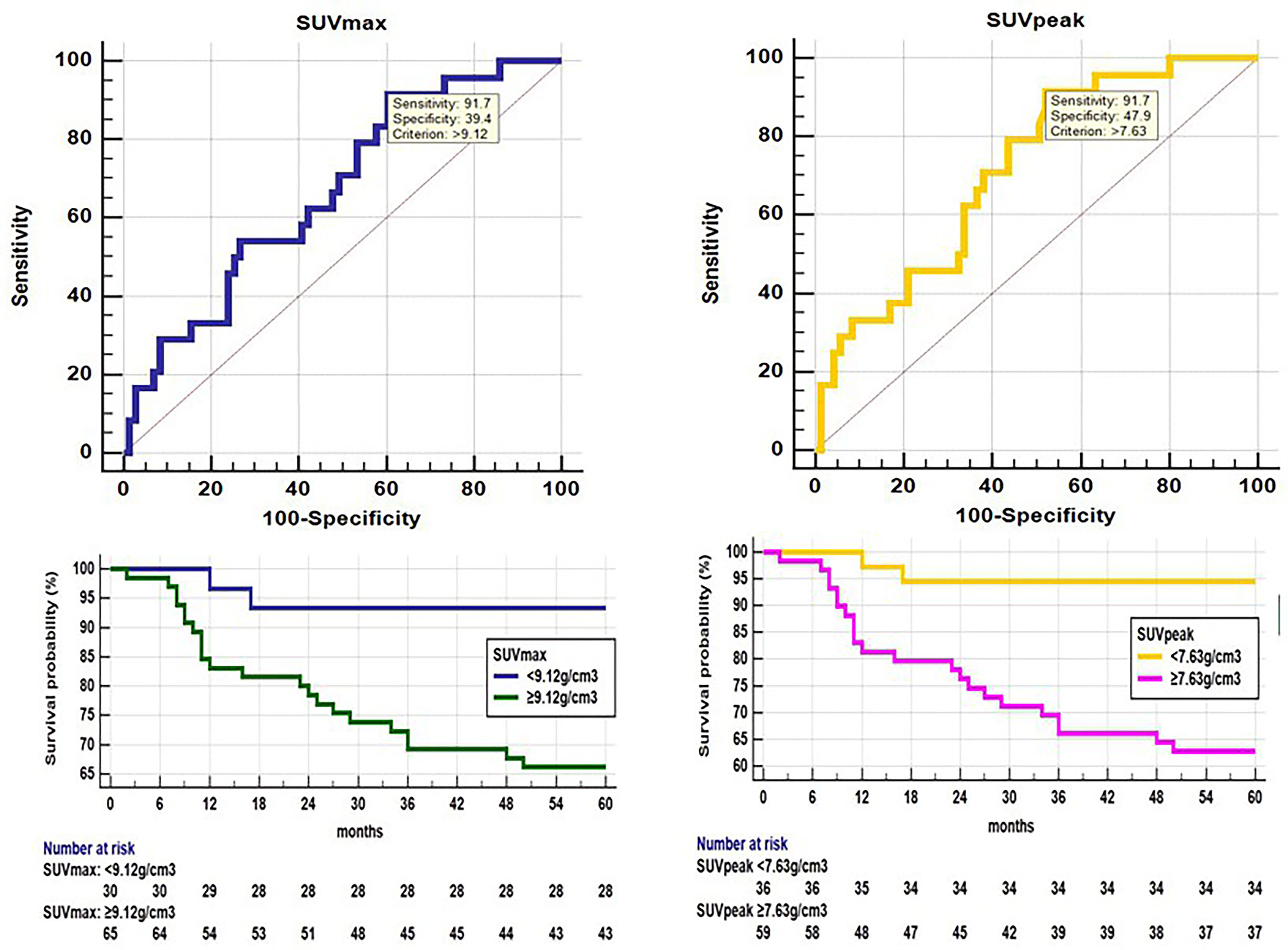
Figure 2 ROC analysis shows that SUVmax(AUC 0.668, 95% confidence interval (CI) 0.564-0.761, p=0.006) and SUVpeak (AUC 0.716, 95% CI 0.615-0.804, p<0.001) had a positive effect on disease progression. The optimal cut-off threshold values for SUVmax and SUVpeak were 9.12 g/cm3 (sensitivity 91.67, specificity 39.44), 7.63 g/cm3 (sensitivity 91.67, specificity 47.89), respectively. Kaplan-Meier survival graph shows significantly different PFS between the groups categorized by SUVmax and SUVpeak above and below cut-off value (p<0.05, log-rank test).
Univariate Cox proportional hazard model showed that cervical stromal invasion depth (p=0.041), LVSI (p=0.018), HPV-16 (p=0.001), Glut1 (p=0.048), D-dimer (p=0.040), SUVmax (p=0.016) and SUVpeak (p=0.005) were associated with PFS. Multivariate Cox proportional hazard model showed that SUVpeak (p=0.012), and HPV-16 (p=0.007) were independent risk factors for PFS (Table 3).
SUVpeak and HPV-16 were incorporated to develop the nomogram for predicting 1-, 2-, and 5-year PFS. The nomogram showed that SUVpeak made the largest contribution to the prognosis, followed by HPV-16 which showed a certain amount of impact on the PFS (Figure 3). ROC analysis showed the AUCs at 1-, 2-, and 5-year PFS reached 0.828, 0.808 and 0.814 in the original model, and 0.814, 0.835 and 0.841 in the validation model, respectively (Figures 4B, D). Whether in the original model or in the validation model, the calibration curves demonstrated considerable agreement between the nomogram and predicted survival (Figures 4A, C). Clinical utility of the nomogram was evaluated by DCA. The nomogram showed enormous positive net benefits across wide ranges of mortality risk in both models, demonstrating its predominant clinical utility in predicting PFS (Figure 5). In particular, it has the greatest clinical utility for 5-year PFS in both models. In addition, we calculated the total scores for the original model to build a risk stratification system based on our nomogram, and then distinguished the patients according to the median quantile of total scores into high-risk subgroups and low-risk subgroups. The PFS in the two subgroups was exactly separated by this system (Figures 6A, B).
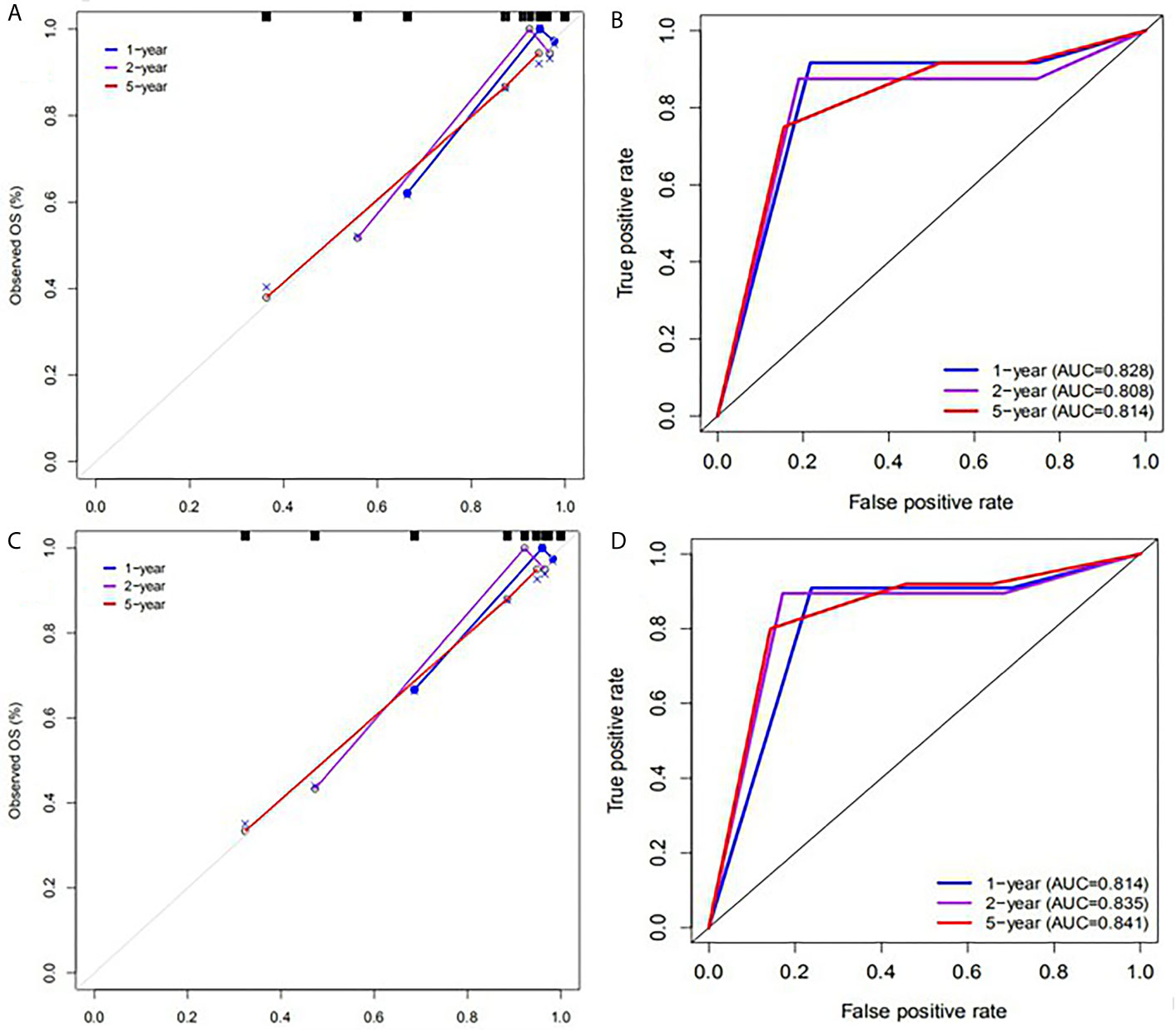
Figure 4 Calibration plots and ROC curves for predicting PFS at 1-, 2-, and 5- year points. (A) The calibration plots for predicting PFS in the original model. (B) ROC curves of the nomogram for predicting PFS in the original model. (C) The calibration plots for predicting PFS in the validation model. (D) ROC curves of the nomogram for predicting PFS in the validation model.
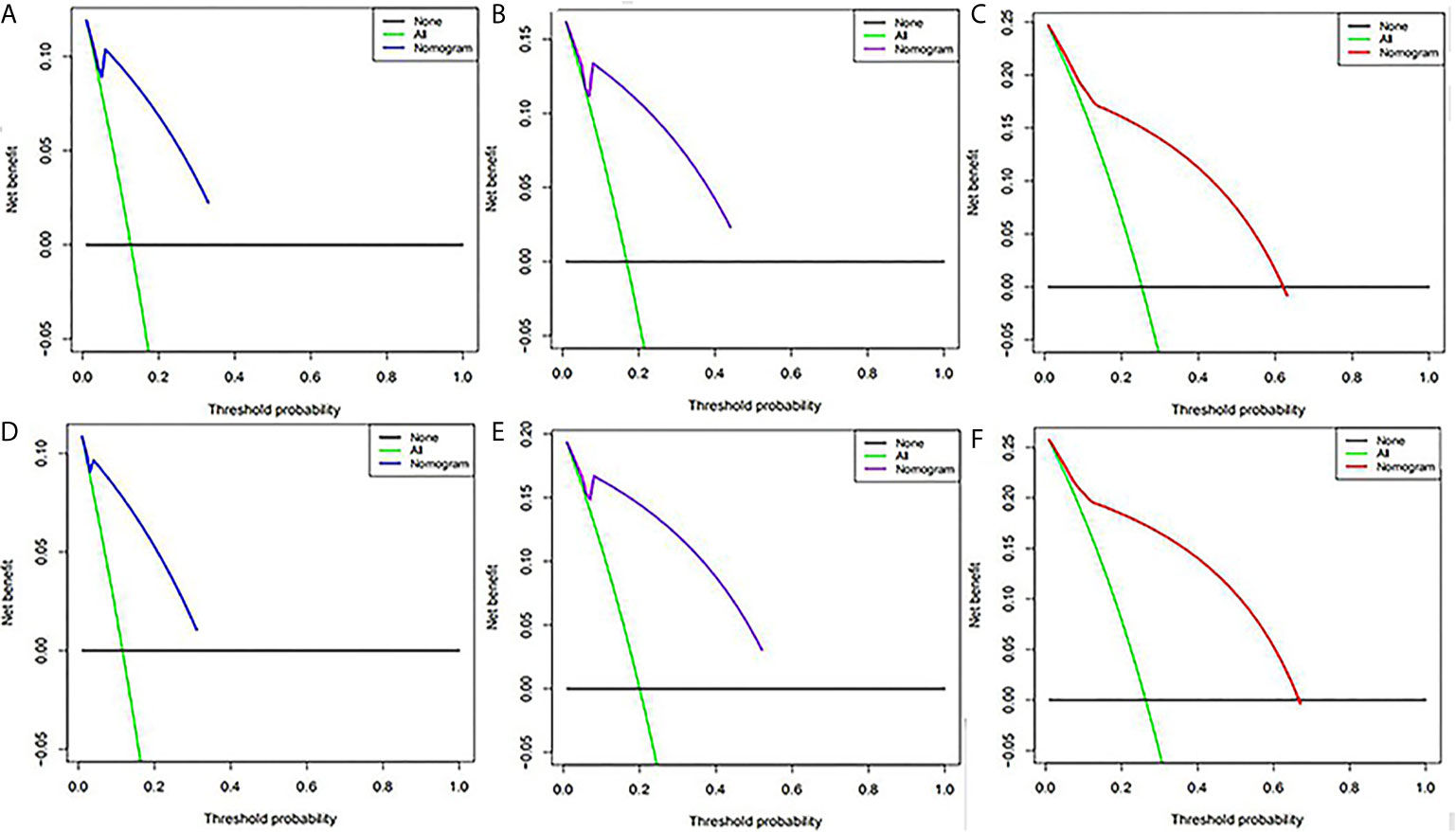
Figure 5 Decision curve analysis of the nomogram for predicting PFS at 1-,(A) 2-,(B) and 5-year (C) points in the original model and PFS at 1-(D), 2-(E) and 5-year (F) points in the validation model. The percentage of threshold probability was represented by the x-axis, whereas the net benefit was represented by the y-axis, calculated by adding the true positives and subtracting the false positives.
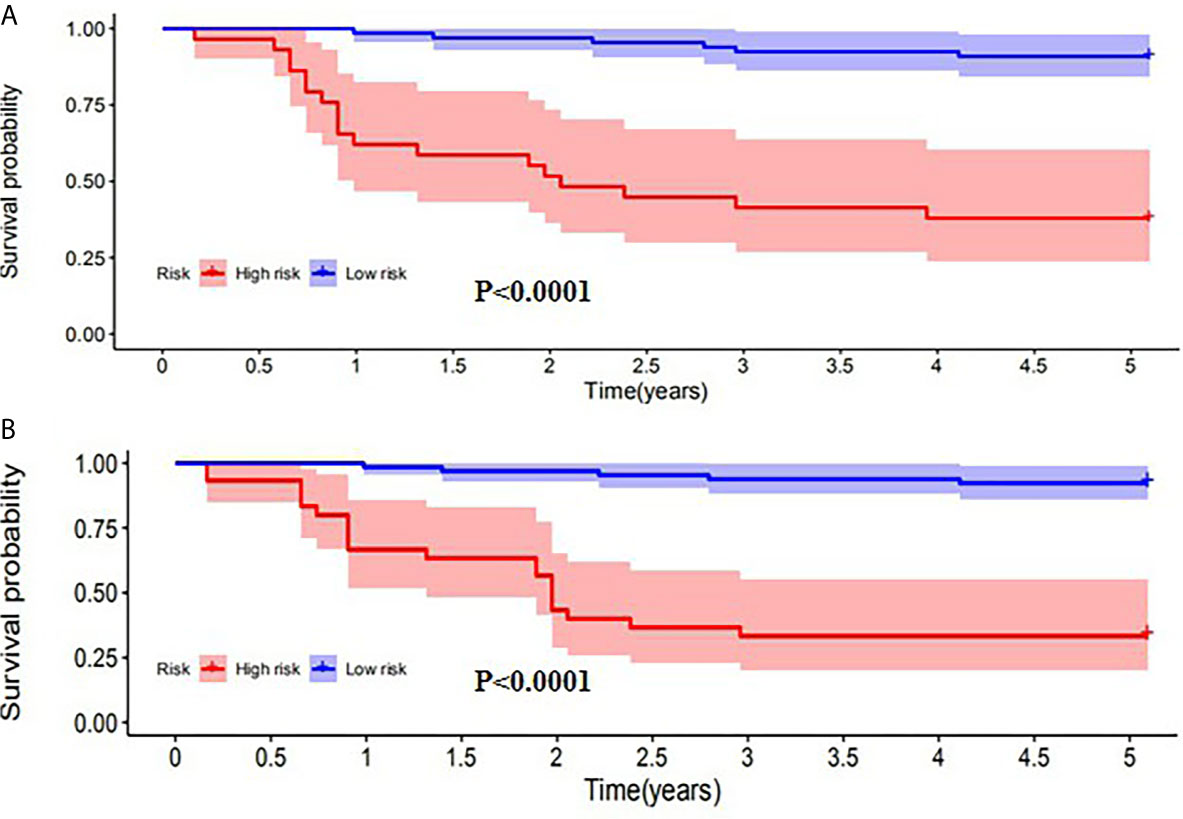
Figure 6 Kaplan-Meier analysis of PFS for patients stratified by the risk stratification system in the original model (A) and validation model (B).
A poor prognosis for patients with cervical cancer is usually associated with a large tumor volume, lymph node metastasis, distant metastasis, and parauterine infiltration. In this study, we explored the prognostic indicators of patients with stage 1B1 and 1B2 cervical cancer without these factors, and established a prognostic nomogram and risk stratification system. Our study showed SUVmax, SUVpeak, Glut1, HPV-16, cervical stromal invasion depth, LVSI and D-dimer were associated with PFS. SUVpeak and HPV-16 were identified as factors independently impacting disease progression.
The basis of PET imaging is the “Warburg effect”, that is, tumor cells mainly produce ATP through a higher rate of glycolysis compared with normal cells. SUVmax, a type of PET parameter, reflects the metabolism of the most active part of the lesion. SUVpeak, another type of SUV, refers to the average SUV within a spherical VOI positioned around the most active metabolism point (30). The glucose uptake of cells is mediated by Glut, and Glut-1 is an important isomer of glucose transporter. Univariate cox analysis shows that SUVmax, SUVpeak and Glut1 have a certain correlation with PFS, but multivariate analysis shows that only SUVpeak is considered to be an independent factor, among these three parameters. SUVmax is the most commonly used PET parameter in the clinic, but it is a single element measurement value and is susceptible to image resolution and noise. Compared with SUVmax, the value of SUVpeak is more stable and accurate because it is not easy affected by tracking bed position, scanning time and the size of the lesion (31, 32). A study on the survival analysis of patients with cervical cancer showed that among the five classic PET parameters: SUVmax, SUVmean, SUVpeak, MTV, TLG and six texture features, SUVpeak is the most accurate parameter in predicting the disease progression (33). Zhang Le et al. showed that SUVpeak had the highest correlation with the clinicopathological features of cervical cancer (34). These all support our results. Compared with other glucose metabolism parameters, SUVpeak is more closely related to the prognosis of patients undergoing surgery. It is worth noting that MTV, TLG and PFS are not related in our study. Yoo et al. conducted a prognostic study of cervical cancer at stage I–IV and showed that MTV and TLG are important PET parameters for predicting disease progression (35). The study of Maura et al. and Sangwon et al. had similar findings (36, 37). This may be because the largest diameter of the cancerous lesions in our study subjects was less than 4cm. However, MTV and TLG reflect the overall metabolic burden of the tumor. Therefore, the parameters MTV and TLG related to the tumor volume did not play a major role in our research.
HPV is a genus of papilloma vacuole virus A, belonging to the papovaviridae family. It is a spherical DNA virus that can cause the proliferation of squamous epithelium of human skin and mucosa. High-risk HPV can be detected in most cervical cancer specimens. HPV16 is a common high-risk type of HPV that is most prone to persistent infection and can be detected in about 40% to 60% of patients with cervical cancer (38). However, a study showed that HPV-negative status is associated with a poor prognosis in patients with cervical cancer, which may be related to WNT/β-catenin signaling and non-synonymous somatic mutations (39). Another Meta-Analysis showed the presence of HPV-16 positivity appears to have no significant association with prognosis cervical cancer in PFS. But, eliminating a study with a strong impact on the outcome from the analysis would lead to a conclusion of a worse prognosis of HPV-16 negative in cervical cancer (40). In addition, one study showed that cervical cancer patients infected with HPV16 had a better prognosis than those with any other HPV type (41). These results are roughly consistent with our study, in which HPV16 negativity was associated with worse PFS for those patients with cervical cancer.
Cervical stromal invasion depth is an essential index in the standard pathological report, and represents the invasion status of tumor cells. Lymphovascular space invasion (LVSI) is a common clinical pathological phenomenon of malignant tumors. For tumors to form distant metastases and spread, tumor cells must first enter the circulatory system and spread through blood or lymphatic vessels. Therefore, LVSI is associated with distant metastasis, from a histological perspective (42). D-dimer is a degradation product produced following fibrinolysis, which participates in the coagulation process. Its plasma level can be used as an evaluation index for blood hypercoagulability and as a measure of whether secondary fibrinolytic hyperfunction occurs (43). Our research showed that the above three indicators were related to prognosis, but they were not independent prognostic factors.
Our combined nomogram developed could effectively identify the patients with progressive disease. It is observed that SUVpeak is of the most value to prognosis, followed by HPV-16. We established a control model using 1000 bootstrapped samples at the same time. The nomogram of the original group and the control group both showed excellent performance, as was indicated by the ROC curves, generate calibration curves, DCA and Kaplan-Meier method. The ROC curves and Kaplan-Meier method showed that the model had good prediction accuracy, and the calibration curves demonstrated consistency. However, even if the model has high accuracy, patients may not necessarily benefit clinically, and there may be false positives and false negatives. Sometimes it is more beneficial to avoid false positives, and at other times more desirable to avoid false negatives. Since neither situation can be avoided, a method with the greatest net benefit is needed. This is what DCA does. The DCA of our model showed great clinical performance, especially for patients with 5 year-PFS. The capability of the combined nomogram for prediction of progressive disease may facilitate personalized treatment decisions.
This study has a number of limitations. On one hand, this is a retrospective study based on the latest FIGO2018 staging. Because the early follow-up was not optimal, the sample size of our study was not sufficiently large, especially for positive cases, thus further future confirmation of the results is needed. Furthermore, there is a lack of data about several important factors, including some functional sequences of magnetic resonance, proteomics and genomics data, and pathway proteins related to prognosis, such as Hive, Caix, etc. Finally, in our original data, compared with patients with squamous cell carcinoma, the proportion of patients with adenocarcinoma is particularly small. In order to prevent extreme value bias, we only selected patients with squamous cell carcinoma as the research object. In the future, additional types of pathology will be included in our research.
In conclusion, SUVpeak level (≥7.63 g/cm3) and HPV16 negative were identified as independent factors and could be associated with poor prognosis for patients with cervical cancer (Stage IB1, IB2). Based on this result, we established a nomogram and risk stratification system, and achieved satisfactory performance and clinical utility. These findings could contribute to test-individualized neoadjuvant treatment. It is worth emphasizing that the stage of cervical cancer of patients selected was only Stage IB1 and IB2. That is, patients with cervical cancer of other stages were not within the scope of this study. At the same time, cervical stromal invasion depth, LVSI, and D-dimer were related to prognosis, but they were not associated with poor DFS in the multivariate analysis. Cautions would be needed when clinical decision-making was made for patients with these risk factors.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
CX and SG designed the research. CX, TM, XL and SG performed the research and analyzed results. CX wrote the paper. CX, XL, HS,TM and SG edited the manuscript and provided critical comments. All authors contributed to the article and approved the submitted version.
This study was funded by LIAONING Science & Technology Project (2017225012), LIAONING Science Natural Science Foundation (2019-MS-373) and 345 Talent Project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank our researchers for their hard work.
1. Tsu V, Jerónimo J. Saving the World’s Women From Cervical Cancer. New Engl J Med (2016) 374:2509–11. doi: 10.1056/NEJMp1604113
2. Signorelli M, Guerra L, Montanelli L, Crivellaro C, Buda A, Dell”Anna T, et al. Preoperative Staging of Cervical Cancer: Is 18-FDG-PET/CT Really Effective in Patients With Early Stage Disease? Gynecol Oncol (2011) 123:236–40. doi: 10.1016/j.ygyno.2011.07.096
3. Susanna L, Mostafa A. 2018 FIGO Staging System for Uterine Cervical Cancer: Enter Cross-sectional Imaging. Radiology (2019) 292:15–24. doi: 10.1148/radiol.2019190088
4. Koji M, Hiroko M, Rachel SM, Ikuo K, Mikio M. Validation of the 2018 FIGO Cervical Cancer Staging System. Gynecol Oncol (2019) 53:87–93. doi: 10.1016/j.ygyno.2018.10.026
5. Ainhoa A, Stanley IG, Ralph RW. Radiotherapy and Immunotherapy for Cancer: From “Systemic” to “Multisite”. Clin Cancer Res (2020) 26:2777–82. doi: 10.2147/CMAR.S239624
6. Pfaendler KS, Tewari KS. Changing Paradigms in the Systemic Treatment of Advanced Cervical Cancer. Am J Obstet Gynecol (2016) 214:22–30. doi: 10.1016/j.ajog.2015.07.022
7. Fendt SM, Lunt SY. Dynamic ROS Regulation by TIGAR: Balancing Anti-cancer and Pro-metastasis Effects. Cancer Cell (2020) 37:141–2. doi: 10.1016/j.ccell.2020.01.009
8. Iwasaki K, Yabushita H, Ueno T, Wakatsuki A. Role of Hypoxia-Inducible Factor-1α, Carbonic anhydrase-IX, Glucose Transporter-1 and Vascular Endothelial Growth Factor Associated With Lymph Node Metastasis and Recurrence in Patients With Locally Advanced Cervical Cancer. Oncol Lett (2015) 10:1970–78. doi: 10.3892/ol.2015.3524
9. Peng J, Qi S, Wang P, Li W, Song L, Liu C, et al. Meta-Analysis of Downregulated E-cadherin as a Poor Prognostic Biomarker for Cervical Cancer. Future Oncol (2016) 12:715–26. doi: 10.2217/fon.15.332
10. Aylas M, Pérez -Regadera GJ. EP-2061: Over-expression of EGFR and/or Cox-2 in Locally Advanced Squamous Cervical Cancer (LASC). Radiotherapy Oncol (2016) 119:S972–3. doi: 10.1016/S0167-8140(16)33312-6
11. Huseyin B, Adem A. Value of Tenascin-C Content and Association With Clinicopathological Parameters in Uterine Cervical Lesions. Int J Cancer (2002) 100:719–22. doi: 10.1002/ijc.10546
12. Lucia F, Miranda O, Abgral R, Bourbonne V, Dissaux G, Pradier O, et al. Use of Baseline 18 F-FDG PET/CT to Identify Initial Sub-Volumes Associated With Local Failure After Concomitant Chemoradiotherapy in Locally Advanced Cervical Cancer. Front Oncol (2020) 10:678. doi: 10.3389/fonc.2020.00678
13. Giacomo ML, Antonella M, Giulio V, Giulia D, Nicoletta N, Eugenia MDC, et al. Prognostic Value of Posttreatment 18F-FDG PET/CT and Predictors of Metabolic Response to Therapy in Patients With Locally Advanced Cervical Cancer Treated With Concomitant Chemoradiation Therapy: An Analysis of Intensity- and Volume-Based PET Parameters. Eur J Nucl Med Mol Imaging (2018) 45:2139–46. doi: 10.1007/s00259-018-4077-1
14. Herrera FG, Prior JO. The Role of PET/CT in Cervical Cancer. Front Oncol (2013) 3:34. doi: 10.3389/fonc.2013.00034
15. Zsuzsanna N, Orsolya H, Julia K, Dorottya V, Larisza L, Bence K, et al. D-Dimer as a Potential Prognostic Marker. Pathol Oncol Res (2012) 18:669–74. doi: 10.1007/s12253-011-9493-5
16. Moreno-Acosta P, Vallard A, Carrillo S, Gamboa O, Romero-Rojas A, Molano M, et al. Biomarkers of Resistance to Radiation Therapy: A Prospective Study in Cervical Carcinoma. Radiat Oncol (2017) 12:120. doi: 10.1186/s13014-017-0856-2
17. Liu Z, Shi H. Prognostic Role of Squamous Cell Carcinoma Antigen in Cervical Cancer: A Meta-Analysis. Dis Markers (2019) 1:1–10. doi: 10.1155/2019/6710352
18. Sun D, An L, Lv G. Albumin-Fibrinogen Ratio and Fibrinogen-Prealbumin Ratio as Promising Prognostic Markers for Cancers: An Updated Meta-Analysis. World J Surg Oncol (2020) 18:9. doi: 10.1186/s12957-020-1786-2
19. Chao B, Ju X, Zhang L, Xu X, Zhao Y. A Novel Prognostic Marker Systemic Inflammation Response Index (SIRI) for Operable Cervical Cancer Patients. Front Oncol (2020) 10:766. doi: 10.3389/fonc.2020.00766
20. Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally Invasive Versus Abdominal Radical Hysterectomy for Cervical Cancer. N Engl J Med (2018) 379:1895–904. doi: 10.1056/NEJMoa1806395
21. Gallotta V, Conte C, Federico A, Vizzielli G, Gueli AS, Tortorella L, et al. Robotic Versus Laparoscopic Radical Hysterectomy in Early Cervical Cancer: A Case Matched Control Study. Eur J Surg Oncol (2018) 44:754–9. doi: 10.1016/j.ejso.2018.01.092
22. Pedone AL, Bizzarri N, Kucukmetin A, Turco LC, Gallotta V, Carbone V, et al. Investigating the Possible Impact of Peritoneal Tumor Exposure Amongst Women With Early Stage Cervical Cancer Treated With Minimally Invasive Approach. Eur J Surg Oncol (2021) 47(5):1090–7. doi: 10.1016/j.ejso.2020.09.038. S0748-7983(20)30829-5.
23. Xu C, Du S, Zhang S, Wang B, Dong C, Sun H. Value of Integrated PET-IVIM MR in Assessing Metastases in Hypermetabolic Pelvic Lymph Nodes in Cervical Cancer: A Multi-Parameter Study. Eur Radiol (2020) 30:2483–92. doi: 10.1007/s00330-019-06611-z
24. Xu C, Yu Y, Li X, Sun H. Value of Integrated PET-IVIM MRI in Predicting Lymphovascular Space Invasion in Cervical Cancer Without Lymphatic Metastasis. Eur J Nucl Med Mol Imaging (2021). doi: 10.1007/s00259-021-05208-3
25. Wang C, He W, Yuan Y, Zhang Y, Li K, Zou R, et al. Comparison of the Prognostic Value of Inflammation-Based Scores in Early Recurrent Hepatocellular Carcinoma After Hepatectomy. Liver Int (2020) 40:229–39. doi: 10.1111/liv.14281
26. Hu B, Yang XR, Xu Y, Sun Y, Sun C, Guo Y, et al. Systemic Immune-Inflammation Index Predicts Prognosis of Patients After Curative Resection for Hepatocellular Carcinoma. Clin Cancer Res (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
27. Kidd EA, Thomas M, Siegel BA, Dehdashti F, Grigsby PW. Changes in Cervical Cancer FDG Uptake During Chemoradiation and Association With Response. Int J Radiat Oncol Biol Phys (2013) 85:116–22. doi: 10.1016/j.ijrobp.2012.02.056
28. Akkas BE, Demirel BB, Dizman A, Vural GU. Do Clinical Characteristics and Metabolic Markers Detected on Positron Emission Tomography/Computerized Tomography Associate With Persistent Disease in Patients With in-Operable Cervical Cancer? Ann Nucl Med (2013) 27:756–63. doi: 10.1007/s12149-013-0745-1
29. Vickers AJ, Elkin EB. Decision Curve Analysis: A Novel Method for Evaluating Prediction Models. Med Decis Making (2006) 26:565–74. doi: 10.1177/0272989X06295361
30. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J Nucl Med (2009) 50:122S–50S. doi: 10.2967/jnumed.108.057307
31. Sher A, Lacoeuille F, Fosse P, Laurent V, Aurélie CV, Djamel D, et al. For Avid Glucose Tumors, the SUVpeak is the Most Reliable Parameter for((18)F)FDG-PET/CT Quantification, Regardless of Acquisition Time. EJNMMI Res (2016) 6:21. doi: 10.1186/s13550-016-0177-8
32. Akamatsu G, Ikari Y, Nishida H, Tomoyuki N, Akihito O, Akira M, et al. Influence of Statistical Fluctuation on Reproducibility and Accuracy of SUVmax and SUVpeak: A Phantom Study. J Nucl Med Technol (2015) 43:222–26. doi: 10.2967/jnmt.115.161745
33. Schernberg A, Reuze S, Orlhac F, Irène B, Laurent D, Roger S, et al. A Score Combining Baseline Neutrophilia and Primary Tumor SUV(peak) Measured From FDG PET is Associated With Outcome in Locally Advanced Cervical Cancer. Eur J Nucl Med Mol Imaging (2018) 45:187–95. doi: 10.1007/s00259-017-3824-z
34. Zhang L, Sun H, Du S, Xu W, Xin J, Guo Q. Evaluation of 18F-FDG PET/CT Parameters for Reflection of Aggressiveness and Prediction of Prognosis in Early-Stage Cervical Cancer. Nucl Med Commun (2018) 9:1045–52. doi: 10.1097/MNM.0000000000000909
35. Yoo J, Choi JY, Moon SH, Bae DS, Park SB, Choe YS, et al. Prognostic Significance of Volume-Based Metabolic Parameters in Uterine Cervical Cancer Determined Using 18F-Fluorodeoxyglucose Positron Emission Tomography. Int J Gynecol Cancer (2012) 22:1226–33. doi: 10.1097/IGC.0b013e318260a905
36. Miccò M, Vargas HA, Burger IA, Marisa AK, Debra AG, Kay JP, et al. Combined Pre-Treatment MRI and 18F-FDG PET/CT Parameters as Prognostic Biomarkers in Patients With Cervical Cancer. Eur J Radiol (2014) 83:1169–76. doi: 10.1016/j.ejrad.2014.03.024
37. Han S, Kim H, Kim YJ, Suh CH, Woo S. Prognostic Value of Volume-Based Metabolic Parameters of 18F-FDG PET/CT in Ovarian Cancer: A Systematic Review and Meta-Analysis. Ann Nucl Med (2018) 32:669–77. doi: 10.2214/AJR.18.19734
38. Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic Classification of Human Papillomavirus Types Associated With Cervical Cancer. N Engl J Med (2003) 348:518–27. doi: 10.1056/NEJMoa021641
39. Banister CE, Liu C, Pirisi L, Creek KE, Buckhaults PJ. Identification and Characterization of HPV-independent Cervical Cancers. Oncotarget (2017) 8:13375–86. doi: 10.18632/oncotarget.14533
40. Chen X, Zhang P, CHen S, Zhu H, Wang K, Ye L, et al. Better or Worse? The Independent Prognostic Role of HPV-16 or HPV-18 Positivity in Patients With Cervical Cancer: A Meta-Analysis and Systematic Review. Front Oncol (2020) 10:1733. doi: 10.3389/fonc.2020.01733
41. Hang D, Jia M, Ma H, Zhou J, Feng X, Lyu Z, et al. Independent Prognostic Role Ofhuman Papillomavirus Genotype in Cervical Cancer. BMC Infect Dis (2017) 17:391. doi: 10.1186/s12879-017-2465-y
42. Tjalling B, Elke EMP, Carien LC, Ina MJS, Jan JJ, Jan WMM, et al. Substantial Lymph-Vascular Space Invasion (LVSI) is a Significant Risk Factor for Recurrence. Eur J Cancer (2015) 51:1742–50. doi: 10.1016/j.ejca.2015.05.015
Keywords: cervical cancer, positron-emission tomography, computed tomography, human papilloma virus, prognosis
Citation: Xu C, Ma T, Sun H, Li X and Gao S (2021) Markers of Prognosis for Early Stage Cervical Cancer Patients (Stage IB1, IB2) Undergoing Surgical Treatment. Front. Oncol. 11:659313. doi: 10.3389/fonc.2021.659313
Received: 27 January 2021; Accepted: 17 May 2021;
Published: 02 June 2021.
Edited by:
Romain-David Seban, Institut Curie, FranceReviewed by:
Jitti Hanprasertpong, Prince of Songkla University, ThailandCopyright © 2021 Xu, Ma, Sun, Li and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohan Li, MjQxMjE3MTUzNEBxcS5jb20=; Song Gao, Z2Fvc0Bzai1ob3NwaXRhbC5vcmc=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.