- 1Department of Urology, University Hospital Frankfurt, Goethe University Frankfurt am Main, Frankfurt, Germany
- 2Cancer Prognostics and Health Outcomes Unit, Division of Urology, University of Montreal Health Center, Montreal, QC, Canada
- 3Martini-Klinik Prostate Cancer Center, University Hospital Hamburg-Eppendorf, Hamburg, Germany
- 4Department of Hematology and Oncology, University Hospital Frankfurt, Frankfurt, Germany
Background: To evaluate the impact of time to castration resistance (TTCR) in metastatic hormone-sensitive prostate cancer (mHSPC) patients on overall survival (OS) in the era of combination therapies for mHSPC.
Material and Methods: Of 213 mHSPC patients diagnosed between 01/2013-12/2020 who subsequently developed metastatic castration resistant prostate cancer (mCRPC), 204 eligible patients were analyzed after having applied exclusion criteria. mHSPC patients were classified into TTCR <12, 12-18, 18-24, and >24 months and analyzed regarding OS. Moreover, further OS analyses were performed after having developed mCRPC status according to TTCR. Logistic regression models predicted the value of TTCR on OS.
Results: Median follow-up was 34 months. Among 204 mHSPC patients, 41.2% harbored TTCR <12 months, 18.1% for 12-18 months, 15.2% for 18-24 months, and 25.5% for >24 months. Median age was 67 years and median PSA at prostate cancer diagnosis was 61 ng/ml. No differences in patient characteristics were observed (all p>0.05). According to OS, TTCR <12 months patients had the worst OS, followed by TTCR 12-18 months, 18-24 months, and >24 months, in that order (p<0.001). After multivariable adjustment, a 4.07-, 3.31-, and 6.40-fold higher mortality was observed for TTCR 18-24 months, 12-18 months, and <12 months patients, relative to TTCR >24 months (all p<0.05). Conversely, OS after development of mCRPC was not influenced by TTCR stratification (all p>0.05).
Conclusion: Patients with TTCR <12 months are at the highest OS disadvantage in mHSPC. This OS disadvantage persisted even after multivariable adjustment. Interestingly, TTCR stratified analyses did not influence OS in mCRPC patients.
Introduction
Prostate cancer is the most common cancer in men and is moreover the second and third most common cause of cancer-specific mortality in the United States and Europe (1–3). Even though survival rates are excellent in localized prostate cancer, metastatic prostate cancer is a palliative situation (4, 5). For several decades, androgen deprivation therapy (ADT) has been the agent of choice in the treatment of metastatic hormone-sensitive prostate cancer (mHSPC) (6). With the publication of the GETUG-AFU 15 trial in 2013, in additional to ADT, several combination therapies, such as abiraterone, docetaxel, apalutamide, or enzalutamide, were approved for treatment in mHSPC; these combination therapies showed benefit according to overall survival (OS) and progression-free survival (PFS), especially in high-volume mHSPC (7–13). In consequence, one aim in the treatment of mHSPC is to delay progression to metastatic castration resistant prostate cancer (mCRPC) (14).
Recently, two Japanese studies focused on the effect of differences in time to castration resistance (TTCR) on OS (15, 16). However, these studies relied exclusively on patients treated with either ADT alone or combination of ADT plus bicalutamide in mHSPC. In consequence, little if anything is known about the impact of differences in TTCR on survival in the era of the above-described combination therapies, especially in European mHSPC patients.
We addressed this void and relied on our institutional metastatic prostate cancer database since the year of the first publication of combination therapy in mHSPC, to investigate the effect of TTCR on OS. We hypothesized that differences in TTCR will result in differences in OS rates but may not influence OS after development of mCRPC even in the era of combination therapies.
Materials and Methods
Study Population
After approval of the local ethics committee, all patients with mHSPC and subsequent mCRPC who were diagnosed since 2013 (year of the first publication of combination therapy in mHSPC) at the Department of Urology, University Hospital Frankfurt, Germany, were retrospectively identified (n=213) (12). All patients who were diagnosed between 01/2013 and 12/2020 were included in the current study. Exclusion criteria were unknown follow-up status or unknown status regarding the time point of progression to mCRPC (n=9). These selection criteria resulted in 204 eligible mHSPC patients.
mCRPC Definition
mCRPC status was defined in accordance with the EAU guidelines (4): PSA progression of three consecutive rises of PSA values or a 50% increase of absolute PSA values over the PSA nadir under mHSPC treatment combined with a testosterone level <50 ng/dl. Additionally, a radiographic progression with at least two new bone metastases or one visceral metastasis was defined as mCRPC (17). For TTCR analyses, the duration from the beginning of the treatment in mHSPC to the first stated date of mCRPC status was calculated. For OS analyses, the duration from the date beginning the treatment in mHSPC or mCRPC to patients’ death of any course was computed.
Statistical Analysis
Descriptive statistics included frequencies and proportions for categorical used variables. Medians and interquartile ranges (IQR) were reported for all used continuously variables. The Chi-square test was used to test for statistical significance in proportions’ differences. The t-test and Kruskal-Wallis test examined the statistical significance of distributions’ differences.
mHSPC patients were stratified into TTCR <12, 12-18, 18-24, and >24 months and accordingly analyzed with regard to OS. In the second set of the analyses, OS analyses were performed in the four TTCR subgroups since development of mCRPC status. Univariable and multivariable Cox regression models were fitted to predict the value of TTCR on OS in both analyses. All variables with p<0.25 in univariable analyses were considered for multivariable analyses, as recently recommended (18).
All tests were two-sided with a level of significance set at p<0.05. R software environment for statistical computing and graphics (version 3.4.3) was used for all analyses (19).
Results
Descriptive Baseline Characteristics
Median follow-up duration was 34 months. Among 204 eligible mHSPC patients, 41.2% (n=84) had a TTCR of <12 months, 18.1% (n=37) of 12-18 months, 15.2% (n=31) of 18-24 months, and 25.5% (n=152) of >24 months (Table 1). Median age and PSA at prostate cancer diagnosis did not differ between the four TTCR groups. Overall, median age was 67 years (IQR 61-73) and median PSA was 61 ng/ml (IQR 15-294, both p≥0.2). Moreover, the Eastern Cooperative Oncology Group (ECOG) distribution did not differ between the TTCR subgroups (p=0.2). Similarly, proportions of primary metastatic patients, high-volume metastatic burden according to CHAARTED criteria, and number and proportions of metastatic sides did not differ between the four TTCR subgroups (all p≥0.2). Patients with TTCR <12 months less frequently received local therapy for primary tumor with radical prostatectomy or radiation therapy than TTCR 12-18, 18-24, and >24 months, without reaching statistical significance (20.2, 27.0, 38.7, and 26.9%, p=0.3). Moreover, differences in combination therapies for mHSPC existed (p=0.01). Overall, in mHSPC patients, 55.4% (n=113) of patients received treatment with ADT vs. 22.5% (n=46) docetaxel vs. 14.2% (n=29) abiraterone vs. 3.4% (n=7) enzalutamide vs. 4.4% (n=9) other treatments. Median treatment therapy lines in mCRPC were 2 (IQR 1-3). Detailed characteristics of all four TTCR subgroups are summarized in Table 1.
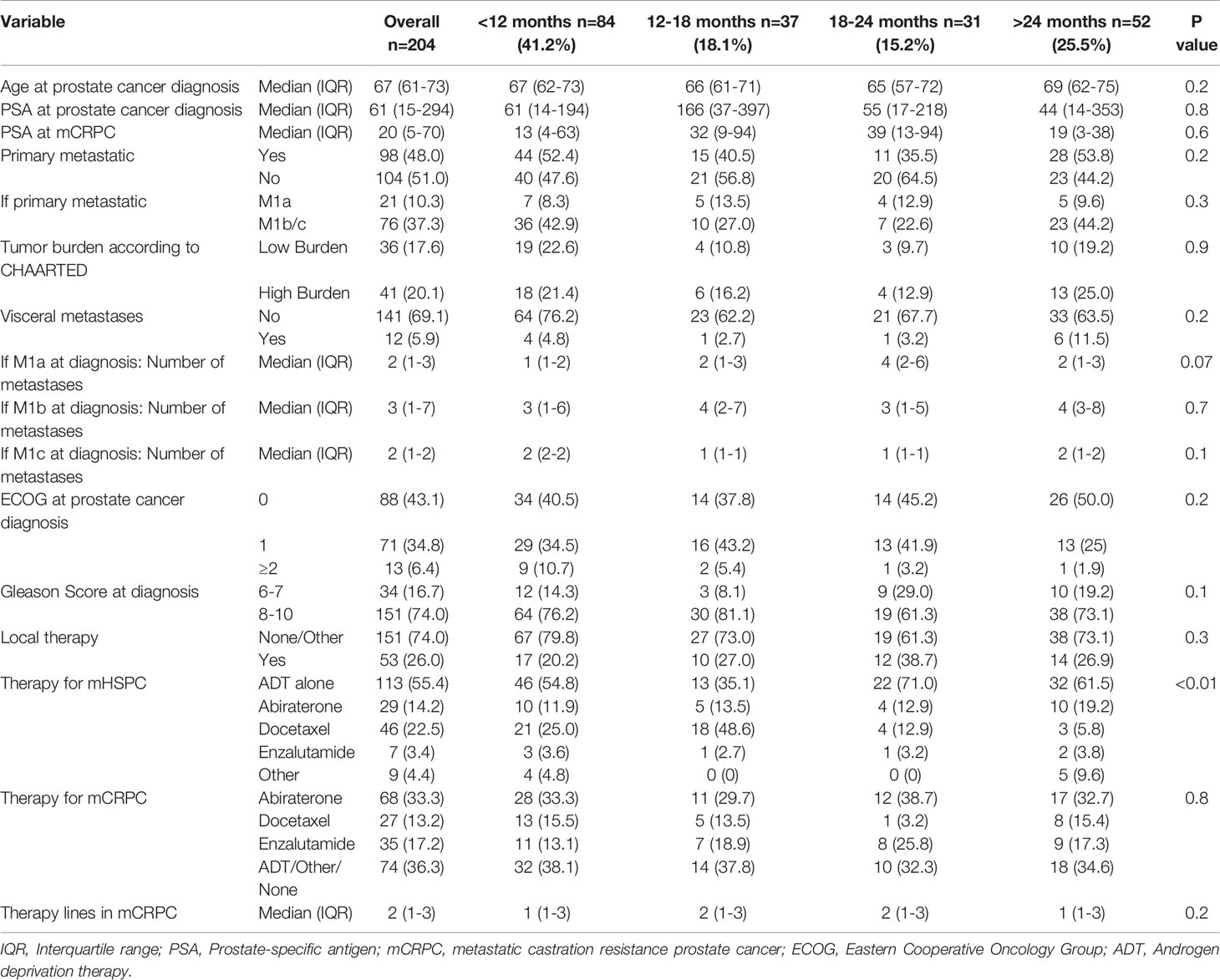
Table 1 Descriptive characteristics of 204 metastatic hormone-sensitive prostate cancer (mHSPC) patients, diagnosed between 2013-2020 at the University Hospital Frankfurt, stratified according to time to castration resistance.
Survival in mHSPC Patients According to TTCR
Patients with TTCR <12 months had the worst OS, followed by TTCR 12-18 months, 18-24 months, and >24 months, in that order (Figure 1, p<0.001). These OS differences translated into a 2.93-fold (confidence interval [CI]: 1.15-7.34, p=0.02), 3.78-fold (CI: 1.47-9.72, p<0.01), and 6.95-fold (CI 3.15-15.32, p<0.001) higher risk of overall mortality for TTCR 18-24 months, 12-18 months, and <12 months patients, relative to TTCR >24 months patients (Table 2).
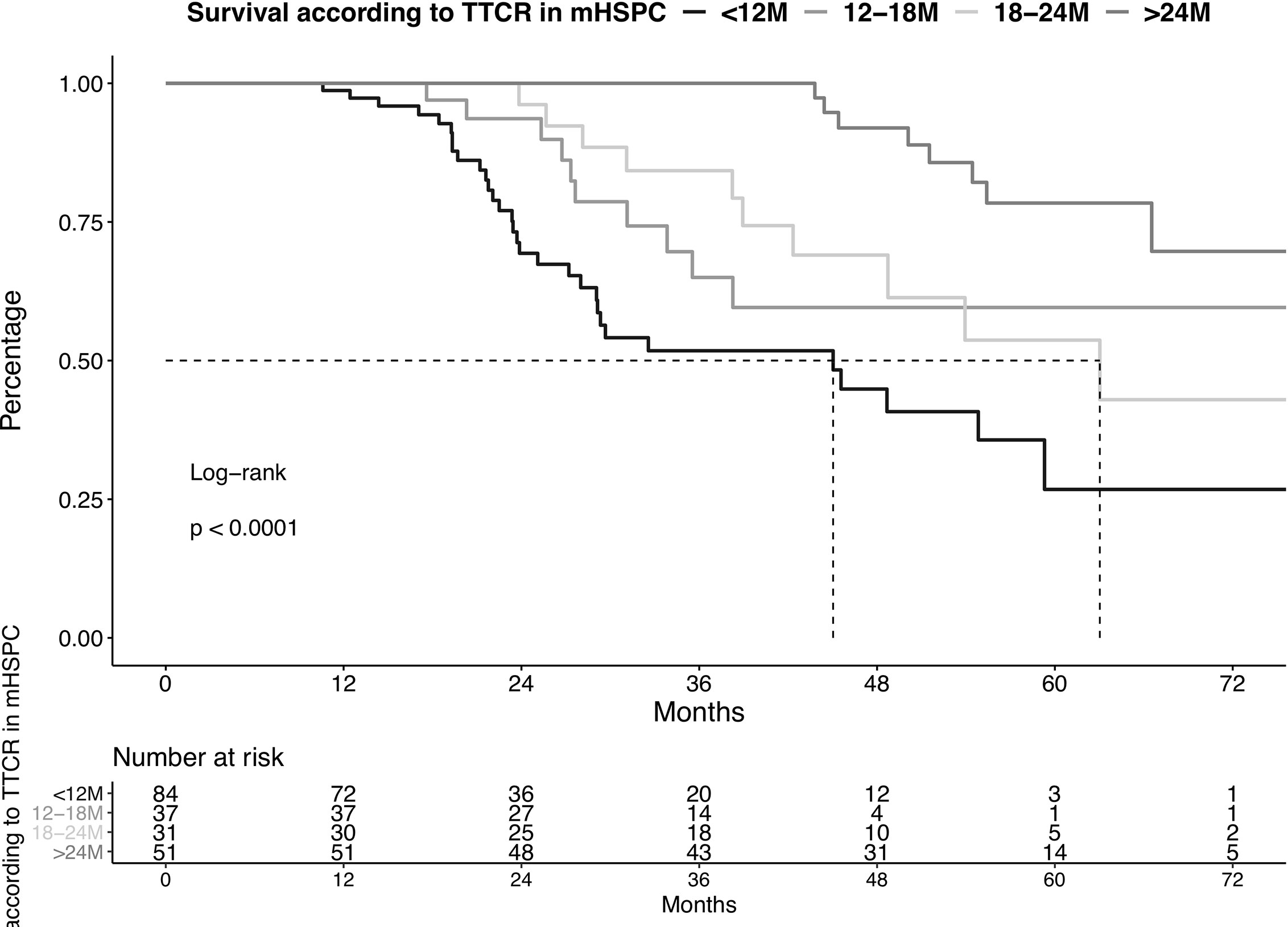
Figure 1 Kaplan-Meier plot illustrating overall survival in metastatic hormone-sensitive prostate cancer (mHSPC) patients diagnosed between 2013-2020, stratified according to time to castration resistance (TTCR). The follow-up starts from the beginning of the therapy in mHSPC. M, Months.

Table 2 Univariable and multivariable Cox regression models predicting overall survival in metastatic hormone-sensitive prostate cancer (mHSPC) patients according to time to castration resistance (TTCR).
After multivariable adjustment for patient and prostate cancer characteristics, the significant OS disadvantages persisted in all three TTCR subgroups. Specifically, hazard ratios (HR) of 3.92 (CI 1.42-10.84, p<0.01), 3.59 (CI 1.28-10.08, p=0.01), and 7.14 (CI 2.85-17.88, p<0.001) were recorded for TTCR 18-24 months, 12-18 months, and <12 months patients, relative to patients with TTCR >24 months patients. Moreover, ECOG ≥2 was an independent predictor for worse OS (HR: 4.45, p<0.001).
Survival in mCRPC Patients According to TTCR
OS did not differ in all four examined TTCR groups after development of mCRPC (Figure 2, p=0.2). Moreover, after multivariable adjustment for patient and prostate cancer characteristics, no statistically significant OS differences were observed between the TTCR subgroups 18-24 months, 12-18 months, and <12 months, relative to TTCR >24 months patients (all p>0.05, Table 3). Conversely, ECOG 1 and ≥2, as well as the number of visceral metastases, were independently associated with worse OS (HRs: 2.95, 9.52, 2.82, all p ≤ 0.02).
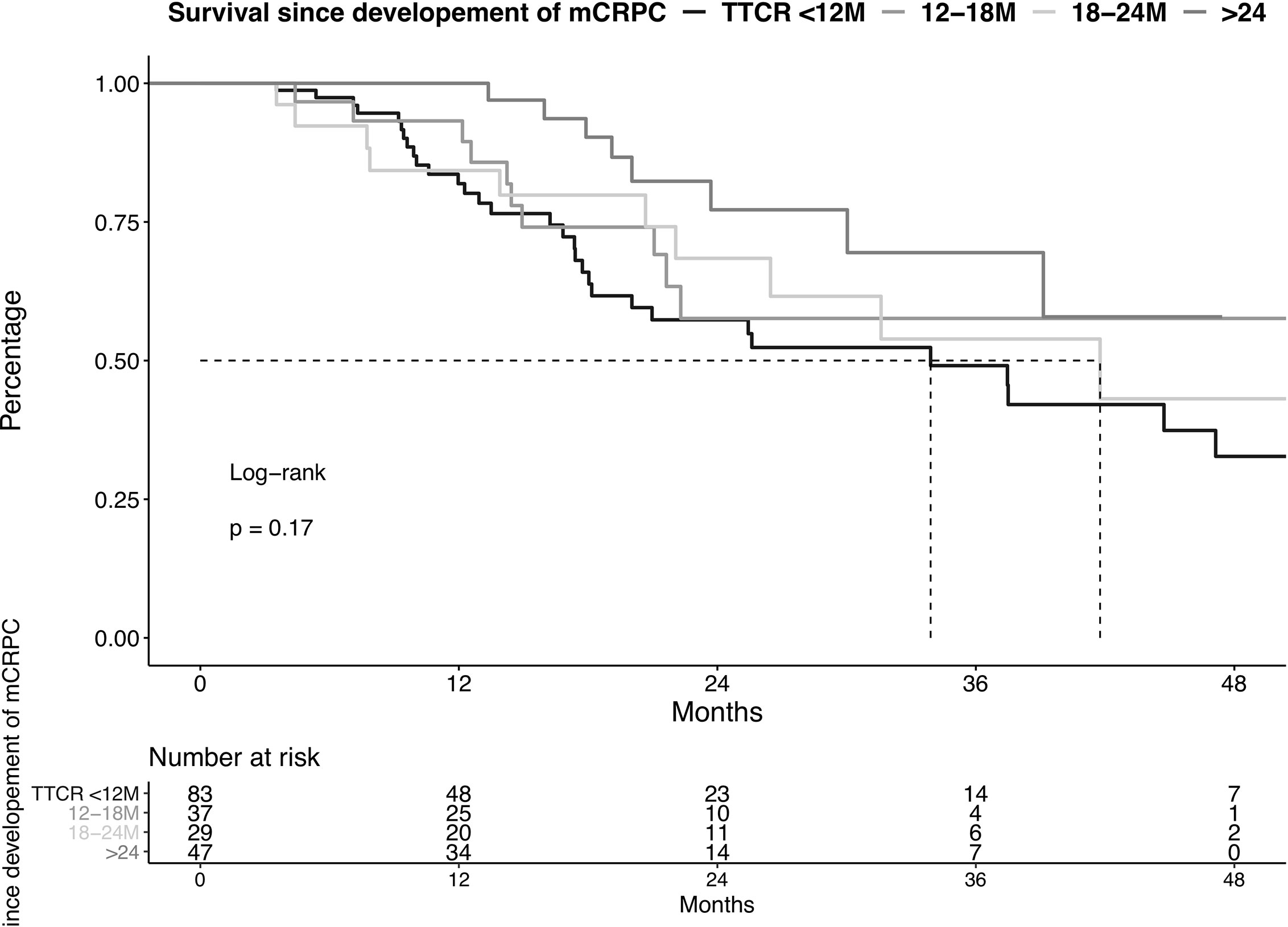
Figure 2 Kaplan-Meier plot illustrating overall survival in metastatic castration resistance prostate cancer (mCRPC) patients diagnosed between 2013-2020, stratified according to the time to castration resistance (TTCR). M, Months.
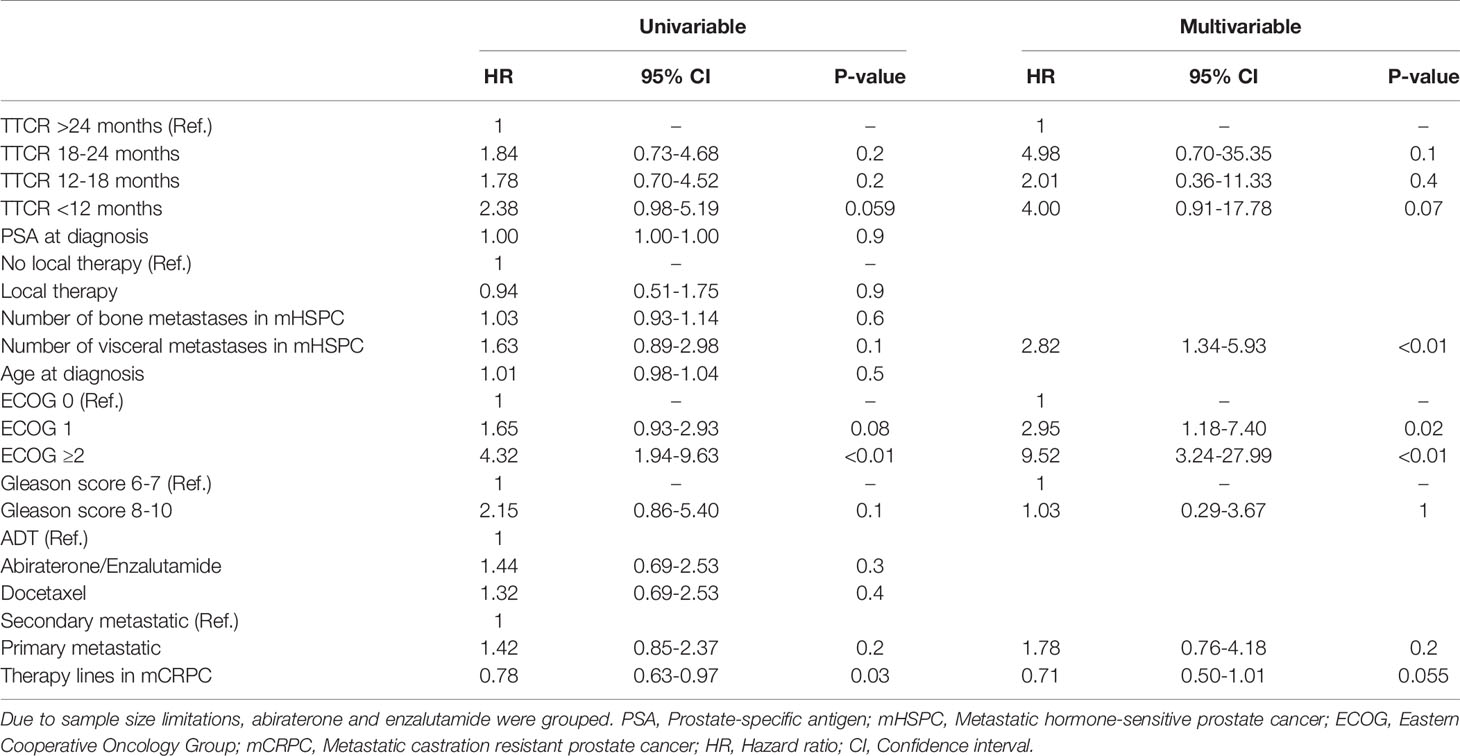
Table 3 Univariable and multivariable Cox regression models predicting overall survival in metastatic castration resistant prostate cancer patients according to time to castration resistance (TTCR).
Discussion
We hypothesized that, even in the era of combination therapies for mHSPC, differences in TTCR may result in differences in OS rates. We tested this hypothesis in our institutional metastatic prostate cancer database and focused exclusively on patients diagnosed since the year of the first publication of combination therapy (2013) in mHSPC and made several noteworthy observations.
First, we observed important differences regarding OS in the four examined TTCR groups. Specifically, TTCR <12 months patients exhibited the worst OS, while TTCR >24 patients exhibited the best OS in mHSPC. Remarkably, patients with TTCR <12 months had a 6.95-fold higher risk of overall mortality relative to patients with TTCR >24 months. It is particularly important to emphasize that this observation was also made after controlling and adjusting for patient and tumor characteristics in multivariable analyses, where all three TTCR groups were at an OS disadvantage relative to TTCR >24 months patients. These observations are in line with the previous publication by Miyake et al. Specifically, Miyake et al. also observed a higher OS in patients with shorter TTCR (15). Moreover, they also found that patients with the longest TTCR had the best OS. However, this study relied on a TTCR stratification of <6 vs. 6-12 vs. 12-18 vs. >18 months. Thus, direct comparisons of OS rates cannot be made. Due to the patients’ TTCR distribution in our cohort, our stratification relied on <12, 12-18, 18-24, and >24 months. This stratification is particularly important, considering that in the study by Miyake et al., all patients received ADT only, while in our cohort approximately 45% received combination therapy in mHSPC. Therefore, compared with the study by Miyake et al., we suspected a longer TTCR in the current study, such as proven in the CHAARTED trial, where combination therapy was used (TTCR for docetaxel and ADT vs. ADT alone: 19.4 vs. 11.7 months). Nonetheless, the clinical implications might be the same in both studies. Moreover, a recent report of 283 mHSPC Japanese patients also observed that TTCR <12 months is associated with worse survival (16). Similarly, Bournakis et al. also investigated an OS disadvantage in patients with TTCR ≤24 months in a more historical cohort (1996-2009) (20). In summary, our observations are particularly important since they validate these previous findings in a large contemporary European metastatic patient cohort of which a large proportion of patients received combination therapy in mHSPC.
Second, we also made important observations according to OS analyses in mCRPC patients stratified according to TTCR. Specifically, we observed no differences in OS between the four examined TTCR subgroups when OS rates were compared after development of mCRPC. It is even more important to emphasize that these observations persisted even after adjustment for patient and tumor characteristics in multivariable analyses. This observation is also in agreement with the publication of Miyake et al., which focused exclusively on ADT patients (15). Specifically, in the current study, no OS differences were observed between all TTCR subgroups since the development of mCRPC. This observation may lead to the assumption that prolonging OS is most effective when TTCR is prolonged in first-line therapy of mHSPC patients. Therefore, clinicians should be aware of the fact that a prolonged TTCR may be a surrogate for OS.
It is also of note that in both mHSPC and mCRPC OS analyses, ECOG ≥2 was independently associated with worse OS after adjusting for patient and tumor characteristics in logistic regression models. These observations are not surprising but validate our survival analyses with regard to patients’ frailty. Moreover, these observations are important since the adjustment for ECOG status equalized differences in the ECOG status distribution between all four examined TTCR subgroups in both OS analyses. Therefore, our multivariable OS results can be interpreted regardless of patients’ frailty that may have led to other cause mortality.
Third, previous publications reported differences in baseline characteristics of patients with different TTCR. For example, Miyake et al. investigated that patients with shorter TTCR had higher proportions of visceral metastasis (15). Additionally, several studies aimed to investigate risk factors for progression to mCRPC in mHSPC patients. Several risk factors such as PSA, Gleason score, or time to PSA nadir in mHSPC treatment were found to be such predictors (21–26). Despite non-significant differences between the four examined TTCR subgroups, possibly due to sample size limitations, we also found interesting trends. For example, as observed in the current study cohort, higher proportions of Gleason score 8-10 were observed in the subgroups TTCR <12 and 12-18 compared to TTCR 18-24 and >24. Moreover, we also found that patients with TTCR <12 months had the worst distribution of tumor burden of visceral metastases, when visceral metastases were found at mHSPC diagnosis. Moreover, number of visceral metastases were a significant predictor for worse OS in mCRPC. Additionally, patients with TTCR <12 months less frequently received local therapies with radical prostatectomy or radiation therapy for the primary tumor, relative to patients with TTCR 12-18, 18-24, or >24 months. Although there was no statistical significance of these variables, these findings may indicate one of the reasons why patients exhibited the shortest TTCR. However, OS differences between TTCR groups cannot be explained by differences in patient or baseline tumor characteristics alone. It is very likely that other factors, such as genetic differences or gene mutations, play a crucial role in TTCR for which we could unfortunately not account for (27, 28).
Our study has several limitations and needs to be considered in the light of its retrospective, single-center design. Although only 4.2% (n=9) of all identified mHSPC patients were excluded after applying inclusion criteria of the current study, a selection bias cannot be completely ruled out. Second, differences between some variables might result in a lack of significance due to limitations in sample size or missing baseline information (e.g., staging modalities) for some patients. Moreover, no treatment-specific TTCR analyses in mHSPC patients could be performed due to limitations in sample size and differences in baseline prostate cancer characteristics between the treatment groups. As the first study analyzing combination therapy in mHSPC – the GETUF-AFU15 study - was published in 2013, the current study focused on the most contemporary patients treated with combination therapy in mHSPC between 2013 to 2020 (12). In consequence, not all patients had directly received new combination therapies upfront since 2013. This circumstance might have led to a heterogenous patient cohort, where approximately half of the included patients received combination therapy in mHSPC, which still reflects current daily practice in many European countries. Moreover, differences in patients’ therapies in mCRPC may have affected OS and might be linked to prior therapies in mHSPC. However, since we aimed to investigate the effect of TTCR on OS regardless of the administered treatment, different treatments may have influenced TTCR, but did not bias the results and the implications of this work. However, to adjust for possible OS differences related to treatments in mHSPC, we included combination therapies as a covariate in all Cox regression models. Finally, since no previous study relied on patients who received combination therapy for mHSPC, the chosen TTCR cut-offs need to be further validated in patient cohorts that were treated with combination therapy.
Taken together, our study demonstrates that TTCR also affects OS in mHSPC patients in the era of combination therapies for mHSPC. More specifically, patients with TTCR <12 months are at highest risk of overall mortality, while TTCR >24 months patients exhibited the longest OS. Moreover, these findings were also observed after controlling for patient and prostate cancer characteristics in multivariable adjustment. Finally, stratification according to TTCR in mCRPC patients did not distinguish patients in separate OS risk levels.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethic committee of the University Hospital Frankfurt. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
MW: Writing – Original Draft, Formal analysis, Methodology, Conceptualization. FP: Methodology, Visualization. BH: Formal analysis. MS: Formal analysis. CW: Conceptualization. TS: Writing – Review and Editing. HH: Writing – Review and Editing. SB: Writing – Review and Editing. MA: Writing – Review and Editing. AB: Writing – Review and Editing. PK: Writing – Review and Editing. FC: Writing – Review and Editing, Supervision. LK: Writing – Review and Editing, Validation. PM: Writing – Review and Editing, Supervision, Validation, Conceptualization. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Eeles RA, Olama AAA, Benlloch S, Saunders J, Leongamornlert DA, Tymrakiewicz M, et al. Identification of 23 New Prostate Cancer Susceptibility Loci Using the iCOGS Custom Genotyping Array. Nat Genet (2013) 45(4):385–91, 391e1-2. doi: 10.1038/ng.2560
2. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551
3. Malvezzi M, Carioli G, Bertuccio P, Lev F, La Veccia C, Negri E. European Cancer Mortality Predictions for the Year 2019 With Focus on Breast Cancer. Ann Oncol (2019) 30(5):781–7. doi: 10.1093/annonc/mdz051
4. Mottet N, Cornford P, Van den Bergh RCN, Lev F, La Veccia C, Negri E, et al. Eau - EANM - ESTRO - ESUR - SIOG. Guidelines on Prostate Cancer. EAU Guidelines. Edn. presented at the EAU Annual Congress Amsterdam 2020. (2020).
5. Patrikidou A, Loriot Y, Eymard J-C, Albiges L, Massard C, Ileana E, et al. Who Dies From Prostate Cancer? Prostate Cancer Prostatic Dis (2014) 17(4):348–52. doi: 10.1038/pcan.2014.35
6. Pagliarulo V, Bracarda S, Eisenberger MA, Mottet N, Schröder FH, Sternberg CN, et al. Contemporary Role of Androgen Deprivation Therapy for Prostate Cancer. Eur Urol (2012) 61(1):11–25. doi: 10.1016/j.eururo.2011.08.026
7. Kyriakopoulos CE, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol (2018) 36(11):1080–7. doi: 10.1200/JCO.2017.75.3657
8. Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone Plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med (2017) 377(4):352–60. doi: 10.1056/NEJMoa1704174
9. Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide With Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med (2019) 381(2):121–31. doi: 10.1056/NEJMoa1903835
10. Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. Arches: A Randomized, Phase Iii Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol (2019) 37(32):2974–86. doi: 10.1200/JCO.19.00799
11. Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes A, Given R, et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med (2019) 381(1):13–24. doi: 10.1056/NEJMoa1903307
12. Gravis G, Fizazi K, Joly F, Oudard S, Priou F, Esterni B, et al. Androgen-Deprivation Therapy Alone or With Docetaxel in non-Castrate Metastatic Prostate Cancer (GETUG-AFU 15): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2013) 14(2):149–58. doi: 10.1016/S1470-2045(12)70560-0
13. Preisser F, Chun FK-H, Banek S, Wenzel M, Graefen M, Steuber T, et al. Management and Treatment Options for Patients With De Novo and Recurrent Hormone-Sensitive Oligometastatic Prostate Cancer. Prostate Int (2021). doi: 10.1016/j.prnil.2020.12.003
14. Frees S, Akamatsu S, Bidnur S, Khalaf D, Chavez-Munoz C, Struss W, et al. The Impact of Time to Metastasis on Overall Survival in Patients With Prostate Cancer. World J Urol (2018) 36(7):1039–46. doi: 10.1007/s00345-018-2236-4
15. Miyake H, Matsushita Y, Watanabe H, Tamura K, Motoyama D, Ito T, et al. Prognostic Significance of Time to Castration Resistance in Patients With Metastatic Castration-Sensitive Prostate Cancer. Anticancer Res (2019) 39(3):1391–6. doi: 10.21873/anticanres.13253
16. Okamoto T, Hatakeyama S, Takahashi S, Narita S, Shida M, Hoshi S, et al. The Impact of Time-to-Castration Resistance on Survival in Patients With Metastatic Hormone-Naïve Prostate Cancer: A Multicenter Retrospective Study. J Clin Oncol (2020) 38(6_suppl):213–3. doi: 10.1200/JCO.2020.38.6_suppl.213
17. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer Oxf Engl 1990 (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
18. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful Selection of Variables in Logistic Regression. Source Code Biol Med (2008) 3:17. doi: 10.1186/1751-0473-3-17
19. Rct. R: A Language and Environment for Statistical Computing (2017). Available at: https://wwwr-projectorg2017.
20. Bournakis E, Efstathiou E, Varkaris A, Wen S, Chrisofos M, Deliveliotis C, et al. Time to Castration Resistance is an Independent Predictor of Castration-Resistant Prostate Cancer Survival. Anticancer Res (2011) 31(4):1475–82.
21. Lin T-T, Chen Y-H, Wu Y-P, Chen SZ, Li XD, Lin Y, et al. Risk Factors for Progression to Castration-Resistant Prostate Cancer in Metastatic Prostate Cancer Patients. J Cancer (2019) 10(22):5608–13. doi: 10.7150/jca.30731
22. Zacho HD, Gade M, Mortensen JC, Bertelsen H, Boldsen S, Barsi T, et al. Bone Scan Index Is an Independent Predictor of Time to Castration-resistant Prostate Cancer in Newly Diagnosed Prostate Cancer: A Prospective Study. Urology (2017) 108:135–41. doi: 10.1016/j.urology.2017.05.058
23. Smaletz O, Scher HI, Small EJ, Verbel DA, McMillan A, Regan K, et al. Nomogram for Overall Survival of Patients With Progressive Metastatic Prostate Cancer After Castration. J Clin Oncol (2002) 20(19):3972–82. doi: 10.1200/JCO.2002.11.021
24. Sim HG, Lau WKO, Cheng CWS. Predictors of Androgen Independence in Metastatic Prostate Cancer. BJU Int (2004) 93(9):1221–4. doi: 10.1111/j.1464-410X.2004.04863.x
25. Divrik RT, Türkeri L, Şahin AF, Akdogan B, Ates F, Cal C, et al. Prediction of Response to Androgen Deprivation Therapy and Castration Resistance in Primary Metastatic Prostate Cancer. Urol Int (2012) 88(1):25–33. doi: 10.1159/000334539
26. Tamada S, Iguchi T, Kato M, Sakawa J, Kita K, Yasuda S, et al. Time to Progression to Castration-Resistant Prostate Cancer After Commencing Combined Androgen Blockade for Advanced Hormone-Sensitive Prostate Cancer. Oncotarget (2018) 9(97):36966–74. doi: 10.18632/oncotarget.26426
27. Wang Z, Shen H, Ma N, Li Q, Mao Y, Wang, et al. The Prognostic Value of Androgen Receptor Splice Variant 7 in Castration-Resistant Prostate Cancer Treated With Novel Hormonal Therapy or Chemotherapy: A Systematic Review and Meta-Analysis. Front Oncol (2020) 10:572590. doi: 10.3389/fonc.2020.572590
Keywords: mortality, survival, castration resistance, metastatic prostate cancer, CRPC
Citation: Wenzel M, Preisser F, Hoeh B, Schroeder M, Würnschimmel C, Steuber T, Heinzer H, Banek S, Ahrens M, Becker A, Karakiewicz PI, Chun FKH, Kluth LA and Mandel P (2021) Impact of Time to Castration Resistance on Survival in Metastatic Hormone Sensitive Prostate Cancer Patients in the Era of Combination Therapies. Front. Oncol. 11:659135. doi: 10.3389/fonc.2021.659135
Received: 27 January 2021; Accepted: 31 March 2021;
Published: 23 April 2021.
Edited by:
Beatrice S. Knudsen, The University of Utah, United StatesReviewed by:
Shingo Hatakeyama, Hirosaki University, JapanClara Hwang, Henry Ford Health System, United States
Jeanny B. Aragon-Ching, Inova Schar Cancer Institute, United States
Copyright © 2021 Wenzel, Preisser, Hoeh, Schroeder, Würnschimmel, Steuber, Heinzer, Banek, Ahrens, Becker, Karakiewicz, Chun, Kluth and Mandel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mike Wenzel, TWlrZS5XZW56ZWxAa2d1LmRl
 Mike Wenzel
Mike Wenzel Felix Preisser
Felix Preisser Benedikt Hoeh
Benedikt Hoeh Maria Schroeder1
Maria Schroeder1 Christoph Würnschimmel
Christoph Würnschimmel Severine Banek
Severine Banek Felix K. H. Chun
Felix K. H. Chun Philipp Mandel
Philipp Mandel