
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 28 May 2021
Sec. Pharmacology of Anti-Cancer Drugs
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.659065
This article is part of the Research Topic Treatment for Non Small Cell Lung Cancer in Distinct Patient Populations View all 32 articles
 Yongdong Feng†
Yongdong Feng† Guangkuo Zhu†
Guangkuo Zhu† Song Lang
Song Lang Ping Hao
Ping Hao Guanghui Li
Guanghui Li Fanglin Chen
Fanglin Chen Wenlei Zhuo
Wenlei Zhuo Yuzhong Duan
Yuzhong Duan Anmei Zhang
Anmei Zhang Zhengtang Chen*
Zhengtang Chen* Jianguo Sun*
Jianguo Sun*Objective: To explore the efficacy and safety of EGFR-TKI combined with thymosin therapy in advanced non-small cell lung cancer (NSCLC) patients harboring active EGFR mutations.
Methods: Patients confirmed as advanced NSCLC with active EGFR mutations were recruited from August 2008 to July 2018 retrospectively. Patients treated with EGFR-TKI were classified as the EGFR-TKI group. And those received EGFR-TKI and thymosin therapy were designated as the EGFR-TKI plus thymosin group. The primary endpoint was progression-free survival (PFS). The secondary endpoints included overall survival (OS), tumor response and adverse effects.
Results: The median PFS was significantly longer in EGFR-TKI plus thymosin group than that in EGFR-TKI group (14.4 months vs. 9.2 months; HR=0.433, 95% CI 0.322 - 0.582, P<0.0001). The median OS was also prolonged in EGFR-TKI plus thymosin group than that in EGFR-TKI group (29.5 months vs. 19.8 months; HR=0.430, 95% CI 0.319 - 0.580, P<0.0001). The objective response rate in EGFR-TKI plus thymosin group and EGFR-TKI group were 60.0% versus 60.8% (P=0.918). The disease control rate was 96.9% in EGFR-TKI plus thymosin group and 97.7% in EGFR-TKI group (P=1.000). There were no significant differences in adverse effects between the two groups. The number of CD3+T cells in peripheral blood decreased significantly after treatment including both CD3+CD4+T and CD3+CD8+T subsets in EGFR-TKI group, but not in EGFR-TKI plus thymosin group.
Conclusions: Combination of EGFR-TKI and thymosin can significantly prolong the PFS and OS compared with EGFR-TKI monotherapy without more adverse events, which offers a new strategy in clinic.
Epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) is currently recommended as a standard first-line therapy for advanced non-small cell lung cancer (NSCLC) patients harboring active EGFR mutations, which was reported to prolong progression-free survival (PFS) compared with standard platinum-based chemotherapy significantly (1–3). However, most of the NSCLC patients with an initial dramatic response to EGFR-TKI treatment developed progression disease after 8.40-13.10 months (4, 5). In order to prolong the survival time of NSCLC patients with active EGFR mutations, novel drugs including osimertinib and crizotinib were developed by targeting resistance mechanisms (6, 7). Besides, combination therapies, such as EGFR-TKI combined with angiogenesis inhibitorwas shown to improve the PFS (8), but not OS (9). EGFR-TKI combined with chemotherapy prolonged PFS and OS but increased toxicity significantly (10). At present, there is still no satisfactory combination therapy.
As reported, sensitive EGFR-TKIs caused obvious tumor microenvironmental changes including the number of immune cells and inflammatory factors in serum (11). EGFR blockade by using erlotinib reduced CD4+T cell proliferation in response to soluble anti-CD3 stimulation (12). Erlotinib demonstrated an immunosuppressive activity on T-cell-mediated immune response both in vitro and in vivo (13). Therefore, the combination of immunomodulators may be a potential method to enhance the efficacy of EGFR-TKI. In clinic, thymosin such as thymosin alpha 1 and thymopentin, have been widely used as immunomodulators in kinds of cancers (14). As reported, thymosin can significantly improve patient’s quality of life by enhancing T-cell function, stimulation of T cell maturation and differentiation in lung cancer (15). In addition to the effect on immunomodulatory, thymosin has been reported to exert synergistic antitumor activity without more adverse effects when combined with chemotherapy in lung cancer (16). Whether thymosin combined with EGFR-TKI can improve patients’ PFS and OS needs to be illustrated.
In this study, we conducted a retrospective study to explore the efficacy and safety of EGFR-TKI plus thymosin in NSCLC patients harboring active EGFR mutations, thereby enhancing the efficacy of EGFR-TKI.
We conducted a retrospective research during August 2008 to July 2018 in three Affiliated Hospitals of Army Medical University (Chongqing, China). Patients over the age of 18 years were histologically or cytologically confirmed as stage IV NSCLC were recruited. TNM classification (tumor, node, and metastasis) of lung cancer was made according to the American Journal of Critical Care (AJCC) 7th edition of Lung Cancer. Those patients with active EGFR mutations, including exon 19 deletion or exon 21 L858R mutation, were detected by amplification refractory mutation system or next-generation sequence. Patients received first or second-line EGFR-TKI therapy, whose Eastern Cooperative Oncology Group (ECOG) performance status (PS) score were 0-2, had one or more measurable target lesions and follow-up time >3 months were included. Exclusion criteria included incomplete medical records, EGFR-TKI treatment less than 8 weeks and thymosin therapy less than 4 weeks, loss to follow-up. This trial had been approved by the Ethics Committee, Xinqiao Hospital, Army Medical University (2018–302–01). All procedures were conducted in accordance with the Declaration of Helsinki.
Patients treated with EGFR-TKI (erlotinib 150mg daily, gefitinib 250mg daily or icotinib 125mg three times a day) alone were classified as the EGFR-TKI group. Patients received EGFR-TKI (erlotinib 150mg daily, gefitinib 250mg daily or icotinib 125mg three times a day) and thymosin (thymosin α1 1.6mg twice a week or thymopentin 10mg daily) concurrently were designated as the EGFR-TKI plus thymosin group.
The data collected included the demographics, clinical characteristics, treatment, tumor response, adverse events (AEs) and T lymphocyte subsets in peripheral blood. Tumor response was assessed by two independent senior physicians in oncology according to RECIST 1.1. The initial response was assessed after 4 weeks of treatment, and tumor evaluation was repeated every 2 months. If the assessments were inconsistent, the controversial results were reassessed by the third oncologist. AEs were assessed according to the National Cancer Institute Common Toxicity Criteria version 3.0. The data of peripheral blood T lymphocyte subsets before EGFR-TKI treatment were gathered within one month before EGFR-TKI treatment. Peripheral blood T lymphocyte subsets after EGFR-TKI treatment were collected from receiving EGFR-TKI treatment to progressive disease, and data after EGFR-TKI plus thymosin treatment were acquired from patients receiving EGFR-TKI plus thymosin treatment to progressive disease, and then taking the average of all these data. 2mL of venous blood was collected in EDTA anticoagulant tube from each patient. Peripheral blood T lymphocytes subsets were detected by flow cytometry by trained technicians in Clinical Laboratory of Xinqiao Hospital in Army Medical University. The samples were stained with antibody to human CD45, CD3, CD4, CD8 or isotype control conjugated with PerCP, FITC, APC and PE for 30 min, respectively. The indicated antibodies were obtained from Agilent Technologies (China Inc). Subsequently, stained samples were measured on a flow cytometer, NovoCyte D2040R (Agilent Technologies). The data were analyzed by NovoExpress software (Agilent Technologies).
The primary endpoint was PFS, which was defined as the time from the initiation of EGFR-TKI treatment to the first documentation of progressive disease or death for any reason. The secondary endpoints included OS, objective response rate (ORR), disease control rate (DCR) and AEs. OS was defined as the time from the first dose of EGFR-TKI to cancer-related death or the last follow-up time.
When comparing the baseline, brain metastasis, bone metastasis and first- or second-line EGFR-TKI treatment had significantly differences in EGFR-TKI group and EGFR-TKI plus thymosin group. Propensity score matching was applied at the ratio of 2:1 in EGFR-TKI group and EGFR-TKI plus thymosin group with these three items as covariates to avoid bias. The Chi-square test or fisher exact test was used to analyze the intergroup difference in clinical features, ORR, DCR and AEs. And the Wilcoxon rank sum test was used to analyze age difference. The Kaplan-Meier estimator was used to estimate the survival curves. The log-rank test was performed for intergroup comparison. Cox proportional hazards regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association of combination of EGFR-TKI and thymosin with the risk of disease progression and death. The changes of peripheral blood T lymphocyte subsets between EGFR-TKI and EGFR-TKI plus thymosin group before and after treatment were assessed by paired-samples T test. All statistical analyses were performed using R software (Version 3.6.1). The statistical significance level was set at P < 0.05 under a two-tailed test.
From August 2008 to July 2018, a total of 908 patients were confirmed as NSCLC with active EGFR mutations. Of these patients, 495 subjects met the inclusion criteria. There were 22 patients excluded for incomplete medical records. 36 cases of the patients received EGFR-TKI therapy for less than 8 weeks. 89 patients were treated with thymosin for less than 4 weeks. And there were 16 patients lost to follow-up. Finally, 267 patients received EGFR-TKI monotherapy and 65 patients received EGFR-TKI plus thymosin treatment (Supplementary Figure 1).
When conducted the comparison of demographic and clinical characteristics between EGFR-TKI group and EGFR-TKI plus thymosin group, we found that brain metastasis, bone metastasis and first- or second-line EGFR-TKI treatment in two groups had significantly differences, then propensity score matching analysis was applied at a ratio of 2:1. Finally, 130 patients took EGFR-TKI monotherapy and 65 patients received EGFR-TKI plus thymosin treatment were included (Supplementary Figure 1). Of the 195 patients, the median age was 57.5 years old (range, 21 to 81 years old) and 120 cases were female. All of them were diagnosis as adenocarcinoma histologically. There were 144 patients received EGFR-TKI as the first line therapy and 51 patients received the second line therapy. 93 patients received gefitinib therapy, 76 patients took received erlotinib therapy and 26 patients were treated with icotinib. The baseline of EGFR-TKI group and EGFR-TKI plus thymosin group were balanced after propensity score matching (Table 1).
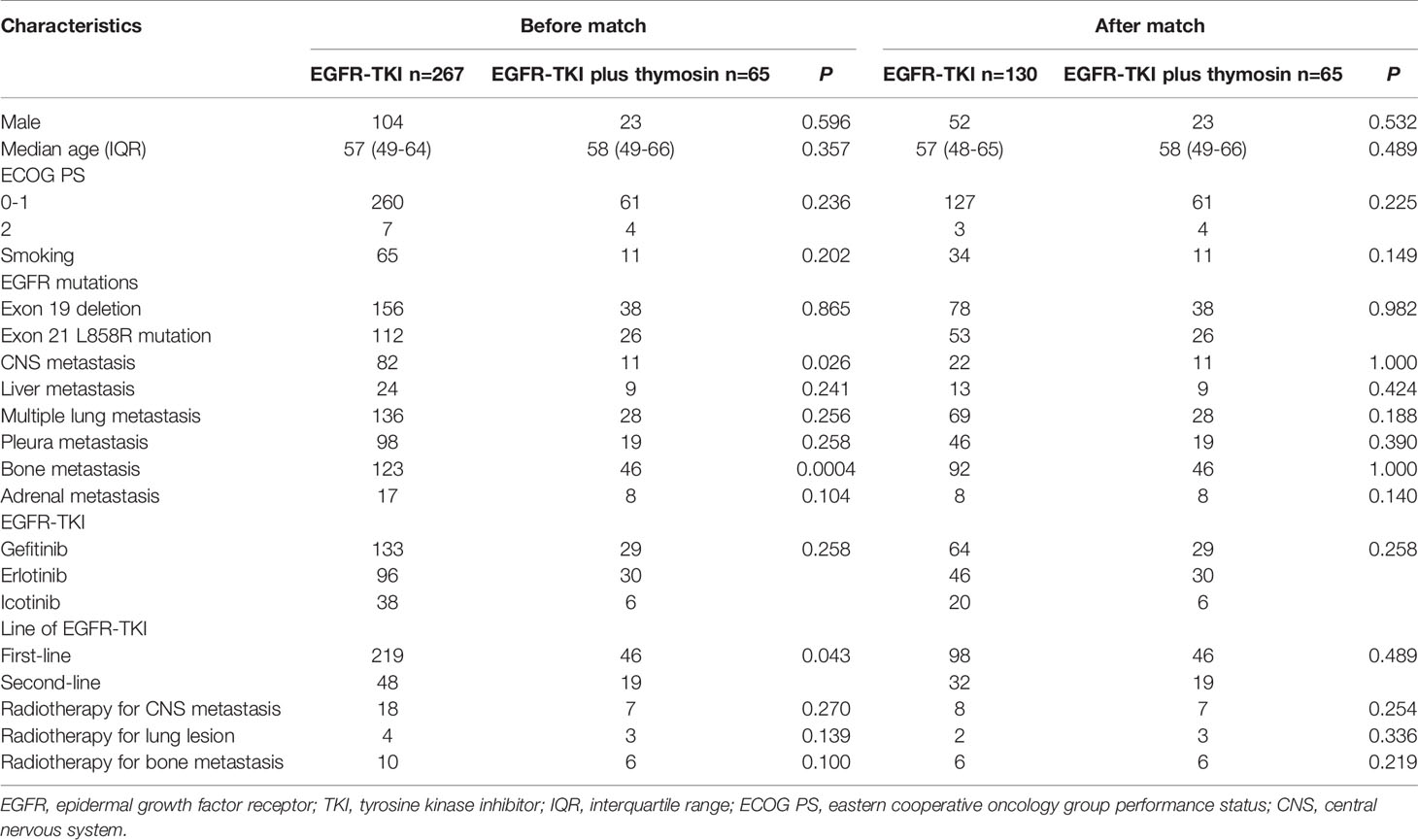
Table 1 Patients’ demographic and clinical characteristics in the EGFR-TKI and EGFR-TKI plus thymosin group before and after propensity score matching.
The median PFS was 14.4 months (95% CI, 11.7-17.1) in EGFR-TKI plus thymosin group, which was significantly improved than 9.2 months (95% CI, 7.9-10.3) in EGFR-TKI group (HR=0.433, 95% CI 0.322 - 0.582, P<0.0001, Figure 1A). The median OS were 29.5 months (95% CI, 21.5-37.5) in EGFR-TKI plus thymosin group and 19.8 months (95% CI, 18.2-21.4) in EGFR-TKI group, respectively (HR=0.430, 95% CI 0.319 - 0.580, P<0.0001, Figure 1B).
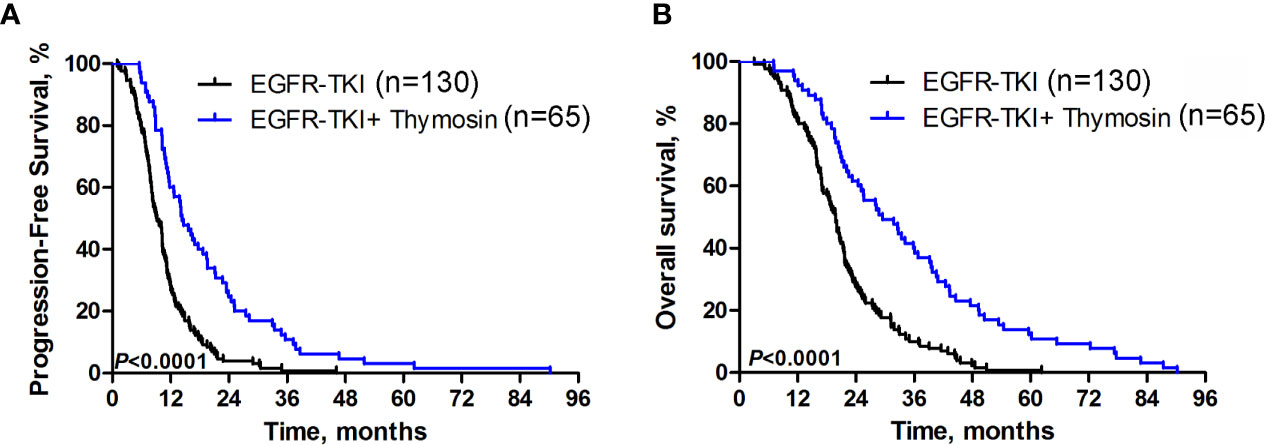
Figure 1 Progression-free Survival and Overall Survival. Kaplan-Meier estimated (A) progression-free survival (PFS) and (B) overall survival (OS) in EGFR-TKI plus thymosin group and EGFR-TKI group.
A consistent benefit of EGFR-TKI plus thymosin over EGFR-TKI with respect to PFS (Figure 2) and OS (Figure 3) were shown across most subgroups that were assessed, including the subgroups based on age, EGFR mutation type (exon 19 deletion vs. exon 21 L858R mutation), the presence or absence of CNS metastases, multiple lung metastasis, pleura metastasis, bone metastasis and adrenal metastasis, treated by gefitinib or erlotinib. The advantages of PFS in EGFR-TKI plus thymosin group were not observed in males, patients whose ECOG score was 2 points, who had smoking history, suffered from liver metastasis, treated with icotinib, received the EGFR-TKI as the second line therapy and received radiotherapy (Figure 2). And OS in EGFR-TKI plus thymosin group had no advantage in patients who suffered from liver metastasis, treated with icotinib, received the EGFR-TKI as the second line therapy. Patients who received radiotherapy for CNS metastasis had a tendency to benefit from the combination therapy (P=0.079) (Figure 3).
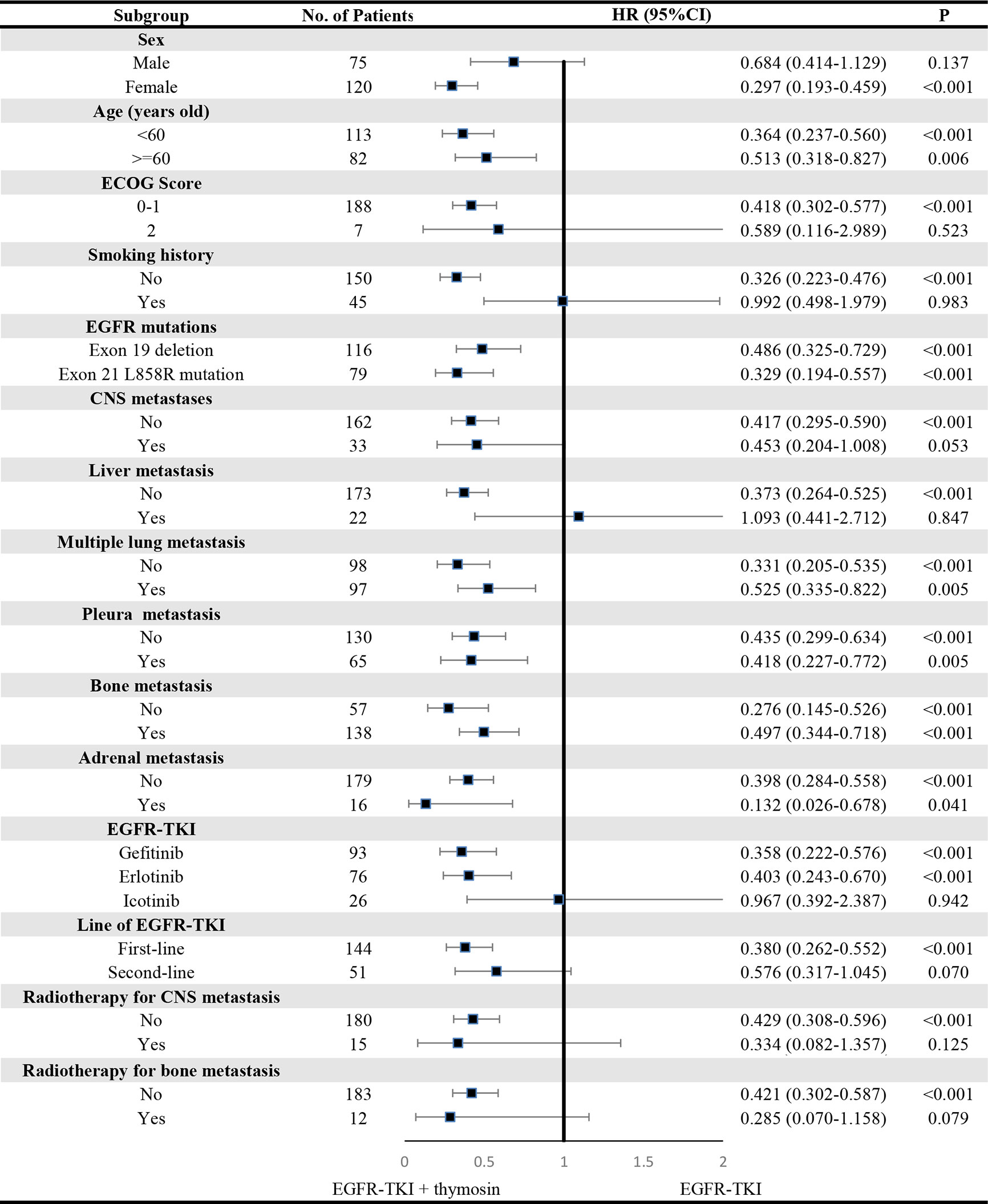
Figure 2 Subgroup Analyses of Progression-free Survival. P showed the significance of the HRs. It was used to test the significance of PFS between EGFR-TKI plus thymosin group and EGFR-TKI group in a certain variable. HR, hazard ratio; CI, confidence interval; ECOG, eastern cooperative oncology group; CNS, central nervous system.
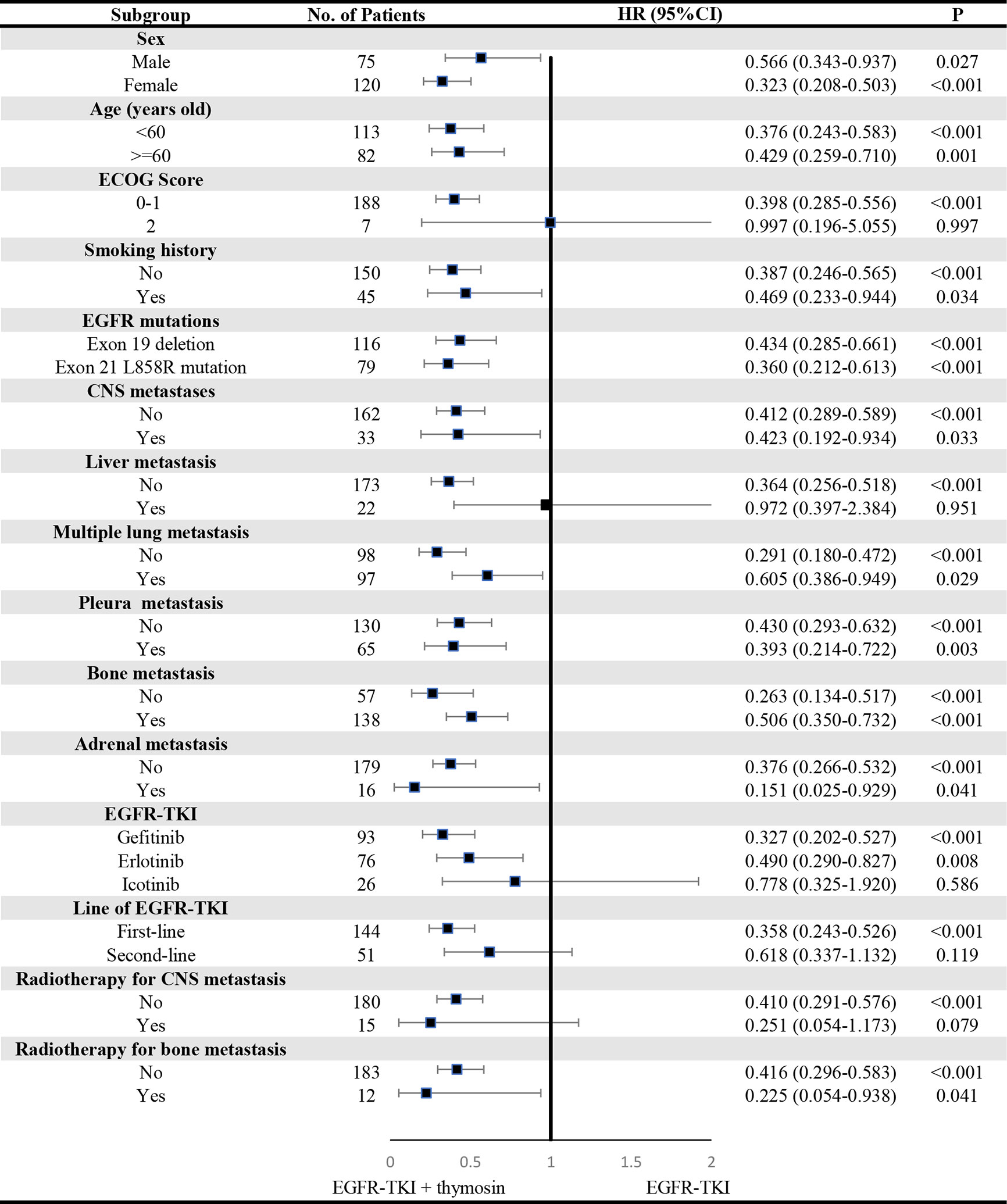
Figure 3 Subgroup Analyses of Overall Survival. P showed the significance of the HRs. It was used to test the significance of OS between EGFR-TKI plus thymosin group and EGFR-TKI group in a certain variable. HR, hazard ratio; CI, confidence interval. ECOG, eastern cooperative oncology group; CNS, central nervous system.
In EGFR-TKI plus thymosin group, there were 29 patients treated with EGFR-TKI plus thymosin α1 whose median PFS were 14.7 months and 36 patients treated with EGFR-TKI plus thymopentin with the median PFS of 14.1 months (P>0.05). And there was no difference in the median OS between the two groups.
The objective response rate (ORR) was 60.0% in EGFR-TKI plus thymosin group and 60.8% in EGFR-TKI group (P=0.918). The disease control rate (DCR) was 96.9% in EGFR-TKI plus thymosin group and 97.7% in EGFR-TKI group (P=1.000). There were no differences in ORR and DCR between the two groups. More details about response rates were shown in Supplementary Figure 2.
The most common adverse events were rash (40.0% in the EGFR-TKI plus thymosin group and 38.5% in the EGFR-TKI group), diarrhea (23.1% and 25.4%, respectively), dry skin (20.0% and 20.8%, respectively) and anorexia (9.2% and 10.0%, respectively). Adverse events of grade 3 or higher occurred in 2 cases in the EGFR-TKI plus thymosin group and 5 cases in the EGFR-TKI group. No fatal adverse events were found in the two groups. There were no significant differences in adverse effects between the two groups (Table 2). Together, the combination of EGFR-TKI and thymosin would not increase the incidences of adverse events.
There were 23 patients detected the peripheral blood T lymphocyte subsets before and after EGFR-TKI monotherapy, and 11 patients had detected the peripheral blood T lymphocyte subsets before and after EGFR-TKI plus thymosin therapy. In the EGFR-TKI group, the number of CD3+T cells decreased after treatment (P<0.05, Figure 4A) including both the CD3+CD4+T and CD3+CD8+T subsets (P<0.05, Figures 4B, C). However, the ratio of CD3+CD4+T to CD3+CD8+T did not change (Figure 4D). In the EGFR-TKI plus thymosin group, both the number of CD3+T cells and the ratio of CD3+CD4+T to CD3+CD8+T had no obvious changes before and after treatment (Figure 4). It suggested that thymosin combined with EGFR-TKI may reverse the inhibition of T cells.
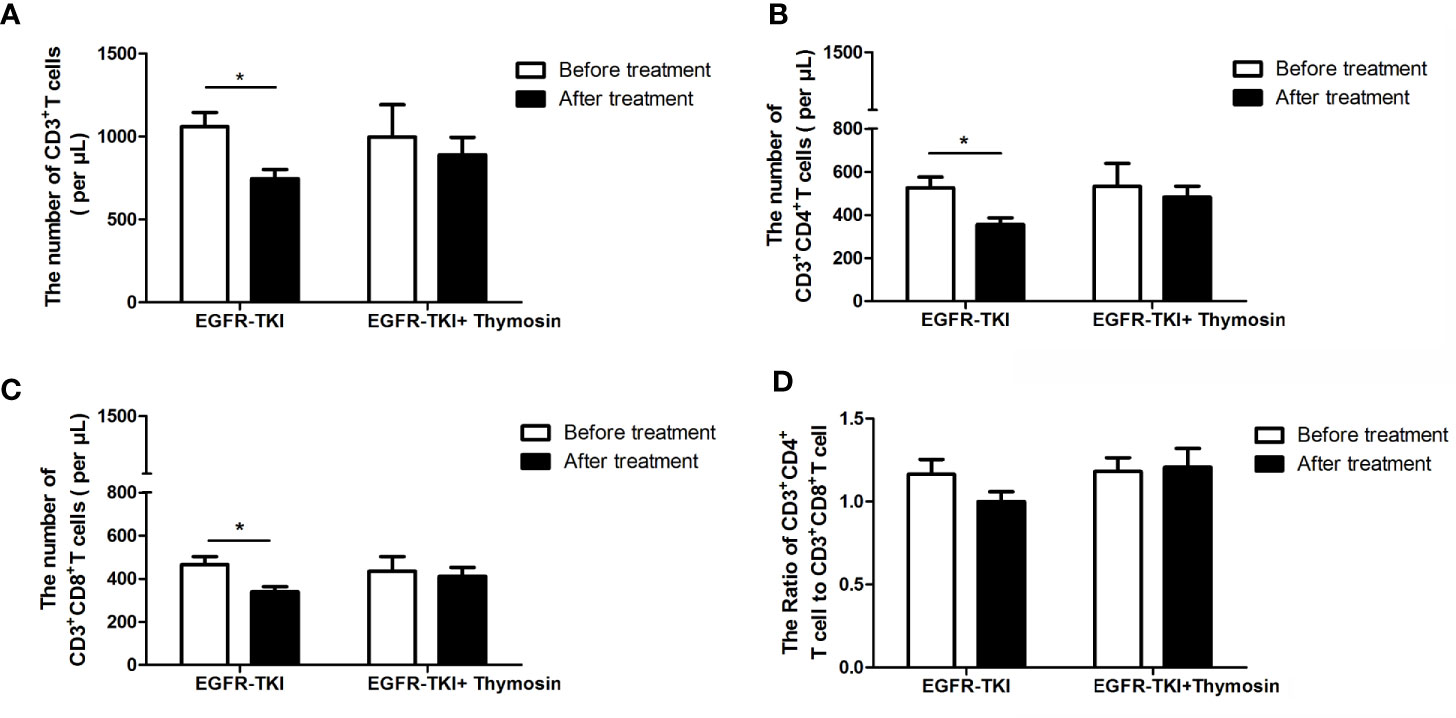
Figure 4 The numbers of T cell subsets in peripheral blood in EGFR-TKI group and EGFR-TKI plus thymosin group before and after treatment. (A) The numbers of CD3+ T cells. (B) The numbers of CD3+ CD4+T cells. (C) The numbers of CD3+ CD8+T cells. (D) The ratio of CD3+ CD4+T cells to CD3+ CD8+T cells. *P < 0.05.
In the current study, our results demonstrated that patients in EGFR-TKI plus thymosin group had significantly prolonged median PFS and OS compared with those in EGFR-TKI group. And the combination of EGFR-TKI and thymosin would not increase the incidences of adverse events. By observing the peripheral blood T lymphocyte subsets before and after treatment, we found thymosin combined with EGFR-TKI may reverse the inhibition of T cells. Therefore, combination of EGFR-TKI and thymosin may be a potential therapy to enhance the efficacy of EGFR-TKI without increasing adverse events.
To avoid the impact of inconsistent baseline, the propensity score matching analysis was applied at the ratio of 2:1 in EGFR-TKI group and EGFR-TKI plus thymosin group. The limitation of propensity score matching analysis is only to control the effect of measurable variables. New bias may appear if there is unobservable selection on variables. However, the results will be less reliable when the confounding factors exist. In this study, there were no differences in demographic and clinical characteristics between the two groups after propensity score matching.
The median PFS in EGFR-TKI plus thymosin group was 14.4 months, with a 57% lower risk of disease progression or death than that in the EGFR-TKI group. The median PFS in the EGFR-TKI group in our research is 9.2 months which is similar with that in previous clinical trials of gefitinib with the median PFS ranges from 8.0 (5) to 10.9 (17) months, erlotinib with the median PFS 13.1 months (4) and icotinib with the median PFS 11.2 months (18). As previously reported, the median OS of EGFR-TKIs (including gefitinib, erlotinib and icotinib) ranged from 21.6 (19) to 30.5 months (2, 18), which was longer than the OS in our study. Zeng et al. (16) had summarized four trials with 269 cases to demonstrate that administration of thymosin with chemotherapy significantly increased the 1-year OS rate. Our study had also showed that combination of EGFR-TKI and thymosin could prolong the PFS and OS and would be a potential therapy to enhance the efficacy of EGFR-TKI.
In the subgroup analysis, consistent benefit of EGFR-TKI plus thymosin over EGFR-TKI with respect to PFS and OS were shown. Patients benefit from EGFR-TKI plus thymosin treatment regardless of the type of EGFR mutations. Fukuoka et al. (19) had reported that PFS was significantly longer for gefitinib versus carboplatin/paclitaxel in both the exon 19 deletions and the exon 21 L858R mutation subgroups. Combined EGFR-TKI with thymosin was more advantageous as first-line treatment compared with who received it as second-line treatment, which was consistent with the result of EGFR-TKI monotherapy (2). OS in EGFR-TKI plus thymosin group had no advantage in patients who suffered from liver metastasis. No comparison stratified by liver metastasis was retrieved in studies about EGFR-TKI combined with angiogenesis inhibitors in NEJ 026 (9) and CTONG 1509 (8). Although patients were stratified by liver metastasis and EGFR mutation, there were still no data about efficacy of EGFR mutation with liver metastasis in immunotherapy combined with angiogenesis inhibitor and chemotherapy in IMpower150 (20). In general, liver metastasis is a negative predictor for EGFR-TKIs therapy in patients with EGFR-mutant NSCLC (21) without satisfactory combination therapies. In our study, the combination of EGFR-TKI and thymosin still showed no superiority. More samples were needed to confirm whether patients who received radiotherapy for CNS metastasis benefit from the combination therapy.
In the Iressa Pan-Asia Study (IPASS) (1), the common adverse events of gefitinib are rash or acne (66.2%), diarrhea (46.2%), dry skin (23.9%), and anorexia (21.9%). Erlotinib was reported to be the most prone to skin diseases in the first-generation of EGFR-TKIs (22). And the adverse events of icotinib were observed: rash (40%), diarrhea (19%), and hepatotoxicity (8%) (23). In our study, there was no statistical comparison of safety data in EGFR-TKI plus thymosin group and EGFR-TKI group. The most common adverse events were rash (40.0% in the EGFR-TKI plus thymosin group and 38.5% in the EGFR-TKI group), diarrhea (23.1% vs 25.4%), dry skin (20.0% vs 20.8%) and anorexia (9.2% vs 10.0%). It suggested that combination of EGFR-TKI and thymosin may be a safe option.
The inhibition of peripheral blood T cell subsets by EGFR-TKI therapy may be a potential reason why combined with thymosin can prolong PFS and OS. In this study, the number of CD3+T cells decreased significantly after treatment including both the CD3+CD4+T cells and CD3+CD8+T subsets decreased in the EGFR-TKI group. However, peripheral blood T cell subsets in the EGFR-TKI plus thymosin group did not change before and after treatment. It has been reported that erlotinib has the effect of immunosuppression while anti-tumor. Erlotinib could damage T-cell-mediated immune response through inhibiting T cell proliferation and activation, and inhibiting the secretion of IL-2 and IFN-γ by activated T lymphocyte cells (13). Treat with gefitinib for 4 weeks can result in a decreased percent of CD4+ T cells (24).
Thymosin, as a non-specific immunomodulator, had been widely used in cancer. It can increase the release of cytokine IL-2 and the expression of IL-2 receptor (25, 26). Thymosin was reported could not only activate T cells, inducing their maturation and differentiation (27), but also enhance the activity of natural killer and dendritic cells (28, 29). In addition, thymosin could enhance the expression of major histocompatibility complex class-I molecule and tumor associated antigens in multiple tumor cells (25, 30), thus tumor cells can be recognized by T lymphocytes easier. Several studies reported that both thymosin α1 and thymopentin could inhibit the growth of tumor cells by decreasing reactive oxygen species levels in tumor cells (31, 32). Together, thymosin plays important roles in both immunomodulatory and anti-tumor. Thus, the combination of EGFR-TKI with thymosin has synergistic effects in NSCLC.
In conclusion, our study revealed that combination of EGFR-TKI and thymosin can prolong the PFS and OS compared with EGFR-TKI monotherapy in NSCLC patients harboring active EGFR mutations without the increasing of adverse events. The combination therapy offers a new strategy for the treatment of NSCLC with active EGFR mutations.
The original contributions presented in the study are included in the Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee, Xinqiao Hospital, Army Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YF and GZ: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing - original draft, Visualization. SL, PH, GL, FC, WZ, YD, and AZ: Data curation, Methodology, Validation. ZC: Conceptualization, Resources, Data curation, Writing - review and editing, Supervision, Project administration. JS: Conceptualization, Resources, Data curation, Writing - review and editing, Supervision, Project administration, Funding acquisition. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China [No. 81672841, 81773245 and 81972858], the talent Foundation of Chongqing [cstccxljrc201910], the Cultivation Program for Clinical Research Talents of Army Medical University in 2018 [2018XLC1010].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to extend our heartfelt gratitude to patients, family members of recruited patients and clinicians for providing some follow-up information.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.659065/full#supplementary-material
AEs, adverse effects; AJCC; American Journal of Critical Care; CI, confidence interval; DCR, disease control rate; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; HR, hazard ratio; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PS, performance status; RECIST, Response Evaluation Criteria In Solid Tumors.
1. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med (2009) 361:947–57. doi: 10.1056/NEJMoa0810699
2. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or Chemotherapy for Non-Small-Cell Lung Cancer With Mutated EGFR. N Engl J Med (2010) 362:2380–8. doi: 10.1056/NEJMoa0909530
3. Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, et al. First-Line Erlotinib Versus Gemcitabine/Cisplatin in Patients With Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer: Analyses From the Phase III, Randomized, Open-Label, ENSURE Study. Ann Oncol (2015) 26:1883–9. doi: 10.1093/annonc/mdv270
4. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib Versus Chemotherapy as First-Line Treatment for Patients With Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (OPTIMAL, CTONG-0802): A Multicentre, Open-Label, Randomised, Phase 3 Study. Lancet Oncol (2011) 12:735–42. doi: 10.1016/S1470-2045(11)70184-X
5. Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: First-Line Single-Agent Iressa Versus Gemcitabine and Cisplatin Trial in Never-Smokers With Adenocarcinoma of the Lung. J Clin Oncol (2012) 30:1122–8. doi: 10.1200/JCO.2011.36.8456
6. Lim SM, Syn NL, Cho BC, Soo RA. Acquired Resistance to EGFR Targeted Therapy in Non-Small Cell Lung Cancer: Mechanisms and Therapeutic Strategies. Cancer Treat Rev (2018) 65:1–10. doi: 10.1016/j.ctrv.2018.02.006
7. Lee CK, Novello S, Rydén A, Mann H, Mok T. Patient-Reported Symptoms and Impact of Treatment With Osimertinib Versus Chemotherapy in Advanced Non-Small-Cell Lung Cancer: The Aura3 Trial. J Clin Oncol (2018) 36:1853–60. doi: 10.1200/JCO.2017.77.2293
8. Zhou Q, Wu Y, Cheng Y, Liu Y, Chen G, Cui J, et al. Ctong 1509: Phase III Study of Bevacizumab Withor Without Erlotinib in Untreated Chinese Patients With Advanced EGFR-Mutatednsclc. Ann Oncol (2019) 30(suppl_5):v602–60. doi: 10.1093/annonc/mdz260.002
9. Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, et al. Erlotinib Plus Bevacizumab Versus Erlotinib Alone in Patients With EGFR-Positive Advanced Non-Squamous Non-Small-Cell Lung Cancer (NEJ026): Interim Analysis of an Open-Label, Randomised, Multicentre, Phase 3 Trial. Lancet Oncol (2019) 20:625–35. doi: 10.1016/S1470-2045(19)30035-X
10. Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, et al. Gefitinib Versus Gefitinib Plus Pemetrexed and Carboplatin Chemotherapy in EGFR-Mutated Lung Cancer. J Clin Oncol (2020) 38:124–36. doi: 10.1200/JCO.19.01154
11. Jia Y, Li X, Jiang T, Zhao S, Zhao C, Zhang L, et al. EGFR-Targeted Therapy Alters the Tumor Microenvironment in EGFR-driven Lung Tumors: Implications for Combination Therapies. Int J Cancer (2019) 145:1432–44. doi: 10.1002/ijc.32191
12. Zeboudj L, Maître M, Guyonnet L, Laurans L, Joffre J, Lemarie J, et al. Selective EGF-Receptor Inhibition in CD4+ T Cells Induces Anergy and Limits Atherosclerosis. J Am Coll Cardiol (2018) 71:160–72. doi: 10.1016/j.jacc.2017.10.084
13. Luo Q, Gu Y, Zheng W, Wu X, Gong F, Gu L, et al. Erlotinib Inhibits T-cell-mediated Immune Response Via Down-Regulation of the c-Raf/ERK Cascade and Akt Signaling Pathway. Toxicol Appl Pharmacol (2011) 251:130–6. doi: 10.1016/j.taap.2010.12.011
14. Wolf E, Milazzo S, Boehm K, Zwahlen M, Horneber M. Thymic Peptides for Treatment of Cancer Patients. Cochrane Database Syst Rev (2011) 2:CD003993. doi: 10.1002/14651858.CD003993.pub3
15. Attapon C, Ruangrong C. Lung Cancer Chemotherapy, New Treatment and Related Patents. Recent Pat Anticancer Drug Discov (2014) 9:372–81. doi: 10.2174/1574892809666140520120806
16. Zeng FL, Xiao Z, Wang CQ, Jiang Y, Shan JL, Hu SS, et al. Clinical Efficacy and Safety of Synthetic Thymic Peptides With Chemotherapy for Non-Small Cell Lung Cancer in China: A Systematic Review and Meta-Analysis of 27 Randomized Controlled Trials Following the PRISMA Guidelines. Int Immunopharmacol (2019) 75:105747. doi: 10.1016/j.intimp.2019.105747
17. Park K, Tan EH, O’Byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib Versus Gefitinib as First-Line Treatment of Patients With EGFR Mutation-Positive Non-Small-Cell Lung Cancer (LUX-Lung 7): A Phase 2B, Open-Label, Randomised Controlled Trial. Lancet Oncol (2016) 17:577–89. doi: 10.1016/S1470-2045(16)30033-X
18. Shi YK, Wang L, Han BH, Li W, Yu P, Liu YP, et al. First-Line Icotinib Versus Cisplatin/Pemetrexed Plus Pemetrexed Maintenance Therapy for Patients With Advanced EGFR Mutation-Positive Lung Adenocarcinoma (CONVINCE): A Phase 3, Open-Label, Randomized Study. Ann Oncol (2017) 28:2443–50. doi: 10.1093/annonc/mdx359
19. Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker Analyses and Final Overall Survival Results From a Phase III, Randomized, Open-Label, First-Line Study of Gefitinib Versus Carboplatin/Paclitaxel in Clinically Selected Patients With Advanced Non-Small-Cell Lung Cancer in Asia (Ipass). J Clin Oncol (2011) 29:2866–74. doi: 10.1200/JCO.2010.33.4235
20. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous Nsclc. N Engl J Med (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
21. Jiang T, Cheng R, Zhang G, Su C, Zhao C, Li X, et al. Characterization of Liver Metastasis and Its Effect on Targeted Therapy in EGFR-mutant Nsclc: A Multicenter Study. Clin Lung Cancer (2017) 18:631–9. doi: 10.1016/j.cllc.2017.04.015
22. Zhou JY, Liu SY, Wu YL. Safety of EGFR-TKIs for EGFR Mutation-Positive Non-Small Cell Lung Cancer. Expert Opin Drug Saf (2020) 19:589–99. doi: 10.1080/14740338.2020.1753697
23. Shi Y, Zhang L, Liu X, Zhou C, Zhang L, Zhang S, et al. Icotinib Versus Gefitinib in Previously Treated Advanced Non-Small-Cell Lung Cancer (ICOGEN): A Randomised, Double-Blind Phase 3 Non-Inferiority Trial. Lancet Oncol (2013) 14:953–61. doi: 10.1016/S1470-2045(13)70355-3
24. Sheng J, Fang W, Liu X, Xing S, Zhan J, Ma Y, et al. Impact of Gefitinib in Early Stage Treatment on Circulating Cytokines and Lymphocytes for Patients With Advanced Non-Small Cell Lung Cancer. Onco Targets Ther (2017) 10:1101–10. doi: 10.2147/OTT.S112158
25. Garaci E. Thymosin alpha1: A Historical Overview. Ann N Y Acad Sci (2007) 1112:14–20. doi: 10.1196/annals.1415.039
26. Sztein MB, Serrate SA. Characterization of the Immunoregulatory Properties of Thymosin Alpha 1 on Interleukin-2 Production and Interleukin-2 Receptor Expression in Normal Human Lymphocytes. Int J Immunopharmacol (1989) 11:789–800. doi: 10.1016/0192-0561(89)90133-1
27. Ahmed A, Wong DM, Thurman GB, Low TL, Goldstein AL, Sharkis SJ, et al. T-Lymphocyte Maturation: Cell Surface Markers and Immune Function Induced by T-lymphocyte Cell-Free Products and Thymosin Polypeptides. Ann N Y Acad Sci (1979) 332:81–94. doi: 10.1111/j.1749-6632.1979.tb47100.x
28. Romani L, Bistoni F, Gaziano R, Bozza S, Montagnoli C, Perruccio K, et al. Thymosin Alpha 1 Activates Dendritic Cells for Antifungal Th1 Resistance Through Toll-Like Receptor Signaling. Blood (2004) 103:4232–9. doi: 10.1182/blood-2003-11-4036
29. Wang Y, Cao Y, Meng Y, You Z, Liu X, Liu Z. The Novel Role of Thymopentin in Induction of Maturation of Bone Marrow Dendritic Cells (Bmdcs). Int Immunopharmacol (2014) 21:255–60. doi: 10.1016/j.intimp.2014.05.011
30. Giuliani C, Napolitano G, Mastino A, Di Vincenzo S, D’Agostini C, Grelli S, et al. Thymosin-Alpha1 Regulates MHC Class I Expression in FRTL-5 Cells At Transcriptional Level. Eur J Immunol (2000) 30:778–86. doi: 10.1002/1521-4141(200003)30:3<778::AID-IMMU778>3.0.CO;2-I
31. Qin Y, Chen FD, Zhou L, Gong XG, Han QF. Proliferative and Anti-Proliferative Effects of Thymosin Alpha1 on Cells are Associated With Manipulation of Cellular ROS Levels. Chem Biol Interact (2009) 180:383–8. doi: 10.1016/j.cbi.2009.05.006
Keywords: NSCLC, EGFR-TKI, thymosin, active EGFR mutations, efficacy, safety
Citation: Feng Y, Zhu G, Lang S, Hao P, Li G, Chen F, Zhuo W, Duan Y, Zhang A, Chen Z and Sun J (2021) The Efficacy and Safety of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Combined With Thymosin in Advanced Non-Small Cell Lung Cancer Patients Harboring Active Epidermal Growth Factor Receptor Mutations. Front. Oncol. 11:659065. doi: 10.3389/fonc.2021.659065
Received: 26 January 2021; Accepted: 03 May 2021;
Published: 28 May 2021.
Edited by:
Olivier Feron, Université catholique de Louvain, BelgiumReviewed by:
Claudio Constantini, University of Perugia, ItalyCopyright © 2021 Feng, Zhu, Lang, Hao, Li, Chen, Zhuo, Duan, Zhang, Chen and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianguo Sun, c3VuamcwOUBhbGl5dW4uY29t; Zhengtang Chen, Y3p0MDVAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.