
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 17 May 2021
Sec. Surgical Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.657955
This article is part of the Research Topic Methods Of Optimizing Surgical Intervention In Esophago-Gastric Cancer View all 19 articles
 LeQi Zhong1†
LeQi Zhong1† JiuDi Zhong1†
JiuDi Zhong1† ZiHui Tan1†
ZiHui Tan1† YiTong Wei2†
YiTong Wei2† XiaoDong Su1
XiaoDong Su1 ZheSheng Wen1
ZheSheng Wen1 TieHua Rong1,3
TieHua Rong1,3 Yi Hu1,3*†
Yi Hu1,3*† KongJia Luo1,3*†
KongJia Luo1,3*†Objective: To explore the comprehensive role of systemic endoscopic intervention in healing esophageal anastomotic leak.
Methods: In total, 3919 consecutive patients with esophageal cancer who underwent esophagectomy and immediate esophageal reconstruction were screened. In total, 203 patients (5.10%) diagnosed with anastomotic leakage were included. The participants were divided into three groups according to differences in diagnosis and treatment procedures. Ninety-four patients received conventional management, 87 patients received endoscopic diagnosis only, and the remaining 22 patients received systematic endoscopic intervention. The primary endpoint was overall healing of the leak after oncologic esophageal surgery. The secondary endpoints were the time from surgery to recovery and the occurrence of adverse events.
Results: 173 (85.2%; 95% CI, 80.3-90.1%) of the 203 patients were successfully healed, with a mean healing time of 66.04 ± 3.59 days (median: 51 days; range: 13-368 days), and the overall healing rates differed significantly among the three groups according to the stratified log-rank test (P<0.001). The median healing time of leakage was 37 days (95% CI: 33.32-40.68 days) in the endoscopic intervention group, 51 days (95% CI: 44.86-57.14 days) in the endoscopic diagnostic group, and 67 days (95% CI: 56.27-77.73 days) in the conventional group. The overall survival rate was 78.7% (95% CI: 70.3 to 87.2%) in the conventional management group, 89.7% (95% CI: 83.1 to 96.2%) in the endoscopic diagnostic group and 95.5% (95% CI: 86.0 to 100%) in the systematic endoscopic intervention group. Landmark analysis indicated that the speed of wound healing in the endoscopic intervention group was 2-4 times faster at any period than that in the conservative group. There were 20 (21.28%) deaths among the 94 patients in the conventional group, 9 (10.34%) deaths among the 87 patients in the endoscopic diagnostic group and 1 (4.55%) death among the 22 patients in the endoscopic intervention group; this difference was statistically significant (Fisher exact test, P < 0.05).
Conclusion: Tailored endoscopic treatment for postoperative esophageal anastomotic leakage based on endoscopic diagnosis is feasible and effective. Systematic endoscopic intervention shortened the treatment period and reduced mortality and should therefore be considered in the management of this disease.
As the seventh most commonly diagnosed cancer, esophageal carcinoma (EC) is associated with a dismal fatality rate, ranking as the sixth most common cause of cancer-related death (1). Once esophageal cancer is confirmed, radical resection is typically recommended, as it is of the most effective therapeutic approaches for select patients. Despite the considerable improvement in surgical conditions and skills, however, esophagectomies are still associated with various complications, of which anastomotic leakage is a disastrous postoperative complication that seriously affects patient quality of life due to both its high incidence (5-40%) and associated mortality (2-60%) (2–9). As a consequence, improvements in leak management are of vital necessity to reduce overall mortality.
Leaks after esophagectomy are defined as full-thickness gastrointestinal defects involving the esophagus, anastomosis, staple line, or conduit irrespective of the presentation or method of identification. Along with the development of esophageal anastomotic leakage (EAL), one consequence followed close on the heels of another. Firstly, EAL is the greatest risk factor for perioperative complication-related death, with up to 60% mortality rates, and the risk of death for patients with EAL is 3 times higher than that for those without EAL (7–11). Moreover, in the short run, it increases the length of hospital stay, prolongs the oral feeding time, contributes to the risk of anastomotic hemorrhage, and increases risk of reoperation. In the long run, a positive association between the occurrence of anastomotic stricture and the development of EAL was observed. EAL can also impair long-term survival, negatively impact surgical and oncologic outcomes and be related to cancer recurrence after surgical resection for esophageal malignancy (5, 7, 9–11).
The diagnosis or interference time of EAL explains the severity of this complication; more specifically, the most predominant risk factors for the subsequent clinical outcome are the patients’ delay as well as the delay of diagnosis or even the absence of any interference, so it is undisputed that prompt diagnosis and immediate intervention are of vital significance to prevent further damage and to control the ensuing clinical development. Throughout the course of intervention, the use of a multidisciplinary diagnostic and treatment approach is undoubtedly highly important.
Traditionally, there are several methods used to detect EAL, of which routine contrast medium esophagography is widely utilized and has gained international recognition. In addition, direct surgical exploration, oral administration of methylene blue, and CT scans with or without oral contrast are extensively used. However, there is no consensus within the literature with regard to whether, when or which strategy should be used, even though their limitations are well documented. Some researchers have suggested that the routine use of contrast radiography be suspended, since it can be unreliable in the detection of anastomotic leaks, with a reported sensitivity between 40% and 66%, and aspiration pneumonia due to aspiration of the contrast agent was noted (12, 13). Meanwhile, operative exploration is limited by its high mortality rate; oral methylene blue may not be proper for diagnosing late EAL, as adhesions formed after esophagectomy may result in localized collection of the dye, making it difficult to identify EAL; and computed tomography (CT) scanning does not provide information about gastric conduit viability, so early ischemic or necrotic areas could be missed (12–16). As endoscopic techniques have begun to be applied clinically over the past decade, they have shown clear advantages (e.g., direct visualization and quantification of the defect, ability to determine gastric conduit viability, and both the sensitivity and specificity could reach up to 95-100%) (14–16).
In terms of treatment, the therapeutic strategies for this issue range from palliative treatment such as antibiotics and nutritional support to operative exploration and endoscopic management using stents, clips, glues, etc., or their combination. All of these efforts share the same goal: to close the breach and eliminate contamination. Traditional surgical repair has certain disadvantages, such as increased hospitalization costs and mortality and extended hospital stays, which obviously conflict with the notion of rapid rehabilitation surgery. Fortunately, minimally invasive endoscopic therapies may have advantages such as enhanced safety, minimal invasiveness, quicker recovery, lower treatment cost, etc., when compared with traditional open surgical methods (14–16).
However, clinicians remain reluctant to perform endoscopy after esophagectomy because of the theoretical risk of disrupting the anastomosis or worsening the EAL (17–19). In our cases, endoscopic intervention was found to aid in making a precise diagnosis and in deciding the most appropriate clinical strategy without increasing the incidence of complications and mortality. As highlighted in a recently published Position Statement of the European Society of Gastrointestinal Endoscopy, it is important to have a systematic approach for the diagnosis and treatment of GI perforations (20). Therefore, this investigation proceeded with the aim of evaluating the safety and efficacy of this new approach to diagnosing and treating anastomotic fistula and to assess the role of endoscopic intervention throughout the entire rehabilitation process of EAL.
This was a single-center retrospective study conducted at our Thoracic Surgery Department. We analyzed our clinical databank and screened out all suspected EAL patients who had undergone esophagectomy between January 2012 and August 2019 at the Sun Yat-Sen University Cancer Center. To improve the homogeneity between the study groups, only patients with anastomotic leakage after esophagectomy due to malignant esophageal tumors were included. Other esophageal leaks, such as iatrogenic leakage, EAL from benign esophageal disease or following gastrectomy, were excluded. Other exclusion criteria were a prior history of esophageal surgery, cases managed by primary surgery, operation performed at another institution and incomplete medical records. The specific process of patient enrollment is shown in the flow chart (Figure 1).
Records were reviewed to collect patient demographics, tumor characteristics, preoperative chemoradiotherapy information, surgical procedures, diagnostic methods, leakage therapy regimens, clinical outcomes, mortality and complications.
A total of 3919 patients underwent esophagectomy during the evaluation period, of whom 203 were confirmed to have EAL and were included in the analysis (Figure 1). Among this population, 138 patients underwent open surgery, including 57 patients who underwent surgery according to the Sweet procedure, 61 patients who underwent surgery according to the McKeown procedure, and 20 patients who underwent Ivor-Lewis surgery. The remaining 65 patients underwent minimally invasive esophagectomy procedures such as the mediastinoscopic transmediastinal approach (n=3), thoraco-laparoscopic McKeown (n=42) and Ivor-Lewis (n=3) esophagectomy, and robot-assisted McKeown (n=17) with the aid of the da Vinci® system (Intuitive Surgical, Sunnyvale, CA). Construction was completed in 201 patients by gastric conduit and in 2 patients by colon interposition. The decision of surgical modality was made at the discretion of the surgeon performing the operation according to the patient’s actual condition.
Radiological contrast studies or endoscopy were routinely performed to screen for the existence of possible leakage at approximately day 7 after surgery. Once EAL was confirmed, the surgeon responsible for the respective case would decide on a treatment plan and initiate intervention. The specific diagnoses and intervention procedures of the 203 included patients are presented in Figure 1.
Conservative approaches included nutritional support, gastrointestinal decompression through an intraoperatively placed gastric tube, perianastomotic drainage via a surgically placed prophylactic chest tube and systemic antibiotics. Supplemental nutritional support was generally provided through a preplaced jejunal nutrition tube during esophagectomy and occasionally through total parenteral nutrition support. Proton pump inhibitor (PPIS)-aided therapy was also included for gastrointestinal decompression, and the intraoperative indwelling gastric tubes were not pulled out until the anastomotic leakage healed. The leak cavity was flushed several times with irrigation fluids containing gentamycin in saline, with the same purpose as thoracic drainage to clear most of the pus. In accordance with the irrigation regimen, all patients received intravenous broad-spectrum antibiotics (2, 5, 9, 11).
Endoscopic interventions were performed by veteran endoscopic surgeons. The endoscopic strategy was subdivided into two types: diagnostic and therapeutic. The location of the anastomosis and the lesion, the extent of the orifice, and the presence of pus were confirmed and evaluated during the diagnostic phase. Then, the leaks were subdivided into the following categories according to the Esophagectomy Complications Consensus Group (ECCG) classification (21):
Type I: Local defect requiring no change in therapy or treated medically.
Type II: Localized defect requiring interventional but not surgical therapy.
Type III: Localized defect requiring surgical therapy.
Then, the therapeutic phase was carried out at the discretion of the responsible surgeon according to the EAL characteristics found above. First of all, in the course of endoscopic intervention, whether the EAL was infected or not would be one of our major focuses. If the EAL was infected, we would irrigate the pus cavity with normal saline under the navigation of ultrafine gastroscopy, and then immediate suction and irrigation of the abscess cavity would be established by Endoscopic Trans-nasal Inner Drainage. Generally, the pus would exterminate in about 7-14 days, subsequent systematic endoscopic therapies would be carried out based on the status of patients and the results of endoscopic reexamination, the processes of drainage was not counted in the number of endoscopic sessions. If the EAL was not infected, the systematic endoscopic treatment would administrate directly. Treatment strategies included a ‘wait and see’ strategy (endoscopic diagnostic group), administration of tissue sealant, the use of an endoscopic clip or the application of combined therapy (endoscopic intervention group). Endoscopic treatment was systematically performed until an effective outcome was achieved or the patient died (6).
Details of intervention strategies in systematic endoscopic group is described as follows.
During the diagnostic procedure or the reexamination process, the depth of lesion was confirmed. If the depth of the wound was less than 1cm, the patient would be treated by endoscopic clips; otherwise, by biological tissue sealants to avoid the formation of residual cavity.
The first clip is proposed to place through the most distal part of the leak to the oral side successively as this prevents accidental snagging and drooping. Then, flushing the anastomosis with normal saline and observing whether there are bubbles, so as to judge whether it is closed completely. Lastly, checking the tension of the anastomosis. If the tension is high or the closure is incomplete, endoscopic review and following sessions will be administrated 7 days later.
Firstly, we use a small endoscopic brush to clean the wound and make it bleed slightly, then spray sealants to fill the fistula and to stop the bleeding. The biological sealants consist two components, one component consists of the antifibrinolytic solution (aprotinin) and a protein concentrate (fibrinogen) derived from human plasma, and the other component includes human thrombin (or a bovine thrombin) and a calcium chloride solution. The two solutions are delivered in a dual-barrel syringe and combined at the site of desired application, through a double lumen catheter, to form a firm, white, rubber-like mass with strong adhesive properties within few seconds of being mixed.
The primary endpoint was the overall healing of leakages after oncologic esophageal surgery. Complete healing of the EAL was defined as patient recovery (no abnormality after oral feeding) after assessment with endoscopy or via follow-up X-ray or CT contrast study. The secondary endpoint was the time (in days) from surgery to recovery and the occurrence of adverse events (sinus formation, bleeding, anastomotic stenosis, etc.). Failure was defined as death or loss to follow-up.
Primary data were managed and extracted from the hospital data management system and then analyzed by IBM SPSS Statistics version 25.0 (Inc, Chicago, Illinois, USA). Continuous variables are presented as the means ± standard deviations (SD), and categorical data are presented as numbers and percentages. Multivariable analyses with the Cox proportional hazards model were used to estimate the simultaneous effects of prognostic factors on healing. All eligible patients were included in the analysis of overall healing by the Kaplan–Meier method and the log-rank test to calculate corresponding P values. First, a univariate analysis using various factors associated with EAL healing time was performed. Next, to identify significant independent factors related to the time needed for EAL healing, multivariate Cox regression analysis was performed using factors identified as significant variables and selected potential confounding factors from the univariate analysis. Given that all patients in the endoscopic intervention group had healed within 90 days after surgery (except for one death on day 90), to explore the role of endoscopic technology in the healing process of EAL at different time periods from esophagectomy to rehabilitation, an exploratory analysis based on the landmark analysis method was performed according to landmark points of 30 days, 60 days, 90 days, and post-90 days, with the hazard ratio calculated separately for events that occurred each month after grouping and events that occurred between 90 days and the end of the follow-up period (22–24). We then performed a test for the interaction between treatment and time. In all time-to-event analyses (i.e., overall and landmark), for each type of event, data were censored at the time of the first event that occurred in a patient. Additionally, all patients were included in the complication assessment. Differences were considered to be statistically significant when the P value was 0.05 or less. All statistical tests were two-sided.
A total of 224 patients were suspected of having EAL due to esophagectomy during the study period, of whom 21 were excluded from the study (Figure 1). The remaining 203 patients were included in the analysis (Table 1 shows the baseline clinical data). Among these 203 patients, 94 patients received conventional diagnosis and treatment procedures (conventional group); of the other 109 patients, 87 patients (including one patient for whom endoscopic clipping was attempted but failed) were diagnosed endoscopically but received conservative treatment (endoscopic diagnostic group), and 22 patients were diagnosed and treated by endoscopy directly (endoscopic intervention group) (Figure 1). There was no significant difference in clinical baseline among the three groups except age (Table 2 shows the comparison of clinical characteristics according to the diagnosis and treatment procedures).
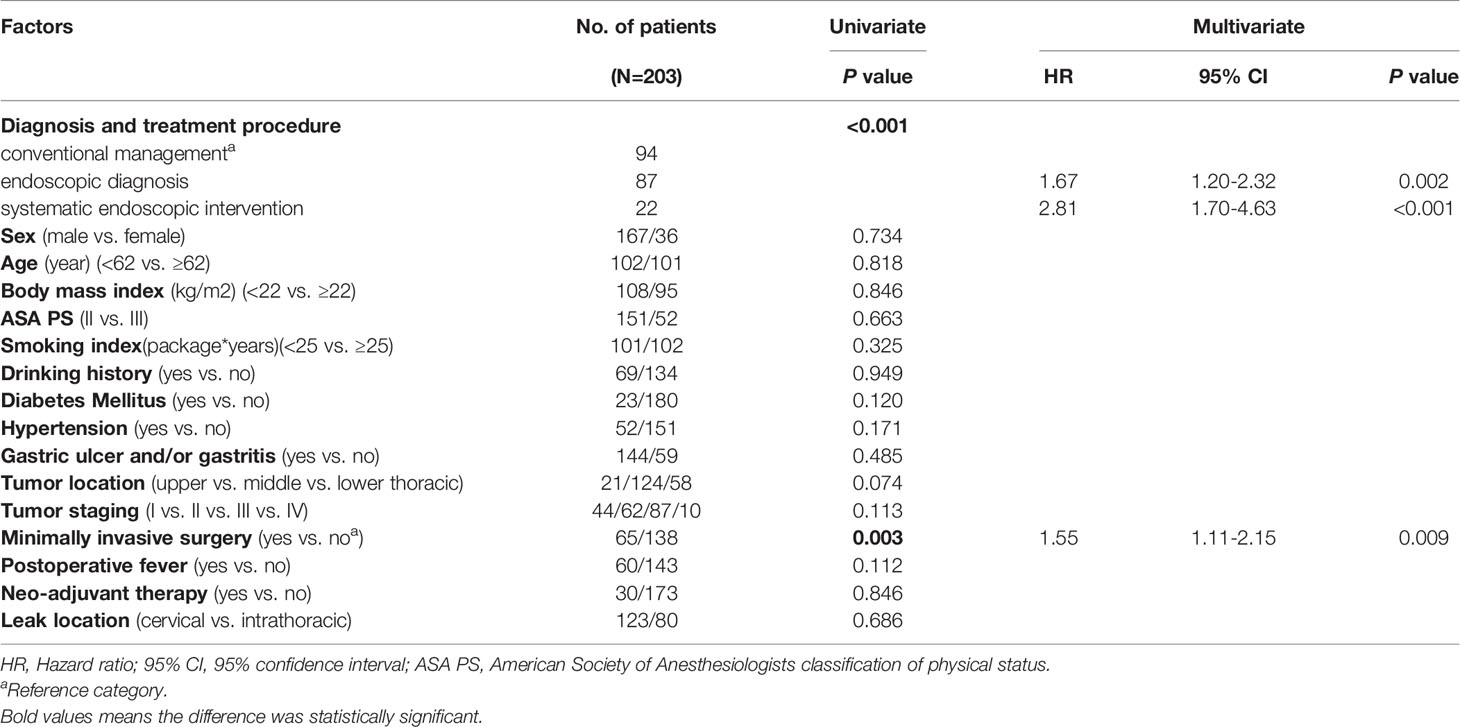
Table 1 Basic characteristics of the 203 patients with EAL and Determinants of EAL postoperative overall healing in patients with EAL.
Traditional radiological contrast studies (n=124) resulted in 30 missed diagnoses (omission diagnostic rate=24.19%) and 5 misdiagnoses among the EAL patients; hence, the sensitivity of traditional diagnostic methods was 75.81%. Comparatively, endoscopy correctly diagnosed the remaining 79 patients who underwent endoscopic examination directly due to suspected EAL with 100% accuracy. Moreover, endoscopy not only correctly identified the 5 false-positive patients from the radiological contrast study but also detected the 30 leaks that were missed.
EAL was treated during hospitalization for all patients, and 173 (85.2%; 95% CI: 80.3-90.1%) of them successfully healed, with a mean healing time of 66.04 ± 3.59 days (median: 51 days; range: 13−368 days). The overall healing rates in the three groups differed significantly based on the results of the stratified log-rank test (P<0.001).
Table 3 shows the characteristics of EAL of the 22 study patients who underwent systematic endoscopic intervention.
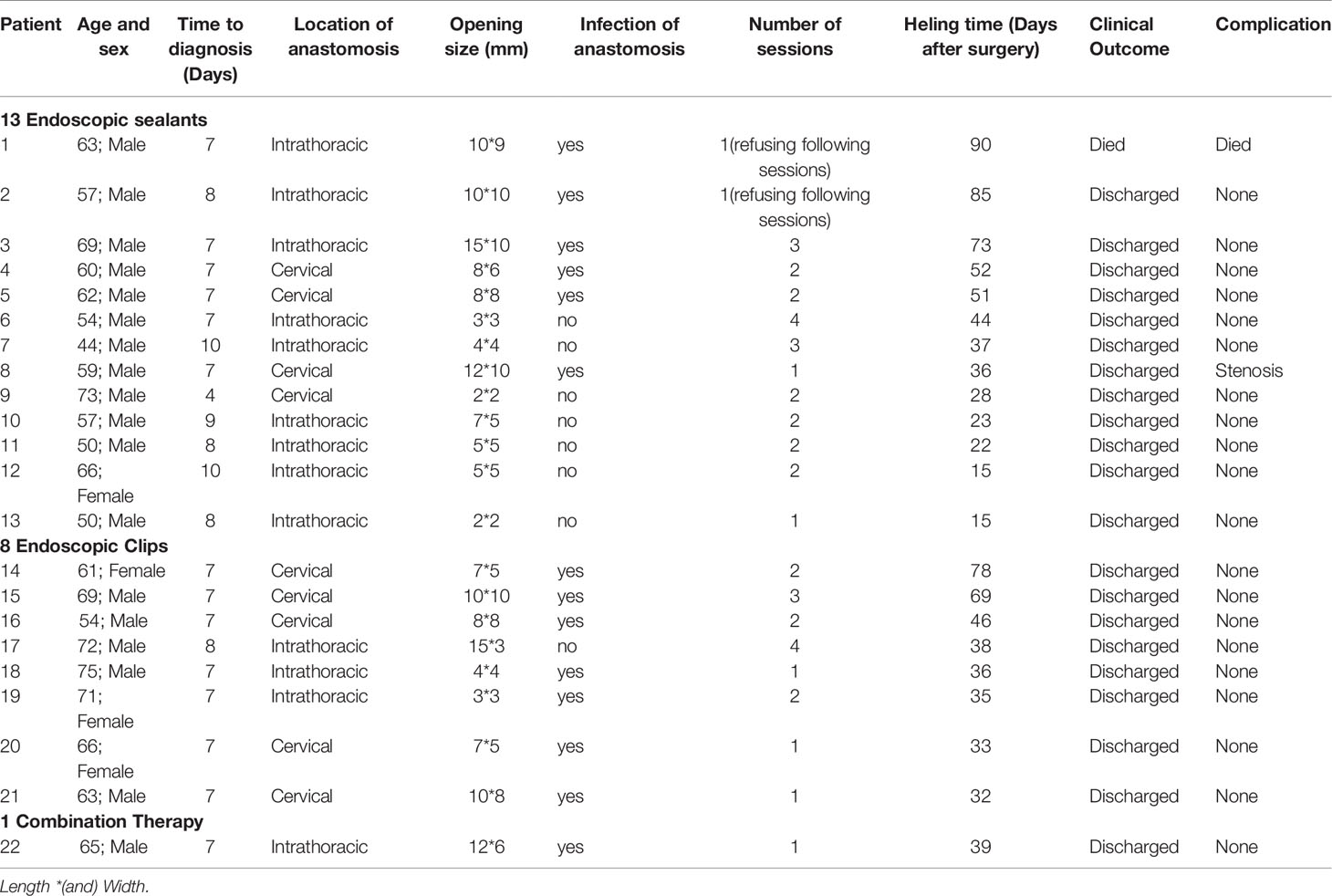
Table 3 Characteristics of EAL of the 22 study patients who underwent systematic endoscopic intervention.
The median healing time of EAL was 37 days (95% CI: 33.32-40.68 days) in the endoscopic intervention group, 51 days (95% CI: 44.86-57.14 days) in the endoscopic diagnostic group, and 67 days (95% CI: 56.27-77.73 days) in the conventional group (Table 4).
The univariate analysis showed a significant relationship between diagnosis and treatment procedure (conventional management vs. endoscopic diagnosis vs. systematic endoscopic intervention) and minimally invasive surgery (yes vs. no) (Table 1). Cumulative healing rates after surgery calculated with the Kaplan−Meier method and stratified by these significant factors are shown in Figures 2A, B.
The multivariate analysis results demonstrated that diagnostic and treatment procedures (conventional management vs. endoscopic diagnosis vs. systematic endoscopic intervention) and minimally invasive surgery (yes vs. no) were significant independent factors for EAL healing time (P<0.001 and P=0.009, respectively) (Table 1).
The landmark analysis results indicated that the speed of wound healing in the endoscopic intervention group was faster than that in the conservative group at any period. The healing characteristics of the different groups at various landmark periods are illustrated in Figures 3 and 4. It was not difficult to find that the healing speed of the endoscopic intervention group was superior to that of the endoscopic diagnostic group, and the advantage was more prominent when compared with the conventional group, whose healing velocity was only one-third of its counterpart.

Figure 4 Overall healing of patients with EAL based on landmark analysis and corresponding hazard ratios. The number of unhealed patients with EAL and the corresponding hazard ratios are shown at various time points for the groups. A total of 94 patients in the conventional group, 87 in the endoscopic diagnosis group, and 22 in the endoscopic intervention group; the corresponding numbers at 60 days were 48, 24, and 5, and the corresponding numbers at 90 days were 28, 11, and 0.
Patients in the systematic endoscopic group had significantly lower rates of death than those in the endoscopic diagnostic group and the conventional group, while no obvious difference in fatality was observed between the endoscopic diagnostic group and the conventional group. In the weighted Cox proportional hazard regression model, the adjusted hazard ratio (HR) for healing in the endoscopic intervention group compared with the conventional group was 1.94 (95% CI, 0.68-5.51; P=.038), and that in the diagnostic group compared with the conventional group was 1.40 (95% CI, 0.66-3.00; P=.021). In this analysis, the differences among the 3 groups were not statistically significant with regard to healing (Figures 3 and 4).
Again, the possibility of death in the systematic interventional group was significantly lower than that of the conventional group; meanwhile, a similar advantage was found in the endoscopic diagnostic group when compared with the conventional group. Moreover, when compared with the traditional group, the endoscopic intervention group and endoscopic diagnostic group showed not only a significant reduction in the mortality rate but also a statistically significant increase in the recovery rate; the hazard ratios for healing were 3.86 (95% CI, 1.93-7.75; P<0.001) and 2.57 (95% CI, 1.56-4.25; P<0.001), respectively (Figures 3 and 4).
A lower mortality rate was found in the endoscopic diagnostic group than in the conventional group, which had 4 fatal cases, yet the mortality rate seemed to be higher in endoscopic interventional group than in the remaining two groups; notably, only 5 patients were in the endoscopic interventional group during this period, which should be taken into consideration. During this period, the HRs for healing were 2.69 (95% CI, 0.89-8.10; P=0.08) in the endoscopic interventional group and 1.50 (95% CI, 0.71-3.18; P=0.29) in the endoscopic diagnostic group when compared with their counterparts (Figures 3 and 4).
It should be noted that all patients in the systematic endoscopic group reached the study endpoints. As illustrated in Figures 3 and 4, during the period 3 months after surgery, the endoscopic diagnostic group and conventional group healed at very similar speeds, and the mortality rates were 3/11 (27.3%) and 6/28 (21.4%), respectively.
Of the 203 enrolled patients, there were 20 (21.28%) fatal cases among the 94 patients in the conventional group, 9 (10.34%) fatal cases among the 87 patients in the endoscopic diagnostic group and 1 (4.55%) fatal case among the 22 cases in the endoscopic intervention group; this difference was statistically significant (Fisher exact test, P=0.049<0.05).
Regarding compilations, 24 (25.53%) complications occurred in the 94 patients in the conventional group, 19 (21.84%) occurred in the 87 patients in the endoscopic diagnostic group, and 1 (4.55%) occurred in the 22 patients in the endoscopic intervention group, but the differences among the three groups were not statistically significant (Fisher’s exact test, P=0.089>0.05).
Therefore, in conclusion, 30 patients died, and 44 patients developed EAL-related complications. The overall mortality and complication rates were 14.78% and 21.67%, respectively. The overall survival rate was 78.7% (95% CI: 70.3 to 87.2%) in the conventional management group, 89.7% (95% CI: 83.1 to 96.2%) in the endoscopic diagnostic group and 95.5% (95% CI: 86.0 to 100%) in the systematic endoscopic intervention group (Table 5).
Post-esophagectomy anastomotic leakage or fistula is a serious and common complication in patients with esophageal carcinoma (2–5, 7, 9–11). Over the past decade, few studies have adequately assessed and evaluated the status of endoscopic technology for the diagnosis and treatment of EAL, and to the best of our knowledge, this paper is the first to discuss the relationship between EAL healing and the timeframe in which healing occurred, not just whether it was healed or not. We found that patients with EAL after endoscopic intervention may have the fastest healing speed at 30-60 days (1-2 months) after surgery based on the landmark analysis results (compared with the conventional management group and the endoscopic diagnosis group, HR values were 3.86 and 2.57, respectively). This may provide a reference to help clinicians make better clinical decisions at different time periods.
EAL can affect the operative efficacy of esophageal cancer, prolong hospital stays and increase postoperative mortality (2, 5–11). EAL can even impair patient quality of life, long-term survival of esophageal cancer and subsequent treatment of esophageal masses using strategies such as adjuvant chemoradiotherapy (2, 6, 8, 10, 25). Finally, because EAL potentially causes subsequent critical postoperative complications, such as intrathoracic abscess, tracheoesophageal fistula and hemorrhage, both predicting and treating EAL are clinically significant issues. Therefore, it is of vital importance to explore a safe and effective treatment model for EAL. The present study focused on the role of systematic endoscopic intervention in postoperative EAL detection and rehabilitation. Our study included patients with EAL following surgery for esophageal cancer at a specialized cancer center, representing a larger, more homogenous patient population.
Given the high incidence of anastomotic leakage and the severe harm it causes, most centers prefer to assess the anastomosis diagnostically before starting oral intake after esophagectomy. The use of endoscopy, however, has been questioned due to the theoretical threat of disrupting the anastomosis or aggravating EAL (17–19). At present, many surgeons in China still pay little attention to or are reluctant to attempt to address EAL by endoscopic means for fear of the possible complications mentioned above. Our findings show that properly performed endoscopic intervention does not cause injury to the anastomosis, and a certain number of studies have proven the safety of endoscopy (14–16); although an intraluminal pressure greater than 80 cmH2O is known to be required to disrupt the anastomosis, the intraluminal maximum insufflation at the anastomosis never exceeds 9 cmH2O and thus rarely disturbs blood flow in the conduit (14, 26–28).
Patients who underwent endoscopic diagnosis and/or intervention had lower probabilities of death and complications than the conventional group in our study (Table 5). It was found that the overall mortality was 14.78%. By comparison, the mortality rates presented in previous studies have ranged from 2.1% to 35.7% (2, 8, 9, 11, 29, 30).
Patients in the endoscopic diagnostic group vs. conventional group had a lower risk of death (odds ratio (OR) =0.43; 95% CI, 0.18-1.00); after adjustment by the Bonferroni method, however, there were no statistically significant differences between the groups with regard to mortality (P=0.067>0.01667). Patients in the endoscopic intervention group vs. conventional group also had a lower risk of death (odds ratio (OR) =0.18; 95% CI, 0.02-1.40), but again, no statistically significant difference was observed (P=0.119>0.01667). Regarding compilations, 24 (25.53%) complications occurred in the 94 patients in the conventional group, 19 (21.84%) occurred in the 87 patients in the endoscopic diagnostic group, and 1 (4.55%) occurred in the 22 patients in the endoscopic intervention group, but the differences among the three groups were not statistically significant (Fisher’s exact test, P=0.089>0.05).
Moreover, the sensitivity and specificity of endoscopic assessment were superior to those of traditional methods. In our study, the sensitivity of traditional diagnostic methods was 75.81%, close to the previously reported CT diagnostic sensitivity of 71.4-80% (5, 12, 13, 26, 27), which is unsatisfactory. While endoscopy not only correctly identified the 5 false-positive patients evaluated by radiological contrast study but also determined 30 leaks that were missed in the radiological contrast study, and both the reported sensitivity and specificity of endoscopic diagnosis could reach 100% (16).
Additionally, the procedure is convenient, as it can be conducted at the bedside, even for patients on ventilation, without worsening an existing EAL. More remarkably, endoscopy is the only approach with the capacity to determine the viability of the gastric conduit and to grade the EAL according to the results of endoscopic observation, which will be highly valuable in making more accurate clinical decisions based on each individual, including the adjustment of the drainage strategy, the need for surgical treatment, the use of antibiotic regimens, adequate nutritional support, and so on. In summary, endoscopic diagnosis offers the advantages of possibly avoiding repetitive examinations, aiding in early diagnosis, guiding further treatments, improving the sensitivity and specificity, and reducing complications, which could make the treatment process more smoothly and accurately, and then enable patients to achieve better clinical outcomes.
With regard to the treatment, although we were interested in determining whether the EAL heals, we were more curious about when. To our knowledge, this paper is the first to discuss the outcome of anastomotic leaks in association with healing time rather than whether it healed based on the results of landmark analysis. Previous studies have reported 55.8-100.0% healing rates for EAL when treated with endoscopic strategies (31–50), while our research suggests that the healing rates could reach up to 95.5% if endoscopic management methods were implemented, and the number would still be near 90% if only endoscopic diagnosis was implemented [Table 2 and Figure 5 (31–50)].
Moreover, we elucidated the actual healing time and successfully identified two statistically significant independent factors associated with the time needed for healing EAL, of which different endoscopic strategies were included (Table 1).
Regarding how to reduce the healing time, endoscopy offered a satisfactory result. The goal of the landmark analysis method was to estimate the healing probabilities in each group at the landmark time in an unbiased way (22–24).The landmark analysis revealed that once the endoscopic intervention was administered, the superiority of endoscopic intervention compared with conventional management persisted until the leisure healed, and this advantage is most pronounced 1 to 2 months after surgery, which indicated that early intervention is of vital importance to the recovery process of EAL. Patients with EAL were found to heal faster than conservative patients even when only endoscopic diagnosis was conducted without systematic endoscopic intervention at the early stage; however, the superiority of the endoscopic diagnostic group compared with the traditional group before 90 days of follow-up was lost after 90 days. Of course, the healing time of EAL would be shorter if endoscopic intervention was added. Interestingly, it was found that if the patients in the endoscopic diagnosis group did not achieve clinical cure at an early stage when there was a healing advantage, their merits of rapid rehabilitation would nowhere to be seem as time goes by, put it another way, they would be found to have similar clinical outcomes as those in the conventional group at later stages of the follow-up, which provides a new perspective on the importance of early diagnosis of EAL, and suggests that patients with EAL may benefit from remedial endoscopic managements.
To summarize, our study provides new evidence that endoscopic therapy can offer an important prognostic benefit to EAL patients. Endoscopic intervention could be considered superior to other regimens in managing anastomotic leakage at any period after esophagectomy. The landmark analysis results suggested that for EAL rehabilitation, endoscopic therapy can be attempted as a remedial measure at any period, even if endoscopic intervention was not employed at the early stage, since remedial endoscopy could shorten the healing time of EAL.
In terms of the clinical application of the results of this study, it is important to take into account the merits of a shorter healing time. Shortening the time needed for EAL healing has some potential clinical advantages, including reducing the incidence of subsequent critical postoperative complications and decreasing the cost of hospitalization due to the shortened hospitalization period. In addition, a shorter healing time allows for smoother coordination of the administration of adjuvant therapy when patients have cancers for which adjuvant therapy is indispensable.
The present study has several limitations. First, endoscopic vacuum-assisted closure (E-VAC) therapy was not carried out in our hospital; more specifically, E-VAC technology has not been widely used throughout China. E-VAC technology was first introduced in 2008 by Weidenhagen et al. (51) and has been proven to be safe and effective in some studies, with encouraging healing rates (93.3-93.5%) (52, 53). We look forward to using E-VAC technology in our hospital to help patients who have suffered from EAL. The second limitation is that the data for the present study were from a single institution, which may produce some bias in the preoperative management of patients, such as operative methods. In the future, these results should be validated in a multi−institutional, prospective, randomized, controlled trial using certain criteria, as mentioned above.
In summary, the results of this study suggest that systematic endoscopic intervention is an effective and safe method for the diagnosis and treatment of postsurgical leaks. This intervention leads to higher success rates and faster anastomotic healing and has the potential to reduce overall mortality. These findings could provide guidance for clinicians to promote earlier recovery from EAL.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
These authors contributed equally and share the first authorship. All authors contributed to the article and approved the submitted version.
This study was supported by the grant from National Key R&D Program of China (2018YFC0910600). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Biere SS, Maas KW, Cuesta MA, van der Peet DL. Cervical or Thoracic Anastomosis After Esophagectomy for Cancer: A Systematic Review and Meta-Analysis. Dig Surg (2011) 28(1):29–35. doi: 10.1159/000322014
3. Law S, Fok M, Chu KM, Wong J. Comparison of Hand-Sewn and Stapled Esophagogastric Anastomosis After Esophageal Resection for Cancer: A Prospective Randomized Controlled Trial. Ann Surg (1997) 226(2):169–73. doi: 10.1097/00000658-199708000-00008
4. Saluja SS, Ray S, Pal S, Sanyal S, Agrawal N, Dash NR, et al. Randomized Trial Comparing Side-to-Side Stapled and Hand-Sewn Esophagogastric Anastomosis in Neck. J Gastrointestinal Surg Off J Soc Surg Aliment Tract (2012) 16(7):1287–95. doi: 10.1007/s11605-012-1885-7
5. Gonzalez R, Sarr MG, Smith CD, Baghai M, Kendrick M, Szomstein S, et al. Diagnosis and Contemporary Management of Anastomotic Leaks After Gastric Bypass for Obesity. J Am Coll Surg (2007) 204(1):47–55. doi: 10.1016/j.jamcollsurg.2006.09.023
6. Siddiqi S, Schraufnagel DP, Siddiqui HU, Javorski MJ, Mace A, Elnaggar AS, et al. Recent Advancements in the Minimally Invasive Management of Esophageal Perforation, Leaks, and Fistulae. Expert Rev Med Devices (2019) 16(3):197–209. doi: 10.1080/17434440.2019.1582329
7. Walther B, Johansson J, Johnsson F, Von Holstein CS, Zilling T. Cervical or Thoracic Anastomosis After Esophageal Resection and Gastric Tube Reconstruction: A Prospective Randomized Trial Comparing Sutured Neck Anastomosis With Stapled Intrathoracic Anastomosis. Ann Surg (2003) 238(6):803–12. doi: 10.1097/01.sla.0000098624.04100.b1
8. Alanezi K, Urschel JD. Mortality Secondary to Esophageal Anastomotic Leak. Ann Thorac Cardiovasc Surg Off J Assoc Thorac Cardiovasc Surg Asia (2004) 10(2):71–5.
9. Urschel JD. Esophagogastrostomy Anastomotic Leaks Complicating Esophagectomy: A Review. Am J Surg (1995) 169(6):634–40. doi: 10.1016/s0002-9610(99)80238-4
10. Lagarde SM, de Boer JD, ten Kate FJ, Busch OR, Obertop H, van Lanschot JJ. Postoperative Complications After Esophagectomy for Adenocarcinoma of the Esophagus are Related to Timing of Death Due to Recurrence. Ann Surg (2008) 247(1):71–6. doi: 10.1097/SLA.0b013e31815b695e
11. Parekh K, Iannettoni MD. Complications of Esophageal Resection and Reconstruction. Semin Thorac Cardiovasc Surg (2007) 19(1):79–88. doi: 10.1053/j.semtcvs.2006.11.002
12. Cools-Lartigue J, Andalib A, Abo-Alsaud A, Gowing S, Nguyen M, Mulder D, et al. Routine Contrast Esophagram has Minimal Impact on the Postoperative Management of Patients Undergoing Esophagectomy for Esophageal Cancer. Ann Surg Oncol (2014) 21(8):2573–9. doi: 10.1245/s10434-014-3654-1
13. Jones CM, Clarke B, Heah R, Griffiths EA. Should Routine Assessment of Anastomotic Integrity be Undertaken Using Radiological Contrast Swallow After Oesophagectomy With Intra-Thoracic Anastomosis? Best Evidence Topic (BET). Int J Surg (London England) (2015) 20:158–62. doi: 10.1016/j.ijsu.2015.06.076
14. Page RD, Asmat A, McShane J, Russell GN, Pennefather SH. Routine Endoscopy to Detect Anastomotic Leakage After Esophagectomy. Ann Thorac Surg (2013) 95(1):292–8. doi: 10.1016/j.athoracsur.2012.09.048
15. Nederlof N, de Jonge J, de Vringer T, Tran TC, Spaander MC, Tilanus HW, et al. Does Routine Endoscopy or Contrast Swallow Study After Esophagectomy and Gastric Tube Reconstruction Change Patient Management? J Gastrointestinal Surg Off J Soc Surg Aliment Tract (2017) 21(2):251–8. doi: 10.1007/s11605-016-3268-y
16. Song P, Li J, Zhang Q, Gao S. Ultrathin Endoscopy Versus Computed Tomography in the Detection of Anastomotic Leak in the Early Period After Esophagectomy. Surg Oncol (2020) 32:30–4. doi: 10.1016/j.suronc.2019.10.019
17. Maish MS, DeMeester SR, Choustoulakis E, Briel JW, Hagen JA, Peters JH, et al. The Safety and Usefulness of Endoscopy for Evaluation of the Graft and Anastomosis Early After Esophagectomy and Reconstruction. Surg Endoscopy (2005) 19(8):1093–102. doi: 10.1007/s00464-004-8816-y
18. Griffin SM, Shaw IH, Dresner SM. Early Complications After Ivor Lewis Subtotal Esophagectomy With Two-Field Lymphadenectomy: Risk Factors and Management. J Am Coll Surg (2002) 194(3):285–97. doi: 10.1016/s1072-7515(01)01177-2
19. Early DS, Ben-Menachem T, Decker GA, Evans JA, Fanelli RD, Fisher DA, et al. Appropriate Use of GI Endoscopy. Gastrointestinal Endoscopy (2012) 75(6):1127–31. doi: 10.1016/j.gie.2012.01.011
20. Paspatis GA, Arvanitakis M, Dumonceau JM, Barthet M, Saunders B, Turino SY, et al. Diagnosis and Management of Iatrogenic Endoscopic Perforations: European Society of Gastrointestinal Endoscopy (Esge) Position Statement - Update 2020. Endoscopy (2020) 52(9):792–810. doi: 10.1055/a-1222-3191
21. Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, D'Journo XB, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (Eccg). Ann Surg (2015) 262(2):286–94. doi: 10.1097/sla.0000000000001098
22. Dafni U. Landmark Analysis At the 25-Year Landmark Point. Circ Cardiovasc Qual Outcomes (2011) 4(3):363–71. doi: 10.1161/circoutcomes.110.957951
23. Deharo P, Ducrocq G, Bode C, Cohen M, Cuisset T, Mehta SR, et al. Timing of Angiography and Outcomes in High-Risk Patients With non-St-Segment-Elevation Myocardial Infarction Managed Invasively: Insights From the TAO Trial (Treatment of Acute Coronary Syndrome With Otamixaban). Circulation (2017) 136(20):1895–907. doi: 10.1161/circulationaha.117.029779
24. De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional Flow Reserve-Guided PCI Versus Medical Therapy in Stable Coronary Disease. New Engl J Med (2012) 367(11):991–1001. doi: 10.1056/NEJMoa1205361
25. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. New Engl J Med (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088
26. Grimminger PP, Goense L, Gockel I, Bergeat D, Bertheuil N, Chandramohan SM, et al. Diagnosis, Assessment, and Management of Surgical Complications Following Esophagectomy. Ann New York Acad Sci (2018) 1434(1):254–73. doi: 10.1111/nyas.13920
27. Low DE. Diagnosis and Management of Anastomotic Leaks After Esophagectomy. J Gastrointestinal Surg Off J Soc Surg Aliment Tract (2011) 15(8):1319–22. doi: 10.1007/s11605-011-1511-0
28. Nishikawa K, Fujita T, Yuda M, Yamamoto S, Tanaka Y, Matsumoto A, et al. Early Postoperative Endoscopy for Targeted Management of Patients At Risks of Anastomotic Complications After Esophagectomy. Surgery (2016) 160(5):1294–301. doi: 10.1016/j.surg.2016.06.022
29. Junemann-Ramirez M, Awan MY, Khan ZM, Rahamim JS. Anastomotic Leakage Post-Esophagogastrectomy for Esophageal Carcinoma: Retrospective Analysis of Predictive Factors, Management and Influence on Longterm Survival in a High Volume Centre. Eur J Cardiothorac Surg Off J Eur Assoc Cardiothorac Surg (2005) 27(1):3–7. doi: 10.1016/j.ejcts.2004.09.018
30. van Workum F, van der Maas J, van den Wildenberg FJ, Polat F, Kouwenhoven EA, van Det MJ, et al. Improved Functional Results After Minimally Invasive Esophagectomy: Intrathoracic Versus Cervical Anastomosis. Ann Thorac Surg (2017) 103(1):267–73. doi: 10.1016/j.athoracsur.2016.07.010
31. Gonzalez JM, Servajean C, Aider B, Gasmi M, D’Journo XB, Leone M, et al. Efficacy of the Endoscopic Management of Postoperative Fistulas of Leakages After Esophageal Surgery for Cancer: A Retrospective Series. Surg Endoscopy (2016) 30(11):4895–903. doi: 10.1007/s00464-016-4828-7
32. Suzuki T, Siddiqui A, Taylor LJ, Cox K, Hasan RA, Laique SN, et al. Clinical Outcomes, Efficacy, and Adverse Events in Patients Undergoing Esophageal Stent Placement for Benign Indications: A Large Multicenter Study. J Clin Gastroenterol (2016) 50(5):373–8. doi: 10.1097/mcg.0000000000000500
33. Persson S, Rouvelas I, Kumagai K, Song H, Lindblad M, Lundell L, et al. Treatment of Esophageal Anastomotic Leakage With Self-Expanding Metal Stents: Analysis of Risk Factors for Treatment Failure. Endoscopy Int Open (2016) 4(4):E420–6. doi: 10.1055/s-0042-102878
34. van Halsema EE, van Hooft JE. Clinical Outcomes of Self-Expandable Stent Placement for Benign Esophageal Diseases: A Pooled Analysis of the Literature. World J Gastrointestinal Endoscopy (2015) 7(2):135–53. doi: 10.4253/wjge.v7.i2.135
35. Freeman RK, Ascioti AJ, Dake M, Mahidhara RS. An Analysis of Esophageal Stent Placement for Persistent Leak After the Operative Repair of Intrathoracic Esophageal Perforations. Ann Thorac Surg (2014) 97(5):1715–9. doi: 10.1016/j.athoracsur.2014.01.011
36. El H II, Imperiale TF, Rex DK, Ballard D, Kesler KA, Birdas TJ, et al. Treatment of Esophageal Leaks, Fistulae, and Perforations With Temporary Stents: Evaluation of Efficacy, Adverse Events, and Factors Associated With Successful Outcomes. Gastrointestinal Endoscopy (2014) 79(4):589–98. doi: 10.1016/j.gie.2013.08.039
37. Lee HL, Cho JY, Cho JH, Park JJ, Kim CG, Kim SH, et al. Efficacy of the Over-the-Scope Clip System for Treatment of Gastrointestinal Fistulas, Leaks, and Perforations: A Korean Multi-Center Study. Clin Endoscopy (2018) 51(1):61–5. doi: 10.5946/ce.2017.027
38. Haito-Chavez Y, Law JK, Kratt T, Arezzo A, Verra M, Morino M, et al. International Multicenter Experience With an Over-the-Scope Clipping Device for Endoscopic Management of GI Defects (With Video). Gastrointestinal Endoscopy (2014) 80(4):610–22. doi: 10.1016/j.gie.2014.03.049
39. Mennigen R, Colombo-Benkmann M, Senninger N, Laukoetter M. Endoscopic Closure of Postoperative Gastrointestinal Leakages and Fistulas With the Over-the-Scope Clip (Otsc). J Gastrointestinal Surg Off J Soc Surg Aliment Tract (2013) 17(6):1058–65. doi: 10.1007/s11605-013-2156-y
40. Dişibeyaz S, Köksal A, Parlak E, Torun S, Şaşmaz N. Endoscopic Closure of Gastrointestinal Defects With an Over-the-Scope Clip Device. A Case Series and Review of the Literature. Clinics Res Hepatol Gastroenterol (2012) 36(6):614–21. doi: 10.1016/j.clinre.2012.04.015
41. Voermans RP, Le Moine O, von Renteln D, Ponchon T, Giovannini M, Bruno M, et al. Efficacy of Endoscopic Closure of Acute Perforations of the Gastrointestinal Tract. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc (2012) 10(6):603–8. doi: 10.1016/j.cgh.2012.02.005
42. Baron TH, Song LM, Ross A, Tokar JL, Irani S, Kozarek RA. Use of an Over-the-Scope Clipping Device: Multicenter Retrospective Results of the First U.S. Experience (With Videos). Gastrointestinal Endoscopy (2012) 76(1):202–8. doi: 10.1016/j.gie.2012.03.250
43. Laukoetter MG, Mennigen R, Neumann PA, Dhayat S, Horst G, Palmes D, et al. Successful Closure of Defects in the Upper Gastrointestinal Tract by Endoscopic Vacuum Therapy (EVT): A Prospective Cohort Study. Surg Endoscopy (2017) 31(6):2687–96. doi: 10.1007/s00464-016-5265-3
44. Bludau M, Hölscher AH, Herbold T, Leers JM, Gutschow C, Fuchs H, et al. Management of Upper Intestinal Leaks Using an Endoscopic Vacuum-Assisted Closure System (E-VAC). Surg Endoscopy (2014) 28(3):896–901. doi: 10.1007/s00464-013-3244-5
45. Schniewind B, Schafmayer C, Voehrs G, Egberts J, von Schoenfels W, Rose T, et al. Endoscopic Endoluminal Vacuum Therapy is Superior to Other Regimens in Managing Anastomotic Leakage After Esophagectomy: A Comparative Retrospective Study. Surg Endoscopy (2013) 27(10):3883–90. doi: 10.1007/s00464-013-2998-0
46. Wedemeyer J, Brangewitz M, Kubicka S, Jackobs S, Winkler M, Neipp M, et al. Management of Major Postsurgical Gastroesophageal Intrathoracic Leaks With an Endoscopic Vacuum-Assisted Closure System. Gastrointestinal Endoscopy (2010) 71(2):382–6. doi: 10.1016/j.gie.2009.07.011
47. Ahrens M, Schulte T, Egberts J, Schafmayer C, Hampe J, Fritscher-Ravens A, et al. Drainage of Esophageal Leakage Using Endoscopic Vacuum Therapy: A Prospective Pilot Study. Endoscopy (2010) 42(9):693–8. doi: 10.1055/s-0030-1255688
48. Kotzampassi K, Eleftheriadis E. Tissue Sealants in Endoscopic Applications for Anastomotic Leakage During a 25-Year Period. Surgery (2015) 157(1):79–86. doi: 10.1016/j.surg.2014.06.002
49. Lippert E, Klebl FH, Schweller F, Ott C, Gelbmann CM, Schölmerich J, et al. Fibrin Glue in the Endoscopic Treatment of Fistulae and Anastomotic Leakages of the Gastrointestinal Tract. Int J Colorectal Dis (2011) 26(3):303–11. doi: 10.1007/s00384-010-1104-5
50. Karvonen JA, Grönroos JM, Nikulainen V, Gullichsen R, Salminen P. Endoscopic Treatment of Internal Gastrointestinal Fistulas With Fibrin Glue. Surg Laparosc Endoscopy Percutan Tech (2013) 23(1):37–40. doi: 10.1097/SLE.0b013e318277d3cb
51. Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW. Endoscopic Vacuum-Assisted Closure of Anastomotic Leakage Following Anterior Resection of the Rectum: A New Method. Surg Endoscopy (2008) 22(8):1818–25. doi: 10.1007/s00464-007-9706-x
52. Mennigen R, Harting C, Lindner K, Vowinkel T, Rijcken E, Palmes D, et al. Comparison of Endoscopic Vacuum Therapy Versus Stent for Anastomotic Leak After Esophagectomy. J Gastrointestinal Surg Off J Soc Surg Aliment Tract (2015) 19(7):1229–35. doi: 10.1007/s11605-015-2847-7
Keywords: esophageal cancer, anastomotic leak, endoscopic intervention, clips, sealants, perioperative complications
Citation: Zhong L, Zhong J, Tan Z, Wei Y, Su X, Wen Z, Rong T, Hu Y and Luo K (2021) An Approach to Accelerate Healing and Shorten the Hospital Stay of Patients With Anastomotic Leakage After Esophagectomy: An Explorative Study of Systematic Endoscopic Intervention. Front. Oncol. 11:657955. doi: 10.3389/fonc.2021.657955
Received: 29 January 2021; Accepted: 19 April 2021;
Published: 17 May 2021.
Edited by:
Alberto Di Leo, Ospedale San Camillo, ItalyReviewed by:
Luigi Bonavina, University of Milan, ItalyCopyright © 2021 Zhong, Zhong, Tan, Wei, Su, Wen, Rong, Hu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Hu, aHV5aXNjaUAxNjMuY29t; KongJia Luo, bHVva2pAc3lzdWNjLm9yZy5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.