
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 17 March 2021
Sec. Pharmacology of Anti-Cancer Drugs
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.655856
This article is part of the Research TopicClinical Therapeutic Development Against Cancers Resistant to Targeted TherapiesView all 31 articles
 Xiao-Hong Xie1,2†
Xiao-Hong Xie1,2† Ze-Jiang Zhan2,3†
Ze-Jiang Zhan2,3† Yin-Yin Qin1,2
Yin-Yin Qin1,2 Ju-Hong Jiang2,4
Ju-Hong Jiang2,4 Wei-Qiang Yin2,5
Wei-Qiang Yin2,5 Rong-Hui Zheng2,3
Rong-Hui Zheng2,3 Shi-Yue Li1,2
Shi-Yue Li1,2 Cheng-Zhi Zhou1,2*
Cheng-Zhi Zhou1,2*The treatment of anaplastic lymphoma kinase (ALK)-positive locally advanced non-small-cell lung cancer (NSCLC) is challenging because there is no randomized controlled trial has been reported. The value of neoadjuvant and adjuvant targeted therapy remains unclear. Herein, we show that systemic treatment with ALK inhibitor crizotinib before surgery can provide the potential to cure the initially inoperable tumor. A 27-year-old man was diagnosed with a stage IIIAcT3N2M0 (7thUICC/AJCC) upper left lung adenocarcinoma harboring EML4-ALK fusion gene. Clinically, the patient had a large primary lesion adjacent to the pericardium and regional lymph node metastasis at the ipsilateral mediastinum. Poor tumor response was observed after 3 cycles of chemotherapy (gemcitabine plus cisplatin), and upon multidisciplinary discussion, the patient was started with 250 mg crizotinib twice daily. Successive clinical examinations showed a progressive reduction of the lesions. After 2 months of therapy, the patient was downstaged to cT2aN2M0, then video-assisted thoracic surgery was performed and the final histopathological stage was ypT2aN2M0. The treatment with crizotinib (250 mg, qd) was continued more than 30 months post surgery and stopped until intracranial oligometastasis. The patient’s overall survival (OS) time is 68 months at last follow-up. This case presented here supports the use of neoadjuvant and adjuvant treatment with ALK inhibitors in ALK positive locally advanced NSCLC.
According to the report of the International Agency for Research on Cancer, 2,093,876 cases of lung cancer were newly diagnosed worldwide in 2018, accounting for 11.6% of newly diagnosed cancers in the same year (1). Approximately 85% of lung cancers are classified as non-small-cell lung cancer (NSCLC), and 30% of which are defined as stage III at diagnosis (2, 3).
Stage III NSCLC is considered highly heterogeneous. Most patients have lost the opportunity for radical surgery at the time of initial diagnosis. The overall clinical efficacy of treatment for stage III NSCLC is not satisfactory, with 5-year survival rates of only 13~36% despite multimodality treatments (4, 5). In recent years, as small-molecule tyrosine kinase inhibitors (TKIs) have been widely used in patients with advanced NSCLC with genetic mutations, the survival outcomes of patients have become highly promising, and the benefits are obvious (6–9), reflecting the need to explore the application of small-molecule TKIs in locally advanced NSCLC with genetic mutations.
For locally advanced NSCLC with epidermal growth factor receptor (EGFR) mutation, previous studies (10–12) have focused on the application of neoadjuvant and adjuvant targeted therapy, and the results are promising. Among these patients, neoadjuvant targeted therapy is significantly superior to chemotherapy, and the regression of the primary tumor is more pronounced, rendering patients more likely to be eligible for radical surgery (10–12). Adjuvant targeted therapy offers a convenient treatment option, and patients can also benefit from survival outcomes (10). However, no randomized controlled trial for patients with anaplastic lymphoma kinase (ALK)-positive locally advanced NSCLC has been reported. The value of neoadjuvant and adjuvant targeted therapy remains unclear and requires further exploration. This case report describes initial crizotinib use as neoadjuvant and adjuvant targeted therapy for an ALK-positive locally advanced NSCLC patient.
In April 2015, a 27-year-old male who presented with cough and phlegm was diagnosed with left upper lung adenocarcinoma by biopsy, with a performance status score of 1. The histological features were characterized by solid growth with mucus production (60%) and acinar growth (40%). The patient’s clinical stage was cT3N2M0 (stage IIIA, 7th UICC/AJCC). After preoperative evaluation, the initial tumor was considered unresectable since it was adjacent to the pericardium, and regional lymph node metastasis at the ipsilateral mediastinum was observed.
Three cycles of gemcitabine (1250mg/m2, day 1 and day 8) plus cisplatin (75 mg/m2, day 1) were given after diagnosis. Simultaneously, the positive ALK expression was detected by Ventana immunohistochemistry (IHC) staining (Figure 1). Contrast chest computed tomography (CT) after 3 cycles of gemcitabine plus cisplatin showed that the primary tumor remained stable according to the RECIST 1.1 criteria compared with the initial CT scan (Figures 2A, B). Thereafter, crizotinib (250 mg, bid) was adopted, replacing the initial treatment. Encouragingly, the patient was downstaged to cT2aN2M0 within 2 months (Figure 2C).
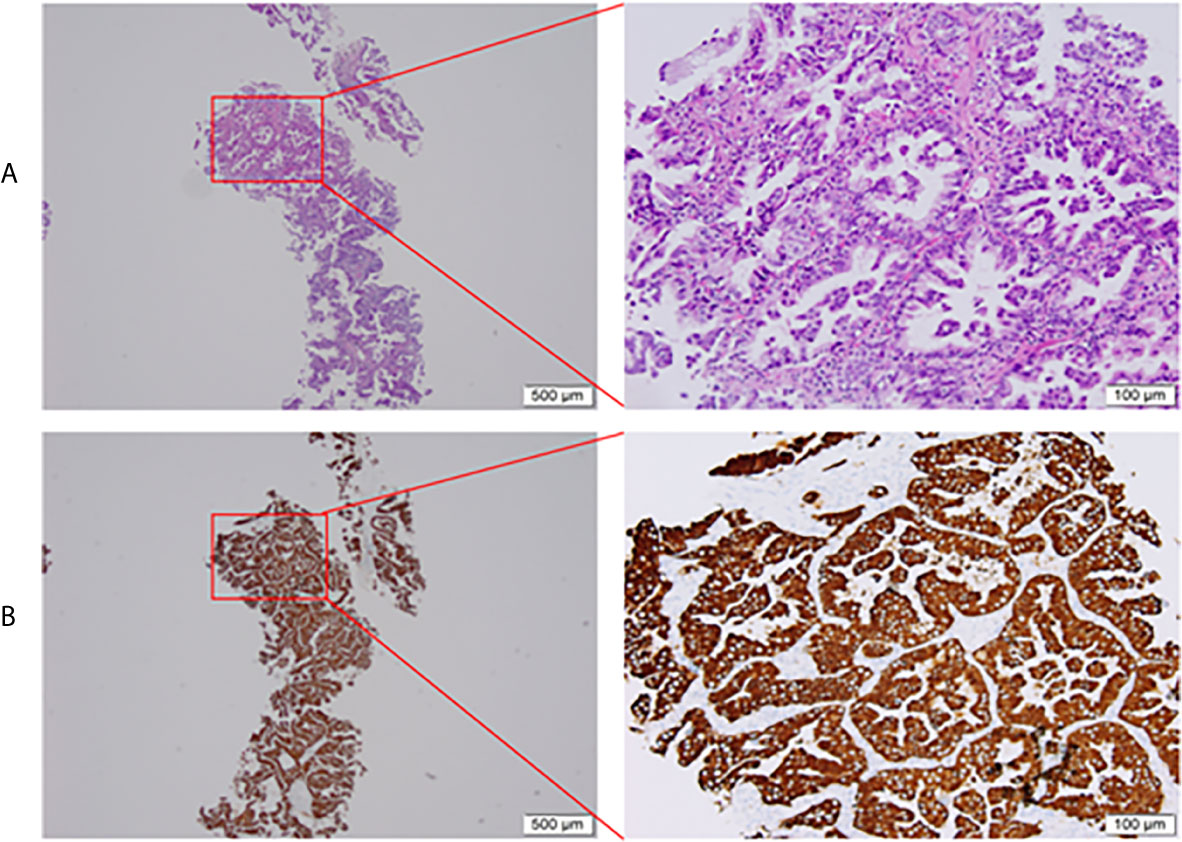
Figure 1 Pretreatment biopsy. (A) Histological aspect of lung adenocarcinoma (HE staining), (B) Intense cytoplasmic ALK protein expression on immunohistochemistry. HE, hematoxylin-eosin; ALK, anaplastic lymphoma kinase.
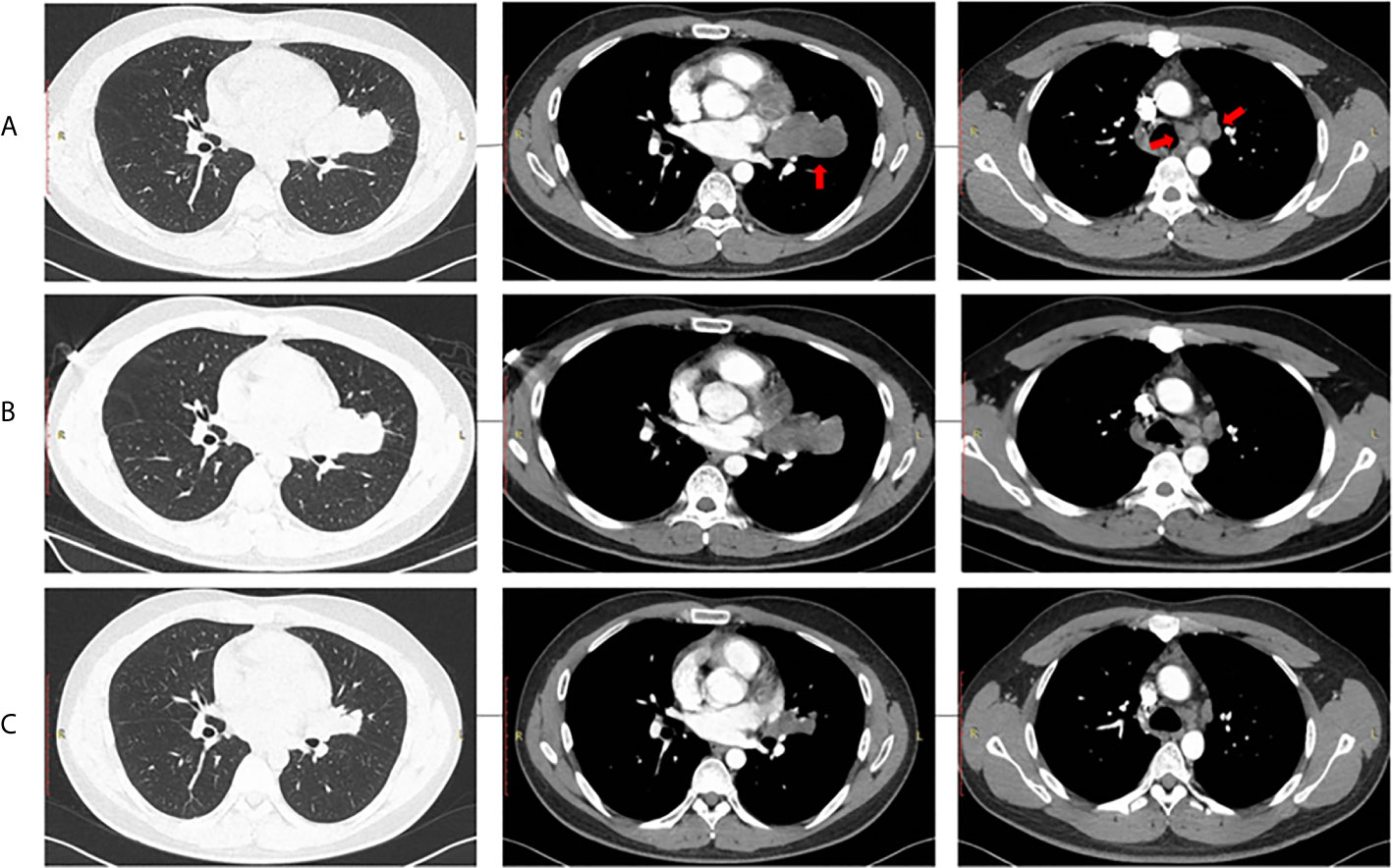
Figure 2 Radiological evaluation of the primary tumor and lymph nodes. (A) Baseline, (B) After 3 chemotherapy cycles, (C) After 2 months of crizotinib treatment.
In September 2015, video-assisted thoracic surgery (VATS) with left upper lobectomy plus lymph node dissection and partial pericardial resection was performed. The pathological stage was ypT2aN2M0. Then, postoperative radiotherapy (PORT) was performed with a total dose of 50 Gy in 25 fractions. Given the limitation of IHC detection, genomic sequencing was performed for the patient’s resected tumor tissue sample. Next-generation sequencing (NGS) analysis was carried out using a commercially available capture-based targeted sequencing panel, targeting 8 genes (ALK, BRAF, EGFR, ERBB2, KRAS, MET, RET and ROS1) (Burning Rock Biotech, Guangzhou, China). EML4-ALK (E13:A20) fusion oncogene was found by NGS assay. Crizotinib (250 mg, qd) was administered as adjuvant targeted therapy until an intracranial oligometastasis was first detected in July 2018, with a first progression-free survival (PFS 1) time of 39 months. Subsequently, stereotactic radiosurgery (SRS) of the intracranial oligometastasis followed by crizotinib treatment (250 mg, bid) was performed. Five months after SRS, the intracranial oligometastasis showed a complete response based on the RECIST 1.1 criteria (Figure 3). At the final follow-up in December 2020, no grade 3/4 adverse events or disease progression had occurred. Remarkably, a second PFS time has not been reached. The patient’s overall survival (OS) time is currently 68 months.
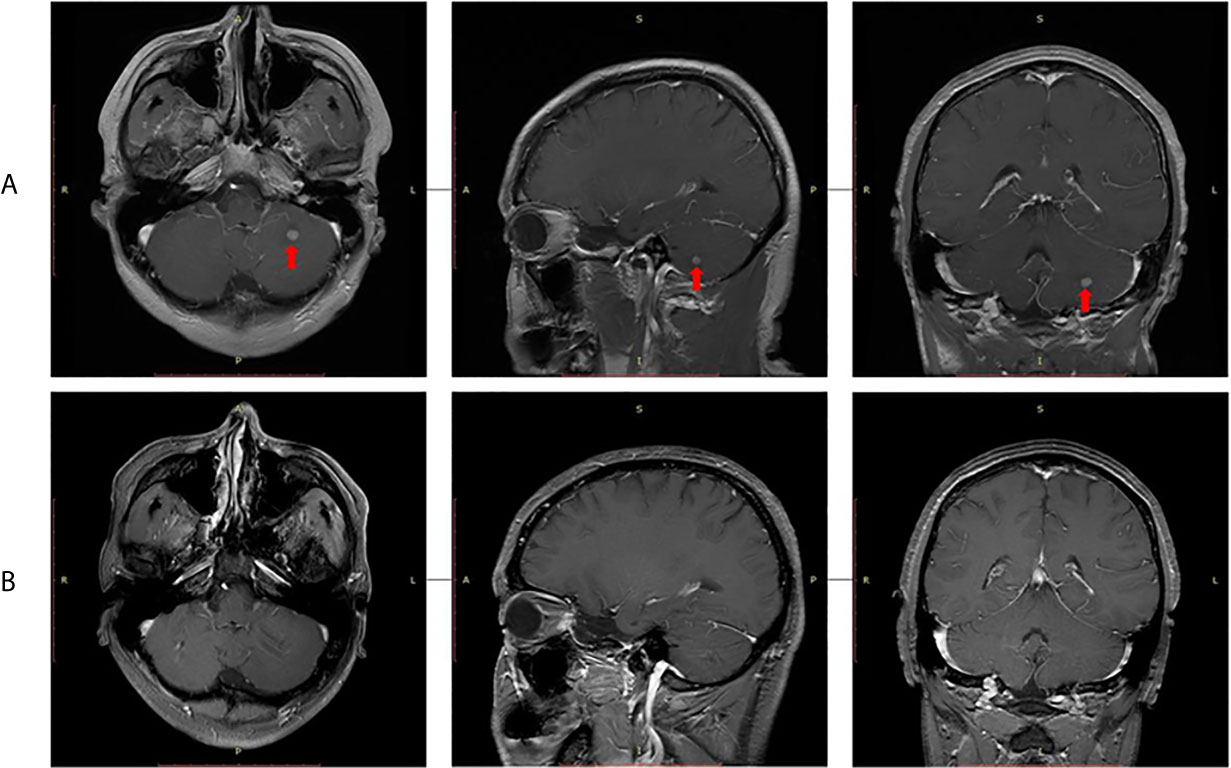
Figure 3 Radiological evaluation of intracranial oligometastasis. (A) Initial detection, (B) Five months after stereotactic radiosurgery.
Based on NCCN guidelines, stage III NSCLC patients should receive multimodality therapies. Radical resection is recommended for operable patients. Potentially operable patients can gain opportunities for radical resection through neoadjuvant therapies including chemotherapy, radiotherapy, or a combination of both. Additionally, concurrent radical chemoradiotherapy followed by immunotherapy maintenance is preferred for inoperable patients. Notably, for locally advanced NSCLC patients with gene mutations, the NCCN guidelines do not indicate whether targeted therapy is superior to traditional chemotherapy as neoadjuvant or adjuvant treatment. This case report describes the application of neoadjuvant and adjuvant targeted therapy in a patient with stage IIIA-N2 ALK-positive NSCLC for the first time.
Approximately 7% of NSCLCs have ALK gene fusions, and EML4 is the most common ALK fusion partner, accounting for 90~95% of ALK rearrangements (13). A variety of rare ALK fusion partners, such as KIF5B, KLC1, TFG, TPR, HIP1, STRN, DCTN1, SQSTM1, NPM1, BCL11A and BIRC6, have also been detected, summarized in a study by Du Xue et al. and colleagues (14). EML4-ALK is further divided into diverse fusion subtypes, among which V1 (E13:A20) and V3 (E6:A20) account for the highest proportion, both at approximately 32%, and other EML4-ALK fusion subtypes are relatively rare (each accounts for less than 10%) (13). The identification of these mutations enables target-specific therapies. The application of ALK inhibitors indeed prolongs patient survival; however, a certain proportion of patients will inevitably acquire resistance to ALK inhibitors (15, 16). Therefore, it is of great significance to identify predictors of response or resistance to ALK inhibitors. In addition, NGS of dynamic plasma or tissue gene mutation abundance detection is effective in predicting the response to ALK inhibitors, as illustrated by Zhang Chao et al. (17). Hence, future clinical research in the era of precision targeted therapy should take dynamic NGS into account.
Crizotinib is an oral small-molecule TKI of ALK, MET, and ROS1 kinases (18) and is a Category 1 recommendation for advanced ALK-positive NSCLC according to the NCCN guidelines since previous studies have demonstrated its superior efficacy over chemotherapy (9, 19). In a profile 1014 study (9), crizotinib was associated with better progression-free survival (PFS) compared to pemetrexed plus cisplatin or carboplatin chemotherapy in patients with advanced ALK-positive NSCLC (median, 10.9 months vs. 7.0 months; P<0.001). A similar outcome was also observed in a profile 1029 study (20). Thus, the potential clinical benefits of targeted therapy adoption for ALK-positive locally advanced NSCLC should also be explored. In this case, the patient was not sensitive to first-line chemotherapy with gemcitabine plus cisplatin and was therefore switched to crizotinib based on his gene mutations, which enabled radical surgery. Kaoru Kaseda et al. and colleagues (21) reported the first case of crizotinib application before surgical resection in ALK-positive NSCLC and showed promising disease reduction. Zhang et al. showed that neoadjuvant crizotinib can achieve complete resection (22). Moreover, the SAKULA trial demonstrated that ceritinib contributed to a high pathologic response rate in ALK-positive resectable locally advanced NSCLC (23). Dumont and colleagues (24) explored crizotinib as a second-line neoadjuvant treatment when chemotherapy failed to achieve satisfactory regression of the tumor burden and observed no disease recurrence at 18 months post-surgery. However, whether the benefit of neoadjuvant ALK inhibitors can transform into long-term survival is controversial. In the present case, unlike the studies of Kaoru Kaseda and Delphine Dumont, a long-term follow-up of 68 months was performed (21, 24).
Previous studies (25, 26) have demonstrated that stage N2 NSCLC patients benefit from PORT. However, in the CTONG 1103 trial (10), PORT was not administered. Nevertheless, PORT was adopted for this patient. In terms of adjuvant targeted therapy, earlier trials (11, 12) explored the application of oral TKIs for EGFR-positive NSCLC patients, and the results were encouraging. ADJUVANT (11) is the first prospective study comparing gefitinib with vinorelbine plus cisplatin as adjuvant treatments in patients with EGFR-positive stage II-IIIA (N1-N2) NSCLC, which showed that gefitinib significantly prolongs PFS (median, 28.7 months vs.18.0 months; P = 0.0054). The EVAN study (12) explored erlotinib vs. vinorelbine plus cisplatin as adjuvant treatments in patients with EGFR-positive stage IIIA NSCLC and demonstrated substantially higher 2-year PFS rates of 81.2% in the erlotinib group and 44.6% in the chemotherapy group with oral TKI administration (P<0.001), reflecting the reliability of adopting oral TKIs as adjuvant treatment for certain groups. No relevant study has described ALK-positive NSCLC patients in this setting. Ultimately, the patient was given adjuvant crizotinib targeted therapy and has achieved considerable efficacy in terms of both PFS and OS, with a PFS 1 of 39 months and an OS of 68 months thus far. Notably, clinical stage IIIB NSCLC is associated with a poor survival outcome, with a median survival time of approximately 14.1 months (5), which is substantially shorter than the PFS 1 of 39 months in this case.
To date, this is the first case of stage IIIA-N2 ALK-positive NSCLC treated with neoadjuvant and adjuvant targeted therapy combined with surgery and PORT to show remarkable clinical efficacy. Further exploration of this treatment model for stage III ALK-positive NSCLC is urgently needed.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
1. Guarantor of integrity of the entire study: C-ZZ. 2. Study concepts and design: Y-YQ, J-HJ. 3 Literature research: W-QY, R-HZ, S-YL. 4. Manuscript preparation: X-HX, Z-JZ. 5. Manuscript editing: C-ZZ. All authors contributed to the article and approved the submitted version.
This report was supported by grants from the State Key Laboratory of Respiratory Disease- The open project [grant number: SKLRD-OP-2018011], the State Key Laboratory of Respiratory Disease- The Independent project [grant number: SKLRD-QN-201720], Wu Jieping Fund-Ministry of Health Project [grant number: 320.6750.18125], Guangdong High Level University Clinical Cultivation Project [grant number: 2017-21020].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin (2012) 62:220–41. doi: 10.3322/caac.21149
3. van Meerbeeck JP. Staging of non-small cell lung cancer: consensus, controversies and challenges. Lung Cancer (2001) 34(Suppl 2):S95–S107. doi: 10.1016/S0169-5002(01)00356-7
4. Garrido P, Rosell R, Massutí B, Cardenal F, Alberola V, Dómine M, et al. Predictors of Long-Term Survival in Patients with Lung Cancer Included in the Randomized Spanish Lung Cancer Group 0008 Phase II Trial Using Concomitant Chemoradiation with Docetaxel and Carboplatin plus Induction or Consolidation Chemotherapy. Clin Lung Cancer (2009) 10:180–6. doi: 10.3816/CLC.2009.n.025
5. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
6. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol (2012) 13:239–46. doi: 10.1016/S1470-2045(11)70393-X
7. Zhou C, Wu Y-L, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol (2011) 12:735–42. doi: 10.1016/S1470-2045(11)70184-X
8. Soria J-C, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in UntreatedEGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N Engl J Med (2018) 378:113–25. doi: 10.1056/NEJMoa1713137
9. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med (2014) 371:2167–77. doi: 10.1056/NEJMoa1408440
10. Zhong WZ, Chen KN, Chen C, Gu CD, Wang J, Yang XN, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non-small-cell lung cancer (EMERGING-CTONG 1103): A randomized phase II study. J Clin Oncol (2019) 37(25):2235–45. doi: 10.1200/JCO.19.00075
11. Zhong W-Z, Wang Q, Mao W-M, Xu ST, Wu L, Shen Y, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1–N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol (2018) 19:139–48. doi: 10.1016/S1470-2045(17)30729-5
12. Yue D, Xu S, Wang Q, Li X, Shen Y, Zhao H, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): a randomised, open-label, phase 2 trial. Lancet Resp Med (2018) 6:863–73. doi: 10.1016/S2213-2600(18)30277-7
13. Lin JJ, Zhu VW, Yoda S, Yeap BY, Schrock AB, Dagogo-Jack I, et al. Impact of EML4-ALK Variant on Resistance Mechanisms and Clinical Outcomes in ALK-Positive Lung Cancer. J Clin Oncol (2018) 36:1199–206. doi: 10.1200/JCO.2017.76.2294
14. Du X, Shao Y, Qin HF, Tai YH, Gao HJ. ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac Cancer (2018) 9:423–30. doi: 10.1111/1759-7714.12613
15. Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, et al. Mechanisms of Acquired Crizotinib Resistance in ALK-Rearranged Lung Cancers. Sci Trans Med (2012) 4:1–12. doi: 10.1158/1538-7445.AM2012-5593
16. Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res (2012) 18:1472–82. doi: 10.1158/1078-0432.CCR-11-2906
17. Gower A, Wang Y, Giaccone G. Oncogenic drivers, targeted therapies, and acquired resistance in non-small-cell lung cancer. J Mol Med (Berl) (2014) 92:697–707. doi: 10.1007/s00109-014-1165-y
18. Christensen JG, Zou HY, Arango ME, Li L, Lee JH, McDonnell SR, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther (2007) 6:3314–22. doi: 10.1158/1535-7163.MCT-07-0365
19. Frampton JE. Crizotinib: A Review of Its Use in the Treatment of Anaplastic Lymphoma Kinase-Positive, Advanced Non-Small Cell Lung Cancer. Drugs (2013) 73:2031–51. doi: 10.1007/s40265-013-0142-z
20. Wu Y-L, Lu S, Lu Y, Zhou J, Shi YK, Sriuranpong V, et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non–Small Cell Lung Cancer. J Thorac Oncol (2018) 13:1539–48. doi: 10.1016/j.jtho.2018.06.012
21. Kaseda K, Watanabe K, Asakura K, Kazama A. Surgical resection of lung adenocarcinoma after crizotinib treatment: report of a case. World J Surg Oncol (2015) 13:74. doi: 10.1186/s12957-015-0480-2
22. Zhang C, Li SL, Nie Q, Dong S, Shao Y, Yang XN, et al. Neoadjuvant Crizotinib in Resectable Locally Advanced Non-Small Cell Lung Cancer with ALK Rearrangement. J Thorac Oncol (2019) 14:726–31. doi: 10.1016/j.jtho.2018.10.161
23. Zenke Y, Yoh K, Sakakibara-Konishi J, Daga H, Hosomi Y, Nogami N, et al. P1.18-04 Neoadjuvant Ceritinib for Locally Advanced Non-Small Cell Lung Cancer with ALK Rearrangement: SAKULA Trial. J Thorac Oncol (2019) 14:S626–27. doi: 10.1016/j.jtho.2019.08.1320
24. Dumont D, Do P, Lerouge D, Planchard G, Riffet M, Dubos-Arvis C, et al. Off-Label Use of Crizotinib as a Neoadjuvant Treatment for a Young Patient When Conventional Chemotherapy Gave No Benefits in Stage IIIA Non-Small Cell Lung Cancer. Am J Case Rep (2017) 18:890–3. doi: 10.12659/AJCR.903528
25. Corso CD, Rutter CE, Wilson LD, Kim AW, Decker RH, Husain ZA. Re-evaluation of the Role of Postoperative Radiotherapy and the Impact of Radiation Dose for Non–Small-Cell Lung Cancer Using the National Cancer Database. J Thorac Oncol (2015) 10:148–55. doi: 10.1097/JTO.0000000000000406
26. Mikell JL, Gillespie TW, Hall WA, Nickleach DC, Liu Y, Lipscomb J, et al. Postoperative radiotherapy is associated with better survival in non-small cell lung cancer with involved N2 lymph nodes: results of an analysis of the National Cancer Data Base. J Thorac Oncol (2015) 10:462–71. doi: 10.1097/JTO.0000000000000411
Keywords: locally advanced non-small-cell lung cancer, ALK inhibitor, neoadjuvant, adjuvant, targeted therapy
Citation: Xie X-H, Zhan Z-J, Qin Y-Y, Jiang J-H, Yin W-Q, Zheng R-H, Li S-Y and Zhou C-Z (2021) Case Report: Neoadjuvant and Adjuvant Crizotinib Targeted Therapy in Stage IIIA-N2 ALK-Positive Non-Small-Cell Lung Cancer. Front. Oncol. 11:655856. doi: 10.3389/fonc.2021.655856
Received: 19 January 2021; Accepted: 26 February 2021;
Published: 17 March 2021.
Edited by:
Fanfan Zhou, The University of Sydney, AustraliaReviewed by:
Rafael Rosell, Catalan Institute of Oncology, SpainCopyright © 2021 Xie, Zhan, Qin, Jiang, Yin, Zheng, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng-Zhi Zhou, emhvdWNoZW5nemhpQGdpcmQuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.