
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 22 March 2021
Sec. Hematologic Malignancies
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.654854
 Huan Chen1†
Huan Chen1† Tao Pan1,2†
Tao Pan1,2† Yizi He1
Yizi He1 Ruolan Zeng1
Ruolan Zeng1 Yajun Li1
Yajun Li1 Liming Yi3
Liming Yi3 Hui Zang4
Hui Zang4 Siwei Chen5
Siwei Chen5 Qintong Duan5
Qintong Duan5 Ling Xiao5*
Ling Xiao5* Hui Zhou1*
Hui Zhou1*Primary mediastinal large B-cell lymphoma (PMBCL) is a distinct clinicopathologic disease from other types of diffuse large B-cell lymphoma (DLBCL) with unique prognostic features and limited availability of clinical data. The current standard treatment for newly diagnosed PMBCL has long been dependent on a dose-intensive, dose-adjusted multi-agent chemotherapy regimen of rituximab plus etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (DA-R-EPOCH). Recent randomized trials have provided evidence that R-CHOP followed by consolidation radiotherapy (RT) is a valuable alternative option to first-line treatment. For recurrent/refractory PMBCL (rrPMBCL), new drugs such as pembrolizumab and CAR-T cell therapy have proven to be effective in a few studies. Positron emission tomography-computed tomography (PET-CT) is the preferred imaging modality of choice for the initial phase of lymphoma treatment and to assess response to treatment. In the future, baseline quantitative PET-CT can be used to predict prognosis in PMBCL. This review focuses on the pathology of PMBCL, underlying molecular basis, treatment options, radiotherapy, targeted therapies, and the potential role of PET-CT to guide treatment choices in this disease.
Primary mediastinal large B-cell lymphoma (PMBCL) is a rare subtype of non-Hodgkin lymphoma (NHL), but it shares histologic features with nodular sclerosing Hodgkin lymphoma (NSHL) (1, 2). Due to the distinct clinical, morphological, and immunophenotypic characteristics, it is recognized as a unique entity in the latest World Health Organization classification of lymphoid tumors (3). Optimal treatment is not fully defined and there is no single standard of care. However, several retrospective studies have found that patients with PMBCL have a high survival rate with chemotherapy using dose-adjusted multi-agent chemotherapy regimen of rituximab plus etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (DA-R-EPOCH) or a combination of rituximab and CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone [R-CHOP]) (4). The survival rate of PMBCL is significantly higher than diffuse large B-cell lymphoma (DLBCL) (5). Over the past 20 years, several studies have been carried out to understand its clinical behavior and optimize its management.
PMBCL accounts for 2% to 4% of all NHL. The incidence of the disease in women is higher than in men (4, 6, 7). In more than two-thirds of cases, it appears as a large anterior mediastinal mass, often accompanied by superior vena cava syndrome and airway obstruction (8). Approximately one-third of patients present B symptoms (fever, weight loss, night sweats). Additional, clinical features consist of pleural and pericardial effusions, elevated serum lactate dehydrogenase (LDH) concentrations, and a trend toward recurrence at uncommon sites. Patients often present with cough and bout a quarter of patients present with advanced disease (9). The primary appearance can be nodular or extranodal; however, recurrences are often extranodal and may involve the gastrointestinal tract, liver, kidneys, and ovaries (10). Bone marrow involvement has been observed in only 1–5% of cases. Central nervous system (CNS) involvement in the form of leptomeningeal or intraparenchymal disease is uncommon and is especially seen in extranodal disease and in recurrences (11, 12). In addition to the above common symptoms, chylous pleural and pericardial effusions are present in some children (13). Moreover, PMBCL cases have been found in non-mediastinal areas, which makes the correct diagnosis of PMBCL difficult (14, 15). The poor prognostic characteristics of patients treated with R-CHOP include higher International Prognostic Index (IPI) score, advanced stage, advanced age, and multiple extranodal sites (16, 17).
PMBCL is an aggressive B-cell lymphoma that arises in the mediastinum and has a putative thymic B-cell origin. Tumor cells have an immunophenotype compatible with B-cell characteristics, such as the positive expression of CD20, CD45, CD79a, while the expression of cellular immunoglobulins, HLA I/II, CD5, CD3, CD21, and CD10 is negative. Furthermore, CD30 expression is observed in over 80% of PMBCL, although it is weaker and more heterogeneous than that observed in classical Hodgkin Lymphoma (cHL) (18–20). Tumor cells are usually positive for nuclear transcription regulators such as BOB1, PU.1, OCT2, PAX5, BCL6, and IRF4. Bcl-6 protein has been detected in over 50% of tumor cells and is considered a favorable prognostic factor (21, 22) (Table 1).
PMBCL has unique genetic characteristics that affect the biological behavior of tumors. Studies defining the gene-expression profiles of PMBCL could help predict the prognosis of patients. However, sometimes it is difficult to make the final diagnosis of PMBCL, as it may overlap with other types of lymphoma. Compared with DLBCL, the identified driver genes were found to be significantly more frequently mutated in PMBCL, while only a restricted number of genes were significantly different between PMBCL and cHL (23–25). Recent studies have established that PMBCL pathobiology is reliant on molecular pathways involving REL, JAK-STAT, PD-L1/PD-L2, and Nuclear factor-κB (NFκB) (23). Through comprehensive genomic analysis, Mottok et al. found that highly duplicated oncogenic mutations in genes belonging to the JAK-STAT and NF-kB pathways (CIITA, CD58, B2M, CD274, PDCD1LG2) were closely associated with immune evasion (25). This study also highlighted the frequent mutations in ITPKB, MFHAS1, XPO1, and NFKBIE in PMBCL, and also found that multiple members of the pathway were frequently mutated in the IRF pathway (IRF2BP2, IRF4, IRF8) (25–27). Hao et al. found that disease-specific chromosome 9p24.1/JAK2 amplification increased JAK2 expression and activity in PMBCL (28). DD1 et al. reported that the programmed death ligand-1 (PD-L1) locus (9p24.1) was frequently and specifically rearranged in PMBCL (20%) when compared with DLBCL, follicular lymphoma (FL), and Hodgkin lymphoma (HL) among 571 different B-cell lymphomas samples (29). Gene expression profiling studies showed that tumor necrosis factor (TNF) family members and TRAF1 are overexpressed in PMBCL (23). In PMBCL, this overactivation leads to activation of downstream anti-apoptotic genes, activation of caspases, and transcription of cell cycle regulators, resulting in malignant proliferation (30). The two most common genetic changes in PMBCL are CIITA rearrangement and chromosome amplification of 9p24.1 (PDL1/PDL2) and 2p14 p16 (24, 31, 32). Steidl et al. found that the presence of CIITA rearrangement was significantly associated with shorter disease-specific survival rates (33, 34). Relapsing somatic mutations in the NF-kB and JAK-STAT6 signaling pathways lead to their abnormal activation and constituted a hallmark of the disease. Somatic IL4R mutations in PMBCL resulted in constitutive activation of JAK-STAT signaling (35). In fact, gene expression profiles of characteristic genes in HL and PMBCL indicated that PMBCL is one-third identical to NSHL. These characteristic genes are not expressed in other types of DLBCL, but high expression of PDL2 is common to both PMBCL and HL (24). Chapuy et al. analyzed the genomes of 37 newly diagnosed PMBCL patients. They identified nine major genetic drivers in each PMBCL patient. The most obvious mutant gene in this study was B2M, which encodes β2-microglobulin, and is responsible for the correct translocation of MHC class I molecules to the intracellular plasma membrane and is essential for the expression of endogenously degraded autoantigens and non-autoantigens (36, 37). In addition, ZNF217 mutations are often present in patients with PMBCL. These newly identified mutations may increase susceptibility to programmed cell death-1 (PD-1) receptor blockade, including high tumor mutational load, microsatellite instability, and an APOBEC mutational signature (36).
The use of gene expression profiles can develop more precise molecular diagnostics for PMBCL. Studies have identified molecular pathways of PMBCL dependent on REL, JAK-STAT, PD-L1/PD-L2, and NF-κB (38). These molecular pathways may become targets for rational therapeutic strategies for PMBCL (Figure 1).
The prognosis of most patients with PMBCL is very impressive as most patients respond to treatment, but some patients still present substantial sclerosis in the mediastinal after treatment, which is translated after therapy into the presence of residual tumor masses in most cases. Ordinary imaging studies such as computed tomography (CT) and X-ray imaging are unable to recognize residual lesions. Positron emission tomography-computed tomography using 2-deoxy-2-[18F] fluoro-D-glucose (PET-CT) can be used to distinguish between benign and malignant tumors. Therefore, when selecting tumor biopsy sites and staging, PET-CT is recommended over CT (39). According to Ujjani et al. PET-CT was highly accurate for detecting bone marrow involvement (BMI) at diagnosis in DLBCL and HL, and highly specific in FL at diagnosis and relapse. It can even detect BMI in patients with negative biopsy (40). Furthermore, RHM et al. investigated 56 patients and reported being able to discriminate between mediastinal Hodgkin’s disease and PMBCL by combining LDH levels with PET-CT findings (41).
In general, PET-CT imaging plays a central role in the disease staging and response assessment of lymphoma patients and has become an important part of successful treatment management strategies (39, 42). To avoid the lack of objectivity in the results of PET-CT, a five-point scale (Deauville criteria) was designed to assess the patient’s condition. The International Conference on Malignant Lymphomas Imaging Working Group considers negative PET-CT (Deauville scores 1–3) may represent a complete response (CR) in a lymphoma patient treated with standard therapy (43, 44).
The prospective IELSG-26 study reported using liver uptake as the cutoff for PET-CT positivity (the boundary of a score, 3 to 4) to distinguish between high failure risk and low failure risk, with 5-year progression-free survival (PFS) of 99% versus 68% (P<0.001) and the 5-year overall survival rate (OS) of 100% versus 83% (P<0.001) (45). The PET-CT scan findings of 88 patients with PMBCL who received immunochemotherapy and radiation therapy (RT) were reported in the IELSG-26 study. Patients with a complete metabolic response (Deauville score 3) did not progress after 5 years, confirming the value of the Lugano classification criteria in the assessment of PMBCL response after RT. After combining RT, the complete metabolic response (CMR) (Deauville score < 4) can be substantially increased (from 74% to 89%). Therefore, PET-CT can identify patients with a high risk of progression after RT (46). The PETAL trial reported that two doses of rituximab following six cycles of R-CHOP did not improve outcomes, according to PET-CT results (47). Several studies have proved the Deauville score of PET-CT can be used to guide the subsequent treatment of PMBCL, and even predict the survival rate after chemoimmunotherapy for PMBCL (45, 48, 49). The International Study Group of Extranodal Lymphoma (IELSG37) study found that the Deauville scoring standard was used, and the final PET-CT scan results were more consistent, which was also recognized by the Lugano classification standard (50). An observational retrospective single-center study reported the experience of PMBCL patients treated with the third-generation chemotherapy regimen R-MACOP-B (rituximab, methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin). After treatment with R-MACOP-B, 82.4% of the patients achieved CR and the 10-years OS was 82%. The PFS rate was 87.6% and the disease-free survival rate (DFS) was 90.5% (median follow-up 4 years). There was no statistically significant difference in the DFS between the two groups in the final observation group (PET-negative) for patients with PET-positive RT consolidation treatment: 90.7% and 90%, respectively (p = 0.85). The study’s results indicated that PET-CT could be used as a guide for patients to undergo RT consolidation therapy in the later disease stages, and it is also convenient to reduce the use of RT. Due to a lack of long-term follow-up data and prospective head-to-head trials, the study could not firmly establish the best treatment plan (51). Nonetheless, treatments must strike a balance between maximizing cure rates and minimizing long-term toxicity (52).
A recent study of 159 PMBCL patients with 94% of them receiving R-CHOP treatment showed that the 5-year time to progression (TTP) and OS of the entire cohort was 80% and 89%, respectively. A total of 113 patients underwent PET-CT scans: 63% were negative and 37% were positive, the 5-year TTP was 90% vs. 71% and the 5-year OS was 97% vs. 88%, respectively. For patients using the Deauville score PET-CT scan (n = 103), the percentage of PET-negative cases (Deauville score 1–3) was 91%. Moreover, the difference in 5-year TTP prognosis between Deauville score 4 and Deauville score 5, was 33% and 87%, respectively, P = 0.0002). This study clearly demonstrated that PMBCL patients could achieve considerable curative effects through the R-CHOP regimen, and using PET-CT adaptive therapy could reduce exposure to RT for most PET-negative patients (53). A limited number of patients Deauville score 4 in IELSG-26 study also achieved satisfactory outcomes (46). Filippi et al. performed a series of combined treatments on 51 patients with PMBCL and obtained similar results. The results showed that 17 patients with Deauville score 4 posterior branch had a good prognosis and no recurrence (54). These false-positive results were thought to be due to residual inflammation thymic rebound after mediastinal treatment or residual 18f-fluoro-D-glucose uptake may not have reflected persistent lymphoma (45, 46).
Despite the possibility of PET-CT substantially increasing the specificity, of diagnosis, there is the potential of false positivity in predicting prognostic. Newer studies are enhancing the prognosis value of PET-CT. For example, PET-CT baseline quantitative parameters, the maximum normalized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) are powerful predictors of PMBCL prognosis (55–57). Ceriani et al. determined that functional PET-CT parameters such as TLG could be used to predict patient outcomes. On multivariate analysis, only TLG retained a significant association with OS (P = 0.01) and PFS (P < 0.01). At 5 years, the OS of patients with low TLG was 100%, while the OS of patients with high TLG was 80% (P = 0.001), while the PFS was 99% and 64%, respectively (P < 0.001) (58). Similar results can be reflected in the study by Pinnix et al. A retrospective analysis was performed in 65 newly diagnosed patients with PMBCL. These patients were evaluated by PET-CT during first-line treatment with DA-R-EPOCH. Evaluation factors included evaluation of MTV and TLG. The median follow-up time was 36.6 months (95% confidence interval [CI] = 28.1–45.1). The 2-year PFS and OS rates of these 65 patients were 81.4% and 98.4%, respectively. The statistical analysis showed that the baseline MTV and TLG thresholds were associated with poor PFS. However, other pretreatment clinical factors, including the IPI and large tumor mass (> 10 cm) disease, were not statistically different (56). In the multivariate analysis, only TLG retained statistical significance (P = 0.049). Univariate analysis of post-treatment variables showed that residual CT tumor volume, maximum standardized uptake value, and the Deauville score were related to PFS. In the multivariate analysis, the Deauville score of 5 was still significant (P =0.006). A model that combines the evaluation of baseline TLG and the Deauville score at the end of treatment could identify patients with a high risk of progression (56, 57). Therefore, this prognostic model may screen high-risk patients for more intensive treatment or even combine with new targeted treatments such as pembrolizumab (53). In the future, baseline quantitative PET-CT may be used to provide an earlier definition of a risk-adapted therapeutic strategy in PMBCL through this new tumor metabolizing biomarker.
The treatment of this PMBCL remains an area of active research. Previously, most clinical management to support clinical care was inferred from retrospective studies. Before the rituximab-era, some retrospective studies suggested that outcomes of patients with V/MACOP-B (etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, bleomycin) were superior to CHOP (5, 9, 59, 60). A retrospective multicenter report in Italy showed a 51.1% reduction in CR in the CHOP group and an 80% reduction in the V/MACOP-B group (P < 0.001). The recurrence rate was 22.7% in patients receiving CHOP and 9.2% in patients receiving V/MACOP-B. CHOP was 39.5% in patients without events and 75.7% in the V/MACOP-B group (P < 0.001) (59). Historically, some European centers have strongly supported the above view, although the efficacy of CHOP was relatively poor (61, 62). However, the CHOP regimen is more common for the treatment of PMBCL in the United States of America, possibly due to the early classification of PMBCL into the DLBCL subtype (63, 64).
Since adding rituximab to CHOP, the benefits of V/MACOP-B are no longer clear (65). Savage et al. reported the 5-year OS rate in patients aged < 65 years old treated with V/MACOP-B and R-CHOP was 87% and 81%, respectively. In a pair-wise survival comparison, there was no significant difference between the V/MACOP-B and R-CHOP regimens. A retrospective study compared 45 patients who received the V/MACOPB-plus-rituximab regimen with mediastinal radiotherapy. There were no statistical differences in CR and relapse-free survival (RFS) when compared with historical data for rituximab-free V/MACOP-B (66). However, cure rates improved dramatically with CHOP plus rituximab. The purpose of the Rituximab International Trial Group was to evaluate the effects of CHOP in combination with rituximab on PMBCL; the authors concluded that rituximab increased the CR rate of PMBCL (67). At the same time, another retrospective study confirmed satisfactory results of R-CHOP (16, 65, 68).
Tai et al. reported the OS and PFS for R-CHOP and CHOP treated patients were 87% vs. 57% and 88% vs. 36%, respectively (after a median follow-up of 31.2 months) (69). Conversely, Soumverain et al. raised the concern that the incidence of primary refractory diseases in PMBCL patients treated with R-CHOP was unacceptably high, particularly for patients with advanced-stage or high IPI risk scores, and thus R-CHOP appeared to be inadequate for chemotherapy. Low-risk patients receiving R-CHOP may require RT consolidation at a later stage (16). A recent long-term retrospective study that investigated the clinical effects of a 12-cycle V/MACOP-B regimen with or without rituximab in 151 PMBCL patients, 120 patients (79.5%) achieved a CR, and 12 patients (7.9%) a partial remission (Objective Response Rate ORR: 87.4%). The 21-year OS was 82.6%; the PFS and DFS rates were 69.3% and 86.4%, respectively. This study also presented long-term follow-up data, indicating that third-generation chemotherapy such as MACOP-B is feasible in the treatment of PMBCL (70).
In 2013, the NCI conducted a single-arm, phase II, prospective study of 51 patients with untreated PMBCL and without combined radiotherapy. The Mean follow-up of 5 years revealed 93% event-free survival and 97% OS (71). Based on these results, this regimen has been considered a standard of care by many centers around the world. However, there is still a lack of prospective studies to verify these results. Similarly, Wilson et al. agreed that DA-R-EPOCH chemotherapy has high efficacy in previously untreated B-cell lymphomas (72, 73). Some studies have shown that R-CHOP may replace DA-R-EPOCH in the treatment of PMBCL patients. Although the CR rate of DA-R-EPOCH is higher than that of R-CHOP (84% vs. 70%, P = 0.046), patients receiving DA-R-EPOCH were more likely to experience treatment-related toxicity. The 2-year OS of 89% versus 91%, indicated that there was no significant difference between these two chemotherapy regimens (74). It has also been observed that grade I–II cardiac complications in the DA-R-EPOCH group were more frequent. Another study including 53 cases of PMBCL treated with R-CHOP (n = 21) and DA-R-EPOCH (n = 28), indicated there was no difference in the 1-year PFS and OS between the two groups (17, 75). In conclusion, treatments for PMBCL have evolved over time. Although, patients who receive the DA-R-EPOCH regimen are more likely to experience short-term toxic side effects (17).
The early, lymphoma tumor group recommended combined therapy consisting of RT followed 6 cycles of CHOP-type chemotherapy. Some previous reports have indicated that consolidation RT induces a good remission rate. The National Cancer Database suggested superior 5-year OS for the no-RT and RT groups, 83% versus 93%, respectively (76). However, a retrospective review failed to show any additional benefit in the RT group with regard to PFS or OS among the 45 patients treated with CHOP and R-CHOP (38). Similar results were found in the Savage et al. report, whereby the conventional addition of radiotherapy did not improve survival (5). Further, Messmera et al. reported there was no significant difference in PFS or OS between the R-CHOP group and the R-CHOP plus radiation group (74).
Given the good results achieved by the incorporation of rituximab and dose-enhanced chemotherapy, RT may be avoidable in most patients (52, 71, 77). A single-center retrospective study advanced the proposal that therapy with DA-R-EPOCH could avoid the need for radiotherapy in PMBCL (71). Most patients who received dose-enhanced chemotherapy could abandon consolidative mediastinal RT without any compromise in long-term outcomes. Similar results were obtained by Malenda et al. study (71, 75, 78). More recently, Jiang et al. and others have made alternative proposals. They analyzed 474 patients with PMBCL, which included 65.8% of patients aged 18–39 years old and 34.2% of patients aged 40–59 years old; of these 45.8% received RT. Univariate analysis revealed that exposure to RT was associated with prognosis in patients aged 40–59 years (after adjusting for tumor stage and race). However, RT treatment could not be administered to patients aged 18–39 years (79). In addition, Chan et al. reported a 5-year PFS of 88% in PMBCL patients receiving either R-CHOP (n = 41), R-CHOP + RT (n = 37) or DA-R-EPOCH (n = 46); a minority of patients in the DA-R-EPOCH arm received radiation. PFS was superior in patients treated with R-CHOP + RT or DA-R-EPOCH than in patients treated with R-CHOP alone, with the 5-year PFS of 90% versus 88.5% versus 56%, respectively (P = 0.02). These findings indicated that both R-CHOP+RT and DA-R-EPOCH could provide an excellent prognosis for PMBCL patients. In particular, patients receiving R-CHOP treatment, especially in those patients with larger disease size, non-radiotherapy consolidation treatment led to poorer PFS (80). In addition, Use of RT in PMBCL may predispose young patients to cardiopulmonary toxicity and secondary malignancies (81). RT therapy for PMBCL is a subject of ongoing debate, with no accepted standard of care. It is undeniable that consolidation RT is particularly valuable for patients with residual disease (Deauville score > 3), as RT can convert a partial response (PR) into full response CR after chemotherapy (59, 62). However, it is still uncertain whether CR patients need to follow-up with RT or not. Therefore, further studies are needed to establish the precise role of RT. The disadvantage of the R-CHOP or MACOP sequential RT regimen is that RT increases the patient’s medical expenses and also increases the risk of secondary tumors (82). Currently, there is no universally accepted standard of care for the initial treatment of PMBCL and there have been only retrospective analyses of data that we will discuss below (Figure 2; Table 2).
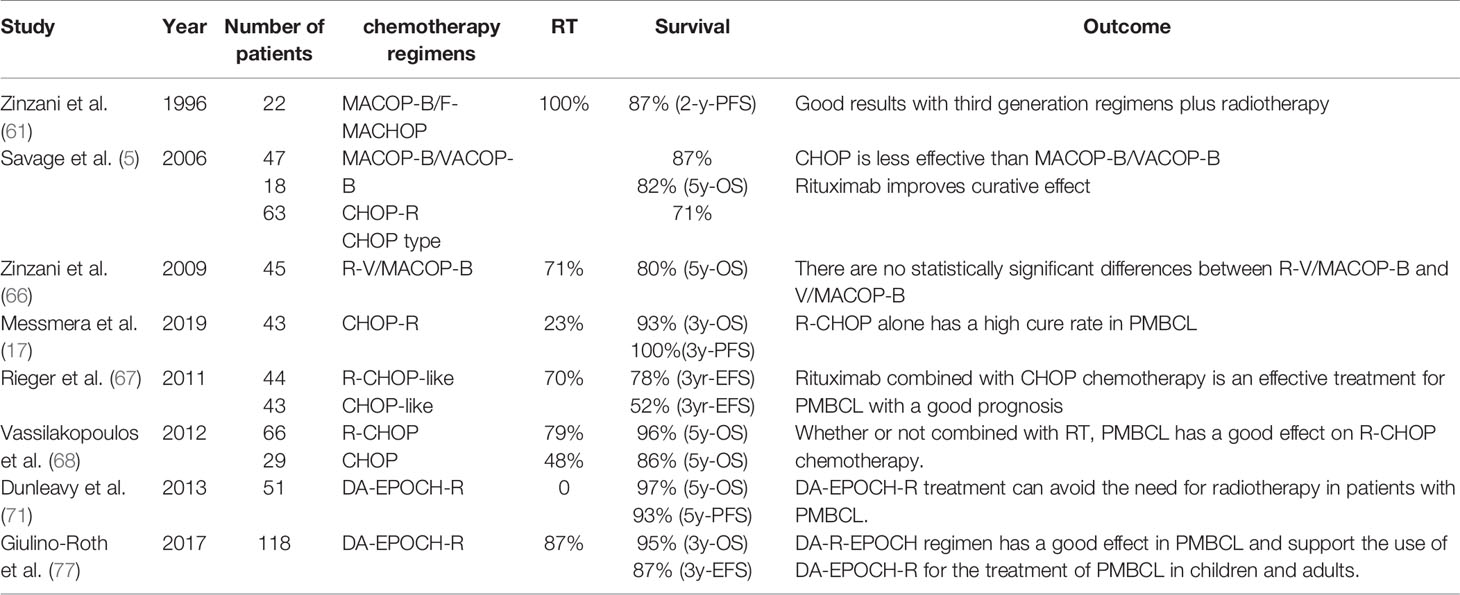
Table 2 Results of chemotherapy data for primary mediastinal large B-cell lymphoma reported in various studies.
Despite the high efficacy of immunochemotherapy regimens, a minority of patients with PMBCL experience relapsed/refractory disease. The prognosis among cases of rrPMBCL is poor (59, 83). The standard treatment for rrPMBCL is salvage therapy followed by hematopoietic stem cell transplantation (HSCT). A report by the European Society of Blood and Bone Marrow Transplantation in PMBCL sensitive to chemotherapy indicated that autogenic stem-cell transplantation (auto-SCT) with or without RT exhibits a good prognosis, while, its benefits seem to be limited in patients with rrPMBCL (84). Another multicenter study reported the results of allogeneic transplantation in patients with rrPMBC (n = 28). Approximately 79% of patients were sensitive to high-dose chemotherapy before transplantation and in this population, the 5-year PFS, 5-year OS, and non-recurring population, and the cumulative incidence of death and recurrence were 34%, 45%, 32%, and 33%, respectively. Compared with refractory patients with a 2-year PFS and OS of 0% each, the outcome of patients was significantly better. Nonetheless, the major morbidity and mortality risks cannot be ignored and the associated high medical costs are not conducive to popularizing this approach (85). The pathobiological and genetic characteristics of PMBCL include motivate and contribute to the hope for the development of new therapeutic drugs for rrPMBCL in the future.
PMBCL is associated with 9p24 genetic abnormalities and overexpression of PD-1 ligand (PD-L1), thus it has been speculated that PMBCL may be susceptible to PD-1 blockade (28, 29, 32). Pembrolizumab is an effective and well-tolerated treatment option that has been approved for use in a variety of cancer types, from advanced melanoma to relapsed/refractory cHL (rr-cHL) (86, 87). Several agencies are currently evaluating the role of pembrolizumab in the treatment of rrPMBCL. There are phase II trials of pembrolizumab in rrPMBCL currently ongoing (KEYNOTE-013/KEYNOTE-170). An interim assessment of this trial reported that the ORR was 48% (7 complete responses; 33%) among the 21 patients in KEYNOTE-013 and 45% (7 complete responses; 13%) among the 53 patients in KEYNOTE-170. Pembrolizumab was also reported to be well tolerated, with treatment-related adverse events in most patients being of low grade. There have been no treatment-related deaths reported to date. Pembrolizumab has exhibited a high response rate, long-lasting activity, and manageable safety in patients with rrPMBCL (88, 89).
As mentioned above, another potential therapeutic target for PMBCL is the JAK/STAT signaling pathway (90). Both the JAK2 inhibitor ruxolitinib and the JAK2/FLT3 inhibitor SB518 have been evaluated in HL and PMBCL (91). However, due to the currently small number of cases evaluated, their efficacy cannot be definitely determined.
Brentuximab vedotin (BV) is an antibody-drug conjugate targeting the CD30 antigen, which is highly expressed in cHL and in systemic anaplastic large cell lymphomas (sALCL). In 2012, the Food and Drug Administration (FDA) approved the use of BV in cHL and sALCL (92). Other studies have confirmed the efficacy of BV in patients expressing high levels of CD30 (93–95). PMBCL is also characterized by an elevated expression of CD30 (20). Therefore, a single-arm phase II trial was conducted to observe the use of BV in patients with rrPMBCL. In this study, the expression of CD30 (usually weak in PMBCL) was not associated with response rates. The mid-term evaluation showed that the ORR was unexpectedly low and the study was terminated prematurely (88). Recently, a multi-center research study reported that the combination of nivolumab (anti-PD-1 checkpoint inhibitor) and BV may exert synergistic activity in rrPMBCL. Among 30 patients treated, ORR (95% CI) was 73%, with a 37% CR rate per investigator, and an ORR of 70%, with a 43% complete metabolic response rate per independent review (a median follow-up of 11.1 months) (96).
CAR-T cell therapy is a cellular therapy that redirects T cells against tumor-associated antigens bypassing the tumor escape mechanism. The success and side effects of this new therapy may depend on the expansion of CAR-T cells in the body. The results of ZUMA-1 (NCT02348216) suggest that CAR-T can induce durable responses and with a median OS of more than 2 years, and has a manageable long-term safety profile in patients with relapsed or refractory DLBCL (97). In NCI trials of autologous anti-CD19 chimeric antigen receptor T cells (anti-CD19 CAR-T) in NHLs, responses to 4 patients with PMBCL included CR (50%), stable disease (25%), and unevaluable (25%), with a duration of response (DOR) of more than 12–22 months (98). Another clinical study showed among the final evaluable patients receiving CAR-T treatment, the achieved DOR percentage over 3 years was 51%, of which DLBCL/PMBCL reached 48% and low-grade lymphoma reached 63%. Finally, the median event-free (EFS) survival of all 45 evaluable patients was 55 months. Except for B cell exhaustion and low blood globulin, long-term adverse reactions were reported to be rare (99). On the basis of this therapy, a digital polymerase chain reaction assay (dPCR) assay was derived to detect transgenic CAR-T cells, which is very conducive to the clinical monitoring of anti-CD19 CAR-T cell therapy (100). As mentioned above, the therapeutic effect of CAR-T is considerable. The FDA has approved CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm581216.htm). The efficacy of CAR-T in PMBCL has not been adequately confirmed and still requires extensive research (101). Although these early clinical trials have included only a small number of patients with PMBCL, these studies have provided a novel therapeutic strategy for the effective treatment of rrPMBCL.
Bispecific antibodies (bs-mAbs) are engineered antibodies presenting two binding sites, in which one binds CD3-positive T cells to CD19-positive B cells, while the other is directed against a co-stimulator on T cells (CD3). This bispecific binding brings B cells and T cells into close proximity, causing T cells to temporarily come into contact with tumor cells (102). The clinical efficacy of blinatumomab (a bispecific CD19-directed CD3 T-cell engager) in the treatment of patients with R/R NHL has been confirmed in various phase I/II trials (103). In the NCT01741792 clinical trial, among 21 evaluable relapsed/refractory diffuse large B-cell lymphoma (rrDLBCL) patients, the total response rate after a single cycle of blinatumomab was 43%, and the CR was 19%. In total, 22% of patients who received progressive dose treatment-experienced Grade 3 neurological events, and 2 patients who received fixed-dose treatment all experienced Grade 3 neurological events (104). Recently, the results of the MT103-104 Phase 1 trial indicated that among the 38 patients subjected to a single-center long-term follow-up analysis, there was no evidence of long-term toxicity, and specifically, there was no evidence of neurocognitive impairment caused by blinatumomab. For the entire study population, the median OS was 4.6 years. In particular, patients who experienced a drug reaction to blinatumomab had a median OS of up to 7.7 years when receiving higher doses (60 mg/m2 per day) (103). Epcoritamab is a novel subcutaneously injected bs-mAbs, which has exhibited good safety in phase I/II trials (NCT03625037) and has demonstrated antitumor activity in preliminary studies involving relapsed/refractory B-Non-Hodgkin lymphoma (rrB-NHL). Epcoritamab (GEN3013) is a new bispecific IgG1 antibody that can direct T cells to CD20+ tumor cells. Epcoritamab has been reported to exert strong anti-tumor activity against primary tumor cells present in lymph node biopsies of patients with rrB-NHL, even if this group of patients has received CD20 monoclonal antibody treatment. The results of this study allow us to speculate that epcoritamab may be used for the treatment of newly diagnosed or rrB-NHL patients (105). In summary, studies investigating bs-mAbs in lymphoma mostly involve rrB-NHL. Although there are no specific data reported relative to the application of bs-mAbs in PMBCL, it is undeniable that rrPMBCL presents the typical characteristics of B-NHL. In the future, bs-mAbs may become a novel strategy for rrPMBCL treatment.
Significant progress has been achieved in the treatment of PMBCL over the last decades. Available studies have shown excellent outcomes with DA-EPOCH-R treatment, which usually allow avoidance of routine mediastinal RT. Alternatively, it is possible to utilize R-CHOP along with end-of-therapy PET-CT evaluation, whose findings with help guide the use of consolidative RT. Nonetheless, the prognosis of rrPMBCL remains dismal. Our primary goal is to improve the treatment regimen of rrPMBCL, to prolong remission and improve outcomes. Recent insight into PMBCL biology has provided the basis for designing treatments that incorporate target agents. In particular, pembrolizumab and CAR-T cell therapy have demonstrated the most promise in PMBCL outcomes. However, it is unlikely that a novel agent could be curative as monotherapy, while a rational combination with chemotherapy drugs might paint a brighter therapeutic perspective.
HZh and LX conceived and designed the study and reviewed the manuscript. HC and TP collected, arranged, and wrote the manuscript. YH, RZ, YL, LY, and HZa revised the manuscript. SC and QD designed and prepared the figures and tables. All authors contributed to the article and approved the submitted version.
This study was supported by grants from Fundamental Research Funds for the Central Universities of Central South University [No: 2019zzts1002], [No: 2019zzts1060], and [No: 2020zzts785]; the National Natural Science Foundation of China [No.82000200]; Natural Science Foundation of Hunan Provincial Health Commission [No. 20201659]; The Research program of Hunan provincial health and family planning commission [No: B20180496]; "Scientific Research Climbing Plan" of Hunan Cancer Hospital [No. ZX2020003].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Andreas R, George W, Karen L, Xin Y, Philippe G, GR D, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med (2003) 198(6):851–62. doi: 10.1084/jem.20031074
2. RS J, Jing O, Przemyslaw J, Treeve C, Kenneth L, ND S, et al. AP1-dependent galectin-1 expression delineates classical hodgkin and anaplastic large cell lymphomas from other lymphoid malignancies with shared molecular features. Clin Cancer Res (2008) 14(11). doi: 10.1158/1078-0432.CCR-07-4709
3. Swerdlow Steven H, Campo E, Pileri Stefano A, Harris Nancy L, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood (2016) 127(20):2375–90. doi: 10.1182/blood-2016-01-643569
4. Jackson MW, Rusthoven CG, Jones BL, Kamdar M, Rabinovitch R. Improved Survival With Radiation Therapy in Stage I-II Primary Mediastinal B Cell Lymphoma: A Surveillance, Epidemiology, and End Results Database Analysis. Int J Radiat Oncol Biol Phys (2016) 94(1):126–32. doi: 10.1016/j.ijrobp.2015.09.017
5. Savage KJ, Al-Rajhi N, Voss N, Paltiel C, Klasa R, Gascoyne RD, et al. Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: the British Columbia experience. Ann Oncol (2006) 17(1):123–30. doi: 10.1093/annonc/mdj030
6. Pan-Pan Liu K-FW, Yi X, Xi-Wen B, Peng S, Yu Wang Z-ML, Wen-Qi J. Racial patterns of patients with primary mediastinal large B-cell lymphoma: SEER analysis. Medicine (2016) 95(27):e4054. doi: 10.1097/MD.0000000000004054
7. Avigdor A, Sirotkin T, Kedmi M, Ribakovsy E, Berkowicz M, Davidovitz Y, et al. The impact of R-VACOP-B and interim FDG-PET/CT on outcome in primary mediastinal large B cell lymphoma. Ann Hematol (2014) 93(8):1297–304. doi: 10.1007/s00277-014-2043-y
8. By Ashraf A, Abou-Elella DDW, Vose JM, Kollath JP, James C. Primary Mediastinal Large B-Cell Lymphoma: A Clinicopathologic Study of 43 Patients From the Nebraska Lymphoma Study Group. J Clin Oncol (1999) 17(3):784–90. doi: 10.1200/JCO.1999.17.3.784
9. Luigi ZP, Maurizio M, Marilena B, GA M, Liliana D, Massimo F, et al. Induction chemotherapy strategies for primary mediastinal large B-cell lymphoma with sclerosis: a retrospective multinational study on 426 previously untreated patients. Haematologica (2002) 87(12):1258–64.
10. Papageorgiou SG, Diamantopoulos P, Levidou G, Angelopoulou MK, Economopoulou P, Efthimiou A, et al. Isolated central nervous system relapses in primary mediastinal large B-cell lymphoma after CHOP-like chemotherapy with or without Rituximab. Hematol Oncol (2013) 31(1):10–7. doi: 10.1002/hon.2012
11. Xu L-M, Li Y-X, Fang H, Jin J, Wang W-H, Wang S-L, et al. Dosimetric Evaluation and Treatment Outcome of Intensity Modulated Radiation Therapy After Doxorubicin-Based Chemotherapy for Primary Mediastinal Large B-Cell Lymphoma. Int J Radiat Oncol Biol Phys (2013) 85(5):1289–95. doi: 10.1016/j.ijrobp.2012.10.037
12. Bishop PC, Wilson WH, Pearson D, Janik J, Jaffe ES, Elwood PC. CNS involvement in primary mediastinal large B-cell lymphoma. J Clin Oncol (1999) 17(8):2479–85. doi: 10.1200/JCO.1999.17.8.2479
13. Trivedi M, Chandar R, Nair M, Jacob PM, Thankamony P. Primary Mediastinal Large B-Cell Lymphoma in a Child Presenting With Superior Mediastinal Syndrome and Chylous Pleural and Pericardial Effusion. J Pediatr Hematol/Oncol (2019) 42(5):e369–72. doi: 10.1097/MPH.0000000000001472
14. Yuan J, Wright G, Rosenwald A, Steidl C, Gascoyne RD, Connors JM, et al. Identification of Primary Mediastinal Large B-cell Lymphoma at Nonmediastinal Sites by Gene Expression Profiling. Am J Surg Pathol (2015) 39(10):1322–30. doi: 10.1097/PAS.0000000000000473
15. Pilichowska M, Pittaluga S, Ferry JA, Hemminger J, Chang H, Kanakry JA, et al. Clinicopathologic consensus study of gray zone lymphoma with features intermediate between DLBCL and classical HL. Blood Adv (2017) 1(26):2600–9. doi: 10.1182/bloodadvances.2017009472
16. Soumerai JD, Hellmann MD, Feng Y, Sohani AR, Toomey CE, Barnes JA, et al. Treatment of primary mediastinal B-cell lymphoma with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone is associated with a high rate of primary refractory disease. Leuk Lymphoma (2014) 55(3):538–43. doi: 10.3109/10428194.2013.810738
17. Messmer M, Tsai HL, Varadhan R, Swinnen LJ, Jones RJ, Ambinder RF, et al. R-CHOP without radiation in frontline management of primary mediastinal B-cell lymphoma. Leuk Lymphoma (2019) 60(5):1261–5. doi: 10.1080/10428194.2018.1519812
18. Yamamoto W, Nakamura N, Tomita N, Ishii Y, Takasaki H, Hashimoto C, et al. Clinicopathological analysis of mediastinal large B-cell lymphoma and classical Hodgkin lymphoma of the mediastinum. Leuk Lymphoma (2013) 54(5):967–72. doi: 10.3109/10428194.2012.733881
19. Higgins JP, Warnke RA. CD30 expression is common in mediastinal large B-cell lymphoma. Am J Clin Pathol (1999) 112(2):241–7. doi: 10.1093/ajcp/112.2.241
20. Aggarwal R, Rao S, Dhawan S, Bhalla S, Kumar A, Chopra P. Primary mediastinal lymphomas, their morphological features and comparative evaluation. Lung India (2017) 34(1):19–24. doi: 10.4103/0970-2113.197115
21. Kolonić SO, Džebro S, Kušec R, Planinc-Peraica A, Dominis M, Jakšić B. Primary Mediastinal Large B-Cell Lymphoma: A Single-Center Study of Clinicopathologic Characteristics. Int J Hematol (2006) 83(4):331–6. doi: 10.1532/IJH97.E0529
22. Pileri SA, Zinzani PL, Gaidano G, Falini B, Gaulard P, Zucca E, et al. Pathobiology of primary mediastinal B-cell lymphoma. Leuk Lymphoma (2003) 44 Suppl 3:S21–6. doi: 10.1080/10428190310001623810
23. Savage KJ, Monti S, Kutok JL, Cattoretti G, Neuberg D, De Leval L, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood (2003) 102(12):3871–9. doi: 10.1182/blood-2003-06-1841
24. Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med (2003) 198(6):851–62. doi: 10.1084/jem.20031074
25. Mottok A, Hung SS, Chavez EA, Woolcock B, Telenius A, Chong LC, et al. Integrative genomic analysis identifies key pathogenic mechanisms in primary mediastinal large B-cell lymphoma. Blood (2019) 134(10):802–13. doi: 10.1182/blood.2019001126
26. Dubois S, Viailly PJ, Mareschal S, Bohers E, Bertrand P, Ruminy P, et al. Next-Generation Sequencing in Diffuse Large B-Cell Lymphoma Highlights Molecular Divergence and Therapeutic Opportunities: a LYSA Study. Clin Cancer Res (2016) 22(12):2919–28. doi: 10.1158/1078-0432.CCR-15-2305
27. Mansouri L, Noerenberg D, Young E, Mylonas E, Abdulla M, Frick M, et al. Frequent NFKBIE deletions are associated with poor outcome in primary mediastinal B-cell lymphoma. Blood (2016) 128(23):2666–70. doi: 10.1182/blood-2016-03-704528
28. Hao Y, Chapuy B, Monti S, Sun HH, Rodig SJ, Shipp MA. Selective JAK2 inhibition specifically decreases Hodgkin lymphoma and mediastinal large B-cell lymphoma growth in vitro and in vivo. Clin Cancer Res (2014) 20(10):2674–83. doi: 10.1158/1078-0432.CCR-13-3007
29. Twa DD, Chan FC, Ben-Neriah S, Woolcock BW, Mottok A, Tan KL, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood (2014) 123(13):2062–5. doi: 10.1182/blood-2013-10-535443
30. Hinz M, Loser P, Mathas S, Krappmann D, Dorken B, Scheidereit C. Constitutive NF-kappaB maintains high expression of a characteristic gene network, including CD40, CD86, and a set of antiapoptotic genes in Hodgkin/Reed-Sternberg cells. Blood (2001) 97(9):2798–807. doi: 10.1182/blood.v97.9.2798
31. Bea S, Zettl A, Wright G, Salaverria I, Jehn P, Moreno V, et al. Diffuse large B-cell lymphoma subgroups have distinct genetic profiles that influence tumor biology and improve gene-expression-based survival prediction. Blood (2005) 106(9):3183–90. doi: 10.1182/blood-2005-04-1399
32. Joos S, Otano-Joos MI, Ziegler S, Bruderlein S, du Manoir S, Bentz M, et al. Primary mediastinal (thymic) B-cell lymphoma is characterized by gains of chromosomal material including 9p and amplification of the REL gene. Blood (1996) 87(4):1571–8. doi: 10.1182/blood.V87.4.1571.bloodjournal8741571
33. Steidl C, Shah SP, Woolcock BW, Rui L, Kawahara M, Farinha P, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature (2011) 471(7338):377–81. doi: 10.1038/nature09754
34. Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin’s lymphoma: increasing evidence of the importance of the microenvironment. J Clin Oncol (2011) 29(14):1812–26. doi: 10.1200/JCO.2010.32.8401
35. Vigano E, Gunawardana J, Mottok A, Van Tol T, Mak K, Chan FC, et al. Somatic IL4R mutations in primary mediastinal large B-cell lymphoma lead to constitutive JAK-STAT signaling activation. Blood (2018) 131(18):2036–46. doi: 10.1182/blood-2017-09-808907
36. Chapuy B, Stewart C, Dunford AJ, Kim J, Wienand K, Kamburov A, et al. Genomic analyses of PMBL reveal new drivers and mechanisms of sensitivity to PD-1 blockade. Blood (2019) 134(26):2369–82. doi: 10.1182/blood.2019002067
37. Reichel J, Chadburn A, Rubinstein PG, Giulino-Roth L, Tam W, Liu Y, et al. Flow sorting and exome sequencing reveal the oncogenome of primary Hodgkin and Reed-Sternberg cells. Blood (2015) 125(7):1061–72. doi: 10.1182/blood-2014-11-610436
38. Steidl C, Gascoyne RD. The molecular pathogenesis of primary mediastinal large B-cell lymphoma. Blood (2011) 118(10):2659–69. doi: 10.1182/blood-2011-05-326538
39. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol (2014) 32(27):3059–68. doi: 10.1200/JCO.2013.54.8800
40. Ujjani CS, Hill EM, Wang H, Nassif S, Esposito G, Ozdemirli M, et al. (18) F-FDG PET-CT and trephine biopsy assessment of bone marrow involvement in lymphoma. Br J Haematol (2016) 174(3):410–6. doi: 10.1111/bjh.14071
41. Alkhawtani RHM, Noordzij W, Glaudemans A, van Rijn RS, van der Galien HT, Balink H, et al. Lactate dehydrogenase levels and 18F-FDG PET/CT metrics differentiate between mediastinal Hodgkin’s lymphoma and primary mediastinal B-cell lymphoma. Nucl Med Commun (2018) 39(6):572–8. doi: 10.1097/MNM.0000000000000840
42. Hosein PJ, Lossos IS. The evolving role of F-FDG PET scans in patients with aggressive non-Hodgkin’s lymphoma. Eur J Clin Med Oncol (2010) 2(1):131–8.
43. Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol (2014) 32(27):3048–58. doi: 10.1200/JCO.2013.53.5229
44. Younes A, Hilden P, Coiffier B, Hagenbeek A, Salles G, Wilson W, et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol (2017) 28(7):1436–47. doi: 10.1093/annonc/mdx097
45. Martelli M, Ceriani L, Zucca E, Zinzani PL, Ferreri AJ, Vitolo U, et al. [18F]fluorodeoxyglucose positron emission tomography predicts survival after chemoimmunotherapy for primary mediastinal large B-cell lymphoma: results of the International Extranodal Lymphoma Study Group IELSG-26 Study. J Clin Oncol (2014) 32(17):1769–75. doi: 10.1200/JCO.2013.51.7524
46. Ceriani L, Martelli M, Gospodarowicz MK, Ricardi U, Ferreri AJ, Chiappella A, et al. Positron Emission Tomography/Computed Tomography Assessment After Immunochemotherapy and Irradiation Using the Lugano Classification Criteria in the IELSG-26 Study of Primary Mediastinal B-Cell Lymphoma. Int J Radiat Oncol Biol Phys (2017) 97(1):42–9. doi: 10.1016/j.ijrobp.2016.09.031
47. Granados U, Fuster D, Pericas JM, Llopis JL, Ninot S, Quintana E, et al. Diagnostic Accuracy of 18F-FDG PET/CT in Infective Endocarditis and Implantable Cardiac Electronic Device Infection: A Cross-Sectional Study. J Nucl Med (2016) 57(11):1726–32. doi: 10.2967/jnumed.116.173690
48. Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, Belhadj K, et al. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood (2005) 106(4):1376–81. doi: 10.1182/blood-2005-01-0272
49. Dupuis J, Gaulard P, Hemery F, Itti E, Gisselbrecht C, Rahmouni A, et al. Respective prognostic values of germinal center phenotype and early (18)fluorodeoxyglucose-positron emission tomography scanning in previously untreated patients with diffuse large B-cell lymphoma. Haematologica (2007) 92(6):778–83. doi: 10.3324/haematol.10895
50. Ceriani L, Barrington S, Biggi A, Malkowski B, Metser U, Versari A, et al. Training improves the interobserver agreement of the expert positron emission tomography review panel in primary mediastinal B-cell lymphoma: interim analysis in the ongoing International Extranodal Lymphoma Study Group-37 study. Hematol Oncol (2017) 35(4):548–53. doi: 10.1002/hon.2339
51. Zinzani PL, Broccoli A, Casadei B, Stefoni V, Pellegrini C, Gandolfi L, et al. The role of rituximab and positron emission tomography in the treatment of primary mediastinal large B-cell lymphoma: experience on 74 patients. Hematol Oncol (2015) 33(4):145–50. doi: 10.1002/hon.2172
52. Giri S, Bhatt VR, Pathak R, Bociek RG, Vose JM, Armitage JO. Role of radiation therapy in primary mediastinal large B-cell lymphoma in rituximab era: A US population-based analysis. Am J Hematol (2015) 90(11):1052–4. doi: 10.1002/ajh.24172
53. Hayden AR, Tonseth P, Lee DG, Villa D, Gerrie AS, Scott DW, et al. Outcome of primary mediastinal large B-cell lymphoma using R-CHOP: impact of a PET-adapted approach. Blood (2020) 136(24):2803–11. doi: 10.1182/blood.2019004296
54. Filippi AR, Piva C, Levis M, Chiappella A, Caracciolo D, Bello M, et al. Prognostic Role of Pre-Radiation Therapy (18)F-Fluorodeoxyglucose Positron Emission Tomography for Primary Mediastinal B-Cell Lymphomas Treated with R-CHOP or R-CHOP-Like Chemotherapy Plus Radiation. Int J Radiat Oncol Biol Phys (2016) 95(4):1239–43. doi: 10.1016/j.ijrobp.2016.02.057
55. Ceriani L, Milan L, Martelli M, Ferreri AJM, Cascione L, Zinzani PL, et al. Metabolic heterogeneity on baseline 18FDG-PET/CT scan is a predictor of outcome in primary mediastinal B-cell lymphoma. Blood (2018) 132(2):179–86. doi: 10.1182/blood-2018-01-826958
56. Pinnix CC, Ng AK, Dabaja BS, Milgrom SA, Gunther JR, Fuller CD, et al. Positron emission tomography-computed tomography predictors of progression after DA-R-EPOCH for PMBCL. Blood Adv (2018) 2(11):1334–43. doi: 10.1182/bloodadvances.2018017681
57. Ceriani L, Milan L, Johnson PWM, Martelli M, Presilla S, Giovanella L, et al. Baseline PET features to predict prognosis in primary mediastinal B cell lymphoma: a comparative analysis of different methods for measuring baseline metabolic tumour volume. Eur J Nucl Med Mol Imaging (2019) 46(6):1334–44. doi: 10.1007/s00259-019-04286-8
58. Ceriani L, Martelli M, Zinzani PL, Ferreri AJ, Botto B, Stelitano C, et al. Utility of baseline 18FDG-PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B-cell lymphoma. Blood (2015) 126(8):950–6. doi: 10.1182/blood-2014-12-616474
59. Todeschini G, Secchi S, Morra E, Vitolo U, Orlandi E, Pasini F, et al. Primary mediastinal large B-cell lymphoma (PMLBCL): long-term results from a retrospective multicentre Italian experience in 138 patients treated with CHOP or MACOP-B/VACOP-B. Br J Cancer (2004) 90(2):372–6. doi: 10.1038/sj.bjc.6601460
60. Lazzarino M, Orlandi E, Paulli M, Strater J, Klersy C, Gianelli U, et al. Treatment outcome and prognostic factors for primary mediastinal (thymic) B-cell lymphoma: a multicenter study of 106 patients. J Clin Oncol (1997) 15(4):1646–53. doi: 10.1200/JCO.1997.15.4.1646
61. Zinzani PL, Bendandi M, Frezza G, Gherlinzoni F, Merla E, Salvucci M, et al. Primary Mediastinal B-cell lymphoma with sclerosis: clinical and therapeutic evaluation of 22 patients. Leuk Lymphoma (1996) 21(3-4):311–6. doi: 10.3109/10428199209067612
62. Zinzani PL, Martelli M, Bertini M, Gianni AM, Devizzi L, Federico M, et al. Induction chemotherapy strategies for primary mediastinal large B-cell lymphoma with sclerosis: a retrospective multinational study on 426 previously untreated patients. Haematologica (2002) 87(12):1258–64.
63. Abou-Elella AA, Weisenburger DD, Vose JM, Kollath JP, Lynch JC, Bast MA, et al. Primary mediastinal large B-cell lymphoma: a clinicopathologic study of 43 patients from the Nebraska Lymphoma Study Group. J Clin Oncol (1999) 17(3):784–90. doi: 10.1200/JCO.1999.17.3.784
64. Rodriguez J, Pugh WC, Romaguera JE, Luthra R, Hagemeister FB, McLaughlin P, et al. Primary mediastinal large cell lymphoma is characterized by an inverted pattern of large tumoral mass and low beta 2 microglobulin levels in serum and frequently elevated levels of serum lactate dehydrogenase. Ann Oncol (1994) 5(9):847–9. doi: 10.1093/oxfordjournals.annonc.a059016
65. Avigdor A, Sirotkin T, Kedmi M, Ribakovsy E, Berkowicz M, Davidovitz Y, et al. The impact of R-VACOP-B and interim FDG-PET/CT on outcome in primary mediastinal large B cell lymphoma. Ann Hematol (2014) 93(8):1297–304. doi: 10.1007/s00277-014-2043-y
66. Zinzani PL, Stefoni V, Finolezzi E, Brusamolino E, Cabras MG, Chiappella A, et al. Rituximab combined with MACOP-B or VACOP-B and radiation therapy in primary mediastinal large B-cell lymphoma: a retrospective study. Clin Lymphoma Myeloma (2009) 9(5):381–5. doi: 10.3816/CLM.2009.n.074
67. Rieger M, Osterborg A, Pettengell R, White D, Gill D, Walewski J, et al. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Ann Oncol (2011) 22(3):664–70. doi: 10.1093/annonc/mdq418
68. Vassilakopoulos TP, Pangalis GA, Katsigiannis A, Papageorgiou SG, Constantinou N, Terpos E, et al. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone with or without radiotherapy in primary mediastinal large B-cell lymphoma: the emerging standard of care. Oncologist (2012) 17(2):239–49. doi: 10.1634/theoncologist.2011-0275
69. Tai WM, Quah D, Yap SP, Tan SH, Tang T, Tay KW, et al. Primary mediastinal large B-cell lymphoma: optimal therapy and prognostic factors in 41 consecutive Asian patients. Leuk Lymphoma (2011) 52(4):604–12. doi: 10.3109/10428194.2010.550073
70. Casadei B, Argnani L, Morigi A, Lolli G, Broccoli A, Pellegrini C, et al. Treatment and outcomes of primary mediastinal B cell lymphoma: a three-decade monocentric experience with 151 patients. Ann Hematol (2020). doi: 10.1007/s00277-020-04364-0
71. Dunleavy K, Pittaluga S, Maeda LS, Advani R, Chen CC, Hessler J, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med (2013) 368(15):1408–16. doi: 10.1056/NEJMoa1214561
72. Wilson WH, Grossbard ML, Pittaluga S, Cole D, Pearson D, Drbohlav N, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood (2002) 99(8):2685–93. doi: 10.1182/blood.v99.8.2685
73. Ganesan P, Ganesan TS, Atreya H, Kannan K, Radhakrishnan V, Dhanushkodi M, et al. DA-EPOCH-R in Aggressive CD 20 Positive B Cell Lymphomas: Real-World Experience. Indian J Hematol Blood Transfus (2018) 34(3):454–9. doi: 10.1007/s12288-017-0901-1
74. Shah NN, Szabo A, Huntington SF, Epperla N, Reddy N, Ganguly S, et al. R-CHOP versus dose-adjusted R-EPOCH in frontline management of primary mediastinal B-cell lymphoma: a multi-centre analysis. Br J Haematol (2018) 180(4):534–44. doi: 10.1111/bjh.15051
75. Malenda A, Kolkowska-Lesniak A, Pula B, Dlugosz-Danecka M, Chelstowska M, Konska A, et al. Outcomes of treatment with dose-adjusted EPOCH-R or R-CHOP in primary mediastinal large B-cell lymphoma. Eur J Haematol (2019) 104(1):59–66. doi: 10.1111/ejh.13337
76. Jackson MW, Rusthoven CG, Jones BL, Kamdar M, Rabinovitch R. Improved survival with combined modality therapy in the modern era for primary mediastinal B-cell lymphoma. Am J Hematol (2016) 91(5):476–80. doi: 10.1002/ajh.24325
77. Giulino-Roth L, O’Donohue T, Chen Z, Bartlett NL, LaCasce A, Martin-Doyle W, et al. Outcomes of adults and children with primary mediastinal B-cell lymphoma treated with dose-adjusted EPOCH-R. Br J Haematol (2017) 179(5):739–47. doi: 10.1111/bjh.14951
78. Goldschmidt N, Kleinstern G, Orevi M, Paltiel O, Ben-Yehuda D, Gural A, et al. Favorable outcome of primary mediastinal large B-cell lymphoma patients treated with sequential RCHOP-RICE regimen without radiotherapy. Cancer Chemother Pharmacol (2016) 77(5):1053–60. doi: 10.1007/s00280-016-3024-8
79. Jiang S, Zhen H, Jiang H. Role of Radiation Therapy in Younger and Older Adults with Primary Mediastinal Large B Cell Lymphoma in Rituximab Era: A U.S. Population-Based Analysis. J Adolesc Young Adult Oncol (2019) 8(5):623–7. doi: 10.1089/jayao.2019.0018
80. Chan EHL, Koh LP, Lee J, De Mel S, Jeyasekharan A, Liu X, et al. Real world experience of R-CHOP with or without consolidative radiotherapy vs DA-EPOCH-R in the first-line treatment of primary mediastinal B-cell lymphoma. Cancer Med (2019) 8(10):4626–32. doi: 10.1002/cam4.2347
81. Smith G, Raj BV, Ranjan P, Gregory BR, VJ M, AJ O. Role of radiation therapy in primary mediastinal large B-cell lymphoma in rituximab era: A US population-based analysis. Am J Hematol (2015) 90(11):1052–4. doi: 10.1002/ajh.24172
82. De Sanctis V, Alfo M, Di Rocco A, Ansuinelli M, Russo E, Osti MF, et al. Second cancer incidence in primary mediastinal B-cell lymphoma treated with methotrexate with leucovorin rescue, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin regimen with or without rituximab and mediastinal radiotherapy: Results from a monoinstitutional cohort analysis of long-term survivors. Hematol Oncol (2017) 35(4):554–60. doi: 10.1002/hon.2377
83. Kuruvilla J, Pintilie M, Tsang R, Nagy T, Keating A, Crump M. Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B-cell lymphoma compared with diffuse large B-cell lymphoma. Leuk Lymphoma (2008) 49(7):1329–36. doi: 10.1080/10428190802108870
84. Avivi I, Boumendil A, Finel H, Nagler A, de Sousa AB, Santasusana JMR, et al. Autologous stem cell transplantation for primary mediastinal B-cell lymphoma: long-term outcome and role of post-transplant radiotherapy. A report of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant (2018) 53(8):1001–9. doi: 10.1038/s41409-017-0063-7
85. Herrera AF, Chen L, Khajavian S, Chase M, Darrah J, Maloney D, et al. Allogeneic Stem Cell Transplantation Provides Durable Remission in Patients with Primary Mediastinal Large B Cell Lymphoma. Biol Blood Marrow Transplant (2019) 25(12):2383–7. doi: 10.1016/j.bbmt.2019.07.041
86. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
87. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med (2015) 372(26):2521–32. doi: 10.1056/NEJMoa1503093
88. Zinzani PL, Pellegrini C, Chiappella A, Di Rocco A, Salvi F, Cabras MG, et al. Brentuximab vedotin in relapsed primary mediastinal large B-cell lymphoma: results from a phase 2 clinical trial. Blood (2017) 129(16):2328–30. doi: 10.1182/blood-2017-01-764258
89. Armand P, Rodig S, Melnichenko V, Thieblemont C, Bouabdallah K, Tumyan G, et al. Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma. J Clin Oncol (2019) 37(34):3291–9. doi: 10.1200/JCO.19.01389
90. Twa DD, Steidl C. Structural genomic alterations in primary mediastinal large B-cell lymphoma. Leuk Lymphoma (2015) 56(8):2239–50. doi: 10.3109/10428194.2014.985673
91. Younes A, Romaguera J, Fanale M, McLaughlin P, Hagemeister F, Copeland A, et al. Phase I study of a novel oral Janus kinase 2 inhibitor, SB1518, in patients with relapsed lymphoma: evidence of clinical and biologic activity in multiple lymphoma subtypes. J Clin Oncol (2012) 30(33):4161–7. doi: 10.1200/JCO.2012.42.5223
92. de Claro RA, McGinn K, Kwitkowski V, Bullock J, Khandelwal A, Habtemariam B, et al. U.S. Food and Drug Administration approval summary: brentuximab vedotin for the treatment of relapsed Hodgkin lymphoma or relapsed systemic anaplastic large-cell lymphoma. Clin Cancer Res (2012) 18(21):5845–9. doi: 10.1158/1078-0432.CCR-12-1803
93. Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood (2016) 128(12):1562–6. doi: 10.1182/blood-2016-02-699850
94. Kim SJ, Yoon DH, Kim JS, Kang HJ, Lee HW, Eom HS, et al. Efficacy of Brentuximab Vedotin in Relapsed or Refractory High-CD30-Expressing Non-Hodgkin Lymphomas: Results of a Multicenter, Open-Labeled Phase II Trial. Cancer Res Treat (2019) 52(2):374–87. doi: 10.4143/crt.2019.198
95. Fukuhara N, Yamamoto G, Tsujimura H, Chou T, Shibayama H, Yanai T, et al. Retreatment with brentuximab vedotin in patients with relapsed/refractory classical Hodgkin lymphoma or systemic anaplastic large-cell lymphoma: a multicenter retrospective study. Leuk Lymphoma (2019) 61(1):176–80. doi: 10.1080/10428194.2019.1654100
96. Zinzani PL, Santoro A, Gritti G, Brice P, Barr PM, Kuruvilla J, et al. Nivolumab Combined With Brentuximab Vedotin for Relapsed/Refractory Primary Mediastinal Large B-Cell Lymphoma: Efficacy and Safety From the Phase II CheckMate 436 Study. J Clin Oncol (2019) 37(33):3081–9. doi: 10.1200/JCO.19.01492
97. Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol (2019) 20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7
98. Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol (2015) 33(6):540–9. doi: 10.1200/JCO.2014.56.2025
99. Cappell KM, Sherry RM, Yang JC, Goff SL, Vanasse DA, McIntyre L, et al. Long-Term Follow-Up of Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy. J Clin Oncol (2020) 38(32):3805–15. doi: 10.1200/JCO.20.01467
100. Fehse B, Badbaran A, Berger C, Sonntag T, Riecken K, Geffken M, et al. Digital PCR Assays for Precise Quantification of CD19-CAR-T Cells after Treatment with Axicabtagene Ciloleucel. Mol Ther Methods Clin Dev (2020) 16:172–8. doi: 10.1016/j.omtm.2019.12.018
101. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med (2017) 377(26):2531–44. doi: 10.1056/NEJMoa1707447
102. Zimmerman Z, Maniar T, Nagorsen D. Unleashing the clinical power of T cells: CD19/CD3 bi-specific T cell engager (BiTE(R)) antibody construct blinatumomab as a potential therapy. Int Immunol (2015) 27(1):31–7. doi: 10.1093/intimm/dxu089
103. Goebeler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, et al. Bispecific T-Cell Engager (BiTE) Antibody Construct Blinatumomab for the Treatment of Patients With Relapsed/Refractory Non-Hodgkin Lymphoma: Final Results From a Phase I Study. J Clin Oncol (2016) 34(10):1104–11. doi: 10.1200/JCO.2014.59.1586
104. Viardot A, Goebeler ME, Hess G, Neumann S, Pfreundschuh M, Adrian N, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood (2016) 127(11):1410–6. doi: 10.1182/blood-2015-06-651380
105. van der Horst HJ, de Jonge AV, Hiemstra IH, Gelderloos AT, Berry D, Hijmering NJ, et al. Epcoritamab induces potent anti-tumor activity against malignant B-cells from patients with DLBCL, FL and MCL, irrespective of prior CD20 monoclonal antibody treatment. Blood Cancer J (2021) 11(2):38. doi: 10.1038/s41408-021-00430-6
Keywords: primary mediastinal large B-cell lymphoma, chemotherapy, targeted therapy, mediastinal radiation, positron emission tomography-computed tomography
Citation: Chen H, Pan T, He Y, Zeng R, Li Y, Yi L, Zang H, Chen S, Duan Q, Xiao L and Zhou H (2021) Primary Mediastinal B-Cell Lymphoma: Novel Precision Therapies and Future Directions. Front. Oncol. 11:654854. doi: 10.3389/fonc.2021.654854
Received: 17 January 2021; Accepted: 01 March 2021;
Published: 22 March 2021.
Edited by:
Stefano Luminari, University of Modena and Reggio Emilia, ItalyReviewed by:
Narendranath Epperla, The Ohio State University, United StatesCopyright © 2021 Chen, Pan, He, Zeng, Li, Yi, Zang, Chen, Duan, Xiao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Xiao, eGlhb2xpbmdjc3VAY3N1LmVkdS5jbg==; Hui Zhou, emhvdWh1aTk0MDNAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.