
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 24 May 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.652378
This article is part of the Research Topic Peritoneal Metastasis of Gastric Cancer: From Basic Research to Clinical Application View all 5 articles
Gastric cancer has a high rate of metastasis, during which pre-metastatic niches (PMN) provide a supportive environment for the upcoming tumor cells. Exosomes are bilayer vesicles secreted by cells containing biological information that mediates communication between cells. Using exosomes, gastric cancer cells establish PMN remotely in multifarious perspectives, including immunosuppression, stroma remodeling, angiogenesis, mesothelial mesenchymal transformation, and organotropism. In turn, the cell components in PMN secrete exosomes that interact with each other and provide onco-promoting signals. In this review, we highlight the role of exosomes in PMN formation in gastric cancer and discuss their potential values in gastric cancer metastasis diagnosis, prevention, and treatment.
Gastric cancer (GC) is one of the most common cancers globally, ranking the fifth in cancer incidence and the third in cancer-related death (1). Despite that surgery and perioperative radiotherapy or chemotherapy are the primary treatments for early-stage gastric cancer, more than half of patients with radical resection suffered local recurrence or distant metastasis (2). Moreover, many patients were initially diagnosed with metastatic gastric cancer that is unresectable (3). Due to high heterogeneity and drug resistance, the median survival rate of metastatic gastric cancer rarely exceeds one year, and the 5-year survival rate is less than 10% (4). In particular, peritoneal metastasis is the most common metastatic pattern of gastric cancer and has several negative features, including high incidence, high mortality, difficult diagnosis, and poor prognosis (5). It occurs in 55%-60% of advanced gastric cancers (6) and has a 5-year survival rate of only 2% (7).
One of the crucial steps during metastases is the formation of a pre-metastatic niche (PMN), which provides a receptive and supportive environment in terms of nutrients, extracellular matrix (ECM), stromal cell, and immune cells for cancer cells to seed in distant organs (8–11). The PMN is initiated and educated by PMN-promoting molecules secreted by the primary tumors, tumor-mobilized myeloid cells, and local stromal cells of the host. These niche-promoting molecules include tumor-secreted factors, cytokines, chemokines, inflammatory factors, microvesicles, oncosomes, and exosomes (11).
Recently, studies revealed that exosomes play essential roles in PMN formation (12). Ranging from 40 to 150 nm in size and enveloped by lipid bilayers, exosomes are a class of extracellular vesicles released by various cells (13). They contain a variety of cell components, including DNA, RNA, lipids, and proteins, that can be transported to and can regulate recipient cells (13). Importantly, they carry a variety of cytokines, such as TGF-β, TNF-α, IL-6, IL-8, and IL-10, to maintain their stability and transfer to distant recipient cells (14). In particular, tumor-derived exosomes (TDEs) travel from their original site to distant potential metastatic sites and educate the PMN components with their cargo, thereby facilitating tumor cell arrival and colonization (15). In addition, exosomes from non-tumor cells may also participate in this process (15–18). In this review, we summarize the roles of exosomes in PMN formation in gastric cancer, as well as their implications in gastric cancer metastasis, prevention, and treatment.
Exosomes contribute to malignity through their roles in gastric cancer growth, drug resistance, and metastasis (Figure 1).
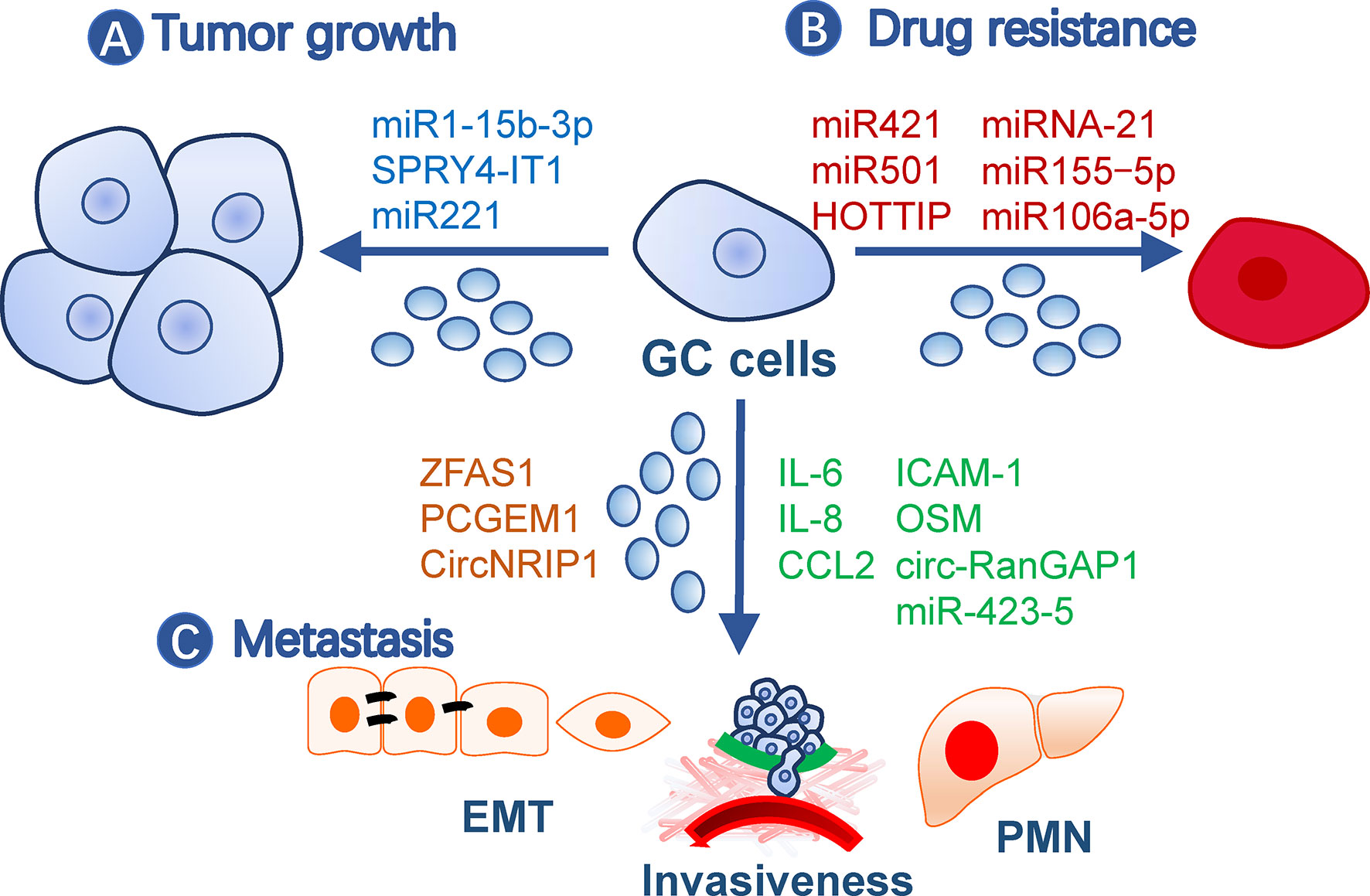
Figure 1 Roles of exosomes in gastric cancer malignity. Exosomes from gastric cancer cells or other cells contribute to malignity in (A) tumor growth, (B) drug resistance, and (C) metastasis. Exosomes may affect all processes of metastasis, such as EMT, invasiveness, and pre-metastatic niche formation. The small circles represent exosomes, and effective molecules were listed by the arrows. EMT, Epithelial-mesenchymal transition; GC, gastric cancer; PMN, pre-metastatic niche.
Gastric cancer TDE contents, including proteins and nucleic acids, have a broad impact on tumor growth. For example, TDEs secreted by BGC-823 cells can transfer miR-15b-3p to SGC-7901 or GES-1 cells to enhance their development by inhibiting the DYNLT1/Caspase-3/Caspase-9 signaling pathway (19). SPRY4-IT1, a long noncoding RNA (lncRNA), promotes gastric cancer proliferation and migration by sponging miR-101-3p, is upregulated in serum exosomes of gastric cancer patients, and is correlated with patient outcomes (20). In addition, exosomes from non-tumor cells may also impact tumor growth. For example, exosomes from gastric cancer tissue-derived mesenchymal stem cells (MSCs) are onco-promoting by transmitting miR-221 (21), while gastric mucosal epithelial cells induce apoptosis of gastric cancer cells through exosomal proteins (22).
Gastric cancer cells may transmit drug resistance to other sensitive clones by communication with exosomes. For instance, doxorubicin-resistant SGC7901 cells conferred the same drug resistance in drug-sensitive cells via exosomal miR-501 that targets BLID (23). Cisplatin resistance was transmitted by exosomal lncRNA HOTTIP, which targets the miR-218/HMGA1 axis in cisplatin-sensitive cells (24). M2 macrophage-derived exosomes also transferred cisplatin-resistance through miRNA-21 targeting PETN in recipient cells (25). A paclitaxel-resistant gastric cancer cell line, MGC803R, can induce chemoresistance in paclitaxel−sensitive cells, MGC803S, by exosomal delivery of miR−155−5p, which further suppresses GATA3 and TP53INP1 in the latter (26). In addition, TFAP2E hypermethylation facilitates packaging of miR-106a-5p and miR-421 into gastric cancer exosomes, which subsequently induce 5−fluorouracil resistance in tumor cells (27).
Metastasis is a multistep process including cancer cell motility, local infiltration, intravasation, transit in the blood or lymph, extravasation, and proliferation in competent organs (28). Epithelial-mesenchymal transition (EMT) is a biological process associated with increased cell motility, resistance to apoptosis and senescence, and suppressed immune reaction during the initial step of metastasis (29) and is closely regulated by TDEs (30). LncRNA ZFAS1 and PCGEM1 are highly enriched in gastric cancer TDEs and are capable of inducing EMT phenotypes among cancer cells during metastasis, in which PCGEM1 stabilizes SNAI1 (31, 32). CircNRIP1, a circular RNA, can also be transmitted among gastric cancer cells through exosomes and regulate EMT through a circNRIP1-miR-149-5p-AKT1/mTOR axis (33). In addition, exosomal TRIM3 was found to be an anti-EMT factor, and its levels were downregulated in gastric cancer TDEs (34). Besides TDEs, malignant ascites-derived exosomes also played essential roles in enhancing the EMT signaling in gastric cancer cells during peritoneal metastasis (35). Invasiveness is also critical during the initial and terminal processes of tumor metastasis and is easy to be quantified by in vitro and in vivo methods. This ability of gastric cancer cells can be elevated by exosomal miR-423-5p, which is remarkably correlated with lymph node metastasis (36). In SGC cells, CD97 facilitated cell invasions through packaging miRNAs into exosomes, which mediated the MAPK signaling pathway (37). circ-RanGAP1 is elevated in plasma exosomes from gastric cancer patients and facilitates gastric cancer invasiveness by upregulating VEGFA expression (38). Besides, omentum may play an active role in enhancing gastric cancer cell invasiveness by exosomal proteins (i.e., IL-6, IL-8, ICAM-1, CCl2, and OSM) (39).
Besides these processes, PMN formation is also indispensable for distant metastasis and is closely regulated by exosomes. Next, we will focus on the roles of exosomes in gastric cancer PMN formation.
A PMN has distinct characters from normal tissue environments, such as immunosuppression, angiogenesis, and organotropism (11). Cells involved in shaping these features are fine-tuned by tumor-secreted factors, including TDEs (Figure 2). They not only act on various immune cells for immunosuppression, but also remodel stromal components into tumor-supporting types in PMN. Additionally, exosomes target endothelial cells for angiogenesis and organ-specific cells, such as peritoneal mesothelial cells (PMCs), for organotropism. In turn, the immune and stromal cells secrete exosomes that benefit tumor cells and communicate among different cells (40).
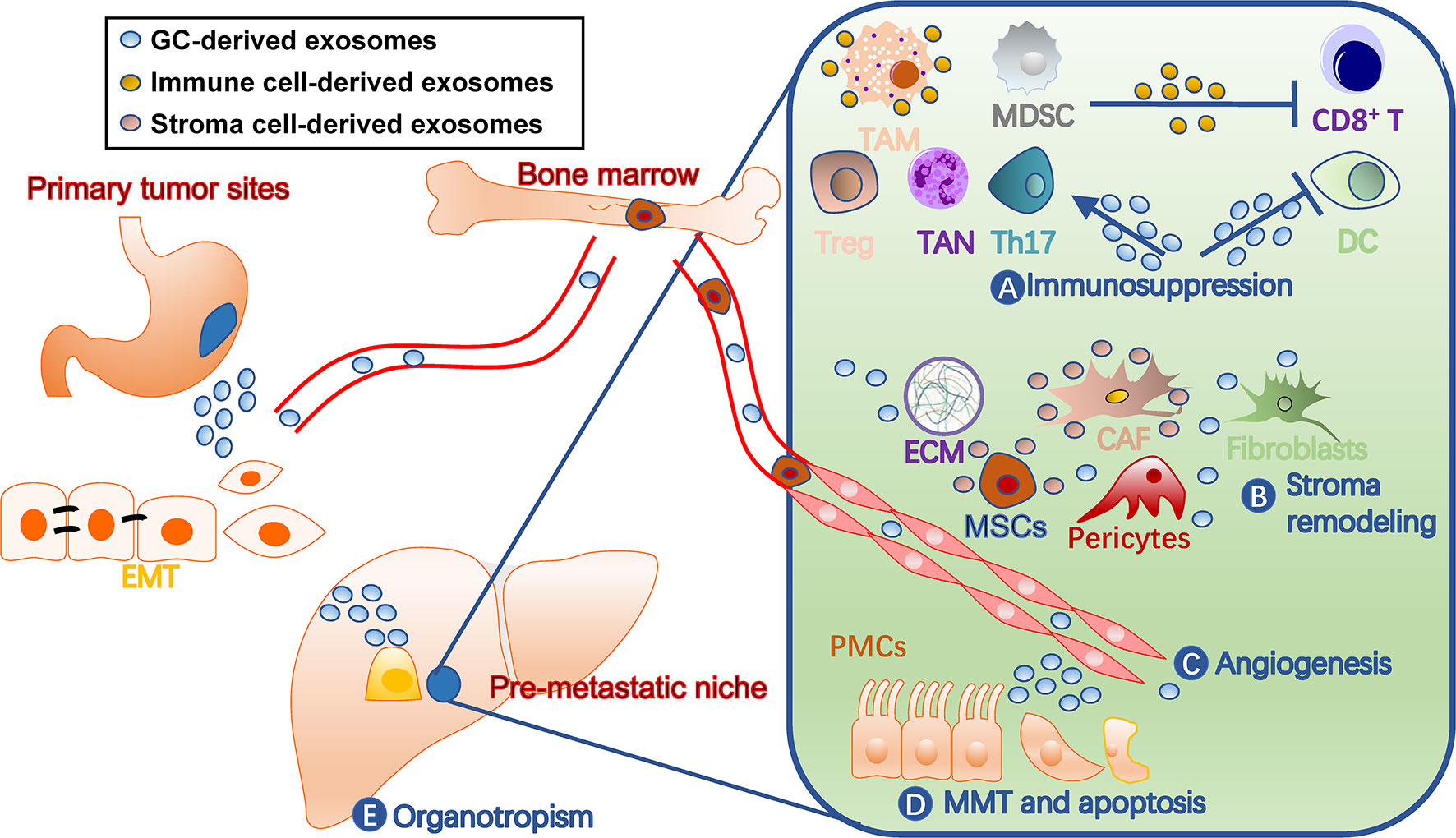
Figure 2 Roles of exosomes in gastric cancer pre-metastatic niches. Exosomes contribute to pre-metastatic niche formation through multiple mechanisms, including (A) immunosuppression by facilitating TAM and TAN polarization, inhibiting dendritic cell maturation and T cell activation, and inducing MDSCs, (B) stroma remodeling by acting on stromal cells such as CAF and MSCs and ECM balance, (C) angiogenesis, (D) MMT and apoptosis of tissue-specific cells, such as PMCs, and (E) organotropisms. The liver is drawn to represent a metastatic target. CAF, cancer-associated fibroblast; DC, dendritic cell; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; GC, gastric cancer; MDSCs, myeloid-derived suppressor cells; MMT, mesothelial-mesenchymal transition; MSC, mesenchymal stem cell; PMC, peritoneal mesothelial cell; TAM, tumor-associated macrophage; TAN, tumor-associated neutrophil; Th17, T-helper 17 cell; Treg, regulatory T cell.
To metastasize, tumor cells must evade immune surveillance and killing in the seeding organ. Thus, the PMN must orchestrate immunosuppression of all types of immune cells to protect colonizing tumor cells from immune attack. Exosomes are one of key factors that orchestrate this process among cancer cells, PMN, and immune cells (Figure 3). Specifically, exosomes polarize tumor-associated macrophages (TAMs), induce tumor-associated neutrophils (TANs), inhibit dendritic cell maturation, regulate T cell differentiation and function, and induce myeloid-derived suppressor cells (MDSCs).
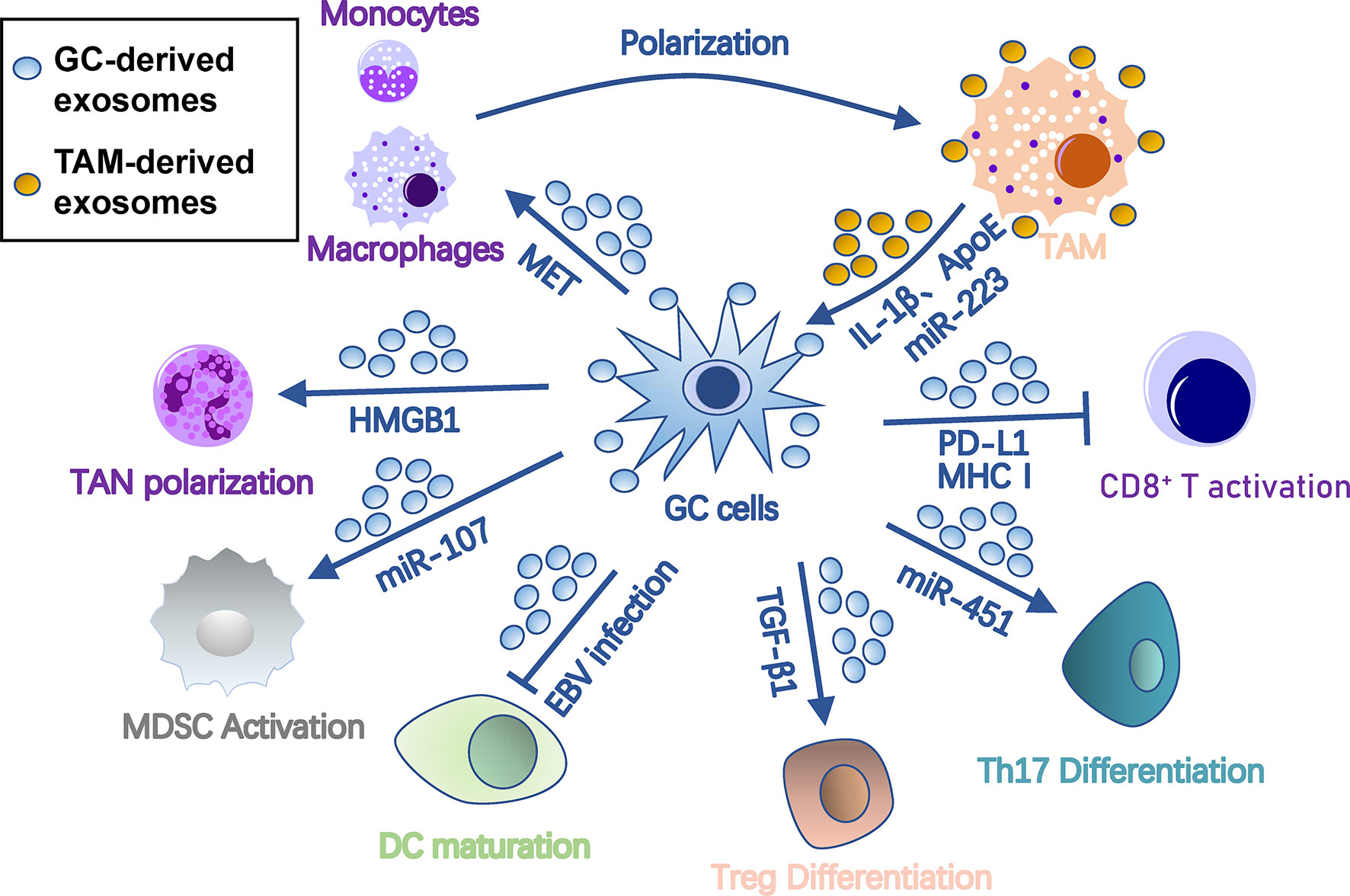
Figure 3 Roles of exosomes in immunosuppression in gastric cancer pre-metastatic niches. Gastric cancer-derived exosomes cause immunosuppression by suppressing DC/CD8+ T cell activation and DC maturation and inducing differentiation of TAM, TAN, MDSC, Treg, and Th17 cells. In turn, TAM provides onco-promoting signals to tumors and stromal cells through its exosomes. Arrows represent activation, whereas bar-headed arrows represent inhibition. CAF, cancer-associated fibroblast; DC, dendritic cells; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; MDSC, myeloid-derived suppressor cell; MMT, mesothelial-mesenchymal transition; MSC, mesenchymal stem cell; PMC, peritoneal mesothelial cell; TAM, tumor-associated macrophage; TAN, tumor-associated neutrophil; Th17, T-helper 17 cell; Treg, regulatory T cell.
Macrophages are the most abundant immune cells with two types of polarized cells, the tumor-suppressing M1 and tumor-promoting M2 (41). TAMs are primarily M2 polarized, capable of promoting tumors by regulating tumor malignancy, angiogenesis, and anti-tumor immunity (42). In gastric cancer, TDEs promote M2 transformation and facilitate production of pro-inflammatory factors by stimulating NF-κB pathways in macrophages (43). Gastric cancer TDEs also induce monocytes to differentiate into PD-1+ macrophages, which exhibit an M2-like surface profile and impair CD8+ T cell function (44). In Helicobacter pylori-infected gastric cancer, TDEs are enriched with activated MET, which educates the macrophages towards an M2 phenotype with high IL-1β expression (45).
In turn, TAM-derived exosomes may act on gastric cancer cells. ApoE is a highly specific protein in TAM-derived exosomes that can activate the PI3K-Akt signaling pathway in gastric cancer cells to rebuild the cytoskeleton for migration (46). Exosomal miR-223 derived from macrophages can provide proliferation/EMT signals for metastasis via the PTEN-PI3K/AKT pathway (47). Interestingly, during peritoneal PMN formation, TAM can transfer TDEs from gastric cancer cells to surrounding stromal cells, including peritoneal mesothelial cells, fibroblasts, and endothelial cells, and induce their conversion into cancer-associated fibroblasts (CAF)-like cells (48).
Like macrophages, neutrophils can also polarize into N1 or N2 phenotypes. N1 cells possess anti-tumor activity due to their immune-activating cytokines/chemokines that recruit and activate CD8+ T cells (49), while most TANs appear to have “pro-tumorigenic” N2 phenotypes (50). TANs release multifarious pro-PMN substrates, including reactive oxygen species and reactive nitrogen species that cause genetic instability and carcinogenesis (51), elastase that degrades tumor-suppressing proteins (52), prostaglandin E2 that drives wound inflammation-mediated pre-neoplastic cell proliferation (53), VEGF and MMP9 that facilitate angiogenesis (54), and CCL2/17 that recruits other immunosuppression cells (55, 56). In gastric cancer, membrane HMGB1 on TDEs interacts with TLR4 on neutrophils, which induces TAN polarization through NF-κB and an autophagic response to reshape the metastatic niche (57).
Dendritic cells are the central antigen-presenting cells in anti-tumor immunity. Epstein-Barr virus (EBV)-positive gastric cancer is characterized by an abundance of infiltrated-immune cells, including dendritic cells, but with suppressed anti-tumor immunity. Hinata et al. revealed that EBV-positive gastric cancer has more TDE secretion than EBV-negative gastric cancer (58). These TDEs suppress dendritic cell maturation, represented with low CD86 expression, and inhibit activation of other immune cells (58).
Anti-tumor immunity is largely imposed by T cells, which are potential targets of TDEs for immunosuppression. Gastric cancer TDEs can induce T cell apoptosis by mediating PI3K proteasome degradation and caspases 3, 8, and 9 activation (59). TDE-bound PD-L1 is an immune response “brake” that is more stable than soluble PD-L1 and is co-expressed with MHC-I. Thus, TDE-bound PD-L1 is able to lead to much stronger T cell dysfunction than soluble PD-L1 in gastric cancer (60).
A group of immunomodulatory T cells, such as regulatory T (Treg) cells, are essential in coordinating distinct immunoregulatory programs and creating an immunologically permissive environment for tumor metastasis (61). Gastric cancer TDEs also induce FOXP3+ Treg cell differentiation from naive T cells through exosomal TGF-β1 (62). In addition, glucose deprivation can induce an exosome-mediated miR-451 redistribution from tumor to T cells, resulting in T-helper 17 cell differentiation in gastric cancer (63). A lncRNA, RP11-323N12.5, is found upregulated in gastric cancer cells as well as tumor-infiltrated lymphocytes (64). Upregulated RP11-323N12.5 in tumor-infiltrated lymphocytes is derived from TDEs and contributes to Treg differentiation by upregulating the Hippo pathway effector, YAP1 (64).
In gastric cancer, TDEs may also regulate T cell functions indirectly by a third cell. They can regulate immunomodulation function of MSCs through the NF-κB signaling pathway, which further activates CD69 and CD25 on the surface of T cells (65). Furthermore, gastric cancer TDEs also have immunogenicity to induce dendritic cell maturation for tumor-specific T cell responses (66).
MDSCs are a heterogeneous population of immature myeloid cells that suppress immunity during tumor progression, inflammation, or infection (67). They are indispensable for PMN formation due to their roles in immunosuppression, vascular permeability, and collagen remodeling (68). Many immune cells in PMNs, including cytotoxic T cells, Treg cells, NKT cells, dendritic cells, and macrophages, are regulated by MDSCs during tumor metastasis (69). TDEs have been shown to transform bone marrow myeloid cells into MDSCs (70). In gastric cancer, TDEs also help MDSC expansion by delivering miRNA-107 targeting DICER1 and PTEN-PI3K signaling in the recipient MDSC (71).
The survival of cancer cells in the metastatic site highly depends on the stromal microenvironment, which is composed of fibroblasts, pericytes, MSC, endothelial cells, ECM, and vasculature (72). TDEs have been shown to remodel stroma by reprograming these stromal cells and ECMs for tumor colonization (Figure 4).
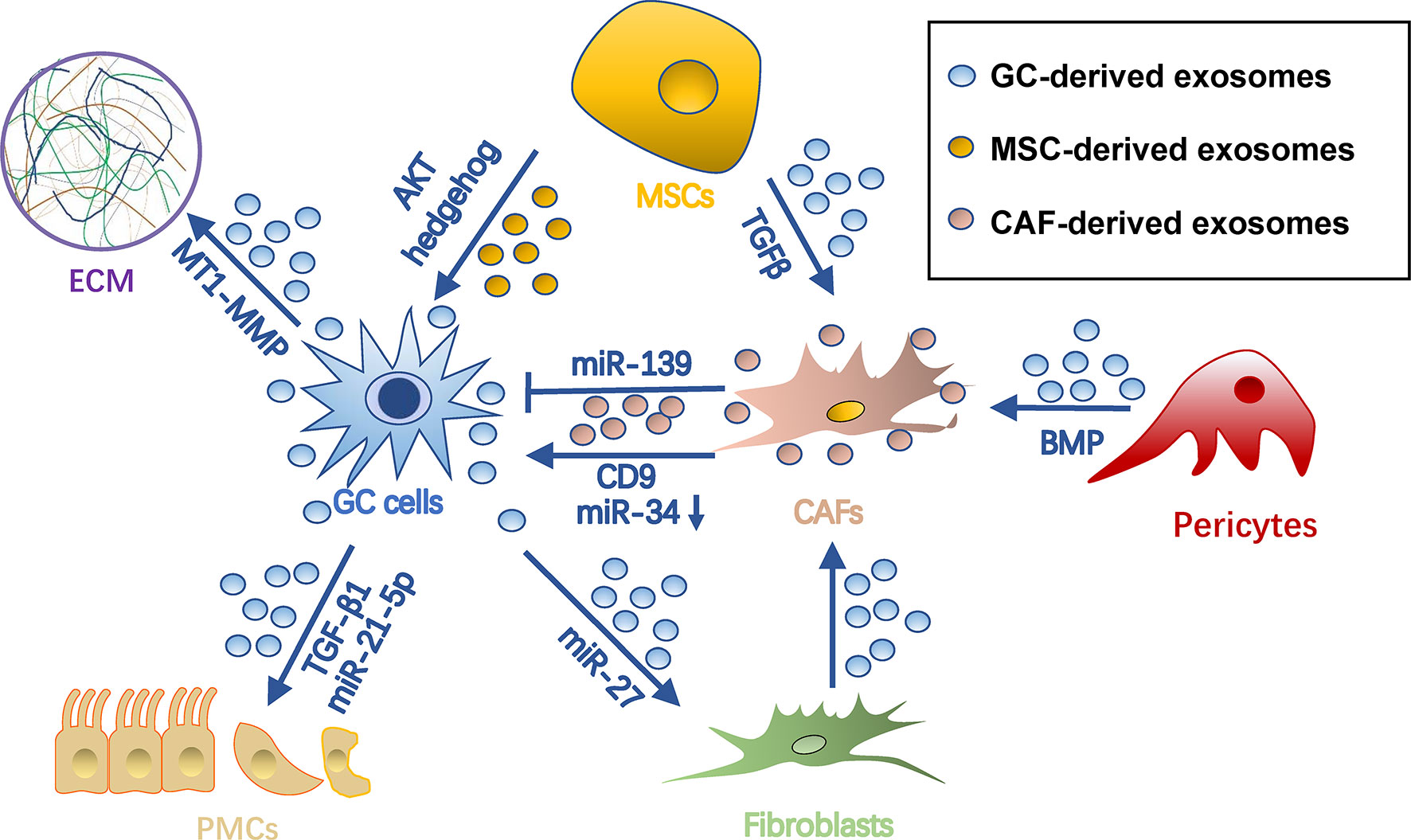
Figure 4 Roles of exosomes in stroma remodeling in gastric cancer pre-metastatic niches. Gastric cancer cell-derived exosomes can convert pericytes, fibroblasts, and MSCs into CAFs. Also, they remodel ECM and induce MMT and apoptosis of PMCs through different cargo during PMN formation. Meanwhile, exosomes from MSCs and CAFs can in turn promote tumor cell progression. Arrows represent activation, whereas bar-headed arrows represent inhibition. CAF, cancer-associated fibroblast; ECM extracellular matrix; MSC, mesenchymal stem cell; PMN, pre-metastatic niche.
CAFs are a group of heterogeneous cells that may differentiate from multiple origins (73) and are potent regulators of tumors and tumor microenvironments (74, 75). Exosomes are important for inducing pro-tumorigenic/metastatic CAFs. For example, gastric cancer TDEs contain high levels of miRNA-27a and can deliver them into fibroblasts for CAF reprogramming in a CSRP2-dependent manner (76). Conversely, CAFs with miRNA-27a provide a favorable environment pleiotropically for malignant behavior of gastric cancer cells (76). TGF-β on gastric cancer TDEs triggers differentiation of umbilical cord-derived MSC into CAF by activating the Smad pathway (77). In addition, gastric cancer TDEs induce transition of pericytes into CAFs by exosome-mediated BMP transfer and PI3K/AKT and MEK/ERK pathway activation (78).
Exosomes from CAF in turn impact gastric cancer cells. CAF-derived CD9+ exosomes potently stimulate MMP2 expression and migration in scirrhous-type gastric cancer cells (OCUM-12 and NUGC-3 cells) (79). This process was hindered by adding CD9 neutralizing antibodies or siRNAs targeting CD9 (79). In addition, CAF-derived exosomes are internalized by gastric cancer cells and facilitate gastric cancer proliferation and invasion (80). This process was mediated by exosomal miRNA-34, which targets 16 onco-promoting molecules in gastric cancer (80). Notably, not all CAF-derived exosomes are onco-promoting. For instance, the exosomal miR-139 derived from gastric cancer CAFs is anti-metastatic, decreasing MMP11 in tumor microenvironments (81).
MSCs are another origin of tumor stromal cells, which are able to differentiate into several types of mesenchymal cells, including adipocytes, CAFs, pericytes, and endothelial-like cells (82). They orchestrate an environment associated with tumor survival, angiogenesis, and immunosuppression, which all contribute to tumor growth and metastasis (83). MSC-derived exosomes have been proven indispensable during this process. In GC, they promote tumor growth and migrations via activation of the AKT (84) or hedgehog pathway (85). Besides, bone marrow-derived MSC exosomes are able to activate the ERK1/2 pathway in gastric cancer cells to upregulate VEGF expression, promoting angiogenesis (86). In a gastric cancer precancerous model, p53 deficient bone marrow-MSCs could secrete UBR2-rich exosomes, which activate the Wnt/catenin pathway in tumor cells and result in tumor growth, motility, and stemness (87). MSC exosomes could also induce chemoresistance in gastric cancer cells by antagonizing 5-fluorouracil-induced apoptosis and enhancing expression of multidrug resistance-associated proteins (88). This process is mediated by the calcium/calmodulin-dependent protein kinases and Raf/MEK/ERK kinase cascades (88).
ECM is a dynamic extracellular environment with continuous degradation, deposition, and modification and functions to fine-tune the elasticity and compressive or tensile strength of tissues (89). Dysregulated ECM remodeling includes irreversible proteolysis and crosslinking, which in turn influences microenvironmental cues, angiogenesis, and tissue biomechanics, being crucial for PMN formation (90). As far as we know, exosomes not only influence ECM dynamics directly, but also induce imbalances of metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) released by tumor or stromal cells. Being rich in stable MMPs, TDEs help degrade collagen, laminin, and fibronectin to reshape ECM (91, 92). mRNA of membrane type 1-MMP is contained in and protected by gastric cancer TDEs and is highly associated with lymphatic metastasis (93). CD63 is an exosome marker as well as a receptor for TIMP1 (94). CD63 positivity in gastric cancer cells or gastric cancer stromal cells is significantly correlated with lymph node metastasis, so it is inferred that CD63-positive exosomes of gastric cancer might also be associated with metastatic niche formation (95), probably by binding with and inhibiting TIMP1. CD9+ exosomes derived from CAF also potently stimulate MMP expression in tumor environments (79).
The peritoneum is composed of a layer of PMCs and connective tissues and is the first barrier to tumor attachment and invasion in peritoneal metastasis (96, 97). Mesothelial-mesenchymal transition (MMT) has been observed to occur early in intraperitoneal dissemination, thus being an important process of peritoneal PMN formation (98, 99). Through MMT, mesothelial cells acquire a migratory phenotype expressing pro-inflammatory cytokines, angiogenetic factors, and a specialized ECM to disintegrate the peritoneum (100). Gastric cancer TDEs are able to induce MMT of PMCs (35, 101–103). For example, TDE-derived miR-21-5p can promote MMT of PMCs by suppressing SMAD7 (101). In addition, internalization of gastric cancer TDEs upregulates expression of adhesion-related molecules, including fibronectin-1 and laminin-γ1, in PMCs and promotes PMC-gastric cancer cell adhesion, which favors tumor settlement (102). Additionally, gastric cancer TDEs also elicit mesothelial barrier disruption and fibrosis by inducing concurrent apoptosis and MMT (103). Ascites-derived exosomes also potently facilitated TGF-β1-induced MMT of PMCs during gastric cancer peritoneal metastasis (104).
Angiogenesis is an essential step in the establishment of PMN by providing oxygen and nutrients for tumor growth and allowing circulating tumor cells to arrive (105). Furthermore, angiogenic factor VEGF has an immunosuppressive effect during PMN establishment (106, 107). Gastric cancer TDEs can activate angiogenesis by delivering several types of miRNAs to vascular endothelial cells, including miR-130a targeting c-MYB (108), miR-135b targeting FOXO1 (109), miR-155 targeting FOXO3 (110) and c-MYB (111), miR-23a targeting PTEN (112) (Figure 5). Transmembrane protein Tetraspanin 8 on gastric cancer TDEs can also activate vascular endothelial cells by stimulating its ERK/MAPK pathway (113). Exosomal YB-1 from gastric cancer also promotes angiogenesis from endothelial cells by upregulating specific angiogenic factors (114). Irradiation-treated gastric cancer can produce TDEs with enhanced ability to induce angiogenesis of vascular endothelial cells (115). Consequently, VEGFR inhibitor, Apatinib, inhibits this process and could be used in combination with radiotherapy for better results (115). In addition, exosomes derived from non-tumor cells, such as MSCs, may also be angiogenic (86).
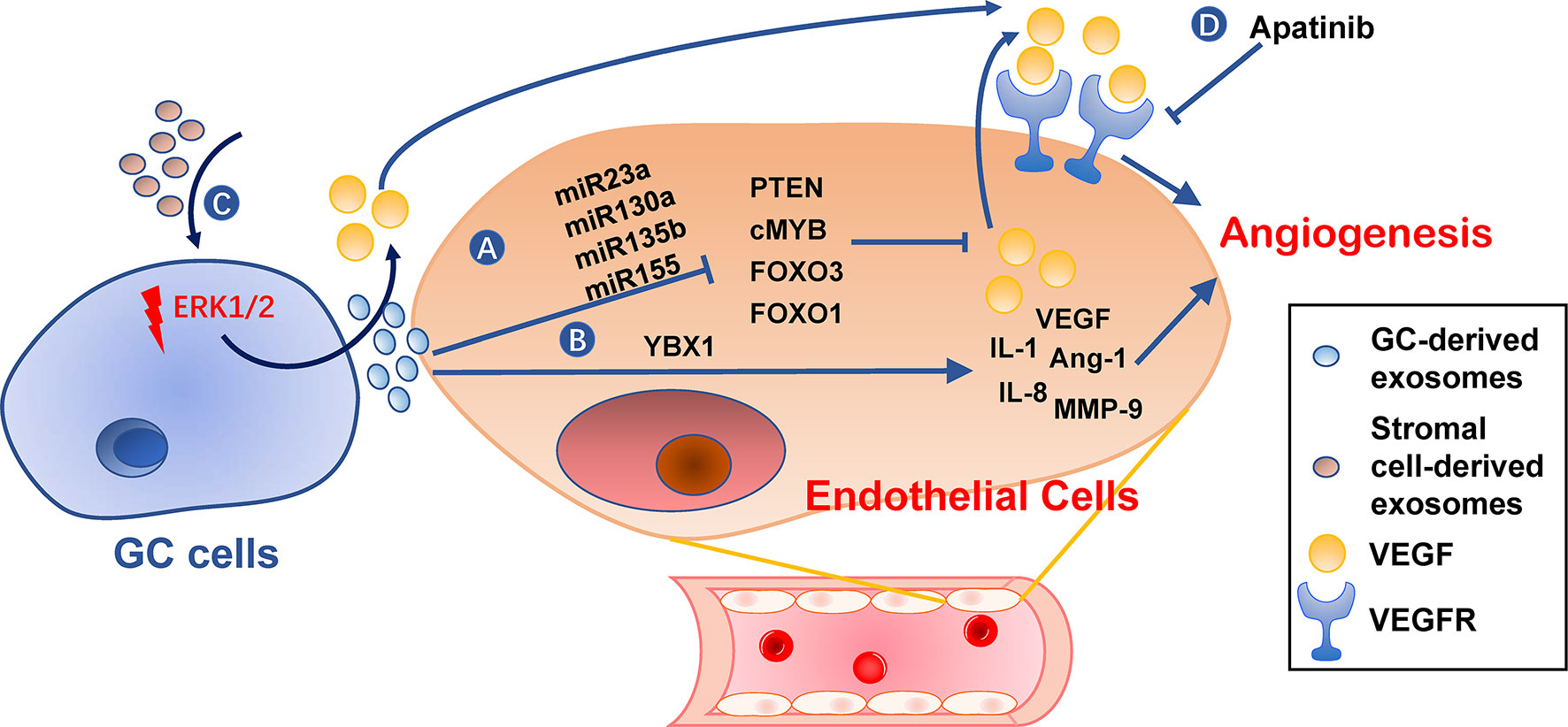
Figure 5 Roles of exosomes in angiogenesis. (A, B) Gastric cancer cell-derived exosomes can induce expression of pro-angiogenesis molecules, such as VEGF, IL-1, IL-8, Ang-1, and MMP-9, in endothelial cells by delivering miRNAs (A) or YBX1 (B). (C) Stromal cell-derived exosomes or tumor-derived exosomes also promote VEGF expression in gastric cancer cells. (D) VEGFR inhibitors, such as Apatinib, can hinder angiogenesis caused by these exosomes. GC, gastric cancer.
Metastatic organotropism is one of the characteristics of PMN (11). Organotropism results from an active selection and education by the primary gastric cancer cells of a specific distant microenvironment into a PMN (116). As reported by Hoshino and colleagues, integrin patterns in TDEs determine the organotropic metastases through an integrin-dependent uptake of exosomes by different organs (117). For example, exosomal integrins α6β4 and α6β1 are lung-tropic, while exosomal integrin αvβ5 was liver-tropic (117). The uptake of exosomal integrin by local cells activates Src phosphorylation and pro-inflammatory factors to allow PMN establishment (117).
In gastric cancer liver metastasis, TDEs deliver membrane EGFR to liver stromal cells prior to liver metastasis (118). Recipient cells then activate hepatocyte growth factor paracrine, which provides a favorable environment for tumor landing by binding to their c-MET receptor (118). The lymphotropic gastric cancer cells produce CD97-enriched TDEs, which effectively aid gastric cancer metastasis by creating distant lymphatic PMN (119). Additionally, PMCs are peritoneum-specific cells and, therefore, the PMC-targeting exosomes mentioned above are considered peritoneum-tropic (101–103). Before gastric cancer peritoneal metastasis, Wnt3a-containing TDEs induce PMC infiltration into the gastric wall to create PMN in these sites, which in turn promoted subserosal invasions of gastric cancer cells and further dissemination (120). In addition, three miRNAs, namely miR-10b-5p, miR-101-3p, and miR-143-5p, are proposed biomarkers for gastric cancer lymph node, ovarian, and liver metastasis, respectively (121).
The lipid bilayer membrane structure of the exosome maintains the stability of its cargo well, so exosomes have great potential as non-invasive biopsy specimens for cancer detection and prognosis (122, 123). So far, we may see potential roles of exosomes as biomarkers in gastric cancer metastasis prediction, prevention, and treatment. Some studies have shown evidence of clinical relevance between exosome and gastric cancer metastasis (summarized in Table 1). For example, exosomal PSMA3 and PSMA6 are explicitly enriched in serum during metastatic gastric cancer, but not primary gastric cancer, thus being a potential biomarker for gastric cancer metastasis (124). Further, exosomal miR-10b-5p, miR-101-3p, and miR-143-5p were proposed biomarkers for gastric cancer lymph node, ovarian, and liver metastasis, respectively, helping distinguish gastric cancer patients with various types of metastasis (121). Exosomal miRNAs from peritoneum lavage fluid, including miR-21 and miR-1225-5p, are specifically elevated in gastric cancer peritoneal metastasis after curative gastric cancer resection, thus providing a novel approach to early diagnosis of peritoneal dissemination of gastric cancer (125). However, evidence is limited up to date.

Table 1 Evidence of clinical relevance between exosomal contents and metastatic sites in gastric cancer.
The characteristics of exosomes, including high biocompatibility, safety, and nano-sized diameters, allow effective drug-loading capacity and long blood circulation half-life. Thus, they serve as an ideal system to deliver cytokines, DNA, RNA, adjuvants, and even vaccines for treatment (126, 127). For example, HGF siRNA packed in exosomes can be transported into gastric cancer cells, where it decreases tumor growth rates and blood vessels in vivo (128). Exosomes from heat-treated gastric cancer malignant ascites have improved immunogenicity, being able to promote dendritic cell maturation and induce a tumor-specific cytotoxic T cell response (66). The organotropic factors, such as integrins on the exosome surface, could be used to improve targeting specificity and deliver drugs to specific tissues (129). However, it remains unknown whether exosome-based drug delivery could be used to prevent or treat gastric cancer metastasis to a specific organ.
Despite immature clinical usage of exosomes in cancer, we believe it will be an important utility in metastasis assessment, prevention, and treatment in the future. First, methods such as nano-plasmonic sensors (14), microfluidic exosome analysis (130), and surface plasmon resonance imaging (131) were developed for exosome analysis with a small amount of sample, compared with routine methods. These techniques will allow exosomes to be a good tool for liquid biopsy. Second, because cancer cells, immune cells, and stromal cells all produce exosomes into the circulation, they will provide comprehensive information from tumor environments and PMN, as long as we have effective ways of exosome classification (132). An interesting technique utilizes a proximity barcoding method to profile surface proteins on individual exosomes, which allows both tissue origination and quantification of exosomes in mixed samples (133). Further classification based on exosome function, organophilicity, biological distribution, and immunogenicity, at single exosome levels, may be considered (134). Third, besides exosome-based drug delivery, another direction is to target PMN-promoting exosomes, based on their essential role in coordinating PMN formation. Although not feasible currently, targeting exosomes may come true in the future with fine characterizations of specific cell-derived exosomes and membrane markers for targeting. Fourth, some chemotherapeutics can induce certain exosome secretions and alters exosome composition, which degrades ECM and activates macrophage to release TNF-α (135). Therefore, whether these “chemo-exosomes” are PMN-promoting may be taken into consideration when choosing therapeutic regimens. Last but not least, despite of insufficient evidence, some studies have reported how oncogenes, cytokines, and exosomes interacted with each other in cancer, as reported in a squamous cancer cell line that cortactin promotes exosome secretion (136). Targeting genes or cytokines that regulate exosome packaging and secretion would be another way for exosome elimination and PMN inhibition.
Exosomes play a vital role in establishing PMN formation by immunosuppression, angiogenesis, stroma remodeling, PMC MMT, and organotropism and have great potential in metastasis prediction, prevention, and treatment. Yet, their clinical usage is limited, and further studies are needed to validate the translational value of exosomes in PMNs of gastric cancer.
JG searched databases and collected studies. JG and SL summarized the contents, wrote the manuscript, and drew the figures. QX, XZ, MH, and XD helped in data collection and figure design. LL designed this review, edited the article, and supervised the work. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (81172487 to LL and 81500092 to SL) and Natural Science Foundation of Shandong Province (ZR201702180008 to LL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The manuscript was edited by Alexandra H. Marshall (Marshall Medical Communications).
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric Cancer. Lancet (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
3. Digklia A, Wagner AD. Advanced Gastric Cancer: Current Treatment Landscape and Future Perspectives. World J Gastroenterol (2016) 22:2403–14. doi: 10.3748/wjg.v22.i8.2403
4. Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, et al. Treatment of Gastric Cancer. World J Gastroenterol (2014) 20:1635–49. doi: 10.3748/wjg.v20.i7.1635
5. Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. Peritoneal Carcinomatosis of Gastric Origin: A Population-Based Study on Incidence, Survival and Risk Factors. Int J Cancer (2014) 134:622–8. doi: 10.1002/ijc.28373
6. Kanda M, Kobayashi D, Tanaka C, Iwata N, Yamada S, Fujii T, et al. Adverse Prognostic Impact of Perioperative Allogeneic Transfusion on Patients With Stage II/III Gastric Cancer. Gastri Cancer (2016) 19:255–63. doi: 10.1007/s10120-014-0456-x
7. Kanda M, Kodera Y. Molecular Mechanisms of Peritoneal Dissemination in Gastric Cancer. World J Gastroenterol (2016) 22:6829–40. doi: 10.3748/wjg.v22.i30.6829
8. Kaplan RN, Rafii S, Lyden D. Preparing the “Soil”: The Premetastatic Niche. Cancer Res (2006) 66:11089–93. doi: 10.1158/0008-5472.CAN-06-2407
9. Chin AR, Wang SE. Cancer Tills the Premetastatic Field: Mechanistic Basis and Clinical Implications. Clin Cancer Res (2016) 22:3725–33. doi: 10.1158/1078-0432.CCR-16-0028
10. Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-Positive Haematopoietic Bone Marrow Progenitors Initiate the Pre-Metastatic Niche. Nature (2005) 438:820–7. doi: 10.1038/nature04186
11. Liu Y, Cao X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell (2016) 30:668–81. doi: 10.1016/j.ccell.2016.09.011
12. Meehan K, Vella LJ. The Contribution of Tumour-Derived Exosomes to the Hallmarks of Cancer. Crit Rev Clin Lab Sci (2016) 53:121–31. doi: 10.3109/10408363.2015.1092496
13. Kalluri R. The Biology and Function of Exosomes in Cancer. J Clin Invest (2016) 126:1208–15. doi: 10.1172/JCI81135
14. Im H, Shao H, Park YI, Peterson VM, Castro CM, Weissleder R, et al. Label-Free Detection and Molecular Profiling of Exosomes With a Nano-Plasmonic Sensor. Nat Biotechnol (2014) 32:490–5. doi: 10.1038/nbt.2886
15. Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-Mediated Metastasis: Communication From a Distance. Dev Cell (2019) 49:347–60. doi: 10.1016/j.devcel.2019.04.011
16. Ingenito F, Roscigno G, Affinito A, Nuzzo S, Scognamiglio I, Quintavalle C, et al. The Role of Exo-miRNAs in Cancer: A Focus on Therapeutic and Diagnostic Applications. Int J Mol Sci (2019) 20:4687. doi: 10.3390/ijms20194687
17. Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, et al. Circular RNA is Enriched and Stable in Exosomes: A Promising Biomarker for Cancer Diagnosis. Cell Res (2015) 25:981–4. doi: 10.1038/cr.2015.82
18. Théry C, Ostrowski M, Segura E. Membrane Vesicles as Conveyors of Immune Responses. Nat Rev Immunol (2009) 9:581–93. doi: 10.1038/nri2567
19. Shuchun W, Lei P, Jiajia Y, Huaiming S, Duochen J, Xuan L, et al. Exosomal Transfer of miR-15b-3p Enhances Tumorigenesis and Malignant Transformation Through the DYNLT1/Caspase-3/Caspase-9 Signaling Pathway in Gastric Cancer. J Exp Clin Cancer Res (2020) 39:32. doi: 10.1186/s13046-019-1511-6
20. Cao S, Lin L, Xia X, Wu H. Lncrna SPRY4-IT1 Regulates Cell Proliferation and Migration by Sponging miR-101-3p and Regulating Ampk Expression in Gastric Cancer. Mol Ther Nucleic Acids (2019) 17:455–64. doi: 10.1016/j.omtn.2019.04.030
21. Wang M, Zhao C, Shi H, Zhang B, Zhang L, Zhang X, et al. Deregulated microRNAs in Gastric Cancer Tissue-Derived Mesenchymal Stem Cells: Novel Biomarkers and a Mechanism for Gastric Cancer. Br J Cancer (2014) 110:1199–210. doi: 10.1038/bjc.2014.14
22. Yoon JH, Ham IH, Kim O, Ashktorab H, Smoot DT, Nam SW, et al. Gastrokine 1 Protein is a Potential Theragnostic Target for Gastric Cancer. Gastri Cancer (2018) 21:956–67. doi: 10.1007/s10120-018-0828-8
23. Liu X, Lu Y, Xu Y, Hou S, Huang J, Wang B, et al. Exosomal Transfer of miR-501 Confers Doxorubicin Resistance and Tumorigenesis Via Targeting of BLID in Gastric Cancer. Cancer Lett (2019) 459:122–34. doi: 10.1016/j.canlet.2019.05.035
24. Wang J, Lv B, Su Y, Wang X, Bu J, Yao L. Exosome-Mediated Transfer of Lncrna HOTTIP Promotes Cisplatin Resistance in Gastric Cancer Cells by Regulating Hmga1/miR-218 Axis. Onco Targets Ther (2019) 12:11325–38. doi: 10.2147/OTT.S231846
25. Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, et al. Exosomal Transfer of Tumor-Associated Macrophage-Derived miR-21 Confers Cisplatin Resistance in Gastric Cancer Cells. J Exp Clin Cancer Res (2017) 36:53. doi: 10.1186/s13046-017-0528-y
26. Wang M, Qiu R, Yu S, Xu X, Li G, Gu R, et al. Paclitaxel−Resistant Gastric Cancer MGC−803 Cells Promote Epithelial−to−Mesenchymal Transition and Chemoresistance in Paclitaxel−Sensitive Cells Via Exosomal Delivery of Mir−155−5p. Int J Oncol (2019) 54:326–38. doi: 10.3892/ijo.2018.4601
27. Jingyue S, Xiao W, Juanmin Z, Wei L, Daoming L, Hong X. TFAP2E Methylation Promotes 5−Fluorouracil Resistance Via Exosomal miR−106a−5p and miR−421 in Gastric Cancer MGC−803 Cells. Mol Med Rep (2019) 20:323–31. doi: 10.3892/mmr.2019.10237
28. Sahai E. Illuminating the Metastatic Process. Nat Rev Cancer (2007) 7:737–49. doi: 10.1038/nrc2229
29. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-Mesenchymal Transitions in Development and Disease. Cell (2009) 139:871–90. doi: 10.1016/j.cell.2009.11.007
30. Syn N, Wang L, Sethi G, Thiery JP, Goh BC. Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape From Immunosurveillance. Trends Pharmacol Sci (2016) 37:606–17. doi: 10.1016/j.tips.2016.04.006
31. Pan L, Liang W, Fu M, Huang ZH, Li X, Zhang W, et al. Exosomes-Mediated Transfer of Long Noncoding RNA ZFAS1 Promotes Gastric Cancer Progression. J Cancer Res Clin Oncol (2017) 143:991–1004. doi: 10.1007/s00432-017-2361-2
32. Piao HY, Guo S, Wang Y, Zhang J. Exosome-Transmitted Lncrna PCGEM1 Promotes Invasive and Metastasis in Gastric Cancer by Maintaining the Stability of SNAI1. Clin Transl Oncol (2021) 23:246–56. doi: 10.1007/s12094-020-02412-9
33. Xing Z, Sen W, Haixiao W, Jiacheng C, Xiaoxu H, Zheng C, et al. Circular RNA circNRIP1 Acts as a microRNA-149-5p Sponge to Promote Gastric Cancer Progression Via the AKT1/mTOR Pathway. Mol Cancer (2019) 18:20. doi: 10.1186/s12943-018-0935-5
34. Fu H, Yang H, Zhang X, Wang B, Mao J, Li X, et al. Exosomal TRIM3 is a Novel Marker and Therapy Target for Gastric Cancer. J Exp Clin Cancer Res (2018) 37:162. doi: 10.1186/s13046-018-0825-0
35. Hu Y, Qi C, Liu X, Zhang C, Gao J, Wu Y, et al. Malignant Ascites-Derived Exosomes Promote Peritoneal Tumor Cell Dissemination and Reveal a Distinct miRNA Signature in Advanced Gastric Cancer. Cancer Lett (2019) 457:142–50. doi: 10.1016/j.canlet.2019.04.034
36. Yang H, Fu H, Wang B, Zhang X, Mao J, Li X, et al. Exosomal miR-423-5p Targets SUFU to Promote Cancer Growth and Metastasis and Serves as a Novel Marker for Gastric Cancer. Mol Carcinog (2018) 57:1223–36. doi: 10.1002/mc.22838
37. Li C, Liu DR, Li GG, Wang HH, Li XW, Zhang W, et al. CD97 Promotes Gastric Cancer Cell Proliferation and Invasion Through Exosome-Mediated MAPK Signaling Pathway. World J Gastroenterol (2015) 21:6215–28. doi: 10.3748/wjg.v21.i20.6215
38. Lu J, Wang YH, Yoon C, Huang XY, Xu Y, Xie JW, et al. Circular RNA circ-RanGAP1 Regulates VEGFA Expression by Targeting miR-877-3p to Facilitate Gastric Cancer Invasion and Metastasis. Cancer Lett (2020) 471:38–48. doi: 10.1016/j.canlet.2019.11.038
39. Kersy O, Loewenstein S, Lubezky N, Sher O, Simon NB, Klausner JM, et al. Omental Tissue-Mediated Tumorigenesis of Gastric Cancer Peritoneal Metastases. Front Oncol (2019) 9:1267. doi: 10.3389/fonc.2019.01267
40. Whiteside TL. Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv Clin Chem (2016) 74:103–41. doi: 10.1016/bs.acc.2015.12.005
41. Zheng X, Turkowski K, Mora J, Brüne B, Seeger W, Weigert A, et al. Redirecting Tumor-Associated Macrophages to Become Tumoricidal Effectors as a Novel Strategy for Cancer Therapy. Oncotarget (2017) 8:48436–52. doi: 10.18632/oncotarget.17061
42. Qian BZ, Pollard JW. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell (2010) 141:39–51. doi: 10.1016/j.cell.2010.03.014
43. Wu L, Zhang X, Zhang B, Shi H, Yuan X, Sun Y, et al. Exosomes Derived From Gastric Cancer Cells Activate NF-kappaB Pathway in Macrophages to Promote Cancer Progression. Tumor Biol (2016) 37:12169–80. doi: 10.1007/s13277-016-5071-5
44. Wang F, Li B, Wei Y, Zhao Y, Wang L, Zhang P, et al. Tumor-Derived Exosomes Induce PD1(+) Macrophage Population in Human Gastric Cancer That Promotes Disease Progression. Oncogenesis (2018) 7:41. doi: 10.1038/s41389-018-0049-3
45. Che Y, Geng B, Xu Y, Miao X, Chen L, Mu X, et al. Helicobacter Pylori-Induced Exosomal MET Educates Tumour-Associated Macrophages to Promote Gastric Cancer Progression. J Cell Mol Med (2018) 22:5708–19. doi: 10.1111/jcmm.13847
46. Zheng P, Luo Q, Wang W, Li J, Wang T, Wang P, et al. Tumor-Associated Macrophages-Derived Exosomes Promote the Migration of Gastric Cancer Cells by Transfer of Functional Apolipoprotein E. Cell Death Dis (2018) 9:434. doi: 10.1038/s41419-018-0465-5
47. Zheng PM, Gao HJ, Li JM, Zhang P, Li G. Effect of Exosome-Derived miR-223 From Macrophages on the Metastasis of Gastric Cancer Cells. Zhonghua Yi Xue Za Zhi (2020) 100:1750–5. doi: 10.3760/cma.j.cn112137-20200425-01309
48. Umakoshi M, Takahashi S, Itoh G, Kuriyama S, Sasaki Y, Yanagihara K, et al. Macrophage-Mediated Transfer of Cancer-Derived Components to Stromal Cells Contributes to Establishment of a Pro-Tumor Microenvironment. Oncogene (2019) 38:2162–76. doi: 10.1038/s41388-018-0564-x
49. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-beta: “N1” Versus “N2Tan” Cancer Cell (2009) 16:183–94. doi: 10.1016/j.ccr.2009.06.017
50. Hurt B, Schulick R, Edil B, El Kasmi KC, Barnett C Jr. Cancer-Promoting Mechanisms of Tumor-Associated Neutrophils. Am J Surg (2017) 214:938–44. doi: 10.1016/j.amjsurg.2017.08.003
51. Wu L, Saxena S, Singh RK. Neutrophils in the Tumor Microenvironment. Adv Exp Med Biol (2020) 1224:1–20. doi: 10.1007/978-3-030-35723-8_1
52. Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil Elastase-Mediated Degradation of IRS-1 Accelerates Lung Tumor Growth. Nat Med (2010) 16:219–23. doi: 10.1038/nm.2084
53. Antonio N, Bønnelykke-Behrndtz ML, Ward LC, Collin J, Christensen IJ, Steiniche T, et al. The Wound Inflammatory Response Exacerbates Growth of Pre-Neoplastic Cells and Progression To Cancer. EMBO J (2015) 34:2219–36. doi: 10.15252/embj.201490147
54. Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, et al. Peritumoral Neutrophils Link Inflammatory Response to Disease Progression by Fostering Angiogenesis in Hepatocellular Carcinoma. J Hepatol (2011) 54:948–55. doi: 10.1016/j.jhep.2010.08.041
55. Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, et al. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology (2016) 150:1646–58.e17. doi: 10.1053/j.gastro.2016.02.040
56. Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, et al. Neutrophils Recruit Regulatory T-cells Into Tumors Via Secretion of CCL17–a New Mechanism of Impaired Antitumor Immunity. Int J Cancer (2014) 135:1178–86. doi: 10.1002/ijc.28770
57. Zhang X, Shi H, Yuan X, Jiang P, Qian H, Xu W. Tumor-Derived Exosomes Induce N2 Polarization of Neutrophils to Promote Gastric Cancer Cell Migration. Mol Cancer (2018) 17:146. doi: 10.1186/s12943-018-0898-6
58. Hinata M, Kunita A, Abe H, Morishita Y, Sakuma K, Yamashita H, et al. Exosomes of Epstein-Barr Virus-Associated Gastric Carcinoma Suppress Dendritic Cell Maturation. Microorganisms (2020) 8:1776. doi: 10.3390/microorganisms8111776
59. Qu JL, Qu XJ, Qu JL, Qu XJ, Zhao MF, Teng YE, et al. The Role of Cbl Family of Ubiquitin Ligases in Gastric Cancer Exosome-Induced Apoptosis of Jurkat T Cells. Acta Oncol (2009) 48:1173–80. doi: 10.3109/02841860903032817
60. Fan Y, Che X, Qu J, Hou K, Wen T, Li Z, et al. Exosomal PD-L1 Retains Immunosuppressive Activity and is Associated With Gastric Cancer Prognosis. Ann Surg Oncol (2019) 26:3745–55. doi: 10.1245/s10434-019-07431-7
61. Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, Klebanoff CA, et al. Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche. Cell (2016) 166:1117–31.e14. doi: 10.1016/j.cell.2016.07.032
62. Yen EY, Miaw SC, Yu JS, Lai IR. Exosomal TGF-β1 is Correlated With Lymphatic Metastasis of Gastric Cancers. Am J Cancer Res (2017) 7:2199–208.
63. Liu F, Bu Z, Zhao F, Xiao D. Increased T-helper 17 Cell Differentiation Mediated by Exosome-Mediated microRNA-451 Redistribution in Gastric Cancer Infiltrated T Cells. Cancer Sci (2018) 109:65–73. doi: 10.1111/cas.13429
64. Wang J, Huang F, Shi Y, Zhang Q, Xu S, Yao Y, et al. Rp11-323N12.5 Promotes the Malignancy and Immunosuppression of Human Gastric Cancer by Increasing YAP1 Transcription. Gastri Cancer (2021) 24:85–102. doi: 10.1007/s10120-020-01099-9
65. Shen Y, Xue C, Li X, Ba L, Gu J, Sun Z, et al. Effects of Gastric Cancer Cell-Derived Exosomes on the Immune Regulation of Mesenchymal Stem Cells by the NF-kB Signaling Pathway. Stem Cells Dev (2019) 28:464–76. doi: 10.1089/scd.2018.0125
66. Zhong H, Yang Y, Ma S, Xiu F, Cai Z, Zhao H, et al. Induction of a Tumour-Specific CTL Response by Exosomes Isolated From Heat-Treated Malignant Ascites of Gastric Cancer Patients. Int J Hyperther (2011) 27:604–11. doi: 10.3109/02656736.2011.564598
67. Talmadge JE, Gabrilovich DI. History of Myeloid-Derived Suppressor Cells. Nat Rev Cancer (2013) 13:739–52. doi: 10.1038/nrc3581
68. Wang Y, Ding Y, Guo N, Wang S. Mdscs: Key Criminals of Tumor Pre-Metastatic Niche Formation. Front Immunol (2019) 10:172. doi: 10.3389/fimmu.2019.00172
69. Motallebnezhad M, Jadidi-Niaragh F, Qamsari ES, Bagheri S, Gharibi T, Yousefi M. The Immunobiology of Myeloid-Derived Suppressor Cells in Cancer. Tumour Biol (2016) 37:1387–406. doi: 10.1007/s13277-015-4477-9
70. Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, et al. Induction of Myeloid-Derived Suppressor Cells by Tumor Exosomes. Int J Cancer (2009) 124:2621–33. doi: 10.1002/ijc.24249
71. Ren W, Zhang X, Li W, Feng Q, Feng H, Tong Y, et al. Exosomal miRNA-107 Induces Myeloid-Derived Suppressor Cell Expansion in Gastric Cancer. Cancer Manag Res (2019) 11:4023–40. doi: 10.2147/CMAR.S198886
72. Guo Y, Ji X, Liu J, Fan D, Zhou Q, Chen C, et al. Effects of Exosomes on Pre-Metastatic Niche Formation in Tumors. Mol Cancer (2019) 18:39. doi: 10.1186/s12943-019-0995-1
73. Tao L, Huang G, Song H, Chen Y, Chen L. Cancer Associated Fibroblasts: An Essential Role in the Tumor Microenvironment. Oncol Lett (2017) 14:2611–20. doi: 10.3892/ol.2017.6497
74. Micke P, Ostman A. Tumour-Stroma Interaction: Cancer-Associated Fibroblasts as Novel Targets in Anti-Cancer Therapy. Lung Cancer (2004) 45 Suppl 2:S163–75. doi: 10.1016/j.lungcan.2004.07.977
75. Ma Z, Chen M, Yang X, Xu B, Song Z, Zhou B, et al. The Role of Cancer-associated Fibroblasts in Tumorigenesis of Gastric Cancer. Curr Pharm Des (2018) 24:3297–302. doi: 10.2174/1381612824666180601094056
76. Wang J, Guan X, Zhang Y, Ge S, Zhang L, Li H, et al. Exosomal Mir-27a Derived From Gastric Cancer Cells Regulates the Transformation of Fibroblasts Into Cancer-Associated Fibroblasts. Cell Physiol Biochem (2018) 49:869–83. doi: 10.1159/000493218
77. Gu J, Qian H, Shen L, Zhang X, Zhu W, Huang L, et al. Gastric Cancer Exosomes Trigger Differentiation of Umbilical Cord Derived Mesenchymal Stem Cells to Carcinoma-Associated Fibroblasts Through TGF-β/Smad Pathway. PloS One (2012) 7:e52465. doi: 10.1371/journal.pone.0052465
78. Ning X, Zhang H, Wang C, Song X. Exosomes Released by Gastric Cancer Cells Induce Transition of Pericytes Into Cancer-Associated Fibroblasts. Med Sci Monit (2018) 24:2350–9. doi: 10.12659/msm.906641
79. Miki Y, Yashiro M, Okuno T, Kitayama K, Masuda G, Hirakawa K, et al. CD9-Positive Exosomes From Cancer-Associated Fibroblasts Stimulate the Migration Ability of Scirrhous-Type Gastric Cancer Cells. Br J Cancer (2018) 118:867–77. doi: 10.1038/bjc.2017.487
80. Shi L, Wang Z, Geng X, Zhang Y, Xue Z. Exosomal miRNA-34 From Cancer-Associated Fibroblasts Inhibits Growth and Invasion of Gastric Cancer Cells In Vitro and In Vivo. Aging (Albany NY) (2020) 12:8549–64. doi: 10.18632/aging.103157
81. Xu G, Zhang B, Ye J, Cao S, Shi J, Zhao Y, et al. Exosomal miRNA-139 in Cancer-Associated Fibroblasts Inhibits Gastric Cancer Progression by Repressing MMP11 Expression. Int J Biol Sci (2019) 15:2320–9. doi: 10.7150/ijbs.33750
82. Roorda BD, ter Elst A, Kamps WA, de Bont ES. Bone Marrow-Derived Cells and Tumor Growth: Contribution of Bone Marrow-Derived Cells to Tumor Micro-Environments With Special Focus on Mesenchymal Stem Cells. Crit Rev Oncol Hematol (2009) 69:187–98. doi: 10.1016/j.critrevonc.2008.06.004
83. Yang X, Hou J, Han Z, Wang Y, Hao C, Wei L, et al. One Cell, Multiple Roles: Contribution of Mesenchymal Stem Cells to Tumor Development in Tumor Microenvironment. Cell Biosci (2013) 3:5. doi: 10.1186/2045-3701-3-5
84. Gu H, Ji R, Zhang X, Wang M, Zhu W, Qian H, et al. Exosomes Derived From Human Mesenchymal Stem Cells Promote Gastric Cancer Cell Growth and Migration Via the Activation of the Akt Pathway. Mol Med Rep (2016) 14:3452–8. doi: 10.3892/mmr.2016.5625
85. Qi J, Zhou Y, Jiao Z, Wang X, Zhao Y, Li Y, et al. Exosomes Derived From Human Bone Marrow Mesenchymal Stem Cells Promote Tumor Growth Through Hedgehog Signaling Pathway. Cell Physiol Biochem (2017) 42:2242–54. doi: 10.1159/000479998
86. Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, et al. Exosomes Derived From Human Bone Marrow Mesenchymal Stem Cells Promote Tumor Growth In Vivo. Cancer Lett (2012) 315:28–37. doi: 10.1016/j.canlet.2011.10.002
87. Mao J, Liang Z, Zhang B, Yang H, Li X, Fu H, et al. Ubr2 Enriched in P53 Deficient Mouse Bone Marrow Mesenchymal Stem Cell-Exosome Promoted Gastric Cancer Progression Via Wnt/β-Catenin Pathway. Stem Cells (2017) 35:2267–79. doi: 10.1002/stem.2702
88. Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan Y, et al. Exosomes Derived From Human Mesenchymal Stem Cells Confer Drug Resistance in Gastric Cancer. Cell Cycle (2015) 14:2473–83. doi: 10.1080/15384101.2015.1005530
89. Lu P, Takai K, Weaver VM, Werb Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb Perspect Biol (2011) 3:a005058. doi: 10.1101/cshperspect.a005058
90. Mohan V, Das A, Sagi I. Emerging Roles of ECM Remodeling Processes in Cancer. Semin Cancer Biol (2020) 62:192–200. doi: 10.1016/j.semcancer.2019.09.004
91. Łukaszewicz-Zając M, Szmitkowski M, Litman-Zawadzka A, Mroczko B. Matrix Metalloproteinases and Their Tissue Inhibitors in Comparison to Other Inflammatory Proteins in Gastric Cancer (Gc). Cancer Invest (2016) 34:305–12. doi: 10.1080/07357907.2016.1197237
92. Mu W, Rana S, Zöller M. Host Matrix Modulation by Tumor Exosomes Promotes Motility and Invasiveness. Neoplasia (2013) 15:875–87. doi: 10.1593/neo.13786
93. Dong Z, Sun X, Xu J, Han X, Xing Z, Wang D, et al. Serum Membrane Type 1-Matrix Metalloproteinase (Mt1-MMP) Mrna Protected by Exosomes as a Potential Biomarker for Gastric Cancer. Med Sci Monit (2019) 25:7770–83. doi: 10.12659/MSM.918486
94. Grünwald B, Harant V, Schaten S, Frühschütz M, Spallek R, Höchst B, et al. Pancreatic Premalignant Lesions Secrete Tissue Inhibitor of Metalloproteinases-1, Which Activates Hepatic Stellate Cells Via CD63 Signaling to Create a Premetastatic Niche in the Liver. Gastroenterology (2016) 151:1011–24.e7. doi: 10.1053/j.gastro.2016.07.043
95. Miki Y, Yashiro M, Okuno T, Kuroda K, Togano S, Hirakawa K, et al. Clinico-Pathological Significance of Exosome Marker CD63 Expression on Cancer Cells and Stromal Cells in Gastric Cancer. PloS One (2018) 13:e0202956. doi: 10.1371/journal.pone.0202956
96. Chen KB, Chen J, Jin XL, Huang Y, Su QM, Chen L. Exosome-Mediated Peritoneal Dissemination in Gastric Cancer and its Clinical Applications. BioMed Rep (2018) 8:503–9. doi: 10.3892/br.2018.1088
97. Yeung TL, Leung CS, Yip KP, Au Yeung CL, Wong ST, Mok SC. Cellular and Molecular Processes in Ovarian Cancer Metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am J Physiol Cell Physiol (2015) 309:C444–56. doi: 10.1152/ajpcell.00188.2015
98. Sandoval P, Jiménez-Heffernan JA, Rynne-Vidal Á, Pérez-Lozano ML, Gilsanz Á, Ruiz-Carpio V, et al. Carcinoma-Associated Fibroblasts Derive From Mesothelial Cells Via Mesothelial-to-Mesenchymal Transition in Peritoneal Metastasis. J Pathol (2013) 231:517–31. doi: 10.1002/path.4281
99. Nakamura M, Ono YJ, Kanemura M, Tanaka T, Hayashi M, Terai Y, et al. Hepatocyte Growth Factor Secreted by Ovarian Cancer Cells Stimulates Peritoneal Implantation Via the Mesothelial-Mesenchymal Transition of the Peritoneum. Gynecol Oncol (2015) 139:345–54. doi: 10.1016/j.ygyno.2015.08.010
100. Yáñez-Mó M, Lara-Pezzi E, Selgas R, Ramírez-Huesca M, Domínguez-Jiménez C, Jiménez-Heffernan JA, et al. Peritoneal Dialysis and Epithelial-to-Mesenchymal Transition of Mesothelial Cells. N Engl J Med (2003) 348:403–13. doi: 10.1056/NEJMoa020809
101. Li Q, Li B, Li Q, Wei S, He Z, Huang X, et al. Exosomal miR-21-5p Derived From Gastric Cancer Promotes Peritoneal Metastasis Via Mesothelial-to-Mesenchymal Transition. Cell Death Dis (2018) 9:854. doi: 10.1038/s41419-018-0928-8
102. Arita T, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Ogino S, et al. Tumor Exosome-Mediated Promotion of Adhesion to Mesothelial Cells in Gastric Cancer Cells. Oncotarget (2016) 7:56855–63. doi: 10.18632/oncotarget.10869
103. Deng G, Qu J, Zhang Y, Che X, Cheng Y, Fan Y, et al. Gastric Cancer-Derived Exosomes Promote Peritoneal Metastasis by Destroying the Mesothelial Barrier. FEBS Lett (2017) 591:2167–79. doi: 10.1002/1873-3468.12722
104. Wei M, Yang T, Chen X, Wu Y, Deng X, He W, et al. Malignant Ascites-Derived Exosomes Promote Proliferation and Induce Carcinoma-Associated Fibroblasts Transition in Peritoneal Mesothelial Cells. Oncotarget (2017) 8:42262–71. doi: 10.18632/oncotarget.15040
105. Aslan C, Maralbashi S, Salari F, Kahroba H, Sigaroodi F, Kazemi T, et al. Tumor-Derived Exosomes: Implication in Angiogenesis and Antiangiogenesis Cancer Therapy. J Cell Physiol (2019) 234:16885–903. doi: 10.1002/jcp.28374
106. Tamura R, Tanaka T, Akasaki Y, Murayama Y, Yoshida K, Sasaki H. The Role of Vascular Endothelial Growth Factor in the Hypoxic and Immunosuppressive Tumor Microenvironment: Perspectives for Therapeutic Implications. Med Oncol (2019) 37:2. doi: 10.1007/s12032-019-1329-2
107. Hegde PS, Wallin JJ, Mancao C. Predictive Markers of anti-VEGF and Emerging Role of Angiogenesis Inhibitors as Immunotherapeutics. Semin Cancer Biol (2018) 52:117–24. doi: 10.1016/j.semcancer.2017.12.002
108. Yang H, Zhang H, Ge S, Ning T, Bai M, Li J, et al. Exosome-Derived Mir-130a Activates Angiogenesis in Gastric Cancer by Targeting C-MYB in Vascular Endothelial Cells. Mol Ther (2018) 26:2466–75. doi: 10.1016/j.ymthe.2018.07.023
109. Bai M, Li J, Yang H, Zhang H, Zhou Z, Deng T, et al. Mir-135b Delivered by Gastric Tumor Exosomes Inhibits FOXO1 Expression in Endothelial Cells and Promotes Angiogenesis. Mol Ther (2019) 27:1772–83. doi: 10.1016/j.ymthe.2019.06.018
110. Zhou Z, Zhang H, Deng T, Ning T, Liu R, Liu D, et al. Exosomes Carrying Microrna-155 Target Forkhead Box O3 of Endothelial Cells and Promote Angiogenesis in Gastric Cancer. Mol Ther Oncol (2019) 15:223–33. doi: 10.1016/j.omto.2019.10.006
111. Deng T, Zhang H, Yang H, Wang H, Bai M, Sun W, et al. Exosome Mir-155 Derived From Gastric Carcinoma Promotes Angiogenesis by Targeting the C-MYB/VEGF Axis of Endothelial Cells. Mol Ther Nucleic Acids (2020) 19:1449–59. doi: 10.1016/j.omtn.2020.01.024
112. Du J, Liang Y, Li J, Zhao JM, Wang ZN, Lin XY. Gastric Cancer Cell-Derived Exosomal Microrna-23a Promotes Angiogenesis by Targeting Pten. Front Oncol (2020) 10:326. doi: 10.3389/fonc.2020.00326
113. Anami K, Oue N, Noguchi T, Sakamoto N, Sentani K, Hayashi T, et al. TSPAN8, Identified by Escherichia Coli Ampicillin Secretion Trap, is Associated With Cell Growth and Invasion in Gastric Cancer. Gastri Cancer (2016) 19:370–80. doi: 10.1007/s10120-015-0478-z
114. Xue X, Huang J, Yu K, Chen X, He Y, Qi D, et al. YB-1 Transferred by Gastric Cancer Exosomes Promotes Angiogenesis Via Enhancing the Expression of Angiogenic Factors in Vascular Endothelial Cells. BMC Cancer (2020) 20:996. doi: 10.1186/s12885-020-07509-6
115. Li G, Lin H, Tian R, Zhao P, Huang Y, Pang X, et al. Vegfr-2 Inhibitor Apatinib Hinders Endothelial Cells Progression Triggered by Irradiated Gastric Cancer Cells-Derived Exosomes. J Cancer (2018) 9:4049–57. doi: 10.7150/jca.25370
116. Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-Metastatic Niches: Organ-Specific Homes for Metastases. Nat Rev Cancer (2017) 17:302–17. doi: 10.1038/nrc.2017.6
117. Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature (2015) 527:329–35. doi: 10.1038/nature15756
118. Zhang H, Deng T, Liu R, Bai M, Zhou L, Wang X, et al. Exosome-Delivered EGFR Regulates Liver Microenvironment to Promote Gastric Cancer Liver Metastasis. Nat Commun (2017) 8:15016. doi: 10.1038/ncomms15016
119. Liu D, Li C, Trojanowicz B, Li X, Shi D, Zhan C, et al. CD97 Promotion of Gastric Carcinoma Lymphatic Metastasis is Exosome Dependent. Gastri Cancer (2016) 19:754–66. doi: 10.1007/s10120-015-0523-y
120. Tanaka M, Kuriyama S, Itoh G, Maeda D, Goto A, Tamiya Y, et al. Mesothelial Cells Create a Novel Tissue Niche That Facilitates Gastric Cancer Invasion. Cancer Res (2017) 77:684–95. doi: 10.1158/0008-5472.CAN-16-0964
121. Zhang Y, Han T, Feng D, Li J, Wu M, Peng X, et al. Screening of non-Invasive miRNA Biomarker Candidates for Metastasis of Gastric Cancer by Small RNA Sequencing of Plasma Exosomes. Carcinogenesis (2020) 41:582–90. doi: 10.1093/carcin/bgz186
122. Wang J, Liu Y, Sun W, Zhang Q, Gu T, Li G. Plasma Exosomes as Novel Biomarker for the Early Diagnosis of Gastric Cancer. Cancer Biomark (2018) 21:805–12. doi: 10.3233/CBM-170738
123. Tang S, Cheng J, Yao Y, Lou C, Wang L, Huang X, et al. Combination of Four Serum Exosomal MiRNAs as Novel Diagnostic Biomarkers for Early-Stage Gastric Cancer. Front Genet (2020) 11:237. doi: 10.3389/fgene.2020.00237
124. Ding XQ, Wang ZY, Xia D, Wang RX, Pan XR, Tong JH. Proteomic Profiling of Serum Exosomes From Patients With Metastatic Gastric Cancer. Front Oncol (2020) 10:1113. doi: 10.3389/fonc.2020.01113
125. Tokuhisa M, Ichikawa Y, Kosaka N, Ochiya T, Yashiro M, Hirakawa K, et al. Exosomal miRNAs From Peritoneum Lavage Fluid as Potential Prognostic Biomarkers of Peritoneal Metastasis in Gastric Cancer. PLoS One (2015) 10:e0130472. doi: 10.1371/journal.pone.0130472
126. Kim OY, Lee J, Gho YS. Extracellular Vesicle Mimetics: Novel Alternatives to Extracellular Vesicle-Based Theranostics, Drug Delivery, and Vaccines. Semin Cell Dev Biol (2017) 67:74–82. doi: 10.1016/j.semcdb.2016.12.001
127. Tan A, De La Peña H, Seifalian AM. The Application of Exosomes as a Nanoscale Cancer Vaccine. Int J Nanomed (2010) 5:889–900. doi: 10.2147/IJN.S13402
128. Zhang H, Wang Y, Bai M, Wang J, Zhu K, Liu R, et al. Exosomes Serve as Nanoparticles to Suppress Tumor Growth and Angiogenesis in Gastric Cancer by Delivering Hepatocyte Growth Factor Sirna. Cancer Sci (2018) 109:629–41. doi: 10.1111/cas.13488
129. Jiang K, Dong C, Yin Z, Li R, Wang Q, Wang L. The Critical Role of Exosomes in Tumor Biology. J Cell Biochem (2019) 120:6820–32. doi: 10.1002/jcb.27813
130. He M, Crow J, Roth M, Zeng Y, Godwin AK. Integrated Immunoisolation and Protein Analysis of Circulating Exosomes Using Microfluidic Technology. Lab Chip (2014) 14:3773–80. doi: 10.1039/c4lc00662c
131. Zhu L, Wang K, Cui J, Liu H, Bu X, Ma H, et al. Label-Free Quantitative Detection of Tumor-Derived Exosomes Through Surface Plasmon Resonance Imaging. Anal Chem (2014) 86:8857–64. doi: 10.1021/ac5023056
132. Mathai RA, Vidya R, Reddy BS, Thomas L, Udupa K, Kolesar J, et al. Potential Utility of Liquid Biopsy as a Diagnostic and Prognostic Tool for the Assessment of Solid Tumors: Implications in the Precision Oncology. J Clin Med (2019) 8:373. doi: 10.3390/jcm8030373
133. Wu D, Yan J, Shen X, Sun Y, Thulin M, Cai Y, et al. Profiling Surface Proteins on Individual Exosomes Using a Proximity Barcoding Assay. Nat Commun (2019) 10:3854. doi: 10.1038/s41467-019-11486-1
134. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomed (2020) 15:6917–34. doi: 10.2147/IJN.S264498
135. Bandari SK, Purushothaman A, Ramani VC, Brinkley GJ, Chandrashekar DS, Varambally S, et al. Chemotherapy Induces Secretion of Exosomes Loaded With Heparanase That Degrades Extracellular Matrix and Impacts Tumor and Host Cell Behavior. Matrix Biol (2018) 65:104–18. doi: 10.1016/j.matbio.2017.09.001
Keywords: exosome, gastric cancer, pre-metastatic niche, metastasis, tumor microenvironment
Citation: Gao J, Li S, Xu Q, Zhang X, Huang M, Dai X and Liu L (2021) Exosomes Promote Pre-Metastatic Niche Formation in Gastric Cancer. Front. Oncol. 11:652378. doi: 10.3389/fonc.2021.652378
Received: 12 January 2021; Accepted: 05 May 2021;
Published: 24 May 2021.
Edited by:
Zhang Xiaotian, Peking University Cancer Hospital, ChinaReviewed by:
Prabhash Dadhich, Cellf Bio LLC, United StatesCopyright © 2021 Gao, Li, Xu, Zhang, Huang, Dai and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lian Liu, bGlhbmxpdXN1YkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.