- 1Institute of Medical Technology, Peking University Health Science Center, Beijing, China
- 2Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Key Laboratory for Research and Evaluation of Radiopharmaceuticals (National Medical Products Administration), Department of Nuclear Medicine, Peking University Cancer Hospital & Institute, Beijing, China
This study aims to further explore dynamic 68Ga-FAPI-04 PET/CT imaging of healthy Chinese subjects and lung cancer patients. Moreover, the variability of 68Ga-FAPI-04 uptake in normal organs was measured to provide a basis for analyzing its biological distribution, interpreting auxiliary images, determining the reliability of image quantification, and monitoring treatment. Six patients (3 subjects without tumors and 3 lung cancer patients) separately underwent 68Ga-FAPI-04 and 2-[18F]FDG PET/CT imaging within 1 week. The biodistribution and internal radiation dosimetry were reported and compared with data previously obtained from Caucasian patients. Moreover, the mean SUV (standardized uptake value) was normalized to body mass or to lean body mass (SUL), and the coefficients of variation (CVs) were calculated and compared for each volume of interest. The average whole-body effective dose was calculated to be 1.27E-02 mSv/MBq, which was comparable with previously reported results of 68Ga-FAPI-04 probes. Furthermore, the SUVmean was slightly higher than the SULmean in most organs; however, the CV of the SULmean for most organs was higher than that of the SUVmean at later time points. In the liver, the CV of the SUVmean was lower (12.7%) than that of the SULmean and was similar to the CV for corresponding 2-[18F]FDG PET/CT value (11.8%). In addition, 68Ga-FAPI-04 PET/CT showed good efficacy for diagnosing lung cancer patients in this study. A comparison of the radiation dosimetry obtained before from a Caucasian population demonstrated no clinically significant differences between these two populations after 68Ga-FAPI-04 injection. The variability in most organs was slightly lower for SUVmean than for SULmean, suggesting that SUVmean may be the preferable parameter for quantifying images obtained with 68Ga-FAPI-04. In addition, 68Ga-FAPI-04 PET/CT imaging is expected to be a promising tool for diagnosing lung cancer.
Introduction
Fibroblast activation protein (FAP), or seprase, is a type II transmembrane glycoprotein that possesses serine protease activity and is closely associated with the invasion and metastasis of human cancers (1). The expression of FAP in normal tissue is highly restricted, but this protein becomes overactive in the tumor stroma, which comprises many cancer-associated fibroblasts (2). Furthermore, the expression of FAP is associated with poor prognosis in several kinds of cancer (3–5). Hence, FAP is a promising specific theranostic target for cancer.
In recent years, a variety of small-molecule inhibitors of FAP, or FAPIs, have been synthesized, and their antitumor activity has been studied in detail (6–8). Among these inhibitors, (4-quinolinoyl)glycyl-2-cyanopyrrolidine-based FAPIs fabricated by Koen et al. showed the highest affinity for FAP and were further modified and developed by scientists into cancer theranostic probes (9). The application of radiolabeled 68Ga-FAPI-04 for PET/CT imaging in patients with breast cancer was first demonstrated by Lindner et al. in 2018 (10). More recently, the application of 68Ga-FAPI-04 PET/CT was further extended to the clinical detection of 28 different kinds of cancer (11, 12). Moreover, the potential of FAPIs for cancer detection and radionuclide therapy has been fully revealed in several recent studies (13, 14).
To the best of our knowledge, no previous study has focused on dynamic PET/CT imaging with 68Ga-FAPI-04 in Chinese patients. Furthermore, the intrinsic variability of 68Ga-FAPI-04 distribution in normal organs needs to be investigated before the ability of this probe to detect and monitor different types of cancer can be evaluated; head-to-head comparisons between 68Ga-FAPI-04 PET/CT and 2-[18F]FDG PET/CT in both healthy volunteers and cancer patients are lacking. Therefore, this study aims to further evaluate dynamic imaging with 68Ga-FAPI-04 in healthy patients and cancer patients. The clinical safety, biodistribution and internal radiation dosimetry assessments of 68Ga-FAPI-04 have been performed previously with a Caucasian cohort of patients (11), and these data were compared with the results obtained from this work. Because ethnicity may affect the expression of transporters and metabolizing enzymes that determine pharmacokinetics and biodistribution, it is essential to confirm interethnic comparability for global use of this PET drug.
Materials and Methods
Subjects
This study was approved by the Ethics Committee of Peking University Cancer Hospital (2019 KT95), and registered in Chinese Clinical Trial Registry (ChiCTR2000038080). Written informed consent was obtained from each participant prior to 68Ga-FAPI-04 PET/CT imaging. The inclusion criteria included the following: age older than 18 years, ability to provide informed written consent, and normal liver and kidney function. The exclusion criteria included the following: liver and renal dysfunction and pregnancy or current lactation, and patients diagnosed with wound healing, arthritis, inflammation or cirrhosis liver. Finally, two groups of subjects (three subjects without tumors and three lung cancer patients) were enrolled in this study. The demographic data of these subjects are shown in Table 1.
Radiopharmaceuticals
68Ga-FAPI-04 was synthesized through the modified method described in previously reported literature (10). Briefly, 68GaCl3 (3 mL, 0.05 M HCl) eluted from a 68Ge-68Ga generator (1.85 GBq, ITG Co., Ltd, Germany) and 20 μg of the DOTA-FAPI-04 (HUAYI TECHNOLOGY Co., Ltd, China) precursor were mixed. The reaction was carried out at 95°C for 10 min after the pH was adjusted to 4.0 by sodium acetate. The radiochemical purity of the final product was tested by radio-HPLC. Before intravenous administration to the patients, the 68Ga-labeled product was purified by solid-phase extraction with Sep-Pak Light C18 (Waters, USA), diluted with saline and further processed with a polytetrafluoroethylene filter (0.2 μm).
Toxicity Monitoring
All subjects were monitored by a medical technician between tracer application and up to 30 min after the PET/CT examination was finished. During this period, all subjects were asked to report any abnormalities.
Examination Procedures
No specific preparation was required for any of the subjects on the day of 68Ga-FAPI-04 PET/CT scanning. A low-dose CT scan (120 kV, 35 mA, slice: 0.6 mm, matrix: 512 × 512) was performed before the 68Ga-FAPI-04 injection; then, a whole-body dynamic PET scan was performed immediately after the intravenous injection of 68Ga-FAPI-04 for all subjects and continued for 6 frames (frame 1, 5 mm/s; frame 2, 2 mm/s; frame 3, 2 mm/s; frame 4, 1.5 mm/s; frame 5, 1.5 mm/s; and frame 6, 1 mm/s), covering a period up to 60 min after the injection. All subjects underwent a follow-up 2-[18F]FDG PET/CT static scan within a week after 68Ga-FAPI-04 imaging. The 68Ga-FAPI-04 and 2-[18F]FDG PET/CT scans were acquired using a Biograph mCT Flow 64 scanner (Siemens, Erlangen, Germany) [120 kV, 146 mAs, slice: 3 mm, matrix: 200 × 200, full width at half maximum (FWHM): 5 mm, filter: Gaussian, field of view (FOV): 256 (head), 576 (body)], which continuously moved the patient bed to cover the entire body of each subject (from the top of the skull to the middle of the femur).
Data Analysis
A Siemens workstation (syngo.via Client 4.1) was used for postprocessing. Two experienced nuclear medicine physicians independently reviewed all images, and any discordant results were resolved by consensus. To analyze the biodistribution and variability of 68Ga-FAPI-04, volumes of interest (VOIs) were manually drawn on a section of each major organ/tissue within the edges of each organ. Data, including SUVmax, SUVmean, SULmax, SULmean, and average activity concentration (Bq/mm3), were obtained to determine the organ biodistribution and to calculate the human organ dosimetry. The dynamic PET/CT images and biodistribution of 68Ga-FAPI-04 in KM mice were also analyzed as described in the Supplementary Information.
For dosimetry analysis, heart content, lung, liver, spleen, pancreas, kidneys, uterus, urinary bladder content and body remainder were selected as source organs. The volumes of the source organs on CT images were calculated, and their mean counts/mL (kBq/mL) were determined from PET images at different time points. Whole-organ activity (MBq) was calculated by multiplying organ volume by mean counts/mL and dividing by 1,000. The percentage of injected activity was calculated to generate the activity concentration-time curve of each source organ. Biexponential curve fitting was applied to activity concentration-time curves of the uterus and urinary bladder content, while monoexponential curve fitting was applied to other source organs, to calculate the areas under the curve (AUCs), and human organ dosimetry was further estimated using OLINDA/EXM software (version 2.0; Hermes Medical Solutions AB). Organ-absorbed doses and total effective doses, as well as residence time for each patient, were obtained using reference adult male and female models.
For the SUVs within the tumor lesions, an elliptical VOI was placed over each metabolically active lesion that was suspected to be malignant with a 50% threshold isocontour. Compared to the uptake in the adjacent normal tissue, focal uptake in the tumor lesion was considered positive. To compute the tumor-to-non-tumor SUV ratio (SUR), we obtained the non-tumor SUV from a disease-free area within the surrounding or contralateral normal tissue.
Statistical Analysis
In our study, all statistical analyses were conducted using SPSS software (version 25.0; IBM Corp.). The data are presented as the mean ± SD for each organ. The paired samples T-test was used to compare differences between SUVmean and SULmean. P < 0.05 were considered statistically significant.
Results
Tracer Administration and PET/CT Imaging
68Ga-FAPI-04 was achieved with high radiolabeling yield of over 90%, and the radiochemical purity was higher than 99%, as detected by radio-HPLC. Furthermore, the specific activity of radiopharmaceuticals applied in this study was calculated to be 32.2–48.3 MBq/nmol. All patients received a bedside administration of 68Ga-FAPI-04 with an injected activity ranging from 103.2 to 257.5 MBq. No adverse effects were reported by any subject during the whole imaging period. The representative dynamic PET maximum intensity projections (MIPs) of subject 1 (female) and subject 4 (male) are shown in Figures 1A,B. The PET image of a representative healthy volunteer is presented in Supplementary Figure 1.
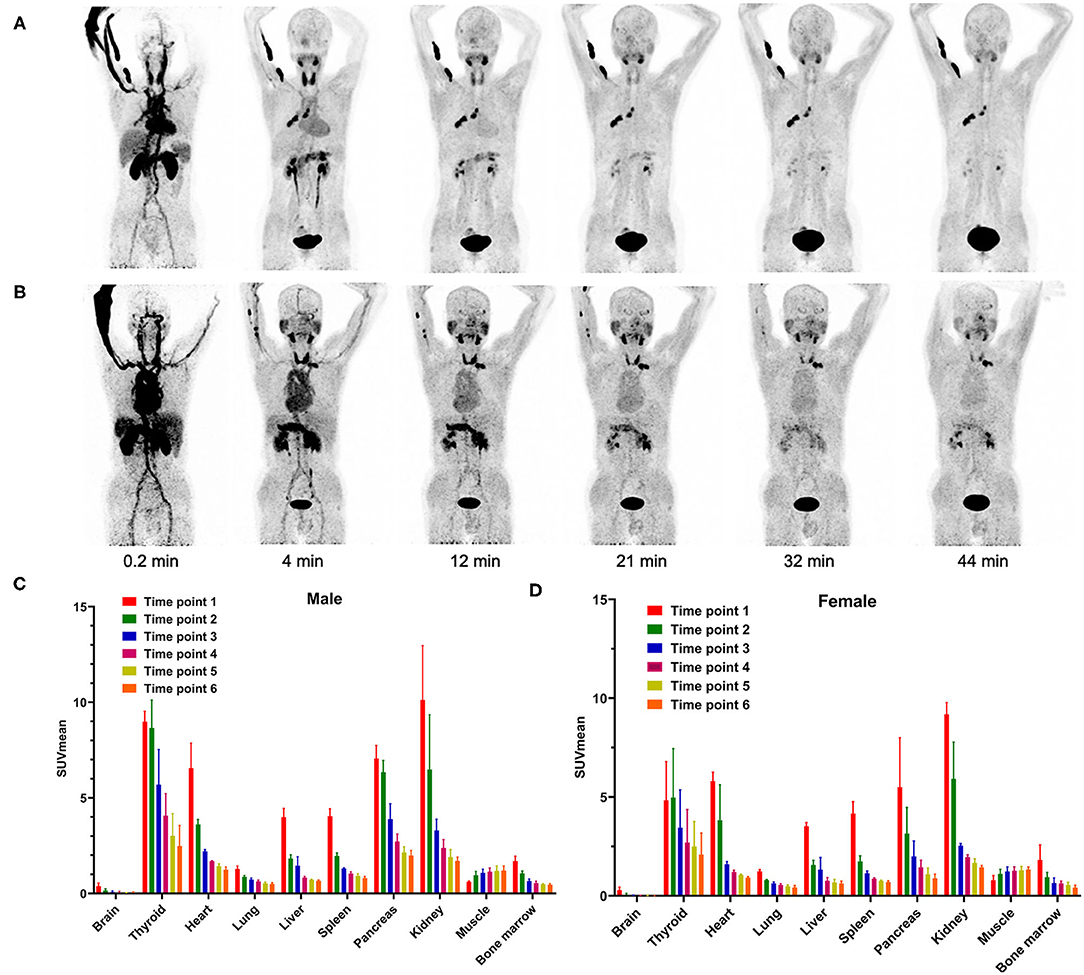
Figure 1. Representative PET MIP images of (A) female patient 1 and (B) male patient 4 at different time points. Dynamic 68Ga-FAPI-04 PET/CT-based biodistribution analysis of (C) male and (D) female Chinese subjects.
Biodistribution
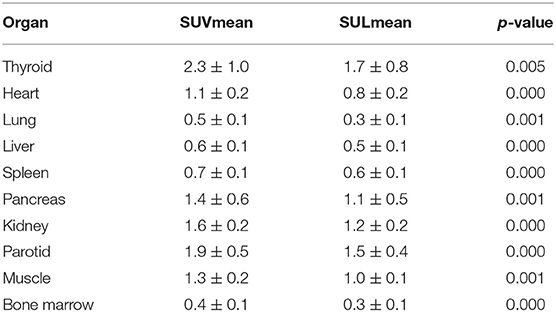
In our study, the SUVmean and SUVmax measurements of the organs of interest at six different time points (0.2 min, 4 min, 12 min, 21 min, 32 min, 44 min) were measured and summarized. The in vivo biodistribution of 68Ga-FAPI-04 in male and female subjects is presented in Figures 1C,D. According to the PET maximum intensity projections (MIPs), the blood pool in these patients was clearly visualized at early time points but quickly vanished over time. The submaxillary glands and parotid glands showed physiological uptake, while the uteruses of female patients remained apparent during the whole imaging period. In most organs, except for muscle and uterus, the SUVmean decreased over time. Radio signals in the bladders of patients increased as early as 4 min postinjection, and the highest SUVmean measurements were observed in the bladder at later time points because of the rapid renal excretion of the tracer. The average organ SUVmean variation over time in healthy volunteers and cancer patients is presented in Figure 2. The SUVmean in most organs decreased with time, and there were no differences between healthy volunteers and cancer patients. Interestingly, we found that the SUVmean of the thyroid in male patients was higher than that in female patients at earlier time points (as shown in Figure 3A), while the SUVmean of the uterus in female patients increased slowly within 1 h postinjection (Figure 3B). Furthermore, the SUVmean and SULmean of 68Ga-FAPI-04 in each organ were compared to investigate the variability in normal organ uptake. As shown in Tables 2, 3, the SULmean was slightly lower than the SUVmean in most organs across all patients at time point six. However, the CV of the SULmean for most organs was higher than that of the SUVmean. The comparison of SUVmean and SULmean in the lung and liver across all patients at time point 6 is presented in Figure 4. Similar to the dynamic images of humans, the dynamic 68Ga-FAPI-04 PET/CT images of KM mice indicated that 68Ga-FAPI-04 was cleared rapidly from the body (Supplementary Figure 2). In the biodistribution analysis, almost all normal organs of KM mice showed low uptake of 68Ga-FAPI-04, and the uptake reached a plateau within 30 min. However, the organs that showed the highest uptake of 68Ga-FAPI-04 were bone and muscle. The uptake value of 68Ga-FAPI-04 in the muscle of mice remained stable within the detection period. In contrast to the results from the human study, 68Ga-FAPI-04 uptake in the bone of mice increased over time (Supplementary Figure 3), which has not been revealed in any previous study.
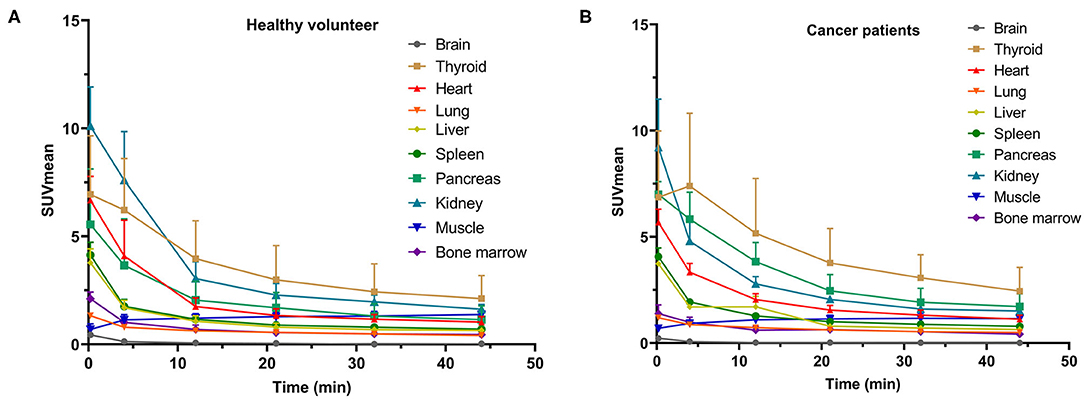
Figure 2. SUVmean of various organs on dynamic 68Ga-FAPI-04 PET/CT for (A) subjects without tumors and (B) cancer patients at different time points.
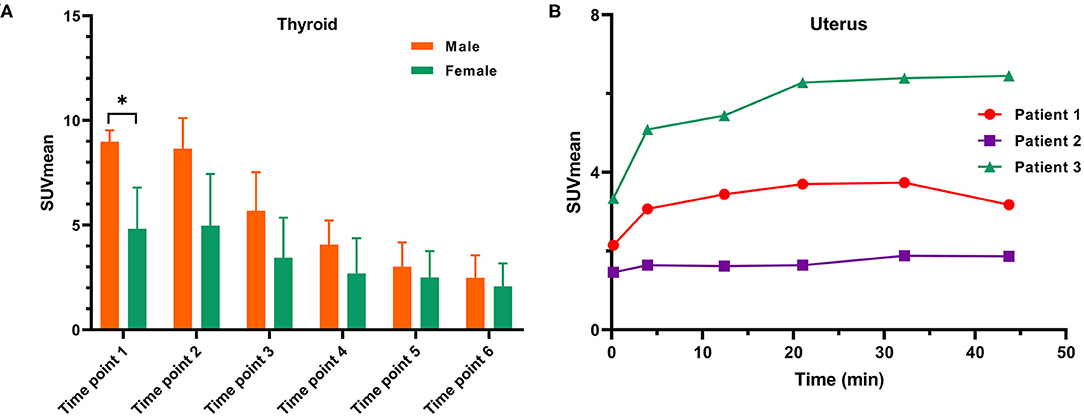
Figure 3. (A) A comparison of SUVmean variation on dynamic 68Ga-FAPI-04 PET/CT in the thyroids of male and female subjects and (B) SUVmean measurements of the uterus in three female subjects on dynamic 68Ga-FAPI-04 PET/CT. *p < 0.05.
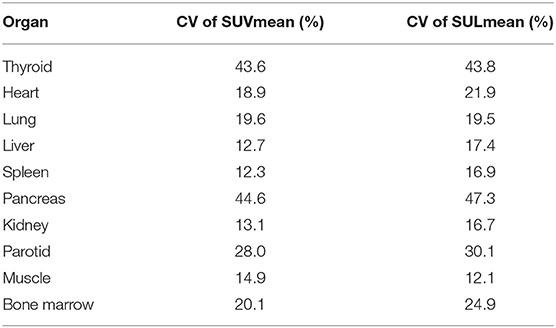
Table 3. Coefficient of variation of the SUVmean and SULmean for each organ across all subjects at time point 6.
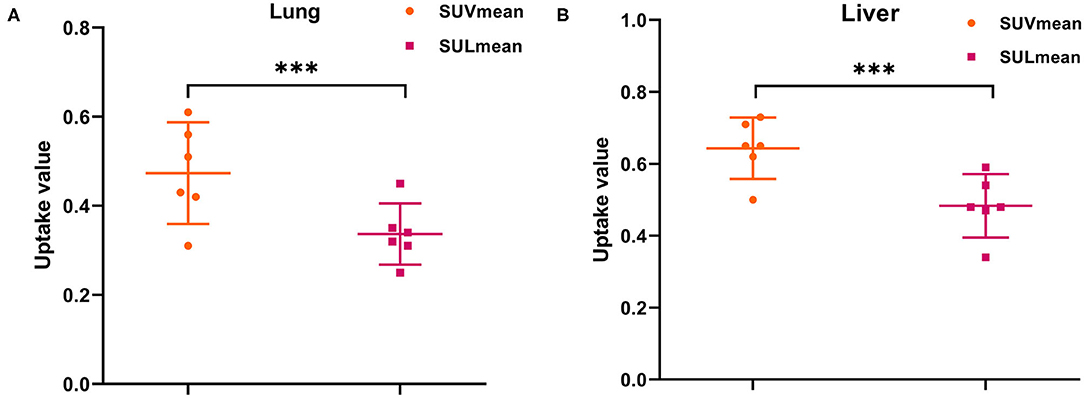
Figure 4. Comparison of SUVmean with SULmean for the (A) lung and (B) liver across all subjects at time point 6 (mean with 95% CI). ***p < 0.001.
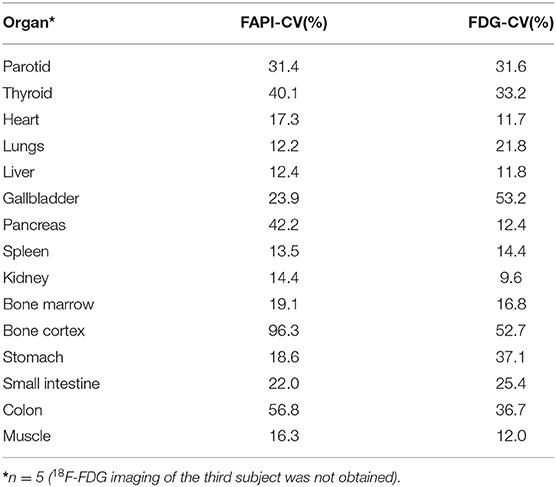
The Differences in CV Between 68Ga-FAPI-04 and 2-[18F]FDG in Normal Organs
The calculated CVs of the SUVmean measurements of 17 organs/tissues on 68Ga-FAPI-04 and 2-[18F]FDG images are shown in Table 4. The lungs had the lowest variability in 68Ga-FAPI-04 uptake of the studied organs with a CV of 12.2%, whereas the bone cortex had the highest variability (96.3%). The CV of liver uptake on 68Ga-FAPI-04 images was 12.4%; for reference, the CV of the liver with 2-[18F]FDG was 11.8%. The CV of 68Ga-FAPI-04 uptake in the pancreas was 42.2%, which was higher than that of 2-[18F]FDG (12.4%).
Comparison of Internal Radiation Dosimetry With a Caucasian Population
The pooled subject dosimetry results from OLINDA/EXM 2.0 are shown in Table 5, and the results for each subject are shown in Supplementary Table 1. In this study, the urinary bladder wall showed the highest average absorbed dose, followed by the uterus, kidneys, lungs, spleen, heart wall, pancreas, rectum and ovaries. The absorbed doses of other organs were below 8.12E-03 mGy/MBq. The organ with the highest average effective dose was also the urinary bladder wall, followed by the lungs, stomach wall and red marrow, as shown in Supplementary Table 2. The average whole-body effective dose was calculated to be 1.27E-02 mSv/MBq, which was comparable with previously reported results of 68Ga-FAPI-04 probes (11) (Table 5). Thus, the administration of 100 MBq 68Ga-FAPI-04 would result in a 1.27 mSv whole-body effective dose. Together with an approximately 3.7 mSv from one low-dose CT attenuation scan (15), a single 68Ga-FAPI-04 PET/CT imaging procedure would result in an estimated total effective dose of 4.97 mSv in adult Chinese subjects. Furthermore, the residence times of the source organs in each subject are summarized in Supplementary Table 3.
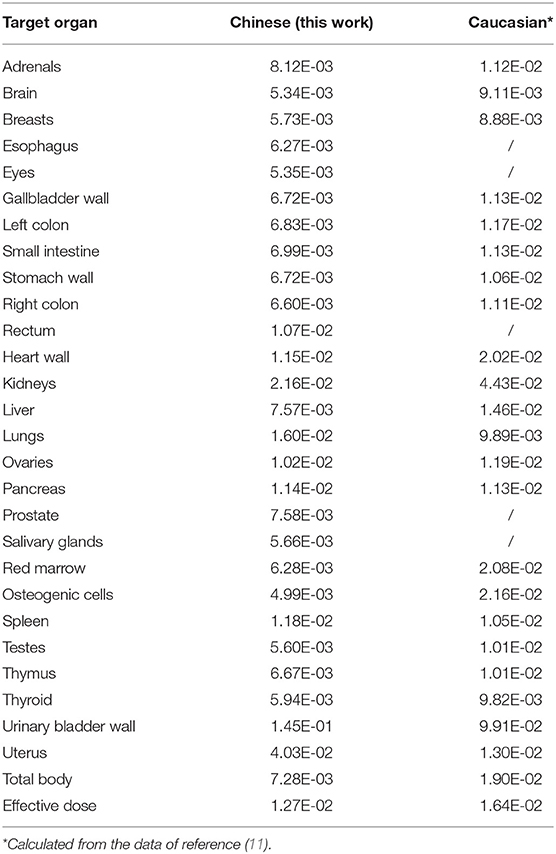
Table 5. Mean organ-absorbed doses (mGy/MBq) and whole-body effective doses (mSv/MBq) were compared between Chinese and Caucasian subjects.
Characteristics of Dynamic 68Ga-FAPI-04 PET/CT Images of Lung Cancer Patients
Upon visual inspection of the 68Ga-FAPI-04 PET/CT images, all pathologically confirmed lung cancer could be seen at the first phase. However, the images acquired in the late phase offered greater visualization (contrast) of the positive 68Ga-FAPI-04 uptake in all tumors than those acquired in the early phase (Figures 1A,B).
Quantitatively, in this dynamic study, we found that the SUVpeak of 68Ga-FAPI-04 in the lung tumors was apparent in the late phases. The SURs (tumor uptake to background ratios) of lung cancer (SUR4min = 5.5; SUR12min = 8.2; SUR21min = 8.5; SUR44min = 11.7) increased from the early-phase acquisitions to the late-phase acquisitions. Variations in 68Ga-FAPI-04 uptake (SUVmax) in the lesions of lung cancer patients are presented in Figure 5, and the SUVmax and SUR values of lung cancer patients at different time points are shown in Supplementary Table 5.
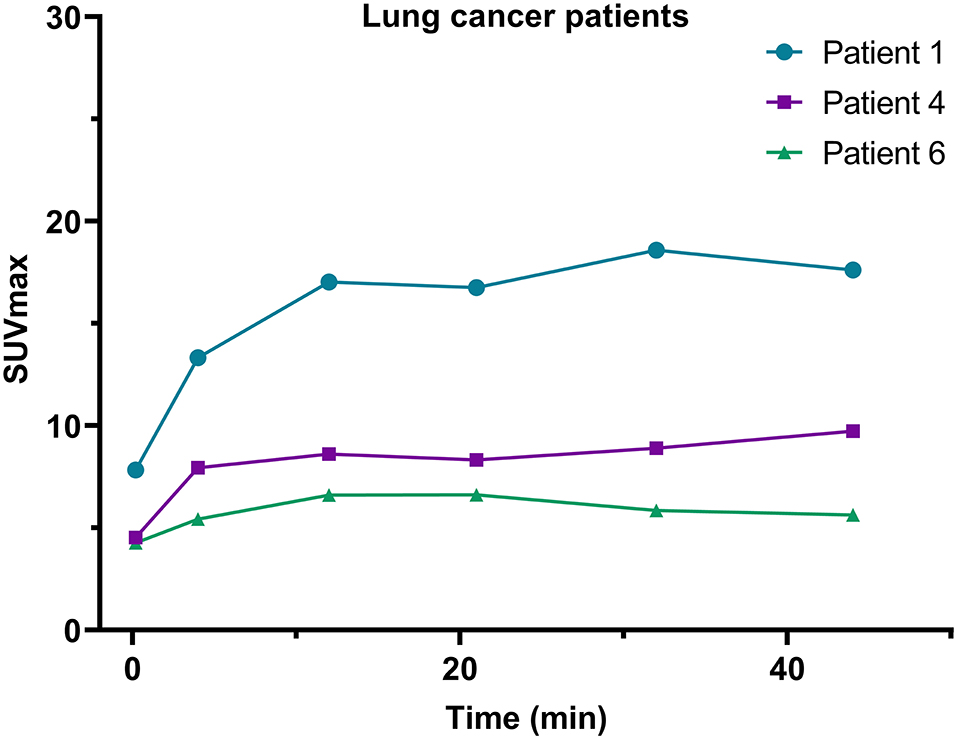
Figure 5. Variations in SUVmax on 68Ga-FAPI-04 PET/CT images of the 3 lung cancer patients included in this study.
The patients involved in this study demonstrated representative 68Ga-FAPI-04 uptake in lung cancer. The 62-year-old male patient with right lung cancer underwent paired 2-[18F]FDG and 68Ga-FAPI-04 PET/CT examinations. The tumor exhibited high uptake of 2-[18F]FDG (SUVmax = 16) and high uptake of 68Ga-FAPI-04 (SUVmax = 9.8). The tumor was confirmed as adenocarcinoma by resected specimen (Figure 6), demonstrating the good imaging efficacy of 68Ga-FAPI-04 for lung cancer.
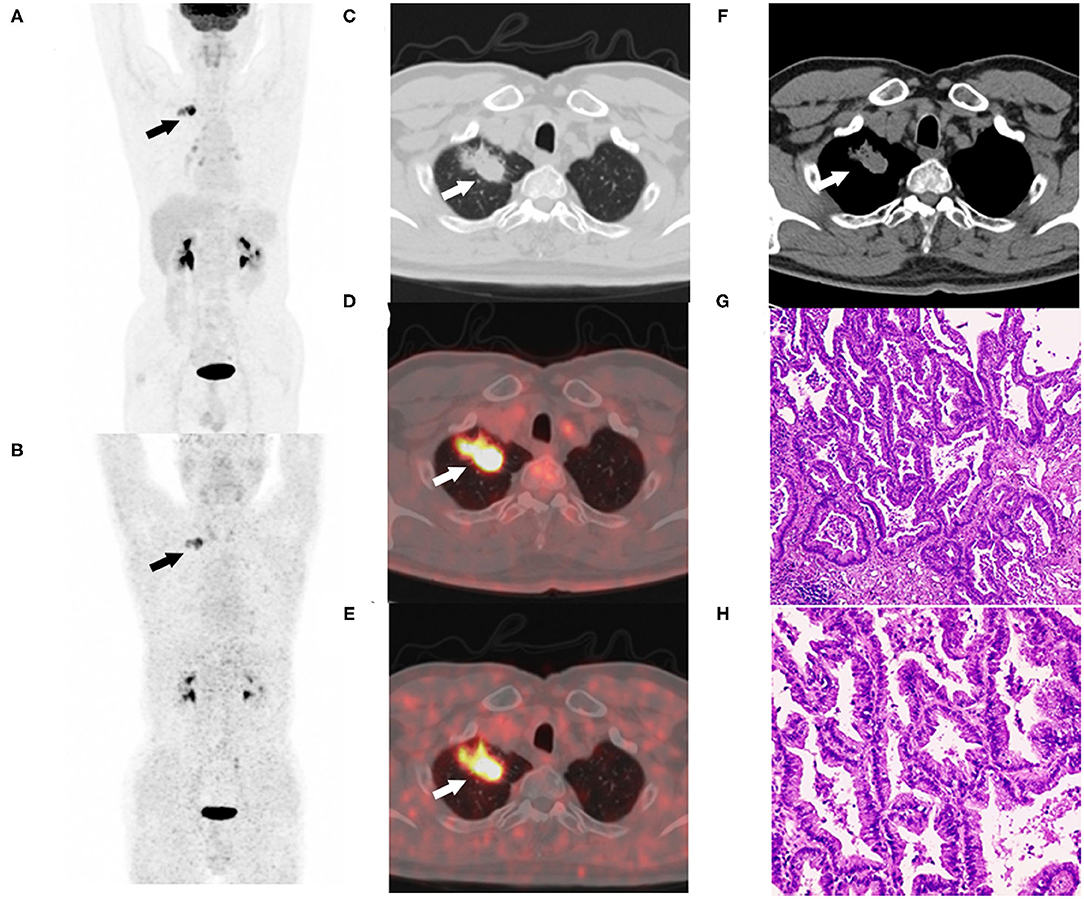
Figure 6. Sixty-two-year-old male with adenocarcinoma of the right upper lung (patient 4). Maximum intensity projection of 2-[18F]FDG (A) and 68Ga-FAPI-04 PET/CT examinations (B). The lobulated and spiculated nodule of the right upper lung were observed in CT images (C,F). The nodule exhibited high uptake of 2-[18F]FDG (SUVmax = 16) (D) and high uptake of 68Ga-FAPI-04 (SUVmax = 9.8) (E). The resected specimens were confirmed as adenocarcinoma by hematoxylin-eosin staining (G) ×40, (H) ×100.
Discussion
Fibroblast activation protein (FAP) is closely associated with the invasion and metastasis of human cancers and has been revealed as a promising theranostic target for tumors. In the past several years, many small-molecule FAPIs based on different chemical structures have been fabricated, and their utility as a tumor theranostic has been evaluated. To further investigate the biodistribution, tumor uptake and retention of 68Ga-FAPI-04, the results of a series of dynamic PET/CT imaging studies of both healthy subjects and oncological patients after an intravenous injection of 68Ga-FAPI-04 were reported and analyzed.
In this work, similar to other clinical trials with FAPIs (16–18), the 68Ga-FAPI-04 probe was cleared rapidly from the human body through renal excretion, rendering low background uptake in most normal organs (Table 2). This clearance was especially noticeable in the lung (SUVmean = 0.4 ± 0.1), liver (SUVmean = 0.6 ± 0.1), and brain (SUVmean = 0.02 ± 0.03) at the later time points, indicating that 68Ga-FPAI-04 is quite suitable for the imaging of tumor lesions in these organs. Regarding the in vivo biodistribution of 68Ga-FAPI-04 in most organs, there was no significant difference between male and female patients. We found that the SUVmean of the thyroid of Chinese male patients was higher than that in female patients at time point 1 (9.0 ± 0.5 vs. 4.8 ± 2.0, p = 0.05), while there was no difference at later time points (2.4 ± 1.1 vs. 2.1 ± 1.1, not statistically significant). This might be because of individual differences, since the CV of the SUVmean of the thyroid of female subjects at time point 1 was 40.7%, while the CV of the SUVmean of the thyroid of male subjects was only 5.6%. Similar to a previous study using 68Ga-FAPI-46 as an imaging agent for cancer patients, local uptake in the uterus of female subjects increased slowly within 1 h postinjection, which might be because of the higher physiological expression of FAP in the uterus (16). Since none of the patients drank water or were administered furosemide after the injection, the clearance of the 68Ga-FAPI-04 probe from the muscle would be slower in these patients than in patients administered furosemide in a previous study (SUVmean = 1.0, SUVmax = 1.4) (12), leading to higher uptake (SUVmean = 1.3 and SUVmax = 2.0) in the muscle at later time points.
Furthermore, the SUL has been preferred in the evaluation of biodistribution of 18F-radiolabeled probes, since the SUL was not correlated with body mass (19). In this study, we also compared the SUVmean with the SULmean in most organs across all patients, as shown in Tables 2, 3. At approximately 40 min postinjection, we found that the SUVmean was slightly higher than the SULmean in most organs, with no significant difference; however, the CV of the SULmean was higher than that of the SUVmean in most organs. Although the SULmean of more patients was within the 95% confidence interval with respect to the lung and liver, as shown in Figure 4, the SUVmean was applied in our study to evaluate the biodistribution of 68Ga-FAPI-04 in normal organs. The variations in the uptake in normal organs on 68Ga-FAPI-04 PET scans, as were measured by the CV, need to be understood to allow for uptake changes in malignant lesions to be confidently attributed to changes in disease or treatment response rather than inherent variability among scans. For these reasons, we analyzed the quantitative aspects of 68Ga-FAPI-04 imaging as they relate to normal organ variability. The liver has a moderate level of 2-[18F]FDG uptake in the normal parenchyma and a low uptake variability relative to other organs. This organ was chosen for further evaluations of quantitative reliability among PET images (20). In this study, liver uptake showed a low variability (CV = 12.4%) when using SUVmean, which was similar to the results of 2-[18F]FDG (11.8%). This finding implies that 68Ga-FAPI-04 PET images can be reliably quantified, laying the groundwork for future studies involving therapeutic monitoring.
In our study, the absorbed dose of all organs was determined based on dynamic PET/CT imaging. During the whole imaging period, no patients voided their bladders, thus causing the radioactivity in the bladder to increase continuously. Hence, biexponential curve fitting was applied to the activity concentration-time curves of urinary bladder content. Similar to the dosimetry results in other studies on 68Ga radiolabeled probes, the urinary bladder had the highest absorbed dose as well as the highest effective dose. Interestingly, we noticed that, other than the urinary bladder wall, among all other normal organs, the lung had the highest organ effective dose (1.92E-03 mSv/MBq), which might be because the three subjects included in this study were lung cancer patients. The absorbed doses of most organs, except the uterus and the whole-body effective dose of 68Ga-FAPI-04 PET imaging in our study (1.26E-02 mSv/MBq), were lower than the previously reported results (1.46E-02 mSv/MBq) in Caucasian patients (11), which might be because of individual differences between these two cohorts. An administration of 100 MBq of 68Ga-FAPI-04 would result in only a 1.26 mSv effective dose for adult patients, which is much lower than the 3.7 mSv of a whole-body scan and 1.8 mSv of a scan of the abdomen at 1 bed position with low-dose CT in adults (15, 21). Such a low effective dose would allow patients to receive 2–4 repeated 68Ga-FAPI-04 PET/CT imaging procedures per year and up to 8 PET/MRI examinations without exceeding the maximum dose of 10 mSv (21). Even so, the kidney absorbed dose would remain well below the threshold for the human kidney acute dose (7 Gy) (22). Furthermore, the absorbed dose in the red marrow was only 0.65 mGy/100 MBq, and even with up to 380 examinations, the absorbed dose in the red marrow would still be <0.25 Gy per year, which would not result in any damaging effects (22).
According to the statistics from OLINDA/EXM 2.0, body remainder had the longest residence time (0.584 h), followed by the urinary bladder (0.102 h), lungs (0.037 h), liver (0.018 h) and kidneys (0.013 h). Compared with a previously reported study of a 68Ga radiolabeled probe, the residence times in this study for most organs were shorter than the values reported for the 68Ga-DOTAZOL probe, especially for the liver (0.018 vs. 0.040) and spleen (0.003 vs. 0.005), indicating that 68Ga-FAPI-04 was quite stable in the human body, with less free/unbound 68Ga than the 68Ga-DOTAZOL probe (23). However, all these results might be limited by the subjects included in our study, since there were only 3 cancer patients and 3 healthy volunteers in this study. Furthermore, the expression of FAP due to wound healing, arthritis, inflammation and cirrhotic liver would interfere with the results (24–26).
In addition, in this study, we found that the SUVmax in lesions of lung cancer patients increased during this dynamic scan, and the background organ/tissue ratio decreased over time. Therefore, these results suggest that late imaging should be used for lung cancer patients, although the significance of 68Ga-FAPI-04 imaging for lung cancer needs to be investigated with more subjects.
Conclusion
The first biodistribution and internal radiation dosimetry profiles in the adult Chinese population following the administration of a 68Ga-FAPI-04 injection have been presented. A comparison of these data with those obtained before in the Caucasian population demonstrated no clinically significant differences between these two populations after 68Ga-FAPI-04 injections. In particular, the variability of 68Ga-FAPI-04 uptake in normal lungs was lower than that of 2-[18F]FDG. This finding implies that 68Ga-FAPI-04 PET images of lung cancer patients can be reliably quantified, thus laying the groundwork for future studies involving therapeutic monitoring. Moreover, a high SUR can be achieved as early as 12 min postinjection of 68Ga-FAPI-04, indicating that high-quality diagnostic images of lung cancer patients can be acquired at earlier time points than with 2-[18F]FDG. Furthermore, the variability in SUVmean for most organs was marginally lower than that in SULmean, favoring the adoption of SUVmean for 68Ga-FAPI-04 PET scans. In addition, 68Ga-FAPI-04 PET/CT imaging is expected to be a promising tool for diagnosing lung cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Peking University Cancer Hospital. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Peking University Cancer Hospital Animal Care and Use Committee.
Author Contributions
ZY, HZ, and NL conceived and designed this research. SW, XZ, and XX were responsible to the recruitment of patients, conducting of clinical patients experiments, data collection and analysis, and they also wrote the manuscript. JD and TL were involvode in the preparation of radiopharmaceuticals in this study. JJ took part in most of the animal experiments. All of the authors joined in the embellishment of the article.
Funding
This work was financially supported by National Natural Science Foundation of China projects (Nos. 81671733, 81871386, and 81871387) and Science Foundation of Peking University Cancer Hospital-2020-18.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully appreciate all the chemists, nurses, and technicians from the Department of Nuclear Medicine, Peking University Cancer Hospital for their contributions to 2-[18F]FDG and 68Ga-FAPI-04 production, tracer administration and PET/CT imaging.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.651005/full#supplementary-material
References
1. Sánchez-Garrido MA, Habegger KM, Clemmensen C, Holleman C, Müller TD, Perez-Tilve D, et al. Fibroblast activation protein (FAP) as a novel metabolic target. Mol Metab. (2016) 5:1015–24. doi: 10.1016/j.molmet.2016.07.003
2. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. (2016) 16:582–98. doi: 10.1038/nrc.2016.73
3. Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. (2020) 20:174–86. doi: 10.1038/s41568-019-0238-1
4. Wen Z, Liu Q, Wu J, Xu B, Liang J, Wang L, et al. Fibroblast activation protein α-positive pancreatic stellate cells promote the migration and invasion of pancreatic cancer by CXCL1-mediated Akt phosphorylation. Ann Transl Med. (2019) 7:532. doi: 10.21037/atm.2019.09.164
5. Zhang Y, Tang H, Cai J, Zhang T, Guo J, Feng D, et al. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. (2011) 303:47–55. doi: 10.1016/j.canlet.2011.01.011
6. Tsai TY, Yeh TK, Chen X, Hsu T, Jao YC, Huang CH, et al. Substituted 4-carboxymethylpyroglutamic acid diamides as potent and selective inhibitors of fibroblast activation protein. J Med Chem. (2010) 53:6572–83. doi: 10.1021/jm1002556
7. Jansen K, Heirbaut L, Cheng JD, Joossens J, Ryabtsova O, Cos P, et al. Selective inhibitors of fibroblast activation protein (FAP) with a (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine scaffold. ACS Med Chem Lett. (2013) 4:491–6. doi: 10.1021/ml300410d
8. Xu J, Huang S, Zhang T, Wu N, Kang H, Cai S, et al. The SAR studies on FAP inhibitors as tumor-targeted agents. Med Chem Res. (2015) 24:1744–52. doi: 10.1007/s00044-014-1128-4
9. Jansen K, Heirbaut L, Verkerk R, Cheng JD, Joossens J, Cos P, et al. Extended structure-activity relationship and pharmacokinetic investigation of (4-quinolinoyl)glycyl-2-cyanopyrrolidine inhibitors of fibroblast activation protein (FAP). J Med Chem. (2014) 57:3053–74. doi: 10.1021/jm500031w
10. Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. (2018) 59:1415–22. doi: 10.2967/jnumed.118.210443
11. Giesel FL, Kratochwil C, Lindner T, Marschalek MM, Loktev A, Lehnert W, et al. 68Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med. (2019) 60:386–92. doi: 10.2967/jnumed.118.215913
12. Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: Tracer uptake in 28 different kinds of cancer. J Nucl Med. (2019) 60:801–5. doi: 10.2967/jnumed.119.227967
13. Lindner T, Altmann A, Kraemer S, Kleist C, Loktev A, Kratochwil C, et al. Design and development of 99mTc labeled FAPI-tracers for SPECT-imaging and 188Re therapy. J Nucl Med. (2020) 61:1507–13. doi: 10.2967/jnumed.119.239731
14. Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. (2020) 47:1820–32. doi: 10.1007/s00259-020-04769-z
15. Huang B, Law MW, Khong PL. Whole-body PET/CT scanning: estimation of radiation dose and cancer risk. Radiology. (2009) 251:166–74. doi: 10.1148/radiol.2511081300
16. Meyer C, Dahlbom M, Lindner T, Vauclin S, Mona C, Slavik R, et al. Radiation dosimetry and biodistribution of 68Ga-FAPI-46 PET imaging in cancer patients. J Nucl Med. (2019) 61:1171–7. doi: 10.2967/jnumed.119.236786
17. Luo Y, Pan Q, Zhang W. IgG4-related disease revealed by 68Ga-FAPI and 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. (2019) 46:2625–6. doi: 10.1007/s00259-019-04478-2
18. Chen H, Zhao L, Ruan D, Sun L, Lin Q. 68Ga-FAPI PET/CT improves therapeutic strategy by detecting a second primary malignancy in a patient with rectal cancer. Clin Nucl Med. (2020) 45:468–70. doi: 10.1097/RLU.0000000000003000
19. Li X, Rowe SP, Leal JP, Gorin MA, Allaf ME, Ross AE, et al. Semiquantitative parameters in PSMA-targeted PET imaging with 18F-DCFPyL: variability in normal-organ uptake. J Nucl Med. (2017) 58:942–6. doi: 10.2967/jnumed.116.179739
20. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med. (2009) 50(Suppl. 1):122S−150S. doi: 10.2967/jnumed.108.057307
21. Boss M, Buitinga M, Jansen TJP, Brom M, Visser EP, Gotthardt M. PET-based human dosimetry of 68Ga-NODAGA-Exendin-4, a tracer for β-cell imaging. J Nucl Med. (2020) 61:112–6. doi: 10.2967/jnumed.119.228627
22. Stewart FA, Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, Macvittie TJ, et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs–threshold doses for tissue reactions in a radiation protection context. Ann ICRP. (2012) 41:1–322. doi: 10.1016/j.icrp.2012.02.001
23. Khawar A, Eppard E, Roesch F, Ahmadzadehfar H, Kürpig S, Meisenheimer M, et al. Preliminary results of biodistribution and dosimetric analysis of [68Ga]Ga-DOTAZOL: a new zoledronate-based bisphosphonate for PET/CT diagnosis of bone diseases. Ann Nucl Med. (2019) 33:404–13. doi: 10.1007/s12149-019-01348-7
24. Keane FM, Yao TW, Seelk S, Gall MG, Chowdhury S, Poplawski SE, et al. Quantitation of fibroblast activation protein (FAP)-specific protease activity in mouse, baboon and human fluids and organs. FEBS Open Bio. (2013) 4:43–54. doi: 10.1016/j.fob.2013.12.001
25. Yazbeck R, Jaenisch SE, Abbott CA. Potential disease biomarkers: dipeptidyl peptidase 4 and fibroblast activation protein. Protoplasma. (2018) 255:375–86. doi: 10.1007/s00709-017-1129-5
Keywords: dynamic PET/CT, fibroblast activation protein, 68Ga-FAPI-04, lung cancer, variability
Citation: Wang S, Zhou X, Xu X, Ding J, Liu T, Jiang J, Li N, Zhu H and Yang Z (2021) Dynamic PET/CT Imaging of 68Ga-FAPI-04 in Chinese Subjects. Front. Oncol. 11:651005. doi: 10.3389/fonc.2021.651005
Received: 08 January 2021; Accepted: 15 February 2021;
Published: 11 March 2021.
Edited by:
Haibin Shi, Soochow University, ChinaReviewed by:
Jianguo Lin, Jiangsu Institute of Nuclear Medicine, ChinaXiaohua Zhu, Huazhong University of Science and Technology, China
Copyright © 2021 Wang, Zhou, Xu, Ding, Liu, Jiang, Li, Zhu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Li, cmFpbmJvdzYyODMmI3gwMDA0MDtzaW5hLmNvbQ==; Hua Zhu, emh1aHVhbmFuamluZyYjeDAwMDQwOzE2My5jb20=; Zhi Yang, cGVreXomI3gwMDA0MDsxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Shuailiang Wang
Shuailiang Wang Xin Zhou2†
Xin Zhou2† Teli Liu
Teli Liu Hua Zhu
Hua Zhu Zhi Yang
Zhi Yang