
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 13 May 2021
Sec. Cancer Imaging and Image-directed Interventions
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.648658
Aims: The aim of this study was to determine whether 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) parameters might be prognostic markers for patients with differentiated thyroid carcinoma (DTC).
Methods: We searched for eligible articles in PubMed, EMBASE (Ovid), Cochrane Library, and ClinicalTrials.gov from inception to February 2021. We included studies addressing the association between 18F-FDG PET/CT parameters and clinical outcomes among patients with DTC. Quality assessment was performed using the Quality in Prognosis Studies (QUIPS) tool.
Results: A total of 25 studies including 2,954 patients (1,994 females, 67.5%) were included; 2,416 patients (81.8%) had papillary thyroid carcinoma (PTC), and the mean or median follow-up time ranged from 19.1 months to 17.1 years. Thirteen (52.0%) studies were assessed as “unclear” for the domain of study participation. The most common timing of PET/CT scans was after thyroidectomy (in 20 of 25 studies, 80%), especially in patients with an elevated thyroglobulin (Tg) and a negative radioiodine whole-body scan (WBS). The most common PET parameter was FDG uptake. Twelve of 17 (70.6%) and 12 of 12 (100%) studies showed an association between PET/CT parameters and disease progression and survival in patients with DTC, respectively.
Conclusion: 18F-FDG PET/CT parameters alone or combined with other variables can serve as prognostic markers to identify DTC patients with poor outcomes, especially in the setting of an elevated Tg and a negative WBS. Future research is needed to confirm these findings and to examine the prognostic value of PET/CT parameters for DTC patients, considering the heterogeneity in PET/CT parameters, unclear information of patients, and PET/CT-adapted treatment modifications.
Differentiated thyroid carcinoma (DTC) is the most common endocrine tumor with an increasing incidence worldwide. DTC has a generally good prognosis, with an overall mortality rate of <10% (1, 2). However, ~10–30% of DTC patients develop metastatic or recurrent diseases, among whom 33–50% eventually progress into radioiodine-refractory (RAI-R) diseases (1, 2). The identification of predictors of clinical outcomes for DTC patients is of immense clinical value (3, 4).
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT), combining functional and anatomic information, has become a valuable tool for the staging, treatment response assessment, prognosis prediction, and surveillance of patients with various malignancies (5). The American Thyroid Association (ATA) and the National Comprehensive Cancer Network (NCCN) guidelines have recommended that PET/CT should be considered for detecting metastasis or recurrence in patients with elevated thyroglobulin (Tg) and negative whole-body scans (WBS) during follow-up (6, 7). Recently, it has been widely illustrated that PET/CT parameters at different times are associated with established prognostic variables, such as age, the level of Tg, tumor size, BRAF mutation, etc. (8). Thus, PET/CT may provide additional prognostic information compared with clinical prognostic variables for DTC patients. Although the diagnostic and staging value of PET/CT in DTC patients has been examined in several studies (9, 10), limited data are available to evaluate the potential of PET/CT parameters as prognostic variables in DTC patients (11).
Therefore, the aim of this systematic review was to report the available evidence on the value of 18F-FDG PET/CT parameters to predict outcomes in patients with DTC.
This systematic review was performed according to the PRISMA statement (12). The PRISMA checklist is provided in Supplementary Table 1.
We included retrospective or prospective cohort studies assessing 18F-FDG PET or PET/CT parameters as prognostic factors in univariate or multivariate analyses to predict outcomes in DTC patients. At least 10 patients were involved and sufficient survival data, including overall/progression/recurrence/disease/event-free survival (OS/PFS/RFS/DFS/EFS, respectively), were reported.
We performed a comprehensive literature search to identify English language studies published in the PubMed, EMBASE (Ovid), Cochrane Library, and ClinicalTrials.gov from inception to March 2020. We used the following search strategy: (thyroid carcinoma OR thyroid cancer) AND (PET OR positron emission tomography OR FDG) AND (Prognos* OR survival OR outcome). The references cited in the retrieved studies were also explored to include potentially eligible studies.
Two reviewers independently screened titles, abstracts, and full texts for eligibility and extracted the following information from each included study: (1) general information of the study (author, publication year, country, and study type); (2) patient characteristics and clinical outcomes (sample size, age, gender, histology, treatment, outcomes, and follow-up); and (3) prediction results/prediction efficiency (univariate and multivariate analysis results).
The quality of the studies was independently assessed by two reviewers using the Quality in Prognosis Studies (QUIPS) tool (Supplementary Table 2) (13). Any disagreement was resolved through discussion with a third reviewer.
A total of 1,238 papers were found and the full texts of 62 papers were screened. Among these articles, 37 studies were excluded. Ultimately, 25 studies (14–38) were included in this systematic review (Figure 1). Thirteen (52.0%) studies (16–20, 22, 23, 25, 26, 31, 32, 35, 37) were assessed as “unclear” for the domain of study participation, mostly due to a lack of information about the source population and the population of interest (TNM stage, histology, etc.), or ambiguous inclusion and exclusion criteria. The risk of bias for outcome measurement was assessed as “unclear” in four studies (14, 15, 25, 32) due to a lack of outcome definition. Three studies (16, 24, 36) were assessed to have a “moderate” risk of bias in the domain of other prognostic factors (covariates) because they did not consider other clinical variables (Figure 2).
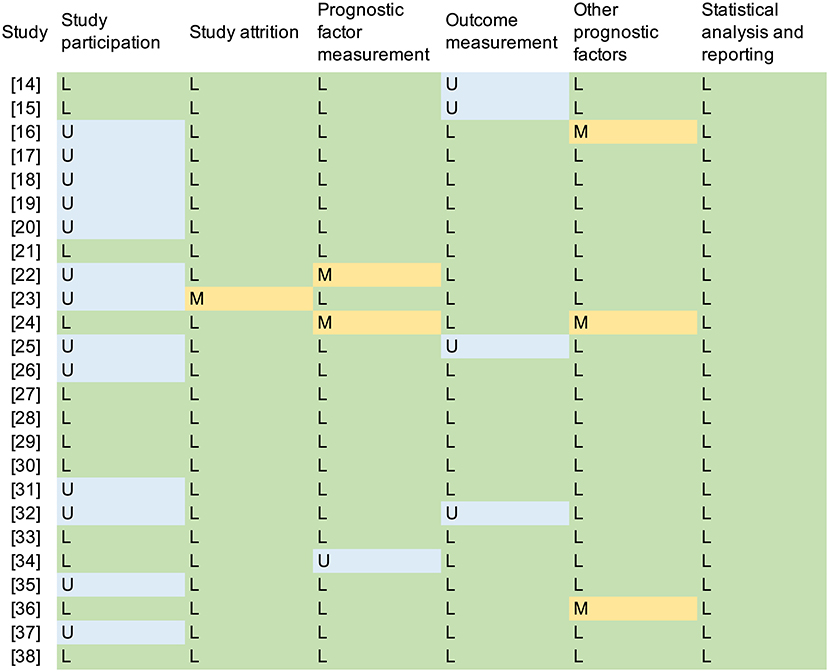
Figure 2. Quality assessment according to the QUIPS. L, low risk; M, moderate risk; H, high risk; U, unclear.
Table 1 shows the characteristics of the included studies. Six, 12, six, and one studies assessed European, Asian, North American, and South American populations, respectively. The study periods ranged from 1983 to 2018, and 21 studies (84%) were developed before 2015. The sample sizes ranged from 17 to 412. A total of 2,954 patients (1,994 females, 67.5%) were included. Their ages ranged from 8 to 89 years; 2,416 (81.8%) and 227 (7.6%) patients had papillary thyroid carcinoma (PTC) and follicular thyroid cancer (FTC), respectively. The most common PET/CT parameter was fluorodeoxyglucose (FDG) uptake (in 16 studies). The mean or median follow-up time ranged from 19.1 months to 17.1 years. The end point was overall survival (OS) in 12 studies and PFS/RFS/DFS/EFS in 17 studies. The results of the included studies are shown in Table 2.
Five studies (14–18) investigated the prognostic value of PET/CT parameters in patients with DTC before thyroidectomy. Three studies (15, 17, 18) suggested the potential prognostic value of PET/CT parameters in this setting. In a study of DTC patients with bone metastases (18), FDG uptake of bone lesions was an independent predictor of OS [hazard ratio (HR) = 4.13, 95% CI = 3.96–4.27, p = 0.009] according to multivariate analysis. In contrast, two studies did not find associations between the tumor-to-liver uptake ratio (TLR) and disease-free survival (DFS) (14) or between the FDG uptake of primary lesion/lateral neck node metastasis and recurrence-free survival (RFS) (16).
Twenty studies (19–38) explored the association between the PET/CT parameters after thyroidectomy and the outcomes of DTC patients. The common indications of PET/CT before radioactive iodine (131-I) therapy included an elevated Tg, abnormal imaging (WBS, US, and CT), high-risk histopathology, and suspicion or proven metastases. Four studies did not report the indication (20–22, 34).
For DFS/PFS/disease-specific survival (DSS), 10 studies (21–23, 27, 28, 30, 32, 34–36) reported associations between the PET/CT parameters and DFS/PFS/DSS using univariate analysis. Five (21, 25, 27, 28, 30) studies further performed a multivariate analysis, four of which (21, 25, 28, 30) reported that the FDG uptake, maximum standardized uptake value (SUVmax), peak standardized uptake value corrected for lean body mass (SULpeak), and number of lesions were associated with DFS/DSS. In contrast, three studies (29, 37, 38) reported no association between the location of FDG-avid lesions, number of FDG-avid lesions, SUVmax (29), FDG uptake (37), baseline SUVmax or reductions in SUVmax of lesions (38), and disease progression.
Eleven studies (19, 20, 23–27, 30–33) explored whether the PET/CT parameters were associated with the survival of DTC patients, and all found an association in univariate analysis. Seven studies (20, 24–27, 31, 33) performed a multivariate analysis, and FDG uptake (20, 26, 27, 31, 33), volume of lesions (24), number of lesions (26), and SUVmax (26) were associated with OS, with a higher predictive value than age (24, 26, 33), sex (24, 33), or metastasis status (24, 26) alone. Only one study (20) reported that FDG uptake of lesions was not a significant predictor of survival in multivariate analysis.
This is the first systematic review about the prognostic value of 18F-FDG PET/CT parameters for the clinical outcomes of patients with DTC. Most studies suggested PET/CT parameters as promising prognostic markers: 12 of 17 (70.6%) and 12 of 12 (100%) studies showed an association between the PET/CT parameters and disease progression and survival in patients with DTC, respectively. However, the potential confounders caused by the heterogeneity in PET/CT parameters, unclear information on patients, and PET/CT-adapted treatment modifications should be considered. The prognostic value of 18F-FDG PET/CT in DTC is not yet generalizable and should be explained with caution.
Primarily, the role of PET/CT has been limited to detecting lesions responsible for elevated Tg in patients with a negative WBS or to determining disease extent in patients with elevated Tg along with positive WBS (6, 7). We found that the PET/CT parameters in this clinical setting can provide additional prognostic information as well. For instance, Pace et al. (21) found that patients with negative FDG uptake had a better progression-free survival (PFS) either in the whole group or in those with elevated Tg (both >2 and >10 ng/ml); only Tg and FDG uptake were independent predictors of PFS in DTC patients. In patients with lung metastasis (31), extrathyroidal invasion, FDG-avid lesions, and metachronous diagnosis of metastasis were independent predictors of OS; age, sex, the moment of diagnosis of lung metastasis, tumor diameter, and the RAI cumulative doses were not. The combination of RAI and FDG uptake was supposed to identify patients with poorer outcomes (24, 26–28), and FDG positivity seems to have a larger influence on prognosis than does RAI uptake (24, 26–28). In a cohort of 64 patients, reduced DSS was observed in patients with FDG (+)/RAI (–) metastatic lesions compared with the FDG (+)/RAI (+) and FDG (–)/RAI (+) groups (28). Deandreis et al. (27) reported that the 2-year survival rates were 60% for PET-positive and 100% for PET-negative patients with metastatic DTC, with no difference between RAI (–)/FDG (+) and RAI (+)/FDG (+) patients. Several studies also reported similar results (24, 26).
Recently, tyrosine kinase inhibitor (TKI) therapy for RAI-R DTC has become a hot topic. The survival of RAI-R DTC was poor, and a study with a median follow-up of 11.1 years (32) reported that, after the diagnosis of metastatic RAI-R disease, the 5-year OS probability of patients was 34%, and the median OS was 3.56 years. The 5-year PFS probability was 19%, and the median PFS was 1.31 years. Not all patients benefit from TKI therapy, and the early identification of subjects with poor response and prognosis is considerably meaningful. A few small-sample studies have explored whether PET/CT parameters can be used as predictors, and the results are controversial (32, 36–38). In a cohort of 20 RAI-R DTC patients treated with apatinib (36), a significant difference between patients with partial response (PR) and stable disease (SD) was observed with respect to ΔMTV% and ΔTLG%; a significant difference in PFS was observed between patients with ΔMTV% at one and two cycles (less than −45% and −45% or greater) and between patients with ΔTLG% at one and two cycles (less than −80% and −80% or greater). In patients who underwent sorafenib therapy (37), the RAI (+) or FDG (+) in lesions did not affect PFS, while larger target lesions (>1.5 cm) and the shortest tumor doubling time (≤6 months) had worse outcomes. Another study (38) reported that baseline SUVmax and early reductions in SUVmax were higher and more robust in patients who showed disease progression than in patients who responded to sorafenib, but no significant association with PFS was found.
Preoperative PET/CT is not a routine modality in DTC because the incidence of distant metastasis is very low, and a high FDG uptake in tumors makes it difficult to detect adjacent metastatic lymph nodes (39, 40). According to current evidence (14, 16, 17), the FDG uptake of primary tumors before thyroidectomy could not predict disease progression or recurrence, although FDG avidity was more common in patients with confirmed prognostic factors, such as larger tumor size, extrathyroidal extension, and high Tg levels (14, 16, 17). FDG uptake in metastatic lesions before thyroidectomy was associated with poor outcomes; for instance, an SUVmax >2.3 of the N1b lymph node was associated with shorter RFS (p = 0.025) among 96 PTC patients (15). The FDG uptake of bone lesions was an independent predictor of OS (HR = 4.13, 95% CI = 3.96–4.27, p = 0.009) (18).
The most common PET/CT parameter was FDG uptake, visually identifiable FDG activity with a higher intensity than the surrounding tissues and no normal or physiological uptake was considered to be positive. Semiquantitative parameters, such as SUVmax, metabolic tumor volume (MTV), and total lesion glycolysis (TLG), have also been described in some studies. We noticed considerable differences in the cutoff values of semiquantitative parameters among studies; the cutoff values of SUVmax were 10 (19, 24, 27, 30, 35), 2.9 in N1b lymph nodes (16), and 3.6 in distant metastatic lesions (28). The cutoff values of MTV were 9.08 ml (32), 10 and 50 ml (30), and 125 ml (24). The cutoff values of TLG were 49.1 (32) and 154 (30). The different study populations, target lesions, or cutoff measurements (based on previous studies, median values, receiver operating characteristic curves, or log-rank test results) may have led to this difference. Additionally, the semiquantitative parameters did not present higher prognostic values than the conventional parameters in the studies. Masson-Deshayes et al. (30) evaluated the PET/CT scans of 37 patients with metastatic DTC. In the univariate analysis, the prognostic factors for PFS and OS were SUVmax, SULpeak, and TLG. The number of FDG-avid lesions was significantly associated with PFS, but not MTV. The number of FDG-avid lesions and the SULpeak were independent prognostic factors in the multivariate analysis. Dichotomizing patients into two groups of risk could introduce measurement errors and reduce the ability to detect a correlation; keeping variables continuous with linear regression may be relevant (41).
One point raises concern that the effect of PET may be misestimated considering the favorable outcomes attributed to PET/CT-adapted treatment modifications (e.g., dose modification of 131-I, targeted therapy) (42). A retrospective analysis of the likely impact of PET/CT on treatment may be biased. For instance, in 77 patients with recurrent/metastatic DTC (29), lesional SUVmax, the number or location of FDG-avid lesions, and the TNM stage did not correlate with DSS. This study did not include non-FDG-avid recurrent tumors, and the presence of surgically amenable recurrence/metastasis was considered as a predictor. The prognostic value of PET/CT might be confounded by the type of treatment that is known to be associated with the prognosis. Only one study (25) stated that the results of PET/CT before 131-I therapy did not have any impact on the treatment decision of the patients; they found that FDG uptake (χ2 = 26.3, p < 0.0001) and Tg were independent predictors of DFS, while Tg was the only variable associated with OS.
This systematic review had some limitations. Firstly, only published English language articles were included, which may lead to publication bias. Secondly, all studies included were retrospective, and, as discussed above, a retrospective analysis of the likely impact of PET/CT on treatment may be biased. Thirdly, we did not contact the authors of the included studies to acquire detailed information of patients. Lastly, we did not perform a cost-effectiveness analysis.
Current evidence suggests that 18F-FDG PET/CT parameters alone or in combination with other variables can serve as prognostic markers to identify DTC patients with poor outcomes, especially when Tg is elevated with a negative WBS. The heterogeneity in PET/CT parameters, unclear information on patients, and PET/CT-adapted treatment modifications may cause potential bias and influence the repeatability of the results. Therefore, larger randomized and prospective research is needed to confirm these findings and to examine the effectiveness of PET/CT parameters at different timings for prognosis assessment in DTC patients. The datasets generated for this study are available on request from the corresponding author.
The datasets generated for this study are available on request to the corresponding author.
Conceptualization: RT, HW, and LS. Investigation: HW and HD. Methodology and Validation: GS and QL. Project administration: HW and RT. Supervision: RT and GS. Visualization: RT and LS. Writing—original draft: HW and HD. Writing—review and editing: GS and RT. All authors contributed to the article and approved the submitted version.
This study was supported by the Science and Technology Department of Sichuan Province (Grant 2019YFS0373).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.648658/full#supplementary-material
1. Cho SW, Choi HS, Yeom GJ, Lim JA, Moon JH, Park DJ, et al. Long-term prognosis of differentiated thyroid cancer with lung metastasis in Korea and its prognostic factors. Thyroid. (2014) 24:277–86. doi: 10.1089/thy.2012.0654
2. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. (1994) 97:418–28. doi: 10.1016/0002-9343(94)90321-2
3. Suh S, Goh TS, Kim YH, Oh SO, Pak K, Seok JW, et al. Development and validation of a risk scoring system derived from meta-analyses of papillary thyroid cancer. Endocrinol. Metab. (2020) 35:435–42. doi: 10.3803/EnM.2020.35.2.435
4. Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for papillary thyroid carcinoma: a review and comparison. Ann. Surg. (2007) 245:366–78. doi: 10.1097/01.sla.0000250445.92336.2a
5. Bomanji JB, Costa DC, Ell PJ. Clinical role of positron emission tomography in oncology. Lancet Oncol. (2001) 2:157–64. doi: 10.1016/S1470-2045(00)00257-6
6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
7. Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, et al. NCCN guidelines insights: thyroid carcinoma, version 2.2018. J. Natl. Compr. Cancer Netw. (2018) 16:1429–40. doi: 10.6004/jnccn.2018.0089
8. Kim MH, Ko SH, Bae JS, Lee SH, Jung CK, Lim DJ, et al. Non-FDG-avid primary papillary thyroid carcinoma may not differ from FDG-avid papillary thyroid carcinoma. Thyroid. (2013) 23:1452–60. doi: 10.1089/thy.2013.0051
9. Schütz F, Lautenschläger C, Lorenz K, Haerting J. Positron emission tomography (PET) and PET/CT in thyroid cancer: a systematic review and meta-analysis. Eur. Thyroid J. (2018) 7:13–20. doi: 10.1159/000481707
10. Treglia G, Villani MF, Giordano A, Rufini V. Detection rate of recurrent medullary thyroid carcinoma using fluorine-18 fluorodeoxyglucose positron emission tomography: a meta-analysis. Endocrine. (2012) 42:535–45. doi: 10.1007/s12020-012-9671-6
11. Treglia G, Giovanella L. Prognostic role of FDG-PET/CT in differentiated thyroid carcinoma: where are we now? J. Med. Imaging Radiat. Oncol. (2015) 59:278–80. doi: 10.1111/1754-9485.12317
12. Zorzela L, Loke YK, Ioannidis JP, Golder S, Santaguida P, Altman DG, PRISMAHarms Group, et al. PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ. (2016) 352:i157. doi: 10.1136/bmj.i157
13. Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann. Intern. Med. (2006) 144:427–37. doi: 10.7326/0003-4819-144-6-200603210-00010
14. Kwon SY, Choi EK, Kong EJ, Chong A, Ha JM, Chun KA, et al. Prognostic value of preoperative 18F-FDG PET/CT in papillary thyroid cancer patients with a high metastatic lymph node ratio: a multicenter retrospective cohort study. Nucl. Med. Commun. (2017) 38:402–6. doi: 10.1097/MNM.0000000000000657
15. Lee CH, Lee SW, Son SH, Hong CM, Jeong JH, Jeong SY, et al. Prognostic value of lymph node uptake on pretreatment F-18 FDG PET/CT in patients with N1B papillary thyroid carcinoma. Endocr. Pract. (2019) 25:787–93. doi: 10.4158/EP-2018-0607
16. Kim H, Na KJ, Choi JH, Ahn BC, Ahn D, Sohn JH. Feasibility of FDG-PET/CT for the initial diagnosis of papillary thyroid cancer. Eur. Arch. Otorhinolaryngol. (2016) 273:1569–76. doi: 10.1007/s00405-015-3640-7
17. Kim SK, So Y, Chung HW, Yoo YB, Park KS, Hwang TS, et al. Analysis of predictability of F-18 fluorodeoxyglucose-PET/CT in the recurrence of papillary thyroid carcinoma. Cancer Med. (2016) 5:2756–62. doi: 10.1002/cam4.867
18. Qiu ZL, Xue YL, Song HJ, Luo QY. Comparison of the diagnostic and prognostic values of 99mTc-MDP-planar bone scintigraphy, 131I-SPECT/CT and 18F-FDG-PET/CT for the detection of bone metastases from differentiated thyroid cancer. Nucl. Med. Commun. (2012) 33:1232–42. doi: 10.1097/MNM.0b013e328358d9c0
19. Pryma DA, Schöder H, Gönen M, Robbins RJ, Larson SM, Yeung HW. Diagnostic accuracy and prognostic value of 18F-FDG PET in Hürthle cell thyroid cancer patients. J. Nucl. Med. (2006) 47:1260–6.
20. Nagamachi S, Wakamatsu H, Kiyohara S, Nishii R, Mizutani Y, Fujita S, et al. Comparison of diagnostic and prognostic capabilities of 18F-FDG-PET/CT, 131I-scintigraphy, and diffusion-weighted magnetic resonance imaging for postoperative thyroid cancer. Jpn. J. Radiol. (2011) 29:413–22. doi: 10.1007/s11604-011-0572-z
21. Pace L, Klain M, Salvatore B, Nicolai E, Zampella E, Assante R, et al. Prognostic role of 18F-FDG PET/CT in the postoperative evaluation of differentiated thyroid cancer patients. Clin. Nucl. Med. (2015) 40:111–5. doi: 10.1097/RLU.0000000000000621
22. Salvatore B, Klain M, Nicolai E, D'Amico D, De Matteis G, Raddi M, et al. Prognostic role of FDG PET/CT in patients with differentiated thyroid cancer treated with 131-iodine empiric therapy. Medicine. (2017) 96:e8344. doi: 10.1097/MD.0000000000008344
23. Zhu X, Wu S, Yuan X, Wang H, Ma C. Progression free survival related to 18F-FDG PET/CT uptake and 131I uptake in lung metastases of differentiated thyroid cancer. Hell J. Nucl. Med. (2019) 22:123–30. doi: 10.1967/s002449911005
24. Gaertner FC, Okamoto S, Shiga T, Ito YM, Uchiyama Y, Manabe O, et al. FDG PET performed at thyroid remnant ablation has a higher predictive value for long-term survival of high-risk patients with well-differentiated thyroid cancer than radioiodine uptake. Clin. Nucl. Med. (2015) 40:378–83. doi: 10.1097/RLU.0000000000000699
25. Wang W, Larson SM, Fazzari M, Tickoo SK, Kolbert K, Sgouros G, et al. Prognostic value of [18F]fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J. Clin. Endocrinol. Metab. (2000) 85:1107–13. doi: 10.1210/jcem.85.3.6458
26. Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J. Clin. Endocrinol. Metab. (2006) 91:498–505. doi: 10.1210/jc.2005-1534
27. Deandreis D, Al Ghuzlan A, Leboulleux S, Lacroix L, Garsi JP, Talbot M, et al. Do histological, immunohistochemical, and metabolic (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocr. Relat. Cancer. (2011) 18:159–69. doi: 10.1677/ERC-10-0233
28. Hong CM, Ahn BC, Jeong SY, Lee SW, Lee J. Distant metastatic lesions in patients with differentiated thyroid carcinoma. Clinical implications of radioiodine and FDG uptake. Nuklearmedizin. (2013) 52:121–9. doi: 10.3413/Nukmed-0541-12-11
29. Akkas BE, Demirel BB, Vural GU. Prognostic factors affecting disease-specific survival in patients with recurrent and/or metastatic differentiated thyroid carcinoma detected by positron emission tomography/computed tomography. Thyroid. (2014) 24:287–95. doi: 10.1089/thy.2013.0195
30. Masson-Deshayes S, Schvartz C, Dalban C, Guendouzen S, Pochart JM, Dalac A, et al. Prognostic value of (18)F-FDG PET/CT metabolic parameters in metastatic differentiated thyroid cancers. Clin. Nucl. Med. (2015) 40:469–75. doi: 10.1097/RLU.0000000000000780
31. Marcus C, Antoniou A, Rahmim A, Ladenson P, Subramaniam RM. Fluorodeoxyglucose positron emission tomography/computerized tomography in differentiated thyroid cancer management: importance of clinical justification and value in predicting survival. J. Med. Imaging Radiat. Oncol. (2015) 59:281–8. doi: 10.1111/1754-9485.12286
32. Manohar PM, Beesley LJ, Bellile EL, Worden FP, Avram AM. Prognostic value of FDG-PET/CT metabolic parameters in metastatic radioiodine-refractory differentiated thyroid cancer. Clin. Nucl. Med. (2018) 43:641–7. doi: 10.1097/RLU.0000000000002193
33. Pitoia F, Bueno F, Cross G. Long-term survival and low effective cumulative radioiodine doses to achieve remission in patients with 131Iodine-avid lung metastasis from differentiated thyroid cancer. Clin. Nucl. Med. (2014) 39:784–90. doi: 10.1097/RLU.0000000000000507
34. Sabra MM, Ghossein R, Tuttle RM. Time course and predictors of structural disease progression in pulmonary metastases arising from follicular cell-derived thyroid cancer. Thyroid. (2016) 26:518–24. doi: 10.1089/thy.2015.0395
35. Kang JH, Jung DW, Pak KJ, Kim IJ, Kim HJ, Cho JK, et al. Prognostic implication of fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in patients with recurrent papillary thyroid cancer. Head Neck. (2018) 40:94–102. doi: 10.1002/hed.24967
36. Wang C, Zhang X, Yang X, Li H, Cui R, Guan W, et al. PET response assessment in apatinib-treated radioactive iodine-refractory thyroid cancer. Endocr. Relat. Cancer. (2018) 25:653–63. doi: 10.1530/ERC-18-0007
37. Kim MJ, Kim SM, Lee EK, Hwangbo Y, Lee YJ, Cho SW, et al. Tumor doubling time predicts response to sorafenib in radioactive iodine-refractory differentiated thyroid cancer. Endocr. J. (2019) 66:597–604. doi: 10.1507/endocrj.EJ18-0488
38. Marotta V, Ramundo V, Camera L, Del Prete M, Fonti R, Esposito R, et al. Sorafenib in advanced iodine-refractory differentiated thyroid cancer: efficacy, safety and exploratory analysis of role of serum thyroglobulin and FDG-PET. Clin. Endocrinol. (2013) 78:760–7. doi: 10.1111/cen.12057
39. Lee JW, Lee SM, Lee DH, Kim YJ. Clinical utility of 18F-FDG PET/CT concurrent with 131I therapy in intermediate-to-high-risk patients with differentiated thyroid cancer: dual-center experience with 286 patients. J. Nucl. Med. (2013) 54:1230–6. doi: 10.2967/jnumed.112.117119
40. Helal BO, Merlet P, Toubert ME, Franc B, Schvartz C, Gauthier-Koelesnikov H, et al. Clinical impact of (18)F-FDG PET in thyroid carcinoma patients with elevated thyroglobulin levels and negative (131)I scanning results after therapy. J. Nucl. Med. (2001) 42:1464–9.
41. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. (2006) 332:1080. doi: 10.1136/bmj.332.7549.1080
Keywords: 18F-FDG PET/CT, differentiated thyroid carcinoma, outcome, systematic review, prediction
Citation: Wang H, Dai H, Li Q, Shen G, Shi L and Tian R (2021) Investigating 18F-FDG PET/CT Parameters as Prognostic Markers for Differentiated Thyroid Cancer: A Systematic Review. Front. Oncol. 11:648658. doi: 10.3389/fonc.2021.648658
Received: 01 January 2021; Accepted: 16 February 2021;
Published: 13 May 2021.
Edited by:
Haibin Shi, Soochow University, ChinaReviewed by:
Ran Zhu, Soochow University, ChinaCopyright © 2021 Wang, Dai, Li, Shen, Shi and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Tian, cm9uZ3RpYW5udWNsZWFyQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.