- 1Department of Oncology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 2Department of Ultrasound, Aero Space Central Hospital, Beijing, China
Background: We performed a systematic review and meta-analysis to evaluate the risks of cardiac adverse events in solid tumor patients treated with monotherapy of immune checkpoint inhibitors (ICIs) or combined therapy of ICIs plus chemotherapy.
Methods: Eligible studies were selected through the following databases: PubMed, Embase and clinical trials (https://clinicaltrials.gov.) and included phase III/IV randomized controlled trials (RCTs) involving patients with the solid tumor treated with ICIs. The data was analyzed by using Review Manager (version5.3), Stata (version 15.1).
Results: Among 2,551 studies, 25 clinical trials including 20,244 patients were qualified for the meta-analysis. Compared with PD-1 inhibitor (nivolumab) or CTLA-4 inhibitor (ipilimumab), PD-1 inhibitor (nivolumab) plus CTLA-4 inhibitor (ipilimumab) combined therapy showed significant increase in grade 5 arrhythmology (OR 3.90, 95% CI: 1.08–14.06, p = 0.603). PD-1 inhibitor plus chemotherapy show significant increase in grades 1–5 myocardial disease (OR 5.09, 95% CI: 1.11–23.32, p = 1.000). Compared with chemotherapy, PD-1 inhibitor (nivolumab) or CTLA-4 inhibitor (ipilimumab), PD-1 inhibitor (nivolumab) plus CTLA-4 inhibitor (ipilimumab) combined therapy show significant increase in grades 1–5 arrhythmology (OR 2.49, 95% CI: 1.30–4.78, p = 0.289).
Conclusions: Our meta-analysis demonstrated that PD-1 inhibitor plus CTLA-4 inhibitor can result in a higher risk of grade 5 arrhythmology in comparison with PD-1/CTLA-4 inhibitor alone, and a higher risk of grade 5 arrhythmology in comparison with chemotherapy. PD-1 inhibitor plus chemotherapy treatment could increase the risk of all-grade myocardial disease compared with chemotherapy. However, in most cases, there was no significant increase of risks of cardiovascular toxicity in PD-1/PD-L1 inhibitor monotherapy or PD-1/PD-L1 inhibitor plus chemotherapy compared with chemotherapy alone.
Introduction
The progression of immunotherapy have considerably changed cancer treatment, and improved the clinical prognosis of many cancer patients. Researches showed that monoclonal antibodies against cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) and programmed cell death protein 1 (PD-1)/programmed cell death protein 1 ligand 1 (PD-L1) are beneficial for solid tumor patients during treatment (1). Thus far, a list of ICIs have been approved by Food and Drug Administration(FDA), including PD-L1 inhibitors (atezolizumab, durvalumab, and avelumab), CTLA4 inhibitor (ipilimumab), and PD-1 inhibitors (nivolumab and pembrolizumab) etc., which were applied to treatable tumors including melanoma, Hodgkin’s lymphoma, non-small-cell lung cancer, renal cell carcinoma, bladder, head and neck cancer, liver, gastric cancer and microsatellite instability high or DNA mismatch repair-deficient colorectal cancer (2), Nevertheless, since immune checkpoints also play pivotal roles in maintaining homeostasis of normal tissues, their therapeutic blockade can unavoidably cause side effects to the patients, termed as immune-related adverse events (irAEs). IrAEs have been shown to affect many systems and cause lesions of differential severity, like pneumonitis and pancreatitis. Although relatively less, cardiotoxicity has also been found following cancer-related treatments such as chemotherapy and targeted therapy, which sometimes, like the myocarditis, can be fatal. However, irAEs concerning cardiovascular toxicity has not been well recognized or reported (3). In the current study, the risks of immune-related cardiac toxicity in cancer patients treated with ICIs were systematically evaluated, which was supposed to help better understand the cardiac harmness of ICIs and provide a reference for the rational use and safety evaluation of ICIs in clinical practice.
Methods
Search Methods and Study Selection
The systematic review with meta-analysis was conducted according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions, and reported according to the PRISMA Statement (4).
We searched the literatures related to the efficacy of immunosuppressive agents in solid tumors from the following databases: PubMed, Embase and clinicaltrials (https://clinicaltrials.gov) up to Oct, 2020. The medical subject heading (MeSH) terms included for searching the relevant studies containing the following keywords and terms: one term that refers to cancer (neoplasm, carcinoma, cancer, or tumor), one term indicating the ICIs (anti-CTLA-4, anti-PD-1, ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, or avelumab), and one term related to randomized controlled trials, connected by “and”. After the initial search, we further selected studies according to the following criteria: (1) phase III/IV RCTs with primary endpoints such as overall survival (OS), progression-free survival (PFS), or objective response rate (ORR); (2) histologically confirmed solid cancers such as lung cancer, and others; (3) containing the information of ICIs and cardiotoxicity; and (4) sharing some similarity in experimental method across different studies. Moreover, studies were excluded if they were (1) reviews, duplicate reports, letters, unfinished studies, or conference reports; (2) studies where cardiotoxicity cannot be confirmed due to insufficient data; (3) papers in languages other than English; (4) studies conducted with cell lines, animal models, or on nonsolid cancers; (5) studies whose experimental method was substantially different from other selected RCTs; and (6) RCTs in phase I/II. This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline.
Data Extraction
Two reviewers (JXH and RYT) independently searched all the relevant studies and read the titles, abstracts, and full texts of the identified studies. The eligibility of the extracted studies was assessed using the PICO (patient, intervention, comparison and outcome) chart. Two reviewers extracted the following information from the selected study: year of publication, name of the journal, the last name of the first author, treatment arms, the primary endpoint, type of underlying solid tumor, number of patients in the ICIs treatment groups, number of patients in control groups, number of patients bearing pneumonitis or pneumonia of all-grade (grades 1–5), high-grade (grades 3–5), and death. Disagreements in the assessment were resolved via discussion with the third reviewer (QS).
Cardiac toxicity related AEs are of a large variety yet the incidence of each type is small. We classified irAEs into six major categories (5):
1. arrhythmology, namely atrial fibrillation, atrial flutter, atrial tachycardia, atrioventricular block, arrhythmia supraventricular, atrioventricular block complete, bradycardia, bifascicular block, sinus bradycardia, sinus tachycardia, supraventricular tachycardia, tachycardia, ventricular arrhythmia, ventricular tachycardia, ventricular fibrillation;
2. cardiac failure, which refers to cardiac failure, acute cardiac failure, chronic cardiac failure, congestive, cardiopulmonary failure, right ventricular failure;
3. coronary artery disease, such as arteriospasm coronary, acute coronary syndromes, angina unstable, angina pectoris, acute myocardial infarction, myocardial infarction, myocardial ischemia;
4. pericardial disease, including cardiac tamponade, pericardial effusion, pericarditis);
5. cardiac arrest, for example cardio-respiratory arrest;
6. myocardial disease, comprising of autoimmune myocarditis, eosinophilic myocarditis, myocarditis).
Data Analysis
We used pooled odds ratios (ORs) with 95% CIs to evaluate the risk of cardiac adverse events of ICIs. We mainly collect the drugs used in clinical trials, major types of adverse cardiac reactions, number of patients in experimental and control groups. Heterogeneity was evaluated using the Q test and Higgin’s and Thompson’s I2 (I2) statistics. Defined as the percentage of variations in the effect sizes that is not the cause of sampling errors, I2 statistics is robust and not sensitive to the number of studies under analysis. We consider I2 values greater than 50% as moderate to high heterogeneity. We used random-effects model (DerSimonian and Laird method) if the I2 value was more than 50%, and used Mantel Haenszel Model to assess the heterogeneity. P <0.05 was considered statistically significant. The quality of the included studies was assessed by Cochrane risk of bias tool. We performed subgroup analysis (based on drugs, treatments and irAEs) and sensitivity analysis to find the source of heterogeneity. Specifically, we sequentially removed individual studies one by one to evaluate the stability of the results. The incidence of each type Cardiac toxicity related AEs is small, we used the continuous correction method to correct our data. Finally, we applied the Begger test, Egger test and funnel plot to assess publication bias. All analyses were performed using Review Manager (version 5.3), Stata (version 15.1), RStudio.
Results
Eligible Studies and Characteristics
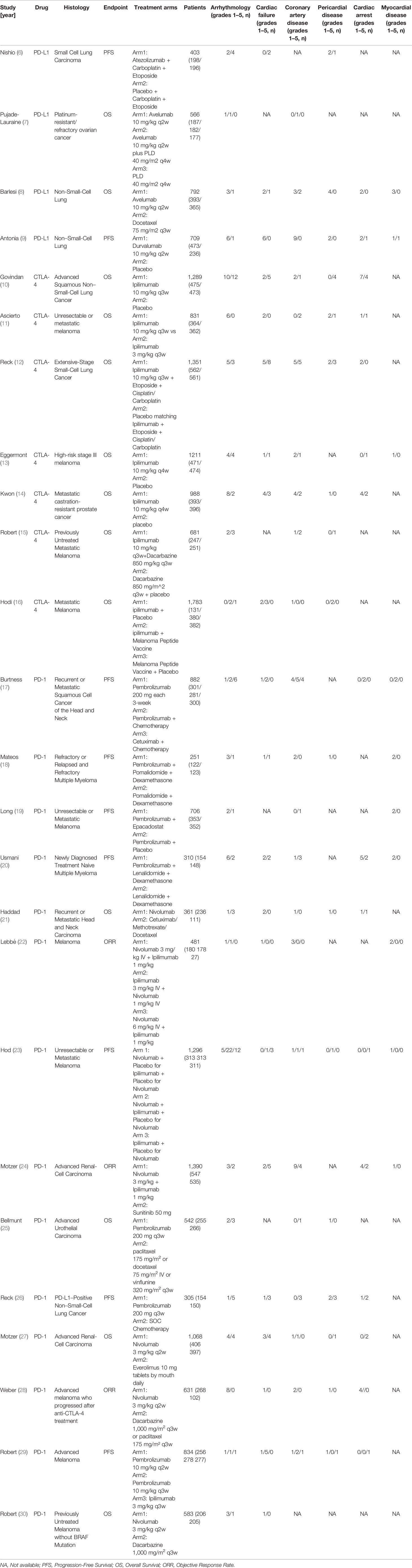
Using the search terminology, we initially identified 2,551 studies from the three databases. Among those studies, 25 RCTs met our rigorous inclusion criteria (Table 1) (6–30). The detailed research flow chart is shown in Figure 1. All 25 trials evaluated and compared the side effects of ICI with other control treatments. A total of 20,244 patients were included in the 25 trials, including seven studies related to CTLA-4 (ipilimumab: seven cohorts, n = 8,134), four studies related to PD-L1 (atezolizumab: one cohort, n = 403; avelumab: two cohorts, n = 1,358; durvalumab: one cohort, n = 709), 14 studies related to PD-1 (nivolumab: seven cohorts, n = 5,810; pembrolizumab: seven cohorts, n = 3,830) and 11 studies compared combined therapy of chemotherapy plus ICIs with chemotherapy. In terms of patients’ tumor type, four trials evaluated non-small-cell lung cancer (NSCLC), one trial evaluated small-cell lung cancer, 10 evaluated melanoma, two studies evaluated advanced renal-cell carcinoma, two trials evaluated multiple myeloma, one trial evaluated advanced urothelial carcinoma, one evaluated prostate cancer, and one trial evaluated ovarian cancer.
Risk of Cardiotoxicity in Patients With PD-1/PD-L1 and CTLA-4 Inhibitors
As shown in Figure 2, compared with control treatments, there was no significant increase of risk of cardiotoxicity in PD-L1 inhibitors. Specifically, the odd ratios (ORs) of different cardiotoxicity were as follows: grades 1–5 arrhythmology, 2.90 (95% CI: 0.72–11.72, p = 0.999); grades 1–5 cardiac failure, 3.13, (95% CI: 0.49–19.8, p = 0.495); grades 1–5 coronary artery, 2.76 (95% CI: 0.40–19.26, p = 0.227); grades 1–5 pericardial disease, 4.71 (95% CI: 0.57–38.78, p = 0.570), grades 1–5 cardiac arrest, 1.81 (95% CI: 0.27–11.92, p = 0.430) and myocardial disease, 1.71 (95% CI: 0.13–22.62, p = 0.204). Similar results were found in patients with CTLA-4/PD-1 inhibitors (Figure 2).
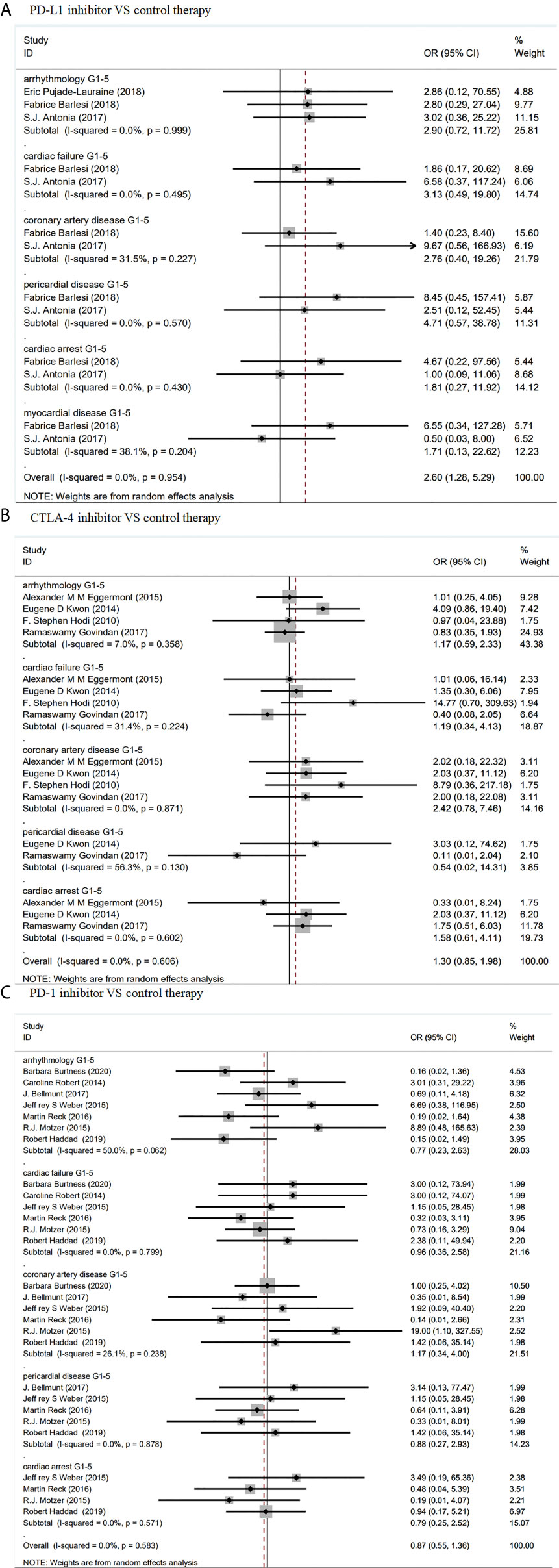
Figure 2 Forest plot analysis of cardiotoxicity in patients treated with ICIS compared with control therapy (A) Forest plot analysis of cardiotoxicity in patients treated with PD-L1 inhibitor compared with control therapy: G1–5, grades 1–5. (B) Forest plot analysis of cardiotoxicity in patients treated with CTLA-4 inhibitor compared with control therapy: G1–5, grades 1–5. (C) Forest plot analysis of cardiotoxicity in patients treated with PD-1 inhibitor compared with control therapy, G1–5: grades 1–5.
Risk of Serious Adverse Events of Cardiotoxicity in ICIs Therapy
Since the studies we selected did not explicitly present cardiac-related adverse events, we mainly extracted the information through the https://clinicaltrials.gov. Cardiac-related adverse events were generally recorded as serious adverse events, which were put into four grades according to the severity in our analysis. Death was also considered to be cardiac-related serious adverse events here.
As shown in Figure 3, compared with PD-1 inhibitor (nivolumab) or CTLA-4 inhibitor (ipilimumab) monotherapy, PD-1 inhibitor (nivolumab) plus CTLA-4 inhibitor (ipilimumab) combined therapy showed significant increase in grade 5 arrhythmology (OR 3.90, 95% CI: 1.08–14.06, p = 0.603), but not in grade 5 myocardial disease (OR 3.00, 95% CI: 0.31–28.92, p = 0.998).
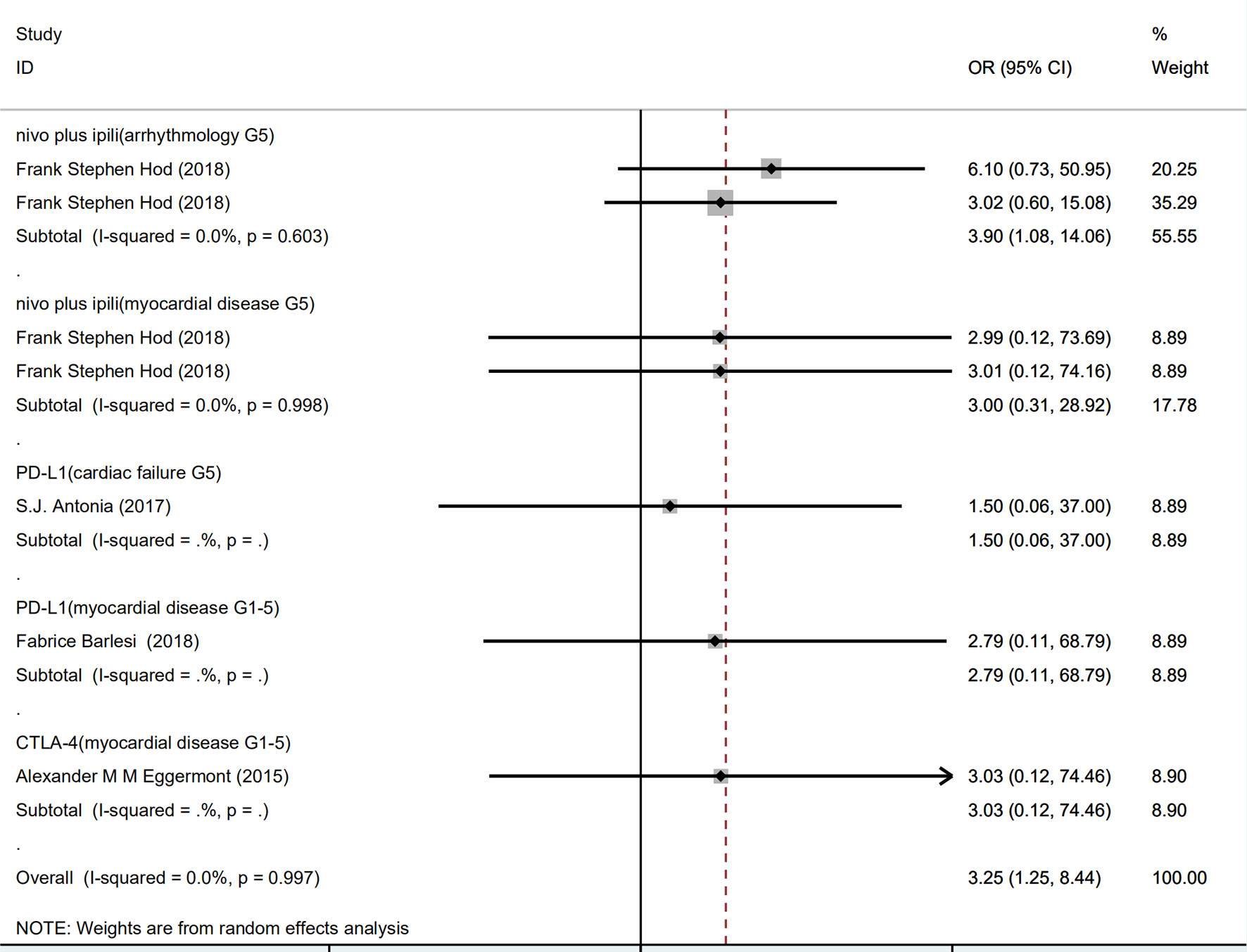
Figure 3 Forest plot analysis of serious adverse events: nivo plus ipili, PD-1 inhibitor (nivolumab) plus CTLA-4 inhibitor (ipilimumab) combination therapies; PD-L1, PD-L1 inhibitor(avelumab) compared with chemotherapy; CTLA-4, CTLA-4 inhibitor (ipilimumab) compared with chemotherapy; G5, grade 5.
There was no significant increase of risk of PD-L1 in grade 5 cardiac failure (OR 1.50, 95% CI: 0.06–37.00) and grade 5 myocardial disease (OR 2.79, 95% CI: 0.11–68.79), compared with chemotherapy. Similarly, CTLA-4 inhibitor did not significantly increase risk of grade 5 myocardial disease (OR 3.03, 95% CI: 0.12–74.46).
Risk of Cardiotoxicity in Combined Therapies
As shown in Figure 4, PD-1 inhibitor plus chemotherapy show significant increase in grades 1–5 myocardial disease (OR 5.09, 95% CI: 1.11–23.32, p = 1.000), compared with chemotherapy alone. In contrast, CTLA-4 inhibitor (ipilimumab) plus chemotherapy showed no significant increase in grades 1–5 arrhythmology (OR 1.29, 95% CI: 0.47–3.57, p = 0.685), grades 1–5 cardiac failure (OR 1.43, 95% CI: 0.14–14.46, p = 0.124), grades 1–5 coronary artery disease (OR 0.86, 95% CI: 0.29–2.61, p = 0.623), or grades 1–5 pericardial disease (OR 0.89, 95% CI: 0.22–3.60, p = 0.420). (Figure 4)
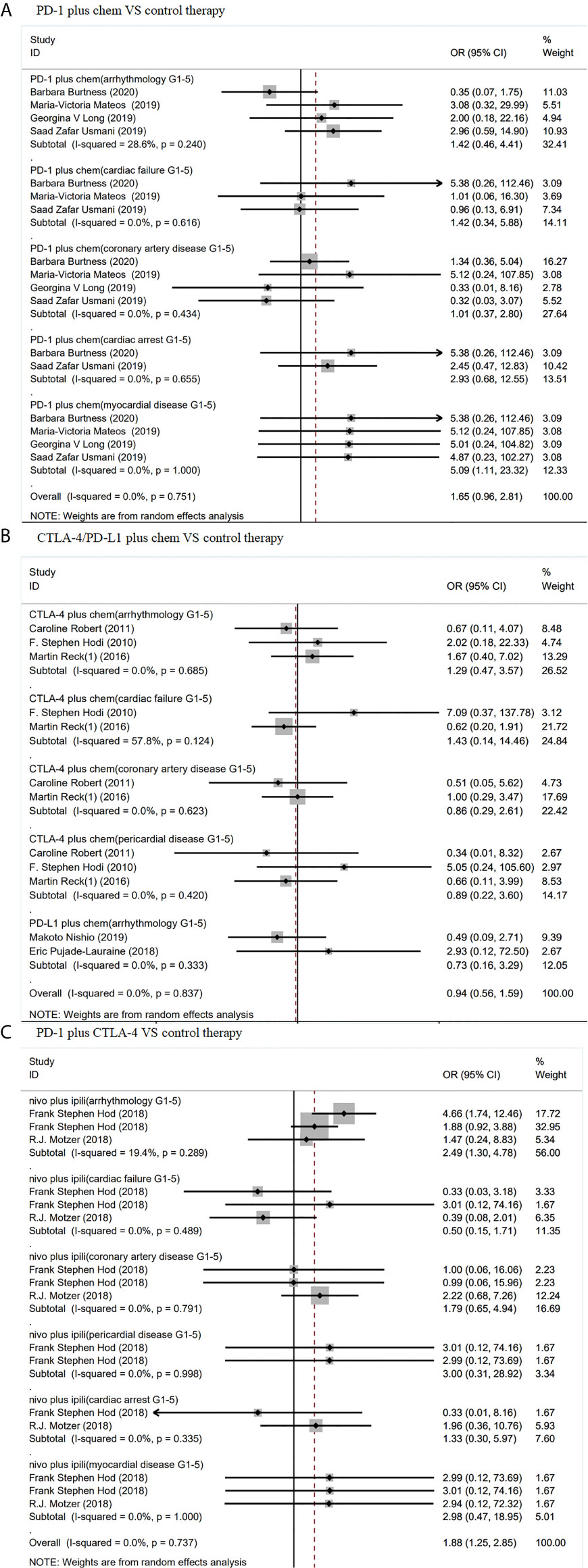
Figure 4 Forest plot analysis of cardiotoxicity in patients treated with combination therapy compared with control therapy (A) Forest plot analysis of cardiotoxicity in patients treated with PD-1 inhibitor plus chemotherapy combination therapy compared with control therapy, PD-1 plus chem, PD-1 inhibitor plus chemotherapy combination therapy; G1–5, grades 1–5. (B) Forest plot analysis of cardiotoxicity in patients treated with CTLA-4/PD-L1 plus chemotherapy combination therapy compared with control therapy, CTLA-4 plus chem, CTLA-4 plus chemotherapy combination therapy; PD-L1 plus chem, PD-L1 plus chemotherapy combination therapy; G1–5, grades 1–5. (C) Forest plot analysis of cardiotoxicity in patients treated with PD-1 plus CTLA-4 combination therapy compared with control therapy: nivo plus ipili, PD-1 inhibitor (nivolumab) plus CTLA-4 inhibitor (ipilimumab) combination therapies; G1–5, grades 1–5.
Compared with chemotherapy and PD-1 inhibitor (nivolumab) or CTLA-4 inhibitor (ipilimumab), PD-1 inhibitor(nivolumab) plus CTLA-4 inhibitor (ipilimumab) combined therapy showed significant increase in grades 1–5 arrhythmology only (OR 2.49, 95% CI: 1.30–4.78, p = 0.289). Nevertheless, these ICIs did not show difference from chemotherapy in grades 1–5 cardiac failure (OR 0.50, 95% CI: 0.15–1.71, p = 0.489), grades 1–5 coronary artery disease (OR 1.79, 95% CI: 0.65–4.94, p = 0.791), grades 1–5 pericardial disease (OR 3.00, 95% CI: 0.31–28.92, p = 0.998), grades 1–5 cardiac arrest (OR 1.33, 95% CI: 0.30–5.96, p = 0.335), or grades 1–5 myocardial disease (OR 2.98, 95% CI: 0.47–18.95, p = 1.000). (Figure 4).
Risk of Cardiotoxicity in Avelumab, Ipilimumab, Pembrolizumab and Nivolumab, Durvalumab
As shown in eFigure 2, there was no significant difference between PD-L1 inhibitor (avelumab) and chemotherapy in risk of in grades 1–5 arrhythmology (OR 2.82, 95% CI: 0.44–17.95, p = 0.992), grades 1–5 cardiac failure (OR 2.80, 95% CI: 0.29–27.04), grades 1–5 coronary artery disease (OR 1.40, 95% CI: 0.23–8.40), grades 1–5 pericardial disease (OR 8.45, 95% CI: 0.45–157.41), grades 1–5 cardiac arrest (OR 4.67, 95% CI: 0.22–97.56), or grades 1–5 myocardial disease (OR 4.67, 95% CI: 0.22–97.56).
Similarly, another PD-L1 inhibitor (durvalumab) did not show significant difference from chemotherapy in terms of grades 1–5 arrhythmology (OR 3.02, 95% CI: 0.36–25.22), grades 1–5 cardiac failure (OR 6.58, 95% CI: 0.37–117.24), grades 1–5 coronary artery disease (OR 9.67, 95% CI: 0.56–166.93), and grades 1–5 myocardial disease (OR 0.50, 95% CI: 0.03–7.98) (eFigure 3).
Likewise, compared with chemotherapy, CTLA-4 inhibitor (ipilimumab) did not show significant increase in risk of grades 1–5 arrhythmology (OR 1.28, 95% CI: 0.52–3.13, p = 0.200), grades 1–5 cardiac failure (OR 0.80, 95% CI: 0.27–2.16, p = 0.551), grades 1–5 coronary artery disease (OR 2.02, 95% CI: 0.61–6.71, p = 1.000), grades 1–5 pericardial disease (OR 0.54, 95% CI: 0.02–14.31, p = 0.130), or grades 1–5 cardiac arrest (OR 1.58, 95% CI: 0.61–4.11, p = 0.602) (eFigure 4).
Also, PD-1 inhibitors (pembrolizumab, nivolumab) show no significant increase of any risk compared with chemotherapy (eFigures 5, 6).
Risk of Cardiotoxicity in Different Doses/Cancer Types
As shown in eFigure 7, compared with a dose of 3 mg/kg q3w, a higher dose (10 mg/kg q3w) of CTLA-4 inhibitor (ipilimumab) showed no significant increase of risks in grades 1–5 arrhythmology (OR 13.15, 95% CI: 0.74–234.20). A dose of nivolumab 3 mg/kg plus ipilimumab 1 mg/kg also showed no significant increase of risks in grades 1–5 coronary artery disease (OR 7.04, 95% CI: 0.36–137.28) compared with a dose of ipilimumab 3 mg/kg plus nivolumab 1 mg/kg.
Similarly, compared with a dose of 10 mg/kg q2w, a dose of 10mg/kg q3w PD-1 inhibitor (pembrolizumab) did not show significantly increase risks of grades 1–5 cardiac failure (OR 0.21, 95% CI: 0.02–1.85).
In lung cancer and melanoma, there was no significant differences between ICIs and chemotherapy in risks of in grades 1–5 arrhythmology (OR 0.95, 95% CI: 0.50–1.81, p = 0.366; OR 1.29, 95% CI: 0.52–3.19, p = 0.467), grades 1–5 cardiac failure (OR 0.64, 95% CI: 0.30–1.36, p = 0.461; OR 3.84, 95% CI: 0.70–21.02, p = 0.431), grades 1–5 coronary artery disease (OR 1.24, 95% CI: 0.49–3.17, p = 0.347;OR 0.77, 95% CI: 0.22–2.66, p = 0.650), grades 1–5 pericardial disease (OR 0.86, 95% CI: 0.27–2.76, p = 0.296; OR 1.05, 95% CI: 0.15–7.20, p = 0.384), grades 1–5 cardiac arrest (OR 1.62, 95% CI: 0.65–4.00, p = 0.709) (eFigures 8, 9).
Quality Assessment and Publication Bias
Cochrane risk of bias tool was used to measure the quality of the included studies and the results were shown in eFigures 1A, B, which showed that the selected literatures are satisfactory. According to the Cochrane Handbook, the Q test and I2 statistics were employed to assess the heterogeneity among the RCTs. I2 values of lower than 30, 30–59, 60–75, and higher than 75% were classified as low, moderate, substantial, and considerable heterogeneity, respectively. There was a moderate heterogeneity (I2 values = 50%) in the PD-1 inhibitor compared with chemotherapy subgroup. Sensitivity analyses were also performed to assess the stability of the included studies. After excluding one study at a time, no significant difference in the results was found from the initial analysis. Moreover, the funnel plot, Egger and Begg test results showed a low risk of publication bias (Egger’s p-value = 0.155; Begg’s = 0.368) (eFigures 10, 11).
Discussion
Immunotherapy has been approved as a first-line treatment for metastatic melanoma and non-small cell lung cancer, a second-line treatment for Hodgkin’s lymphoma, head and neck cancer, renal cell cancer, and bladder cancer (31). Immunosuppressants inhibit tumor growth and metastasis by blocking the immune escape of tumor cells, which inevitably expose patients to different degrees of adverse events, and cardiotoxic-related adverse events are relatively infrequent (32). In 2014, adverse events related to cardiac toxicity were first reported by Heery et al. (33) since then, more and more cardiac toxicity related adverse events have been found during immunotherapy. Cardiac toxicity from immunotherapy is diversified, covering almost all parts of the heart. The most common adverse events are myocarditis and heart failure, which could lead to severe consequences including death. In a study of 964 patients by Mahmood et al., about 1.14% of patients developed myocarditis, and 0.52% experienced severe cardiovascular adverse events (e.g. complete heart block cardiac arrest) (32). A 2017 study on immunotherapy for non-small cell lung cancer found that the incidences of heart failure, cardiopulmonary arrest and myocardial infarction are 2, 1 and 1%, respectively, all of which were severe cardiovascular events (34).
This study is thus far the most comprehensive report on the impacts of immunotherapy on the heart, where all cardiotoxic-related adverse reactions were covered. The purpose of the study is to provide guidelines for the applications of immunosuppressants to clinical practice, which did not find significant differences in cardiac toxicity between many immunotherapies and chemotherapy. However, the meta-analysis showed that, compared with chemotherapy and PD-1 inhibitor (nivolumab) or CTLA-4 inhibitor (ipilimumab), PD-1 inhibitor (nivolumab) plus CTLA-4 inhibitor (ipilimumab) combined therapies showed significant increase in grades 1–5 arrhythmology (OR 2.49, 95% CI: 1.30–4.78, p = 0.006) and grade 5 arrhythmology (OR 3.90, 95% CI: 1.06–14.06, p = 0.04). Additionally, PD-1 inhibitor plus chemotherapy lead to more grades 1–5 myocardial disease (OR 5.09, 95% CI: 1.11–23.32, p = 0.04) than chemotherapy alone.
Consistent with our findings, some other existing studies showed that the combined treatment of nivolumab and ipilimumab has a high risk of heart-related adverse events. A phase one study of untreated melanoma showed that at least one fatal ventricular arrhythmia occurred in the early nivolumab and ipilimumab combined treatment group, and the probability of arrhythmia was higher (2.12%) than that of ipilimumab alone (0.32%). A phase three study included in our meta-analysis found that 16.3% of patients in the nivolumab group had grade 3 or grade 4 treatment-related adverse events while the number was 27.3 and 55.0% in the ipilimumab and combined group of nivolumab with ipilimumab, respectively. Meanwhile, the incidence of at least one severe arrhythmia in the combined group (1.6%) was even greater than that in the single-drug group (0.32%). The European medicines agency’s Opdivo public evaluation report revealed the incidence of heart-related adverse events in combination treatment of nivolumab and ipilimumab were: tachycardia (1.3%), arrhythmia (0.4%), and atrial fibrillation (0.2%) (35). Statistics also indicated that when ipilimumab and nivolumab were used simultaneously, the incidence of IrAEs can increase by 96%, and chances of grade 3 or grade 4 adverse events grow by 55%. All these events typically occur in the first 15 weeks of the treatment cycle and require less than 9 weeks of remission. Consequently, the combination of ipilimumab and nivolumab is currently inappropriate for 40% of patients.
In one case study, following three injections of combined immunotherapy (nivolumab and ipilimumab), a patient with left ventricular dysfunction developed heart failure symptoms where the left ventricular ejection fraction was dropped from 50 to 15% (36). The patient was diagnosed as immune-related myocarditis after biopsy. Our meta-analysis also found that most severe (including fatal) adverse events were almost all related to myocarditis. Fatal myocarditis has an incidence of 0.32% in the phase three study we analyzed. Bristol-Myers Squibb also evaluated the safety of its ipilimumab and nivolumab, and the data showed that ipilimumab and nivolumab combined treatment had a higher incidence of myocarditis (0.27%) than nivolumab monotherapy (37). In a clinical trial of combined therapy with ipilimumab and nivolumab, two fatal myocarditis cases occurred. Biopsy revealed infiltration of CD4+T, CD8+T cells and macrophages along with rhabdomyolysis in the myocardial tissue of these two patients (38). Furthermore, patients may experience chest pain, shortness of breath, fatigue, palpitations, edema of the lower limbs, or syncope. For patients without heart risk factors yet only hypertension history, the adverse reactions might be diagnosed as autoimmune-related myocarditis. Norwood et al. reported a myocarditis case 17 days after receiving ipilimumab plus nivolumab combined therapy. The patient’s Troponin I levels were 13 times higher than the normal, and myocardial biopsy revealed fibrosis with inflammation infiltrated by CD8+T cells. Myocarditis is also considered to be the earliest cardiotoxic reaction, occurring on average 17 days after initial treatment (39).
In addition, our study reported fatal myocarditis cases in patients with two other ICIs, namely, PD-L1 (avelumab) chemotherapy group (OR 2.79, 95% CI: 0.11–68.79, p = 0.53) and CTLA-4 (ipilimumab) chemotherapy group (OR 3.03, 95% CI: 0.12–74.46, p = 0.50). In total, patients with myocarditis treated with ICIs were 11 times more likely to develop myocarditis, compared with those treated without ICIs. Among the 964 patients treated by ICI at Massachusetts general hospital between 2013 and 2017, 1.14% (11 patients) developed myocarditis. A study on ipilimumab treatment (10 mg q3w) of a large dose also showed a fatal myocarditis following the treatment of the single drug (29). Myocarditis is the first adverse immune reaction that affected about 50% of patients. These patients often have no risk factors for heart disease before treatments, and 46% of them develop severe cardiovascular sequelae after cancer treatments (32). Corticosteroid medications can help to alleviate symptoms of most patients with nonfatal myocarditis.
The underlying physiological mechanism of myocardial injury caused by ICIs treatment has not been documented. In several cardiac biopsies of fatal myocarditis patients, the cardiac tissue was found to be infiltrated with CD4+/CD8+T lymphocytes and a few number of macrophages. Tarrio et al. found that myocardial injury was caused by PD-1-CD4+ T cells and PD-1-CD8+ T cells, and both T cell subsets needed PD-1 to maintain their tolerance to the components of the myocardium itself (40). Studies have found that the consumption of PD-1 can lead to impaired systolic function of the heart, leading to severe myocarditis and congestive heart failure. Lichtman et al. induced myocarditis by cytotoxic T cells and found that the genetic loss of PD-1 ligand, PD-L1 and PD-L2, as well as the treatment of PD-L1 inhibitors, can make transient myocarditis develop into a fatal disease. This demonstrated that PD-1 protects cardiomyocytes from their own T-lymphocytes. Another study in a mouse model found that the PD-1 knockout mice had the potential to develop spontaneous lupus arthritis and glomerulonephritis (41). In a preclinical model of Murphy Roths rats, downregulation of PD-L1 also leads to fatal autoimmune myocarditis. In cancer treatments, ICIs indirectly inhibits the protective effect of PD-1 and PD-L1 on their own cardiac tissue (42).
CTLA-4 has also been found to be closely related with cardiac homeostasis in mouse models, and the absence of CTLA-4 can lead to fatal myocarditis and pancreatitis. In the transplant response, blocking CTLA-4 can accelerate the acute rejection of heart transplantation. In a study by the German heart failure network, patients in the G/G genotype variant of Thr17Ala CTLA-4 group suffered higher risk of dilated cardiomyopathy (14.7%) than the control group (7%), suggesting that uncontrolled activation of T cells may promote tissue damage in the heart. Lichtman et al. found that CTLA-4 plays a vital role in regulating the functions of CD8+T cells in myocarditis, and absence of CTLA-4 increases the cardiac infiltration of CD8 + T cells driven by IL-12 (43). Initial T lymphocyte activation requires dendritic cells (DC) to present antigens to T cells through interaction of T cell receptors (TCR) and the major histocompatibility complexes (MHC). CTLA-4 regulates T cell activation as a transmissive inhibitory signal. The primary mechanism of ICIs is to release the inhibition of T cells by tumor cells. If the inhibition of T cells by cardiomyocytes is also blocked, the over-activated T cells may cause damage to the myocardium. CTLA-4 can competitively bind to CD80 (B7-2)/CD86 (B7-2) on antigen presenting cells (APCs) in the body, inhibit the activation of CD28, down-regulate the activity of helper CD4+T cell but enhance the activity of regulatory T cells (Treg) to achieve immunosuppression. Blocking CTLA-4 production can promote the proliferation and infiltration of CD4+T and CD8+T cells in the heart and the occurrence of myocarditis. PD-1 regulates the tolerance of myocardial tissue to the invasion of T lymphocytes, while CTLA-4 inhibits the over-activation of T lymphocytes. When used to treat the immunosuppressive effect of tumor, ICIs were thus inevitably cause immune abnormalities in the myocardium, leading to the occurrence of autoimmune myocarditis.
Currently, no standard management guidelines on ICI-related cardiotoxicity have been established, due to its low incidence and limited data on its manifestation, diagnosis, therapy, and outcomes (44). However, a prospective assessment of potential cardiotoxicity is warranted. From the clinical perspective, there are many potential risk factors, such as autoimmune diseases, pre-existing heart disease. Some studies have found that patients with underlying autoimmune diseases may be more susceptible to cardiotoxicity. For example, patients with systemic autoimmune diseases are more likely to develop subclinical myocarditis than patients without.3This suggests that a patient’s autoimmune status needs to be evaluated before initiating ICI treatment. Most of the patients with autoimmune cardiotoxicity had pre-existing heart disease or peripheral artery disease (45). In addition, individual differences in intestinal microbiota represent another source of heterogeneity in the efficacy of cancer immunotherapy and the toxicity of ICIs (46). The study of intestinal microbiota and the cardiotoxicity of ICIs is also of great clinical significance. From the genetic perspective, omics data can help us find biomarkers associated with cardiotoxicity. In glioma, multi-layer network biomarkers found by single-cell RNA sequencing technology can be applied to the prognosis and adverse reactions of tumors, and the prediction accuracy is better than that of traditional genetic biomarkers (47, 48). This method is novel and available, and may help us to find more suitable biomarkers for early prediction of cardiac toxicity.
In order to manage the cardiotoxicity associated with immunotherapy, we recommend that ECG and cardiac biomarkers should be monitored prior to the start of treatment to obtain baseline treatment information, and clinicians need to be vigilant for this complication.
Limitations
Our meta-analysis examined the adverse cardiac effects of ICIs.
But this meta-analysis has limitations. Because of a small number yet diverse heart diseases reported, we have grouped these reactions according to internal medicine guidelines, which may omit details of specific heart diseases. Moreover, the clustering of research may result in the analysis inaccuracy and some subjective errors.
Conclusions
In summary, our meta-analysis has clearly demonstrated that, compared with chemotherapy, 1) PD-1 inhibitor (nivolumab) or CTLA-4 inhibitor (ipilimumab) showed significant increase in grades 1–5 arrhythmology and grade 5 arrhythmology; 2) PD-1 inhibitor plus chemotherapy can significantly increase risks of grades 1–5 myocardial disease. According to the existing research, ICIs does cause cardiac adverse reactions when treating cancer patients. Therefore, it is very important for patients with heart disease history or/and high-risk factors to choose the appropriate program and dosage. A more heart friendly immunotherapy treatment is of high value in future research.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
JH and RT had access to all the data included in the study and are responsible for the completeness of the data and the accuracy of our analysis. YM and HZ helped to design the study. JH and XM contributed to the statistical analysis and the revision of this manuscript. QS and BC approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Beijing Natural Science Foundation Program and Scientific Research Key Program of Beijing Municipal Commission of Education (KZ202010025047) and Training Program of the Major Research Plan of the National Natural Science Foundation of China (92046015).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.645245/full#supplementary-material
References
1. Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol (2017) 35(1):40–7. doi: 10.1200/JCO.2016.69.1584
2. Zhang J-C, Chen W-D, Alvarez JB, Jia K, Shi L, Wang Q, et al. Cancer Immune Checkpoint Blockade Therapy and its Associated Autoimmune Cardiotoxicity. Acta Pharmacol Sin (2018) 39(11):1693–8. doi: 10.1038/s41401-018-0062-2
3. Varricchi G, Galdiero MR, Marone G, Criscuolo G, Triassi M, Bonaduce D, et al. Cardiotoxicity of Immune Checkpoint Inhibitors. ESMO Open (2017) 2(4):e000247. doi: 10.1136/esmoopen-2017-000247
4. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol (2009) 62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005
5. Bloom MW, Hamo CE, Cardinale D, Ky B, Nohria A, Baer L, et al. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure: Part 1: Definitions, Pathophysiology, Risk Factors, and Imaging. Circ Heart Fail (2016) 9(1):e002661. doi: 10.1161/CIRCHEARTFAILURE.115.002661
6. Nishio M, Sugawara S, Atagi S, Akamatsu H, Sakai H, Okamoto I, et al. Subgroup Analysis of Japanese Patients in a Phase III Study of Atezolizumab in Extensive-Stage Small-Cell Lung Cancer (Impower133). Clin Lung Cancer (2019) 20(6):469–76. doi: 10.1016/j.cllc.2019.07.005
7. Pujade-Lauraine E, Fujiwara K, Dychter SS, Devgan G, Monk BJ. Avelumab (anti-PD-L1) in Platinum-Resistant/Refractory Ovarian Cancer: JAVELIN Ovarian 200 Phase III Study Design. Future Oncol (2018) 14(21):2103–13. doi: 10.2217/fon-2018-0070
8. Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab Versus Docetaxel in Patients With Platinum-Treated Advanced non-Small-Cell Lung Cancer (JAVELIN Lung 200): An Open-Label, Randomised, Phase 3 Study. Lancet Oncol (2018) 19(11):1468–79. doi: 10.1016/S1470-2045(18)30673-9
9. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med (2017) 377(20):1919–29. doi: 10.1056/NEJMoa1709937
10. Govindan R, Szczesna A, Ahn M-J, Schneider C-P, Gonzalez MPF, Barlesi F, et al. Phase III Trial of Ipilimumab Combined With Paclitaxel and Carboplatin in Advanced Squamous Non-Small-Cell Lung Cancer. J Clin Oncol (2017) 35(30):3449–57. doi: 10.1200/JCO.2016.71.7629
11. Ascierto PA, Del Vecchio M, Robert C, Mackiewicz A, Chiarion-Sileni V, Arance A, et al. Ipilimumab 10 Mg/Kg Versus Ipilimumab 3 Mg/Kg in Patients With Unresectable or Metastatic Melanoma: A Randomised, Double-Blind, Multicentre, Phase 3 Trial. Lancet Oncol (2017) 18(5):611–22. doi: 10.1016/S1470-2045(17)30231-0
12. Reck M, Luft A, Szczesna A, Havel L, Kim S-W, Akerley W, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol (2016) 34(31):3740–8. doi: 10.1200/JCO.2016.67.6601
13. Eggermont AMM, Chiarion-Sileni V, Grob J-J, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant Ipilimumab Versus Placebo After Complete Resection of High-Risk Stage III Melanoma (EORTC 18071): A Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol (2015) 16(5):522–30. doi: 10.1016/S1470-2045(15)70122-1
14. Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJM, et al. Ipilimumab Versus Placebo After Radiotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer That had Progressed After Docetaxel Chemotherapy (CA184-043): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol (2014) 15(7):700–12. doi: 10.1016/S1470-2045(14)70189-5
15. Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab Plus Dacarbazine for Previously Untreated Metastatic Melanoma. N Engl J Med (2011) 364(26):2517–26. doi: 10.1056/NEJMoa1104621
16. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival With Ipilimumab in Patients With Metastatic Melanoma. N Engl J Med (2010) 363(8):711–23. doi: 10.1056/NEJMoa1003466
17. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab Alone or With Chemotherapy Versus Cetuximab With Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet (2019) 394(10212):1915–28. doi: 10.1016/S0140-6736(19)32591-7
18. Mateos M-V, Blacklock H, Schjesvold F, Oriol A, Simpson D, George A, et al. Pembrolizumab Plus Pomalidomide and Dexamethasone for Patients With Relapsed or Refractory Multiple Myeloma (KEYNOTE-183): A Randomised, Open-Label, Phase 3 Trial. Lancet Haematol (2019) 6(9):e459–69. doi: 10.1016/S2352-3026(19)30110-3
19. Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat Plus Pembrolizumab Versus Placebo Plus Pembrolizumab in Patients With Unresectable or Metastatic Melanoma (ECHO-301/KEYNOTE-252): A Phase 3, Randomised, Double-Blind Study. Lancet Oncol (2019) 20(8):1083–97. doi: 10.1016/S1470-2045(19)30274-8
20. Usmani SZ, Schjesvold F, Oriol A, Karlin L, Cavo M, Rifkin RM, et al. Pembrolizumab Plus Lenalidomide and Dexamethasone for Patients With Treatment-Naive Multiple Myeloma (KEYNOTE-185): A Randomised, Open-Label, Phase 3 Trial. Lancet Haematol (2019) 6(9):e448–58. doi: 10.1016/S2352-3026(19)30109-7
21. Haddad R, Concha-Benavente F, Blumenschein G, Fayette J, Guigay J, Colevas AD, et al. Nivolumab Treatment Beyond RECIST-Defined Progression in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck in CheckMate 141: A Subgroup Analysis of a Randomized Phase 3 Clinical Trial. Cancer (2019) 125(18):3208–18. doi: 10.1002/cncr.32190
22. Lebbé C, Meyer N, Mortier L, Marquez-Rodas I, Robert C, Rutkowski P, et al. Evaluation of Two Dosing Regimens for Nivolumab in Combination With Ipilimumab in Patients With Advanced Melanoma: Results From the Phase Iiib/IV CheckMate 511 Trial. J Clin Oncol (2019) 37(11):867–75. doi: 10.1200/JCO.18.01998
23. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Cowey CL, et al. Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipili Mumab Alone in Advanced Melanoma (CheckMate 067): 4-Year Outcomes of a Multicentre, Ran Domised, Phase 3 Trial. Lancet Oncol (2018) 19(11):1480–92. doi: 10.1016/S1470-2045(18)30700-9
24. Motzer RJ, Tannir NM, McDermott DF, Arén FO, Melichar B, Choueiri TK, et al. Nivolumab Plus Ipilimumab Versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med (2018) 378(14):1277–90. doi: 10.1056/NEJMoa1712126
25. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee J-L, Fong L, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med (2017) 376(11):1015–26. doi: 10.1056/NEJMoa1613683
26. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
27. Motzer RJ, Escudier B, George S, Hammers HJ, Srinivas S, Tykodi SS, et al. Nivolumab Versus Everolimus in Patients With Advanced Renal Cell Carcinoma: Updated Results With Long-Term Follow-Up of the Randomized, Open-Label, Phase 3 CheckMate 025 Trial. Cancer (2020) 126(18):4156–67. doi: 10.1002/cncr.33033
28. Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab Versus Chemotherapy in Patients With Advanced Melanoma Who Progressed After anti-CTLA-4 Treatment (CheckMate 037): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol (2015) 16(4):375–84. doi: 10.1016/S1470-2045(15)70076-8
29. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab Versus Ipilimumab in Advanced Melanoma. N Engl J Med (2015) 372(26):2521–32. doi: 10.1056/NEJMoa1503093
30. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in Previously Untreated Melanoma Without BRAF Mutation. N Engl J Med (2015) 372(4):320–30. doi: 10.1056/NEJMoa1412082
31. Tadokoro T, Keshino E, Makiyama A, Sasaguri T, Ohshima K, Katano H, et al. Acute Lymphocytic Myocarditis With Anti-Pd-1 Antibody Nivolumab. Circ Heart Fail (2016) 9(10):e003514. doi: 10.1161/CIRCHEARTFAILURE.116.003514
32. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol (2018) 71(16):1755–64. doi: 10.1016/j.jacc.2018.02.037
33. Heery CR, O'Sullivan CGH, Madan RA, Schlom J, von Heydebreck A, Cuillerot JM, et al. Phase I Open-Label, Multiple Ascending Dose Trial of MSB0010718C, an anti-PD-L1 Monoclonal Antibody, in Advanced Solid Malignancies. Am Soc Clin Oncol (2014) 36(15):3064–4. doi: 10.1200/jco.2014.32.15
34. Hu Y-B, Zhang Q, Li H-J, Michot JM, Liu H-B, Zhan P, et al. Evaluation of Rare But Severe Immune Related Adverse Effects in PD-1 and PD-L1 Inhibitors in Non-Small Cell Lung Cancer: A Meta-Analysis. Transl Lung Cancer Res (2017) 6(Suppl 1):S8–S20. doi: 10.21037/tlcr.2017.12.10
35. Hassel JC, Heinzerling L, Aberle J, Bähr O, Eigentler TK, Grimm M, et al. Combined Immune Checkpoint Blockade (Anti-PD-1/Anti-CTLA-4): Evaluation and Management of Adverse Drug Reactions. Cancer Treat Rev (2017) 57:36–49. doi: 10.1016/j.ctrv.2017.05.003
36. Heinzerling L, Ott PA, Hodi FS, Husain AN, Tajmir-Riahi A, Tawbi H, et al. Cardiotoxicity Associated With CTLA4 and PD1 Blocking Immunotherapy. J Immunother Cancer (2016) 4(1):50. doi: 10.1186/s40425-016-0152-y
37. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant Myocarditis With Combination Immune Checkpoint Blockade. N Engl J Med (2016) 375(18):1749–55. doi: 10.1056/NEJMoa1609214
38. Wang DY, Okoye GD, Neilan TG, Johnson DB, Moslehi JJ. Cardiovascular Toxicities Associated With Cancer Immunotherapies. Curr Cardiol Rep (2017) 19(3):21. doi: 10.1007/s11886-017-0835-0
39. Delgobo M, Frantz S. Heart Failure in Cancer: Role of Checkpoint Inhibitors. J Thorac Dis (2018) 10(Suppl 35):S4323–34. doi: 10.21037/jtd.2018.10.07
40. Tarrio ML, Grabie N, Bu D-X, Sharpe AH, Lichtman AH. PD-1 Protects Against Inflammation and Myocyte Damage in T Cell-Mediated Myocarditis. J Immunol (2012) 188(10):4876–84. doi: 10.4049/jimmunol.1200389
41. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science (2001) 291(5502):319–22. doi: 10.1126/science.291.5502.319
42. Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelley VR. Programmed Death Ligand 1 Regulates a Critical Checkpoint for Autoimmune Myocarditis and Pneumonitis in MRL Mice. J Immunol (2008) 181(4):2513–21. doi: 10.4049/jimmunol.181.4.2513
43. Love VA, Grabie N. Duramad Paurene, Stavrakis George, Sharpe Arlene, Lichtman Andrew. CTLA-4 Ablation and interleukin-12 Driven Differentiation Synergistically Augment Cardiac Pathogenicity of Cytotoxic T Lymphocytes. Circ Res (2007) 101(3):248–57. doi: 10.1161/CIRCRESAHA.106.147124
44. Zhou Y-W, Zhu Y-J, Wang M-N, Xie Y, Chen C-Y, Zhang T, et al. Immune Checkpoint Inhibitor-Associated Cardiotoxicity: Current Understanding on Its Mechanism, Diagnosis and Management. Front Pharmacol (2019) 10:1350. doi: 10.3389/fphar.2019.01350
45. Hsu C-Y, Su Y-W, Chen S-C. Sick Sinus Syndrome Associated With Anti-Programmed Cell Death-1. J Immunother Cancer (2018) 6(1):72. doi: 10.1186/s40425-018-0388-9
46. Pitt JM, Vétizou M, Daillère R, Roberti MP, Yamazaki T, Routy B, et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity (2016) 44(6):1255–69. doi: 10.1016/j.immuni.2016.06.001
47. Zhang J, Zhu W, Wang Q, Gu J, Huang LF, Sun X. Differential Regulatory Network-Based Quantification and Prioritization of Key Genes Underlying Cancer Drug Resistance Based on Time-Course RNA-Seq Data. PLoS Comput Biol (2019) 15(11):e1007435. doi: 10.1371/journal.pcbi.1007435
Keywords: cardiovascular toxicity, PD-1/PD-L1 inhibitors, chemotherapy, solid tumors, meta-analysis
Citation: Hu J, Tian R, Ma Y, Zhen H, Ma X, Su Q and Cao B (2021) Risk of Cardiac Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-Analysis. Front. Oncol. 11:645245. doi: 10.3389/fonc.2021.645245
Received: 22 December 2020; Accepted: 06 April 2021;
Published: 27 May 2021.
Edited by:
Jun Chen, Sun Yat-Sen University, ChinaReviewed by:
Xiaoqiang Sun, Sun Yat-Sen University, ChinaChen Yang, Southern Medical University, China
Copyright © 2021 Hu, Tian, Ma, Zhen, Ma, Su and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Su, cWlhbmcuc3VAY2NtdS5lZHUuY24=; Bangwei Cao, b25jb2xvZ3lAY2NtdS5lZHUuY24=
 Jiexuan Hu1
Jiexuan Hu1 Xiao Ma
Xiao Ma Bangwei Cao
Bangwei Cao