- 1Azienda Ospedaliero-Universitaria di Bologna, Istituto di Ematologia “Seràgnoli”, Bologna, Italy
- 2Department of Experimental, Diagnostic and Specialty Medicine (DIMES), University of Bologna, Bologna, Italy
- 3Dipartimento di Medicina Clinica e Chirurgia, Università di Napoli Federico II, Napoli, Italy
- 4IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, Meldola, Italy
- 5Division of Hematology & Internal Medicine, Department of Clinical & Biological Sciences of the University of Turin, ‘San Luigi Gonzaga’ University Hospital, Orbassano, Italy
- 6 Hematology, Department of Translational and Precision Medicine, Sapienza University, Rome, Italy
The treatment of chronic myeloid leukemia (CML) has been advanced by the development of small-molecule tyrosine kinase inhibitors (TKIs), which target the fusion protein BCR-ABL1 expressed by the Philadelphia chromosome. Ponatinib is a 3rd generation TKI that binds BCR-ABL1 with high affinity and inhibits most BCR-ABL1 mutants, including the T315I mutation. The approved starting dose of ponatinib is 45 mg once daily (full dose), however, the need for a full dose, especially in patients with dose adjustments due to tolerability problems, remains undemonstrated. Lower starting doses of ponatinib (30 mg or 15 mg once daily) for patients “with lesser degrees of resistance or multiple intolerances, especially those with an increased cardiovascular risk profile” has been recommended by the 2020 European LeukemiaNet. However, the available literature and guidance on the use of ponatinib at low dosage are limited. The objective of this paper is to describe how we select ponatinib dosage for CML patients in chronic phase in our clinical practice based on the available evidence and our clinical experience. We propose dosing regimens for the optimal starting dose for six generic cases of CML patients in chronic phase eligible for the switch to ponatinib and provide an algorithm to guide ponatinib dosing during treatment.
Introduction
The development, over the past two decades, of small-molecule tyrosine kinase inhibitors (TKIs) targeting the fusion protein BCR-ABL1 expressed by the Philadelphia chromosome (Ph) has considerably advanced the treatment of chronic myeloid leukemia (CML) and Ph+ acute lymphoblastic leukemia (ALL). Among several potential mechanisms underlying the emergence of resistance to 1st or 2nd generation TKIs, point mutations in the BCR-ABL1 active site is one of the most relevant and actionable: the presence of mutation can impair the activity of specific TKIs, leading to treatment failure (1).
Ponatinib, a 3rd generation TKI, was designed to bind the kinase domain of unmutated and mutated BCR-ABL1 with high affinity (2). Ponatinib also inhibits the T315I mutation of BCR-ABL1, which occurs in 5%–10% of resistant patients and suppresses the action of all other approved TKIs (2, 3).
Based on the initial efficacy results of the pivotal phase 2 PACE (Ponatinib Ph+ ALL and CML Evaluation) trial (2), ponatinib was approved in December 2012 by the United States Food and Drug Administration (FDA) via an accelerated approval program (4). However, due to an increased incidence of arterial occlusion events reported in the ongoing PACE trial, ponatinib was temporarily removed from the market in October 2013 and recommendations for dose reduction were issued (4). Regulatory approval of ponatinib, albeit with restrictions on the patient indication, was re-established in January 2014. Patients with pre-existing risk factors were shown to have a higher risk of cardiovascular events; in particular, patients with ≥2 risk factors had the highest relative risk [2.2 (95% confidence interval, 1.5–3.3)] (5).
Ponatinib is currently indicated by the FDA for the treatment of adult patients with chronic phase, accelerated phase, or blast phase CML or with Ph+ ALL for whom no other TKI is indicated, and for all adult patients with CML or Ph+ ALL carrying the T315I mutation (4). In Europe, ponatinib is approved by the European Medicines Agency (EMA) in adult patients with chronic phase, accelerated phase, or blast phase CML or with Ph+ ALL who are resistant or intolerant to dasatinib or nilotinib and for whom imatinib is not clinically appropriate, and for all patients carrying the T315I mutation (6). The 2020 updated recommendations issued by the European LeukemiaNet (ELN) recommend ponatinib in patients with resistance to a 2nd generation TKI (dasatinib, nilotinib, and bosutinib), even without specific mutations, unless its use is precluded by the presence of cardiovascular risk factors (7).
The approved starting dose of ponatinib is 45 mg once daily (full dose) (4, 6). This dose was established in a phase 1, dose-escalation study, the primary objective of which was to determine the maximum tolerated dose, defined as the dose causing a dose-limiting toxic effect in no more than one in six patients (3). This study also found that at daily doses of ≥30 mg, the trough plasma concentration was >40 nM, a concentration at which ponatinib inhibited all BCR-ABL1 mutants tested and suppressed the emergence of BCR-ABL1 mutations in preclinical studies. However, as stated in the FDA prescribing information, the optimal dose of ponatinib has not yet been identified (4), and the need for a full dose, especially in patients with dose adjustments due to tolerability problems, remains undemonstrated (8). The 2020 ELN guidelines recommend a lower starting dose, 30 mg or 15 mg once daily, for the great majority of patients in chronic phase, especially those with an increased cardiovascular risk profile, with dose increases only if needed; the 45 mg once daily starting dose is recommended only for patients with the T315I mutation, compound mutations, or patients with progression to advanced-phase disease (7).
Both the FDA and EMA labels state that dose reductions should be considered in patients with a major cytogenetic response (4, 6).
In the treatment of CML it is common practice to administer TKIs indefinitely, which can be associated with a substantial burden to patients in terms of drug-related adverse events and decreased quality of life; elevated treatment costs are also an issue. Recently available evidence suggests that TKI dose-reduction strategies, in patients with a stable major molecular response (MMR) who are regularly monitored for BCR-ABL1, are safe and may constitute a valuable option for optimizing TKI-based therapy (9, 10). However, studies evaluating dose-reduction strategies with ponatinib are limited and practical indications to guide clinicians in the selection of the starting dose of ponatinib are lacking.
The objective of this paper is to describe how we select ponatinib dosage for CML patients in chronic phase in our clinical practice, based on the available evidence and our experience. To this purpose, we will first review the available data on ponatinib dose reduction then discuss the optimal starting dose for six generic cases of CML patients in chronic phase eligible for the switch to ponatinib. An algorithm for the treatment of these cases with ponatinib will also be provided.
Reported Outcomes With Low-Dose Ponatinib
The available literature on the use of ponatinib at a low dosage is limited and there are only a few clinical publications available, mainly concerning real-life experiences. The optimal risk-benefit profile of ponatinib at doses lower than the licensed starting dose of 45 mg/day was evaluated in the PACE trial (5, 11), and is under investigation in the ongoing open-label phase 2 OPTIC (Optimizing Ponatinib Treatment In CML) trial (NCT02467270) (12, 13).
In the PACE trial, ponatinib dose reductions from the starting dose of 45 mg/day to 30 mg/day or 15 mg/day were implemented per protocol to manage adverse events or proactively in October 2013 to decrease the risk of arterial occlusive events (5). For chronic-phase CML patients with a major cytogenetic response, a decrease to 15 mg/day was recommended; for chronic-phase CML patients without a major cytogenetic response, and for patients with accelerated- and blast-phase CML, a decrease to 30 mg/day was recommended. A post hoc analysis found that major cytogenetic responses or MMRs were sustained for 40 months in ≥90% of 270 patients with chronic-phase CML, suggesting that, irrespective of dose reductions, ponatinib offers durable and clinically meaningful outcomes in heavily pretreated chronic-phase CML patients (5).
A pooled multivariate analysis of three clinical trials (phase 1 dose-escalating study (3) phase 2 PACE trial (2), phase 3 EPIC trial (14); 671 patients in total), which investigated the impact of ponatinib dose intensity on adverse events of interest including arterial occlusive events, found that the risk of an arterial occlusive event decreased by approximately 33% for each daily 15 mg decrease of ponatinib dose (11). This analysis identified significant associations between higher ponatinib doses and higher rates of most of the adverse events considered, suggesting the existence of a causal relationship between ponatinib dose intensity and toxicity, and supporting the need for prospective studies to determine optimal ponatinib dosing regimens.
The interim analysis (IA) of the OPTIC trial reinforced the concept that an initial treatment with 45 mg daily may induce higher response rates, especially in resistant patients, but suggest that higher dose may increase the incidence of cardiovascular events, even with a short treatment duration (12, 13). OPTIC is an ongoing, multicenter, randomized phase 2 trial which is investigating three starting doses of ponatinib (45 mg, 30 mg, 15 mg) in 282 patients, 94 patients in each treatment cohort, with chronic-phase CML resistant or intolerant to at least two TKIs, or carrying the T315I mutation. Ponatinib dose was reduced to 15 mg/day for patients who initially received a 45 mg or 30 mg daily dose and achieved a ≤1% BCR-ABL1 response. At the OPTIC IA cut-off of 21 months, the median duration of exposure varied from 12 to 14 months in the three arms of the study. For patients with 12 months of follow-up, 38.7%, 27.4%, and 26.5% of patients receiving 45, 30, and 15 mg/day starting dose achieved a ≤1% BCR-ABL1 response (primary endpoint). Responses achieved at starting dose of 45 or 30 mg/day were maintained despite the dose reduction to 15 mg/day. The dose-dependency of efficacy and safety suggested an optimal benefit-risk profile may be achieved when treatment is initiated at 45 mg/day and de-escalated to 15 mg/day when BCR-ABL1 is ≤1%. The rate of adjudicated arterial occlusive events at 21 months showed a decreasing dose-dependent trend from 5.3%, 4.3%, and 1.1%, respectively, in the 45 mg/day, 30 mg/day, and 15 mg/day starting dose cohorts.
An indirect comparison between the PACE trial (n=257) and the 45 mg starting dose of the OPTIC IA (n=93), recently presented at the 2020 ASH Meeting, confirmed that a prompt dose reduction to 15 mg after achievement of MR2 does not jeopardize the efficacy of ponatinib and remarkably reduced the incidence of treatment-emergence arterial occlusive events (15). Both PACE and the OPTIC IA showed an increase over time in the ≤1% BCR-ABL1 response rate (from 42.0% to 47.1%, respectively, at 12 and 60 months for PACE, and from 47.3% to 51.6%, respectively, at 12 and 24 months in the OPTIC IA). Serious treatment-emergent adverse events (31.2% vs. 63.4%) and adjudicated arterial occlusive events (5.4% vs. 20.2%) were lower in the OPTIC IA than in the PACE trial.
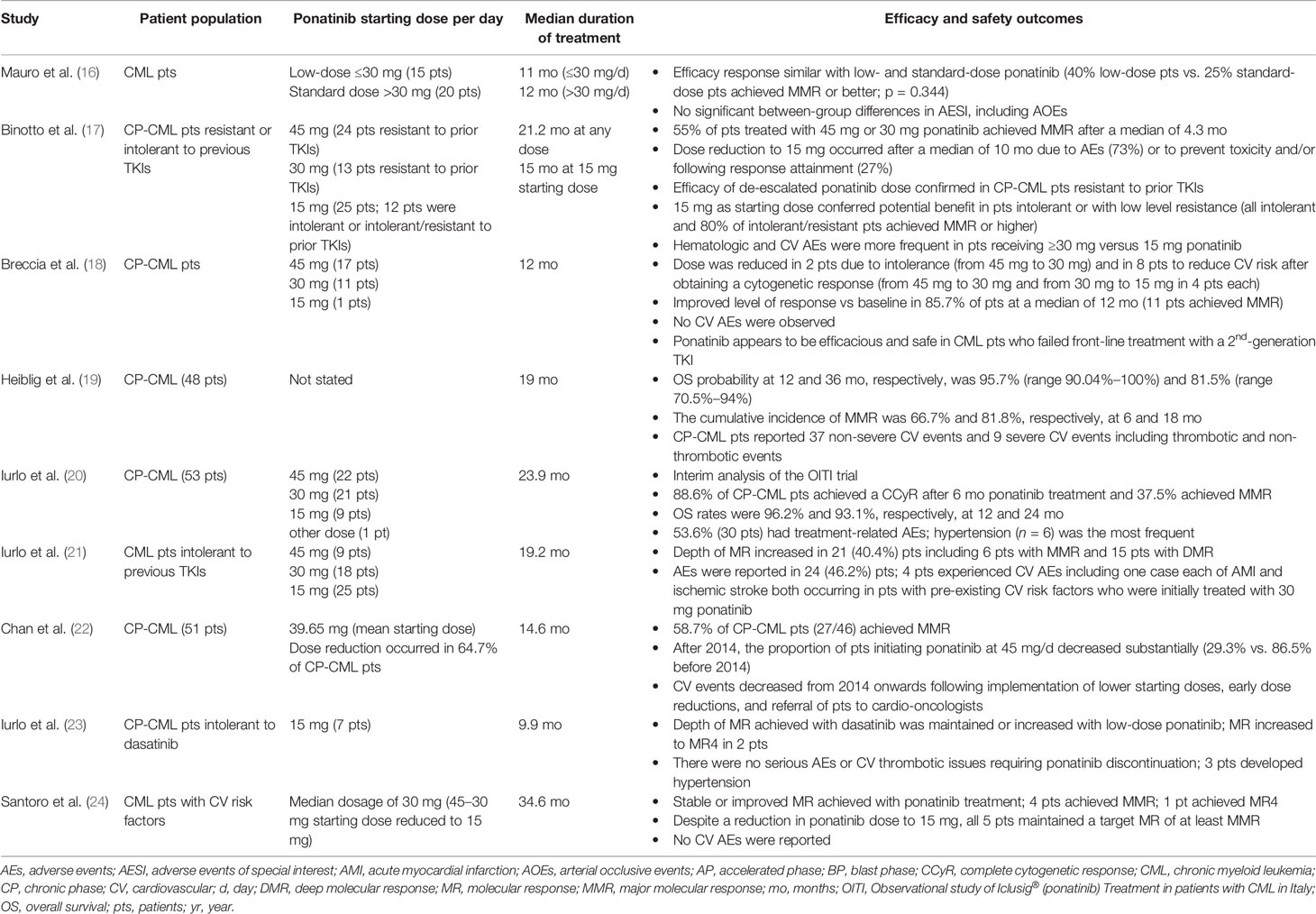
The efficacy and safety profile of low-dose ponatinib in CML patients in chronic phase emerging from real-life observations appears to confirm the results of clinical trials (Table 1) (16–24). A lower ponatinib starting dose appears to be a beneficial strategy in selected CML patients, such as those intolerant or with a low level of resistance to other TKIs, with reported lower incidences of adverse events, no unexpected adverse events, and efficacy data in line with those from clinical trials. Notably, a retrospective analysis of 78 CML patients treated with ponatinib in the US between 2011 and 2017 revealed a decrease of cardiovascular events beginning in 2014 when the study institution implemented lower starting ponatinib doses, early dose reductions, and referral of patients to cardio-oncologists (22). Hence, dose optimization appears to be a reality that can be achieved in clinical practice.
Collectively, the available data indicate that the treatment of CML with ponatinib can be optimized by dose modification. The evidence suggests that lowering the dose of ponatinib up to 15 mg/day is a feasible strategy for improving treatment safety and tolerability while maintaining response. Based on this evidence, and our experience in the treatment of CML, in the following practice-oriented section we propose dosing regimens for initiating ponatinib in eligible patients and provide an algorithm for guiding ponatinib dosing during treatment.
Suggested Strategies for Initiating Treatment With Ponatinib in CML Patients in Chronic Phase
In agreement with the approved indication of ponatinib and to current ELN recommendations (4, 6, 7), we generally consider CML patients in chronic phase eligible for ponatinib when they are resistant and/or intolerant to a 2nd generation TKI; in particular, we consider a patient resistant to 2nd generation TKIs if they can be defined at least as “warning” according to the 2020 ELN recommendations (BCR-ABL1 transcript >10% at 3 months, >1% at 6 months, >0.1% at 12 months or later), or when they carry the T315I mutation. Along with the response to previous TKI therapy, patient characteristics (age, comorbidities, and cardiovascular risk) should also be taken into account when selecting the starting ponatinib dose. Figure 1 shows the algorithm we propose for the treatment of CML patients in chronic phase eligible for ponatinib and summarizes the starting dose of ponatinib that we use in six generic CML patient cases that can be encountered in clinical practice. The terms “optimal response”, “failure”, and “warning response” are defined according to the ELN 2020 recommendations (7).
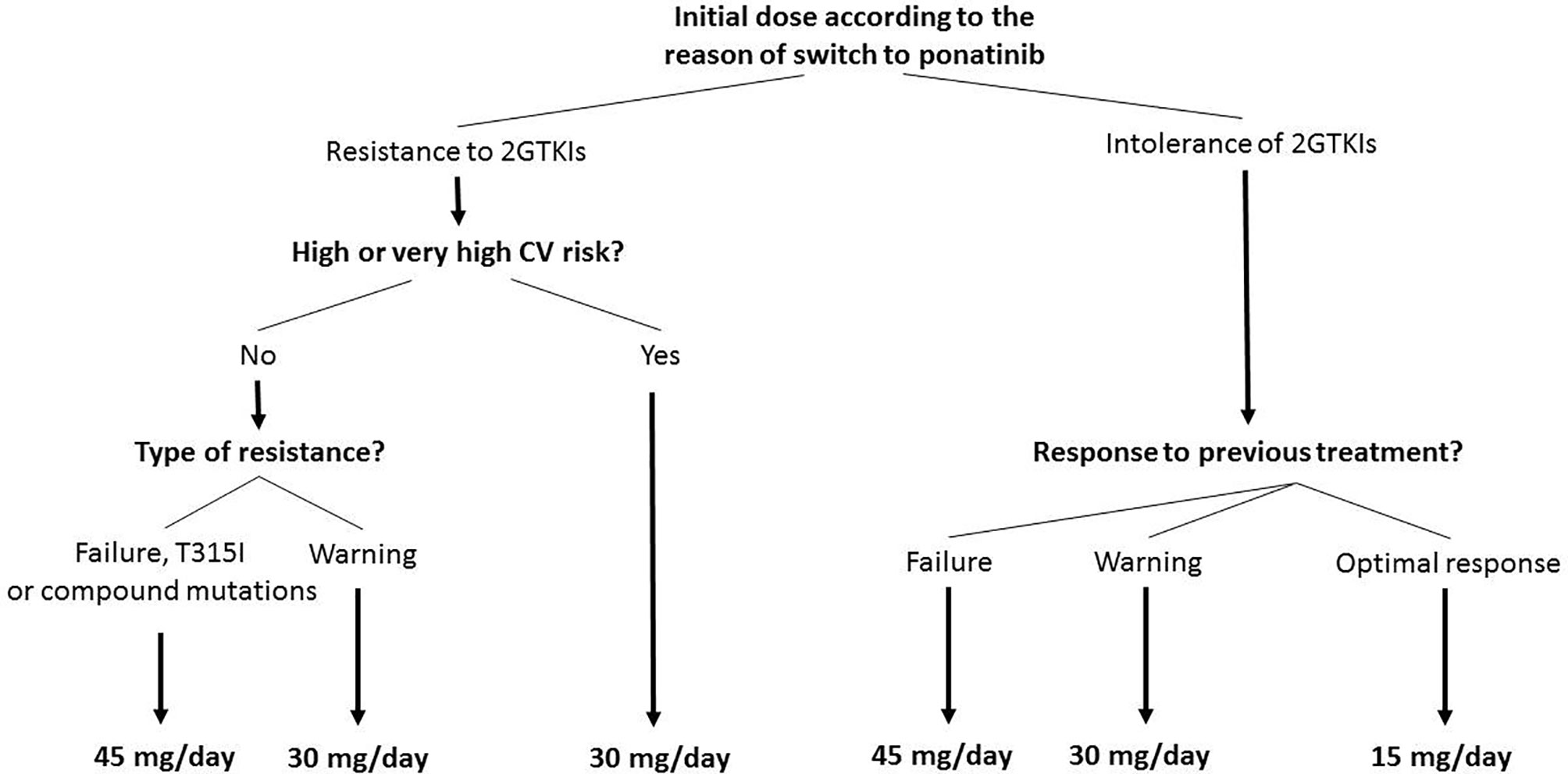
Figure 1 Treatment algorithm for starting doses of ponatinib in eligible patients with chronic myeloid leukemia in chronic phase. In case of hematologic toxicity of 2nd generation tyrosine kinase inhibitors (2GTKIs), consider 15 mg/day as initial dose. Patients with a starting dose of 45 mg/day or 30 mg/day, should reduce to 15 mg/day upon achievement of a MR3 (MR2 in case of high or very high cardiovascular [CV] risk). Failure criteria defined according to the European LeukemiaNet (ELN) 2020 recommendations (BCR-ABL1 >10% within 1–3 months, >10% at 6 months, >1% at 12 months, and >1%, resistance mutations, high-risk additional chromosome abnormalities any time after 12 months) (7). Warning response defined according to the ELN 2020 recommendations (BCR-ABL1 >10% at 3 months, >1%–10% at 6 months, > 0.1%–1% at 12 months onwards) (7). Optimal response defined according to the ELN 2020 recommendations (BCR-ABL1 ≤10% at 3 months, ≤1% at 6 months, and ≤0.1% at 12 months onwards) (7). CV risk defined according to the 2016 European Guidelines on CV disease prevention (25).
Starting dose of ponatinib can be 45 mg/day (full dose), 30 mg/day, or 15 mg/day (Figure 1). In the PACE trial, a dose reduction has been performed in patients achieving a MCyR, while in the OPTIC trial the dose was reduced after achievement of MR2. According to most international recommendations MR3 should be considered as the optimal response, protecting patients from disease progression. Consequently, differently from the PACE and OPTIC studies, in patients initiating treatment with 45 mg/day or 30 mg/day, we suggest a dose reduction to 15 mg/day upon achievement of an MR3 response or <0.1% BCR-ABL1. It is important to underline that in the PACE trial the median times to CCyR (approximately corresponding to MR2) and MR3 were 2.9 months and 5.5 months: both PACE and the OPTIC IA highlighted the short time to molecular response on ponatinib, hence dose reduction can be very early. Considering the dose-dependent risk of cardiovascular events on ponatinib, an earlier dose reduction, namely after the achievement of MR2 (BCR-ABL1 transcript 0.1%–1%), should be considered in patients with high or very high cardiovascular risk.
Patients who fail a 2nd generation TKI should start ponatinib at full dose, while patients with a “warning response” to a 2nd generation TKI should start ponatinib at 30 mg/day. In patients carrying the T315I mutation or a compound mutation, ponatinib should be started at the full dose (45 mg/day). Patients who are intolerant to a 2nd generation TKI without optimal response to it, should initiate ponatinib at the same dose as resistant patients, while those with an optimal response can start with ponatinib at 15 mg/day.
Finally, a reduced starting dose of 30 mg/day should be used in all patients with high or very high cardiovascular risk, regardless of the reason for the switch to ponatinib and, therefore, also following the failure of a 2nd generation TKI. Cardiovascular risk must be stratified in accordance with the European Society of Cardiology Guidelines (Table 2) (25) and numerous consensus for the management of cardiovascular risk during treatment have been published (26, 27).
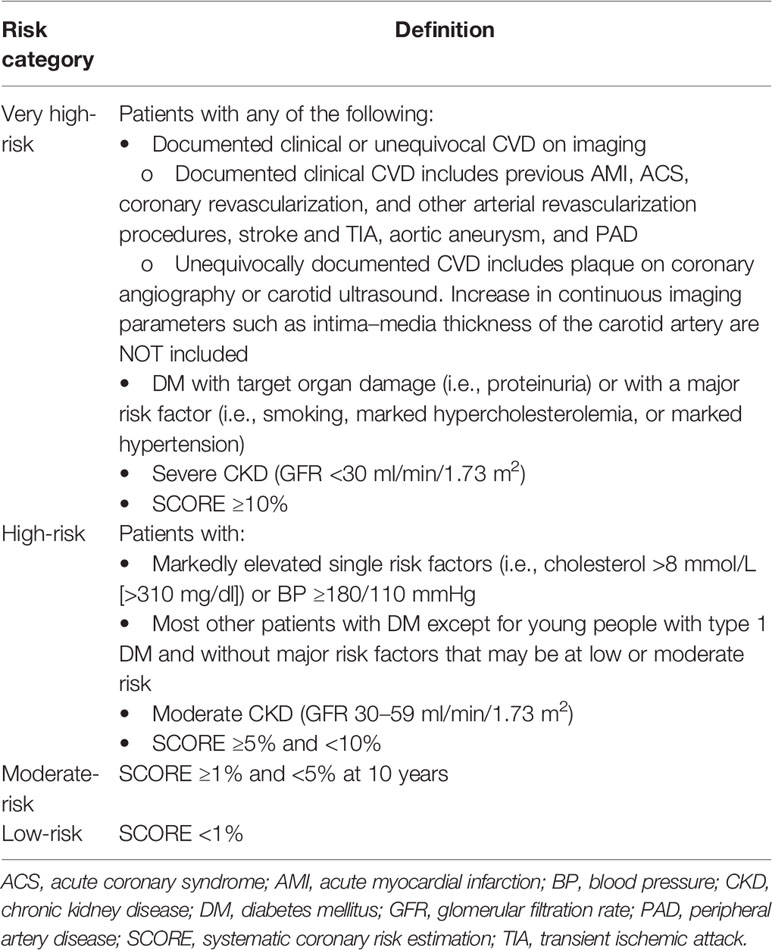
Table 2 Cardiovascular risk categories stratified according to the European Society of Cardiology Guidelines (25).
The proportion of patients who discontinue first- or second-line treatment with 2nd generation TKIs is variable, ranging from approximately 40% to 75% depending mainly on patient characteristics, line of treatment, and the length of follow-up of published studies; around 10% to 40% of patients who switch from 2nd generation TKIs do so due to an unsatisfactory response (28–34). There is no literature data to determine the number of resistant patients who have a warning response and how many have a failure. According to our algorithm, if we consider more recently published studies (where early switches are more frequent), we estimate that only a minority of patients will require 45 mg ponatinib as initial dose because of failure or T315I mutation, followed by prompt dose reduction in case of achievement of MMR.
The proposed dosing strategies and algorithm shown here, except for the starting dose of 45 mg/day, are off-label and are based entirely on the available literature and our clinical experience. Until further randomized, prospective data from clinical trials become available, a risk-benefit analysis should be applied on a case-by-case basis when treating CML patients. Indeed, anecdotal evidence suggests that clinicians start ponatinib at 45 mg/day in only a minority of cases, with most patients beginning therapy at 30 mg/day, hence dose optimization is already a reality in real-life clinical practice.
Recent recommendations from a German expert consensus panel on ponatinib proposed the following criteria supporting a ponatinib starting dose of 30 mg/day in CML patients: chronic phase, good response status, no mutations, resistance to only one TKI, intolerance despite good response, and increased cardiovascular risk (35). The strategy to start ponatinib at doses lower than 45 mg/day should be guided by a thorough evaluation of risk factors, as well as the depth and stability of molecular response, and total exposure to ponatinib; continued monitoring of response is highly recommended (26).
Conclusions
Evidence shows that low-dose ponatinib is effective and that selected patients with CML may not need the full starting dose currently approved. The starting ponatinib dose should be indeed tailored to patient characteristics considering the response to previous TKI therapy. We propose here practical strategies for more flexible dosing of ponatinib from therapy initiation to maintenance, with the ultimate goal of improving the risk-benefit balance and the use of this potent TKI.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
All authors contributed to the study conception and design in equal measure. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that this study received funding from Incyte for medical writing assistance. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
FC consultancy and honoraria: Novartis, Incyte, Pfizer, and BMS. FP received honoraria from Incyte, Novartis, BMS, and Pfizer. GR consultancy and honoraria: Novartis, BMS, Incyte, and Pfizer. GS consultancy and honoraria: Novartis, BMS, Incyte, and Pfizer. MB received honoraria from Novartis, Pfizer, Incyte, BMS/Celgene, and AbbVie.
The reviewer JHL declared a past co-authorship with the authors to the handling editor.
Acknowledgments
Medical writing assistance was provided by Lorenza Lanini and Melanie Gatt (PhD), independent medical writers, on behalf of Springer Healthcare. This assistance was funded by Incyte.
References
1. O’Hare T, Eide CA, Deininger MW. Bcr-Abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood (2007) 110:2242–9. doi: 10.1182/blood-2007-03-066936
2. Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med (2013) 369:1783–96. doi: 10.1056/NEJMoa1306494
3. Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med (2012) 367:2075–88. doi: 10.1056/NEJMoa1205127
4. U.S. Food and Drug Administration. Iclusig® (Ponatinib) tablets for oral use. Prescribing Information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/203469s022lbl.pdf (Accessed 28 September 2020).
5. Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood (2018) 132:393–404. doi: 10.1182/blood-2016-09-739086
6. European Medicines Agency. Iclusig (Ponatinib). Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/iclusig-epar-product-information_en.pdf (Accessed 28 September 2020).
7. Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia (2020) 34:966–84. doi: 10.1038/s41375-020-0776-2
8. Baccarani M, Abruzzese E, Accurso V, Albano F, Annunziata M, Barulli S, et al. Managing chronic myeloid leukemia for treatment-free remission: a proposal from the GIMEMA CML WP. Blood Adv (2019) 3:4280–90. doi: 10.1182/bloodadvances.2019000865
9. Michel C, Burchert A, Hochhaus A, Saussele S, Neubauer A, Lauseker M, et al. Imatinib dose reduction in major molecular response of chronic myeloid leukemia: results from the German Chronic Myeloid Leukemia-Study IV. Haematologica (2019) 104:955–62. doi: 10.3324/haematol.2018.206797
10. Fassoni AC, Baldow C, Roeder I, Glauche I. Reduced tyrosine kinase inhibitor dose is predicted to be as effective as standard dose in chronic myeloid leukemia: a simulation study based on phase III trial data. Haematologica (2018) 103:1825–34. doi: 10.3324/haematol.2018.194522
11. Dorer DJ, Knickerbocker RK, Baccarani M, Cortes JE, Hochhaus A, Talpaz M, et al. Impact of dose intensity of ponatinib on selected adverse events: Multivariate analyses from a pooled population of clinical trial patients. Leuk Res (2016) 48:84–91. doi: 10.1016/j.leukres.2016.07.007
12. Cortes JE, Apperley F, Hochhaus A, Mauro MJ, Rousselot P, Sacha T, et al. Outcome By Mutation Status and Line of Treatment in Optic, a Dose-Ranging Study of 3 Starting Doses of Ponatinib in Patients with CP-CML. 62nd ASH Annual Meeting and Exposition. December 5-8, 2020. [Abstract: 48] (2020). Available at: https://ash.confex.com/ash/2020/webprogram/Paper135883.html (Accessed 11 December 2020).
13. Cortes JE, Lomaia E, Turkina A, Moiraghi B, Sutton MU, Pavlovsky C, et al. Interim analysis (IA) of OPTIC: A dose-ranging study of three ponatinib (PON) starting doses. J Clin Oncol (2020) 38:7502. doi: 10.1200/JCO.2020.38.15_suppl.7502
14. Lipton JH, Chuah C, Guerci-Bresler A, Rosti G, Simpson D, Assouline S, et al. Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. Lancet Oncol (2016) 17:612–21. doi: 10.1016/S1470-2045(16)00080-2
15. Kantarjian HM, Deininger MW, Abruzzese E, Apperley J, Cortes JE, Chuah C, et al. Efficacy and Safety of Ponatinib (PON) in Patients with Chronic-Phase Chronic Myeloid Leukemia (CP-CML) Who Failed One or More Second-Generation (2G) Tyrosine Kinase Inhibitors (TKIs): Analyses Based on PACE and OPTIC. 62nd ASH Annual Meeting and Exposition. December 5-8, 2020. [Abstract: 647] (2020). Available at: https://ash.confex.com/ash/2020/webprogram/Paper133922.html (Accessed 11 December 2020).
16. Mauro M, McGarry L, Inguilizian A. MoulinR. d, Huang H. A Chart Review of Lower Dosing of Ponatinib in Patients with Chronic Myeloid Leukemia (CML): Preliminary Findings. Clin Lymphoma Myeloma Leuk (2017) 16:S57–S8. doi: 10.1016/j.clml.2016.07.083
17. Binotto G, Castagnetti F, Gugliotta G, Abruzzese E, Iurlo A, Stagno F, et al. Ponatinib 15 mg daily, combining efficacy and tolerability. A retrospective survey in Italy. Abstract: PS1122 (2018). 23rd European Hematology Association Congress. Available at: https://learningcenter.ehaweb.org/eha/2018/stockholm/215436/gianni.binotto.ponatinib.15.mg.daily.combining.efficacy.and.tolerability.a.html?f=menu=6*ce_id=1346*ot_id=19052*media=3 (Accessed 28 September 2020).
18. Breccia M, Abruzzese E, Castagnetti F, Bonifacio M, Gangemi D, Sora F, et al. Ponatinib as second-line treatment in chronic phase chronic myeloid leukemia patients in real-life practice. Ann Hematol (2018) 97:1577–80. doi: 10.1007/s00277-018-3337-2
19. Heiblig M, Rea D, Chretien ML, Charbonnier A, Rousselot P, Coiteux V, et al. Ponatinib evaluation and safety in real-life chronic myelogenous leukemia patients failing more than two tyrosine kinase inhibitors: the PEARL observational study. Exp Hematol (2018) 67:41–8. doi: 10.1016/j.exphem.2018.08.006
20. Iurlo A, Annunziata M, Albano F, Luciano L, Spadano R, Scortechini AR, et al. Multicenter, Prospective and Retrospective Observational Cohort Study of Ponatinib in Patients with CML in Italy: Interim Analysis of the OITI Trial. Blood (2019) 134:1652. doi: 10.1182/blood-2019-126098
21. Iurlo A, Cattaneo D, Malato A, Accurso V, Annunziata M, Gozzini A, et al. Low-dose ponatinib is a good option in chronic myeloid leukemia patients intolerant to previous TKIs. Am J Hematol (2020) 95:E260–3. doi: 10.1002/ajh.25908
22. Chan O, Talati C, Isenalumhe L, Shams S, Nodzon L, Fradley M, et al. Side-effects profile and outcomes of ponatinib in the treatment of chronic myeloid leukemia. Blood Adv (2020) 4:530–8. doi: 10.1182/bloodadvances.2019000268
23. Iurlo A, Cattaneo D, Orofino N, Bucelli C, Molica M, Breccia M. Low-Dose Ponatinib in Intolerant Chronic Myeloid Leukemia Patients: A Safe and Effective Option. Clin Drug Investig (2018) 38:475–6. doi: 10.1007/s40261-018-0623-7
24. Santoro M, Accurso V, Mancuso S, Contrino AD, Sardo M, Novo G, et al. Management of Ponatinib in Patients with Chronic Myeloid Leukemia with Cardiovascular Risk Factors. Chemotherapy (2019) 64:205–9. doi: 10.1159/000504664
25. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J (2016) 2016) 37:2315–81. doi: 10.1093/eurheartj/ehw106
26. Breccia M, Pregno P, Spallarossa P, Arboscello E, Ciceri F, Giorgi M, et al. Identification, prevention and management of cardiovascular risk in chronic myeloid leukaemia patients candidate to ponatinib: an expert opinion. Ann Hematol (2017) 96:549–58. doi: 10.1007/s00277-016-2820-x
27. Hochhaus A, Breccia M, Saglio G, Garcia-Gutierrez V, Rea D, Janssen J, et al. Expert opinion-management of chronic myeloid leukemia after resistance to second-generation tyrosine kinase inhibitors. Leukemia (2020) 34:1495–502. doi: 10.1038/s41375-020-0842-9
28. Brümmendorf TH, Gambacorti-Passerini C, Kim D-W, Goh YT, Dyagil I, Pagnano KB, et al. Second-Line Bosutinib in Patients with Chronic Phase Chronic Myeloid Leukemia (CP CML) Resistant or Intolerant to Prior Imatinib: An 8-Year Update [Abstract]. ASH Annual Meeting. Blood (2017) 130:900. doi: 10.1182/blood.V130.Suppl_1.900.900
29. Brümmendorf TH, Giles F, Gambacorti-Passerini C, Roboz GJ, Le Coutre P, Hjorth-Hansen H, et al. Efficacy and safety following dose reduction of bosutinib in previously treated patients with chronic myeloid leukemia: analysis of the phase 4 BYOND trial [Abstract PF415] (2019). European Hematology Association. Available at: https://library.ehaweb.org/eha/2019/24th/266215/tim.h.brmmendorf.efficacy.and.safety.following.dose.reduction.of.bosutinib.in.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D2%2Asearch%3Dtki (Accessed 18 November 2020).
30. Cortes JE, Gambacorti-Passerini C, Deininger MW, Mauro MJ, Chuah C, Kim DW, et al. Bosutinib Versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia: Results From the Randomized BFORE Trial. J Clin Oncol (2018) 36:231–7. doi: 10.1200/JCO.2017.74.7162
31. Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boque C, et al. Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients Trial. J Clin Oncol (2016) 34:2333–40. doi: 10.1200/JCO.2015.64.8899
32. Giles FJ, le Coutre PD, Pinilla-Ibarz J, Larson RA, Gattermann N, Ottmann OG, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia (2013) 27:107–12. doi: 10.1038/leu.2012.181
33. Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia (2016) 30:1044–54. doi: 10.1038/leu.2016.5
34. Shah NP, Rousselot P, Schiffer CA, Rea D, Cortes JE, Milone J, et al. Seven-Year (yr) Follow-up of Patients (pts) with Imatinib-Resistant or -Intolerant Chronic-Phase Chronic Myeloid Leukemia (CML-CP) Receiving Dasatinib in Study CA180-034, Final Study Results. Blood (2014) 124:520. doi: 10.1182/blood.V124.21.520.520
35. Saussele S, Haverkamp W, Lang F, Koschmieder S, Kiani A, Jentsch-Ullrich K, et al. Ponatinib in the Treatment of Chronic Myeloid Leukemia and Philadelphia Chromosome-Positive Acute Leukemia: Recommendations of a German Expert Consensus Panel with Focus on Cardiovascular Management. Acta Haematol (2020) 143:217–31. doi: 10.1159/000501927
Keywords: chronic myeloid leukemia, dosing regimens, low-dose, ponatinib, risk-benefit profile, treatment algorithm
Citation: Castagnetti F, Pane F, Rosti G, Saglio G and Breccia M (2021) Dosing Strategies for Improving the Risk-Benefit Profile of Ponatinib in Patients With Chronic Myeloid Leukemia in Chronic Phase. Front. Oncol. 11:642005. doi: 10.3389/fonc.2021.642005
Received: 15 December 2020; Accepted: 29 January 2021;
Published: 16 March 2021.
Edited by:
Alessandro Isidori, AORMN Hospital, ItalyReviewed by:
Charles Chuah, Singapore General Hospital, SingaporeJeffrey H. Lipton, University Health Network, Canada
Copyright © 2021 Castagnetti, Pane, Rosti, Saglio and Breccia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fausto Castagnetti, ZmF1c3RvLmNhc3RhZ25ldHRpQHVuaWJvLml0
 Fausto Castagnetti
Fausto Castagnetti Fabrizio Pane
Fabrizio Pane Gianantonio Rosti
Gianantonio Rosti Giuseppe Saglio
Giuseppe Saglio Massimo Breccia
Massimo Breccia