
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 11 January 2022
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.641124
This article is part of the Research Topic Gastric Cancer Phenotype and Treatment View all 39 articles
 Tao Pan†
Tao Pan† Xiao-long Chen†
Xiao-long Chen† Kai Liu
Kai Liu Bo-qiang Peng
Bo-qiang Peng Wei-han Zhang
Wei-han Zhang Meng-hua Yan
Meng-hua Yan Rui Ge
Rui Ge Lin-yong Zhao
Lin-yong Zhao Kun Yang
Kun Yang Xin-zu Chen
Xin-zu Chen Jian-kun Hu*
Jian-kun Hu*Background: We aimed to generate and validate a nomogram to predict patients most likely to require intensive care unit (ICU) admission following gastric cancer surgery to improve postoperative outcomes and optimize the allocation of medical resources.
Methods: We retrospectively analyzed 3,468 patients who underwent gastrectomy for gastric cancer from January 2009 to June 2018. Here, 70.0% of the patients were randomly assigned to the training cohort, and 30.0% were assigned to the validation cohort. Least absolute shrinkage and selection operator (LASSO) method was performed to screen out risk factors for ICU-specific care using the training cohort. Then, based on the results of LASSO regression analysis, multivariable logistic regression analysis was performed to establish the prediction nomogram. The calibration and discrimination of the nomogram were evaluated in the training cohort and validated in the validation cohort. Finally, the clinical usefulness was determined by decision curve analysis (DCA).
Results: Age, the American Society of Anesthesiologists (ASA) score, chronic pulmonary disease, heart disease, hypertension, combined organ resection, and preoperative and/or intraoperative blood transfusions were selected for the model. The concordance index (C-index) of the model was 0.843 in the training cohort and 0.831 in the validation cohort. The calibration curves of the ICU-specific care risk nomogram suggested great agreement in both training and validation cohorts. The DCA showed that the nomogram was clinically useful.
Conclusions: Age, ASA score, chronic pulmonary disease, heart disease, hypertension, combined organ resection, and preoperative and/or intraoperative blood transfusions were identified as risk factors for ICU-specific care after gastric surgery. A clinically friendly model was generated to identify those most likely to require intensive care.
Intensive care units (ICUs) provide a limited number of specialized medical services and consume a significant portion of hospital resources for a minority of patients (1). Triage of high-risk surgical patients to ICUs may impact the outcomes of those with the highest probability of postoperative complications and deaths (2). However, in many hospitals, the availability of ICU is often limited (3), which may lead to canceled surgeries, delayed patient transfers (4), and increased morbidity and costs (5). Besides, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection continues to grow across the world, and it is estimated that approximately 15% of patients presenting with SARS-CoV-2 will require ICU admission based on studies from Italy and China (6, 7). Therefore, identifying postoperative patients who need to be admitted to an ICU is a challenging but necessary task, especially during the coronavirus disease 2019 (COVID-19) pandemic.
Gastric cancer is the fifth most common cancer and the third leading cause of cancer-related death worldwide (8). Gastrectomy with curative intent is the most powerful treatment strategy to improve prognosis (9). Despite the advances in surgical and anesthetic techniques over the last decade, gastrectomy is associated with a high postoperative complication rate, ranging from 10.5% to 40.1% (10–12). Many complications require interventions and management that can be provided only in an ICU. As the frequency of elderly gastric cancer patients with more preexisting comorbidities is increasing (13, 14), the number of patients requiring ICU-specific care may inevitably increase. However, for many patients who will be undergoing gastrectomy for gastric cancer, postoperative admission to an ICU is only planned for surveillance purposes. ICU admissions for surveillance is not cost-effective and could lead to overuse of ICU resources (15). Furthermore, inappropriate ICU admission may be responsible for nosocomial infections and delirium (16). So, surgeons, anesthesiologists, and intensivists must identify which patients are most likely to require ICU-specific care by the end of surgery. Risk factors for postoperative ICU admission have been identified for several surgeries, including colon cancer surgery (17), lung resection (15), and total joint arthroplasty (18). Unfortunately, there are no studies that can guide the clinical decision-making of ICU admission after gastric cancer surgery.
Thus, we undertook this study to identify risk factors for ICU-specific care following gastrectomy for gastric cancer. We chose to evaluate preoperative and intraoperative factors because such a model would be more clinically friendly and useful than models based on postoperative complications or factors when ICU-specific admission would be inevitable and imminent. We aimed to use the risk factors to generate a nomogram to identify patients most likely to require ICU-specific care with the goal to provide a tool for optimizing the allocation of health care resources and ultimately improve postoperative outcomes.
A total of 3,468 gastric cancer patients who underwent gastrectomy from January 2009 to June 2018 were included in the study. The inclusion criteria were as follows: 1) histologically confirmed gastric cancer; 2) patients underwent gastrectomy with radical or palliative intent. The exclusion criteria were as follows: 1) gastroenterostomy or exploration; 2) the gastric stump cancer; 3) with emergency surgery; 4) with incomplete medical data. The data of the patients were retrospectively extracted from the database of Surgical Gastric Cancer Patient Registry in West China Hospital under the registration number: WCH-SGCPR-2020-5. The establishment of this database was authorized by the Research Ethics Committee of West China Hospital. Due to the retrospective nature of the study, informed patient consent was waived. However, patient records were de-identified and anonymized before analysis.
Various preoperative and intraoperative variables were retrieved for risk factor selection: age, sex, body mass index (BMI), history of smoking, history of alcoholism, preoperative hemoglobin, preoperative albumin, the American Society of Anesthesiologists (ASA) score, preexisting comorbidities (including chronic pulmonary disease, heart disease, hypertension, diabetes mellitus, liver dysfunction), previous abdominal surgery, neoadjuvant chemotherapy, clinical TNM stage, the extent of surgery (curative gastrectomy or palliative gastrectomy), surgical approach, surgical procedure, reconstruction method, the extent of lymphadenectomy, number of retrieved lymph nodes, combined organ resection, surgery duration, tumor size, macroscopic type, and preoperative and/or intraoperative blood transfusions.
The ASA score was obtained from the anesthesia record sheet and had been determined by the anesthesiologist providing operating room care. The diagnosis of chronic pulmonary disease, heart disease, hypertension, diabetes mellitus, and liver dysfunction were made by physicians and recorded in the patient’s chart. Chronic pulmonary disease included any of the following diseases: chronic obstructive pulmonary disease, asthma, chronic bronchitis, bronchiectasis, emphysema, and occupational lung diseases (19). Heart diseases included any of the following diseases: arrhythmias, hypertensive heart disease, ischemic heart disease, valvulopathies, and heart failure (20). Hypertension was diagnosed according to the hypertension guideline (21). Blood transfusion was administration of packed red blood cells. The indication for blood transfusion was hemoglobin level <80 g/L. For patients with hemoglobin level between 80 and 100 g/L, blood transfusion was adopted based on the risk factors associated with hemodynamic instability and inappropriate oxygenation (22).
The surgery was performed by experienced surgeons according to the Japanese gastric cancer treatment guidelines (23, 24). Intraoperative frozen section was routinely performed. Curative gastrectomy included cases in which an R0 resection was performed. Palliative gastrectomy was adopted only for patients with distant metastases but serious complications of gastric cancer (such as massive bleeding or pyloric obstruction) or for patients with residual tumor (R1 or R2 resections).
Combined organ resection was selectively performed for the purpose of curative resection or for patients with other comorbidities (such as cholecystectomy for gallbladder stone).
According to previous studies (15, 25), postoperative ICU-specific care was defined as the presence of one or more of the following characteristics: myocardial infarction, acute respiratory failure, shock, arrhythmia with hemodynamic instability, use of a variety of vasoactive drugs, reintubation, and maintenance of controlled ventilation longer than 48 h.
So, the ICU-specific group consisted of three groups of patients: 1) ICU treatment group: patients who were admitted to an ICU immediately after surgery and met the criteria of ICU-specific care; 2) Ward-ICU group: patients who were not admitted to ICU immediately after surgery but were admitted for an emergent reason, such as sudden cardiac arrest, acute respiratory failure, and any other situations that required ICU-specific care; 3) Refuse transfer group: patients who were admitted to the general ward after surgery and developed complications that required ICU-specific care; however, they refused to transfer to an ICU.
The Non-ICU-specific group consisted of two groups of patients: 1) ICU surveillance group: patients who were admitted to an ICU immediately after surgery for surveillance purposes and did not meet the criteria of ICU-specific care; 2) Recovery group: patients who were transferred to the general ward after surgery and then discharged without any complications. The patient flowchart is indicated in Figure 1.
Statistical analysis was conducted using R software (Version 3.6.1; https://www.r-project.org) and SPSS 20.0 (SPSS®, Chicago, IL, USA). Categorical variables are represented by number and percentage, while continuous variables are represented by mean ± standard deviation. We randomly assigned 70.0% of the patients to the training cohort and 30.0% to the validation cohort. We used the least absolute shrinkage and selection operator (LASSO) method to screen out the optimal variables with non-zero coefficients as risk factors (26). Then, based on the results of LASSO regression analysis, multivariable logistic regression analysis was used to establish the predictive model, and nomogram was further generated (27, 28). The predictive efficiency of the nomogram was evaluated by Harrell’s concordance index (C-index). Calibration curves were plotted to assess the calibration of the nomogram in both training cohort and validation cohort. A decision curve analysis (DCA) was also generated to determine the clinical usefulness of the nomogram. A p value <0.05 was considered to be statistically significant.
A total of 3,468 gastric cancer patients who underwent gastrectomy from January 2009 to June 2018 were included in the study. There were 129 patients (3.7%) in the ICU-specific care group and 3,335 patients (96.3%) in the Non-ICU-specific care group (Figure 1). All patients were randomly divided into the training cohort (n = 2,428, 70.0% of the total patients) and the validation cohort (n = 1,040, 30.0% of the total patients). The characteristics of patients in the training and validation cohorts are shown in Table 1. There was no significant difference in any of the variables between the training and validation cohorts (all p > 0.05), indicating that the baseline was balanced between them.
We performed a LASSO regression analysis to evaluate the 29 variables in the training cohort (Figure 2). Finally, we retained 7 variables with non-zero coefficients as potential predictors of the prediction model. These predictors included age, the ASA score, chronic pulmonary disease, heart disease, hypertension, combined organ resection, and preoperative and/or intraoperative blood transfusions.
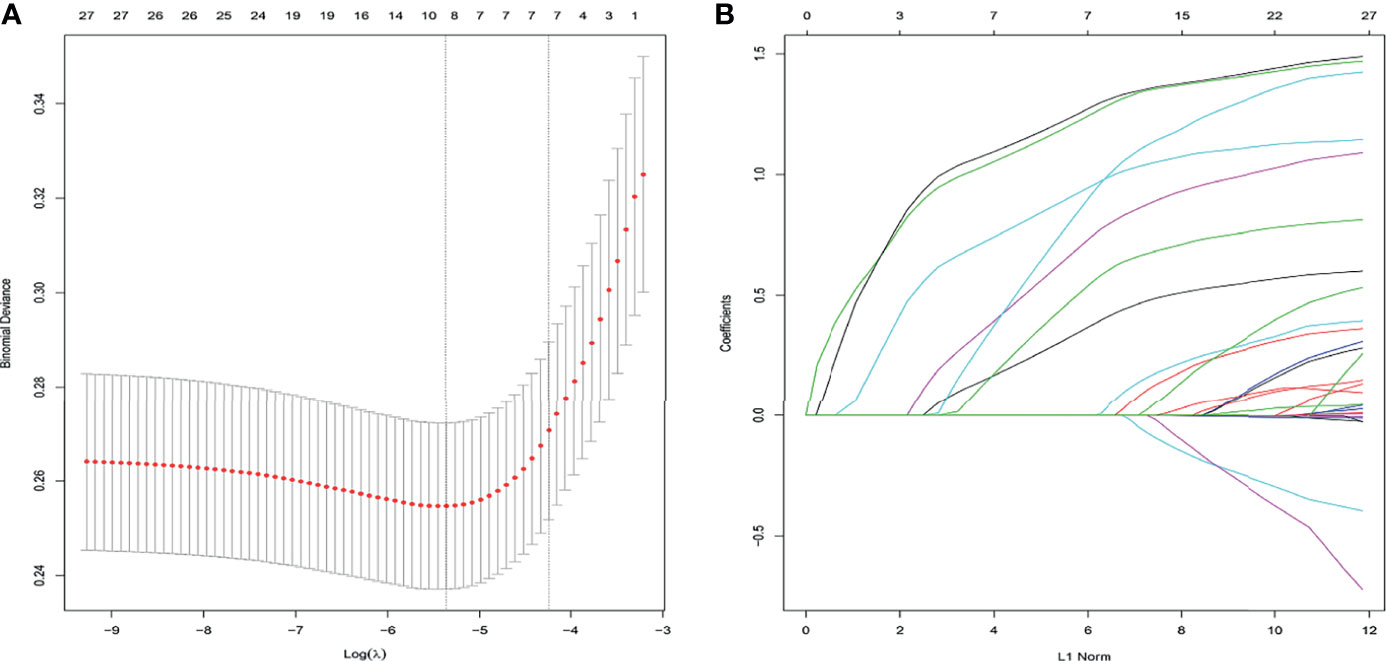
Figure 2 Clinicopathological features selection using the LASSO logistic regression model. Final predictors include age, the ASA score, chronic pulmonary disease, heart disease, combined organ resection, and preoperative and/or intraoperative blood transfusion. (A) Optimal parameter (λ) selection in the LASSO model used 5-fold cross-validation and minimum criteria. The partial likelihood deviance (binomial deviance) curve was plotted vs. log(λ). Dotted vertical lines were drawn at the optimal values by using the minimum criteria and the 1 SE of the minimum criteria (the 1-SE criteria). (B) LASSO coefficient profiles of the 27 features. A coefficient profile plot was plotted against the log(λ) sequence, and the 7 non-zero coefficients were chosen at the values selected using 5-fold cross-validation. ASA, American Society of Anesthesiologists; LASSO, least absolute shrinkage and selection operator; SE, standard error.
To get a more comprehensive view of the relationship between the need for ICU-specific care and these predictors, we further performed a multivariable logistic regression analysis and constructed a predictive model. The results of the logistic regression analysis were given in Table 2 and visualized in the form of a nomogram plot to help practice in the clinic (Figure 3). The C-index of the model was 0.843 in the training cohort and 0.831 in the validation cohort. The calibration curves of the ICU-specific care risk nomogram suggested great agreement in both training cohort and validation cohort (Figures 4A, B).
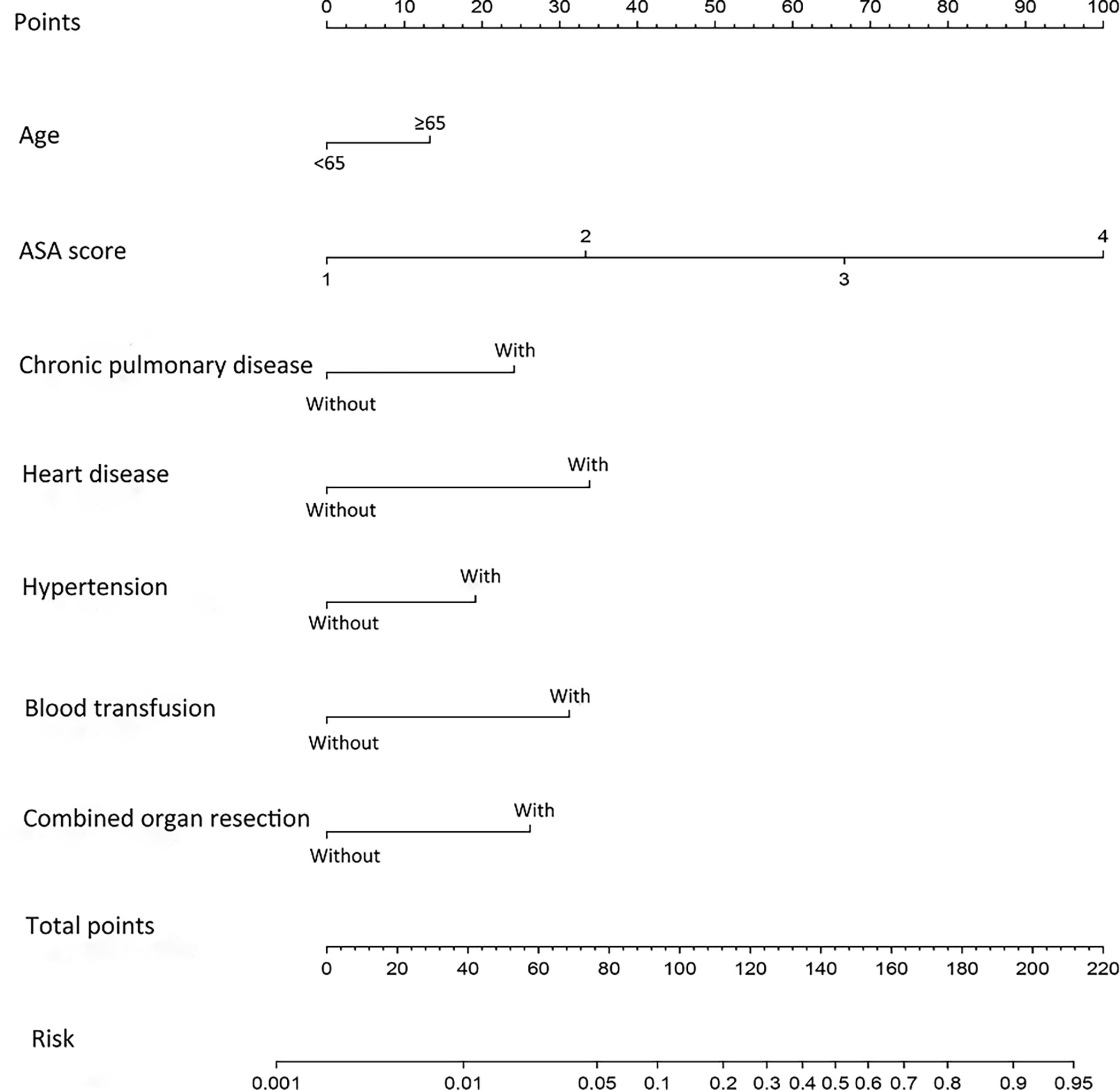
Figure 3 Nomogram for predicting ICU-specific care following gastrectomy for gastric cancer. The prediction nomogram was developed in the training cohort, with age, ASA score, chronic pulmonary disease, hypertension, combined organ resection, and preoperative and/or intraoperative blood transfusions incorporated. ASA, American Society of Anesthesiologists; ICU, intensive care unit. Blood transfusion, preoperative and/or intraoperative blood transfusions.
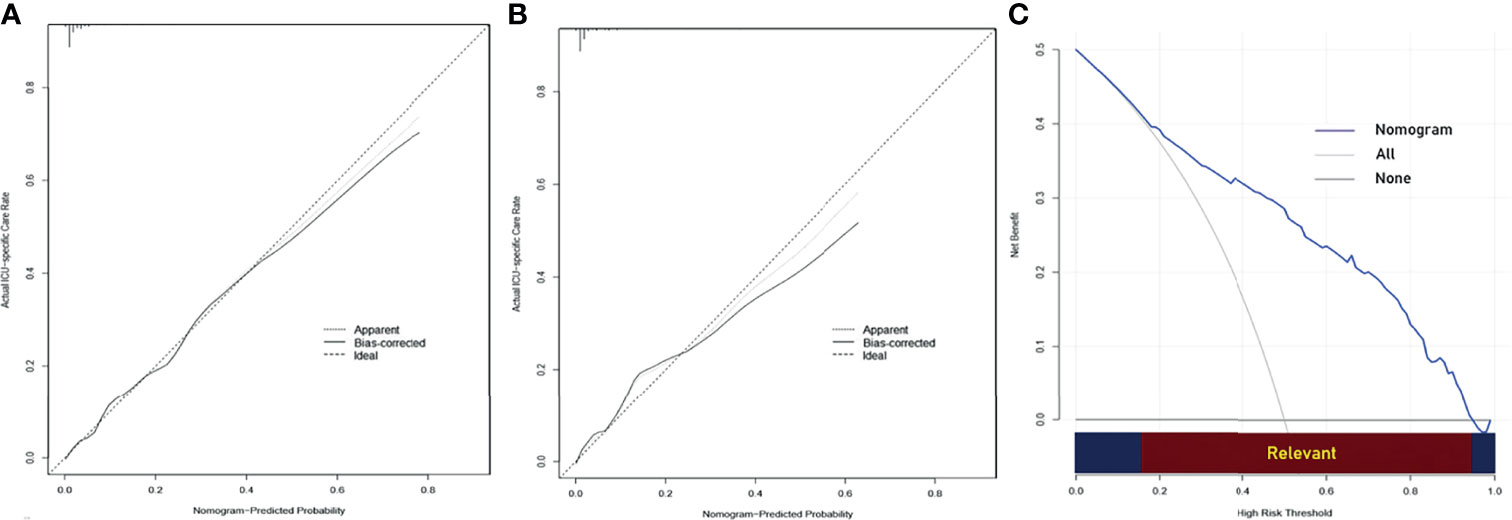
Figure 4 (A) Calibration curve of the nomogram for predicting ICU-specific care following gastrectomy for gastric cancer in the training cohort. (B) Calibration curve of the nomogram for predicting ICU-specific care following gastrectomy for gastric cancer in the validation cohort. (C) Decision curve analysis (DCA) for predicting ICU-specific care following gastrectomy for gastric cancer. The y-axis represents net benefit. The x-axis shows the threshold probability. “All” refers to the assumption that all patients need ICU-specific care, and “None” refers to the assumption that no patient needs ICU-specific care. When the score is within the range 0.14–0.95 (Relevant), using the nomogram to predict ICU-specific care adds more net benefit than the treat-all or treat-none strategies. ICU, intensive care unit.
The DCA for the predictive nomogram is shown in Figure 4C. The analysis indicated that using the nomogram to predict ICU-specific care following gastrectomy for gastric cancer added more net benefit than the treat-all or treat-none strategies when the threshold probability was within the range 0.14–0.95.
This study showed that a small (3.7%) but important proportion of patients required ICU-specific care following gastrectomy for gastric cancer. These patients tended to be older and more likely to have a higher ASA score, chronic pulmonary disease, heart disease, hypertension, combined organ resection, and blood transfusion before and/or during surgery. Recent data have shown that ICU admission after surgery only for surveillance purposes may increase medical costs without the expected additional benefits for patients (29, 30). Therefore, identifying patients at a high risk of postoperative ICU-specific care can help improve postoperative outcomes and optimize the allocation of health care resources, especially during the COVID-19 pandemic. To our knowledge, this was the first study that can guide the clinical decision-making of ICU admission after gastrectomy for gastric cancer. The model can be used to evaluate ICU resource allocation by retrospectively identifying patient groups whose characteristics indicate that they may not have needed ICU admission. The ability to identify low-risk admission patients allows managers to implement protocols and educational programs for providing effective and safe care alternatives in intermediate care units or general wards.
In the present study, older age was identified as a risk factor for postoperative ICU-specific care. Multiple previous studies have demonstrated that older age was independently associated with postoperative complications after gastrectomy (31, 32). Some complications can be managed only in an ICU. Although the incidence of gastric cancer has been declining due to longer life expectancy, the number of aged patients with this disease is continuously increasing (13). So, we can foresee that an increasing number of patients may require ICU-specific care after gastrectomy for gastric cancer.
We also found that several preexisting comorbidities were also associated with postoperative ICU-specific care, such as chronic pulmonary disease, heart disease, and hypertension. All these factors have been identified as risk factors for postoperative morbidity and mortality after gastrectomy for gastric cancer in previous studies (33–37). So, special attention should be paid to patients with these comorbidities, and we believe that prior treatment of these preoperative comorbidities is essential to the postoperative recovery of patients with gastric cancer.
The ASA score was found to have a strong influence on ICU-specific care in the present study. Several studies have reported that it was a risk factor for ICU admission following other surgeries (38, 39). The ASA score has the advantages of simplicity and universality (40) and is an effective risk indicator whether used alone or in combination with other parameters (41). A difficulty in using it in patient assessment is the limited interobserver reliability (42). However, a previous study has confirmed that the ASA score had the greatest validity and highest interobserver reliability when assigned by the responsible anesthesiologist in the operating theater (43). Therefore, we obtained the ASA score from the anesthesiologist chart and had been determined by the anesthesiologist providing operating theater care to maximize its validity and reliability.
Among all the surgical factors, only combined organ resection was identified as a risk factor for ICU-specific care in our study. These findings were supported by a previous study (44), which demonstrated that combined organ resection had an increased risk for postoperative complications and mortality. Our study did not identify any association between surgical approach (open or laparoscopic), surgical procedure (distal, proximal, or total gastrectomy), extent of surgery (radical or palliative), or extent of lymphadenectomy (D1/D1+ or D2/D2+) and postoperative ICU-specific care. Laparoscopic gastrectomy has gained popularity in the treatment of gastric cancer in China, Japan, and Korea (45). Multiple randomized trials have demonstrated that there was no significant difference in postoperative complications and deaths between laparoscopic and open gastrectomy for patients with preoperative stage I gastric cancer (12) and for patients with advanced gastric cancer who underwent distal gastrectomy (45). In terms of surgical procedure, previous studies reported mixed results. Shin et al. (46) reported that surgical procedure was not associated with postoperative complications. However, Lee et al. (34) reported that total gastrectomy was an independent risk factor for postoperative complications. In the present study, the extent of surgery (radical vs. palliative resection) was not identified as a risk factor for ICU-specific care. In a previous study (47), there was no significant difference in mortality and morbidity rate after palliative or radical surgery. The possible explanation is that patients undergoing palliative surgery may be in poorer general condition, but the surgery is less invasive and shorter in duration (47). In terms of the extent of lymphadenectomy, mortality and morbidity rates did not differ significantly between D1 and D2 group whether in retrospective (48) or prospective studies (11). In our personal opinion, D2 lymph node dissection can be safely performed by senior gastric cancer surgeons.
In the present study, blood transfusion was also found to be a risk factor for ICU-specific care. These findings were in accordance with a previous study (49). There is a high incidence of anemia in patients with advanced gastric cancer (50). In addition, gastrectomy with lymphadenectomy sometimes leads to excessive bleeding even performed by experienced surgeons (51). Thus, perioperative blood transfusion is sometimes inevitable when performing gastrectomy for advanced gastric cancer. Although blood transfusion can be lifesaving for gastric cancer patients with severe anemia by improving their oxygen delivery capacity and tissue perfusion, it can also result in systemic inflammation and other transfusion-related adverse events, especially acute lung injury and infection (52, 53). Furthermore, preoperative and intraoperative blood transfusions may reflect the patient’s poor systemic condition or complexity of the surgery (54). So, special attention should be paid to patients who have blood transfusion in the perioperative period.
The endpoint of our study was postoperative ICU-specific care. However, postoperative ICU-specific care has been defined differently in previous studies. Two studies (29, 30) defined at least 24 h in an ICU setting as postoperative ICU-specific care, regardless whether the patients received any active life-supporting treatments (1) or not. Dahm et al. (25) defined ICU-specific care as the presence of one or more of the following characteristics: mechanical ventilation longer than 12 h, continuous intravenous infusion of vasoactive medication, or a postoperative event mandating treatment in an ICU setting (pulmonary embolism, myocardial infarction, or arrhythmia with hemodynamic instability). Kim et al. (15) defined ICU-specific care as the presence of one or more of the following characteristics: reintubation, maintenance of controlled ventilation, hemodynamic instability, shock, acute respiratory failure, use of multiple vasoactive drugs, and cardiac arrhythmia. Patients who were admitted to the ICU and then transferred to the general ward the day after the surgery were deemed as non-specific care group in their study. In the present study, we defined ICU-specific care group as the presence of one or more of the following characteristics: myocardial infarction, acute respiratory failure, shock, arrhythmia with hemodynamic instability, use of a variety of vasoactive drugs, reintubation, and maintenance of controlled ventilation longer than 48 h. This parameter was based on our institutional guidelines that patients in the postoperative ICU are expected to be extubated within 48 h. We also included several life-supporting treatments that are best or unique to performed in an ICU setting. Such a definition may be more comprehensive and clinically relevant (55).
In the present study, we constructed a nomogram to guide the clinical decision-making of ICU admission. Medical providers could make individualized predictions of the probability of receiving intensive care with this easy-to-use model, which is in accordance with the current trend toward personalized medicine (56). Improved health care resource use and reduced costs might be achieved by providing care for these patients in general wards or intermediate care units, especially during the COVID-19 pandemic. The most important argument for the use of the nomogram is based on the need to interpret a patient’s need for additional treatment or care. However, discrimination and calibration cannot capture the clinical consequences of specific levels of discrimination or degrees of miscalibration (57). The DCA showed that using the nomogram to predict the probability of receiving intensive care is more beneficial than the treat-all or the treat-none strategies if the threshold probability of an individual is within 0.14–0.95.
The strengths of the study were that it included a wide range of variables with ICU-specific care from a large cohort. The proposed prediction nomogram was generated based on routinely collected preoperative and intraoperative data to maximize its application and ensure its generalizability. The study also had some limitations. First, the study was conducted retrospectively; there may have been some inherent selection biases. A prospective study should be carried out to validate the prediction model. Second, our study was a monocentric study and the results were validated only internally; further external validation should be performed to make sure whether these results could be applied to other institutions.
Several risk factors for ICU-specific care after gastrectomy for gastric cancer were identified. A clinically friendly model with excellent ability was generated to identify those most likely to require intensive care.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
This study was based on the information gathered from the database of the Surgical Gastric Cancer Patient Registry of West China Hospital (WCH-SGCPR) under registration number WCH-SGCPR-2020-5. The establishment of this database was approved by the Research Ethics Committee of West China Hospital. Informed patient consent was waived because of the retrospective nature of the analysis.
J-KH, TP, and X-LC. Administrative support: J-KH, TP, and X-LC. Provision of study materials or patients: KL, W-HZ, RG, M-HY, L-YZ, KY, and X-ZC. Collection and assembly of data: TP, B-QP and X-LC. Data analysis and interpretation: TP and X-LC. Article writing: All authors. Final approval of article: All authors. All authors contributed to the article and approved the submitted version.
This study was funded by the Foundation of Science & Technology Department of Sichuan Province (2019YFS0255); the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZY2017304); and the Sichuan Province Cadre Health Care Research Project (No. 2017-114).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the substantial work of Volunteer Team of Gastric Cancer Surgery (VOLTGA) West China Hospital, Sichuan University, China, for the establishment and updating of the gastric cancer database.
1. Graf J, Graf C, Janssens U. Analysis of Resource Use and Cost-Generating Factors in a German Medical Intensive Care Unit Employing the Therapeutic Intervention Scoring System (TISS-28). Intensive Care Med (2002) 28(3):324–31. doi: 10.1007/s00134-001-1201-6
2. Sobol JB, Gershengorn HB, Wunsch H, Li G. The Surgical Apgar Score is Strongly Associated With Intensive Care Unit Admission After High-Risk Intraabdominal Surgery. Anesth Analg (2013) 117(2):438–46. doi: 10.1213/ANE.0b013e31829180b7
3. Halpern NA, Pastores SM. Critical Care Medicine in the United States 2000–2005: An Analysis of Bed Numbers, Occupancy Rates, Payer Mix, and Costs. Crit Care Med (2010) 38(1):65–71. doi: 10.1097/CCM.0b013e3181b090d0
4. Liu V, Kipnis P, Rizk NW, Escobar GJ. Adverse Outcomes Associated With Delayed Intensive Care Unit Transfers in an Integrated Healthcare System. J Hosp Med (2012) 7(3):224–30. doi: 10.1002/jhm.964
5. Edbrooke D, Minelli C, Mills G, Iapichino G, Pezzi A, Corbella D, et al. ICU Triage Decisions on Patient Mortality: A Cost-Effectiveness Analysis. Crit Care (2011) 15(1):R56. doi: 10.1186/cc10029
6. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA (2020). doi: 10.1001/jama.2020.5394
7. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients With COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet (2020) 395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3
8. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
9. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric Cancer. Lancet (2016) 388(10060):2654–64. doi: 10.1016/S0140-6736(16)30354-3
10. Lee JH, Park DJ, Kim HH, Lee HJ, Yang HK. Comparison of Complications After Laparoscopy-Assisted Distal Gastrectomy and Open Distal Gastrectomy for Gastric Cancer Using the Clavien-Dindo Classification. Surg Endosc (2012) 26(5):1287–95. doi: 10.1007/s00464-011-2027-0
11. Degiuli M, Sasako M, Ponti A, Vendrame A, Tomatis M, Mazza C, et al. Randomized Clinical Trial Comparing Survival After D1 or D2 Gastrectomy for Gastric Cancer. Br J Surg (2014) 101(2):23–31. doi: 10.1002/bjs.9345
12. Kim H-H, Hyung WJ, Cho GS, Kim M, Han S, Kim W, et al. Morbidity and Mortality of Laparoscopic Gastrectomy Versus Open Gastrectomy for Gastric Cancer. Ann surg (2010) 251(3):417–20. doi: 10.1097/SLA.0b013e3181cc8f6b
13. Orsenigo E, Tomajer V, Palo SD, Carlucci M, Vignali A, Tamburini A, et al. Impact of Age on Postoperative Outcomes in 1118 Gastric Cancer Patients Undergoing Surgical Treatment. Gastric Cancer (2007) 10(1):39–44. doi: 10.1007/s10120-006-0409-0
14. Katai H, Sasako M, Sano T, Fukagawa T. Gastric Cancer Surgery in the Elderly Without Operative Mortality. Surg Oncol (2004) 13(4):235–8. doi: 10.1016/j.suronc.2004.09.007
15. Kim SH, Na S, Park SY, Lee J, Kang Y, Jung H, et al. Perioperative Factors for Predicting the Need for Postoperative Intensive Care After Major Lung Resection. J Clin Med (2019) 8(5). doi: 10.3390/jcm8050744
16. Osman MF, Askari R. Infection Control in the Intensive Care Unit. Surg Clin North Am (2014) 94(6):1175–94. doi: 10.1016/j.suc.2014.08.011
17. Garfinkle R, Abou-Khalil M, Salama E, Marinescu D, Pang A, Morin N, et al. Development and Validation of a Clinical Risk Score for Intensive Care Resource Utilization After Colon Cancer Surgery: A Practical Guide to the Selection of Patients During COVID-19. J Gastrointest Surg (2020). doi: 10.1007/s11605-020-04665-9
18. AbdelSalam H, Restrepo C, Tarity TD, Sangster W, Parvizi J. Predictors of Intensive Care Unit Admission After Total Joint Arthroplasty. J Arthroplasty (2012) 27(5):720–5. doi: 10.1016/j.arth.2011.09.027
19. Varhabhatla N, Zuo Z. The Effects of Chronic Pulmonary Disease on Hospital Length of Stay and Cost of Hospitalization After Neurosurgery. Clinical Article. J Neurosurg (2011) 115(2):375–9. doi: 10.3171/2011.3.JNS101608
20. Herrero Rivera D, Nieto-Guerrero Gomez JM. Cacicedo Fernandez De Bobadilla J Cardiovascular Disease and Survival in non-Small Cell Lung Cancer: A Multicenter Prospective Assessment. Clin Trans Oncol Off Publ Fed Spanish Oncol Soc Natl Cancer Inst Mex (2019) 21(9):1220–30. doi: 10.1007/s12094-019-02047-5
21. James PA, Oparil S, Carter BL, Cushman W, Dennison-Himmelfarb C, Handler J, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8). JAMA (2014) 311(5):507–20. doi: 10.1001/jama.2013.284427
22. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice Guidelines for Perioperative Blood Transfusion and Adjuvant Therapies: An Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology (2006) 105:198–208. doi: 10.1097/00000542-200607000-00030
23. Sano T, Aiko T. New Japanese Classifications and Treatment Guidelines for Gastric Cancer: Revision Concepts and Major Revised Points. Gastric Cancer (2011) 14(2):97–100. doi: 10.1007/s10120-011-0040-6
24. Japanese Gastric Cancer A. Japanese Gastric Cancer Treatment Guidelines 2014 (Ver. 4). Gastric Cancer (2017) 20(1):1–19. doi: 10.1007/s10120-016-0622-4
25. Philipp D, Janet E, Shahid M, Byrne C, Yowell C, Price D, et al. Indications for Admission to the Surgical Intensive Care Unit After Radical Cystectomy and Urinary Diversion. J Urol (2001) 42:614–8.
26. Jerome F, Trevor H, Rob T. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw (2010) 33(1):1–22.
27. Zhao LY, Wang JJ, Zhao YL, Chen X, Yang K, Chen X, et al. Superiority of Tumor Location-Modified Lauren Classification System for Gastric Cancer: A Multi-Institutional Validation Analysis. Ann Surg Oncol (2018) 25(11):3257–63. doi: 10.1245/s10434-018-6654-8
28. Pan T, Galiullin D, Chen XL, Zhang W, Yang K, Liu K, et al. Incidence of Adhesive Small Bowel Obstruction After Gastrectomy for Gastric Cancer and its Risk Factors: A Long-Term Retrospective Cohort Study From a High-Volume Institution in China. Updates Surg (2021). doi: 10.1007/s13304-021-00983-y
29. Cerullo M, Gani F, Chen SY, Canner J, Dillhoff M, Cloyd J, et al. Routine Intensive Care Unit Admission Among Patients Undergoing Major Pancreatic Surgery for Cancer: No Effect on Failure to Rescue. Surg (2019) 165(4):741–6. doi: 10.1016/j.surg.2018.11.005
30. Merath K, Cerullo M, Farooq A, Canner J, He J, Tsilimigras D, et al. Routine Intensive Care Unit Admission Following Liver Resection: What Is the Value Proposition? J Gastrointest Surg (2019) 33(1):1–22. doi: 10.1007/s11605-019-04408-5
31. Nelen SD, Bosscha K, Lemmens V, Hartgrink H, Verhoeven R, Wilt J, et al. Morbidity and Mortality According to Age Following Gastrectomy for Gastric Cancer. Br J Surg (2018) 105(9):1163–70. doi: 10.1002/bjs.10836
32. Park DJ, Lee HJ, Kim HH, Yang HK, Lee KU, Choe KJ. Predictors of Operative Morbidity and Mortality in Gastric Cancer Surgery. Br J Surg (2005) 92(9):1099–102. doi: 10.1002/bjs.4952
33. Persiani R, Antonacci V, Biondi A, Rausei S, La Greca A, Zoccali M, et al. Determinants of Surgical Morbidity in Gastric Cancer Treatment. J Am Coll Surg (2008) 207(1):13–9. doi: 10.1016/j.jamcollsurg.2007.12.050
34. Lee KG, Lee HJ, Yang JY, Oh S, Bard S, Suh Y, et al. Risk Factors Associated With Complication Following Gastrectomy for Gastric Cancer: Retrospective Analysis of Prospectively Collected Data Based on the Clavien-Dindo System. J Gastrointest Surg (2014) 18(7):1269–77. doi: 10.1007/s11605-014-2525-1
35. Coimbra FJF, de Jesus VHF, Franco CP, Calsavara V, Ribeiro H, Diniz A, et al. Predicting Overall and Major Postoperative Morbidity in Gastric Cancer Patients. J Surg Oncol (2019) 120(8):1371–8. doi: 10.1002/jso.25743
36. Kim MC, Kim W, Kim HH, Ryu SW, Ryu SY, Song KY, et al. Risk Factors Associated With Complication Following Laparoscopy-Assisted Gastrectomy for Gastric Cancer: A Large-Scale Korean Multicenter Study. Ann Surg Oncol (2008) 15(10):2692–700. doi: 10.1245/s10434-008-0075-z
37. Kurita N, Miyata H, Gotoh M, Shimada M, Imura S, Kimura W, et al. Risk Model for Distal Gastrectomy When Treating Gastric Cancer on the Basis of Data From 33,917 Japanese Patients Collected Using a Nationwide Web-Based Data Entry System. Ann Surg (2015) 262(2):295–303. doi: 10.1097/SLA.0000000000001127
38. Kamath AF, McAuliffe CL, Baldwin KD, Lucas JB, Kosseim LM, Israelite CL. Unplanned Admission to the Intensive Care Unit After Total Hip Arthroplasty. J Arthroplasty (2012) 27(6):1027–32.e1021-1022. doi: 10.1016/j.arth.2012.01.004
39. Pieretti P, Alifano M, Roche N, Vincenzi M, Forti Parri SN, Zackova M, et al. Predictors of an Appropriate Admission to an ICU After a Major Pulmonary Resection. Respiration (2006) 73(2):157–65. doi: 10.1159/000088096
40. Sutton R, Bann S, Brooks M, Sarin S. The Surgical Risk Scale as an Improved Tool for Risk-Adjusted Analysis in Comparative Surgical Audit. Br J Surg (2002) 42:614–8. doi: 10.1046/j.1365-2168.2002.02080.x
41. Watanabe M, Miyata H, Gotoh M, Baba H, Kimura M, Tomita N, et al. Total Gastrectomy Risk Model. Ann Surg (2014) 260(6):1034–9. doi: 10.1097/SLA.0000000000000781
42. Riley R, Holman C, Fletcher D. Inter-Rater Reliability of the ASA Physical Status Classification in a Sample of Anaesthetists in Western Australia. Anaesth Intensive Care (2014) 42:614–8. doi: 10.1177/0310057X1404200511
43. Sankar A, Johnson SR, Beattie WS, Tait G, Wijeysundera DN. Reliability of the American Society of Anesthesiologists Physical Status Scale in Clinical Practice. Br J Anaesth (2014) 113(3):424–32. doi: 10.1093/bja/aeu100
44. Dias AR, Pereira MA, Ramos M, Oliveira RJ, Ribeiro U, Zilberstein B, et al. Prediction Scores for Complication and Recurrence After Multivisceral Resection in Gastric Cancer. Eur J Surg Oncol (2020) 46(6):1097–102. doi: 10.1016/j.ejso.2020.01.014
45. Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol (2016) 34(12):1350–7. doi: 10.1200/JCO.2015.63.7215
46. Shin HS, Oh SJ, Suh BJ. Factors Related to Morbidity in Elderly Gastric Cancer Patients Undergoing Gastrectomies. J Gastric Cancer (2014) 14(3):173–9. doi: 10.5230/jgc.2014.14.3.173
47. Sinduja R, Anandhi A, Sureshkumar S, Barathi D, Mahalakshmy T, Kate V. Role of Sarcopenia in Predicting the Postoperative Morbidity and Perioperative Mortality in Patients Undergoing Elective Surgery for Gastric Cancer. J Gastrointest Cancer (2021). doi: 10.1007/s12029-021-00715-w
48. Lam S, Tan E, Menezes A, Martin D, Gallagher J, Storey D, et al. A Comparison of the Operative Outcomes of D1 and D2 Gastrectomy Performed at a Single Western Center With Multiple Surgeons: A Retrospective Analysis With Propensity Score Matching. World J Surg Oncol (2018) 16(1):136. doi: 10.1186/s12957-018-1422-6
49. Xiao H, Quan H, Pan S, Yin B, Luo W, Huang G, et al. Impact of Peri-Operative Blood Transfusion on Post-Operative Infections After Radical Gastrectomy for Gastric Cancer: A Propensity Score Matching Analysis Focusing on the Timing, Amount of Transfusion and Role of Leukocyte Depletion. J Cancer Res Clin Oncol (2018) 144(6):1143–54. doi: 10.1007/s00432-018-2630-8
50. Liu X, Ma M, Huang H, Wang Y. Effect of Perioperative Blood Transfusion on Prognosis of Patients With Gastric Cancer: A Retrospective Analysis of a Single Center Database. BMC Cancer (2018) 18(1). doi: 10.1186/s12885-018-4574-4
51. Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 Lymphadenectomy Alone or With Para-Aortic Nodal Dissection for Gastric Cancer. N Engl J Med (2008) 42:614–8. doi: 10.1056/NEJMoa0707035
52. Bueter M, Thalheimer A, Schuster F, Bock M, von Erffa C, Meyer D, et al. Transfusion-Related Acute Lung Injury (TRALI)–an Important, Severe Transfusion-Related Complication. Langenbeck's Arch Surg (2006) 391(5):489–94. doi: 10.1007/s00423-006-0072-2
53. Almeida J, Vincent J, Galas F, Almeida E, Fukushima J, Osawa E, et al. Transfusion Requirements in Surgical Oncology Patients A Prospective, Randomized Controlled Trial. Anesthesiology (2015) 122:29–38. doi: 10.1097/ALN.0000000000000511
54. Weber RS, Jabbour N, Martin RC. 2nd. Anemia and Transfusions in Patients Undergoing Surgery for Cancer. Ann Surg Oncol (2008) 15(1):34–45. doi: 10.1245/s10434-007-9502-9
55. Zimmerman JE, Kramer AA. A Model for Identifying Patients Who May Not Need Intensive Care Unit Admission. J Crit Care (2010) 25(2):205–13. doi: 10.1016/j.jcrc.2009.06.010
56. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in Oncology: More Than Meets the Eye. Lancet Oncol (2015) 16(4):e173–80. doi: 10.1016/S1470-2045(14)71116-7
Keywords: gastric cancer, intensive care medicine, resource allocation, complications, scoring system
Citation: Pan T, Chen X-l, Liu K, Peng B-q, Zhang W-h, Yan M-h, Ge R, Zhao L-y, Yang K, Chen X-z and Hu J-k (2022) Nomogram to Predict Intensive Care Following Gastrectomy for Gastric Cancer: A Useful Clinical Tool to Guide the Decision-Making of Intensive Care Unit Admission. Front. Oncol. 11:641124. doi: 10.3389/fonc.2021.641124
Received: 13 December 2020; Accepted: 13 December 2021;
Published: 11 January 2022.
Edited by:
Alberto Biondi, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Xiaohua Jiang, Tongji University, ChinaCopyright © 2022 Pan, Chen, Liu, Peng, Zhang, Yan, Ge, Zhao, Yang, Chen and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-kun Hu, aHVqa3djaEAxMjYuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.