
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 17 March 2021
Sec. Women's Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.640690
This article is part of the Research Topic Immunotherapy as an Evolving Approach for the Treatment of Breast Cancer View all 13 articles
 Anna R. Schreiber1
Anna R. Schreiber1 Jodi A. Kagihara2
Jodi A. Kagihara2 Jennifer A. Weiss1
Jennifer A. Weiss1 Andrew Nicklawsky3
Andrew Nicklawsky3 Dexiang Gao3
Dexiang Gao3 Virginia F. Borges2
Virginia F. Borges2 Peter Kabos2
Peter Kabos2 Jennifer R. Diamond2*
Jennifer R. Diamond2*Background: Immuno-oncology (IO) agents have demonstrated efficacy across many tumor types and have led to change in standard of care. In breast cancer, atezolizumab and pembrolizumab were recently FDA-approved in combination with chemotherapy specifically for patients with PD-L1-positive metastatic triple-negative breast cancer (TNBC). However, the single agent PD-1/PD-L1 inhibitors demonstrate only modest single agent efficacy in breast cancer. The purpose of this study was to investigate the efficacy of novel IO agents in patients with metastatic breast cancer (MBC), beyond TNBC, treated in phase I clinical trials at the University of Colorado.
Methods: We performed a retrospective analysis using a database of patients with MBC who received treatment with IO agents in phase I/Ib clinical trials at the University of Colorado Hospital from January 1, 2012 to July 1, 2018. Patient demographics, treatments and clinical outcomes were obtained.
Results: We identified 43 patients treated with an IO agent either as a single agent or in combination. The average age was 53 years; 55.8% had hormone receptor-positive/HER2-negative breast cancer, 39.5% TNBC and 4.7% HER2-positive. Patients received an average of 2 prior lines of chemotherapy (range 0-7) in the metastatic setting. Most patients (72.1%) received IO alone and 27.9% received IO plus chemotherapy. Median progression-free survival (PFS) was 2.3 months and median overall survival (OS) was 12.1 months. Patients remaining on study ≥ 6 months (20.9%) were more likely to be treated with chemotherapy plus IO compared to patients with a PFS < 6 months (77.8% v. 14.7%). No differences in number of metastatic sites, prior lines of chemotherapy, breast cancer subtype, absolute lymphocyte count, or LDH were identified between patients with a PFS ≥ 6 months vs. < 6 months.
Conclusions: Our phase I experience demonstrates benefit from IO therapy that was not limited to patients with TNBC and confirms improved efficacy from IO agents in combination with chemotherapy. A subset of patients with MBC treated in phase I clinical trials with an IO agent derived prolonged clinical benefit. Predictors of response to immunotherapy in breast cancer remain uncharacterized and further research is needed to identify these factors.
Breast cancer is the most common cancer in women and patients with metastatic breast cancer (MBC) have a 5-year overall survival of only 27% (1). While prognosis depends on biologic subtype, there remains a critical unmet need for novel therapeutic options to improve survival for patients with MBC.
The development of immuno-oncology (IO) therapeutics has changed the way we treat many cancers, most dramatically with inhibitors of programmed cell death-1 (PD-1), its ligand (PD-L1) and cytotoxic T-lymphocyte-associated antigen (CTLA-4) (2–4). The first approval in metastatic breast cancer came in 2019 with the approval of atezolizumab in combination with nab-paclitaxel for patients with PD-L1-positive (tumor-infiltrating immune cells ≥ 1%) metastatic triple-negative breast cancer (TNBC) (5). This was followed in 2020 by the approval of pembrolizumab in combination with chemotherapy including paclitaxel, nab-paclitaxel or gemcitabine plus carboplatin in patients with PD-L1-positive (combined positive score ≥10) metastatic TNBC (5, 6).
TNBC and human epidermal growth factor receptor 2 (HER2)-positive breast cancers are perceived as being more immunogenic compared to luminal breast cancers based on a higher mutational burden, higher tumor-infiltrating lymphocyte (TIL) rates and higher PD-L1 expression (7–10). Higher TIL expression is associated with increased pathologic complete response (pCR) rates in patients treated with neoadjuvant chemotherapy and improved prognosis in HER2-positive and TNBC (9, 11). PD-L1 is expressed in 20-50% of breast cancers and varies depending on the specific antibody clone and evaluation on tumor cells or immune cells in the tumor microenvironment (10). Expression is higher in TNBC and HER2-positive breast cancer compared to hormone receptor (HR)-positive/HER2-negative tumors (10, 12). In patients with TNBC and HER2-positive breast cancer treated with neoadjuvant chemotherapy, PD-L1 expression correlates with a higher pCR rates and improved clinical outcomes (13–15).
Despite the increased immunogenicity of TNBC, response rates to IO monotherapy with pembrolizumab range from 23% for PD-L1-positive patients treated in the first-line setting to approximately 5% for patients previously treated with chemotherapy regardless of PD-L1 status (16, 17). While there is a subset of patients with TNBC who are exceptional responders to immunotherapy and experience long-term disease control, the efficacy of IO monotherapy generally is no better than palliative chemotherapy and combinations of IO plus chemotherapy are more active (5, 6, 18).
The activity of IO agents in luminal breast cancers is more limited with response rates ranging from 11% to 30% for pembrolizumab in patients with advanced PD-L1 positive, HR-positive HER2-negative breast cancer and 3% with avelumab in a similar patient population (19, 20). In the neoadjuvant setting, the addition of pembrolizumab to an anthracycline and taxane-containing chemotherapy backbone resulted in an increased pCR rate in patients with HR-positive/HER2-negative breast cancer in the I-SPY2 clinical trial (21). There are numerous ongoing clinical trials evaluating IO agents in combination with chemotherapy, radiotherapy, other immune checkpoint inhibitors or cancer vaccines (22).
Over the last decade, there has been a rapid increase in the development of diverse IO agents targeting numerous pathways extending beyond CTLA-4 and PD-1/PD-L1. The recent approval of atezolizumab and pembrolizumab in combination with chemotherapy for patients with PD-L1-positive TNBC allows for an IO option for a subset of patients with metastatic breast cancer. Outside of this limited indication, the opportunity for many patients to receive treatment with an IO agent has been in the setting of a clinical trial. Given the great enthusiasm for IO agents in general for the treatment of cancer and the promise of durable responses for some patients, we observed high enrollment of patients with metastatic breast cancer in phase I clinical trials evaluating IO agents at our site.
The purpose of this study was to evaluate clinical outcomes for patients with metastatic breast cancer who were treated in phase I clinical trials containing at least one IO agent at the University of Colorado Cancer Center. We included patients with all breast cancer subtypes who were treated with many different types of IO agents ranging from PD-1/PD-L1-inhibitors to cancer vaccines. While each phase I trial enrolled a small number of patients with metastatic breast cancer, we sought to combine these patients into one dataset to explore outcomes for IO agents in a phase I breast cancer population.
We performed a retrospective analysis using a database of patients from the electronic medical record system (EMRS) with MBC who received treatment with IO agents in phase I/Ib clinical trials at the University of Colorado Hospital from January 1, 2012 – July 1, 2018. All data was stored in a secure online database and the study was performed in accordance with local IRB guidelines. Phase I trials included all protocols that studied single agent or multi-agent investigational drugs that had phase I or phase Ib in the title. For patients in phase Ib/II trials, only patients enrolled in the phase Ib portion of the study were included for analysis. Patients were included in the study if they received an agent considered to directly target or modulate immune cells or immune cell signaling (an IO agent).
Patient characteristics including age, sex, presence of metastatic disease at diagnosis, number of sites of metastases, lines of prior systemic therapy, HR and HER2 receptor status, Eastern Cooperative Oncology Group Performance Status (ECOG PS), radiation within 30 days of IO and mean lab values were collected via chart review using the EMRS. HR and HER2 receptor status was based on local pathology report also found in the EMRS. Other data collected included: time of treatment discontinuation, disease progression and death. We did not collect PD-L1 status as this was not uniformly performed for all patients with MBC during the time period of this study at our institution.
Investigational treatments were administered at the University of Colorado Hospital as part of a clinical trial that received institutional review board (IRB) approval. All patients provided written informed consent prior to enrollment in these phase I clinical trials.
Cohort characteristics were summarized using counts with percentages for categorical variables and with the mean with quartiles for continuous variables. The association between cohort characteristics and progression-free survival (PFS) was evaluated with the Wilcoxon rank-sum test for continuous variables and the Fisher Exact test for categorical variables due to low cell counts. The Wilcoxon rank-sum test was chosen to account for the non-normal distribution of the continuous variables.
PFS was defined as the time from study enrollment to the date of discontinuation for progressive disease, initiation of a new anti-cancer therapy, or death. Overall survival (OS) was defined as the time from study enrollment to the date of death. Patients lost to follow-up were censored at the last follow-up date. For patients who remained on study, the date of analysis (May 1st, 2019) was used to censor the patient outcomes. The median number of months for OS and PFS were calculated using the Kaplan-Meier method with p-values determined by log-rank test. p-values were reported based on a null hypothesis of no difference against a two-sided alternative. Analyses were performed using SAS 9.4 (SAS Institute; Cary, NC).
A total of 43 patients with MBC were treated with a wide range of IO agents in phase I/Ib clinical trials at the University of Colorado Hospital during the period of our study. The average age was 53 years (range 33-71) and all patients were female (Table 1). ECOG PS was 0 in 53.5% of patients and 1 in 46.5% of patients. Most patients had 3 or more sites of metastasis (51.6%). On average, patients received two prior lines of chemotherapy (range 0-7) in the metastatic setting. Most patients had HR-positive/HER2-negative breast cancer (55.8%), followed by TNBC (39.5%) and HER2-positive disease (4.7%). In the phase I/Ib clinical trials, 72.1% of patients received single or combination immunotherapy and 27.9% received an IO agent plus chemotherapy.
Patients with HR-positive/HER2-negative breast cancer all received hormonal therapy prior to enrollment in the phase I/Ib clinical trials (Supplemental Table 1a). The most common prior therapy administered in the HR-positive/HER2-negative group, included capecitabine (45.8%) and everolimus (45.8%). Around one-fifth (20.8%) of patients with HR-positive/HER2-negative cancers received a cyclin-dependent kinase (CDK) 4/6 inhibitor prior to enrollment in phase I/Ib clinical trials. A little less than half (44.4%) of HR-positive/HER2-negative patients received a CDK 4/6 inhibitor and one patient (5.6%) received alpelisib following progression on phase I/Ib clinical trials (Supplemental Table 1b). In TNBC, the most common prior therapy administered in any setting was carboplatin with gemcitabine (64.7%) and doxorubicin, cyclophosphamide, paclitaxel/docetaxel (47.1%) (Supplemental Table 1c). Around one-quarter (23.5%) of patients with TNBC received sacituzumab govitecan prior to enrollment in phase I/Ib trials. The most common therapy received post-progression in TNBC was eribulin (28.6%) (Supplemental Table 1d). One patient (7.1%) with TNBC received sacituzumab govitecan following progression. Prior therapies received in HER2-positive cancers are listed in Supplemental Table 1e.
In the phase I studies included in this analysis, patients were treated with PD-L1/PD-1 inhibitors without chemotherapy (N=12, 27.9%), IO agents other than PD-L1/PD-1 inhibitors without chemotherapy (N=19, 44.2%) or any IO agent plus chemotherapy (N= 12, 27.9%) (Figure 1). Patients treated with PD-L1/PD-1 inhibitors without chemotherapy also received other agents targeting vascular endothelial growth factor (VEGF), indoleamine 2,3-dioxygenase 1 (IDO), OX40, CD38, and T-cell immunoreceptor with Ig and ITIM domains (TIGIT). Trials containing IO agents other than PD-L1/PD-1 inhibitors without chemotherapy included agents targeting IL-10 inhibitor, toll-like receptor 9 (TLR9) agonist and cancer vaccines. Trials with an IO agent in combination with chemotherapy included agents targeting PD-L1, cancer vaccines, nab-paclitaxel, cyclophosphamide and FOLFOX chemotherapy.
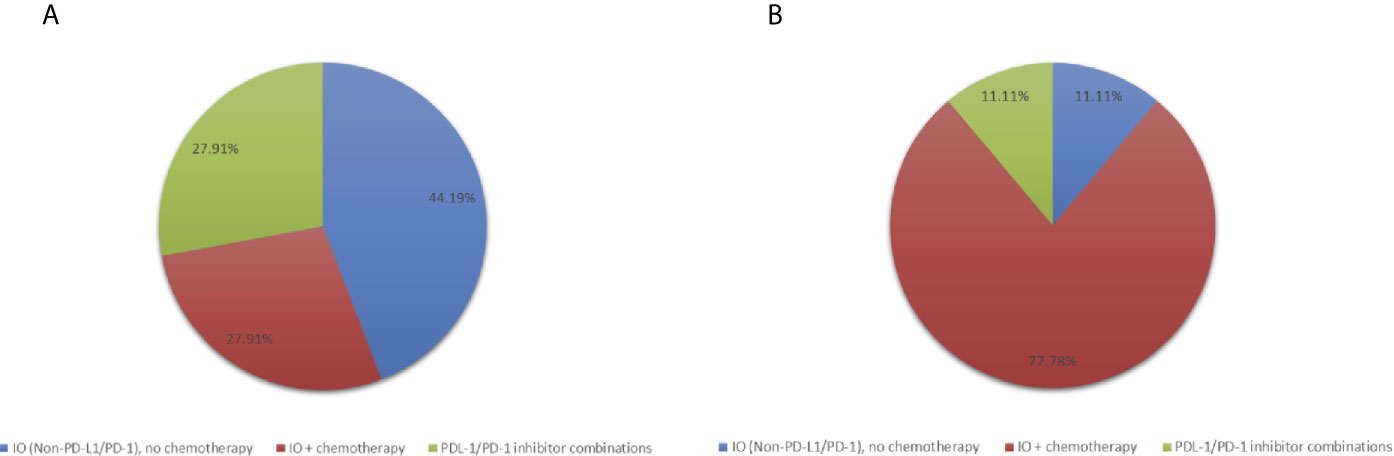
Figure 1 Pie-charts showing (A) Treatment distribution in all patients (N=43) (B) Treatment distribution in patients with PFS ≥ 6 months (N=9).
The median PFS and OS for all patients with MBC enrolled in phase I clinical trials including any IO agent was 2.3 months (95% CI, 2.07-2.60) and 12.1 months (95% CI, 8.35-14.27), respectively (Figure 2). Patients who received an IO agent plus chemotherapy had an improved PFS (5.9 months [95% CI, 2.60-10.45] vs. 2.1 months [95% CI, 1.55-2.30], p<0.001) and OS (18.4 months [95% CI, 11.54-28.60] vs. 9.5 months [95% CI, 5.39-13.84] p=0.015) compared to those who received an IO agent without chemotherapy (Table 2, Figure 3). In subgroup analysis for patients with HR-positive/HER2-negative breast cancer, median PFS was prolonged in patients treated with IO plus chemotherapy compared to IO alone (5.6 months [95% CI, 2.6-8.1] vs. 2.2 months [95% CI, 2.0-2.4], p = 0.0096) (Figure 3). There was also a trend towards improved OS in these patients (17.2 months [95% CI, 11.5-31.0] vs. 11.0 months [95% CI, 5.4-14.8], p= 0.276). Similar findings were observed in patients with TNBC with improved median PFS (10.5 months [95% CI, 2.5- NE (Not Estimable)] vs. 1.8 months [95% CI, 0.6-2.5], p=0.008) and OS (24.2 months [95% CI, 6.6-NE] vs. 6.0 months [95% CI, 0.7-14.4], p=0.0193) in patients treated with IO plus chemotherapy versus IO alone (Figure 3).
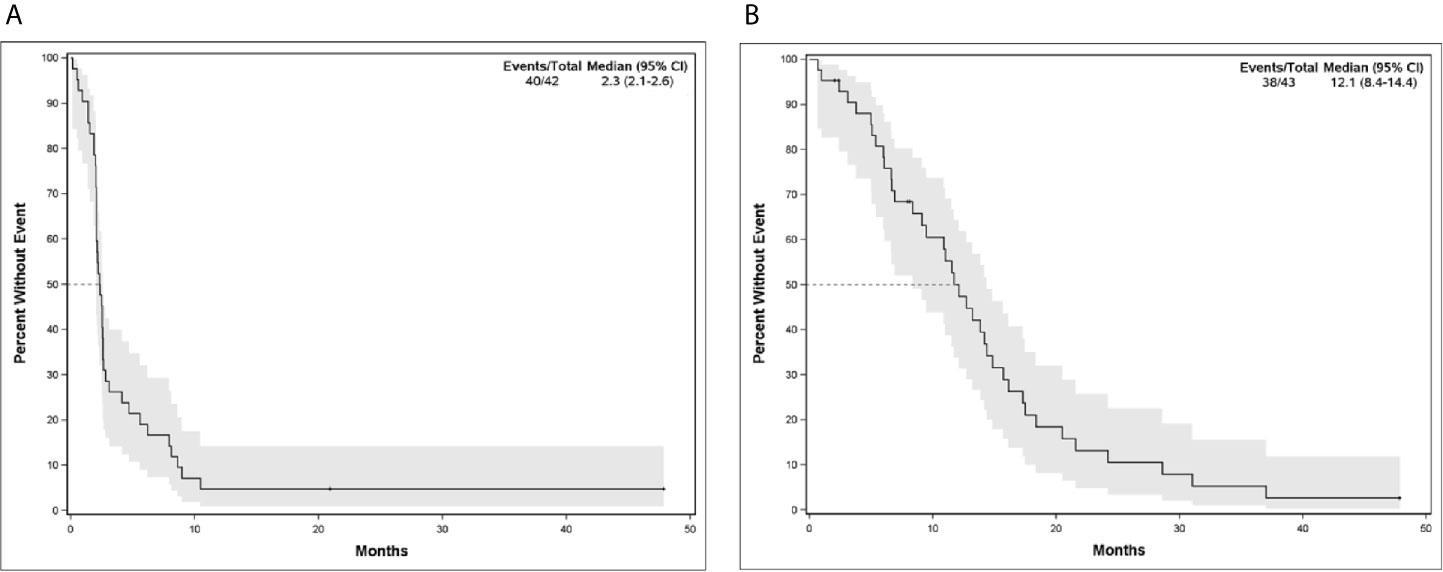
Figure 2 (A) Progression-Free Survival and (B) Overall Survival for Patients with Metastatic Breast Cancer in Phase I/Ib Clinical Trials Treated with IO Agents.

Table 2 Progression-Free Survival (PFS) and Overall Survival (OS) for Patients Who Received IO Plus Chemotherapy Compared to Patients Who Received IO Only.
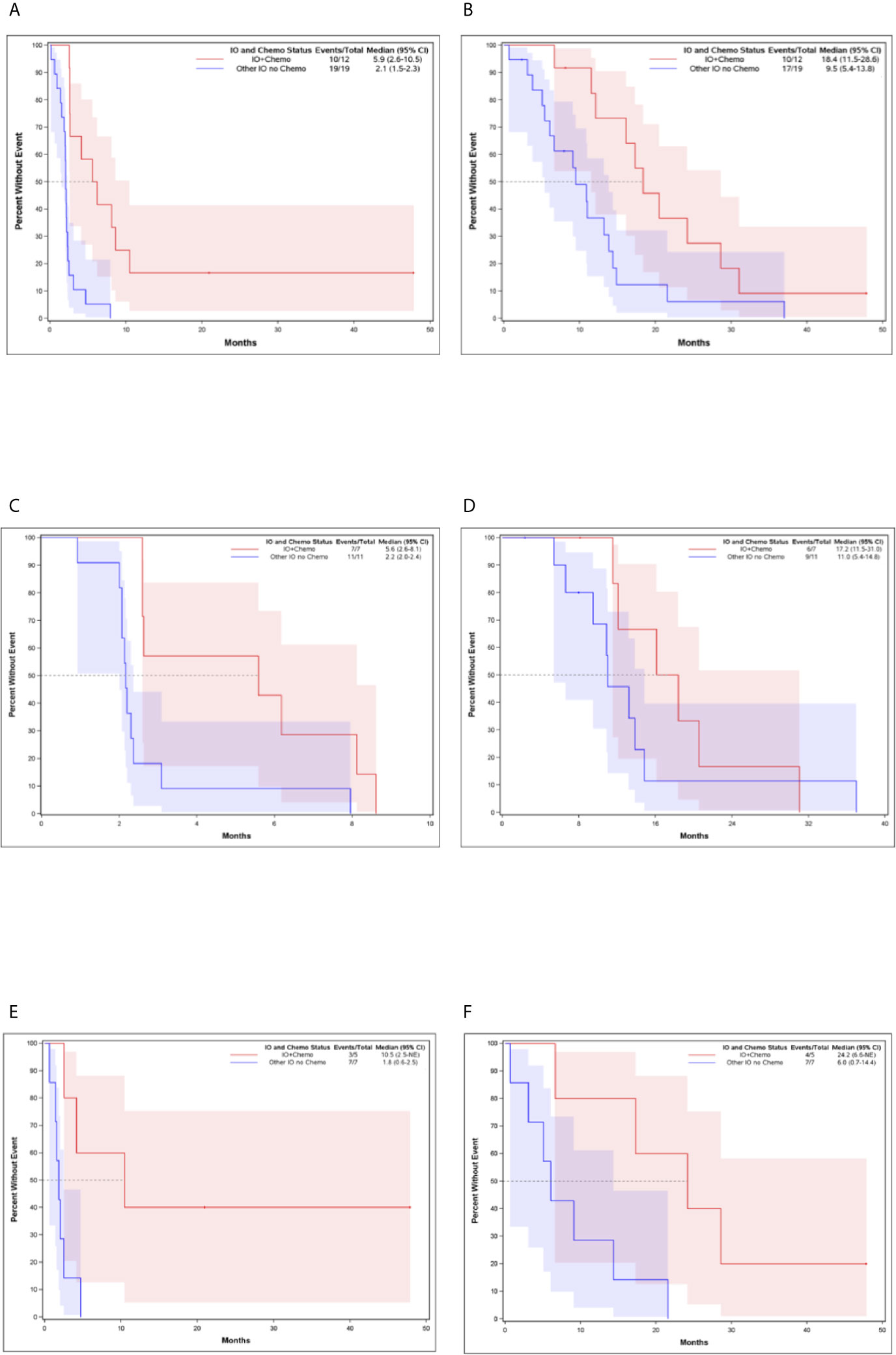
Figure 3 Kaplan-Meier Curves for Patients Who Received IO and Chemotherapy Compared to Patients Who Received IO Without Chemotherapy (A) Progression-Free Survival for all patients (B) Overall Survival for all patients (C) Progression-Free Survival for HR-positive, HER-2 negative breast cancer (D) Overall Survival for HR-positive, HER-2 negative breast cancer (E) Progression-Free Survival for TNBC (F) Overall Survival for TNBC.
We identified 9 patients (20.9%) with PFS ≥ 6 months which we considered to be consistent with clinical benefit (Table 1). Of these, 5 had HR-positive/HER2-negative breast cancer and 4 patients had TNBC. Patients with PFS ≥ 6 months, were treated with IO plus chemotherapy (N=7, 77.8%) and IO alone (N=2, 22.2%) (Table 1, Figure 1). Patients with PFS ≥ 6 months were more likely to receive an IO agent plus chemotherapy compared to those with PFS < 6 months (77.8% vs. 14.7%). No significant differences in prior lines of therapy, lymphocyte count, alkaline phosphatase, or lactate dehydrogenase (LDH) were identified between patients with PFS < 6 months and ≥ 6 months.
Five patients (11.6%) had PFS ≥ 9 months (range 9 months to >36 months) which we considered to be consistent with durable response. All but one of these patients were treated with IO plus chemotherapy and four of the five patients had TNBC. The best response observed in our study was in a 59-year-old woman with TNBC metastatic to her chest wall, lymph nodes and lungs who was treated with anti-PD-L1 and chemotherapy in the second-line setting. Chemotherapy was discontinued after 4 cycles due to neuropathy and she continued on single agent anti-PD-L1 for another 11 cycles before developing immune-mediated pneumonitis requiring discontinuation of immunotherapy. She had a complete clinical response to therapy and remains with no evidence of disease 3.5 years later.
Our study looked at clinical outcomes in patients with previously-treated metastatic breast cancer treated in phase I clinical trials that included an IO agent. We included patients with all breast cancer subtypes treated with many different IO agents targeting PD-1/PD-L1, but also other immune checkpoints and cancer vaccines. Patients were previously treated with an average of 2 prior lines of chemotherapy in the metastatic setting, approximately 20% of patients with HR-positive/HER2-negative disease previously received CDK 4/6 inhibitors and 23.5% of patients with TNBC received prior sacituzumab govitecan. Our study demonstrates that regardless of breast cancer subtype or specific IO target, patients with metastatic breast cancer (TNBC or endocrine-resistant HR-positive/HER2-negative) treated with combinations of IO plus chemotherapy had prolonged PFS and OS compared to patients treated with IO agents alone. Our study found limited efficacy for IO agents administered without chemotherapy, including novel immune checkpoint inhibitor combinations, in patients with previously-treated metastatic breast cancer.
In our study, the median PFS for all patients with previously-treated metastatic breast cancer who received an IO agent in a phase I clinical trial was a modest 2.3 months. This is consistent with other reports of outcomes for similar patients treated in phase I trials (23). However, a unique finding of our study looking specifically at patients receiving IO agents was the observation of durable responses (PFS ≥ 9 months) in 11.6% of patients including one patient who experienced a durable remission lasting many years after stopping therapy for toxicity. Additionally, 20.9% of patients had PFS ≥ 6 months consistent with clinical benefit. Durable responses to immunotherapy observed in our study are consistent with what has been observed in other larger trials of IO agents in breast cancer and other solid tumors where durable remissions can occur even in patients with widespread metastatic disease (24–26).
The majority of patients in our study who experienced durable long-term responses (PFS > 9 months) were patients with metastatic TNBC treated with IO plus chemotherapy consistent with the now proven benefit of FDA-approved regimens in PD-L1-positive metastatic TNBC. Notably, the efficacy of the combination of atezolizumab and nab-paclitaxel in patients with PD-L1-positive TNBC was confirmed in the phase III first-line Impassion130 trial resulting in FDA-approval following the observation of preliminary efficacy in a Phase Ib trial including previously-treated patients (5, 26). Despite our patients being somewhat heavily pretreated, a subset of patients with TNBC still derived long-term benefit from IO + chemotherapy when treated in a phase I clinical trial setting.
There were patients in our study with metastatic HR-positive/HER2-negative breast cancer, resistant to endocrine therapy, that derived benefit from IO plus chemotherapy including one patient with a durable response (PFS > 9 months) and 5 patients with PFS > 6 months. The efficacy of single agent PD-1/PD-L1-inhibitors in HR-positive HER2-negative breast cancer is modest with response rates lower than in TNBC (16, 17, 19, 20, 27, 28). Our results support the many ongoing clinical trials of IO agents in combination with chemotherapy in patients with endocrine-resistant HR-positive/HER2-negative breast cancer (22).
Clinical benefit in our study was greater in patients treated with IO plus chemotherapy and this finding was observed in patients with both endocrine-resistant HR-positive/HER2- breast cancer and TNBC which is also consistent with other studies in breast cancer demonstrating modest response rates with IO agents alone (16, 17, 19, 20, 26, 29, 30). Patients in our study with HR-positive/HER2-negative breast cancers, had a median PFS of 5.6 vs. 2.2 months (p=0.0096) in those treated with IO plus chemotherapy compared to IO monotherapy. OS was also improved however this result was not statistically significant.
There are few studies which have examined IO agents in combination with chemotherapy in a metastatic HR-positive/HER2-negative population. Interestingly, a recently released study examining survival of HR-positive/HER2-negative MBC patients treated with eribulin with or without pembrolizumab did not find improvement in OS or PFS in the IO plus chemotherapy group, which differs from our findings (31). In the I-SPY2 trial, the combination of pembrolizumab with chemotherapy led to a more than doubling of the pCR rate in patients with early stage HR-positive/HER2-negative cancers who had a MammaPrint that was not in the low risk range (21). The limited efficacy of IO monotherapy in HR-positive/HER2-negative breast cancer has been hypothesized to be potentially related to lower PD-L1 expression, tumor-infiltrating lymphocytes (TILs) and tumor mutation burden (TMB) in this disease subset (9, 31–33). Current data suggest that the addition of chemotherapy to IO agents may have multiple favorable effects including stimulation of the immune system by release of tumor neoantigens and recruitment of antigen-presenting cells (22, 34). Moreover, IO plus chemotherapy combination may delay the development of resistance to treatment (35).
Our study relays the importance of phase I clinical trials, often thought as a last resort for patients with advanced malignancy. Enrollment in phase I clinical trials remains a viable option for select patients with previously-treated metastatic breast cancer (23). Of the patients examined, 11.6% had durable response and one patient with metastatic TNBC remains disease free after 3.5 years. It is estimated that only 3-5% of United States adult cancer patients are enrolled in clinical trials (36). However, when comparing breast cancer (BC) patients to the general population, BC patients appear to obtain clinical benefit from phase I therapies with similar toxicity (36). As a result of phase I trials, atezolizumab with nab-paclitaxel is FDA approved for metastatic TNBC as is tucatinib in the treatment of HER2-positive MBC (5, 26, 37, 38). Phase I trials are important for discovering promising therapies and should continue to be utilized.
While our study showed benefit of IO plus chemotherapy in a metastatic TNBC and HR-positive population, there were several limitations. Limitations to our study included the retrospective nature of the analysis and our inability to include PD-L1-expression as a variable. We included patients treated with a diverse range of IO agents making our population heterogeneous and patients were all treated at a single academic center. There were overall very modest patient numbers and very few patients with HER2-positive breast cancer were treated in these studies. Another limitation to our study was that only a fifth of patients received prior therapy with CDK 4/6 inhibitors. As CDK 4/6 inhibitors are standard first line therapies, our study may not be able to be extrapolated to patients who did receive this therapy prior to IO.
The success of the combination of IO plus chemotherapy in TNBC highlights the potential for activity of new therapies in early phase clinical trials in carefully selected patients. Our study demonstrates that the benefit derived from novel IO agents is not limited to a TNBC population. Despite these benefits, larger, multi-center trials are needed in order to better understand the use of IO agents in all breast cancer subtypes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Colorado Multiple Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
AS, JD: manuscript writing and submission. AS, JD, JK, JW: material preparation and data collection. AN, DG: statistical analysis and figure development. JD, JK, VB, PK: study review and monitoring. All authors contributed to the article and approved the submitted version.
This work was supported by the Women’s Cancer Developmental Therapeutics Program at the University of Colorado Cancer Center, Internal Medicine Resident Research Program, University of Colorado School of Medicine, NIH/NCI through 5K23CA172691 (PI Diamond), P30CA046934 (UCCC CCSG), and under Ruth L. Kirschstein National Research Service Award T32CA236734-01 (Kagihara).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to acknowledge the Women’s Cancer Developmental Therapeutics Program at the University of Colorado Cancer Center and the Internal Medicine Resident Research Program, University of Colorado School of Medicine.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.640690/full#supplementary-material
1. National Cancer Institute Surveillance E, End Results Program. Cancer Stat Facts: Female Breast Cancer 2020. Available at: https://seer.cancer.gov/statfacts/html/breast.html.
2. Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol (2019) 30(2):219–35. doi: 10.1093/annonc/mdy551
3. Weber JS. Immuno-oncology comes of age-introduction. Semin Oncol (2014) 41(Suppl 5):S1–2. doi: 10.1053/j.seminoncol.2014.09.002
4. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science (2018) 359(6382):1350–5. doi: 10.1126/science.aar4060
5. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med (2018) 379(22):2108–21. doi: 10.1056/NEJMoa1809615
6. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im S-A, Yusof MM, et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol (2020) 38(15_suppl):1000–. doi: 10.1200/JCO.2020.38.15_suppl.1000
7. Stanton SE, Adams S, Disis ML. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol (2016) 2(10):1354–60. doi: 10.1001/jamaoncol.2016.1061
8. Ahn S, Chung YR, Seo AN, Kim M, Woo JW, Park SY. Changes and prognostic values of tumor-infiltrating lymphocyte subsets after primary systemic therapy in breast cancer. PloS One (2020) 15(5):e0233037. doi: 10.1371/journal.pone.0233037
9. Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol (2018) 19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X
10. Vikas P, Borcherding N, Zhang W. The clinical promise of immunotherapy in triple-negative breast cancer. Cancer Manag Res (2018) 10:6823–33. doi: 10.2147/CMAR.S185176
11. Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol (2013) 31(7):860–7. doi: 10.1200/JCO.2011.41.0902
12. Bertucci F, Gonçalves A. Immunotherapy in Breast Cancer: the Emerging Role of PD-1 and PD-L1. Curr Oncol Rep (2017) 19(10):64. doi: 10.1007/s11912-017-0627-0
13. Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget (2015) 6(7):5449–64. doi: 10.18632/oncotarget.3216
14. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med (2020) 382(9):810–21. doi: 10.1056/NEJMoa1910549
15. Kurozumi S, Inoue K, Matsumoto H, Fujii T, Horiguchi J, Oyama T, et al. Clinicopathological values of PD-L1 expression in HER2-positive breast cancer. Sci Rep (2019) 9(1):16662. doi: 10.1038/s41598-019-52944-6
16. Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol (2019) 30(3):397–404. doi: 10.1093/annonc/mdy517
17. Adams S, Loi S, Toppmeyer D, Cescon DW, De Laurentiis M, Nanda R, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol (2019) 30(3):405–11. doi: 10.1093/annonc/mdy518
18. Keenan TE, Tolaney SM. Role of Immunotherapy in Triple-Negative Breast Cancer. J Natl Compr Canc Netw (2020) 18(4):479–89. doi: 10.6004/jnccn.2020.7554
19. Rugo HS, Delord JP, Im SA, Ott PA, Piha-Paul SA, Bedard PL, et al. Safety and Antitumor Activity of Pembrolizumab in Patients with Estrogen Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer. Clin Cancer Res (2018) 24(12):2804–11. doi: 10.1158/1078-0432.CCR-17-3452
20. Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero-Torres A, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat (2018) 167(3):671–86. doi: 10.1007/s10549-017-4537-5
21. Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol (2020) 6(5):676–84. doi: 10.1001/jamaoncol.2019.6650
22. Adams S, Gatti-Mays ME, Kalinsky K, Korde LA, Sharon E, Amiri-Kordestani L, et al. Current Landscape of Immunotherapy in Breast Cancer: A Review. JAMA Oncol (2019) 5(8):1205–14. doi: 10.1001/jamaoncol.2018.7147
23. Weiss JA, Nicklawsky A, Kagihara JA, Gao D, Fisher C, Elias A, et al. Clinical outcomes of breast cancer patients treated in phase I clinical trials at University of Colorado Cancer Center. Cancer Med (2020) 9(23):8801–8. doi: 10.1002/cam4.3487
24. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
25. Ascierto PA, Long GV, Robert C, Brady B, Dutriaux C, Di Giacomo AM, et al. Survival Outcomes in Patients With Previously Untreated BRAF Wild-Type Advanced Melanoma Treated With Nivolumab Therapy: Three-Year Follow-up of a Randomized Phase 3 Trial. JAMA Oncol (2019) 5(2):187–94. doi: 10.1001/jamaoncol.2018.4514
26. Adams S, Diamond JR, Hamilton E, Pohlmann PR, Tolaney SM, Chang CW, et al. Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer With 2-Year Survival Follow-up: A Phase 1b Clinical Trial. JAMA Oncol (2019) 5(3):334–42. doi: 10.1001/jamaoncol.2018.5152
27. Schmid P, Cruz C, Braiteh FS, Eder JP, Tolaney S, Kuter I, et al. Abstract 2986: Atezolizumab in metastatic TNBC (mTNBC): Long-term clinical outcomes and biomarker analyses. Cancer Res (2017) 77(13 Supplement):2986–. doi: 10.1158/1538-7445.AM2017-2986
28. Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol (2016) 34(21):2460–7. doi: 10.1200/JCO.2015.64.8931
29. Merck. Update on Phase 3 KEYNOTE-119 Study of KEYTRUDA® (pembrolizumab) Monotherapy in Previously-Treated Patients with Metastatic Triple-Negative Breast Cancer May 20th. Merck (2019). Available at: https://www.merck.com/news/merck-provides-update-on-phase-3-keynote-119-study-of-keytruda-pembrolizumab-monotherapy-in-previously-treated-patients-with-metastatic-triple-negative-breast-cancer/.
30. Tolaney S, Kalinsky K, Kaklamani V, Savulsky C, Olivo M, Aktan G, et al. Abstract PD6-13: Phase 1b/2 study to evaluate eribulin mesylate in combination with pembrolizumab in patients with metastatic triple-negative breast cancer. Cancer Res (2018) 78(4 Supplement):PD6–13-PD6-. doi: 10.1158/1538-7445.SABCS17-PD6-13
31. Tolaney SM, Barroso-Sousa R, Keenan T, Li T, Trippa L, Vaz-Luis I, et al. Effect of Eribulin With or Without Pembrolizumab on Progression-Free Survival for Patients With Hormone Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer: A Randomized Clinical Trial. JAMA Oncol (2020) 6(10):1–8. doi: 10.1001/jamaoncol.2020.3524
32. Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res (2014) 2(4):361–70. doi: 10.1158/2326-6066.CIR-13-0127
33. Luen S, Virassamy B, Savas P, Salgado R, Loi S. The genomic landscape of breast cancer and its interaction with host immunity. Breast (2016) 29:241–50. doi: 10.1016/j.breast.2016.07.015
34. Mina LA, Lim S, Bahadur SW, Firoz AT. Immunotherapy for the Treatment of Breast Cancer: Emerging New Data. Breast Cancer (Dove Med Press) (2019) 11:321–8. doi: 10.2147/BCTT.S184710
35. Zhu H, Du C, Yuan M, Fu P, He Q, Yang B, et al. PD-1/PD-L1 counterattack alliance: multiple strategies for treating triple-negative breast cancer. Drug Discovery Today (2020) 25(9):1762–71. doi: 10.1016/j.drudis.2020.07.006
36. Lynce F, Blackburn MJ, Cai L, Wang H, Rubinstein L, Harris P, et al. Characteristics and outcomes of breast cancer patients enrolled in the National Cancer Institute Cancer Therapy Evaluation Program sponsored phase I clinical trials. Breast Cancer Res Treat (2018) 168(1):35–41. doi: 10.1007/s10549-017-4563-3
37. Murthy R, Borges VF, Conlin A, Chaves J, Chamberlain M, Gray T, et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. Lancet Oncol (2018) 19(7):880–8. doi: 10.1016/S1470-2045(18)30256-0
Keywords: immunotherapy, metastatic breast cancer, PD-L1 inhibitors, phase I clinical trials, PD-1 inhibitors
Citation: Schreiber AR, Kagihara JA, Weiss JA, Nicklawsky A, Gao D, Borges VF, Kabos P and Diamond JR (2021) Clinical Outcomes for Patients With Metastatic Breast Cancer Treated With Immunotherapy Agents in Phase I Clinical Trials. Front. Oncol. 11:640690. doi: 10.3389/fonc.2021.640690
Received: 11 December 2020; Accepted: 01 March 2021;
Published: 17 March 2021.
Edited by:
Basel K. Al-Ramadi, United Arab Emirates University, United Arab EmiratesReviewed by:
Deniz Can Guven, Hacettepe University, TurkeyCopyright © 2021 Schreiber, Kagihara, Weiss, Nicklawsky, Gao, Borges, Kabos and Diamond. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer R. Diamond, amVubmlmZXIuZGlhbW9uZEBjdWFuc2NodXR6LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.