Overall Survival in Heart Disease–Related Death in Non-Small Cell Lung Cancer Patients: Nonimmunotherapy Versus Immunotherapy Era: Population-Based Study
- 1School of Automation, Southeast University, Nanjing, China
- 2Jiangsu Chunyu Education Group Co., Ltd., Nanjing, China
by Safi, M., Kanesvaran, R., Alradhi, M., Al-Danakh, A., Ping, F., Al-Sabai, N., et al. (2020). Front. Oncol. 10:572380. doi: 10.3389/fonc.2020.572380
Introduction
Immunotherapy has been advised for advanced-stage non-small-cell lung cancer (NSCLC) patients; however, it could lead to heart disease-related death (HDD) due to cardiovascular toxicity. A recent study (1) compared the overall survival of HDD patients in the immunotherapy era with the non-immunotherapy era in the Surveillance, Epidemiology, and End Results (SEER) dataset. Specifically, advanced-stage NSCLC death cases in 2015 and 2007 were used as a study cohort. By comparing the death cases, it concluded that the HDD risk is significantly higher in the immunotherapy era, with a hazard ratio (HR) of 1.31, 95% CI: 1.099–1.570. Pericardial effusion is sometimes reported in NSCLC patients who receive immunotherapy (2). In very rare cases, immunotherapy could lead to autoimmune myocarditis (3). However, we believe that the HDD risk is overestimated in the commented study. We argue that using the death cases only could lead to potential issues; for example, the differences in age distribution and life span may mislead the conclusions. In this commentary, we reconsidered this problem; however, we found that the HDD risk in the immunotherapy era is actually lower than that in the counterpart by considering the whole advanced-stage NSCLC patient cohort.
Methods and Results
We would argue that the whole advanced-stage NSCLC patients (referred to as a “cohort”) should be considered in the evaluation, rather than focusing on the subtle pattern among cases of confirmed death. For this, the following are considered:
1. There are 243,637 incidences of advanced-stage (IIIB and IV) NSCLC collected in the SEER dataset from 2000 to 2017.
2. Kaplan–Meier (K-M) analysis for HDD risk in the cohort is carried out. Moreover, the competing risk model is also considered in order to avoid overestimating the risk (4).
3. Data on the underlying cause of death in WONDER (5) are collected from 1999 to 2018. We compared the HDD risk in the cohort with that in the general public.
Survival Analyses
In accordance with the previous study, we compared the HDD event between 2007 and 2015; the results of the K-M analysis and competing risk model are given in Figures 1A,B, respectively. At 20 months, the HDD survival ratios of the immunotherapy and non-immunotherapy groups in the K-M analysis are 97.79 and 97.23%, respectively. In the competing risk model, the estimated incidences of HDD are 1.34 and 1.57% in the two groups. From these results, we demonstrated that the HDD risk in the immunotherapy group is lower than that in the counterpart, with a relative hazard ratio of 0.8535 and 0.7978 from K-M and the competing risk model, respectively.
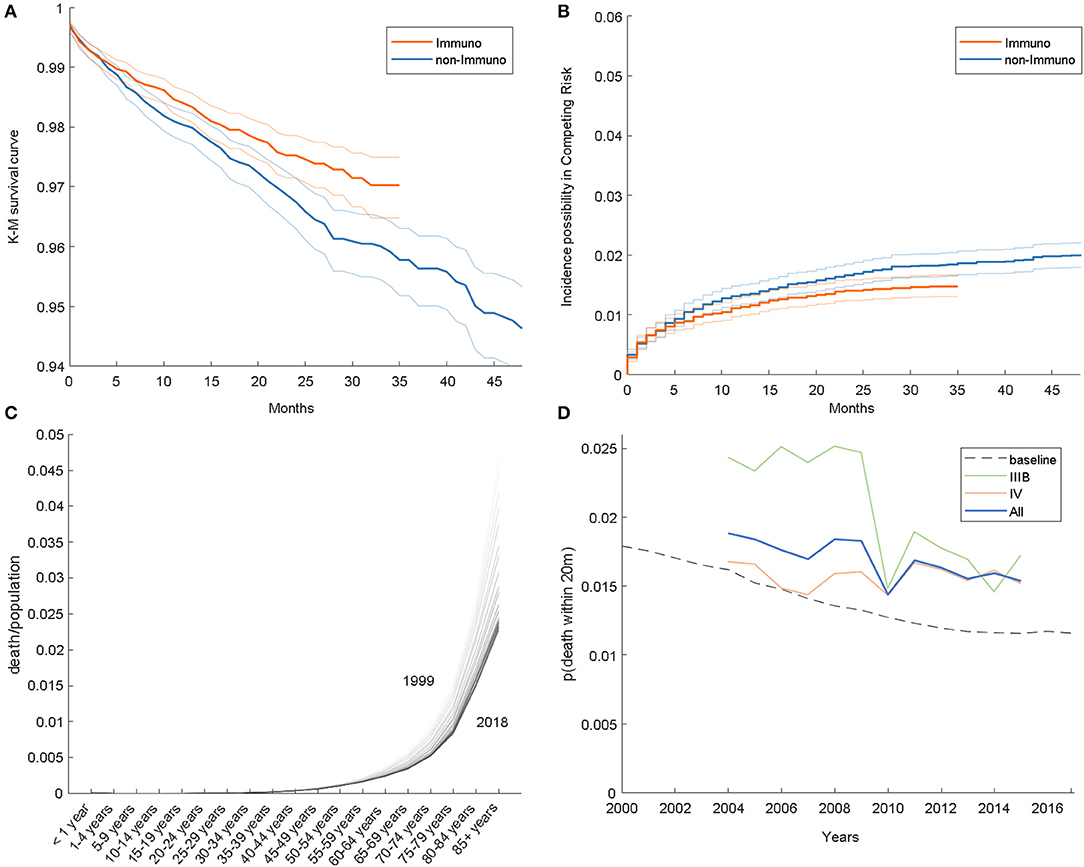
Figure 1. (A) Kaplan–Meier survival curves. (B) Estimated incidence possibility in the competing risk model. (C) Age-specific HDD risk in the general population. (D) HDD event frequency in the SEER dataset.
Comparison With the General Population
It would be interesting to know whether lung cancer itself is correlated with heart disease risk. For this, we calculated the frequency of the event (heart disease-related death within 20 months after NSCLC diagnosis), shown as a solid curve in Figure 1D; the risk in 2015 is actually lower than in 2007, with a relative ratio of 0.718, 1.055, and 0.906 for IIIB, IV, and both stages, respectively. Overall, the frequency of HDD is decreasing among years, which is consistent with Figures 1A,B.
Furthermore, we analyzed HDD data of the general population from WONDER, illustrated in Figure 1C. As shown, the mortality rises as the age becomes older; on the other hand, the overall curve is descending over time. The age distribution of the cohort also varies among years; for example, the cohort is composed of more individuals of age above 55 in 2015 than in 2007 (89.1 vs. 91.9%). To consider these aspects jointly, we estimated a risk baseline for the cohort, which is synthesized by weighting the general HDD risk by the age distribution. The baseline is shown as a dashed line in Figure 1D. As shown, the HDD risk in advanced-stage NSCLC patients is higher than that in the general population. A similar phenomenon was also reported in other types of cancer, for example, endometrial cancer (6).
Conclusion
In this commentary, we reconsidered the HDD risk in advanced-stage NSCLC patients who receive immunotherapy. From the perspective of K-M analysis, competing risk model, and frequency, we found that the HDD risk is decreasing among years. Based on these findings, we argue that there is no sufficient evidence that immunotherapy can lead to a significantly higher HDD risk in NSCLC patients. Nevertheless, the NSCLC patients indeed have a higher HDD risk than the general population, for which further investigation is needed.
Author Contributions
YJ collected and analyzed the data, finished the initial version of the manuscript. CQ finished the survival analysis. SF went through the paper. All the authors participated in the writing and reviewing of the paper.
Conflict of Interest
CQ was employed by Jiangsu Chunyu Education Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the SEER program (https://seer.cancer.gov/) and WONDER database (https://wonder.cdc.gov/) for making the epidemiology and end events record publicly available. For survival analysis, we use lifelines (https://github.com/CamDavidsonPilon/lifelines/), which is an open-source Python library.
References
1. Safi M, Kanesvaran R, Alradhi M, Al-Danakh A, Ping F, Al-Sabai N, et al. Overall survival in heart disease–related death in non-small cell lung cancer patients: nonimmunotherapy versus immunotherapy era: population-based study. Front Oncol. (2020) 10:572380. doi: 10.3389/fonc.2020.572380
2. Canale ML, Camerini A, Casolo G, Lilli A, Bisceglia I, Parrini I, et al. Incidence of pericardial effusion in patients with advanced non-small cell lung cancer receiving immunotherapy. Adv Ther. (2020) 37:3178–84. doi: 10.1007/s12325-020-01386-y
3. Gibson R, Delaune J, Szady A, Markham M. Suspected autoimmune myocarditis and cardiac conduction abnormalities with nivolumab therapy for non-small cell lung cancer. BMJ Case Rep. (2016) 2016:bcr2016216228. doi: 10.1136/bcr-2016-216228
4. van Walraven C, McAlister FA. Competing risk bias was common in Kaplan–Meier risk estimates published in prominent medical journals. J Clin Epidemiol. (2016) 69:170–3.e8. doi: 10.1016/j.jclinepi.2015.07.006
5. WONDER. https://wonder.cdc.gov/.
Keywords: immunotherapy, non-small cell lung cancer, heart diseases, adverse effects, survival analysis
Citation: Jiao Y, Qian C and Fei S (2021) Commentary: Overall Survival in Heart Disease–Related Death in Non-Small Cell Lung Cancer Patients: Nonimmunotherapy Versus Immunotherapy Era: Population-Based Study. Front. Oncol. 11:639042. doi: 10.3389/fonc.2021.639042
Received: 09 December 2020; Accepted: 18 February 2021;
Published: 22 April 2021.
Edited by:
Qing Zhou, Guangdong Provincial People's Hospital Lung Cancer Institute, ChinaReviewed by:
Yu Chen, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandCopyright © 2021 Jiao, Qian and Fei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shumin Fei, c21mZWkmI3gwMDA0MDtzZXUuZWR1LmNu
 Yiping Jiao
Yiping Jiao Chengqi Qian
Chengqi Qian Shumin Fei
Shumin Fei