
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 17 June 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.630837
Intestinal metaplasia refers to the replacement of the differentiated and mature normal mucosal epithelium outside the intestinal tract by the intestinal epithelium. This paper briefly describes the etiology and clinical significance of intestinal metaplasia in Barrett’s esophagus. This article summarizes the impact of intestinal metaplasia on the diagnosis, monitoring, and treatment of Barrett’s esophagus according to different guidelines. We also briefly explore the basis for the endoscopic diagnosis of intestinal metaplasia in Barrett’s esophagus. The identification techniques of goblet cells in Barrett’s esophagus are also elucidated by some scholars. Additionally, we further elaborate on the current treatment methods related to Barrett’s esophagus.
First reported by Norman Barrett, Barrett’s esophagus (BE) can be simply defined as the presence of columnar epithelium in the esophagus (1). Until 1976, Paull et al. (2) classified the presence of columnar epithelium within the esophagus based on histiological subtypes: Gastric fundic type, junctional type, and specialized type with intestinal metaplasia (IM).
IM refers to the replacement of normal mucosal epithelium with the intestinal epithelium outside the intestine, implying the transformation of a well-differentiated and mature tissue into another differentiated mature tissue under abnormal conditions. IM is common in the stomach and esophagus, but it can also occur in the gallbladder, bile ducts, uterus, bladder, pelvis, ureter, and urethra (3–9).
In recent years, there has been much controversy regarding the role of IM in the diagnosis of BE. We hereby summarize the significance of IM and the evidence supporting the fact that the presence of IM is either mandatory or not to establish a diagnosis of BE. IM in BE is often missed, and several other cells can mimic goblet cells. We cover the classification, manifestation and detection rate of IM under different endoscopic techniques, and methods to accurately identify IM in BE based on endoscopic and pathologic findings. Finally, we also discuss the principles regarding the monitoring and management of BE with and without IM.
IM is considered a precancerous lesion and is closely related to the occurrence of cancer. According to the study of Watanabe et al (10), the presence of IM is associated with the degree of severity of the Barrett’s mucosa, regardless of the size of the tumor. Therefore, they speculated that IM might be an epiphenomenon of BE extension rather than a reflection of the tumor’s origin. However, differentiated mature intestinal epithelial cells can acquire additional mutations, then develop into dysplasia and cancer (11). There is also evidence that IM is associated with a higher frequency of cancer-related mutations compared with nongoblet cell metaplasia (12). Similar to the significance of IM in the stomach (13), although there was evidence that tumor cells may not directly originate from goblet cells (11, 14). It has been undoubtedly suggested that the BE’s mucosa is more prone to neoplastic transformation. In the replacement of the normal esophageal stratified squamous epithelium with columnar epithelium, IM is the only type that is clearly prone to malignant transformation (15).
The possible etiologies for BE include the transcommitment of stem cells or progenitor cells, transdifferentiation of differentiated cells, the expansion of residual embryonic cells located at the squamous-columnar junction (SCJ), and the differentiation of circulating bone marrow cells (16–20). It was not until 2017 that Jiang et al. (21) simulated a BE mouse model with real goblet cells. They described a novel transitional columnar epithelium with distinct basal progenitor cells (p63 + KRT5 + KRT7 +) at the SCJ. After the expression of the caudal-type homeobox transcription factor-2 (CDX2), these progenitor cells were differentiated into intestinal-like epithelium, including goblet cells, resulting in BE with IM. Moreover, such epithelium was found to be localized at the subject’s gastroesophageal junction (GEJ). Moreover, Jin et al. (11) proposed the SPEM model of esophageal IM to explain the multiformity of the stem cells involved in esophageal IM. The differentiated cells and stem cells in this model could be transformed into SPEM-like cells (TFF2+ MUC6+); persistent inflammatory injury eventually led to IM, dysplasia, and carcinogenesis of the SPEM.
Replacement of the distal esophageal squamous epithelium by metaplastic columnar epithelium forms the pathological basis for BE. Whether IM must be present or whether the length of the columnar mucosa needs to be larger than 1 cm are the main controversial points. The American Gastroenterological Association (AGA), American College of Gastroenterology (ACG), and European Society of Gastrointestinal Endoscopy (ESGE) all believe that IM is necessary for the diagnosis of BE, while the British Society of Gastroenterology (BSG), Asia-Pacific Working Group (APWG) and Benign Barrett’s and Cancer Taskforce consensus group (BOB CAT) all reckon that IM is not needed for the diagnosis of BE (15, 22–26). However, BOB CAT also stressed that the existence of IM should be noted when diagnosing BE (23) (Table 1).
The main basis supporting the fact that IM is mandatory for the diagnosis of BE includes the following: a large number of population-based cohort studies have shown that the risk of esophageal adenocarcinoma is much lower in patients with columnar metaplasia without IM compared to those with IM (27). Compared with columnar metaplasia without goblet cells, tumorigenesis is most commonly seen in columnar metaplasia with goblet cells (12, 28). In-depth evaluation of esophageal biopsy specimens before EMR and esophageal resection specimens after EMR found that IM was found in the columnar esophagus of all the patients with adenocarcinoma (29). Furthermore, some studies have demonstrated that it might be unwise to diagnose a disease that has a negative impact on insurance status and quality of life in non-IM-CLE (Columnar metaplasia without IM) patients (30, 31). The use of IM as a prerequisite for the diagnosis of BE was conducive to the adoption of a more cost-effective approach to the care of BE patients (26).
Evidence supporting the fact that IM is not necessary for diagnosing BE includes the following: a single endoscopic examination and a small number of biopsy samples were not sufficient to rule out IM (32, 33). In the study of Jankowski et al. (34), all the patients with BE who did not have IM at the beginning of the study developed IM during follow-up. Related retrospective studies have reported that patients with or without IM had a similar risk of developing cancer (35). Studies carried out on columnar mucosa resected endoscopically in early cancer subjects have confirmed that cancer may also occur in non-IM columnar epithelium (36). There was also evidence that columnar epithelium without goblet cells may contain similar molecular abnormalities to columnar epithelium with goblet cells (28, 37–39). Lavery et al. (40) reconstructed the cloned ancestor of EAC and provided direct genetic evidence for the malignant tendency of metaplastic columnar epithelium without goblet cells. This is the first direct demonstration that the clonal expansion and precancerous progression of BE are not limited to the metaplastic columnar epithelium with goblet cells.
With or without IM, it was uncertain whether columnar mucosa with a length smaller than 1 cm at the GEJ would progress to cancer. Current research has also depicted that the rate of carcinogenesis in this group of patients is significantly lower than in those with BE (Columnar mucosa more than 1 cm). Thus, most guidelines explicitly require that the diagnosis of BE must include a columnar mucosa exceeding 1 cm at the GEJ (22, 24–26) (Table 2). Although BOB CAT did not specify that the columnar mucosa at the GEJ must be more than 1 cm to establish a diagnosis of BE. BOB CAT also indicated that an irregular and ≤ 1 cm lesion at the GEJ may be a natural phenomenon (26). Besides, according to a recent prospective multicenter cohort study, patients with BE lesions smaller than 1 cm will not develop high-grade dysplasia (HGD) or esophageal cancer within 5 years of endoscopic examination (41).
In Japan, the 1 cm criteria regarding the morphological changes of BE’s mucous membrane is not mandatory, and such lesions are called ultra-short-segment BE (USSBE). The true meaning of cardiac cancer refers to cancer that occurs in the intestinal metaplastic area of the anatomical cardia or esophagogastric junction (42). By definition, the anatomical position of the cardia and USSBE basically coincided. IM can occur in the cardia and USSBE, and the cardiac mucosa with IM is considered a precancerous lesion of cardiac carcinoma. Over the past few years, with the increasing incidence of cardiac cancer, more attention has been paid to USSBE. Nonetheless, due to the above reasons, the concept of USSBE is not widely used and accepted. In a large sample study in 2016, the annual cancer rate of USSBE was only 0.01% (compared with 0.22% and 0.03% for the long and short segments of BE, respectively) (43). A related etiological investigation also pointed out that BE lesion’s length exceeding 1 cm was not the defining factor (44). However, IM is not required in the definition of USSBE in these studies. If we investigate USSBE with IM exclusively, there may be new results. Monitoring may be crucial for these patients’ prognosis, albeit further research is required to confirm this assumption. Given the high incidence of USSBE in the population, this issue should be taken into account when calculating and comparing the incidence of BE.
Conventional endoscopy has not been satisfactory in the diagnosis of BE, especially in the detection of precancerous BE lesions. Biopsy of BE lesions is routinely performed from four quadrants superior to the GEJ at intervals of 1-2 cm. According to previous studies, only 10–79% of doctors take biopsy specimens according to this protocol. The higher the length of BE lesions, the lower the application rate of the four-quadrant biopsy principle (45–47). Also, obtaining multiple biopsy specimens and pathological interpretation both imply a significant financial burden (48). BE with IM is a precancerous lesion, which has more significant clinical significance (49). Relevant investigations conducted in Japan suggested that similar to the most common site of occurrence of esophageal adenocarcinoma (EAC), IM often appeared in the 0–3 o’clock area (50). This finding indicates that this area should be the focus of attention and that biopsy specimens taken from this area may increase the detection rate of IM. With the advancement of endoscopic techniques, there are more means for endoscopic detection of IM (Table 3, Figure 1).
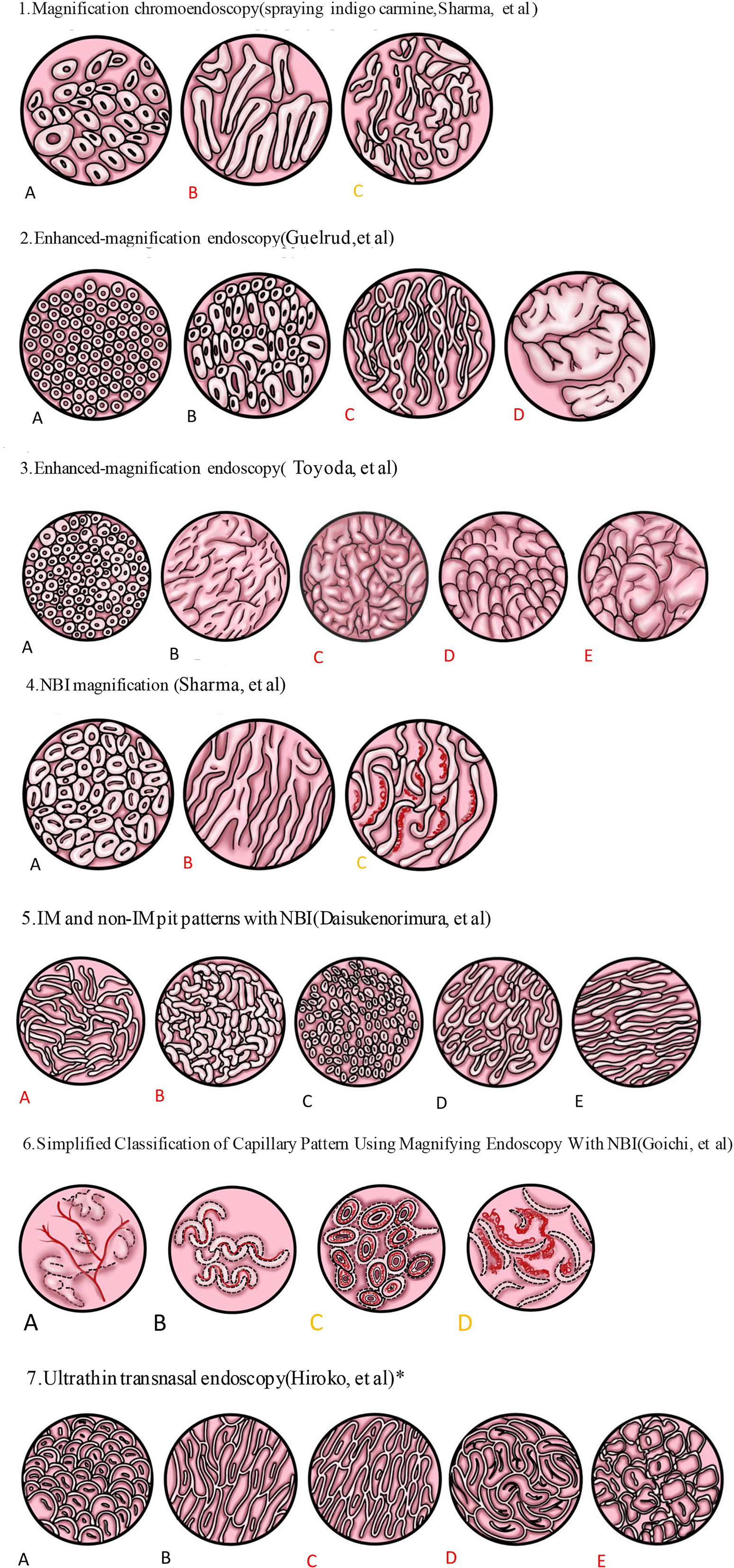
Figure 1 Spraying indigo carmine. (A) Circular pattern: a regularly arranged circular or oval area. (B) Ridge/villous pattern: a regular arrangement of tortuous and thick villi, such as sausages or cerebriform. (C) Irregular/distorted pattern: the villous pattern was obviously distorted and irregular. 2. Enhanced magnifying endoscopy. (A) Pattern I: A regularly arranged pattern of circular dots with round pits. (B) Pattern II: Circular or oval pits of regular shape and arrangement. (C) Pattern III: A regular arrangement of fine villiform appearance with no pits. (D) Pattern IV: The thick villi were curled into cerebriform but arranged regularly. 3. Another classification by enhanced magnifying endoscopy. (A) Small round pits of uniform size and shape (type 1, corpus). (B) Slit and reticular pattern (type 2, cardiac). (C) Gyrus pattern (type 3, IM). (D) Villous pattern (type 3, IM). (E) Mixed gyrus and villous pattern (type 3, IM). 4. NBI images. (A) Circular pattern. (B) Ridge/villous pattern. (C) Irregular and distorted pattern. 5. Another classification by NBI. The pit pattern of BE could be divided into IM and non-IM pit patterns. IM pit patterns included two subtypes: (A) tubular, and (B) villous types. Non-IM pit patterns included three subtypes: (C) round, (D) oval, and (E) straight types. 6. Simplified classification of capillary pattern in BE by ME-NBI. The capillary pattern was divided into two types: type I: (A, B) a branched or rattan-shaped pattern that was clearly shaped and could be tracked smoothly, and type II: (C, D) a curly or spiral pattern whose shape was disordered and could not be fully tracked. All dysplasia regions were type II, but there was no significant difference in intestinal phenotype between type I and type II. 7. Pit pattern of SSBE was divided into closed and open types by ultrathin transnasal endoscopy with ME-NBI. Closed pit patterns included two subtypes: (A) oval or round pattern, and (B) long straight pattern. Open-pit patterns included three subtypes: (C) villous pattern; (D) cerebriform pattern; and (E) irregular pattern. IM was more common in the open type. Black indicated no IM or dysplasia. Red indicated that IM is more likely to be found. Yellow indicated that dysplasia or cancer was more likely to be detected. *indicated an observation of SSBE.
Methylene blue is a dye that can be absorbed by IM cells giving them a blue coloration, whereas the normal esophageal mucosa does not stain with methylene blue. Studies have shown that methylene blue can improve the detection rate of IM, but subsequent multivariate analysis showed that, compared with the traditional four-quadrant biopsy, the methylene blue stain had no obvious advantage in the diagnosis of BE with IM, BE with dysplasia, and even early cancer. Methylene blue also has a carcinogenic effect (48, 49, 58, 59). Three types of mucosal patterns were noted by Sharma et al. (51) within the columnar mucosa after spraying mucicarmine and using high magnification endoscopy: eidged/villous pattern, circular pattern, and irregular/distorted pattern. The sensitivity, specificity, and positive predictive value of the ridge/villous pattern for detecting IM were 97%, 76%, and 92%, respectively. The sensitivity (100% vs 92%) and specificity (100% vs 69%) of long-segment BE were better than those of short-segment BE. However, there is a lack of sufficient research data.
EME is a diagnostic method combining acetic acid with magnifying endoscopy. Guelrud (52) first used EME to study the relationship between various histological types and fine structures of mucous membranes and divided the mucosal image into four types: I, round pits; II, reticular; III, villous; and IV, ridged. They found that the IM corresponding to round pits, reticular, villous, and ridged types were 0%, 11%, 87%, and 100%, respectively. In 2003, Toyoda et al. (53) found that pattern III included both villous and slit like patterns, and a new classification was devised as follows: type 1, small round pits of uniform size and shape (“corpus” type); type 2, slit reticular pattern with horizontally elongated mucosal pits (“cardia” type); and type 3, gyrus, villous, or mixed gyrus-villous patterns (“IM” type). The sensitivity, specificity, and diagnostic accuracy of type 3 in predicting IM were 85.5%, 92.2%, and 90.0%, respectively. Even though the specific mucosal classification is different, a large number of studies have demonstrated that EME can accurately identify IM (52, 53, 60). Eight prospective studies in a recent meta-analysis provided data for the diagnosis of IM (54). The combined sensitivity, specificity, positive likelihood ratio (LR), and negative LR for IM were 96%, 69%, 3.0, and 0.06, respectively. This finding suggested that EME is an adequate screening method for IM, and histological confirmation remains key considering its low specificity.
ME-NBI allows for detailed examination of mucosal morphology without the use of stains (61). ME-NBI is widely used to diagnose and monitor BE, not only by expert endoscopists but also by non-experts (62). Sharma et al. (55) used ME-NBI to observe 51 patients with BE and divided the BE mucosa into ridge/villous, circular, and irregular/distorted patterns. The sensitivity, specificity, and positive predictive value of the ridge/villous pattern for the diagnosis of IM without HGD were 93.5%, 86.7%, and 94.7%, respectively. Norimura et al. (56) classified pits in BE into IM and non-IM pit patterns. The IM pit pattern comprised two subtypes: Tubular and villous types. Meanwhile, the non-IM pit patterns consisted of three subtypes: round, oval, and straight types. Through ME-NBI, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the IM pit pattern for the diagnosis of IM were 92%, 77%, 76%, 92%, and 83%, respectively. The study also delineated that the appearance of light blue crests (LBC) can be an accurate sign to predict IM in BE. The sensitivity, specificity, and accuracy of LBC for IM were 79%, 97%, and 89%, respectively. Electron microscopy revealed brush borders on the metaplastic epithelium extracted from LBC-positive BE lesions. The appearance of LBC in BE may be closely related to the CD10 antigen present at the brush border, which is also expressed within the LBC-positive mucosa. Researchers speculate that the LBC’s morphology can be explained by the difference in the reflectance of the light at the surface of the ciliated tissue structure (63). Hence, if LBC was detected on NBI, we would always find evidence of IM on histology. However, if LBC was not found on NBI, IM could not be excluded. A meta-analysis (57) elucidated that the targeted biopsy performed by NBI had high sensitivity and specificity for IM and HGD per patient and each lesion. Targeted biopsies by NBI have the same IM detection rate as high-definition white light endoscopy under the Seattle protocol, but require fewer biopsies (64). NBI targeted biopsy could detect more atypical areas of hyperplasia, and the normal surface pattern did not include HGD or EAC. Based on this fact, a biopsy could be avoided in these normal areas. Similar results were obtained from the study conducted by Pascarenco et al. (65), which found that the villus type (According to Shama et al.) had higher sensitivity and specificity for the diagnosis of IM.
To establish a simplified classification, Goichi Uno et al. (61) proposed a new capillary pattern (CP) classification based on the shape and microvessel occupied areas. They divided CP into the following two categories: type I, uniform branched or vine-like pattern with a clear shape that is able to be traced smoothly, and type II, coiled or spiral pattern with a nonuniform shape that cannot be traced sufficiently and with increased vascularity. In this study, all the regions of dysplasia were type II, but there was no significant difference in the intestinal phenotype between type I and type II.
The combination of magnifying endoscopy and NBI makes it easier to detect IM. Although the detection rate of IM has not been greatly improved compared with random biopsy under white light, it has greatly reduced the number of biopsies, which also reduces the number of specimens needed for pathological observation, generating a certain financial benefit. Compared to chromoendoscopy, NBI does not require additional staining, and many large-capacity endoscopy centers use it for routine esophageal observations at no additional cost. This finding indicates its application prospects in the diagnosis and monitoring of BE, but it is still necessary to establish a uniform and reliable standard.
In recent years, numerous reports have been published on the application of LCI and BLI in gastrointestinal examinations. Despite the fact that some medical centers have used it to observe the esophagus, there are very few reports on BE. These studies concluded that compared with WLI, LCI improved the visibility of short-segment BE lesions (Esophageal columnar epithelium was less than 3 cm, more than 1 cm, and did not require IM), especially for trainees (66). BLI was deemed extremely helpful for the early detection of synchronous adenocarcinoma in BE patients (67). Reports on the observation of IM in BE with LCI and BLI have not been retrieved. Upper gastrointestinal examination entails continuous observation. Moreover, LCI and BLI have obvious advantages over WLI with respect to the detection of lesions within the stomach To optimize the system for complete detection of lesions in the upper digestive tract, the detection rate for esophageal lesions requires more clinical data.
Multiphoton microscopy (MPM) based on 2-photon excitation fluorescence and second-harmonic generation allows for simultaneous visualization of cellular details and extracellular matrix components of fresh, unfixed, and unstained tissue (68). MPM can easily identify several cells in the GEJ. For this purpose, the mucosa was classified into a squamous type, columnar stomach type, and metaplastic columnar intestinal-type/BE based on the type of cells identified. Chen et al. (68) showed that goblet cells were identified in 10 out of 25 patients examined by MPM, of which only 7 were diagnosed pathologically as IM. Of the 35 biopsy specimens, 3 (9%) delineated clear goblet cells by MPM observation, which could not be seen in histopathological sections. This study showed that MPM can accurately identify goblet cells, opening a new door for the identification of IM and diagnosis of BE, but its diagnostic efficiency in combination with endoscopy and clinical application still necessitates more exploration.
CLE is a combination of traditional video endoscopy and confocal laser microscopy. The addition of confocal laser microscopy enabled us to dynamically identify different cell structures in real-time and make histological observations in vivo. In 2006, Kiesslich et al. (69) divided the distal esophagus into three types according to the appearance of different vessels and cell structures: Gastric-type epithelium, Barrett’s epithelium, and neoplastic changes. The predictive sensitivities of BE (with IM) and related tumors were 98.1% and 92.9%, respectively, whereas the specificities were 94.1% and 98.4%, respectively. Studies performed by Richardson et al. (70) also revealed that significantly more IM patients were detected by early CLE users compared with the conventional Seattle protocol. Regarding the detection of BE-related neoplasia, both a clinical randomized controlled trial and meta-analysis showed that CLE combined with targeted biopsy was superior to high-definition white light endoscopy combined with four-quadrant biopsy (71, 72). A recent meta-analysis concluded that CLE is superior to NBI in the detection rate of single lesions (73). However, due to the need to inject a fluorescent agent, the high cost of equipment, and the need for additional learning, the clinical application of CLE is still limited.
Sugimoto et al. (74) believed that, although present endoscopies used high vision or magnified endoscopy, these endoscopes were of larger caliber and needed sedation. Thus, these endoscopes were not suitable for endoscopic examination. Consequently, they used a newly developed ultrathin transnasal endoscope to closely observe the mucosal structure and investigated the incidence of BE and the usefulness of mucosal structural pattern classification. The study suggested that the new ultrathin transnasal endoscopy is a useful technique for monitoring BE, especially SSBE. The pit pattern was divided into closed (Oval/round and long straight patterns) and open (Villous, cerebriform, and irregular patterns) types. Their results revealed that IM was more prevalent in the open type.
With the emergence of artificial intelligence, the use of computer-aided identification has increased in the field of endoscopy. The use of artificial intelligence and machine learning techniques have become major aids to cope with pattern prognosis associated with BE, and there have been significantly more research projects on automatic stage classification of BE using different endoscopic techniques (75). Recently, Ghatwary et al. (76) proposed an automatic classification method for the stage classification of BE, which focused on improving the classification of IM. This classification method divides the mucous tissue into four types: Normal scaly tissue, gastric metaplastic tissue, IM, and tumor. This method’s sensitivity and specificity for IM detection were as high as 0.97 and 0.96, respectively. The sensitivity and specificity for the diagnosis of dysplasia and tumors were also over 90%, and the overall accuracy was 96.05%. These aided systems can be used as a second opinion to assist physicians in the diagnosis, as well as training beginners to improve the detection rate of IM. Withal, it should be noted that a huge image data set must be constructed in order to enhance the computer-aided identification system’s overall diagnostic accuracy.
The presence of goblet cells in the CLM is the pathological diagnosis of IM in BE. However, some non-goblet columnar epithelial cells can mimic goblet cells and impair the diagnosis of IM. Panarelli et al. (77)delineated the characteristics of these cells and their differences from goblet cells. Damaged foveolar epithelial cells containing a copious amount of cytoplasmic mucin can mimic the globular structure of goblet cells and are therefore known as “pseudo-goblet cells,” which can be distinguished from goblet cells due to their pink cytoplasm. A foveal cell that produces enough acid mucin to cause the blue discoloration of the cytoplasm can mimic the blue tone of goblet cells and become a “columnar blue cell.” These cells are cylindrical and can be distinguished from goblet cells due to their difference in shape. The multilayered epithelium is composed of immature-appearing squamoid cells and superficial clusters of columnar cells containing acid mucin. The multilayered epithelium can also simulate goblet cells. Furthermore, all three cell types simulating goblet cells continually fill the surface epithelium diffusely. In contrast, goblet cells are typically individually dispersed in the foveal epithelium background, which is a distinctive feature.
Immunohistochemical staining is commonplace nowadays. Alkaline blue, high iron diamine, Alcian blue, periodic acid Schiff (PAS), CDX2, MUC 2, Heppar1, etc. can all stain goblet cells. However, numerous studies have shown that these types of stains can also stain non-goblet cells, entailing a decrease in specificity (78–86). Although the expression of some immuno-labels in non-goblet columnar epithelium may suggest that IM is more likely to be found elsewhere, it is still not possible to identify goblet cells and non-goblet cells. As a result, the relationship between the staining characteristics of non-goblet epithelium and the risk of dysplasia and/or cancer remains unclear as of yet (78).
Besides, Maskacci et al. (87) demonstrated the combined use of descriptive endoscopy, the suspected esophageal metaplasia range of the Prague standard, and diagnostic charts all conveniently improved the consistency of the CLE interpretation of the esophageal biopsy, which resulted in improved consistency in the diagnosis of IM.
Although there is a difference in the need for IM in BE diagnosis, only some of the guidelines are specified in the relationship between BE monitoring and treatment strategies and IM (Table 2). To optimize the detection rate of IM, ACG (24) suggested that patients who were suspected of having BE (but lacked IM) should undergo a repeat endoscopy within 1 to 2 years, patients with suspected BE should have at least eight random biopsies, patients having short (1–2 cm) segments of suspected BE in which eight biopsies may be unfeasible should have at least four biopsies per cm of circumferential BE, and one biopsy per cm in tongues with BE. BSG (22) suggested that surveillance should consider the presence of IM and the length of BE. For BE, without IM or dysplasia and BE shorter than 3 cm, repeated endoscopic biopsies are recommended to confirm the diagnosis, and if no IM is confirmed repeatedly, de-surveillance is indicated. For patients with BE shorter than 3 cm and accompanied with IM, endoscopic monitoring should be performed every 3–5 years. For patients with BE ≥ 3 cm, endoscopy should be performed every 2–3 years.
The treatment of BE mainly includes drug therapy, anti-reflux surgery, esophagectomy, endoscopic treatment, etc. The main advantage of drug treatment and anti-reflux surgery is that they can alleviate the symptoms of reflux. For simple BE, with or without IM, drug therapy is recommended (23, 24), mainly to bring reflux symptoms under control. If the reflux symptoms are severe and the curative effect of drugs is lacking, anti-reflux surgery can also be considered. Routine endoscopic treatment of BE without dysplasia is not recommended unless the length of BE exceeds 10 cm (26) or is accompanied by nodules (24).
Some breakthroughs in the drug therapy of BE have been made. Initial studies suggest that proton pump inhibitors (PPIs) have a protective effect on the malignant progression of BE (87–89). However, a case-control study accompanied with the follow-up of 9883 patients with BE for up to 10 years showed that the use of PPIs increased the risk of developing EAC or HGD (90). But, a recent large-scale randomized controlled trial of patients with BE showed that high doses of PPIs protect against a composite endpoint of all-cause mortality, EAC, and HGD (34). The study also showed a coupled treatment of PPIs and Aspirin was more effective than PPIs alone, and high doses of PPI (40 mg twice daily) combined with aspirin were the best combination. Aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) have been reported to reduce the risk of malignant progression of BE, and studies have shown that aspirin is more effective than nonsteroidal anti-inflammatory drugs in reducing the risk of malignant progression of BE (69). Recently, Huo et al. (91) provided a theoretical basis for this. Their work showed that aspirin exerts a unique anti-BE protective effect by acting on IKKβ, inhibiting the action of the nuclear factor-k-gene binding (NF-kB) pathway and inhibiting the expression of CDX2. Also, some auxiliary drugs that are often used in the treatment of gastroesophageal reflux disease (GERD), including medicines that promote gastric motility, neutralize bile acids, and inhibit transient relaxation of the lower esophageal sphincter, which reduces the progression of GERD to BE at varying degrees (92–96) (Table 4).
Surgery is still the primary method employed in anti-reflux treatment. Furthermore, laparoscopy gave rise to numerous surgical techniques. The surgical methods mainly include gastric fundus folding, magnetic sphincter augmentation (MSA), electrical stimulation of the lower esophageal sphincter (LES), and bariatric surgery. These procedures all carry merits and shortcomings, but the difficulty of the operation and related complications remain a matter of concern (97–104) (Table 5).
The field of endoscopy is constantly evolving. Examples of endoscopic anti-reflux surgery include trans-oral incision-free fundoplication (TIF), radiofrequency ablation (Stretta), anti-reflux mucosectomy (ARMS), and endoscopic injection or implantation (105–112) (Table 6).
Generally, surgery or endoscopic treatment is only indicated in BE that is more than 10 cm in length or is accompanied by nodules, intraepithelial neoplasia, or cancer (24, 26). Esophagectomy can achieve complete eradication, but the quality of life is seriously impaired, and its mortality rate is high. There are many kinds of endoscopic therapy, including endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), radiofrequency ablation (RFA), argon plasma coagulation (APC), contact cryo-balloon focal ablation system (CbFAS), and so on (Table 7). Compared with other methods, endoscopic treatment possesses the merits of high safety, conserving the normal function of the esophagus and eradicating lesions simultaneously.
Patients suffering from esophageal intramucosal cancer who have undergone EMR and esophagectomy had a similar long-term recurrence rate and mortality (118). RFA is a mature endoscopic technique for the eradication of IM with dysplasia (116). EMR and RFA are usually combined in the treatment of early tumors of BE. According to a recent study (113), the focal EMR followed by RFA has a similar efficiency level as EMR but is safer than EMR in patients with HGD/EAC. However, due to the decreased access to specimens, the uncertainty of the damage to the anatomical layer, and high costs, RFA is not commonplace in Asia. For larger lesions, EMR can only be segmented and resected multiple times. The Japanese view is that the single resection of ESD is the preferred choice regardless of the size of the lesion due to the blind resection line in patients suffering from adenocarcinoma (119). However, due to the long-term learning curve and the high rate of adverse events, the use of ESD in the West is still limited. A study has shown that the complete remission rate from neoplasia of ESD is the same as that of EMR at 3 months. ESD can potentially cause severe adverse events (two cases of perforation) because of its long operation time (114). However, according to the results of a multicenter study from the West, ESD is effective and safe and can achieve a good level of proficiency after approximately 30 operations (120). A recent meta-analysis also shows its effectiveness and safety (115).
The remission rate of APC in the treatment of BE ranged from 64.9%-94.7%. Like previous endoscopic treatments, esophageal stricture was still the most commonly observed complication (121). To prevent postoperative esophageal stricture, hybrid APC was developed based on APC, that is, submucosal fluid injection before APC treatment to reduce the depth of coagulation which in turn reduces the incidence of esophageal stricture (122). In vitro animal experiments showed that the coagulation depth of hybrid APC was shallower than that of conventional APC (123). In the esophageal wall level analysis, the two methods were consistent in the injury of the epithelium, lamina propria, and muscularis mucosae. The number of submucosal injury cases by conventional APC was more significant, and only conventional APC damaged the proper muscle layer. In humans, it was found that the common observation remission rate of hybrid APC for BE was 96%, the pathological observation remission rate was 78%, and the postoperative stenosis rate was only at 2% (122).
Recently, CbFAS has been developed for esophageal mucosal ablation, which freezes the target mucosa to -85 degrees Celsius to achieve ablation of the BE mucosa (124). According to a recent prospective trial (117), the overall 1-year complete eradication of all dysplasia (CE-D) and complete eradication of intestinal metaplasia rates of BE patients with neoplasia was 95% and 88%, respectively. However, the CE-D rate of ultra-long BE, with length ≥ 8 cm, was only at 67%. The main complications were esophageal stricture (9.7%) and upper gastrointestinal bleeding without treatment (2.4%). Based on the current data, CbFAS can be used in the primary or emergency treatment of BE-related tumors and IM, but there is a lack of large-scale clinical research data to evaluate this technique.
This review summarizes BE and IM from the aspects of the definition, endoscopic recognition, pathology, diagnosis, and treatment. It is believed that there will be more detection and treatment in the future.
LZ and BS drafted this review. XZ and QW assisted in table making and sketching. SL and GL edited the grammar, text format, and framework, etc. TL put forward some essential suggestions on the content of the article. ML provided the direction and ideas of writing and made repeated revisions and guidance in the whole process of writing. All authors contributed to the article and approved the submitted version.
This research was supported by the NFSC (No. 81672458 and No. 81972996), Sichuan provincial Science and Technology Department (No. 2017JQ0052), and the Science and Technology Strategic Cooperation Programs of Luzhou Municipal People’s Government and Southwest Medical University (No. 2016LZXNYD-T06). Talent development project of The Affiliated Hospital of Southwest Medical University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
BE, Barrett Esophagus; IM, Intestinal Metaplasia; USSBE, ultra-short-segment BE; GERD, gastroesophageal reflux; HGIN: high-grade intraepithelial neoplasia; HGD: high-grade dysplasia; LGD: Low-grade dysplasia; EAC: Esophageal adenocarcinoma; SCJ, squamous-columnar junction; CDX2, caudal-type homeobox transcription factor-2; EME, Enhanced-magnification endoscopy; ME-NBI, Narrow-band imaging magnification procedure; LCI, Linked Color Imaging; BLI, Blue Laser Imaging; MPM, Multiphoton microscopy; CLE, Confocal laser endoscopy; PPI, proton pump inhibitors; TIF, transoral incisionless fundoplication; RAP, resection and plication; ARMS, antireflux mucosectomy; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; RFA, radiofrequency ablation; APC, argon plasma coagulation; CbFAS, contact cryoballoon focal ablation system.
1. Barrett NR. Chronic Peptic Ulcer of the Oesophagus and ‘Oesophagitis’. Br J Surgery (1950) 38:175–82. doi: 10.1002/bjs.18003815005
2. Paull A, Trier JS, Dalton MD, Camp RC, Loeb P, Goyal RK. The Histologic Spectrum of Barrett’s Esophagus. New Engl J Med (1976) 295:476–80. doi: 10.1056/NEJM197608262950904
3. Kinoshita H, Nagata E, Hirohashi K, Sakai K, Kobayashi Y. Carcinoma of the Gallbladder With an Anomalous Connection Between the Choledochus and the Pancreatic Duct. Report of 10 Cases and Review of the Literature in Japan. Cancer (1984) 54:762–9. doi: 10.1002/1097-0142(1984)54:4<762::AID-CNCR2820540429>3.0.CO;2-K
4. Barron-Rodriguez LP, Manivel JC, Mendez-Sanchez N, Jessurun J. Carcinoid Tumor of the Common Bile Duct: Evidence for its Origin in Metaplastic Endocrine. Cells. Am J Gastroenterol (1991) 86:1073–6.
5. Rubio A, Schuldt M, Guarch R, Laplaza Y, Giordano G, Nogales FF. Pseudomyxoma-Type Invasion in Gastrointestinal Adenocarcinomas of Endometrium and Cervix: A Report of 2 Cases. Int J Gynecol Pathol (2016) 35:118–22. doi: 10.1097/PGP.0000000000000227
6. Dominici A, Castigli M, Mondaini N, Sarti E, Nesi G, Di Cello V. Intestinal Metaplasia of the Bladder. Arch Ital Urol Androl (1999) 71:233–5.
8. Rao DS, Krigman HR, Walther PJ. Enteric Type Adenocarcinoma of the Upper Tract Urothelium Associated With Ectopic Ureter and Renal Dysplasia: An Oncological Rationale for Complete Extirpation of This Aberrant Developmental Anomaly. J Urol (1996) 156:1272–4. doi: 10.1016/S0022-5347(01)65567-8
9. Maekawa S, Hishima T, Yamada Y, Ichikawa H, Natsui S, Shinohara M. A Case of Primary Urethral Adenocarcinoma Accompanied by Vaginal Wall Infiltration in Which the CA19-9 Level was Very High. Hinyokika Kiyo (2009) 55:513–6.
10. Watanabe G, Ajioka Y, Takeuchi M, Annenkov A, Kato T, Watanabe K, et al. Intestinal Metaplasia in Barrett’s Oesophagus may be an Epiphenomenon Rather Than a Preneoplastic Condition, and CDX2-positive Cardiac-Type Epithelium is Associated With Minute Barrett’s Tumour. Histopathology (2015) 66:201–14. doi: 10.1111/his.12486
11. Jin RU, Mills JC. Are Gastric and Esophageal Metaplasia Relatives? The Case for Barrett’s Stemming From SPEM. Dig Dis Sci (2018) 63:2028–41. doi: 10.1007/s10620-018-5150-0
12. Bandla S, Peters JH, Ruff D, Chen SM, Li CY, Song K, et al. Comparison of Cancer-Associated Genetic Abnormalities in Columnar-Lined Esophagus Tissues With and Without Goblet Cells. Ann Surg (2014) 260:72–80. doi: 10.1097/SLA.0000000000000424
13. Graham DY, Zou WY. Guilt by Association: Intestinal Metaplasia Does Not Progress to Gastric Cancer. Curr Opin Gastroenterol (2018) 34:458–64. doi: 10.1097/MOG.0000000000000472
14. Goldenring JR, Nam KT, Wang TC, Mills JC, Wright NA. Spasmolytic Polypeptide-Expressing Metaplasia and Intestinal Metaplasia: Time for Reevaluation of Metaplasias and the Origins of Gastric Cancer. Gastroenterology (2010) 138:2207–10. doi: 10.1053/j.gastro.2010.04.023
15. Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association Medical Position Statement on the Management of Barrett’s Esophagus. Gastroenterology (2011) 140:1084–91. doi: 10.1053/j.gastro.2011.01.031
16. Eberhard D, Tosh D. Transdifferentiation and Metaplasia as a Paradigm for Understanding Development and Disease. Cell Mol Life Sci (2008) 65:33–40. doi: 10.1007/s00018-007-7428-9
17. Wang DH, Souza RF. Transcommitment: Paving the Way to Barrett’s Metaplasia. Adv Exp Med Biol (2016) 908:183–212. doi: 10.1007/978-3-319-41388-4_10
18. Coad RA, Woodman AC, Warner PJ, Barr H, Wright NA, Shepherd NA. On the Histogenesis of Barrett’s Oesophagus and its Associated Squamous Islands: A Three-Dimensional Study of Their Morphological Relationship With Native Oesophageal Gland Ducts. J Pathol (2005) 206:388–94. doi: 10.1002/path.1804
19. Quante M, Bhagat G, Abrams JA, Marache, Good P, Lee MD, et al. Bile Acid and Inflammation Activate Gastric Cardia Stem Cells in a Mouse Model of Barrett-like Metaplasia. Cancer Cell (2012) 21:36–51. doi: 10.1016/j.ccr.2011.12.004
20. Wang X, Ouyang H, Yamamoto Y, Kumar PA, Wei TS, Dagher R, et al. Residual Embryonic Cells as Precursors of a Barrett’s-Like Metaplasia. Cell (2011) 145:1023–35. doi: 10.1016/j.cell.2011.05.026
21. Jiang M, Li H, Zhang Y, Yang Y, Lu R, Liu K, et al. Transitional Basal Cells at the Squamous-Columnar Junction Generate Barrett’s Oesophagus. Nature (2017) 550:529–33. doi: 10.1038/nature24269
22. Fitzgerald RC, di Pietro M, Ragunath K, Yang Y, Lu R, Liu K, et al. British Society of Gastroenterology Guidelines on the Diagnosis and Management of Barrett’s Oesophagus. Gut (2014) 63:7–42. doi: 10.1136/gutjnl-2013-305372
23. Bennett C, Moayyedi P, Corley DA, DeCaestecker J, Falck-Ytter Y, Falk G, et al. Bob Cat: A Large-Scale Review and Delphi Consensus for Management of Barrett’s Esophagus With No Dysplasia, Indefinite for, or Low-Grade Dysplasia. Am J Gastroenterol (2015) 110:662–82; quiz 683. doi: 10.1038/ajg.2015.55
24. Shaheen NJ, Falk GW, Iyer PG, Gerson LB. Acg Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol (2016) 111:30–50; quiz 51. doi: 10.1038/ajg.2015.322
25. Fock KM, Talley N, Goh KL, Sugano K, Katelaris P, Holtmann G, et al. Asia-Pacific Consensus on the Management of Gastro-Oesophageal Reflux Disease: An Update Focusing on Refractory Reflux Disease and Barrett’s Oesophagus. Gut (2016) 65:1402–15. doi: 10.1136/gutjnl-2016-311715
26. Weusten B, Bisschops R, Coron E, Dinis-Ribeiro M, Dumonceau JM, Esteban JM, et al. Endoscopic Management of Barrett’s Esophagus: European Society of Gastrointestinal Endoscopy (Esge) Position Statement. Endoscopy (2017) 49:191–8. doi: 10.1055/s-0042-122140
27. Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, et al. Risk of Malignant Progression in Barrett’s Esophagus Patients: Results From a Large Population-Based Study. J Natl Cancer Inst (2011) 103:1049–57. doi: 10.1093/jnci/djr203
28. Liu W, Hahn H, Odze RD, Goyal RK. Metaplastic Esophageal Columnar Epithelium Without Goblet Cells Shows DNA Content Abnormalities Similar to Goblet Cell-Containing Epithelium. Am J Gastroenterol (2009) 104:816–24. doi: 10.1038/ajg.2009.85
29. Smith J, Garcia A, Zhang R, DeMeester S, Vallone J, Chandrasoma P. Intestinal Metaplasia is Present in Most If Not All Patients Who Have Undergone Endoscopic Mucosal Resection for Esophageal Adenocarcinoma. Am J Surg Pathol (2016) 40:537–43. doi: 10.1097/PAS.0000000000000601
30. Shaheen NJ, Green B, Medapalli RK, Mitchell KL, Wei JT, Schmitz SM, et al. The Perception of Cancer Risk in Patients With Prevalent Barrett’s Esophagus Enrolled in an Endoscopic Surveillance Program. Gastroenterology (2005) 129:429–36. doi: 10.1016/j.gastro.2005.05.055
31. Crockett SD, Lippmann QK, Dellon ES, Shaheen NJ. Health-Related Quality of Life in Patients With Barrett’s Esophagus: A Systematic Review. Clin Gastroenterol Hepatol (2009) 7:613–23. doi: 10.1016/j.cgh.2009.02.024
32. Harrison R, Perry I, Haddadin W, McDonald S, Bryan R, Abrams K, et al. Detection of Intestinal Metaplasia in Barrett’s Esophagus: An Observational Comparator Study Suggests the Need for a Minimum of Eight Biopsies. Am J Gastroenterol (2007) 102:1154–61. doi: 10.1111/j.1572-0241.2007.01230.x
33. Gatenby PA, Ramus JR, Caygill CP, Shepherd NA, Watson A. Relevance of the Detection of Intestinal Metaplasia in non-Dysplastic Columnar-Lined Oesophagus. Scand J Gastroenterol (2008) 43:524–30. doi: 10.1080/00365520701879831
34. Jankowski JAZ, de Caestecker J, Love SB, Reilly G, Watson P, Sanders S, et al. Esomeprazole and Aspirin in Barrett’s Oesophagus (AspECT): A Randomised Factorial Trial. Lancet (2018) 392:400–8. doi: 10.1016/S0140-6736(18)31388-6
35. Kelty CJ, Gough MD, Van Wyk Q, Stephenson TJ, Ackroyd R. Barrett’s Oesophagus: Intestinal Metaplasia is Not Essential for Cancer Risk. Scand J Gastroenterol (2007) 42:1271–4. doi: 10.1080/00365520701420735
36. Takubo K, Aida J, Naomoto Y, Sawabe M, Arai T, Shiraishi H, et al. Cardiac Rather Than Intestinal-Type Background in Endoscopic Resection Specimens of Minute Barrett Adenocarcinoma. Hum Pathol (2009) 40:65–74. doi: 10.1016/j.humpath.2008.06.008
37. Chaves P, Crespo M, Ribeiro C, Laranjeira C, Pereira AD, Suspiro A, et al. Chromosomal Analysis of Barrett’s Cells: Demonstration of Instability and Detection of the Metaplastic Lineage Involved. Mod Pathol (2007) 20:788–96. doi: 10.1038/modpathol.3800787
38. DeMeester SR, Wickramasinghe KS, Lord RV, Friedman A, Balaji NS, Chandrasoma PT, et al. Cytokeratin and DAS-1 Immunostaining Reveal Similarities Among Cardiac Mucosa, CIM, and Barrett’s Esophagus. Am J Gastroenterol (2002) 97:2514–23. doi: 10.1111/j.1572-0241.2002.06033.x
39. Riddell RH, Odze RD. Definition of Barrett’s Esophagus: Time for a Rethink–is Intestinal Metaplasia Dead? Am J Gastroenterol (2009) 104:2588–94. doi: 10.1038/ajg.2009.390
40. Lavery DL, Martinez P, Gay LJ, Cereser B, Novelli MR, Rodriguez-Justo M, et al. Evolution of Oesophageal Adenocarcinoma From Metaplastic Columnar Epithelium Without Goblet Cells in Barrett’s Oesophagus. Gut (2016) 65:907–13. doi: 10.1136/gutjnl-2015-310748
41. Thota PN, Vennalaganti P, Vennelaganti S, Young P, Gaddam S, Gupta N, et al. Low Risk of High-Grade Dysplasia or Esophageal Adenocarcinoma Among Patients With Barrett’s Esophagus Less Than 1 Cm (Irregular Z Line) Within 5 Years of Index. Endoscopy. Gastroenterology (2017) 152:987–92. doi: 10.1053/j.gastro.2016.12.005
42. Siewert JR, Holscher AH, Becker K, Gossner W. Cardia Cancer: Attempt at a Therapeutically Relevant Classification. Chirurg (1987) 58:25–32.
43. Pohl H, Pech O, Arash H, Stolte M, Manner H, May A, et al. Length of Barrett’s Oesophagus and Cancer Risk: Implications From a Large Sample of Patients With Early Oesophageal Adenocarcinoma. Gut (2016) 65:196–201. doi: 10.1136/gutjnl-2015-309220
44. Matsuzaki J, Suzuki H, Asakura K, Saito Y, Hirata K, Takebayashi T, et al. Etiological Difference Between Ultrashort- and Short-Segment Barrett’s Esophagus. J Gastroenterol (2011) 46:332–8. doi: 10.1007/s00535-010-0353-y
45. Curvers WL, Peters FP, Elzer B, Schaap AJ, Baak LC, van Oijen A, et al. Quality of Barrett’s Surveillance in The Netherlands: A Standardized Review of Endoscopy and Pathology Reports. Eur J Gastroenterol Hepatol (2008) 20:601–7. doi: 10.1097/MEG.0b013e3282f8295d
46. Das D, Ishaq S, Harrison R, Kosuri K, Harper E, Decaestecker J, et al. Management of Barrett’s Esophagus in the UK: Overtreated and Underbiopsied But Improved by the Introduction of a National Randomized Trial. Am J Gastroenterol (2008) 103:1079–89. doi: 10.1111/j.1572-0241.2008.01790.x
47. Abrams JA, Kapel RC, Lindberg GM, Saboorian MH, Genta RM, Neugut AI, et al. Adherence to Biopsy Guidelines for Barrett’s Esophagus Surveillance in the Community Setting in the United States. Clin Gastroenterol Hepatol (2009) 7:736–742; quiz 710. doi: 10.1016/j.cgh.2008.12.027
48. Thosani N, Abu Dayyeh BK, Sharma P, Aslanian HR, Enestvedt BK, Komanduri S, et al. Asge Technology Committee Systematic Review and Meta-Analysis Assessing the ASGE Preservation and Incorporation of Valuable Endoscopic Innovations Thresholds for Adopting Real-Time Imaging-Assisted Endoscopic Targeted Biopsy During Endoscopic Surveillance of Barrett’s Esophagus. Gastrointestinal Endosc (2016) 83:684–698.e687. doi: 10.1016/j.gie.2016.01.007
49. Murray L, Watson P, Johnston B, Sloan J, Mainie IM, Gavin A. Risk of Adenocarcinoma in Barrett’s Oesophagus: Population Based Study. BMJ (2003) 327:534–5. doi: 10.1136/bmj.327.7414.534
50. Fukui S, Watari J, Tomita T, Fukui A, Gen Y, Iwai N, et al. Localization of Specialized Intestinal Metaplasia and the Molecular Alterations in Barrett Esophagus in a Japanese Population: An Analysis of Biopsy Samples Based on the “Seattle” Biopsy Protocol. Hum Pathol (2016) 51:32–40. doi: 10.1016/j.humpath.2015.12.013
51. Sharma P, Weston AP, Topalovski M, Cherian R, Bhattacharyya A, Sampliner RE. Magnification Chromoendoscopy for the Detection of Intestinal Metaplasia and Dysplasia in Barrett’s Oesophagus. Gut (2003) 52:24–7. doi: 10.1136/gut.52.1.24
52. Guelrud M, Herrera I, Essenfeld H, Castro J. Enhanced Magnification Endoscopy: A New Technique to Identify Specialized Intestinal Metaplasia in Barrett’s Esophagus. Gastrointestinal Endosc (2001) 53:559–65. doi: 10.1067/mge.2001.114059
53. Toyoda H, Rubio C, Befrits R, Hamamoto N, Adachi Y, Jaramillo E. Detection of Intestinal Metaplasia in Distal Esophagus and Esophagogastric Junction by Enhanced-Magnification Endoscopy. Gastrointestinal endosc (2004) 59:15–21. doi: 10.1016/S0016-5107(03)02527-6
54. Coletta M, Sami SS, Nachiappan A, Fraquelli M, Casazza G, Ragunath K. Acetic Acid Chromoendoscopy for the Diagnosis of Early Neoplasia and Specialized Intestinal Metaplasia in Barrett’s Esophagus: A Meta-Analysis. Gastrointestinal Endosc (2016) 83:57–67.e51. doi: 10.1016/j.gie.2015.07.023
55. Sharma P, Bansal A, Mathur S, Wani S, Cherian R, McGregor D, et al. The Utility of a Novel Narrow Band Imaging Endoscopy System in Patients With Barrett’s Esophagus. Gastrointestinal Endosc (2006) 64:167–75. doi: 10.1016/j.gie.2005.10.044
56. Norimura D, Isomoto H, Nakayama T, Hayashi T, Suematsu T, Nakashima Y, et al. Magnifying Endoscopic Observation With Narrow Band Imaging for Specialized Intestinal Metaplasia in Barrett’s Esophagus With Special Reference to Light Blue Crests. Dig Endosc (2010) 22:101–6. doi: 10.1111/j.1443-1661.2010.00940.x
57. Song J, Zhang J, Wang J, Guo X, Yu S, Wang J, et al. Meta-Analysis of the Effects of Endoscopy With Narrow Band Imaging in Detecting Dysplasia in Barrett’s Esophagus. Dis Esophagus (2015) 28:560–6. doi: 10.1111/dote.12222
58. DeMeester TR. Clinical Biology of the Barrett’s Metaplasia, Dysplasia to Carcinoma Sequence. Surg Oncol (2001) 10:91–102. doi: 10.1016/S0960-7404(01)00030-5
59. Ngamruengphong S, Sharma VK, Das A. Diagnostic Yield of Methylene Blue Chromoendoscopy for Detecting Specialized Intestinal Metaplasia and Dysplasia in Barrett’s Esophagus: A Meta-Analysis. Gastrointestinal Endosc (2009) 69:1021–8. doi: 10.1016/j.gie.2008.06.056
60. Fortun PJ, Anagnostopoulos GK, Kaye P, James M, Foley S, Samuel S, et al. Acetic Acid-Enhanced Magnification Endoscopy in the Diagnosis of Specialized Intestinal Metaplasia, Dysplasia and Early Cancer in Barrett’s Oesophagus. Aliment Pharmacol Ther (2006) 23:735–42. doi: 10.1111/j.1365-2036.2006.02823.x
61. Uno G, Ishimura N, Tada Y, Tamagawa Y, Yuki T, Matsushita T, et al. Simplified Classification of Capillary Pattern in Barrett Esophagus Using Magnifying Endoscopy With Narrow Band Imaging: Implications for Malignant Potential and Interobserver Agreement. Medicine (2015) 94:e405. doi: 10.1097/MD.0000000000000405
62. Alvarez Herrero L, Curvers WL, Bansal A, Wani S, Kara M, Schenk E, et al. Zooming in on Barrett Oesophagus Using Narrow-Band Imaging: An International Observer Agreement Study. Eur J Gastroenterol Hepatol (2009) 21:1068–75. doi: 10.1097/MEG.0b013e3283271e87
63. Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamamoto S, Yamada T, et al. A New Method of Diagnosing Gastric Intestinal Metaplasia: Narrow-Band Imaging With Magnifying Endoscopy. Endoscopy (2006) 38:819–24. doi: 10.1055/s-2006-944632
64. Zhang S, Zhang XQ, Ding XW, Yang RK, Huang SL, Kastelein F, et al. Cyclooxygenase Inhibitors Use is Associated With Reduced Risk of Esophageal Adenocarcinoma in Patients With Barrett’s Esophagus: A Meta-Analysis. Br J Cancer (2014) 110:2378–88. doi: 10.1038/bjc.2014.127
65. Pascarenco OD, Coros MF, Pascarenco G, Boeriu AM, Drasovean SC, Onisor DM, et al. A Preliminary Feasibility Study: Narrow-band Imaging Targeted Versus Standard White Light Endoscopy non-Targeted Biopsies in a Surveillance Barrett’s Population. Dig Liver Dis (2016) 48:1048–53. doi: 10.1016/j.dld.2016.04.017
66. Takeda T, Nagahara A, Ishizuka K, Okubo S, Haga K, Suzuki M, et al. Improved Visibility of Barrett’s Esophagus With Linked Color Imaging: Inter- and Intra-Rater Reliability and Quantitative Analysis. Digestion (2018) 97:183–94. doi: 10.1159/000485459
67. Dohi O, Yagi N, Naito Y, Fukui A, Gen Y, Iwai N, et al. Blue Laser Imaging-Bright Improves the Real-Time Detection Rate of Early Gastric Cancer: A Randomized Controlled Study. Gastrointestinal endosc (2019) 89:47–57. doi: 10.1016/j.gie.2018.08.049
68. Chen J, Wong S, Nathanson MH, Jain D. Evaluation of Barrett Esophagus by Multiphoton Microscopy. Arch Pathol Lab Med (2014) 138:204–12. doi: 10.5858/arpa.2012-0675-OA
69. Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, et al. In Vivo Histology of Barrett’s Esophagus and Associated Neoplasia by Confocal Laser Endomicroscopy. Clin Gastroenterol Hepatol (2006) 4:979–87. doi: 10.1016/j.cgh.2006.05.010
70. Richardson C, Colavita P, Dunst C, Bagnato J, Billing P, Birkenhagen K, et al. Real-Time Diagnosis of Barrett’s Esophagus: A Prospective, Multicenter Study Comparing Confocal Laser Endomicroscopy With Conventional Histology for the Identification of Intestinal Metaplasia in New Users. Surg Endosc (2019) 33:1585–91. doi: 10.1007/s00464-018-6420-9
71. Gupta A, Attar BM, Koduru P, Murali AR, Go BT, Agarwal R. Utility of Confocal Laser Endomicroscopy in Identifying High-Grade Dysplasia and Adenocarcinoma in Barrett’s Esophagus: A Systematic Review and Meta-Analysis. Eur J Gastroenterol Hepatol (2014) 26:369–77. doi: 10.1097/MEG.0000000000000057
72. Canto MI, Anandasabapathy S, Brugge W, Falk GW, Dunbar KB, Zhang Z, et al. In Vivo Endomicroscopy Improves Detection of Barrett’s Esophagus-Related Neoplasia: A Multicenter International Randomized Controlled Trial (With Video). Gastrointestinal endosc (2014) 79:211–21. doi: 10.1016/j.gie.2013.09.020
73. Xiong YQ, Ma SJ, Hu HY, Ge J, Zhou LZ, Huo ST, et al. Comparison of Narrow-Band Imaging and Confocal Laser Endomicroscopy for the Detection of Neoplasia in Barrett’s Esophagus: A Meta-Analysis. Clin Res Hepatol Gastroenterol (2018) 42:31–9. doi: 10.1016/j.clinre.2017.05.005
74. Sugimoto H, Kawai T, Naito S, Yanagizawa K, Yamagishi T, Fukuzawa M, et al. Surveillance of Short-Segment Barrett’s Esophagus Using Ultrathin Transnasal Endoscopy. J Gastroenterol Hepatol (2015) 30(Suppl 1):41–5. doi: 10.1111/jgh.12879
75. de Souza LA Jr., Palm C, Mendel R, Hook C, Ebigbo A, Probst A, et al. A Survey on Barrett’s Esophagus Analysis Using Machine Learning. Comput Biol Med (2018) 96:203–13. doi: 10.1016/j.compbiomed.2018.03.014
76. Ghatwary N, Ahmed A, Grisan E, Jalab H, Bidaut L, Ye X. In-Vivo Barrett’s Esophagus Digital Pathology Stage Classification Through Feature Enhancement of Confocal Laser Endomicroscopy. J Med Imaging (Bellingham) (2019) 6:014502. doi: 10.1117/1.JMI.6.1.014502
77. Panarelli NC, Yantiss RK. Do Ancillary Studies Aid Detection and Classification of Barrett Esophagus? Am J Surg Pathol (2016) 40:e83–93. doi: 10.1097/PAS.0000000000000654
78. Offner FA, Lewin KJ, Weinstein WM. Metaplastic Columnar Cells in Barrett’s Esophagus: A Common and Neglected Cell Type. Hum Pathol (1996) 27:885–9. doi: 10.1016/S0046-8177(96)90213-0
79. Wright CL, Kelly JK. The Use of Routine Special Stains for Upper Gastrointestinal Biopsies. Am J Surg Pathol (2006) 30:357–61. doi: 10.1097/01.pas.0000184808.45661.cb
80. Younes M, Ertan A, Ergun G, Verm R, Bridges M, Woods K, et al. Goblet Cell Mimickers in Esophageal Biopsies are Not Associated With an Increased Risk for Dysplasia. Arch Pathol Lab Med (2007) 131:571–5. doi: 10.5858/2007-131-571-GCMIEB
81. Johnson DR, Abdelbaqui M, Tahmasbi M, Mayer Z, Lee HW, Malafa MP, et al. CDX2 Protein Expression Compared to Alcian Blue Staining in the Evaluation of Esophageal Intestinal Metaplasia. World J Gastroenterol (2015) 21:2770–6. doi: 10.3748/wjg.v21.i9.2770
82. Jeung JA, Coran JJ, Liu C, Cardona DM. Hepatocyte Paraffin 1 Antigen as a Biomarker for Early Diagnosis of Barrett Esophagus. Am J Clin Pathol (2012) 137:111–20. doi: 10.1309/AJCPYOBVGS4CGA8Y
83. Chen YY, Wang HH, Antonioli DA, Spechler SJ, Zeroogian JM, Goyal R, et al. Significance of Acid-Mucin-Positive Nongoblet Columnar Cells in the Distal Esophagus and Gastroesophageal Junction. Hum Pathol (1999) 30:1488–95. doi: 10.1016/S0046-8177(99)90172-7
84. Groisman GM, Amar M, Meir A. Expression of the Intestinal Marker Cdx2 in the Columnar-Lined Esophagus With and Without Intestinal (Barrett’s) Metaplasia. Mod Pathol (2004) 17:1282–8. doi: 10.1038/modpathol.3800182
85. Hahn HP, Blount PL, Ayub K, Das KM, Souza R, Spechler S, et al. Intestinal Differentiation in Metaplastic, Nongoblet Columnar Epithelium in the Esophagus. Am J Surg Pathol (2009) 33:1006–15. doi: 10.1097/PAS.0b013e31819f57e9
86. McIntire MG, Soucy G, Vaughan TL, Shahsafaei A, Odze RD. MUC2 is a Highly Specific Marker of Goblet Cell Metaplasia in the Distal Esophagus and Gastroesophageal Junction. Am J Surg Pathol (2011) 35:1007–13. doi: 10.1097/PAS.0b013e318218940d
87. Umansky M, Yasui W, Hallak A, Brill S, Shapira I, Halpern Z, et al. Proton Pump Inhibitors Reduce Cell Cycle Abnormalities in Barrett’s Esophagus. Oncogene (2001) 20:7987–91. doi: 10.1038/sj.onc.1204947
88. Islami F, Kamangar F, Boffetta P. Use of Proton Pump Inhibitors and Risk of Progression of Barrett’s Esophagus to Neoplastic Lesions. Am J Gastroenterol (2009) 104:2646–8. doi: 10.1038/ajg.2009.369
89. Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-Suppressive Medications and Risk of Oesophageal Adenocarcinoma in Patients With Barrett’s Oesophagus: A Systematic Review and Meta-Analysis. Gut (2014) 63:1229–37. doi: 10.1136/gutjnl-2013-305997
90. Hvid-Jensen F, Pedersen L, Funch-Jensen P, Drewes AM. Proton Pump Inhibitor Use may Not Prevent High-Grade Dysplasia and Oesophageal Adenocarcinoma in Barrett’s Oesophagus: A Nationwide Study of 9883 Patients. Aliment Pharmacol Ther (2014) 39:984–91. doi: 10.1111/apt.12693
91. Huo X, Zhang X, Yu C, Cheng E, Zhang Q, Dunbar KB, et al. Aspirin Prevents NF-kappaB Activation and CDX2 Expression Stimulated by Acid and Bile Salts in Oesophageal Squamous Cells of Patients With Barrett’s Oesophagus. Gut (2018) 67:606–15. doi: 10.1136/gutjnl-2016-313584
92. MacFarlane B. Management of Gastroesophageal Reflux Disease in Adults: A Pharmacist’s Perspective. Integr Pharm Res Pract (2018) 7:41–52. doi: 10.2147/IPRP.S142932
93. Cossentino MJ, Mann K, Armbruster SP, Lake JM, Maydonovitch C, Wong RK. Randomised Clinical Trial: The Effect of Baclofen in Patients With Gastro-Oesophageal Reflux–a Randomised Prospective Study. Aliment Pharmacol Ther (2012) 35:1036–44. doi: 10.1111/j.1365-2036.2012.05068.x
94. Tsoukali E, Sifrim D. The Role of Weakly Acidic Reflux in Proton Pump Inhibitor Failure, has Dust Settled? J Neurogastroenterol Motility (2010) 16:258–64. doi: 10.5056/jnm.2010.16.3.258
95. Onuchina EV, Tsukanov VV, Osipenko MF. Drug UDCA (Ursosan) in Therapeutic Management of Patients Barrett’s Esophagus. Eksp Klin Gastroenterol (2010) (12):96–101.
96. Peng S, Huo X, Rezaei D, Zhang Q, Zhang X, Yu C, et al. In Barrett’s Esophagus Patients and Barrett’s Cell Lines, Ursodeoxycholic Acid Increases Antioxidant Expression and Prevents DNA Damage by Bile Acids. Am J Physiol Gastrointestinal Liver Physiol (2014) 307:G129–39. doi: 10.1152/ajpgi.00085.2014
97. Abu Dayyeh B, Murad MH, Bazerbachi F, Buttar NS, Akshintala V, Canto MI, et al. Efficacy of Laparoscopic Nissen Fundoplication vs Transoral Incisionless Fundoplication or Proton Pump Inhibitors in Patients With Gastroesophageal Reflux Disease: Misleading Ranking Probabilities in Network Meta-Analysis. Gastroenterology (2018) 155:935–6. doi: 10.1053/j.gastro.2018.02.042
98. Glen P, Chasse M, Doyle MA, Nasr A, Fergusson DA. Partial Versus Complete Fundoplication for the Correction of Pediatric GERD: A Systematic Review and Meta-Analysis. PloS One (2014) 9:e112417. doi: 10.1371/journal.pone.0112417
99. Lipham JC, Taiganides PA, Louie BE, Ganz RA, DeMeester TR. Safety Analysis of First 1000 Patients Treated With Magnetic Sphincter Augmentation for Gastroesophageal Reflux Disease. Dis Esophagus (2015) 28:305–11. doi: 10.1111/dote.12199
100. Ganz RA, Edmundowicz SA, Taiganides PA, Lipham JC, Smith CD, DeVault KR, et al. Long-Term Outcomes of Patients Receiving a Magnetic Sphincter Augmentation Device for Gastroesophageal Reflux. Clin Gastroenterol Hepatol (2016) 14:671–7. doi: 10.1016/j.cgh.2015.05.028
101. Rodríguez L, Rodriguez P, Gómez B, Ayala JC, Saba J, Perez-Castilla A, et al. Electrical Stimulation Therapy of the Lower Esophageal Sphincter is Successful in Treating GERD: Final Results of Open-Label Prospective Trial. Surg Endosc (2012) 27:1083–92. doi: 10.1007/s00464-012-2561-4
102. Rodríguez L, Rodriguez PA, Gómez B, Netto MG, Crowell MD, Soffer E. Electrical Stimulation Therapy of the Lower Esophageal Sphincter is Successful in Treating GERD: Long-Term 3-Year Results. Surg Endosc (2015) 30:2666–72. doi: 10.1007/s00464-015-4539-5
103. Parmar CD, Mahawar KK, Boyle M, Schroeder N, Balupuri S, Small PK. Conversion of Sleeve Gastrectomy to Roux-en-Y Gastric Bypass is Effective for Gastro-Oesophageal Reflux Disease But Not for Further Weight Loss. Obes Surg (2017) 27:1651–8. doi: 10.1007/s11695-017-2542-8
104. Borbely Y, Kroll D, Nett PC, Moreno P, Tutuian R, Lenglinger J. Radiologic, Endoscopic, and Functional Patterns in Patients With Symptomatic Gastroesophageal Reflux Disease After Roux-en-Y Gastric Bypass. Surg Obes Relat Dis (2018) 14:764–8. doi: 10.1016/j.soard.2018.02.028
105. Reavis KM, Perry KA. Transoral Incisionless Fundoplication for the Treatment of Gastroesophageal Reflux Disease. Expert Rev Med Devices (2014) 11:341–50. doi: 10.1586/17434440.2014.925394
106. Witteman BP, Conchillo JM, Rinsma NF, Betzel B, Peeters A, Koek GH, et al. Randomized Controlled Trial of Transoral Incisionless Fundoplication vs. Proton Pump Inhibitors for Treatment of Gastroesophageal Reflux Disease. Am J Gastroenterol (2015) 110:531–42. doi: 10.1038/ajg.2015.28
107. Huang X, Chen S, Zhao H, Zeng X, Lian J, Tseng Y, et al. Efficacy of Transoral Incisionless Fundoplication (TIF) for the Treatment of GERD: A Systematic Review With Meta-Analysis. Surg Endosc (2017) 31:1032–44. doi: 10.1007/s00464-016-5111-7
108. Liang W-T, Wang Z-G, Wang F, Yang Y, Hu Z-W, Liu J-J, et al. Long-Term Outcomes of Patients With Refractory Gastroesophageal Reflux Disease Following a Minimally Invasive Endoscopic Procedure: A Prospective Observational Study. BMC Gastroenterol (2014) 14:178–8. doi: 10.1186/1471-230X-14-178
109. Lipka S, Kumar A, Richter JE. No Evidence for Efficacy of Radiofrequency Ablation for Treatment of Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol (2015) 13:1058–67 e1051. doi: 10.1016/j.cgh.2014.10.013
110. Inoue H, Ito H, Ikeda H, Sato C, Sato H, Phalanusitthepha C, et al. Anti-Reflux Mucosectomy for Gastroesophageal Reflux Disease in the Absence of Hiatus Hernia: A Pilot Study. Ann Gastroenterol (2014) 27:346–51.
111. Lo W-K, Mashimo H. Critical Assessment of Endoscopic Techniques for Gastroesophageal Reflux Disease. J Clin Gastroenterol (2015) 49:720–4. doi: 10.1097/MCG.0000000000000389
112. Benias PC, D’Souza L, Lan G, Gluckman C, Inamdar S, Trindade AJ, et al. Initial Experience With a Novel Resection and Plication (RAP) Method for Acid Reflux: A Pilot Study. Endosc Int Open (2018) 6:E443–9. doi: 10.1055/s-0044-101453
113. Desai M, Saligram S, Gupta N, Vennalaganti P, Bansal A, Choudhary A, et al. Efficacy and Safety Outcomes of Multimodal Endoscopic Eradication Therapy in Barrett’s Esophagus-Related Neoplasia: A Systematic Review and Pooled Analysis. Gastrointestinal Endosc (2017) 85:482–95.e484. doi: 10.1016/j.gie.2016.09.022
114. Terheggen G, Horn EM, Vieth M, Gabbert H, Enderle M, Neugebauer A, et al. A Randomised Trial of Endoscopic Submucosal Dissection Versus Endoscopic Mucosal Resection for Early Barrett’s Neoplasia. Gut (2017) 66:783–93. doi: 10.1136/gutjnl-2015-310126
115. Yang D, Zou F, Xiong S, Forde JJ, Wang Y, Draganov PV. Endoscopic Submucosal Dissection for Early Barrett’s Neoplasia: A Meta-Analysis. Gastrointestinal endosc (2018) 87:1383–93. doi: 10.1016/j.gie.2017.09.038
116. Krajciova J, Janicko M, Falt P, Gregar J, Suchanek S, Ngo O, et al. Radiofrequency Ablation in Patients With Barrett’s Esophagus- Related Neoplasia - Long-Term Outcomes in the Czech National Database. J Gastrointestin Liver Dis (2019) 28:149–55. doi: 10.15403/jgld-174
117. Canto MI, Shaheen NJ, Almario JA, Voltaggio L, Montgomery E, Lightdale CJ. Multifocal Nitrous Oxide Cryoballoon Ablation With or Without EMR for Treatment of Neoplastic Barrett’s Esophagus (With Video). Gastrointest Endosc (2018) 88(3):438–446.e2. doi: 10.1016/j.gie.2018.03.024
118. Li C, Yamashita DT, Hawel JD, Bethune D, Henteleff H, Ellsmere J. Endoscopic Mucosal Resection Versus Esophagectomy for Intramucosal Adenocarcinoma in the Setting of Barrett’s Esophagus. Surg Endosc (2017) 31:4211–6. doi: 10.1007/s00464-017-5479-z
119. Otaki F, Iyer PG. Best of Foregut: Esophagus, Stomach, and Duodenum. Gastrointestinal Endosc (2017) 85:48–54. doi: 10.1016/j.gie.2016.10.005
120. Subramaniam S, Chedgy F, Longcroft-Wheaton G, Kandiah K, Maselli R, Seewald S, et al. Complex Early Barrett’s Neoplasia at 3 Western Centers: European Barrett’s Endoscopic Submucosal Dissection Trial (E-Best). Gastrointestinal Endosc (2017) 86:608–18. doi: 10.1016/j.gie.2017.01.027
121. Manner H, Rabenstein T, Pech O, Braun K, May A, Pohl J, et al. Ablation of Residual Barrett’s Epithelium After Endoscopic Resection: A Randomized Long-Term Follow-Up Study of Argon Plasma Coagulation vs. Surveillance (APE Study). Endoscopy (2014) 46:6–12. doi: 10.1055/s-0033-1358813
122. Manner H, May A, Kouti I, Pech O, Vieth M, Ell C. Efficacy and Safety of Hybrid-APC for the Ablation of Barrett’s Esophagus. Surg Endosc (2016) 30:1364–70. doi: 10.1007/s00464-015-4336-1
123. Manner H, Neugebauer A, Scharpf M, Braun K, May A, Ell C, et al. The Tissue Effect of Argon-Plasma Coagulation With Prior Submucosal Injection (Hybrid-APC) Versus Standard APC: A Randomized Ex-Vivo Study. United Eur Gastroenterol J (2014) 2:383–90. doi: 10.1177/2050640614544315
Keywords: Barrett’s esophagus (BE), intestinal metaplasia (IM), endoscopy and pathological identification, monitoring, treatment
Citation: Zhang L, Sun B, Zhou X, Wei Q, Liang S, Luo G, Li T and Lü M (2021) Barrett’s Esophagus and Intestinal Metaplasia. Front. Oncol. 11:630837. doi: 10.3389/fonc.2021.630837
Received: 18 November 2020; Accepted: 31 May 2021;
Published: 17 June 2021.
Edited by:
Shiming Yang, Xinqiao Hospital, ChinaReviewed by:
Qiuming He, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2021 Zhang, Sun, Zhou, Wei, Liang, Luo, Li and Lü. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhan Lü, bHZtdWhhbkBzd211LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.