
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 25 March 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.629132
Purpose: Chemotherapy-induced gastrointestinal (CIGI) toxicity affects the quality of life of patients with colorectal cancer (CRC) and the clinical application of treatment drugs. This review aims to evaluate the efficacy of traditional herbal medicines (HMs) in alleviating symptoms of CIGI toxicity (including nausea and vomiting, anorexia, diarrhea, constipation, oral mucositis, abdominal pain, and abdominal distension), and to explore further individual herb or herbal combinations in alleviating the CIGI toxicity.
Methods: Nine electronic databases were screened from 2010 to 2020. Twenty-two randomized controlled trials with a total of 1,995 patients evaluating the complementary efficacy of HMs with chemotherapy compared with chemotherapy-alone were included. Further, sensitivity analyses of orally administered multi-ingredient HM interventions were explored based on the composition of HM interventions.
Results: The meta-analysis showed that HM treatment combined with chemotherapy significantly alleviated the overall CIGI toxicity (RR = 0.78 [0.72, 0.84], p < 0.001, I2 = 44%), nausea and vomiting (RR = 0.74 [0.66, 0.82], p < 0.001, I2 = 35%), diarrhea (P = 0.02, RR = 0.64, 95% CI = 0.44–0.93, I2 = 50%), oral mucositis (RR = 0.65 [0.48, 0.88], P = 0.005, I2 = 24%), and abdominal distension (RR = 0.36 [0.18, 0.73], P = 0.004, I2 = 0%). However, no statistically significant effects of HMs were shown in studies with a double-blind design for CIGI toxicity. Based on the ingredients of the HMs, further sensitivity analyses identified five herbs [Glycyrrhiza uralensis Fisch., Atractylodes macrocephala Koidz., Astragalus membranaceus (Fisch.) Bge., Codonopsis pilosula (Franch.) Nannf., and the pericarp of Citrus reticulata Blanco.] that were associated with significant reductions in CIGI toxicity.
Conclusion: A statistically significant effect of HMs combined with chemotherapy on alleviating the overall CIGI toxicity, nausea and vomiting, diarrhea, oral mucositis, or abdominal distension is only shown in studies without a double-blind design. Further well-designed, double-blinded, large-scaled randomized controlled trials (RCTs) are warranted to comprehensively evaluate the treatment efficacy. Further clinical research that includes the five herbs with chemotherapy for patients, the safety of the combinations of these herbs, and the potential synergistic effects of these combinations of herbs should be conducted.
Colorectal cancer (CRC) is considered the second most frequently diagnosed carcinoma in women and the third in men worldwide. There are 1.8 million patients newly diagnosed with CRC in 2018 (1). Chemotherapy 5-fluorouracil (FU) has been the backbone of treatment in patients with CRC for more than half a century (2), and the combination of 5-FU with irinotecan and oxaliplatin has become the standard of therapy for patients with metastatic CRC (mCRC) in the late 1990's (3). However, up to 80% of patients with CRC receiving 5-FU based adjuvant therapy develop gastrointestinal (GI) toxicity, which is currently without a widely effective treatment strategy (4, 5). Common symptoms in CIGI include nausea and vomiting, diarrhea, abdominal pain, abdominal bleeding, ulcerative lesions along the GI tract, etc. (6, 7). Some GI symptoms, such as nausea and vomiting caused by chemotherapy, are well-managed by using potent anti-emetic drugs (8). However, other common GI symptoms reported by patients with cancer such as altered taste, anorexia, dysphagia, reflux, regurgitation, borborygmi, bloating, constipation, diarrhea, tenesmus, mucus discharge, steatorrhea, weight loss, etc., are still lacking optimal management (7, 8). These symptoms often lead to a reduction of therapeutic dose, compromised clinical efficacy of treatment drugs, and impinge on the quality of life of patients. Severe complications of CIGI toxicity such as bacteremia and sepsis interfere with chemotherapy prompting dose reduction and, in profound cases, cessation of therapy.
In order to maintain the tolerance of chemotherapy while preserving the quality of life of patients, the hunt for a complementary treatment for the alleviation of CIGI toxicity becomes crucial. Chinese traditional herbal medicine (HM), which refers to the utilization of plants or plant-derived materials, represents one of the most commonly used complementary treatments of GI toxicity. A large number of herbal formulae such as Si-Jun-Zi decoction, Ping-Wei-San, Shen-Ling-Bai-Zhu-San, etc., have been used in China for over 1,800 years for treating GI toxicity. The use of HM is based on the sophisticated theory of TCM and has undergone long-term repeated confirmation (9). Recent studies on cell line, animal, and clinical trials have shown that some HMs are potentially effective in alleviating CIGI toxicity. Previous systematic reviews and meta-analyses have reported that the combination of HMs with chemotherapy, could alleviate CIGI toxicity in CRC (10–13). However, these systematic reviews and meta-analyses mainly focused on single symptoms such as nausea and/or vomiting (10–13), diarrhea (10–13), and anorexia (11). An overall assessment of the CIGI toxicity of HM treatments is necessary since multiple symptoms of CIGI toxicity may occur in individual patients, and usually, one single HM prescription with combinations is used. Besides, studies that assess the individual symptoms usually focus more on the efficacy of HMs across different types of cancers, which may cause high heterogeneity due to the different severity of GI symptoms. In addition, most of the studies that assess the efficacy of HM treatment do not adopt a double-blind design. This may introduce performance bias into a meta-analysis, and thus overestimate the efficacy of HMs in alleviating individual symptoms.
The review aims to assess the efficacy of HMs in alleviating the symptoms of CIGI toxicity by evaluating the complementary efficacy of HMs with chemotherapy compared with chemotherapy alone. An overall analysis of CIGI toxicity and sub-analysis on the following symptoms: nausea and vomiting, diarrhea, anorexia, constipation, oral mucositis, abdominal pain, and abdominal distension will be conducted. In addition, this review will stratify data analysis for CIGI toxicity with a double-blind design and studies without a double-blind design. Further sensitivity analysis of orally administered multi-ingredient HM interventions will be conducted to explore individual herb or herbal combinations in alleviating the CIGI toxicity.
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). This study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42020201981.
Nine electronic databases, including PubMed, Cochrane Library, EMBASE, ISI Web of Science, Comprehensive Journal Index and Additional Resources for Nursing and Allied Health Professionals (CINAHL Plus), AMED, WanFang Data, and China National Knowledge Infrastructure (CNKI) were searched for CIGI toxicity in patients with CRC. There were no restrictions on language. Only randomized controlled trials (RCTs) evaluated the effects of the combination of the HMs with chemotherapy in comparison with the same chemotherapy regimen were included. The complete search strategy used for the bibliographic databases is provided in Appendix 1.
Randomized controlled studies with two or more arms studies were included according to the following criteria: (1) studies examined adults who had been diagnosed with CRC by pathologists; (2) studies assessed at least one of the symptoms of CIGI toxicity, including nausea and vomiting, diarrhea, anorexia, constipation, oral mucositis, abdominal pain, and abdominal distension; (3) studies that used HM, including a single substance or multi-ingredient formulation as an interventional group without administration restriction; (4) studies that used chemotherapy such as folinic acid, fluorouracil and irinotecan (FOLFIRI), folinic acid, fluorouracil and oxaliplatin (FOLFOX), and other regimens combined with HMs in the intervention group, and compared with the same chemotherapy in the control group. Anti-emetic drugs were allowed for use; and (5) studies that measured CIGI toxicity were assessed by toxicity criteria recommended by WHO (14), the National Cancer Institute Common Terminology Criteria for Adverse Events (15), the Guidelines for Clinical Research of New Chinese Medicines (16), or any validated criteria. GI toxicity was either a primary or a secondary outcome of the study.
Two independent reviewers extracted the following information from each study: first author and year of publication; sample size; intervention and control; treatment dosage and duration; and toxicity assessment. Discrepancies were resolved through consensus discussion and the suggestion of a third reviewer.
The RoB of each included trial was evaluated with the Cochrane Collaboration's tool for assessing the RoB in randomized trials. RoB was assessed by two reviewers. The following domains were assessed: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of data sets, selective outcome reporting, and other bias. Each of the domains was judged “low RoB,” “high RoB,” and “unclear RoB.”
Each study was also assessed using the Jadad scale for assessing bias (17). This scale contained five questions; (i) randomization, (ii) appropriate method for randomization, (iii) double-blinding, (iv) appropriate method for double-blinding, and (v) description of dropouts and withdrawals. A score of 0 or 1 was given to each question, with higher scores representing higher methodological quality. Studies with a score of ≥ 3 were considered high-quality clinical trials, and studies with a score of < 3 were considered low-quality (18).
Review Manager (RevMan) 5.1 was used to conduct the meta-analysis. Review methods were based on Cochrane Handbook 6.1 (19). Data were indicated as risk ratio (RR) with 95% confidence interval (95% CI) or mean difference (MD) in a fixed-effects model or a random-effect model. Heterogeneity was measured using I2. A random-effect model was used if I2 is not < 50%. Studies that measured CIGI toxicity assessed by RR were subjected to the meta-analysis. Studies assessed by MD were analyzed separately. Funnel plots were generated to investigate publication bias (19).
To assess the efficacy of HMs for alleviating CIGI toxicity induced by chemotherapy in patients with CRC, subgroup and sensitivity analyses were explored for each symptom of CIGI toxicity: nausea and vomiting, diarrhea, anorexia, constipation, oral mucositis, abdominal pain, and abdominal distension. Data analysis for each symptom was stratified by studies with a double-blind design and studies without a double-blind design.
In order to explore individual herb or herbal combinations in alleviating the CIGI toxicity, further sensitivity analyses of orally administered multi-ingredient HM interventions were explored based on the composition of HM interventions. Chen et al. reasoned that the pooled RR outcomes of multiple studies that employed the same herb or the same herbal combination reflected whether a particular herb or combination in the intervention was effective or not (20). In our review, the method described by Chen et al. was used to explore individual herb or herbal combinations in alleviating the CIGI toxicity. Therefore, herbs or herbal combinations that had RR results smaller than the pooled RR were identified to show potential for further research into interventions.
The approach was described as following: at Level 1, single herbs present in more than one study were identified. Studies that contained the same herbs were considered a subgroup, and the pooled RR (with 95% CI and I2) was calculated for each subgroup of studies. Only subgroups that had significant pooled RRs with I2 < 30% were considered for higher-level combinations. At Level 2, single herbs with significant pooled RRs and I2 < 30% were paired up, and the RRs of pairs were calculated for each pool. Significant results with I2 < 30% were noted. At Level 3 and above, combinations of 3, and more herbs were generated. The RRs of combinations were calculated for each pool, and significant results with I2 < 30% were noted. The pooled RRs at each level were indicated in ascending order. Besides, only advanced combinations of herbs were included. For example, in the review, all the HM interventions that contained G. uralensis + C. lacryma also included C. pilosula, so no contribution to the RR from G. uralensis + C. lacryma was shown at Level 2.
According to Chen et al., the following selection criteria were used in order to select herbs for further research (20): (1) the RR result of the group of more than one study that included the same herb(s) in the HM interventions was significant with I2 < 30%; (2) the RR was equal or lesser than the total pooled RR for the multi-ingredient HM interventions; and (3) the herb was contained at multiple levels of combination with consistently significant RR results, or the herbal combination had RRs that were lower than those of the herbs individually.
The literature search retrieved 646 records; 122 of them were duplicates (Figure 1). After identifying the unduplicated studies, 117 studies were evaluated, and 94 studies were further excluded. Finally, 22 studies involving a total of 1,995 patients were recruited and included in the qualitative synthesis. Included studies were published from 2010 to 2020, and most of them were conducted in Mainland China. Nineteen studies reported nausea and vomiting (21–39); 14 studies reported diarrhea (21, 24, 25, 27, 29, 30, 32–34, 36–40); 9 studies reported anorexia (26, 27, 31–33, 35, 36, 38, 39); 3 studies reported constipation (21, 36, 39); 6 studies reported oral mucositis (21, 24, 27, 29, 39, 41); 3 studies reported abdominal pain (33, 39, 40); and 6 studies reported abdominal distension (26, 32, 33, 39, 40, 42). Study characteristics are presented in Table 1.
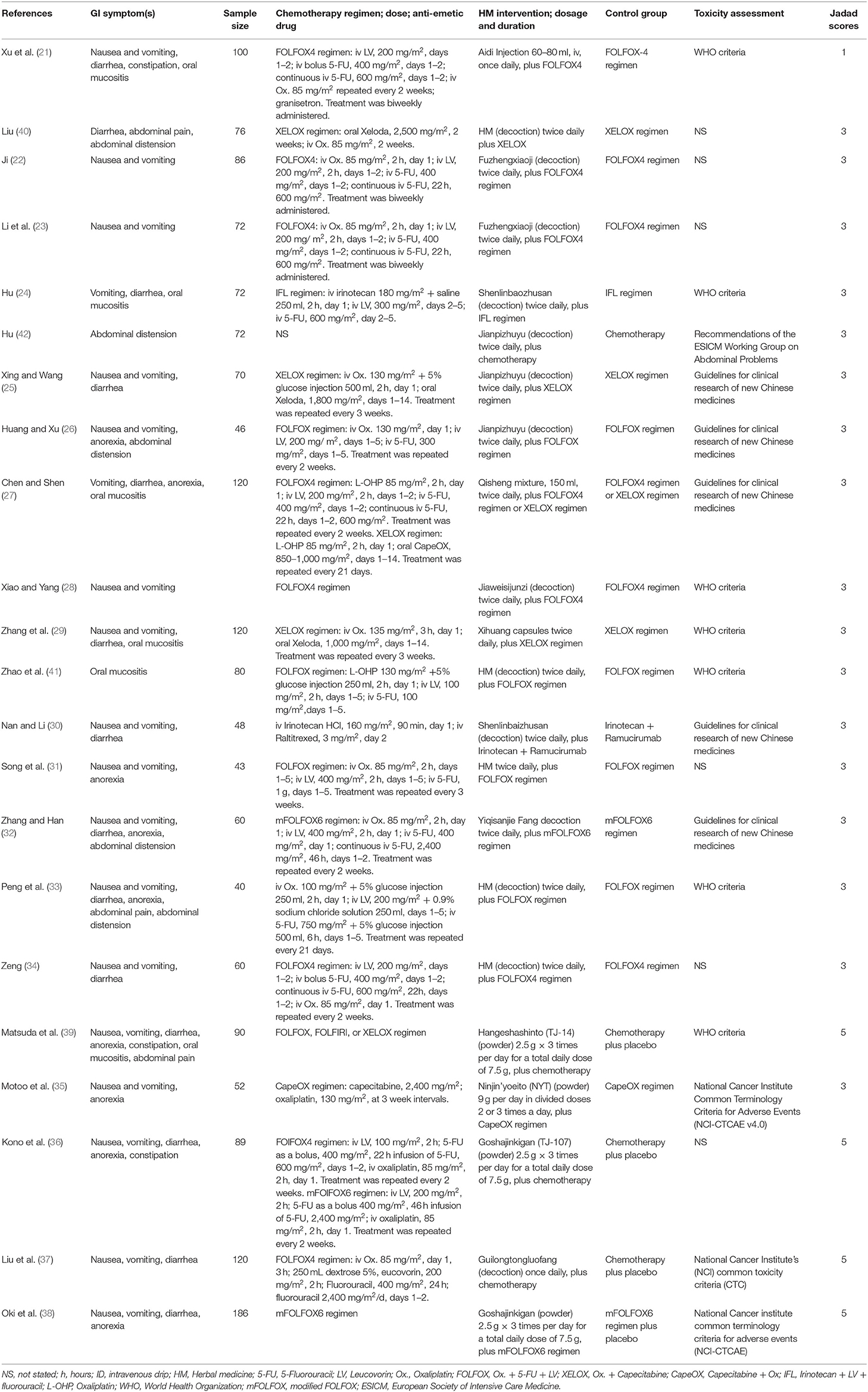
Table 1. Characteristics of randomized controlled trials of HMs combined with chemotherapy for colorectal cancer with GI toxicity incidence as an outcome.
The following study characteristics are recorded in Table 1: CIGI symptoms, sample sizes, interventions, doses, schedules, controls, outcome measures, and Jaded scores. Of the 22 eligible studies, 18 studies originated from China and 4 from Japan. The mean age of included participants was between 44 and 69 years. Most of them had stage III or stage IV cancer. Formulations of HMs used in the 22 studies included decoctions, injection, capsules, powder, and mixture. Among them, one study (21) used injection, one study (29) used capsules, four studies (35, 36, 38, 39) used power, and one study (27) used mixture, while the rest of the fifteen studies used decoctions. Furthermore, 19 out of 22 studies described the components of these medicines. Regimens of chemotherapy were described in all studies. Among them, most of the studies used the FOLFOX regimen and XELOX/CAPOX regimen, while some used the IFL regimen, and irinotecan plus ramucirumab. Among the 16 studies that clarified toxicity assessments, 7 studies assessed outcomes by the toxicity criteria recommended by the WHO (14), 5 studies used the Guidelines for Clinical Research of New Chinese medicines (16), 2 studies used the National Cancer Institute Common Terminology Criteria for Adverse Events (15), 1 study used the National Cancer Institute Common Toxicity Criteria (43), and 1 study used the Recommendations of the European Society of Intensive Care Medicine (ESICM) Working Group on Abdominal Problems (44). Twenty studies that measured GI toxicity assessed by RR were subjected to meta-analysis, while 2 studies that adopted the rating scale (16) were analyzed separately. For some studies that reported the grades of GI toxicity, all grades were included except grade 0.
Figures 2, 3 present the overall RoB assessment and the methodological quality by individual selected studies, respectively. One study (21) did not perform random sequence generation and the rest of the 22 studies employed computer software or random number tables for randomization. However, only two studies (37, 39) described adequate allocation concealment. Performance bias and detection bias are the two primary sources of RoB. Participants in most studies were not blinded, as only four studies (36–39) conducted placebo controls and were judged as “low” RoB for performance and detection biases. All studies had a low RoB for incomplete outcome data or selective reporting due to the adequate description of dropouts. Five studies did not clarify toxicity assessments used for GI toxicity, which accounted for the high risk of other bias. The Jadad scores of the 22 included studies were in the range of 1–5, and the mean scores of studies were 3.27. Twenty-one of the included studies were of high quality (Jadad score ≥ 3).
This meta-analysis was conducted for all grades of CIGI toxicity combined. The treatment group is favored when RR < 1 or MD < 0. A lower RR or MD represents a lower risk of CIGI toxicity. In our meta-analysis, a total of 20 studies with 1,509 patients were reported as RR. Data analysis for GI toxicity was stratified by studies with a double-blind design and studies without a double-blind design. Sixteen studies with 1,028 patients involved in this review did not adopt double-blind procedure, while 4 studies with 481 patients did. Data reported as MD for two studies (26, 27) were analyzed separately.
The overall results showed that, without differentiation of methodological quality, HM treatment combined with chemotherapy significantly alleviated CIGI toxicity, with an effect of 0.78 (95% CI = 0.72–0.84, p < 0.001, I2 = 44%) (Figure 4). The results demonstrated that the treatment groups significantly reduced CIGI toxicity compared to control groups in studies without a double-blind design, with an effect of 0.50 (95% CI = 0.44–0.57, p < 0.001, I2 = 0%); however, no statistically significant difference between treatment groups and control groups in studies with a double-blind design was found. The funnel plot was symmetrically distributed, suggesting that the risk of publication bias was relatively low in the included studies (Figure 5). A minor difference was observed in sensitivity analysis without essential change, indicating that the model is relatively stable. In the two studies that displayed data as mean difference, a statistically significant difference in favor of the treatment groups was shown, with an effect of −1.74 (95% CI = −2.99 to −0.48, I2 = 98%) (Figure 6).
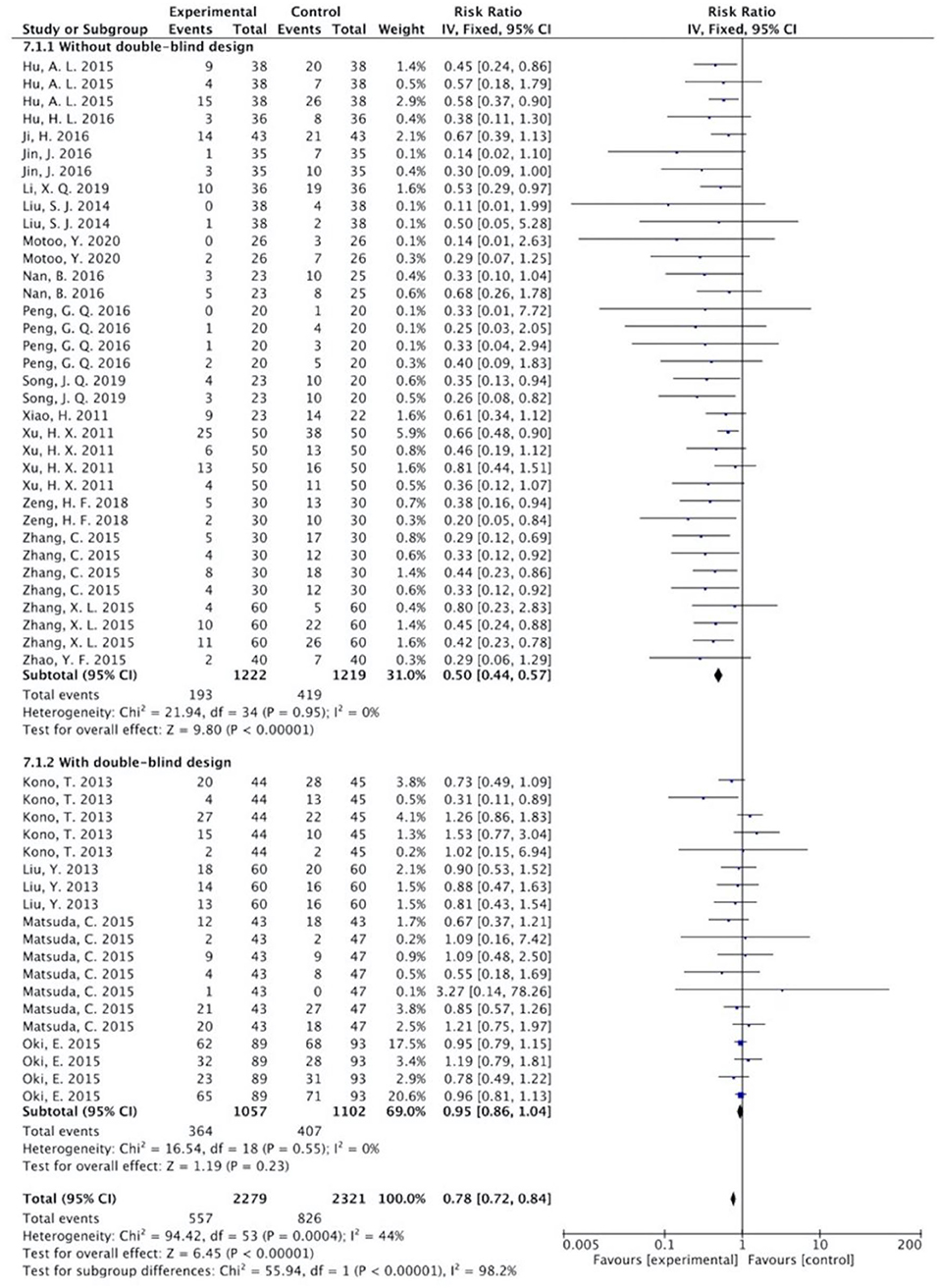
Figure 4. Overall effect of herbal medicine on chemotherapy-induced gastrointestinal toxicity reported as risk ratio.
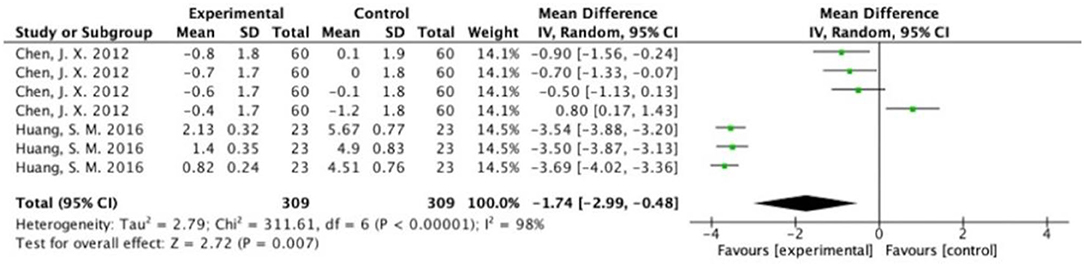
Figure 6. Overall effect of herbal medicine on chemotherapy-induced gastrointestinal toxicity reported as mean difference.
There were 17 studies that analyzed nausea and vomiting (21–25, 28–39), 13 studies that analyzed diarrhea (21, 24, 25, 29, 30, 32–34, 36–40), 7 studies that analyzed anorexia (26, 31–33, 35, 36, 38, 39), 3 studies that analyzed constipation (21, 36, 39), 5 studies that analyzed oral mucositis (21, 24, 29, 39, 41), 3 studies that analyzed abdominal pain (33, 39, 40), and 5 studies that analyzed abdominal distension (32, 33, 39, 40, 42). Sub-analysis of the above symptoms was stratified by studies with a double-blind design, and studies without a double-blind design.
The results (Figures 7–13) showed that for studies assessed without a double-blind design, the occurrence of nausea and vomiting, diarrhea, anorexia, oral mucositis, and abdominal distension significantly decreased in treatment groups. The RRs were 0.55 (95% CI = 0.47–0.66), 0.40 (95% CI = 0.27–0.58), 0.31 (95% CI = 0.17–0.54), 0.43 (95% CI = 0.27–0.70), and 0.32 (95% CI = 0.16–0.66). I2 was found at 0%. For studies with a double-blind design, the results of the occurrence of nausea and vomiting (RR = 0.88, 95% CI = 0.77–1.00), diarrhea (RR = 1.14, 95% CI = 0.85–1.53), and anorexia (RR = 1.04, 95% CI = 0.87–1.23) were not statistically significant.
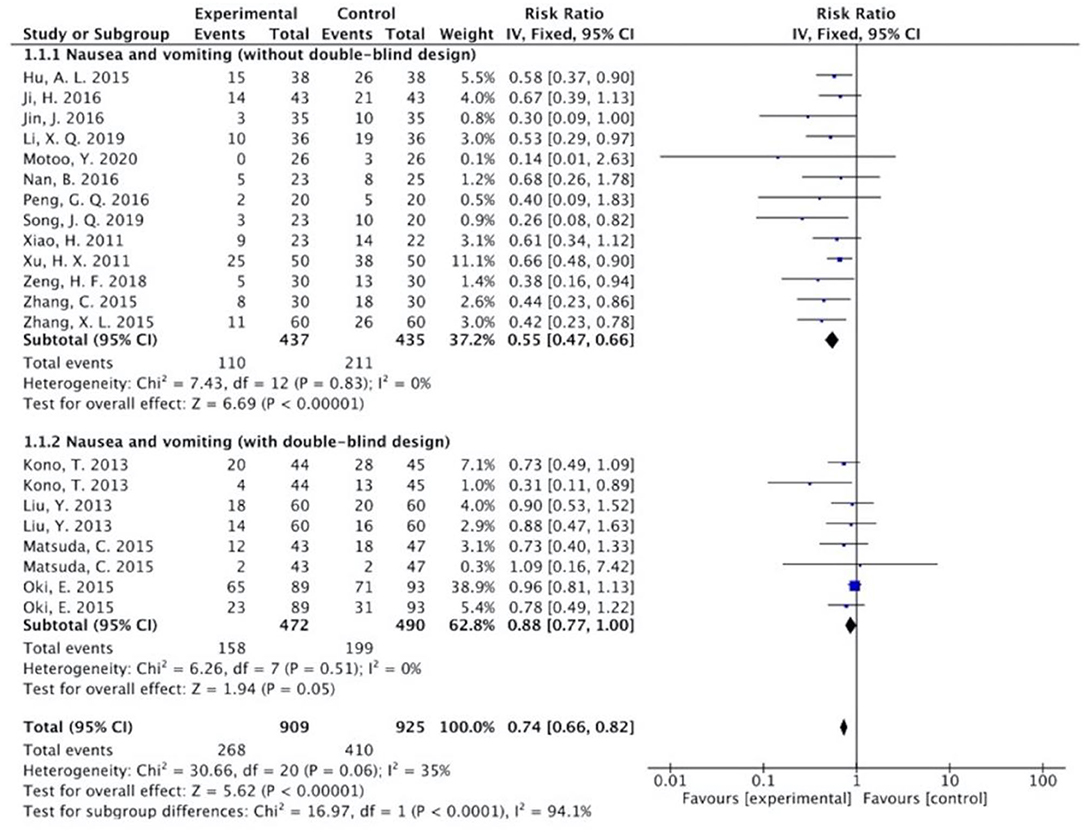
Figure 7. Sub-analysis on the effect of herbal medicine in nausea and vomiting in chemotherapy-induced toxicity.
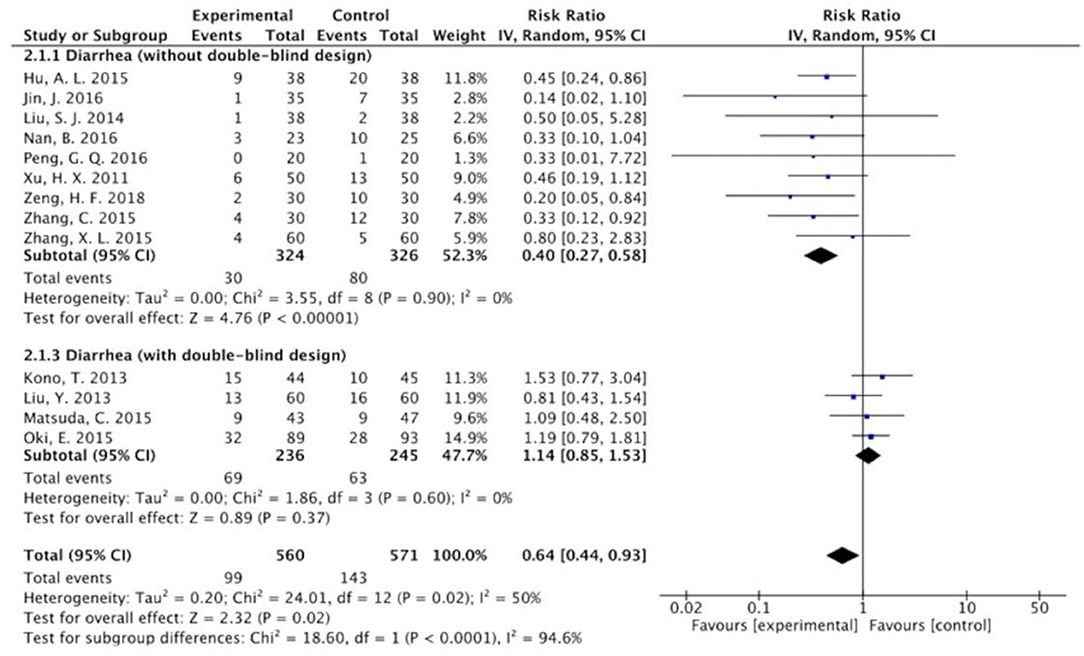
Figure 8. Sub-analysis on the effect of herbal medicine in diarrhea in chemotherapy-induced toxicity.
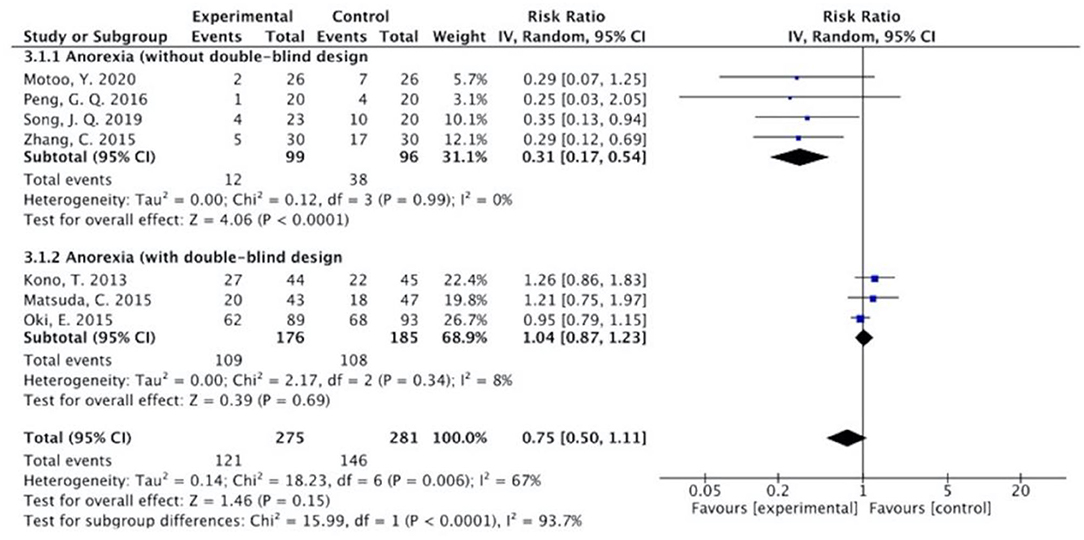
Figure 9. Sub-analysis on the effect of herbal medicine in anorexia in chemotherapy-induced toxicity.
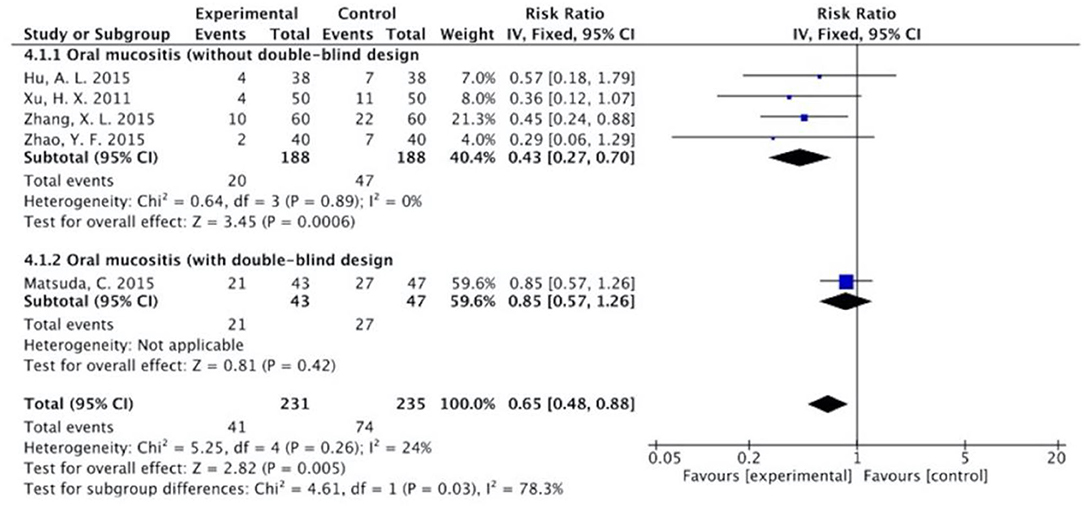
Figure 10. Sub-analysis on the effect of herbal medicine in oral mucositis in chemotherapy-induced toxicity.
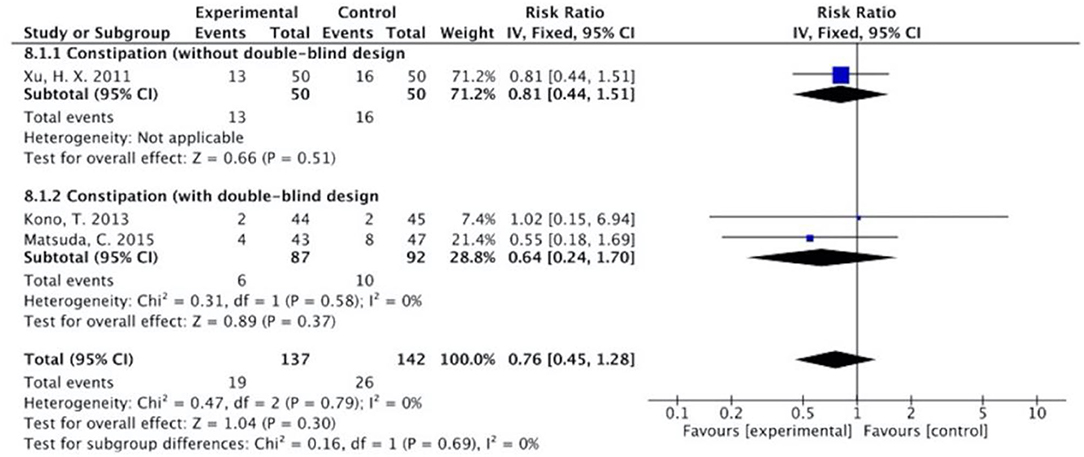
Figure 11. Sub-analysis on the effect of herbal medicine in constipation in chemotherapy-induced toxicity.
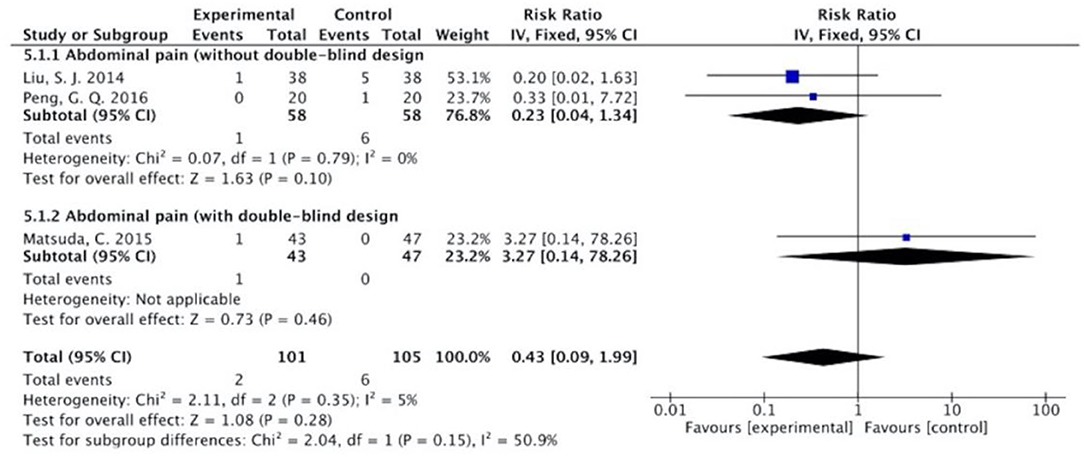
Figure 12. Sub-analysis on the effect of herbal medicine in abdominal pain in chemotherapy-induced toxicity.
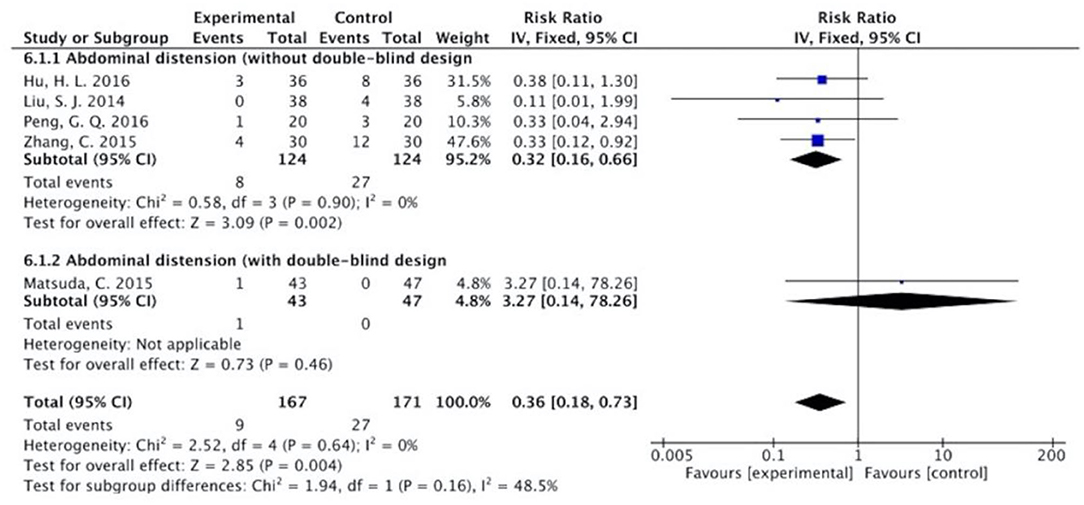
Figure 13. Sub-analysis on the effect of herbal medicine in abdominal distension in chemotherapy-induced toxicity.
Overall, without differentiation of methodological quality, the comparisons of the occurrence of the following symptoms between the two groups were statistically significant: nausea and vomiting (p < 0.001, RR = 0.74, 95% CI = 0.66–0.82, I2 = 35%), diarrhea (P = 0.02, RR = 0.64, 95% CI = 0.44–0.93, I2 = 50%), oral mucositis (P = 0.005, RR = 0.65, 95% CI = 0.48–0.88, I2 = 24%), and abdominal distension (P = 0.004, RR = 0.36, 95% CI = 0.18–0.73, I2 = 0%). No statistically significant results were identified in the occurrence of anorexia (P = 0.15, RR = 0.75, 95% CI = 0.50–1.11, I2 = 67%), constipation (P = 0.30, RR = 0.76, 95% CI = 0.45–1.28, I2 = 0%), and abdominal pain (P = 0.35, RR = 0.43, 95% CI = 0.09–1.99, I2 = 5%).
In multi-ingredient HM treatments, different combinations of the same herbs were used. In order to identify which herbs or herbal combinations that had the greatest contributions to the alleviation of CIGI toxicity, further sensitivity analyses of orally administered multi-ingredient HM interventions were conducted based on the composition of HM interventions.
The orally administered HMs contained 74 different herbs with an average of 10 herbs per treatment. The effects on the alleviation of CIGI toxicity of the herbs were presented at the level of the single herb, pair of herbs, and groups of three up to seven herbs. Thirty-two herbs were used in more than one study. The RR of the group of studies that contained each herb was calculated. Twenty-one of these herbs had significant RRs with low heterogeneity (I2 < 30%). The effects of these herbs (n = 21) that appeared in pairs, triplets, and higher-level combinations were assessed. All significant RR results were reported in Table 2, and only HMs with RR of low heterogeneity that were not greater than the total pooled RR (0.64 [0.55, 0.73]) were shown in the text.
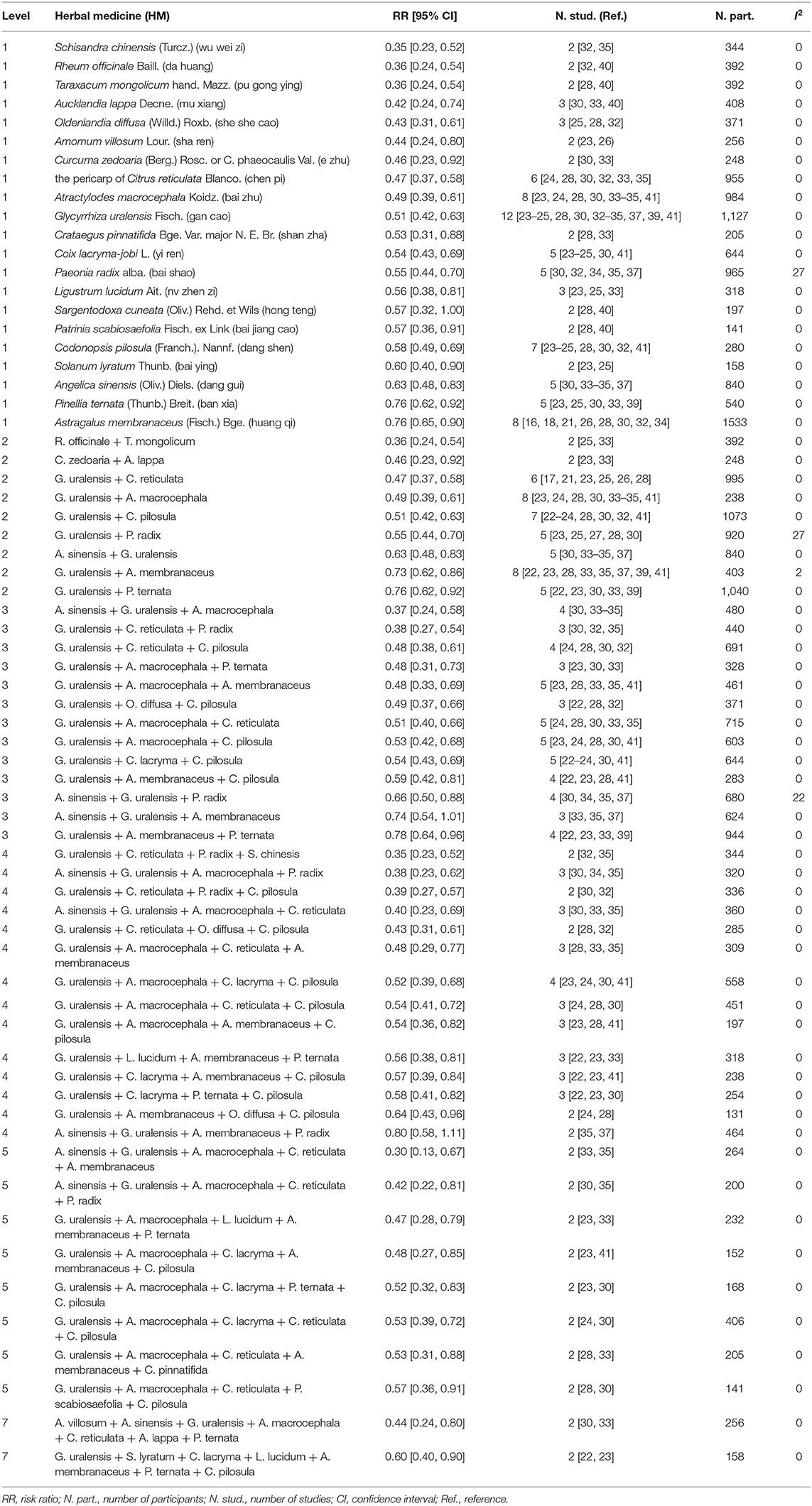
Table 2. Effects of specific HMs on alleviation of chemotherapy-induced gastrointestinal toxicity: single HMs and combinations.
The more frequently used herbs in the HM treatments were: Glycyrrhiza uralensis Fisch. (gan cao) (n = 12), Atractylodes macrocephala Koidz. (bai zhu) (n = 8), Astragalus membranaceus (Fisch.) Bge. (huang qi) (n = 8), Codonopsis pilosula (Franch.) Nannf. (dang shen) (n = 7), and the pericarp of Citrus reticulata Blanco. (chen pi) (n = 6).
Of the 21 plants shown at the Level 1 analysis, two did not combined with another plant. They were Aucklandia lappa Decne. (mu xiang) (n = 3) (RR 0.42 [0.24, 0.74], I2 = 0%), and Glycyrrhiza uralensis Fisch. (gan cao) (n = 12) (RR 0.51 [0.42, 0.63], I2 = 0%). The rest of the plants associated with at least one other plant.
Twenty-one plants were paired with other plants from Level 1, and 9 pairs were generated. Seven pairs had lower RRs when compared with the total pool RR for orally administered HM intervention. Rheum officinale + Taraxacum mongolicum (n = 2) (RR 0.36 [0.24, 0.54], I2 = 0%) had the lowest RR, followed by Curcuma zedoaria + Arctium lappa (n = 2) (RR 0.46 [0.23, 0.92], I2 = 0%), and G. uralensis + C. reticulata (n = 6) (RR 0.47 [0.37, 0.58], I2 = 0%). The most frequent pairs were G. uralensis + A. macrocephala (n = 8) (RR 0.49 [0.39, 0.61], I2 = 0%) and G. uralensis + C. pilosula (n = 7) (RR 0.51 [0.42, 0.63], I2 = 0%).
Compared with the total pool RR, 10 combinations of three plants presented lower RRs. The most frequent combinations were G. uralensis + Coix lacryma + C. pilosula (n = 5) (RR 0.54 [0.43, 0.69], I2 = 0%), G. uralensis + A. macrocephala + C. reticulata (n = 5) (RR 0.51 [0.40, 0.66], I2 = 0%), G. uralensis + A. macrocephala + Astragalus membranaceus (n = 5) (RR 0.48 [0.33, 0.69], I2 = 0%), and G. uralensis + A. macrocephala + C. pilosula (n = 5) (RR 0.53 [0.42, 0.68], I2 = 0%). The combination of G. uralensis + C. reticulata + Paeonia radix (n = 2) had the lowest RR (0.38 [0.27, 0.54], I2 = 0%).
Compared with the total pool RR, 13 combinations of four plants showed lower RRs. G. uralensis + A. macrocephala + C. lacryma + C. pilosula (n = 4) (RR 0.52 [0.39, 0.68], I2 = 0%) was the most frequent combination. G. uralensis + C. reticulata + P. radix + Schisandra chinensis (n = 2) had the lowest RR (0.35 [0.23, 0.52], I2 = 0%), followed by Angelica sinensis + G. uralensis + A. macrocephala + P. radix (n = 3) (0.38 [0.23, 0.62], I2 = 0%).
Compared with the total pool RR, RRs of the eight combinations of five plants were lower. All of the combinations appeared in two studies. The combination of A. sinensis + G. uralensis + A. macrocephala + C. reticulata + A. membranaceus had the lowest RR (0.30 [0.13, 0.67], I2 = 0%), followed by A. sinensis + G. uralensis + A. macrocephala + C. reticulata + P. radix (RR 0.42 [0.22, 0.81], I2 = 0%), and G. uralensis + A. macrocephala + Ligustrum lucidum + A. membranaceus + Pinellia ternata (RR 0.47 [0.28, 0.79], I2 = 0%).
Compared with the total pool RR, RRs of the two combinations of seven plants were lower. They were G. uralensis + Solanum lyratum + C. lacryma + L. lucidum + A. membranaceus + P. ternata + C. pilosula (n = 2) (RR 0.60 [0.40, 0.90], I2 = 0%), and Amomum villosum + A. sinensis + G. uralensis + A. macrocephala + C. reticulata + A. lappa + P. ternata (n = 2) (RR 0.44 [0.24, 0.80], I2 = 0%).
Plants that showed significant RR results that were not greater than the pool total RR, with heterogeneity < 30% at multiple levels, were identified and selected for further research. Five plants were shown at all six levels. They were G. uralensis, C. reticulata, A. sinensis, C. pilosula, and A. macrocephala. Therefore, a clinical benefit for CIGI toxicity was suggested when these five plants were included in HM interventions.
Eleven combinations of plants presented lower RRs compared with those of the plants from Level 1. The following four triplets were included: G. uralensis + C. reticulata + P. radix, A. sinensis + G. uralensis + A. macrocephala, G. uralensis + A. macrocephala + P. ternata, and G. uralensis + A. macrocephala + A. membranaceus; three combinations of four plants: G. uralensis + C. reticulata + P. radix + C. pilosula, A. sinensis + G. uralensis + A. macrocephala + C. reticulata, and A. sinensis + G. uralensis + A. macrocephala + P. radix; four combinations of five plants: A. sinensis + G. uralensis + A. macrocephala + C. reticulata + P. radix, G. uralensis + A. macrocephala + C. lacryma + A. membranaceus + C. pilosula, A. sinensis + G. uralensis + A. macrocephala + C. reticulata + A. membranaceus, and G. uralensis + A. macrocephala + L. lucidum + A. membranaceus + P. ternata. Of these, the combination of A. sinensis + G. uralensis + A. macrocephala + C. reticulata + A. membranaceus (n = 2) had the lowest RR (0.30 [0.13, 0.67], I2 = 0%), followed by A. sinensis + G. uralensis + A. macrocephala (n = 4) (RR 0.37 [0.24, 0.58], I2 = 0%), G. uralensis + C. reticulata + P. radix (n = 3) (RR 0.38 [0.27, 0.54], I2 = 0%), and A. sinensis + G. uralensis + A. macrocephala + P. radix (n = 3) (RR 0.38 [0.23, 0.62], I2 = 0%).
This review and meta-analysis evaluated 22 studies on the combination of the HMs with chemotherapy as an intervention to manage CIGI toxicity in patients with CRC. Evidence was found of an association between HMs and relief from CIGI symptoms. In our meta-analysis, the effects of HMs on nausea and vomiting and diarrhea are generally in line with previous meta-analyses (10–13); however, none of the included studies performed the placebo-controlled, double-blind procedure. In contrast to the previous meta-analyses, our study reported no statistical significance between chemotherapy plus HMs and comparators regarding the effects on anorexia in patients with CRC, while a previous analysis conducted by Zhong et al. showed significant effects favoring HMs (11). The discrepancies of results may arise mainly from the difference in sample size and study quality. Zhong's meta-analysis included only three studies without a placebo-controlled, double-blind design, which likely caused significant bias and influenced the results.
No previous meta-analysis on constipation, oral mucositis, abdominal pain, and abdominal distension that compared the combination of HMs with chemotherapy over the same chemotherapy regimen in patients with CRC was identified. Chung et al. assessed the effects of TCM in conjunction with conventional medicine or chemotherapy for alleviating constipation in patients with cancer by investigating seven RCTs (45); however, data were not pooled because the definition of significant relief among included studies that were not the same. Yuan et al. conducted a meta-analysis evaluating the efficacy of TCM for oral mucositis (46). The study included 8 RCTs with 694 patients and compared the effects of the combination of TCM and concurrent chemoradiotherapy with chemoradiotherapy. Significant reductions in the severity of oral mucositis were shown (RR = 0.52, 95% CI = 0.43–0.64, p < 0.001); however, all studies included in this meta-analysis showed low methodological quality, and the treatment involved both chemotherapy and radiotherapy. CRC was not specified in any of the included studies by Yuan et al. (46).
Our present meta-analysis identified that among studies without a double-blind design, the HMs groups significantly alleviated symptoms of CIGI toxicity, including nausea and vomiting, diarrhea, anorexia, oral mucositis, and abdominal distension. However, among studies with a double-blind design, no statistical differences were found between HMs groups and control groups in all GI symptoms. Our sensitivity analysis between studies with and without a double-blind design showed a statistically significant difference for overall GI toxicity, nausea and vomiting, diarrhea, anorexia, and oral mucositis. The observed differences demonstrated that trials are more likely to show the advantage of the combination of HMs with chemotherapy over chemotherapy alone if a double-blind design is not employed. This difference may arise from a combination of response bias and placebo effect if the outcomes were patient-reported (47). Patients in the intervention groups may have high expectations for HMs treatment and therefore lower the severity of symptoms on self-rating scales. Chen et al. commended that in subjective outcome measurements, such as for the symptom of nausea, may be influenced by the lack of blinding, and a non-specific effect is likely to be produced (20). Moreover, if the outcomes in trials were assessed by non-blinded investigators, the outcomes could also be less reliable and less objective, arising from a favorable reporting toward the intervention groups (20).
Moreover, four studies (36–39) on diarrhea and three studies (36, 38, 39) on anorexia were accessed among the studies with a double-blind design. Positive effects favoring the control groups were shown in the occurrence of both diarrhea and anorexia, suggesting that the prescribed HM combinations including TJ-107 (Goshajinkigan), Guilongtongluofang, and TJ-14 (Hangeshashinto) did not exert a positive effect on alleviating diarrhea or anorexia. It is worth noting that, all of these studies investigated diarrhea or anorexia as secondary outcomes, hence the possibility that the combination therapy caused the CIGI toxicity could not be ruled out. Well-designed RCTs should be further conducted to provide more evidence.
Herbal medicine treatments are composed of a variety of herbs used in different combinations and forms. Although the treatments vary among studies, the herbs are commonly used in multiple studies (20). The following five herbs that had significant pooled RRs, without heterogeneity, were consistently present at multiple levels of combination: G. uralensis (n = 12), C. reticulata (n = 6), A. sinensis (n = 5), C. pilosula (n = 7), and A. macrocephala (n = 8). These herbs were thus considered to show consistent effects on alleviating CIGI toxicity in multiple combinations. Significant alleviation of GI toxicity was also found for other herbs and herb combinations; however, low frequency of the herbs provided insufficient information to assess their effects. For example, A. villosum (n = 2), P. ternata (n = 5), and A. lappa (n = 3) appeared in a subgroup that showed the greatest reduction in GI toxicity at Level 7. It is possible that these herbs also contributed to the results although the subgroup included G. uralensis, C. reticulata, A. membranaceus, and A. sinensis. Therefore, it is essential to note that the herbs selected in the final analyses are not the only herbs that had effects on alleviating CIGI toxicity. Instead, they had consistent effects in multiple combinations.
Although the five herbs are commonly used in combination, there is insufficient information about the efficacy of single herbs in treating CIGI toxicity in patients with CRC. Studies of these five herbs related to CIGI or GI toxicity reduction on experimental models in animals were then explored, which may give an explanation of the effects shown in the meta-analyses to a certain extent. Zhou et al. reported that Radix Codonopsi Polysaccharide significantly decreased the diarrhea scores and the levels of TNF-α, IL-1β, and IL-6 in mice with 5-FU-induced GI mucositis when compared with positive control groups (48). Chen and Zhang investigated the effects of the C. pilosula and A. macrocephala on promoting growth and differentiation of small intestine epithelial IEC-6 cells in normal rats, found that the combination of C. pilosula and A. macrocephala stimulated IEC-6 cells growth and differentiation more evidently than these HMs used singly (49). A study reported by He showed that water extractive of C. reticulata significantly increased the contraction of small intestine smooth muscle in rats with GI motility disorder (50). Du et al. concluded that A. sinensis improved the mucosal atrophy, increased the secretion of colonic mucus, and thus had a statistically significant effect on improving constipation (51).
Potential synergistic effects of the HM combination of three of the five herbs were explored. A HM called Si-Jun-Zi decoction (SJZD), which contains C. pilosula, G. uralensise, and A. macrocephala, is used for treating GI disorders (52). Evidence for an association between SJZD and CIGI symptoms relieve was found. Ni assessed the effects of FOLFOX-7 chemotherapy combined application of modified SJZD on CIGI toxicity (anorexia, abdominal distension, and loose stool) in 70 patients with spleen-stomach Qi deficiency syndrome, and statistically significant alleviation of CIGI toxicity was shown (53). Li et al. conducted a meta-analysis including 8 studies with 483 eligible patients, which compared the effects of chemotherapy combined with SJZD and chemotherapy-alone in patients with CRC (54). They concluded that SJZD showed significant alleviations in CIGI toxicity including nausea and vomiting, and diarrhea, compared to chemotherapy alone. Experimental models in animals have also revealed the efficacy of SJZD on alleviating GI toxicity. Zheng et al. assessed the effects of aqueous extracts of herbs in SJZD, including G. uralensis, C. pilosula, and A. macrocephala on contractile of isolated rat gastric muscle strips (55). They found that G. uralensis, C. pilosula, and A. macrocephala enhanced longitudinal muscle tension in strips from the gastric body, G. uralensis and C. pilosula increased the motility index of pyloric circular muscle, and C. pilosula increased the average amplitude of contraction waves of longitudinal and circular muscles from gastric antrum.
Apart from the efficacy of HMs on alleviating GI toxicity, investigation of the safe use of HMs was another significant focus. Potential adverse effects of the combination of HMs with chemotherapy may appear due to direct toxic effects of HMs, herb–herb, or herb–drug interactions, which are considered major concerns among patients with CRC, especially for those undergoing active chemotherapy (56). However, commonly used HMs such as C. reticulata, A. membranaceus and SJZD are generally perceived as relatively safe treatments with rarely reported adverse effects (57–60). Studies on animal and clinical trials have shown the safety of these HMs. For example, a clinical and preclinical systematic review was conducted by Zheng et al. to investigate the safety of A. membranaceus. Twenty-eight RCTs with 2,522 participants and 16 animal studies with 634 animals were accessed (61). They concluded that A. membranaceus was a relatively safe herb as no statistical difference was found in the incidence of adverse reaction. Ma Jin-Yeul conducted a study on rats to determine the potential toxic effects of SJZD, and the results showed that no direct toxic effects or negative herb–herb interaction among C. pilosula, G. uralensise, A. macrocephala, and Poria cocos (Schw.) Wolf. were found (62). Nevertheless, most of the herbs commonly combined with chemotherapy have not been well-studied, and clinically relevant data on herb–drug interaction is sparse (63).
In our meta-analysis, although none of the included studies reported adverse effects related to HMs, only seven studies (21, 27, 30, 35–37, 39) stated that no adverse effects were caused during the trials; however, evidence was insufficient. Furthermore, RCTs may not reliably explore rare adverse effects or adverse effects with significant latency because of the limited sample size and time (64). Thus, a clear conclusion regarding the safety of HMs requires further investigation. Moreover, nine studies (21–25, 30, 35, 37, 39) showed that HMs intervention alleviated CIGI toxicity without causing a reduction in the response to chemotherapy. Among them, three studies (22, 23, 35) concluded that HMs had statistically significant anti-tumor effects in increasing tumor response. The HMs evaluated in these studies deserve to be further investigated by RCTs.
The following limitations of our meta-analysis are present. First, most of the included studies in our meta-analysis were performed in Chinese populations. Further investigation should be done to assess the efficacy of HMs on CIGI toxicity in other populations. Moreover, our meta-analysis did not include any unpublished study, although an attempt was made to retrieve it. Second, the same studies were used in duplicate in the analysis of the overall effect of HM on CIGI toxicity. This resulted in inflating the total sample size and overestimated the efficacy of HMs on alleviating overall CIGI toxicity. More well-designed RCTs should be conducted to support our conclusion. Third, the RoB occurred in many of the included studies, which limited the credibility of the results. More specifically, the lack of blinding in the control groups was the most significant bias in our meta-analysis. It likely produced a placebo effect, especially for less objective outcomes such as nausea, anorexia, and abdominal distension. Other sources of bias, such as low compliance with protocols and unclarity of HM ingredients in studies, and selective reporting of non-significant outcomes, may have influenced the reliability of the effect sizes. Moreover, most of the studies were carried out in single hospitals. Fourth, the heterogeneity of nausea and vomiting, anorexia, and diarrhea were observed to be medium. Heterogeneity was not eliminated by sensitivity analysis. Various toxicity criteria were used for CIGI toxicity evaluation, contributing to the heterogeneity of this meta-analysis. Differences in sample size, tumor stage and grade, and ingredients and doses of HMs were other possible factors causing heterogeneity.
Although our meta-analysis showed that HMs intervention significantly alleviated overall CIGI toxicity, nausea and vomiting, diarrhea, oral mucositis, and abdominal distension without differentiation of methodological quality, sensitivity analysis of methodological quality revealed that a statistically significant effect of HMs is only shown in studies without a double-blind design. Therefore, further well-designed, double-blinded, large-scaled RCTs are warranted to comprehensively evaluate the treatment efficacy. Based on the ingredients of the HMs, further sensitivity analyses identified five herbs that showed consistent effects on alleviating CIGI toxicity in multiple combinations and multiple studies. However, at present, there are insufficient clinical trials of single herbs investigating the efficacy on treating CIGI toxicity in patients with CRC. Further clinical research includes the five herbs to chemotherapy in patients, the safety of the combinations of these herbs, and the potential synergistic effects of these combinations of herbs should be conducted.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
YF conceived and designed the study. YC and C-sC developed the search terms and drafted the manuscript. H-YT, CT, and NW reviewed the protocol and revised the manuscript. All authors read and approved the final version of the manuscript.
The study was financially supported by Wong's Donation (Project Code: 200006276), the Gaia Family Trust for Modern Oncology of Chinese Medicine (Project Code: 200007008), Research Grant Council, HKSAR (Project Code RGC GRF 17152116), and the Health and Medical Research Fund (Project Codes 15162961, 16172751, and 17181101).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.629132/full#supplementary-material
1. Siegel RL, Miller KD, Jemal A. Cancer statistics 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
2. Pfeiffer P, Köhne CH, Qvortrup C. The changing face of treatment for metastatic colorectal cancer. Expert Rev Anticancer Ther. (2019) 19:61–70. doi: 10.1080/14737140.2019.1543593
3. Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. (2012) 23:2479–516. doi: 10.1093/annonc/mds236
4. Gibson RJ, Bowen JM, Coller JK. What are the predictive factors in the risk and severity of chemotherapy-induced gastrointestinal toxicity? Future Oncol. (2015) 11:2367–70. doi: 10.2217/fon.15.138
5. Mocellin S, Baretta Z, Roqué IFM, Solà I, Martin-Richard M, Hallum S, et al. Second-line systemic therapy for metastatic colorectal cancer. Cochrane Datab Syst Rev. (2017) 1:Cd006875. doi: 10.1002/14651858.CD006875.pub3
6. Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. (2014) 120:1453–61. doi: 10.1002/cncr.28592
7. Boussios S, Pentheroudakis G, Katsanos K, Pavlidis N. Systemic treatment-induced gastrointestinal toxicity: incidence, clinical presentation and management. Ann Gastroenterol. (2012) 25:106–18.
8. Andreyev HJN, Lalji A, Mohammed K, Muls ACG, Watkins D, Rao S, et al. The FOCCUS study: a prospective evaluation of the frequency, severity and treatable causes of gastrointestinal symptoms during and after chemotherapy. Support Care Cancer. (2020) 29:1443–53. doi: 10.1007/s00520-020-05610-x
9. Liu W, Ge T, Pan Z, Leng Y, Lv J, Li B. The effects of herbal medicine on epilepsy. Oncotarget. (2017) 8:48385–97. doi: 10.18632/oncotarget.16801
10. Wang XJ, Zhang M. Meta-analysis on effect and safety of Chinese medicine combined with chemotherapy in the treatment of advenced colorectal cancer. Chin Med Modern Dist Educ China. (2017) 4: 53–56. doi: 10.3969/j.issn.1672-2779.2017.04.023
11. Zhong LLD, Chen HY, Cho WCS, Meng XM, Tong Y. The efficacy of Chinese herbal medicine as an adjunctive therapy for colorectal cancer: a systematic review and meta-analysis. Complement Therap Med. (2012) 20:240–52. doi: 10.1016/j.ctim.2012.02.004
12. McCulloch M, Ly H, Broffman M, See C, Clemons J, Chang R. Chinese herbal medicine and fluorouracil-based chemotherapy for colorectal cancer: a quality-adjusted meta-analysis of randomized controlled trials. Integ Cancer Therap. (2016) 15:285–307. doi: 10.1177/1534735416638738
13. Lin S, An X, Guo Y, Gu J, Xie T, Wu Q, et al. Meta-analysis of astragalus-containing traditional chinese medicine combined with chemotherapy for colorectal cancer: efficacy and safety to tumor response. Front Oncol. (2019) 9:749. doi: 10.3389/fonc.2019.00749
14. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. (1981) 47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::AID-CNCR2820470134>3.0.CO;2-6
15. National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) v3.0 (2006).
16. Zheng XY. Guidelines for Clinical Research of New Chinese Medicines. Beijing:China Medical Science and Technology Press (2002).
17. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
18. Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. (1998) 352:609–13. doi: 10.1016/S0140-6736(98)01085-X
19. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (2020). Available online at: www.training.cochrane.org/handbook
20. Chen MH, May BH, Zhou IW, Zhang AL, Xue CC. Integrative medicine for relief of nausea and vomiting in the treatment of colorectal cancer using oxaliplatin-based chemotherapy: a systematic review and meta-analysis. Phytother Res. (2016) 30:741–53. doi: 10.1002/ptr.5586
21. Xu HX, Huang XE, Li Y, Li CG, Tang JH. A clinical study on safety and efficacy of Aidi injection combined with chemotherapy. Asian Pac J Cancer Prev. (2011) 12:2233–6.
22. Ji H. FOLFOX4 chemotherapy combined with self-made traditional Chinese medicine Fuzheng Xiaoji Decoction in the treatment of advanced colon cancer. Mod J Integrat Tradit Chin Western Med. (2016) 21:2363–5. doi: 10.3969/j.issn.1008-8849.2016.21.031
23. Li X, Zhang M, Zhang B, Wang X, Song J, Gu D. Clinical effect and safety of FOLFOX4 chemotherapy combined with Chinese medicine Fuzheng Xiaoji Decoction in the treatment of patients with advanced colon cancer. Mod J Integrat Tradit Chin Western Med. (2019) 28:635–7. doi: 10.3969/j.issn.1008-8849.2019.06.018
24. Hu AL. Clinical observation of Shenlingbaizhu powder combined with IFL regimen in treatment of advanced colorectal cancer patients with chemotherapy. Pub Med Forum Magazine. (2015) 19:444–6.
25. Xing J, Wang X. A clinical study of XELOX chemotherapy combined with Chinese herbal medicines for obstructive colorectal cancer. Int J Trad Chin Med. (2016) 38:523–6. doi: 10.3760/cma.j.issn.1673-4246.2016.06.011
26. Huang SM, Xu J. Effect of Chinese medicine on gastrointestinal function of patients with colorectal cancer after chemotherapy. Chin Gastroenterol J. (2016) 24:6189–91. doi: 10.3969/j.issn.1005-9202.2016.24.068
27. Chen JX, Shen XH. Effects of qisheng mixture on chemotherapy induced myelosuppression in patients with colorectal cancer. Chin J Integrated Tradit Western Med. (2012) 32:1161–5.
28. Xiao H, Yang J. Immune enhancing effect of modified sijunzi decoction on patients with colorectal cancer undergoing chemotherapy. Chin J Integrated Tradit Western Med. (2011) 31:164–7.
29. Zhang XL, Xing RG, Miao ZG, Guo ZJ, Han GD. Effect of Xihuang Capsules in adjuvant treatment of colon cancer patients with liver metastases and on IL-17 and IL-6 in serum of patients. Chin Tradit Herbal Drugs. (2015) 46:871–4. doi: 10.7501/j.issn.0253-2670.2015.06.016
30. Nan B, Li X. Clinical observation of shenling baishu powder addition and subtraction combined with chemotherapy in the treatment of advanced colorectal cancer. China Pharmacy. (2016) 33. doi: 10.6039/j.issn.1001-0408.2016.33.13
31. Song J, Ding H, Xu X, Tian Z, He L. A clinical study of traditional Chinese medicine Pobi decoction on improving adverse reactions of patients with colorectal cancer chemotherapy. China Pract Med. (2019) 31:117–9. doi: 10.14163/j.cnki.11-5547/r.2019.31.063
32. Zhang C, Han ZG. Clinical effect observation of chemotherapy combined with Chinese herbs treatment after colorectal cancer surgery. China Modern Med. (2015) 22.
33. Peng GQ, Shi KJ, Peng ZG. The clinical adverse reactions of cooperation of Chinese and western medicine treatment for advanced colorectal cancer. Chin J Clin Rational Drug Use. (2016) 5:9–10. doi: 10.15887/j.cnki.13-1389/r.2016.05.005
34. Zeng HF. An effect of chemotherapy plus TCM medicine on colorectal cancer after operation. Clin J Chin Med. (2018) 2:52–3.
35. Motoo Y, Tomita Y, Fujita H. Prophylactic efficacy of ninjin'yoeito for oxaliplatin-induced cumulative peripheral neuropathy in patients with colorectal cancer receiving postoperative adjuvant chemotherapy: a randomized, open-label, phase 2 trial (HOPE-2). Int J Clin Oncol. (2020) 25:1123–9. doi: 10.1007/s10147-020-01648-3
36. Kono T, Hata T, Morita S, Munemoto Y, Matsui T, Kojima H, et al. Goshajinkigan oxaliplatin neurotoxicity evaluation (GONE): a phase 2, multicenter, randomized, double-blind, placebo-controlled trial of goshajinkigan to prevent oxaliplatin-induced neuropathy. Cancer Chemother Pharmacol. (2013) 72:1283–90. doi: 10.1007/s00280-013-2306-7
37. Liu Y, Zhu G, Han L, Liu J, Ma T, Yu H. Clinical study on the prevention of oxaliplatin-induced neurotoxicity with guilongtongluofang: results of a randomized, double-blind, placebo-controlled trial. Evid Based Complement Alternat Med. (2013) 2013:541217. doi: 10.1155/2013/541217
38. Oki E, Emi Y, Kojima H, Higashijima J, Kato T, Miyake Y, et al. Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): a placebo-controlled, double-blind, randomized phase III study. Int J Clin Oncol. (2015) 20:767–75. doi: 10.1007/s10147-015-0784-9
39. Matsuda C, Munemoto Y, Mishima H, Nagata N, Oshiro M, Kataoka M, et al. Double-blind, placebo-controlled, randomized phase II study of TJ-14 (Hangeshashinto) for infusional fluorinated-pyrimidine-based colorectal cancer chemotherapy-induced oral mucositis. Cancer Chemother Pharmacol. (2015) 76:97–103. doi: 10.1007/s00280-015-2767-y
40. Liu SJ. Research on curative effect of combined traditional Chinese medicine and western medicine on advanced colon cancer. Clin J Chin Med. (2014) 6:126–8. doi: 10.3969/j.issn.1674-7860.2014.30.057
41. Zhao Y, Cui H, Shi Q. Observation on the clinical efficacy of combination of Chinese and western medicines in treating colon cancer. World Latest Med Informat. (2015) 15:42–3. doi: 10.3969/j.issn.1671-3141.2015.39.026
42. Hu HL. Clinical effect of TCM of tonifying spleen and removing blood stasis in gastrointestinal function of postoperative patients with chemotherapy of colorectal cancer. Chin J Med Guide. (2016) 18:379–80.
43. National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Toxicity Criteria (CTC) v2.0 (1999).
44. Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, et al. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working group on abdominal problems. Intensive Care Med. (2012) 38:384–94. doi: 10.1007/s00134-011-2459-y
45. Chung VC, Wu X, Lu P, Hui EP, Zhang Y, Zhang AL, et al. Chinese herbal medicine for symptom management in cancer palliative care: systematic review and meta-analysis. Medicine. (2016) 95:e2793. doi: 10.1097/MD.0000000000002793
46. Yuan N, Zhang G, Yan X, Jiang X, Ma M, Ma Y, et al. The efficacy of compound kushen injection in preventing and treating radiation-induced oral mucositis: a systematic review and meta-analysis. Int J Clin Exp Med. (2017) 10:5788–5804.
47. Hróbjartsson A, Emanuelsson F, Skou Thomsen AS, Hilden J, Brorson S. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies. Int J Epidemiol. (2014) 43:1272–83. doi: 10.1093/ije/dyu115
48. Zhou WD, Xiang L, Lu HQ, Chen ZW, Gong QF, Luo R, et al. Radix Codonopsi polysaccharide against 5-fluorouracil-induced gastrointestinal mucositis in mice model. Liaon J Tradit Chin Med. (2016) 43:1495–8.
49. Chen WW, Zhang ZL, Wang JH, Shen XL, Han L, Zhou L, et al. Effects of extracts from codonopsis pilosula and atractylodes macrocephala on growth and differentiation of IEC-6 cells. Chin Pharmacol Bull. (2002) 18:444–7.
50. He ZK, Zhang GL, Tang F, Du JC, Jin WJ. Effects of extracts of pericarpium citri reticulatae and pogostemon cablin on the contraction of gastrointestinal smooth muscle and gastrointestinal hormones in rats with gastrointestinal motility disorder. Tianjin Med J. (2017) 45:1175–9. doi: 10.11958/20170739
51. Du LD, Ren Y, Wu GT, Niu TH, Wang ZW, Shao J. Effect of Angelica sinensis radix on colonic morphology and mucus secretion of experimental XuexuBianmi model mice. Zhongguo Ying Yong Sheng Li Xue Za Zhi. (2018) 34:367–70. doi: 10.12047/j.cjap.5616.2018.084
52. Liu L, Han L, Wong DY, Yue PY, Ha WY, Hu YH, et al. Effects of Si-Jun-Zi decoction polysaccharides on cell migration and gene expression in wounded rat intestinal epithelial cells. Br J Nutr. (2005) 93:21–9. doi: 10.1079/BJN20041295
53. Ni ZQ, Wang YH. Clinical study on Jiawei Sijunzi Decoction on synergism and attenuation of chemotherapy for patients with spleen and stomach qi deficiency syndrome after rectal cancer operation. J Hunan University Chin Med. (2019) 39:532–6.
54. Li YR, Gu YF, Chen YQ, Wang H. Meta-analysis of chemotherapy combined with Sijunzi Tang in treatment of patients with colorectal cancer. Chin J Experi Tradit Med Formul. (2016) 22:204–9.
55. Zheng TZ, Li W, Qu SY, He DY, Ding YH, Wei YL. Action of traditional Chinese medicine of buqi on contractile activity of isolated gastric muscle strops in rats. J Lanzhou Med Coll. (1998) 24:7.
56. Samuels N, Ben-Arye E. Exploring herbal medicine use during palliative cancer care: the integrative physician as a facilitator of pharmacist-patient-oncologist communication. Pharmaceuticals. (2020) 13:455. doi: 10.3390/ph13120455
57. Li S, Yu P, Zhou C, Tong L, Li D, Yu Z, et al. Analysis of pesticide residues in commercially available chenpi using a modified QuEChERS method and GC-MS/MS determination. J Pharm Anal. (2020) 10:60–9. doi: 10.1016/j.jpha.2019.01.005
58. Chan KW, Kwong AS.K, Tsui PN, Cheung SC.Y, Chan GC.W, Choi WF, et al. Efficacy, safety and response predictors of adjuvant astragalus for diabetic kidney disease (READY): study protocol of an add-on, assessor-blind, parallel, pragmatic randomised controlled trial. BMJ Open. (2021) 11:e042686. doi: 10.1136/bmjopen-2020-042686
59. Zhang L, Shergis JL, Yang L, Zhang AL, Guo X, Zhang L, et al. Astragalus membranaceus (Huang Qi) as adjunctive therapy for diabetic kidney disease: an updated systematic review and meta-analysis. J Ethnopharmacol. (2019) 239:111921. doi: 10.1016/j.jep.2019.111921
60. Pan W, Su X, Bao J, Wang J, Zhu J, Cai D, et al. Open randomized clinical trial on JWSJZ decoction for the treatment of ALS patients. Evid Based Complement Alternat Med. (2013) 2013:347525. doi: 10.1155/2013/347525
61. Zheng Q, Zhuang Z, Wang ZH, Deng LH, Jin WJ, Huang ZJ, et al. Clinical and preclinical systematic review of astragalus membranaceus for viral myocarditis. Oxid Med Cell Longev. (2020) 2020:1560353. doi: 10.1155/2020/1560353
62. Ma JY, Young-Beob Y, Hye-Kyung H, Dae-sun H, Hyun-Kyoo S. Subacute toxicity study on Sagunjia-tang(Sijunzi-tang) in SD rats. Korean J Oriental Med. (2007) 13.
63. Fasinu PS, Rapp GK. Herbal Interaction with chemotherapeutic drugs-a focus on clinically significant findings. Front Oncol. (2019) 9:1356. doi: 10.3389/fonc.2019.01356
Keywords: colorecal cancer, herbal medicine, traditional medicine, gastrointestinal toxicity, chemotherapy induced nausea and vomiting, chemotherapy induced diarrhea, chemotherapy induced gastrointestinal toxicity, chemotherapy induced anorexia
Citation: Chen Y, Cheng C-s, Tan H-Y, Tam CW, Wang N and Feng Y (2021) Efficacy of Herbal Medicines Intervention for Colorectal Cancer Patients With Chemotherapy-Induced Gastrointestinal Toxicity — a Systematic Review and Meta-Analysis. Front. Oncol. 11:629132. doi: 10.3389/fonc.2021.629132
Received: 13 November 2020; Accepted: 08 February 2021;
Published: 25 March 2021.
Edited by:
Ziwen Liu, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Chan-Young Kwon, Kyung Hee University, South KoreaCopyright © 2021 Chen, Cheng, Tan, Tam, Wang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yibin Feng, eWZlbmdAaGt1Lmhr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.