- Department of Clinical Laboratory, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Hepatocellular carcinoma (HCC) is often diagnosed at an advanced stage where only systemic treatment can be offered. The emergence of immune checkpoint inhibitors (ICIs) provides hope for the treatment of HCC. In this study, we performed a meta-analysis to provide evidence for the efficacy and safety of ICIs in the treatment of HCC.
Methods: The following databases and websites were searched: Embase, PubMed, Cochrane Library and ClinicalTrials.gov. The primary endpoints were response rate (RR), disease control rate (DCR), progression-free survival (PFS) and overall survival (OS).
Results: Finally, twelve studies were included in this meta-analysis. When the corresponding outcome indicators and their 95% confidence intervals (CIs) were pooled directly, the overall RR, DCR, PFS and OS were 0.17 (0.15-0.19, I2 = 56.2%, P=0.009), 0.58 (0.55-0.61, I2 = 75.9%, P<0.001), 3.27 months (2.99-3.55, I2 = 73.0%, P=0.001), 11.73 months (10.79-12.67, I2 = 90.3%, P<0.001). Compared to the control group, treatment with ICIs significantly improved RR, PFS and OS, the OR and HRs were 3.11 (2.17-4.44, P<0.001), 0.852 (0.745-0.974, P=0.019) and 0.790 (0.685-0.911, P=0.001), respectively. However, no significant improvement in DCR was found in ICIs treatment in this meta-analysis.
Conclusion: HCC patients would benefit from ICIs treatment, however, more studies are needed in the future to provide more useful evidence for the treatment of HCC by programmed death-1 (PD-1) or programmed death ligand 1 (PD-L1) inhibitors.
Introduction
Primary liver cancer is the sixth most common tumor in the world and the fourth leading cause of cancer-related death, of which 75% to 85% are hepatocellular carcinoma (HCC) (1). Chronic infection with hepatitis C virus (HCV) or hepatitis B virus (HBV) is the leading cause of hepatocellular carcinoma (2). Additionally, HCC is often diagnosed at an advanced stage where only systemic treatment can be offered (3). Although many measures have been taken, the incidence of HCC has increased during the last decade globally and increases progressively with advancing age in all populations (4).
For a long time, there has been a lack of effective systemic therapy for advanced HCC. In the past decade, sorafenib was the only approved first-line agent for patients with unresectable or metastatic hepatocellular carcinoma (3, 5, 6). However, the benefits of sorafenib as the first-line standard treatment were limited. In the global and Asian phase III studies, compared with the placebo group, the median overall survival (OS) of patients in the sorafenib group was only extended by about 2 months, and the objective response rate (ORR) was only 2%-3.3%, and it often causes adverse events (7, 8). Targeted agents currently used in patients with HCC, such as sorafenib, regorafenib, and lenvatinib, are multikinase inhibitors, which have lower response rates and higher therapeutic resistance than targeted therapy agents in other cancers (9).
The emergence of immune checkpoint inhibitors (ICIs) provides hope for the treatment of hepatocellular carcinoma. ICIs are designed to block immunosuppressive receptors expressed on the surface of T lymphocytes such as cytotoxic T-lymphocyte-associated antigen 4, programmed death receptor-1 (PD-1), and the programmed death-ligand 1 (PD-L1) expressed on tumor cells and tumor-infiltrating immune cells (10). At present, immunotherapy, together with surgery, radiotherapy, chemotherapy and targeted therapy, has become the mainstay of the treatment of malignant tumors. Therapeutic monoclonal antibodies targeting PD-1 or PD-L1 have demonstrated notable clinical efficacy in the treatment of various advanced cancers, including non-small-cell lung cancer (NSCLC), melanoma, hepatocellular carcinoma et al. (11).
In this study, the existing literature on the treatment of HCC with PD-1 or PD-L1 inhibitors was retrieved, and a meta-analysis was conducted to provide evidence for the efficacy and safety of PD-1/PD-L1 inhibitors in the treatment of HCC.
Materials and Methods
We conducted this meta-analysis according to the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines (PRISMA) (12).
Data Sources and Searches
The following databases and websites were searched: Embase, PubMed, Cochrane Library and ClinicalTrials.gov. Key words used were: hepatocellular carcinoma; PD-1/PD-L1 inhibitors, nivolumab, pembrolizumab, camrelizumab, tislelizumab, atezolizumab. The time limit was from the establishing of the databases to October 2020. References in the eligible articles would also be searched when necessary.
Study Selection
Inclusion criteria: (1) Study design: Randomized controlled trials (RCTs), cohort studies or single-arm studies about the treatment of HCC with PD-1 or PD-L1 inhibitors. (2) Population: patients with HCC. (3) Intervention and comparison: PD-1 or PD-L1 inhibitors were compared with placebo or other non-ICI drugs for HCC, such as sorafenib. (4) Outcomes: response rate [RR, defined as defined as patients with complete or partial response (9)], disease control rate [DCR, defined as patients with complete response, partial response, or stable disease (9)], progression-free survival [PFS, median, defined as the time from the date of first checkpoint inhibitor administration until radiological disease progression or death, whatever came first (3)] and overall survival [OS, median, defined as the time from the date of first checkpoint inhibitor administration until death (3)]. Exclusion criteria: (1) Duplicated articles. (2) Articles with too small sample size to extract data. (3) Articles that did not provide outcomes needed. (4) Articles about the combination of ICIs with other treatments for HCC. (5) Articles in other languages than English.
Data Extraction and Quality Assessment
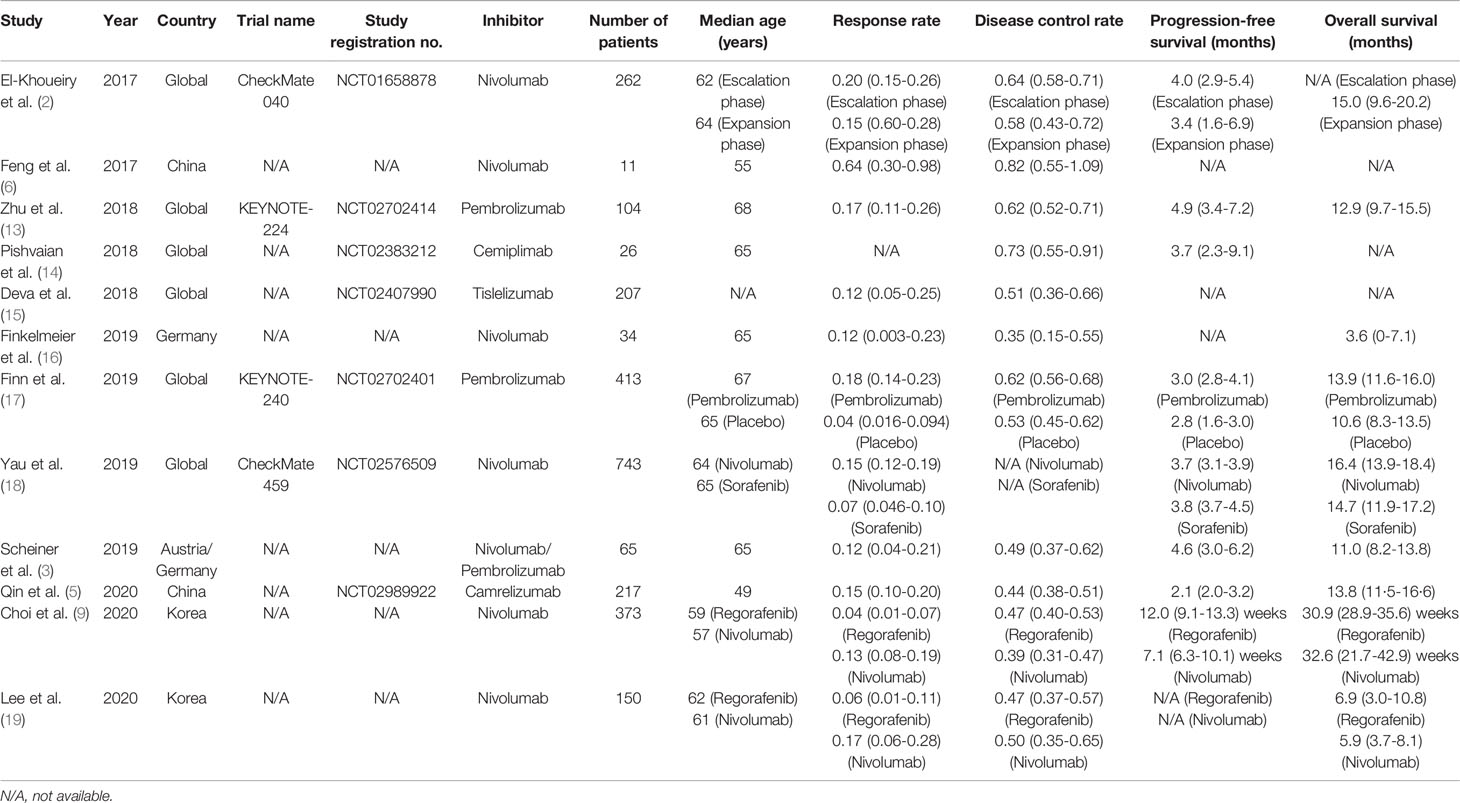
Two independent investigators screened the articles and extracted the data. If there was any disagreement, it would be resolved through discussion between the two investigators or by a third investigator. The data extracted were: publication year, countries, trial names, study registration no., inhibitors used, number of patients and their median ages (years), RR, DCR, PFS and OS.
Statistical Analysis
The data was analyzed by Stata 14.0 (StataCorp), Excel (Microsoft office 2016) and SPSS 21.0 (IBM SPSS Statistics). I2 statistic was used to evaluate the heterogeneity among studies. If I2<50% or P>0.10, then the heterogeneity was considered to be low and fixed-effects model was applied. Otherwise, the random-effects model was applied. For the single-arm study, outcomes were pooled to get overall RR, DCR, PFS and OS. Hazard ratios (HRs) were used to analyze the PFS and OS and odd ratios (ORs) were used to analyze the RR and DCR. P<0.05 indicated that the results were statistically significant.
Results
Search Results and Study Quality Assessment
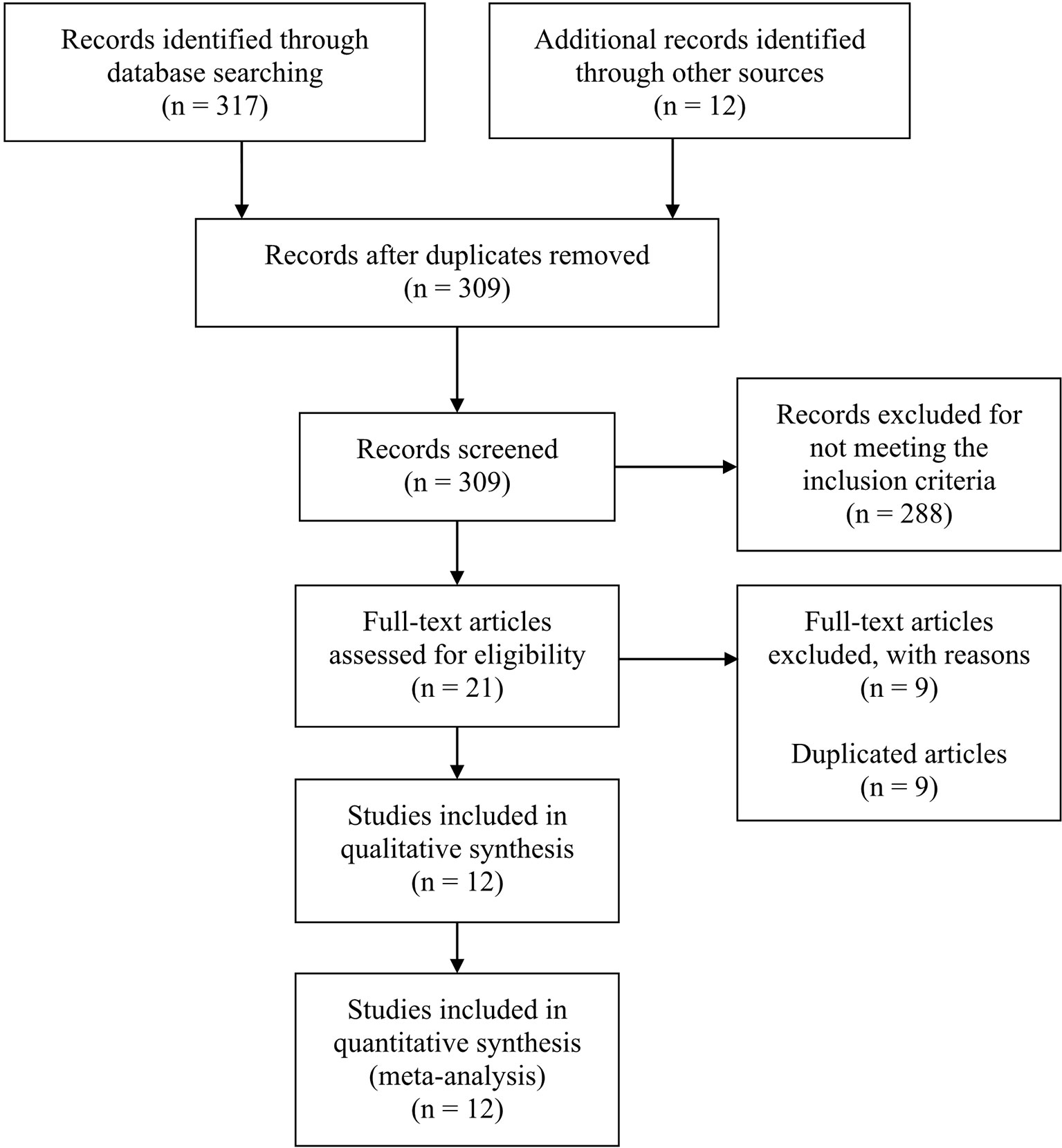
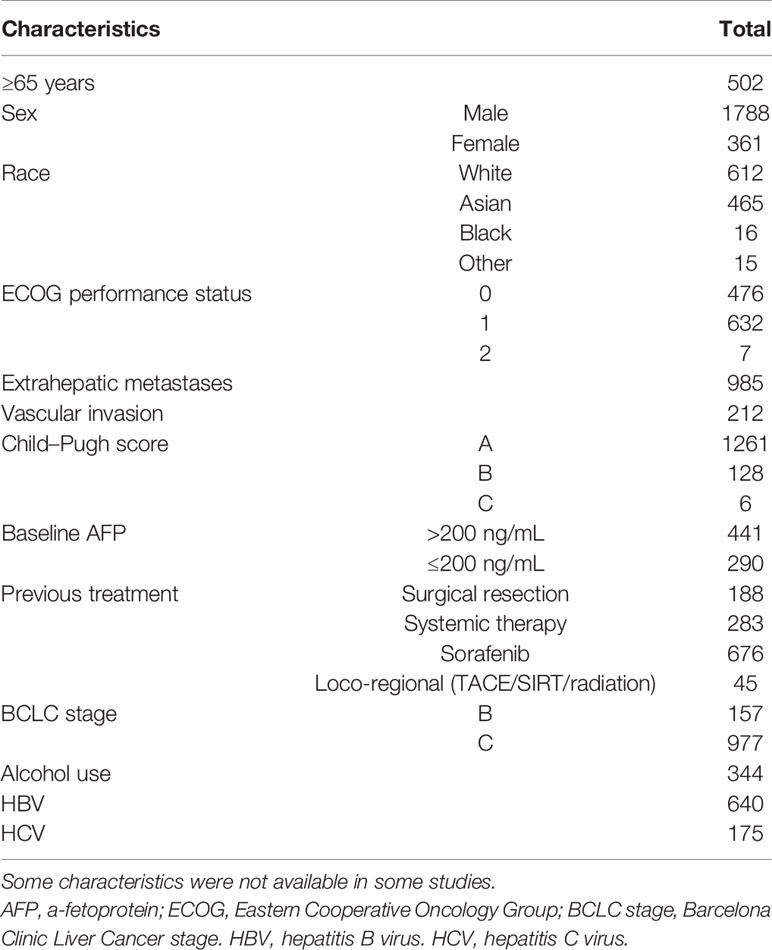
This meta-analysis searched a total of 317 studies, and finally 12 studies were included, among which 8 were single-arm studies, 2 were RCTs and 2 were retrospective cohort studies. Figure 1 displayed the flow chart of study selection. The PD-1 or PD-L1 inhibitors involved in these studies were nivolumab (7 studies), pembrolizumab (3 studies), camrelizumab (1 study), cemiplimab (1 study) and tislelizumab (1 study). The characteristics of included studies were shown in Table 1. Table 2 displayed the characteristics of the patients in the studies included in this meta-analysis.
This meta-analysis focused on 4 outcomes: response rates (RR), disease control rates (DCR), progression-free survival (PFS) and overall survival (OS). For single-arm studies, the corresponding outcome indicators and their 95% confidence intervals (CIs) were pooled directly due to the lack of control group data. Data from RCTs or cohort studies would also be pooled with single-arm studies if they reported the same outcome indicators. There were 11, 11, 7, 8 studies in this meta-analysis reporting corresponding RR, DCR, PFS, OS data and their 95% CIs, respectively. The overall RR, DCR, PFS and OS were 0.17 (0.15-0.19, I2 = 56.2%, P=0.009), 0.58 (0.55-0.61, I2 = 75.9%, P<0.001), 3.27 months (2.99-3.56, I2 = 73.0%, P=0.001), 11.73 months (10.79-12.67, I2 = 90.3%, P<0.001). See Figure 2 for details.

Figure 2 Forest plots of response rates (RR) (upper left), disease control rates (DCR) (upper right), progression-free survival (PFS) (low left) and overall survival (OS) (low right).
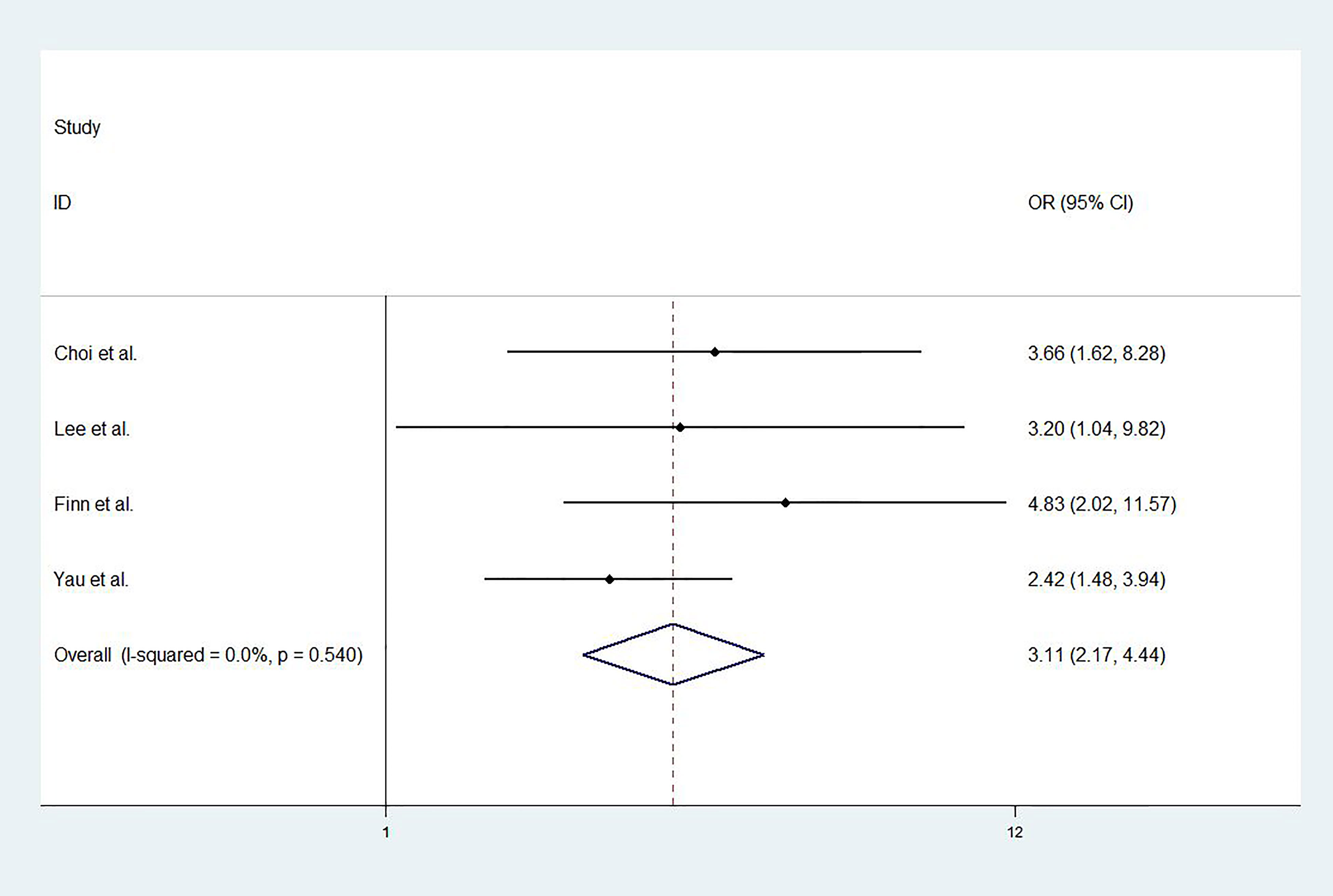
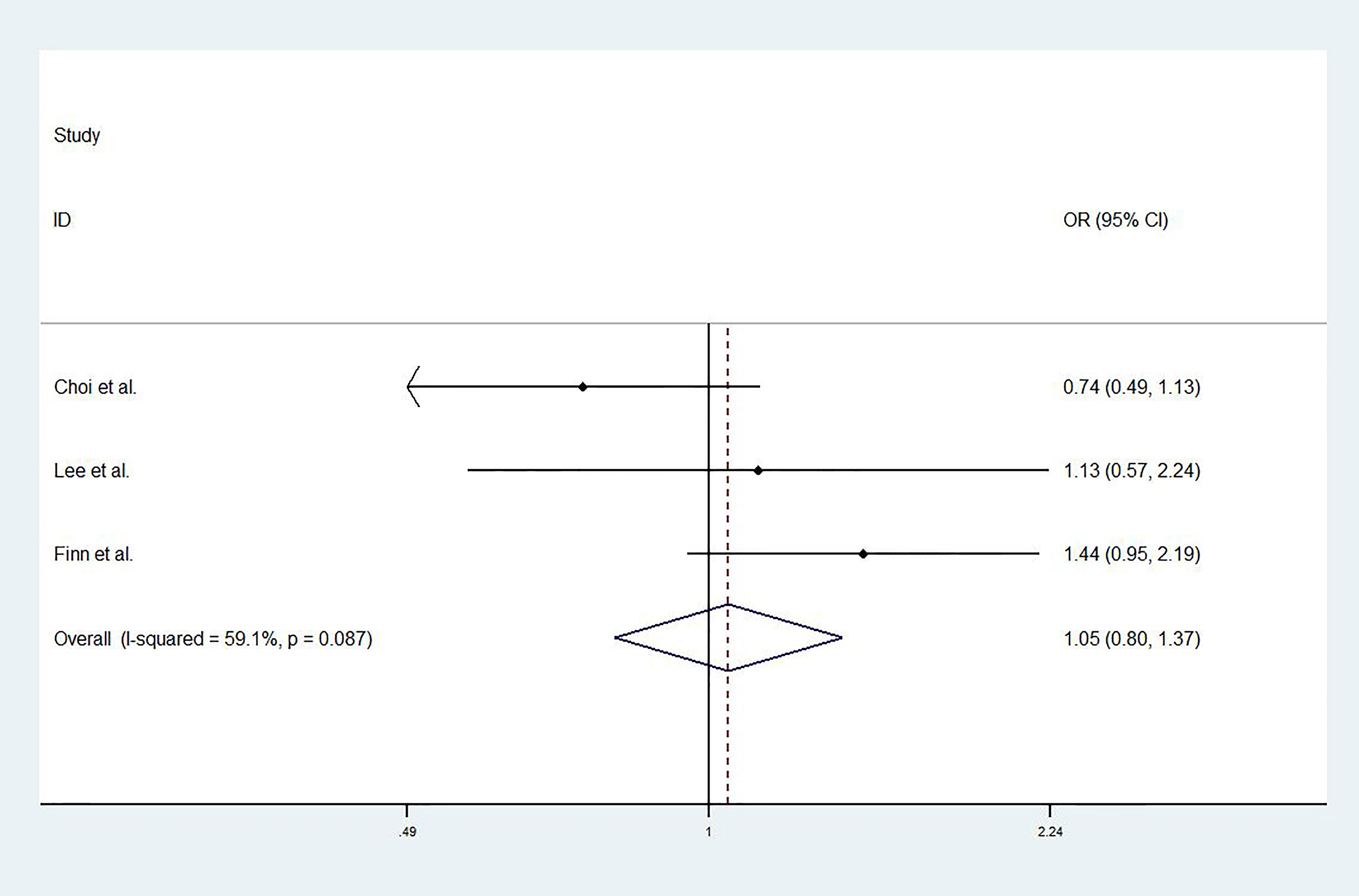
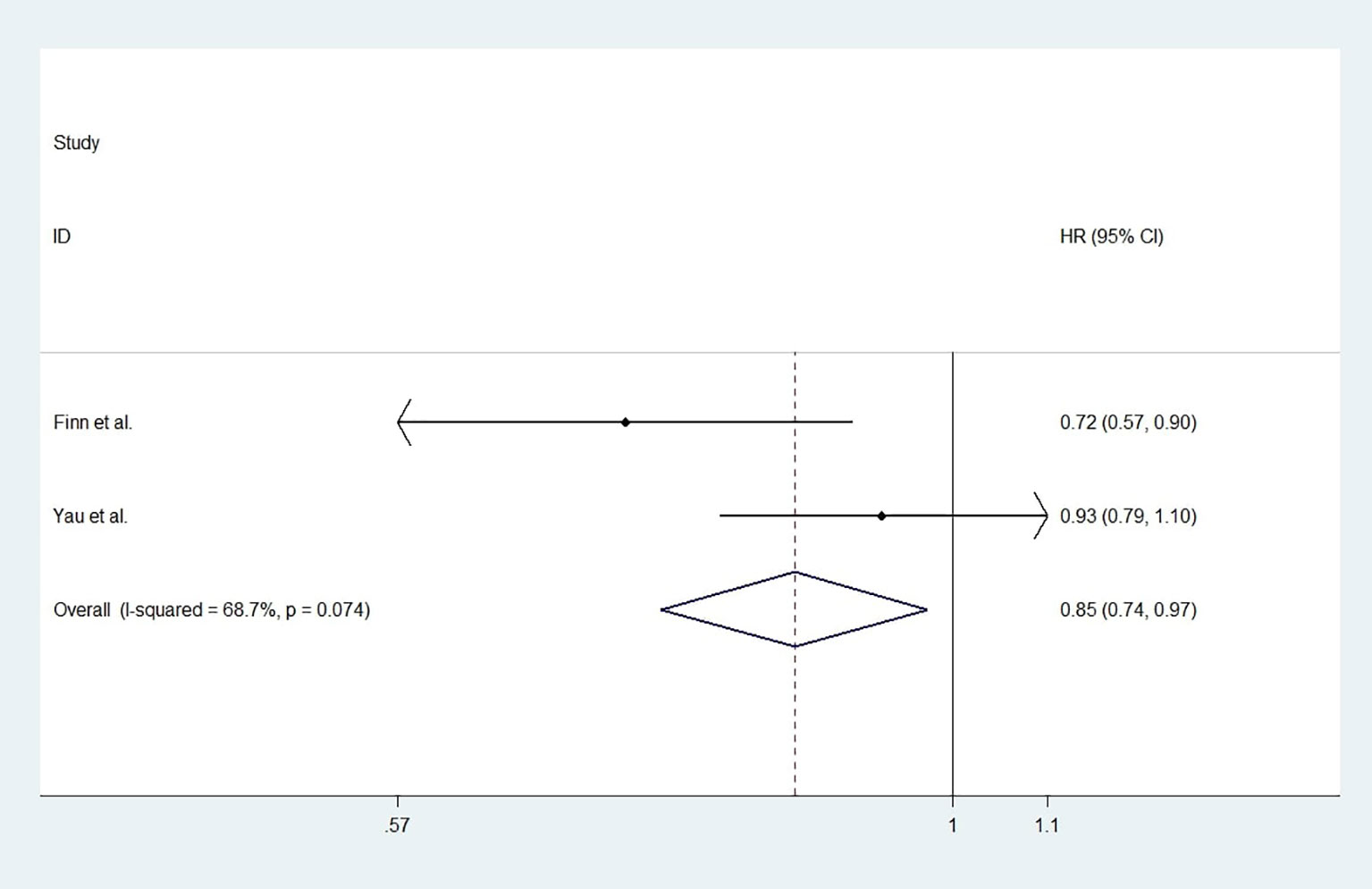
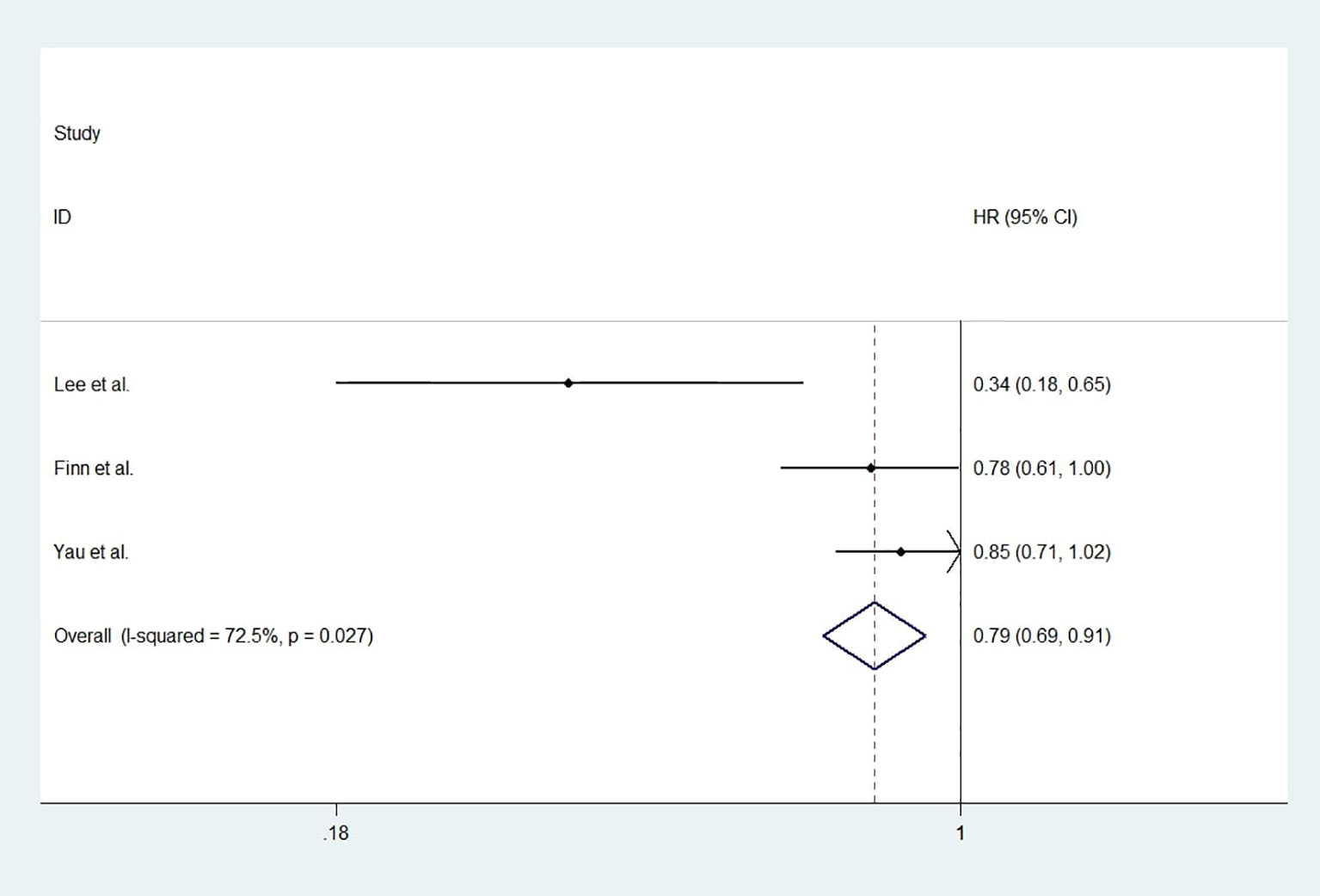
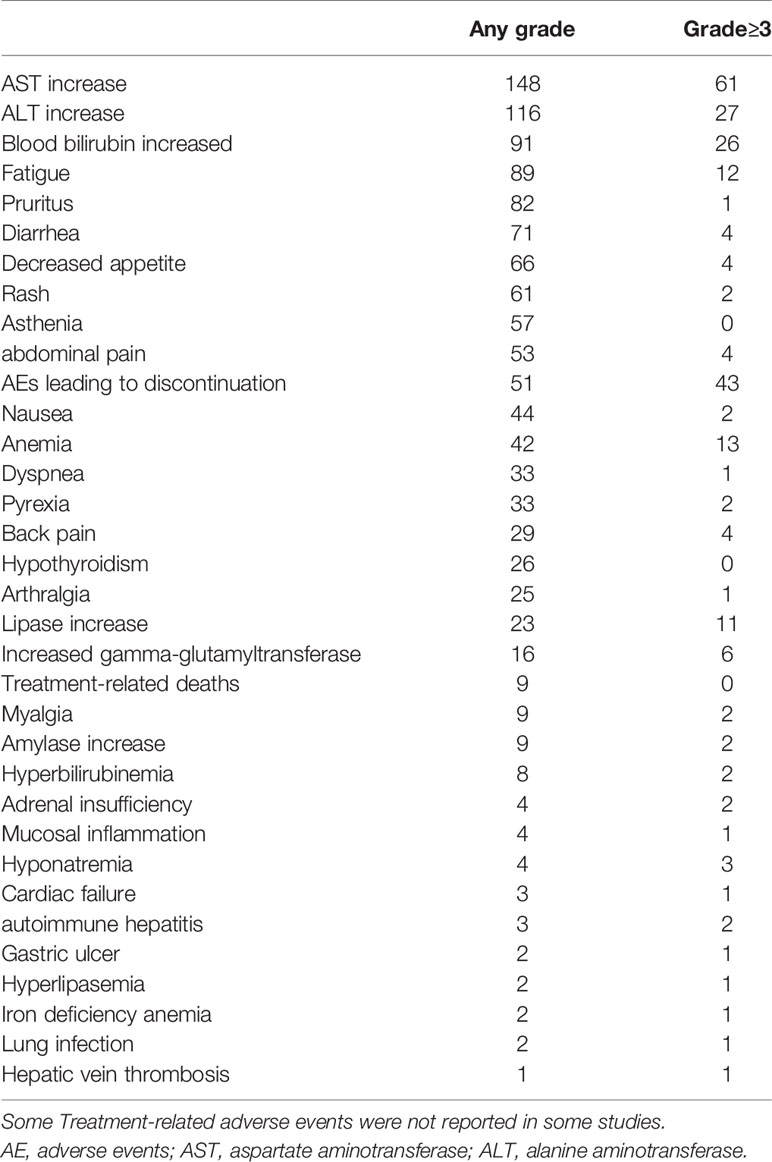
For studies with control group data (RCTs or cohort studies), data from the experimental and control groups were analyzed. For RR and DCR, the corresponding ORs were 3.11 (2.17-4.44, P<0.001) and 1.05 (0.80-1.37, P=0.731), and I2 were 0.0% (P=0.540) and 59.1% (P=0.087). For PFS and OS, HRs were 0.852 (0.745-0.974, P=0.019) and 0.790 (0.685-0.911, P=0.001), respectively. The I2 were 68.7% (P=0.074) and 72.5% (P=0.027). Figures 3–6 displayed the forest plots of RR, DCR, PFS and OS. The treatment-related adverse events in the included studies were shown in Table 3.
Discussion
In recent years, the inhibition of PD-1 and PD-L1 pathway has emerged as one of the most potential therapeutic strategies in a variety of cancers, such as melanoma, lung cancer, renal cell carcinoma, head and neck squamous cell carcinoma, etc. (20). This meta-analysis analyzed the existing studies on the treatment of HCC with PD-1 or PD-L1 inhibitors. The results showed that for patients treated by PD-1 or PD-L1 inhibitors, RR, DCR, PFS, OS were 0.17 (0.15-0.19), 0.58 (0.55-0.61), 3.27 months (2.99-3.55), 11.73 months (10.79-12.67), respectively. Compared to the control group, treatment with ICIs significantly improved RR, PFS and OS, the OR and HRs were 3.11 (2.17-4.44, P<0.001), 0.852 (0.745-0.974, P=0.019) and 0.790 (0.685-0.911, P=0.001), respectively. However, no significant improvement in DCR was found in ICIs treatment in this meta-analysis, which may be due to the small number of RCTs or cohort studies included in this study.
Although immunotherapy has achieved certain results, the efficacy of treating some patients with ICI single drug is not ideal. Therefore, similarly to the treatment strategies that were commonly used against other malignant tumors, researchers are now exploring the use of a combination of immune checkpoint inhibitors with other treatments for HCC therapy (21). For example, Finn et al. combined pembrolizumab (a PD-1 inhibitor) with lenvatinib (a multikinase inhibitor) to treat unresectable HCC (uHCC), and found that lenvatinib plus pembrolizumab has promising antitumor activity in uHCC. Toxicities were manageable, with no unexpected safety signals (22). Xu et al. used apatinib and SHR-1210 (camrelizumab) to treat advanced HCC and results also showed manageable toxicity in patients with HCC (23). Other combinations include ICIs combination, ICIs combined with MTA (molecular targeted agents), ICIs combined with local/systemic therapy (21).
However, immunotherapy, as a drug class, boosts the body’s natural defense against cancer. These drugs have adverse effects, collectively known as immune-related adverse events, that represent immune effects on normal tissue that can result from misdirected stimulation of the immune system (24). There were also a lot of adverse events reported in the included studies, such as infection, rash, pruritus, reactive cutaneous capillary endothelial proliferation (RCCEP), increased aspartate aminotransferase (2, 6, 16).
There were also many deficiencies in this meta-analysis. Firstly, although 12 studies were finally included in this study, 8 of them were single-arm studies and only 2 were RCTs, and the lack of comparison data made it difficult to provide solid evidence of the efficacy and safety in the treatment of HCC with ICIs. Most of the studies included in this meta-analysis were single-arm studies, which only provided information on patients treated with ICIs, but did not have a control group for comparison. The direct merging of the single-arm studies data with the comparative studies data in the article might make the results of this meta-analysis unstable. At the same time, it was very difficult for us to perform the bias analysis due to the lack of studies with comparison data in this meta-analysis. Secondly, all the ICIs used in the included studies were PD-1 inhibitors (nivolumab, pembrolizumab, camrelizumab, cemiplimab and tislelizumab), so that the efficacy and safety of PD-L1 inhibitors in the treatment of HCC could not be analyzed, such as atezolizumab, durvalumab et al. Besides, the classification of HCC, the dosage and method of administration of ICIs were not identical, which could affect the reliability of the meta-analysis results. In general, there were still few studies on the treatment of HCC with PD-1 or PD-L1 inhibitors, especially the high-quality RCT studies that would reveal the efficacy and safety of PD-1 or PD-L1 inhibitors in the treatment of HCC. The good news is that there are a lot of studies going on right now, such as KEYNOTE-394 (25) (pembrolizumab plus best supportive care vs. placebo plus best supportive care), RATIONALE-301 (26) (tislelizumab vs sorafenib), etc. It is hoped that in the future, more objective and rich data can be obtained from these studies, so as to provide more useful evidence for the treatment of HCC by PD-1 or PD-L1 inhibitors.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
XW conceived the idea for the study and assessed the quality of the manuscript. KF was responsible for data acquisition. SH and WJ performed the meta-analysis and co-drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
National Natural Science Foundation of China (NO. 81501828).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to extend our gratitude to all the patients who have participated in this study.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (2017) 389(10088):2492–502. doi: 10.1016/S0140-6736(17)31046-2
3. Scheiner B, Kirstein MM, Hucke F, Finkelmeier F, Schulze K, von Felden J, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther (2019) 49(10):1323–33. doi: 10.1111/apt.15334
4. Chiew Woon L, Joycelyn Jie Xin L, Su Pin C. Nivolumab for the treatment of hepatocellular carcinoma. Expert Opin Biol Ther (2020) 20(7):687–93. doi: 10.1080/14712598.2020.1749593
5. Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol (2020) 21(4):571–80. doi: 10.1016/S1470-2045(20)30011-5
6. Feng D, Hui X, Shi-Chun L, Yan-Hua B, Li C, Xiao-Hui L, et al. Initial experience of anti-PD1 therapy with nivolumab in advanced hepatocellular carcinoma. Oncotarget (2017) 8(57):96649–55. doi: 10.18632/oncotarget.20029
7. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med (2008) 359(4):378–90. doi: 10.1056/NEJMoa0708857
8. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol (2009) 10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7
9. Choi WM, Choi J, Lee D, Shim JH, Lim YS, Lee HC, et al. Regorafenib Versus Nivolumab After Sorafenib Failure: Real-World Data in Patients With Hepatocellular Carcinoma. Hepatol Commun (2020) 4(7):1073–86. doi: 10.1002/hep4.1523
10. Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and Tolerability of PD-1/PD-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis. Oncologist (2017) 22(4):470–9. doi: 10.1634/theoncologist.2016-0419
11. Xia L, Liu Y, Wang Y. PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions. Oncologist (2019) 24(Suppl 1):S31–41. doi: 10.1634/theoncologist.2019-IO-S1-s05
12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
13. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol (2018) 19(7):940–52. doi: 10.1016/S1470-2045(18)30351-6
14. Pishvaian MJ, Weiss GJ, Falchook GS, Yee N, Gil-Martin M, Shahda S, et al. 1151P Cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with advanced or metastatic hepatocellular carcinoma (HCC): Data from an expansion cohort in a phase I study. Ann Oncol (2018) 29(suppl_8). doi: 10.1093/annonc/mdy288.024
15. Deva S, Lee JS, Lin CC, Yen CJ, Millward M, Chao Y, et al. 70OA phase Ia/Ib trial of tislelizumab, an anti-PD-1 antibody (ab), in patients (pts) with advanced solid tumors. Ann Oncol (2018) 29(suppl_10). doi: 10.1093/annonc/mdy487.042
16. Finkelmeier F, Czauderna C, Perkhofer L, Ettrich TJ, Trojan J, Weinmann A, et al. Feasibility and safety of nivolumab in advanced hepatocellular carcinoma: real-life experience from three German centers. J Cancer Res Clin Oncol (2019) 145(1):253–9. doi: 10.1007/s00432-018-2780-8
17. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol (2020) 38(3):193–202. doi: 10.1200/JCO.19.01307
18. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol (2019) 30(suppl_5). doi: 10.1093/annonc/mdz394.029
19. Lee CH, Lee YB, Kim MA, Jang H, Oh H, Kim SW, et al. Effectiveness of nivolumab versus regorafenib in hepatocellular carcinoma patients who failed sorafenib treatment. Clin Mol Hepatol (2020) 26(3):328–39. doi: 10.3350/cmh.2019.0049n
20. Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, et al. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer (2018) 119(5):538–45. doi: 10.1038/s41416-018-0100-3
21. Kang S, Bai X, Chen S, Song Y, Liu L. The potential combinational immunotherapies for treatment of hepatocellular carcinoma. J Intervent Med (2019) 2(2):47–51. doi: 10.1016/j.jimed.2019.09.006
22. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol (2020) 38(26):2960–70. doi: 10.1200/JCO.20.00808
23. Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, et al. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin Cancer Res (2019) 25(2):515–23. doi: 10.1158/1078-0432.CCR-18-2484
24. Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ (2018) 360:k793. doi: 10.1136/bmj.k793
Keywords: hepatocellular carcinoma, immune checkpoint inhibitors, PD-1/PD-L1 inhibitors, immunotherapy, meta-analysis
Citation: He S, Jiang W, Fan K and Wang X (2021) The Efficacy and Safety of Programmed Death-1 and Programmed Death Ligand 1 Inhibitors for the Treatment of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 11:626984. doi: 10.3389/fonc.2021.626984
Received: 07 November 2020; Accepted: 04 March 2021;
Published: 23 March 2021.
Edited by:
Alexandr Bazhin, LMU Munich University Hospital, GermanyReviewed by:
Markus Schoenberg, LMU Munich University Hospital, GermanySze Huey Tan, National Cancer Centre Singapore, Singapore
Copyright © 2021 He, Jiang, Fan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaobei Wang, d3hiNjE3NEBodXN0LmVkdS5jbg==
†These authors have contributed equally to this work
 Shukang He
Shukang He Weichao Jiang†
Weichao Jiang† Kai Fan
Kai Fan