
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 25 March 2021
Sec. Thoracic Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.619376
This article is part of the Research TopicTherapeutic Strategies in EGFR Mutant Lung CancerView all 26 articles
Background: Chemotherapy has been the current standard adjuvant treatment for early-stage non-small-cell lung cancer (NSCLC) patients, while recent studies showed benefits of epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI). We conducted a cost-effectiveness analysis to comprehensively evaluate the benefit of EGFR-TKI compared with chemotherapy for early-stage EGFR-mutant NSCLC patients after resection from the perspective of the Chinese health care system.
Method: A Markov model was established. Clinical data were based on the phase 3, ADJUVANT trial, where stage II-IIIA, EGFR-mutant NSCLC patients were randomized into gefitinib group or chemotherapy group after resection. Cost parameters mainly included costs of drugs, examinations, and adverse events (AEs). Effect parameters were evaluated by quality-adjusted life year (QALY). Outcomes contained incremental cost-effective ratio (ICER), average cost-effective ratio (ACER), and net benefit. The willingness-to-pay threshold was set as 3 times per capita gross domestic product ($30,828/QALY). Sensitivity analyses were also conducted to verify the stability of the model.
Results: Patients who received gefitinib had both a higher cost ($12,057.98 vs. $11,883.73) and a higher QALY (1.55 vs. 1.42) than patients who received chemotherapy. With an ICER of $1,345.62/QALY, adjuvant gefitinib was of economic benefit compared with chemotherapy. The ACER and net benefit were also consistent (gefitinib vs. chemotherapy, ACER: $7,802.30/QALY vs. $8,392.77/QALY; net benefit: $35,584.85 vs. $31,767.17). Sensitivity analyses indicated the stability of the model and the impact of utility.
Conclusion: Adjuvant EGFR-TKI application for early-stage EGFR-mutant NSCLC patients was cost-effective compared with chemotherapy, which might provide a reference for clinical decision-making and medical insurance policy formulation in China.
Non-small-cell lung cancer (NSCLC) is the major type of lung cancer, among which, approximately 25% are diagnosed with early-stage NSCLC and are supposed to undergo surgical resection (1, 2). However, the high postoperative recurrence rate has a negative impact on prognosis, with approximately 30% for stage I patients and up to 75% for stage III patients (3–5). Common relapse after surgery for NSCLC patients highlights the importance of optimizing adjuvant treatment regions to eliminate residual tumors (6). Previous studies have shown that postoperative cisplatin-based chemotherapy could bring survival benefits to NSCLC patients with a 5–10% improvement in 5-year overall survival (OS) rate, furthermore, the combination of vinorelbine and cisplatin is currently the standard adjuvant treatment regimen for resected stage II–III NSCLC patients (7–10). However, the toxicity of chemotherapy reduces the compliance of patients and therapeutic efficacy (4).
Epidermal growth factor receptor (EGFR) mutation is the most common type of genomic alteration in NSCLC, with an incidence range of 10–20% in Caucasians to 50% in Asian populations (11). The efficacy of EGFR-tyrosine kinase inhibitor (EGFR-TKI) for advanced EGFR-mutant NSCLC patients is well accepted, and current studies are exploring the application of EGFR-TKI in the adjuvant setting (12). A meta-analysis confirmed the disease-free survival (DFS) benefit of adjuvant EGFR-TKI compared with both placebo (hazard ratio [HR]: 0.59, 95% confidence interval [CI]: 0.40–0.88, P = 0.009) and chemotherapy (HR: 0.42, 95%CI: 0.19–0.93, P = 0.03) for EGFR-mutant patients, however, OS analysis only showed superior tendency without significant benefit, besides patients administrated with EGFR-TKI had fewer adverse events (AEs) than patients receiving chemotherapy (risk ratio [RR]: 0.26, 95%CI: 0.18–0.38, P <0.00001) (13).
In clinical practice, despite survival benefits, cost and quality of life are also important considerations for treatment decisions. Patient’s quality of life reflects both the physical and psychological status of patients, besides, the impact of AEs is also included. Multi-dimensional assessments are not only conducive to a comprehensive evaluation and decision making but could also improve the compliance of patients. Thus, a cost-effectiveness analysis was conducted to evaluate the benefit of EGFR-TKI compared with chemotherapy as adjuvant therapy for EGFR-mutant NSCLC patients after resection, in order to select the optimal adjuvant therapy comprehensively and provide guidance for both clinical decision making and health insurance policy formulation.
The clinical data was based on a phase 3, randomized, open-label ADJUVANT trial (CTONG1104, NCT01405079), patients who underwent complete resection (R0) and diagnosed with stage II–IIIA (N1–N2), EGFR mutation-positive (exon 19 deletion or exon 21 Leu858Arg) NSCLC were eligible from multi-centers in China (14, 15). After complete resection and randomization, patients were allotted into targeted therapy group (receiving gefitinib 250 mg once daily for 24 months, oral administration) or chemotherapy group (receiving vinorelbine 25 mg/m² on days 1 and 8 plus cisplatin 75 mg/m² on day 1, every 3 weeks for four cycles, intravenous administration). After a median follow-up of 80 months, results showed patients receiving gefitinib achieved a superior DFS (median DFS: 30.8 months vs. 19.8 months, HR: 0.56, 95%CI: 0.40–0.79, P = 0.001) than those administrated with chemotherapy, while OS analysis did not show a significant difference between gefitinib and chemotherapy group (median OS: 75.5 months vs. 62.8 months, HR: 0.92, 95%CI: 0.62–1.36, P = 0.674). Besides, in terms of AEs, patients receiving gefitinib suffered from fewer AEs than patients in the chemotherapy group (AEs: 58% vs. 80%, grades 3–4: 12% vs. 48%). Detailed information was listed in Table 1.
A Markov model was established using Treeage Pro with a 21-day cycle length, a 10-year horizon, a 3% annual discount rate, and three mutually independent Markov states: DFS, progressive disease (PD), and die. All patients were in DFS state initially and transferred into other states according to progressive and survival probabilities. The structure of the Markov model was presented in Figure 1. Progression and survival probabilities were extracted and calculated from DFS and OS Kaplan–Meier curves respectively in the ADJUVANT trial by GetData Graph Digitizer and R software (14, 15). Fitting to the Weibull model, the following formulas were used to calculate progressive or survival probability P and transition probability at time t: P =1 − Exp(−r × t); Pt = 1 – Exp [λ(t − u)γ – λtγ], where r represented for the progressive or survival rate, u was the cycle length, λ and γ were the scale and shape parameter separately (16).
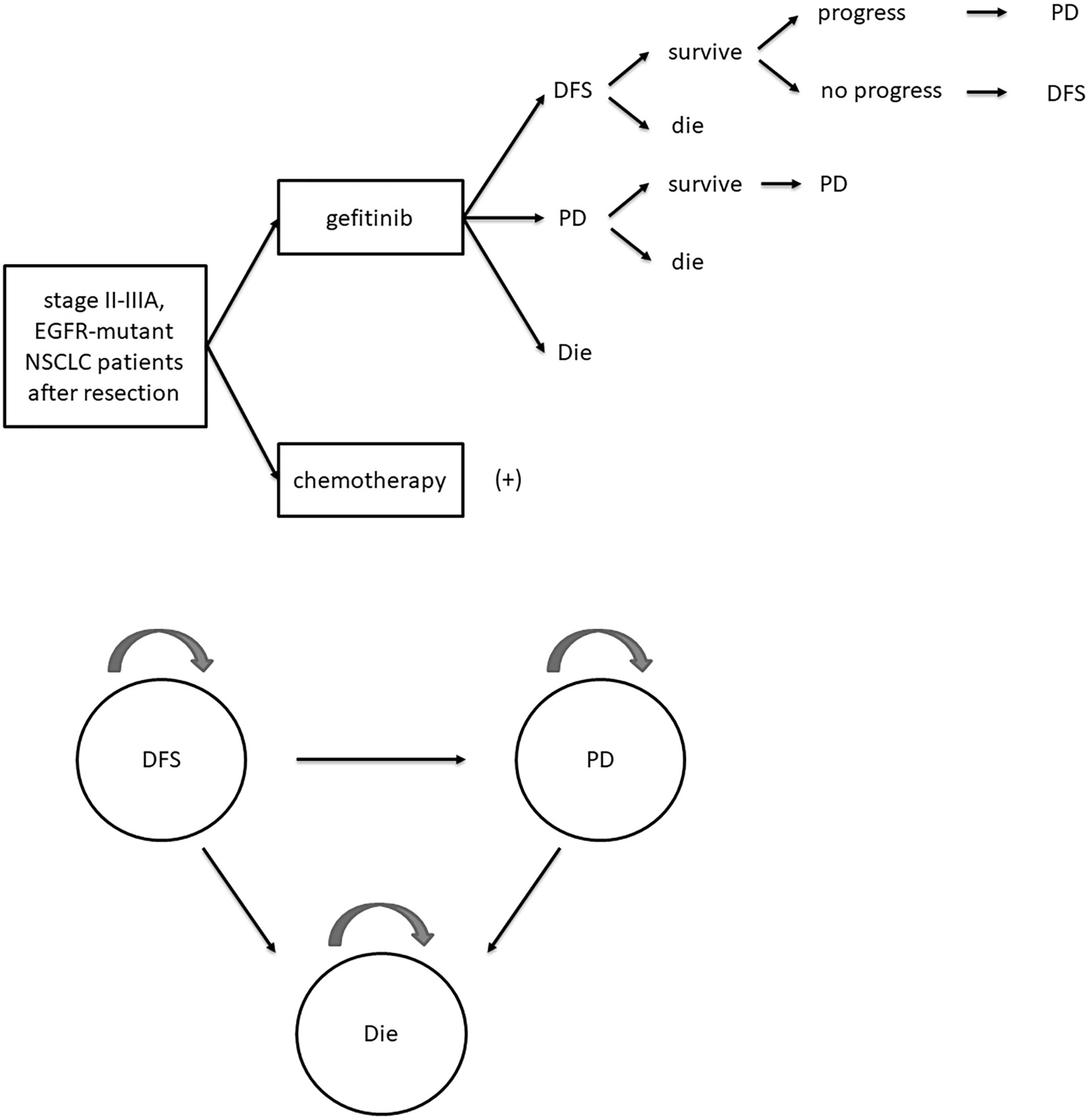
Figure 1 Schematic diagram of Markov model. DFS, disease-free survival; PD, progressive disease; EGFR, epidermal growth factor receptor; NSCLC, non-small-cell lung cancer.
Costs were extracted from local hospitals and published literature. For specific calculation of drug doses and costs, we assumed a typical patient with a 1.64 m height, a 65 kg weight, and a body surface area (BSA) of 1.72 m2 according to previous cost-effectiveness analysis evaluating chemotherapy (17). Direct medical costs of drugs (gefitinib, vinorelbine, and cisplatin), imaging examinations, laboratory tests, follow-up, supportive care, grade ≥3 AEs, and PD state were calculated as US dollars (exchange rate: 6.8409) (17, 18). Common expenses for both groups such as costs of surgery and EGFR tests were not calculated since they did not affect the cost-effectiveness results.
Effects of treatments were representative by quality-adjusted life year (QALY), which is a comprehensive evaluation index of patients’ survival period and quality of life. Health state utility parameters were extracted from published literature and were ranged from 0 to 1 with 1 representing the best physical and psychological conditions. Extracted utilities contained utilities for DFS state, including oral therapy (0.8) and intravenous therapy (0.76) respectively; PD state (0.7); death (0); and AEs of grades 3–4 (−0.0731), thereinto considering the unavailability of accurate utilities of various AEs, the average value was obtained for substitution based on published studies (19, 20). Specific utilities were adjusted based on clinical reports in the ADJUVANT trial. Cost and utility parameters were listed in Table 2.
The primary outcome of the study was the incremental cost-effective ratio (ICER), which is the ratio of incremental cost and incremental effect between the two groups. Secondary outcomes were the average cost-effective ratio (ACER, the ratio of average cost and average effect) and net benefit (willingness-to-pay [WTP] × effect − cost). The cost-effectiveness analysis was conducted in the perspective of the Chinese health care system and the WTP threshold was set as three times per capita gross domestic product (GDP, $30,828/QALY).
Both one-way sensitivity analysis and probabilistic sensitivity analysis were conducted by Treeage Pro. One-way sensitivity analysis was displayed as a Tornado diagram to explore the most influential factor on the Markov model. Cost parameters were evaluated with a range of 30% based on the baseline value, while a 20% range was set for both utilities and survival probabilities. Detailed information was shown in Supplemental Table 1. Probabilistic sensitivity analysis was conducted through Monte Carlo Simulation with 1,000 iterations, cost parameters were hypothesized to fit gamma distribution, while utilities and survival probabilities were assumed to be beta distributed (21). Results were displayed as cost-effectiveness acceptability curve and net monetary benefit acceptability curve in order to represent the cost-effective iterations with various WTP thresholds.
Clinical data in the current manuscript were extracted from a published clinical trial (ADJUVANT trial/CTONG1104/NCT01405079) and therefore ethics approval or specific consent procedures were not required for this study.
Patients receiving gefitinib achieved a better QALY than patients receiving chemotherapy (1.55 vs. 1.42) with an incremental QALY of 0.13, however, the gefitinib group also had a higher cost than the chemotherapy group ($12,057.98 vs. $11,883.73) with an incremental cost of $174.24. The ICER was $1,345.62/QALY, which indicated the administration of gefitinib was cost-effective compared to chemotherapy in the perspective of the Chinese health care system. The cost-effectiveness analysis curve was shown in Figure 2. As for secondary outcomes, the gefitinib group showed a lower ACER and a higher net benefit than the chemotherapy group (ACER: $7,802.30/QALY vs. $8,392.77/QALY; net benefit: $35,584.85 vs. $31,767.17). Specific results were listed in Table 3.
As for sensitivity analyses, one-way sensitivity analysis showed that the utility of patients receiving gefitinib in DFS state was the most dominant influence index, followed by the utility of DFS patients receiving chemotherapy and utility of PD patients receiving gefitinib. The tornado diagram was shown in Supplemental Figure 1. While in terms of probabilistic sensitivity analysis, the cost-effectiveness acceptability curve showed even at a WTP of $1,500, the gefitinib group was of more economic benefit than the chemotherapy group, which was displayed in Figure 3. Net monetary benefit acceptability curve showed advantages gradually expanded with the increase of WTP (Supplemental Figure 2). Probability distributions were listed in Supplemental Table 2.
Although several clinical trials confirmed the superior DFS benefit of adjuvant EGFR-TKI over both chemotherapy and placebo, none of them showed a long-term survival benefit, in addition, it was also suggested that two years of treatment course was not conducive to the adherence of patients due to chronic AEs, hence the adjuvant application of EGFR-TKI was still controversial (22). Thus, we conducted a comprehensive evaluation of adjuvant EGFR-TKI benefits from multi-dimensions including clinical survival benefit, quality of life, and costs. According to the outcomes, administration of EGFR-TKI brought a higher cost of $174.24 and a higher QALY of 0.13, the ICER was $1,345.62/QALY, which showed prominent advantages.
Previous cost-effectiveness analyses demonstrated that the application of adjuvant chemotherapy had superior benefits compared with the observation group for early-stage NSCLC patients in the Canadian health care system perspective (23). While other cost-effectiveness studies showed economic benefits of prognostic tests in guiding adjuvant chemotherapy, which was also from the perspective of the United States and Canada health care systems (24–26). However, there was still a lack of economic evaluations on EGFR-TKI in the adjuvant setting, making this study the first cost-effectiveness analysis to comprehensively evaluate the benefit of adjuvant EGFR-TKI therapy for early-stage EGFR-mutant NSCLC patients.
Currently, only advanced EGFR mutation-positive NSCLC patients receiving first-generation EGFR-TKI are included in the Chinese medical insurance policy. Considering the consistent DFS, safety, and cost-effectiveness benefits of first-generation EGFR-TKI application for early-stage EGFR mutation-positive NSCLC patients, it is suggested that the reimbursement policy could be further expanded. In addition, the cost of gefitinib was based on the ADJUVANT trial, while the cost of domestic gefitinib was cheaper, which could further expand the benefits.
In spite of the positive outcomes, further explorations and developments are still required in this field. Firstly, since the survival benefit of the third-generation EGFR-TKI, osimertinib for advanced EGFR-mutant NSCLC patients has been verified, studies are also exploring the efficacy of osimertinib in the adjuvant setting for EGFR-mutant NSCLC patients after complete resection (27). The phase 3 ADAURA (NCT02511106) trial demonstrated that patients receiving osimertinib achieved a significant superior DFS compared with those receiving placebo (stage II–IIIA patients: HR, 0.17; 99.06%CI, 0.11–0.26; P <0.001; stage IB–IIIA patients: HR, 0.20; 99.12%CI, 0.14–0.30; P <0.001) (28). We did not include osimertinib in the cost-effectiveness analysis due to the immature survival data, further exploration could be conducted with mature data. Secondly, although EGFR-TKI monotherapy could reduce AEs, considering tumor heterogeneity and the efficacy of other treatment regimens, including chemotherapy and radiotherapy, different combination therapies should also be further investigated for assessing the optimal adjuvant therapy (22). Thirdly, there is still a lack of relevant studies for treatments after resistance to EGFR-TKI adjuvant therapy, which should be further explored as well (5).
The main limitation of the study was that the outcomes were restricted to geographical regions and populations. Despite the phase 2, single-arm, SELECT trial also showed efficacy of adjuvant EGFR-TKI therapy based on major non-Asian population (5-year DFS rate: 56%, 5-year OS rate: 86%), studies showed that both EGFR mutation rate and therapeutic efficacy of EGFR-TKI are related to ethnicity with distinctive clinicopathologic characteristics (3, 29). Both the clinical data and cost parameters of this cost-effectiveness analysis were based on Chinese populations, thus it is not suitable to generalize the outcomes to Caucasians or other populations.
In the cost-effectiveness analysis, we comprehensively evaluated the benefit of adjuvant EGFR-TKI application for early-stage EGFR mutation-positive NSCLC patients by synthesizing clinical survival data, quality of life and cost parameters. The ICER was $1,345.62/QALY and demonstrated economic benefits from the perspective of the Chinese health care system. Our results could further propel the development of precision treatment, and provide a reference for clinical decision-making and medical insurance policy formulation in China.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Clinical data in the current manuscript were extracted from published clinical trial (ADJUVANT trial/CTONG1104/NCT01405079) and therefore ethics approval or specific consent procedures were not required for this study.
Guarantor of integrity of the entire study: JC. Study concepts and design: JC and WL. Literature research: WL and HG. Data analysis: WL and LL. Manuscript preparation: WL and JC. Manuscript editing: JC. All authors contributed to the article and approved the submitted version.
This work was supported by Nation Key Research and Development Program of China (Grant No. 2016YFC1303800).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.619376/full#supplementary-material
1. He Q, Xin P, Zhang M, Jiang S, Zhang J, Zhong S, et al. The impact of epidermal growth factor receptor mutations on the prognosis of resected non-small cell lung cancer: a meta-analysis of literatures. Transl Lung Cancer Res (2019) 8(2):124–34. doi: 10.21037/tlcr.2019.03.14
2. Friedlaender A, Addeo A, Russo A, Gregorc V, Cortinovis D, Rolfo CD. Targeted Therapies in Early Stage NSCLC: Hype or Hope? Int J Mol Sci (2020) 21(17):6329. doi: 10.3390/ijms21176329
3. Pennell NA, Neal JW, Chaft JE, Azzoli CG, Jänne PA, Govindan R, et al. SELECT: A Phase II Trial of Adjuvant Erlotinib in Patients With Resected Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer. J Clin Oncol (2019) 37(2):97–104. doi: 10.1200/JCO.18.00131
4. Xu ST, Xi JJ, Zhong WZ, Mao WM, Wu L, Shen Y, et al. The Unique Spatial-Temporal Treatment Failure Patterns of Adjuvant Gefitinib Therapy: A Post Hoc Analysis of the ADJUVANT Trial (CTONG 1104). J Thorac Oncol (2019) 14(3):503–12. doi: 10.1016/j.jtho.2018.11.020
5. Kauffmann-Guerrero D. Adjuvant TKI treatment of EGFR-mutant lung cancer-already ripe for decision? Transl Lung Cancer Res (2020) 9(4):964–6. doi: 10.21037/tlcr.2020.04.13
6. Romero D. Adjuvant TKIs - a long-term matter. Nat Rev Clin Oncol (2019) 16(2):67. doi: 10.1038/s41571-018-0144-6
7. Kris MG, Gaspar LE, Chaft JE, Kennedy EB, Azzoli CG, Ellis PM, et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non-Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol (2017) 35(25):2960–74. doi: 10.1200/JCO.2017.72.4401
8. Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol (2006) 7(9):719–27. doi: 10.1016/S1470-2045(06)70804-X
9. Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol (2008) 26(21):3552–9. doi: 10.1200/JCO.2007.13.9030
10. Douillard JY, Tribodet H, Aubert D, Shepherd FA, Rosell R, Ding K, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol (2010) 5(2):220–8. doi: 10.1097/JTO.0b013e3181c814e7
11. Li WQ, Cui JW. Non-small cell lung cancer patients with ex19del or exon 21 L858R mutation: distinct mechanisms, different efficacies to treatments. J Cancer Res Clin Oncol (2020) 146(9):2329–38. doi: 10.1007/s00432-020-03296-6
12. Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol (2015) 26(9):1883–9. doi: 10.1093/annonc/mdv270
13. Cheng H, Li XJ, Wang XJ, Chen ZW, Wang RQ, Zhong HC, et al. A meta-analysis of adjuvant EGFR-TKIs for patients with resected non-small cell lung cancer. Lung Cancer (2019) 137:7–13. doi: 10.1016/j.lungcan.2019.08.002
14. Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Shen Y, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol (2018) 19(1):139–48. doi: 10.1016/S1470-2045(17)30729-5
15. Wu YL, Zhong W, Wang Q, Mao W, Xu ST, Wu L, et al. CTONG1104: Adjuvant gefitinib versus chemotherapy for resected N1-N2 NSCLC with EGFR mutation—Final overall survival analysis of the randomized phase III trial 1 analysis of the randomized phase III trial. J Clin Oncol (2020) 38:9005. doi: 10.1200/JCO.2020.38.15_suppl.9005
16. Li W, Bai R, Qian L, Chen N, Zhao Y, Han F, et al. Cost-effectiveness of icotinib versus whole-brain irradiation with or without chemotherapy in EGFR-mutant NSCLC patients with brain metastases. Asia Pac J Clin Oncol (2020). doi: 10.1111/ajco.13291
17. Lu S, Ye M, Ding L, Tan F, Fu J, Wu B. Cost-effectiveness of gefitinib, icotinib, and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Oncotarget (2017) 8(6):9996–10006. doi: 10.18632/oncotarget.14310
18. Zeng X, Karnon J, Wang S, Wu B, Wan X, Peng L. The cost of treating advanced non-small cell lung cancer: estimates from the chinese experience. PloS One (2012) 7(10):e48323. doi: 10.1371/journal.pone.0048323
19. Labbé C, Leung Y, Silva Lemes JG, Stewart E, Brown C, Cosio AP, et al. Real-World EQ5D Health Utility Scores for Patients With Metastatic Lung Cancer by Molecular Alteration and Response to Therapy. Clin Lung Cancer (2017) 18(4):388–95.e4. doi: 10.1016/j.cllc.2016.12.015
20. Brown J, Cook K, Adamski K, Lau J, Bargo D, Breen S, et al. Utility values associated with advanced or metastatic non-small cell lung cancer: data needs for economic modeling. Expert Rev Pharmacoecon Outcomes Res (2017) 17(2):153–64. doi: 10.1080/14737167.2017.1311210
21. Handorf EA, McElligott S, Vachani A, Langer CJ, Bristol Demeter M, Armstrong K, et al. Cost effectiveness of personalized therapy for first-line treatment of stage IV and recurrent incurable adenocarcinoma of the lung. J Oncol Pract (2012) 8(5):267–74. doi: 10.1200/JOP.2011.000502
22. Jassem J. Adjuvant EGFR tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: still an investigational approach. Transl Lung Cancer Res (2019) 8(Suppl 4):S387–s90. doi: 10.21037/tlcr.2019.09.02
23. Ng R, Hasan B, Mittmann N, Florescu M, Shepherd FA, Ding K, et al. Economic analysis of NCIC CTG JBR.10: a randomized trial of adjuvant vinorelbine plus cisplatin compared with observation in early stage non-small-cell lung cancer–a report of the Working Group on Economic Analysis, and the Lung Disease Site Group, National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol (2007) 25(16):2256–61. doi: 10.1200/JCO.2006.09.4342
24. Roth JA, Billings P, Ramsey SD, Dumanois R, Carlson JJ. Cost-effectiveness of a 14-gene risk score assay to target adjuvant chemotherapy in early stage non-squamous non-small cell lung cancer. Oncologist (2014) 19(5):466–76. doi: 10.1634/theoncologist.2013-0357
25. Wong KM, Ding K, Li S, Bradbury P, Tsao MS, Der SD, et al. A Cost-Effectiveness Analysis of Using the JBR.10-Based 15-Gene Expression Signature to Guide Adjuvant Chemotherapy in Early Stage Non-Small-Cell Lung Cancer. Clin Lung Cancer (2017) 18(1):e41–e7. doi: 10.1016/j.cllc.2016.06.009
26. Stenehjem DD, Bellows BK, Yager KM, Jones J, Kaldate R, Siebert U, et al. Cost-Utility of a Prognostic Test Guiding Adjuvant Chemotherapy Decisions in Early-Stage Non-Small Cell Lung Cancer. Oncologist (2016) 21(2):196–204. doi: 10.1634/theoncologist.2015-0162
27. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378(2):113–25. doi: 10.1056/NEJMoa1713137
28. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med (2020) 383(18):1711–23. doi: 10.1056/NEJMoa2027071
Keywords: non-small-cell lung cancer, epidermal growth factor receptor-tyrosine kinase inhibitor, cost-effectiveness analysis, adjuvant therapy, chemotherapy
Citation: Li W, Guo H, Li L and Cui J (2021) Comprehensive Comparison Between Adjuvant Targeted Therapy and Chemotherapy for EGFR-Mutant NSCLC Patients: A Cost-Effectiveness Analysis. Front. Oncol. 11:619376. doi: 10.3389/fonc.2021.619376
Received: 20 October 2020; Accepted: 05 March 2021;
Published: 25 March 2021.
Edited by:
Yaxiong Zhang, Sun Yat-sen University Cancer Center (SYSUCC), ChinaCopyright © 2021 Li, Guo, Li and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiuwei Cui, Y3VpandAamx1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.