
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 13 April 2021
Sec. Thoracic Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.598265
This article is part of the Research TopicTherapeutic Strategies in EGFR Mutant Lung CancerView all 26 articles
Objective: The aim of this meta-analysis was to evaluate efficacy and toxicity of epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) in combination with chemotherapy (CT) compared to EGFR-TKI monotherapy as first-line treatment in advanced non-small cell lung cancer (NSCLC) harboring activating EGFR mutation.
Methods: A systematic literature search of randomized controlled trials using Cochrane Library, PubMed, Embase, and Web of Science, was performed up to Jan. 7th, 2020. Hazard ratios (HRs) with 95% confidence intervals (CI) were calculated as effect values for progress-free survival (PFS) and overall survival (OS). Risk ratio (RR) and Odds ratio (OR) were calculated as effect values for objective response rate (ORR) and toxicity, respectively.
Results: A total of eight randomized trials involving 1,349 advanced NSCLC patients with sensitive EGFR mutation were included in the meta-analysis. All patients in both groups received first-generation TKI as first-line treatment. The pooled HR of PFS and OS was 0.56 (95% CI = 0.50–0.64; P <0.00001) and 0.70 (95% CI = 0.54–0.90; P = 0.005), respectively. Subgroup analysis showed significantly higher OS advantages in patients receiving doublet CT (P = 0.02) and concurrent therapy (P = 0.002). The ORR in the EGFR-TKI plus CT group was significantly higher than in the EGFR-TKI monotherapy group (RR = 1.18, 95% CI = 1.10–1.26). The combination regimen showed a higher incidence of chemotherapy-induced toxicities. Subgroup analysis indicated that doublet chemotherapy rather than single-agent chemotherapy significantly increased incidence of grade 3 or higher leukopenia, neutropenia and anemia.
Conclusions: Compared with EGFR-TKI monotherapy, the combination of first-generation EGFR-TKI and CT, especially when applying concurrent delivery of platinum-based doublet chemotherapeutic drugs, significantly improve ORR and prolong PFS and OS in first-line treatment for advanced EGFR-mutated NSCLC. Although increasing incidence of chemotherapy-induced toxicities occurs in the combination group, it is well tolerated and clinically manageable.
Lung cancer is the leading cause of cancer morbidity and mortality worldwide, with 2.1 million new cases and 1.8 million deaths estimated in 2018 (1). Non-small cell lung cancer (NSCLC) accounts for nearly 85% of all cases of lung cancer. Due to ineffective screening method and insidious symptom, lung cancer is usually diagnosed at an advanced stage in a majority of patients. Systematic therapy, therefore, remains the pivotal treatment approach for NSCLC in clinical practice.
Epidermal growth factor receptor (EGFR) is one of the most significant driver genes in lung cancer and its mutated form tempts constitutive activation of the EGFR tyrosine kinase, leading to uncontrolled growth and proliferation of tumor. Approximately, 10–15% of NSCLC patients in Europe and 30–35% of NSCLC patients in Asia harbor activating EGFR mutation (2, 3). An Individual Patient Data Meta-Analysis of six large randomized controlled trials (RCTs) suggested that compared with chemotherapy, first-line EGFR tyrosine kinase inhibitor (TKI) significantly prolonged progression-free survival (PFS) (median PFS = 11.0 vs. 5.6 months; Hazard Ratio (HR) = 0.37, 95% confidence intervals (CI) = 0.32–0.42, P <0.001) in EGFR-mutated NSCLC patients (4). Thus, first-line EGFR-TKI monotherapy, including representative gefitinib and erlotinib, is currently the mainstay treatment for naive advanced EGFR mutation positive NSCLC patients (5).
Inevitably, most patients who initially respond to an EGFR-TKI over 8–12 months, eventually develop resistance to first- or second-generation drugs (6). In order to prolong the survival \outcome, combination therapy of EGFR-TKI with other therapeutic drugs is an emerging promising approach. As one of promising combined strategy, EGFR-TKI plus chemotherapy has long been evaluated to overcome or delay resistance in advanced NSCLC since the early 2000s (7). Due to lack of EGFR-mutation status selection, however, preliminary studies failed to demonstrate the survival benefit of EGFR-TKI in combination with chemotherapy (8, 9). Recently, many phase II-III RCTs have investigated the EGFR-TKI plus chemotherapy combination in selected NSCLC patient with activating EGFR mutation (10). These studies with EGFR sensitive mutation had mixed overall survival (OS) results. Meta-analysis assessing the efficacy and toxicity of EGFR-TKI in combination with chemotherapy as first-line treatment in advanced NSCLC with EGFR activating mutation, has not yet been reported to our best knowledge. Therefore, we synthesized the results of different studies in this meta-analysis, to provide more objective data for the optimal clinical use of EGFR-TKI combined with chemotherapy.
Our study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (11). A comprehensive search of PubMed, Embase, Web of Science, and Cochrane databases was conducted to identify all relevant full-length literatures on the comparison of EGFR-TKI plus chemotherapy to EGFR-TKI alone as first-line treatment in advanced non-small cell lung cancer with activating EGFR mutation, up till Jan. 7th, 2020. Keywords including non-small-cell lung cancer, EGFR, TKI, and chemotherapy were used for initial search of eligible literatures. For instance, the following retrieval strategy was used on PubMed: (lung cancer OR lung carcinoma OR lung neoplasm) AND (epidermal growth factor receptor OR EGFR) AND (tyrosine kinase inhibitor OR TKI OR gefitinib OR erlotinib OR icotinib OR afatinib OR dacomitinib OR osimertinib) AND (chemotherapy OR pemetrexed OR gemcitabine OR paclitaxel OR vinorelbine) AND (first line OR untreat* OR naive). To obtain additional related articles, references cited in the eligible studies were also searched manually.
Inclusion criteria were as follows: (1) the patients were histologically diagnosed with advanced NSCLC; (2) the randomized trials were performed to evaluate the efficacy and safety of compared the EGFR-TKI plus chemotherapy to EGFR-TKI alone as first-line treatment in advanced non-small cell lung cancer with activating EGFR mutation; (3) the studies with affluent data for pooling the survival results, response rate, and toxicity. Exclusion criteria were as follows: (a) nonoriginal research articles with limited data, such as letters, case reports, reviews, comments, and conference abstracts; (b) duplicates of previous publications; and (c) studies with a sample size of less than 30 analyzable lesions.
Basic information of each individual study was extracted by two reviewers (QW and WXL) independently. Any discrepancies were resolved by discussion and consensus during the process of research selection and data extraction or by consulting the third investigator (FX) when necessary. The following information was extracted: name of first author, trial name, publication year, trial phase, treatment arms, participants’ characteristics, number of patients evaluable for analysis and other clinical characteristics. The primary data for calculation were the HR with 95% CI for PFS and OS, the number of patients who experienced a partial response or complete response, the number of patients that developed all grade toxicities.
A specific tool recommended by the Cochrane Collaboration was applied to assess the risk of bias for each identified study. Biases were categorized as selection bias, performance bias, detection bias, attrition bias, and reporting bias (12).
All statistical analysis was performed using the Review Manager 5.3 software (Cochrane Library, Oxford, UK) and STATA 12.0 software (Stata Corp., College Station, TX). Cochrane’s Q statistic and I² (I2 >50% was considered substantially heterogeneous) statistic test were used to evaluate the heterogeneity between the eligible studies (13). The random effect model was used when there was significant heterogeneity between studies; otherwise, the fixed effect model was used. Publication bias was assessed via funnel plot with Begg’s rank correlation. A two-sided p-value of <0.05 was considered to be statistically significant.
A comprehensive literature search yielded 1,732 non-duplicate papers. Of these, 21 full-text articles were screened for assessment of eligibility in the review. Finally, eight studies comparing EGFR-TKI plus chemotherapy with EGFR-TKI alone as first-line treatment in advanced non-small cell lung cancer with activating EGFR mutation were included in this meta-analysis (8, 14–20). The detail excluded studies from the 13 potential literatures was summarized in the supplementary selection of study. The flow diagram of studies selection was summarized in Figure 1.
From eight clinical trials, a total of 1,349 advanced NSCLC patients with sensitive EGFR mutation (704 in EGFR-TKI combination group and 645 in EGFR-TKI monotherapy group), were available for the meta-analysis. The great majority of histological type was adenocarcinoma. Of these EGFR-mutated patients, exon 19 deletion and L858R point mutation accounted for 55.7% (751/1,349) and 40.9% (552/1,349), respectively. As for first-line EGFR-TKI treatment, patients in all trials received first-generation drugs, including gefitinib (six studies), erlotinib (one study) and icotinib (one study). Most of trials involved platinum-based doublet chemotherapy, apart from two trials (8, 16). In addition, concurrent drug delivery of TKI and chemotherapy were engaged in four of these studies, three studies were intercalated, and one study was sequential. The characteristics of the included studies are listed in Table 1.
The median PFS as the primary end point of the studies ranged from 7.2 to 20.9 months in the EGFR-TKI combination arm and ranged from 4.7 to 16.6 months in EGFR-TKI monotherapy arm. The heterogeneity was not significant (I2 = 11%; P = 0.34), and hence a fixed-effects model was used to pool the data (Figure 2A). The pooled HR of PFS in total population with activating EGFR mutation was 0.56 (95% CI = 0.50–0.64; P <0.00001; Figure 2A), which indicated that EGFR-TKI combination therapy significantly reduced the risk of disease progression compared with EGFR-TKIs alone. Furthermore, the pooled HR of PFS in patients with Exon 19 deletion or L858R point mutation was 0.54 (95% CI = 0.45–0.65; P <0.00001; Figure 2B) and 0.52 (95% CI = 0.42–0.65; P <0.00001; Figure 2C), respectively, retrieved from five included studies.
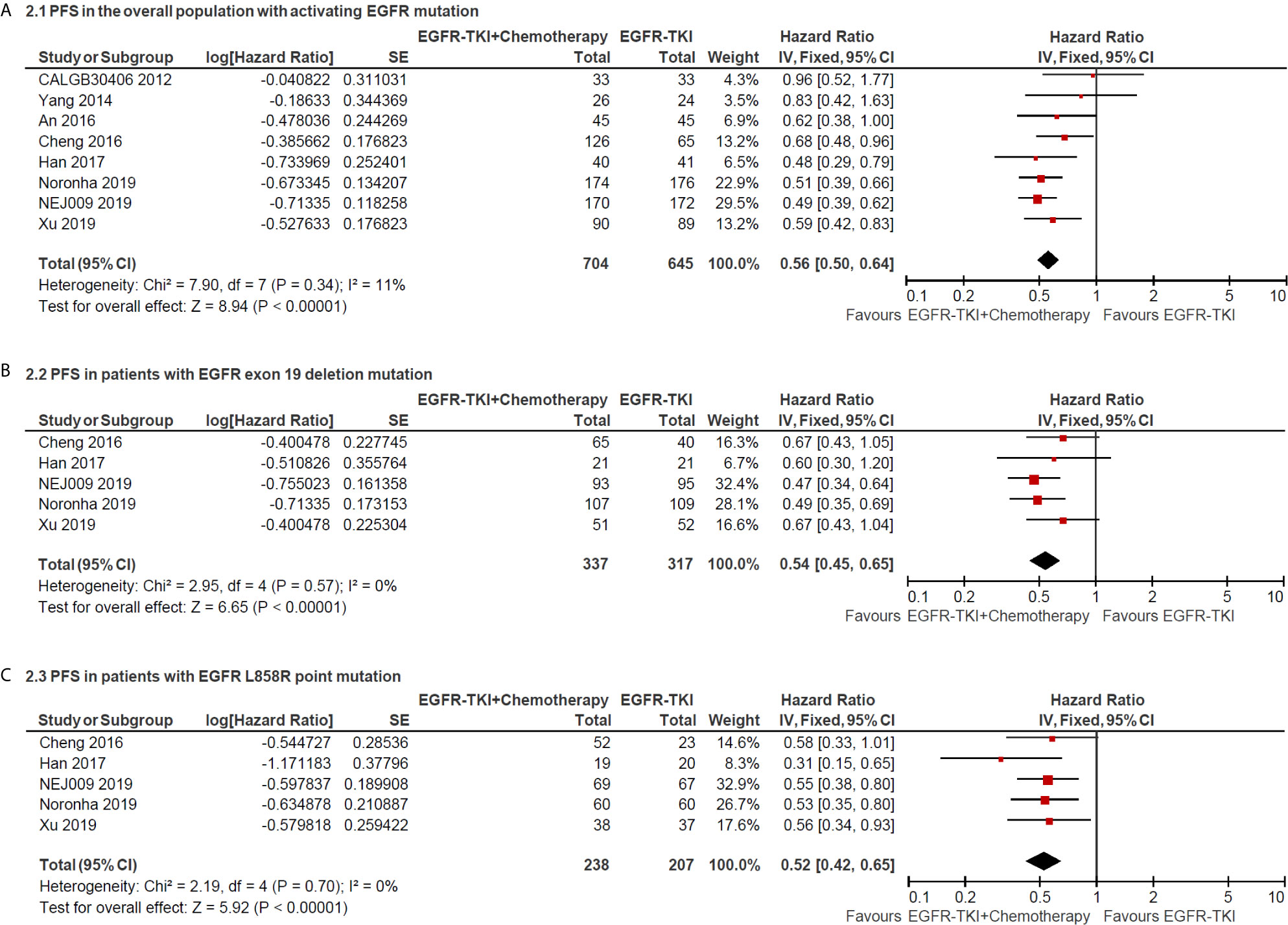
Figure 2 Forest plot of hazard ratio of progress-free survival in overall patients with all sites of positive activating EGFR mutation (A); in patients with positive exon 19 deletion mutation (B) and positive L858R point mutation (C).
Subgroup analysis of chemotherapy drugs revealed that double-agents mighty induced longer PFS (double-agents, HR = 0.54, 95% CI = 0.47–0.62; single-agent, HR = 0.66, 95% CI = 0.50–0.87; Figure 3A). Moreover, subgroup analysis of combination timing indicated statistically significant PFS in concurrent and intercalated therapy (HR = 0.55, 95% CI = 0.47–0.64 and HR = 0.57, 95% CI = 0.45–0.73, respectively; Figure 3A), but was not statistically significant in sequential therapy (HR = 0.83; 95% CI = 0.42–1.63; Figure 3A).
The median OS in the included studies ranged from 18.5 to 50.9 months in the EGFR-TKI combination arm and ranged from 14.2 to 45.7 months in EGFR-TKI monotherapy arm. The pooled HR of OS in total EGFR sensitive mutation sites between two arms was 0.70 (95% CI = 0.54–0.90; P = 0.005; Figure 4A), which indicated that combination therapy significantly improved the OS compared with EGFR-TKIs alone. Furthermore, the pooled HR of OS in patients with exon 19 deletion or L858R point mutation was 0.60 (95% CI = 0.42–0.86; P = 0.005; Figure 4B) and 0.82 (95% CI = 0.57–1.18; P = 0.28), respectively, retrieved from two trials. It revealed that overall survival benefit from EGFR-TKI in combination with chemotherapy might occur in patients with positive 19 deletion mutation other than in L858R mutation.
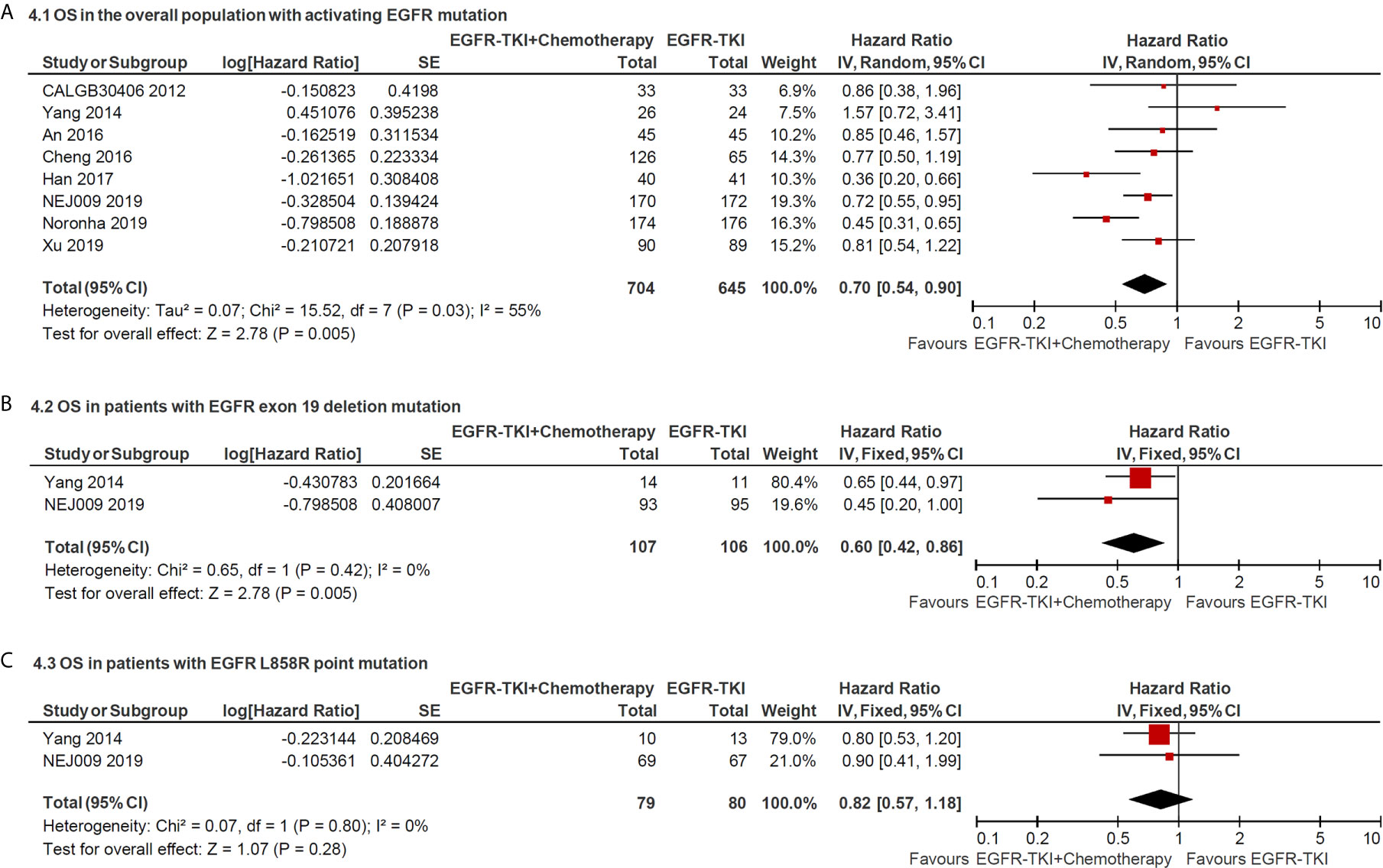
Figure 4 Forest plot of the hazard ratio of overall survival in patients with: (A) all sites of positive activating EGFR mutation; (B) positive exon 19 deletion mutation and (C) positive L858R point mutation.
Subgroup analysis of chemotherapy drugs revealed that double-agents significantly improved PFS (double-agents, HR = 0.67, 95% CI = 0.48–0.94; single-agent, HR = 0.80, 95% CI = 0.56–1.14; Figure 3B). In addition, subgroup analysis of combination timing indicated statistically significant OS in concurrent therapy (HR = 0.65, 95% CI = 0.49–0.86; Figure 3B), but was not statistically significant in intercalated and sequential therapy (HR = 0.64, 95% CI = 0.39–1.07 and HR = 1.57, 95% CI = 0.72–3.41, respectively; Figure 3B).
All of eight studies reported the data of objective response rate (ORR). The heterogeneity was not significant (I2 = 0%; P = 0.66), and hence a fixed-effects model was used to pool the data (Figure 5). The meta-analysis demonstrated that pooled ORR in the EGFR-TKI plus chemotherapy group was significantly higher than in the EGFR-TKI monotherapy group (RR = 1.18, 95% CI = 1.10–1.26; p <0.00001; Figure 5).
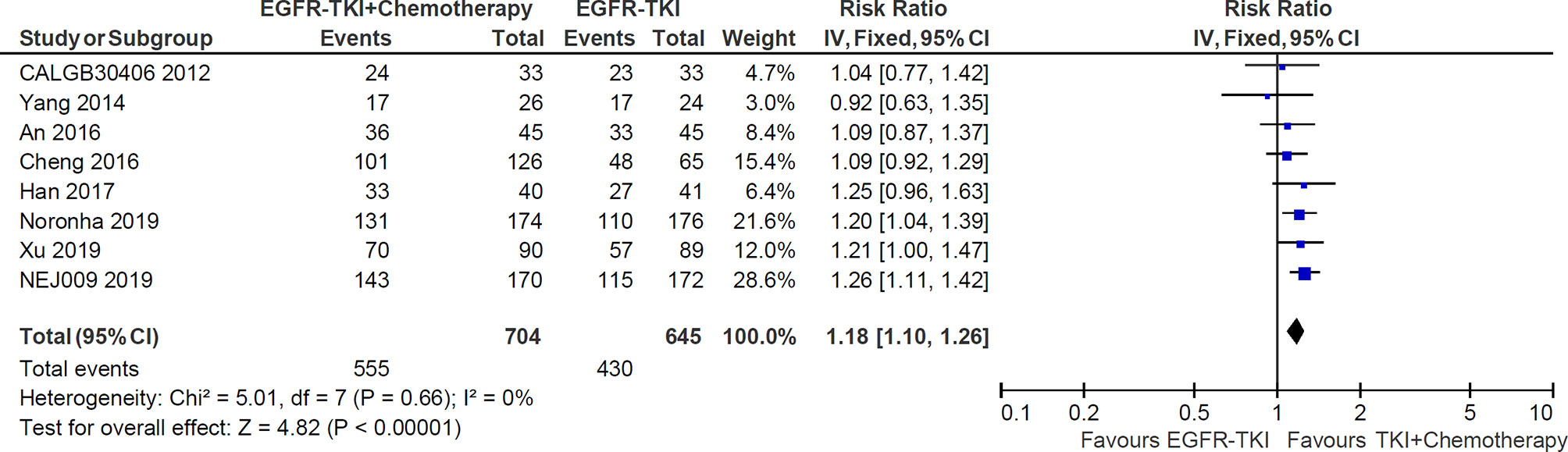
Figure 5 Forest plot of Risk ratio of objective response rate in EGFR-TKI plus chemotherapy group and EGFR-TKI monotherapy group.
Compared with the EGFR-TKI alone, the addition of chemotherapy to TKI was associated with a higher incidence of any grade hematologic toxicities, such as neutropenia (grades 1–2, OR = 16.84, 95% CI = 9.04–31.36; grade 3 or higher, OR = 10.03, 95% CI = 1.04–96.69) and thrombocytopenia (grades 1–2, OR = 7.04, 95% CI = 4.73–10.48; grade 3 or higher, OR = 43.41, 95% CI = 6.01–313.71). Similarly, the combination therapy significantly increased the incidence of chemotherapy-induced toxicities, including any grade fatigue, anorexia, nausea and vomiting, and grade 3 or higher diarrhea. Subgroup analysis indicated that doublet chemotherapy significantly increased incidence of grade 3 or higher leukopenia (OR = 37.30, 95% CI = 7.26–191.63, Figure 6A), neutropenia (OR = 54.79, 95% CI = 13.21–227.24, Figure 6B) and anemia (OR = 14.28, 95% CI = 6.10–33.43, Figure 6C), while those differences were not significant in single-agent chemotherapy subgroup.
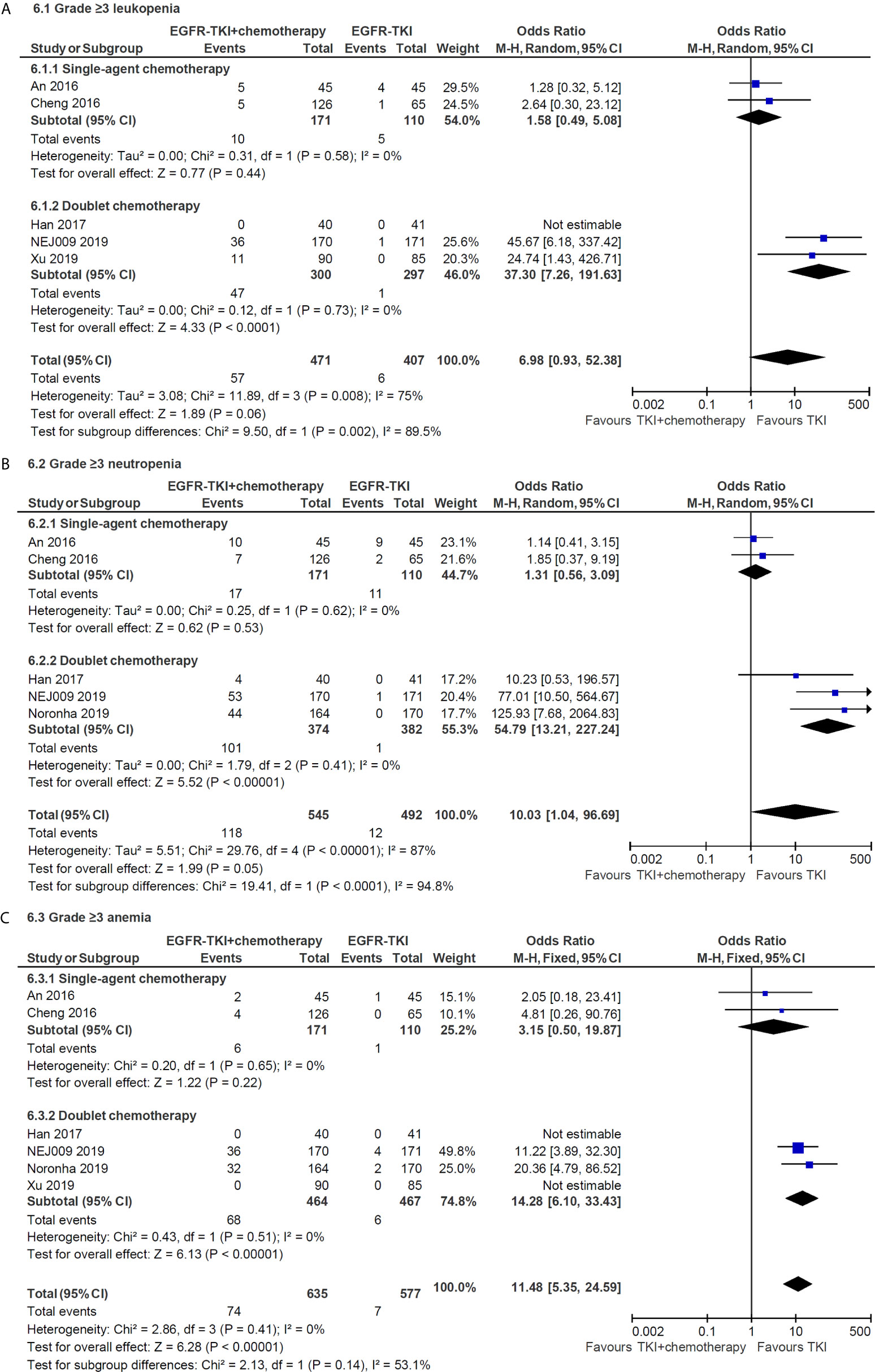
Figure 6 Subgroup analysis of grade 3 or higher hematologic toxicities for leukopenia (A), neutropenia (B) and anemia (C) in single-agent and doublet chemotherapy.
Nevertheless, no significant differences were founded in terms of any grade rash and grade 3 or higher liver dysfunction when applying combined treatment. The detail results are illustrated in Table 2.
As defined by the Cochrane’s manual for systematic reviews, all of included studies had a low risk of bias (Figure S1). In addition, no publication bias for PFS and OS was found based on Begg’s test (P = 0.05 and P = 0.39, respectively; Figure S2).
As the most common driver gene of lung adenocarcinoma, the status of EGFR mutation, has been gradually founded to be the most useful predictor of efficacy for EGFR-TKI over the past decade (21). The addition of chemotherapy to EGFR-TKI as first-line treatment for EGFR-mutated NSCLC has been reevaluated to overcome or delay resistance and prolong survival time (10). To comprehensively assess the effectiveness and toxicity of EGFR-TKI combined with chemotherapy, we systematically reviewed published randomized trials and performed a meta-analysis. The meta-analysis included eight RCTs with a combined total of 1,349 participants with EGFR-mutated NSCLC. Our results demonstrated that compared with EGFR-TKI monotherapy, the combination of first-generation EGFR-TKI and CT, especially when applying concurrent delivery of double-agents CT, significantly improve ORR (RR = 1.18, 95% CI = 1.10–1.26; p <0.00001) and prolong PFS (HR= 0.56 (95% CI = 0.50–0.64; P <0.00001) and OS (HR = 0.70 (95% CI = 0.54–0.90; P = 0.005), in first-line treatment for advanced NSCLC harboring activating EGFR mutation.
Growing evidence suggests that exon 19 deletions and L8585R point mutation are two different disease entities in the matter of response to TKIs and prognosis (22–25). Kuan et al. conducted a meta-analysis of eight trials comparing EGFR-TKI with chemotherapy as first-line treatment in EGFR-mutated NSCLC (24). Their results showed that TKI monotherapy demonstrated PFS benefit in patients with exon 19 deletions (HR = 0.27, 95% CI = 0.21–0.35) more than L858R (HR = 0.45, 95% CI = 0.35–0.58). How about the results when TKI combined with chemotherapy? In our study, the pooled HR of PFS in exon 19 deletion and L858R point mutation from five trials was 0.54 (95% CI = 0.45–0.65) and 0.52 (95% CI = 0.42–0.65), respectively, which were consistent. It indicated that compared with 19 deletion, the PFS of patient with L858R could be more prolonged after TKI combined with chemotherapy. This might be related to increase in ORR of L858R patient after combined chemotherapy.
Currently, first-generation EGFR-TKI monotherapy is still the mainstay of first-line treatment for EGFR-mutated NSCLC, despite the third generation TKI is preferred recommended for first-line therapy. Although the PFS can be substantially prolonged by first-generation TKI compared with platinum-based doublet chemotherapy, none of the first-generation TKIs provide an overall survival benefit revealed by several meta-analyses (4, 26–28). Development of new-generation TKIs or combined therapy is promising strategy to improve OS. Recent ARCHER 1050 (29) and FLAURA (30) trials have shown that second-generation dacomitinib and third-generation osimertinib, significantly prolong the OS and then both of them have been approved for first-line treatment in EGFR-mutated NSCLC (31). As for combined strategy, adding chemotherapy to EGFR-TKI is main approach. Our meta-analysis indicated that first-generation TKI in combination with chemotherapy significantly improved the OS compared with EGFR-TKI alone (HR = 0.70, 95% CI = 0.54–0.90, P = 0.005). Despite head-to-head RCTs are lacking in directly comparing the efficacy, first-generation EGFR-TKI combined with chemotherapy might seem to prolong OS more than dacomitinib (HR = 0.76, 95% CI = 0.58–0.99, P = 0.044) and osimertinib (HR = 0.80, 95% CI = 0.64–1.00, P = 0.046), according to the results of HR. Based on those inspiring results, third-generation osimertinib combined with chemotherapy is speculated as a treatment strategy that could maximize the length of OS in EGFR-mutated NSCLC patients. Studies on the combination of osimertinib with chemotherapy in EGFR-mutated NSCLC, including TAKUMI and FLAURA2 trials, are currently ongoing and eagerly awaited (10).
Preclinical data indicate that the intercalated or sequential combination of EGFR-TKIs with cytotoxic agents has shown more efficacy than in the concurrent way. A possible explanation is that TKI drugs could induce the G1-phase arrest of tumor cells, which conferred a protection against the cytotoxic activity of pemetrexed (32–34). In our subgroup analysis, however, we founded that the benefit of PFS in concurrent administration (HR = 0.55, 95% CI = 0.47–0.64) were consistent with in intercalated administration (HR = 0.57, 95% CI = 0.45–0.73), and that only concurrent administration did confer an OS benefit to patients with EGFR-mutated NSCLC (HR = 0.65, 95% CI = 0.49–0.86). Our indirectly compared results could be proved by the results of NEJ005 trial, which compared concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated NSCLC with sensitive EGFR mutations (35). In addition, our subgroup analysis revealed an OS benefit in doublet chemotherapy combination group (HR: 0.67, 95% CI: 0.48–0.94), not in single-agent chemotherapy combination group (HR: 0.80, 95% CI: 0.56–1.14). But this conclusion should be applied with caution in clinical practice because only two studies adopted single-agent chemotherapy.
Adding chemotherapy to TKI also increased toxicity, notably, while increasing efficacy. Our meta-analysis indicated that most of the increased toxicities were a result of chemotherapy-induced myelosuppression and gastrointestinal toxicity, as may be expected. The incidences of serious (grade 3 or higher) hematologic toxicities from chemotherapy combination group in the meta-analysis, including leukopenia (12.1%), neutropenia (21.7%), thrombocytopenia (7.3%) and anemia (11.7%), were similar with those landmark trials in which platinum-based doublet chemotherapy were used as first-line treatment approach for the control group (36–39). Otherwise, no significant differences were founded in terms of TKI-induced toxicities, such as any grade rash and grade 3 or higher liver dysfunction, when applying combined treatment in our meta-analysis. Meaningfully, TKI combined with chemotherapy did not significantly increase each other’s serious side effects. Therefore, the toxicities of combination therapy are manageable. Our subgroup analysis of toxicity found that compared with doublet chemotherapy, single-agent approach did not significantly increase grade 3 or higher hematologic toxicities, it may because chemotherapeutic drug in the single-agent group both adopted pemetrexed, which is generally considered to have mild toxicity (40, 41).
Admittedly, our meta-analysis has several limitations. First of all, some of results, especially in subgroup analysis, did not cover all enrolled patients due to the deficiency of detailed data, which might have an impact on the conclusion. Moreover, the outcome of OS would be confounded by the low proportion of patients in the controlled group receiving chemotherapy after experiencing progression on first-line TKI monotherapy and our study was underpowered for assessment of such effect. Meantime, the proportion of the third-generation EGFR-TKI osimertinib usage was relatively low, in that osimertinib was used for only 11–15% and 22% of patients after the first TKI treatment in the NEJ009 trial and the study by Noronha et al., respectively. Therefore, the conclusion of overall survival benefit might not be overvalued. Thirdly, the subgroup analyses of different first-generation EGFR-TKI drugs, including gefitinib, erlotinib and icotinib, were not performed due to in lack of sufficient included studies. Thus, it is not clear whether the efficacies of different first-generation drugs will have differences when combined with chemotherapy. What’s more, the fact that this meta-analysis is not based on individual patient data represents a limitation to the interpretation of results, since this approach could tend to overestimate treatment effects. Finally, all included literatures in the meta-analysis were English language publications, which may omit other languages’ studies so as to increase the publication bias.
In conclusion, our results demonstrate that compared with first-generation EGFR-TKI monotherapy, the combination of EGFR-TKI and chemotherapy, especially when applying concurrent delivery of platinum-based doublet chemotherapeutic drugs, significantly improve ORR and prolong PFS and OS of first-line treatment in advanced NSCLC patients harboring activating EGFR mutation. Although increasing incidence of chemotherapy-induced toxicities occurs in the combination group, it is well tolerated and clinically manageable. Thus, the combination of first-generation EGFR-TKI and chemotherapy may represent a new option for first-line treatment in EGFR-mutated NSCLC. Greatly inspired by the promising results of first-generation TKI combination therapy, the results of ongoing randomized trials regarding third-generation EGFR-TKI, such as osimertinib, in combination with chemotherapy, are eagerly awaited.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
QW, WXL and FX conceived the study. QW, WXL, WL and TW performed the systematic review of the literature and FX was consulted for a final decision in case of controversy. LH performed the statistical analyses. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the National Natural Science Foundation of China (No. 81573024).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This manuscript has been released as a pre-print at bioRxiv (42), https://www.biorxiv.org/content/10.1101/2020.04.17.046409v1.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.598265/full#supplementary-material
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR, Threapleton D, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget (2016) 7(48):78985–93. doi: 10.18632/oncotarget.12587
3. Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med (2009) 361(10):958–67. doi: 10.1056/NEJMoa0904554
4. Lee CK, Davies L, Wu YL, Mitsudomi T, Inoue A, Rosell R, et al. Gefitinib or Erlotinib vs Chemotherapy for EGFR Mutation-Positive Lung Cancer: Individual Patient Data Meta-Analysis of Overall Survival. J Natl Cancer Inst (2017) 109(6):djw279. doi: 10.1093/jnci/djw279
5. Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol (2019) 30(2):171–210. doi: 10.1093/annonc/mdy554
6. Yi L, Fan J, Qian R, Luo P, Zhang J. Efficacy and safety of osimertinib in treating EGFR-mutated advanced NSCLC: A meta-analysis. Int J Cancer (2019) 145(1):284–94. doi: 10.1002/ijc.32097
7. Yang JC, Mok T, Han B, Orlando M, Puri T, Park K. A Review of Regimens Combining Pemetrexed With an Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor in the Treatment of Advanced Nonsquamous Non-Small-Cell Lung Cancer. Clin Lung Cancer (2018) 19(1):27–34. doi: 10.1016/j.cllc.2017.06.013
8. An C, Zhang J, Chu H, Gu C, Xiao F, Zhu F, et al. Study of Gefitinib and Pemetrexed as First-Line Treatment in Patients with Advanced Non-Small Cell Lung Cancer Harboring EGFR Mutation. Pathol Oncol Res (2016) 22(4):763–8. doi: 10.1007/s12253-016-0067-4
9. Zhang M, Guo H, Zhao S, Wang Y, Yang M, Yu J, et al. Efficacy of epidermal growth factor receptor inhibitors in combination with chemotherapy in advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. Oncotarget (2016) 7(26):39823–33. doi: 10.18632/oncotarget.9503
10. Rebuzzi SE, Alfieri R, La Monica S, Minari R, Petronini PG, Tiseo M. Combination of EGFR-TKIs and chemotherapy in advanced EGFR mutated NSCLC: Review of the literature and future perspectives. Crit Rev Oncol Hematol (2019) 146:102820. doi: 10.1016/j.critrevonc.2019.102820
11. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
12. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj (2011) 343:d5928. doi: 10.1136/bmj.d5928
13. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
14. Jänne PA, Wang X, Socinski MA, Crawford J, Stinchcombe TE, Gu L, et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol (2012) 30(17):2063–9. doi: 10.1200/JCO.2011.40.1315
15. Yang JC-H, Kang JH, Mok T, Ahn M-J, Srimuninnimit V, Lin C-C, et al. First-line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian patients with locally advanced or metastatic non-squamous non-small cell lung cancer: a randomised, phase 3 trial. Eur J Cancer (Oxford Engl 1990) (2014) 50(13):2219–30. doi: 10.1016/j.ejca.2014.05.011
16. Cheng Y, Murakami H, Yang PC, He J, Nakagawa K, Kang JH, et al. Randomized Phase II Trial of Gefitinib With and Without Pemetrexed as First-Line Therapy in Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancer With Activating Epidermal Growth Factor Receptor Mutations. J Clin Oncol (2016) 34(27):3258–66. doi: 10.1200/JCO.2016.66.9218
17. Han B, Jin B, Chu T, Niu Y, Dong Y, Xu J, et al. Combination of chemotherapy and gefitinib as first-line treatment for patients with advanced lung adenocarcinoma and sensitive EGFR mutations: A randomized controlled trial. Int J Cancer (2017) 141(6):1249–56. doi: 10.1002/ijc.30806
18. Xu L, Qi Q, Zhang Y, Cui J, Liu R, Li Y. Combination of icotinib and chemotherapy as first-line treatment for advanced lung adenocarcinoma in patients with sensitive EGFR mutations: A randomized controlled study. Lung Cancer (2019) 133:23–31. doi: 10.1016/j.lungcan.2019.05.008
19. Hosomi Y, Morita S, Sugawara S, Kato T, Fukuhara T, Gemma A, et al. Gefitinib Alone Versus Gefitinib Plus Chemotherapy for Non-Small-Cell Lung Cancer With Mutated Epidermal Growth Factor Receptor: NEJ009 Study. J Clin Oncol (2020) 38(2):115–23. doi: 10.1200/JCO.19.01488
20. Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, et al. Gefitinib Versus Gefitinib Plus Pemetrexed and Carboplatin Chemotherapy in EGFR-Mutated Lung Cancer. J Clin Oncol (2020) 38(2):124–36. doi: 10.1200/JCO.19.01154
21. Roengvoraphoj M, Tsongalis GJ, Dragnev KH, Rigas JR. Epidermal growth factor receptor tyrosine kinase inhibitors as initial therapy for non-small cell lung cancer: focus on epidermal growth factor receptor mutation testing and mutation-positive patients. Cancer Treat Rev (2013) 39(8):839–50. doi: 10.1016/j.ctrv.2013.05.001
22. Renaud S, Seitlinger J, Guerrera F, Reeb J, Beau-Faller M, Voegeli A-C, et al. Prognostic Value of Exon 19 Versus 21 EGFR Mutations Varies According to Disease Stage in Surgically Resected Non-small Cell Lung Cancer Adenocarcinoma. Ann Surg Oncol (2018) 25(4):1069–78. doi: 10.1245/s10434-018-6347-3
23. Yang JC-H, Wu Y-L, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials.The Lancet. Oncology (2015) 16(2):141–51. doi: 10.1016/S1470-2045(14)71173-8
24. Kuan F-C, Kuo L-T, Chen M-C, Yang C-T, Shi C-S, Teng D, et al. Overall survival benefits of first-line EGFR tyrosine kinase inhibitors in EGFR-mutated non-small-cell lung cancers: a systematic review and meta-analysis. Br J Cancer (2015) 113(10):1519–28. doi: 10.1038/bjc.2015.356
25. Fenchel K, Dale SP, Dempke WCM. Improved overall survival following tyrosine kinase inhibitor (TKI) treatment in NSCLC-are we making progress? Trans Lung Cancer Res (2016) 5(4):373–6. doi: 10.21037/tlcr.2016.07.01
26. Ma H, Tian X, Zeng X-T, Zhang Y, Wang Y, Wang F, et al. The Efficacy of Erlotinib Versus Conventional Chemotherapy for Advanced Nonsmall-Cell Lung Cancer: A PRISMA-Compliant Systematic Review With Meta-Regression and Meta-Analysis. Medicine (2016) 95(2):e2495–5. doi: 10.1097/MD.0000000000002495
27. Guetz GD, Landre T, Uzzan B, Chouahnia K, Nicolas P, Morere J-F. Is There a Survival Benefit of First-Line Epidermal Growth Factor Receptor Tyrosine-Kinase Inhibitor Monotherapy Versus Chemotherapy in Patients with Advanced Non-Small-Cell Lung Cancer?: A Meta-Analysis. Target Oncol (2016) 11(1):41–7. doi: 10.1007/s11523-015-0373-x
28. Gao G, Ren S, Li A, Xu J, Xu Q, Su C, et al. Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is effective as first-line treatment of advanced non-small-cell lung cancer with mutated EGFR: A meta-analysis from six phase III randomized controlled trials. Int J Cancer (2012) 131(5):E822–9. doi: 10.1002/ijc.27396
29. Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J Clin Oncol (2018) 36(22):2244–50. doi: 10.1200/JCO.2018.78.7994
30. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med (2020) 382(1):41–50. doi: 10.1056/NEJMoa1913662
31. Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw (2019) 17(12):1464–72. doi: 10.6004/jnccn.2019.0059
32. Li T, Ling Y-H, Goldman ID, Perez-Soler R. Schedule-dependent cytotoxic synergism of pemetrexed and erlotinib in human non-small cell lung cancer cells. Clin Cancer Res (2007) 13(11):3413–22. doi: 10.1158/1078-0432.CCR-06-2923
33. Giovannetti E, Lemos C, Tekle C, Smid K, Nannizzi S, Rodriguez JA, et al. Molecular mechanisms underlying the synergistic interaction of erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor, with the multitargeted antifolate pemetrexed in non-small-cell lung cancer cells. Mol Pharmacol (2008) 73(4):1290–300. doi: 10.1124/mol.107.042382
34. Feng X, Zhang Y, Li T, Li Y. Sequentially administrated of pemetrexed with icotinib/erlotinib in lung adenocarcinoma cell lines in vitro. Oncotarget (2017) 8(69):114292–9. doi: 10.18632/oncotarget.23224
35. Oizumi S, Sugawara S, Minato K, Harada T, Inoue A, Fujita Y, et al. Updated survival outcomes of NEJ005/TCOG0902: a randomised phase II study of concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated non-small cell lung cancer with sensitive EGFR mutations. ESMO Open (2018) 3(2):e000313. doi: 10.1136/esmoopen-2017-000313
36. Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med (2009) 361(10):947–57. doi: 10.1056/NEJMoa0810699
37. Zhou C, Wu Y-L, Chen G, Feng J, Liu X-Q, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol (2011) 12(8):735–42. doi: 10.1016/S1470-2045(11)70184-X
38. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol (2012) 13(3):239–46. doi: 10.1016/S1470-2045(11)70393-X
39. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med (2010) 362(25):2380–8. doi: 10.1056/NEJMoa0909530
40. Li X, Wei S, Chen J. Critical appraisal of pemetrexed in the treatment of NSCLC and metastatic pulmonary nodules. Onco Targets Ther (2014) 7:937–45. doi: 10.2147/OTT.S45148
41. Pérez-Moreno MA, Galván-Banqueri M, Flores-Moreno S, Villalba-Moreno A, Cotrina-Luque J, Bautista-Paloma FJ. Systematic review of efficacy and safety of pemetrexed in non-small-cell-lung cancer. Int J Clin Pharm (2014) 36(3):476–87. doi: 10.1007/s11096-014-9920-2
42. Wu Q, Luo W, Li W, Wang T, Huang L, Xu F. First-generation EGFR-TKI plus chemotherapy versus EGFR-TKI alone as first-line treatment in advanced NSCLC with EGFR activating mutation: a systematic review and meta-analysis of randomized controlled trials. bioRxiv (2020). 2020.04.17.046409. doi: 10.1101/2020.04.17.046409
Keywords: EGFR-TKI, chemotherapy, first-line, advanced, NSCLC, mutation
Citation: Wu Q, Luo W, Li W, Wang T, Huang L and Xu F (2021) First-Generation EGFR-TKI Plus Chemotherapy Versus EGFR-TKI Alone as First-Line Treatment in Advanced NSCLC With EGFR Activating Mutation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Oncol. 11:598265. doi: 10.3389/fonc.2021.598265
Received: 24 August 2020; Accepted: 04 March 2021;
Published: 13 April 2021.
Edited by:
Wenhua Liang, First Affiliated Hospital of Guangzhou Medical University, ChinaReviewed by:
Rafael Lopez Castro, Hospital Clínico Universitario de Valladolid, SpainCopyright © 2021 Wu, Luo, Li, Wang, Huang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Xu, ZmVuZ3h1c3RlckAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.