- Clinical Laboratory, Huzhou Central Hospital, Affiliated Central Hospital of Huzhou University, Huzhou, China
Background: Previous studies have investigated the role of systemic immune-inflammation index (SII) as a prognostic factor for gastric cancer (GC) patients, although with inconsistent results. Thus, the aim of this study was to identify the prognostic value of SII in GC through meta-analysis.
Methods: We systematically searched the PubMed, Embase, and Web of Science databases for relevant studies investigating the prognostic role of SII in GC up to December 2019. The hazard ratios (HRs) and 95% confidence intervals (CIs) related to overall survival (OS) and disease-free survival (DFS) were combined. Odds ratios (ORs) and 95% CIs were pooled to assess the correlation between SII and clinicopathological features of GC.
Results: A total of eight studies, comprising 4,236 patients, were included in this meta-analysis. Pooled analysis indicated that a high pretreatment SII predicted poor OS (HR=1.40, 95% CI=1.08–1.81, p=0.010) but not poor DFS (HR=1.30, 95% CI=0.92–1.83, p=0.140) in GC. In addition, an elevated SII correlated with an advanced tumor–node–metastasis stage (OR=2.34, 95% CI=1.40–3.92, p=0.001), T3–T4 stage (OR=2.25, 95% CI=1.34–3.77, p=0.002), positive lymph node metastasis (OR=1.79, 95% CI=1.12–2.87, p=0.016), and tumor size ≥ 5 cm (OR=2.28, 95% CI=1.62–3.22, p<0.001) in patients with GC.
Conclusions: A high pretreatment SII significantly associated with poorer survival outcomes as well as several clinical characteristics in GC. We suggest that SII could be monitored to guide prognostication and provide reliable information on the risk of disease progression in GC.
Introduction
Gastric cancer (GC) is the sixth most common malignancy globally (1.2 million new cases in 2017) and remains the fourth leading cause of cancer-related deaths (885,000 deaths annually) worldwide (1). The incidence of GC varies geographically: East Asia; Latin, Central, and South America; and Eastern Europe have the highest incidence, whereas North America, North and East Africa, Australia, and Northern Europe have the lowest incidence (2). As most GC patients are asymptomatic in the early stages, the disease is often diagnosed at an advanced stage (3). Multidisciplinary treatment (MDT) is mandatory for the planning of GC treatment (4). Multiple therapeutic methods, including surgery, chemotherapy, targeted therapy, and immunotherapy, have been applied for the medical management of GC (5, 6). Despite this, the prognosis of patients with advanced disease remains unsatisfactory, with a 5-year overall survival (OS) rate of <5% (5). Therefore, the search for non-invasive and readily accessible prognostic factors is necessary and important for the prediction of prognosis in clinical practice.
Systemic inflammatory responses, which are involved in angiogenesis promotion, tumor development, and metastasis, play a pivotal role in the tumor microenvironment (7). In recent years, several parameters derived from peripheral blood have shown prognostic significance in cancer patients. Examples include neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), lymphocyte–monocyte ratio (LMR), and systemic immune-inflammation index (SII) (8–13). SII, which is calculated as platelet count × neutrophil count/lymphocyte count, has been recently shown to have a powerful prognostic value in several tumors including lung cancer (14), esophageal cancer (15), colorectal cancer (16), and hepatocellular carcinoma (17). Previous studies have also investigated the prognostic impact of SII on GC, but the results have been inconsistent (12, 13, 18–23). For example, some studies reported SII as a useful tool to discriminate high-risk GC patients from low-risk GC patients (12, 13, 18, 21), whereas other investigators did not find a prognostic role for SII (20, 23). Therefore, we conducted this meta-analysis to determine the prognostic significance of pretreatment SII in patients with GC. We also investigated the relationship between SII and the clinicopathological factors of GC.
Materials and Methods
Search Strategy
The meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (24). PubMed, Embase, the Cochrane Library, and Web of Science were systematically searched for papers published in English up to December 2019. The following combined search keywords were used: (“systemic immune-inflammation index” OR “SII” OR “neutrophil× platelets/lymphocyte”) AND (“gastric cancer” OR “gastric carcinoma” OR “gastric neoplasms” OR “stomach cancer”). Moreover, the references of the included studies were manually checked for potential candidate papers. As this meta-analysis was performed by reviewing published papers, ethical approval and informed patient consent were not required.
Selection Criteria
The inclusion criteria were as follows: (1) including patients pathologically diagnosed with GC; (2) SII was measured using serum-based methods prior to treatment; (3) hazard ratios (HRs) and the corresponding 95% confidence intervals (95% CIs) for the association between SII and OS and/or disease-free survival (DFS) were reported or sufficient data were available; (4) a cutoff value to stratify high/low levels of SII was identified; and (5) full-text English articles. The exclusion criteria were as follows: (1) duplicated studies; (2) reviews, case reports, meeting abstracts, or letters; (3) studies with insufficient data to compute survival outcomes (OS or DFS) with HRs and 95% CIs; (4) animal studies; and (5) studies published in languages other than English.
Data Extraction and Quality Assessment
To ensure the validity of the findings, two independent investigators (YQ and ZZ) extracted data from eligible studies, and any discrepancies were resolved following discussion with a third investigator (YC). The following information was extracted from each included study: first author’s name, year of publication, country, study period, sample size, patient’s age, sex distribution, tumor node and metastasis (TNM) stage, treatment method, cutoff value, cutoff selection, end-point, and HRs and 95% CIs of OS and DFS. If HRs and 95% CIs were provided in both univariate and multivariate analyses, the latter was adopted because it was more precise, as it considers confounding factors. The quality of the studies was evaluated in accordance with the Newcastle–Ottawa Scale (NOS) (25). NOS contained three domains: patient selection (0–4 points), comparability (0–2 points), and outcome (0–3 points). NOS scores ranged from 0 to 9 points, and studies with an NOS score ≥ 6 were considered to be of high quality.
Statistical Analysis
The association of SII with OS and DFS was evaluated by pooling HRs and 95% CIs. The heterogeneity among studies was tested using Cochran’s Q test and Higgins I2 statistic method. The random-effects model was used when significant heterogeneity was detected (P <0.10 or I2 > 50%); otherwise, the fixed-effects model was adopted. Pooled odds ratios (ORs) with 95% CIs were calculated to assess the relationship between SII and clinicopathological features in GC patients. Subgroup analysis was performed to explore the potential sources of heterogeneity. Publication bias was estimated using Begg’s funnel plots. All statistical analyses were conducted using Stata software version 12.0 (STATA Corporation, College Station, TX, USA). P < 0.05 was considered significant.
Results
Study Selection
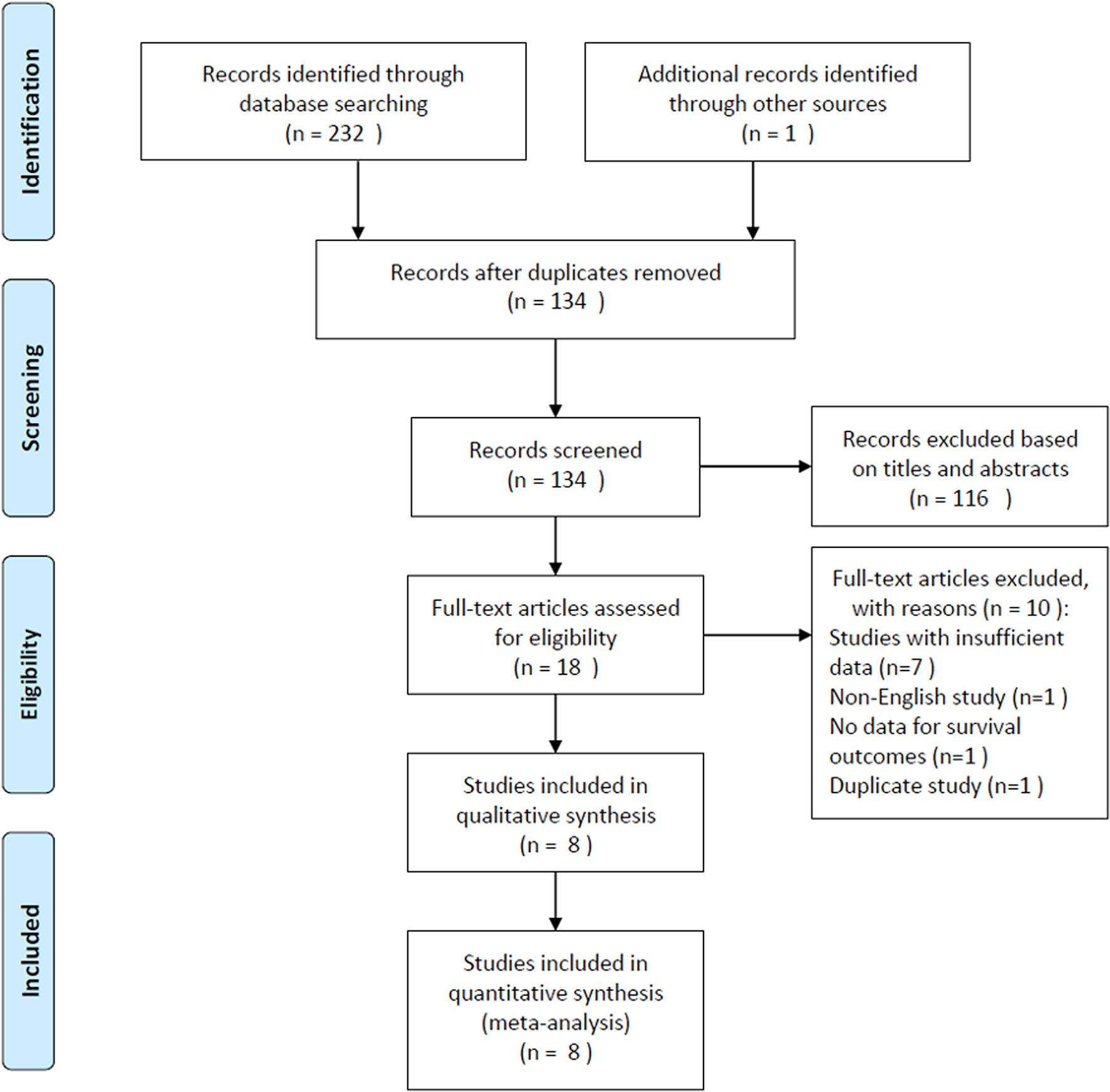
The selection procedure is shown in detail in Figure 1. The initial literature search retrieved a total of 233 records and, after excluding duplicated studies, 134 records remained. A total of 116 studies were excluded after screening of the titles and abstracts, and the full text of the remaining 18 studies were assessed for eligibility. Ten articles were subsequently excluded for the following reasons: seven studies had insufficient data for analysis, one study was published in a non-English language, one study did not report survival, and one study was duplicated. Finally, a total of eight studies (12, 13, 18–23), comprising 4,236 patients, were included in this meta-analysis.
Characteristics of the Included Studies
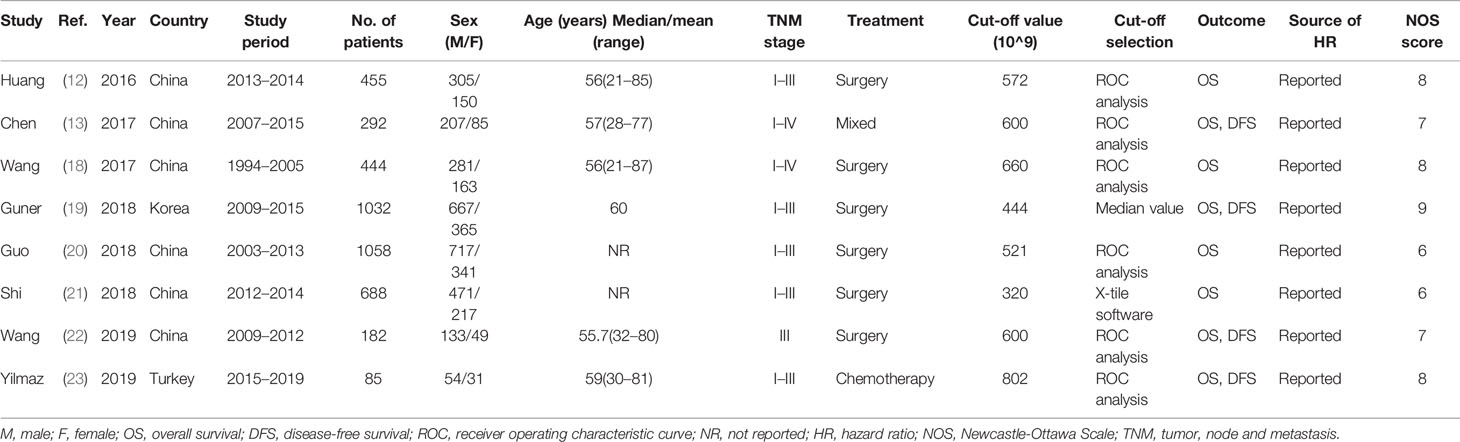
The basic characteristics of the eight included studies are summarized in Table 1. As listed in Table 1, all included articles were published in English between 2016 and 2019. Among them, six studies were conducted in China (12, 13, 18, 20–22), one in Korea (19), and one in Turkey (23). Sample sizes ranged from 85 to 1,058 patients, and the median value was 449.5. Therefore, we selected 450 as the cutoff value for the subgroup analysis of sample size. Five studies recruited patients with GC stages I–III (12, 19–21, 23), two studies recruited patients with GC stages I–IV (13, 18), and one study recruited patients with stage III (22). The cutoff values of SII varied from 320 to 802, with a median value of 586. Using relevant meta-analyses of SII and hepatocellular carcinoma (17) and breast cancer (26) as references, we selected an SII of 600 for subgroup analysis.
Six studies (12, 13, 18, 20, 22, 23) adopted ROC analysis to determine the cutoff value of SII, one study (19) used the median value as the cutoff, and one study (21) applied the X-tile software. All 8 studies (12, 13, 18–23), which included a total of 4,236 patients, reported a correlation between SII and OS. Further, four studies (13, 19, 22, 23), including 1,591 patients, showed an association between SII and DFS in GC. The NOS scores of all included studies ranged from 6 to 9, suggesting that all included studies were of high quality.
Associations Between SII and OS
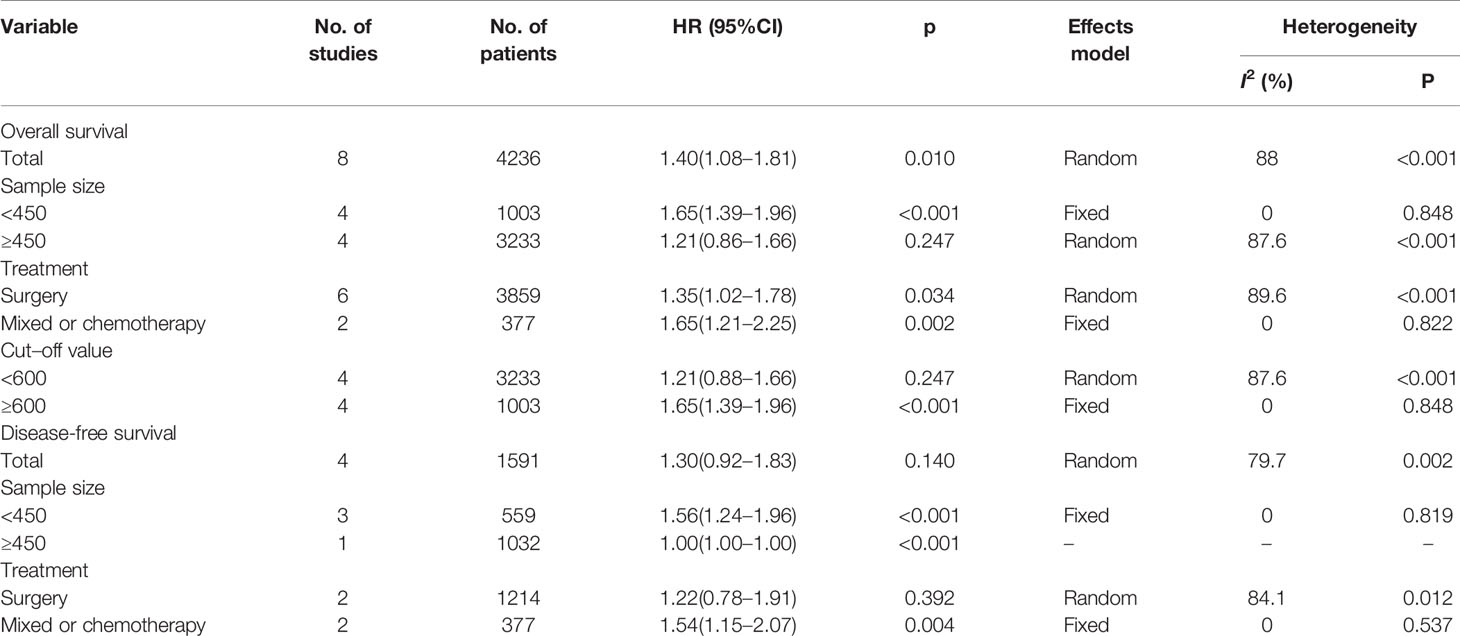
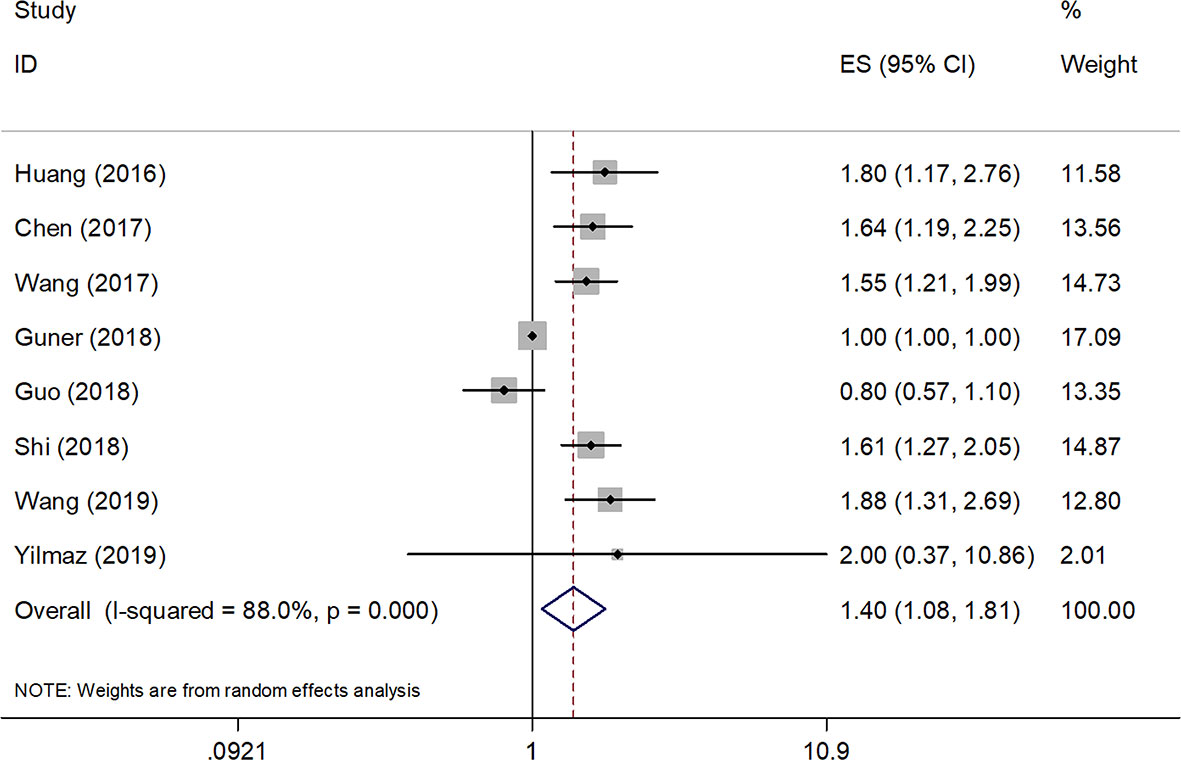
A total of eight studies (4236 patients) (12, 13, 18–23) were included in the analysis of pooled HR for OS. As shown in Table 2 and Figure 2, the combined data demonstrated that compared with a low SII, a high SII was significantly associated with poor OS (HR=1.40, 95% CI=1.08–1.81, p=0.010). Owing to a significant heterogeneity among studies (I2 = 88%, P<0.001), a random-effects model was applied. As shown in Table 2, subgroup analysis was also conducted for further investigation. The HR and 95% CI of OS were HR=1.65, 95% CI=1.39–1.96, p<0.001 for studies with a sample size < 450, whereas SII had non-significant prognostic value for studies with sample size ≥ 450 (HR= 1.21, 95% CI=0.86–1.66, p=0.247). In the context of treatment, a high SII remained a significant prognostic marker in patients undergoing surgery (HR=1.35, 95% CI=1.02–1.78, p=0.034) and in those receiving mixed treatment or chemotherapy (HR=1.65, 95% CI=1.21–2.25, p=0.002). The stratified analysis also indicated that a cutoff value ≥ 600 correlated with a poor OS (HR=1.65, 95% CI=1.39–1.96, p<0.001) in patients with GC.
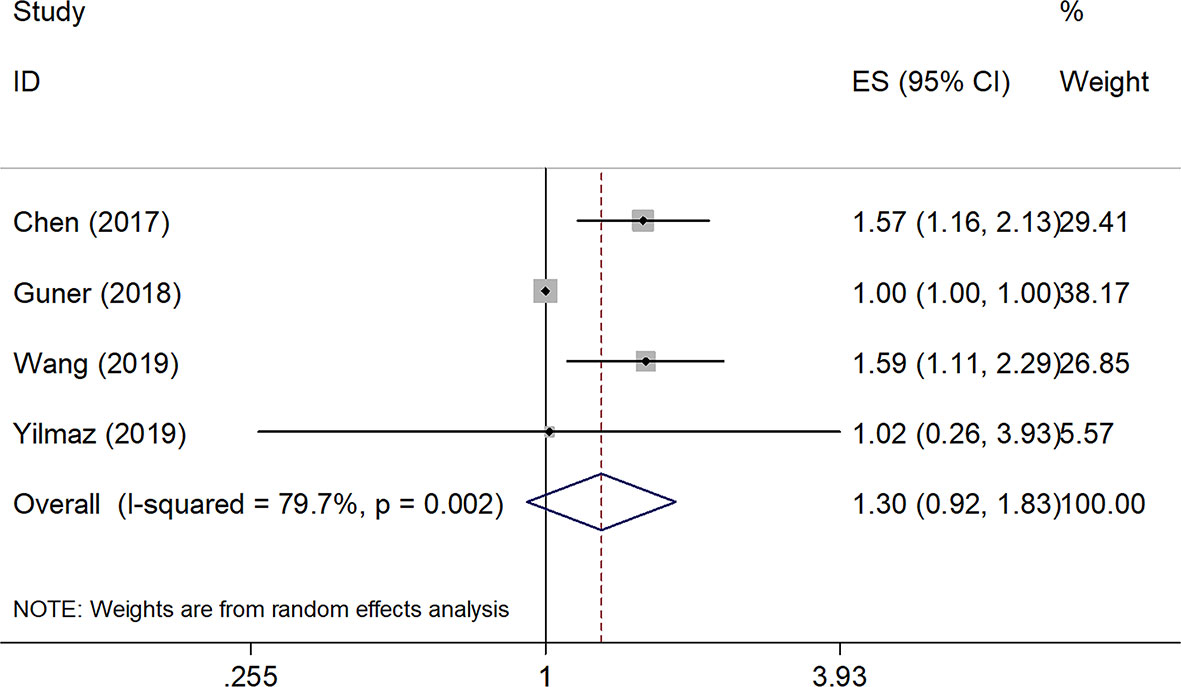
Relationships Between SII and DFS
Four studies, with a total of 1,591 subjects, investigated the relationship between SII and DFS in GC. Results from their analyses suggested that a high pretreatment SII was not significantly correlated with a worse DFS (HR=1.30, 95% CI=0.92–1.83, p=0.140, Table 2 and Figure 3). However, results of the subgroup analysis indicated that an elevated SII was correlated with inferior DFS in studies with sample size < 450 (HR=1.56, 95% CI=1.24–1.96, p<0.001) and in patients receiving mixed treatment or chemotherapy (HR=1.54, 95% CI=1.15–2.07, p=0.004) (Table 2).
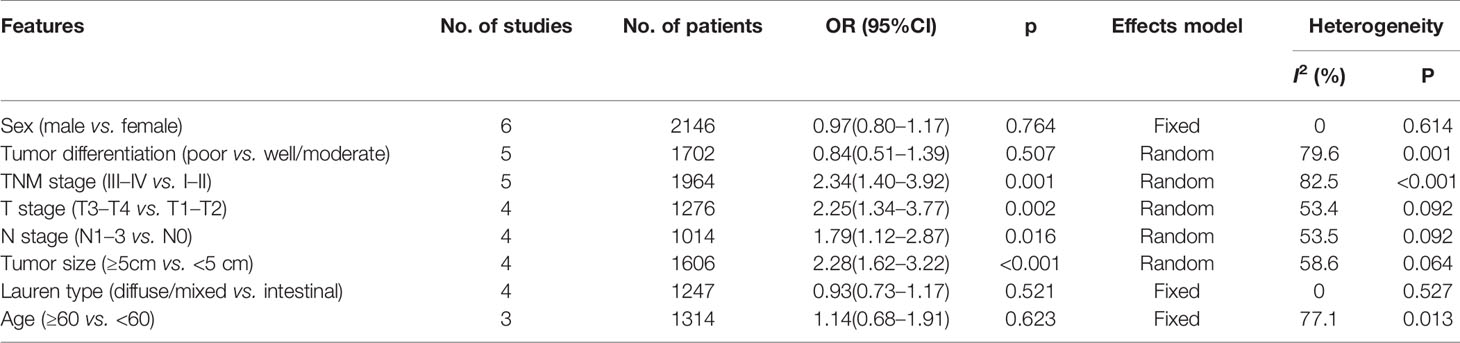
SII and Clinicopathological Features in GC
To explore the association between SII and clinicopathological factors in GC patients, we determined the ORs and 95% CIs of SII and eight clinicopathological features including sex, tumor differentiation, T stage, N stage, TNM stage, tumor size, Lauren type, and age. As shown in Table 3 and Figure 4, the combined results suggested that a high pretreatment SII correlated with an advanced TNM stage (n=5, OR=2.34, 95% CI=1.40–3.92, p=0.001), T3–T4 stage (n=4, OR=2.25, 95% CI=1.34–3.77, p=0.002), N1–3 stage (n=4, OR=1.79, 95% CI=1.12–2.87, p=0.016), and tumor size ≥ 5 cm (n=4, OR=2.28, 95% CI=1.62–3.22, p<0.001). However, pooled data also indicated that there was no significant association of SII with sex (n=6, OR=0.97, 95% CI=0.80–1.17, p=0.764), tumor differentiation (n=5, OR=0.84, 95% CI=0.51–1.39, p=0.507), Lauren type (n=4, OR=0.93, 95% CI=0.73–1.17, p=0.521), or age (n=3, OR=1.14, 95% CI=0.68–1.91, p=0.623) in patients with GC (Table 3, Figure 4).
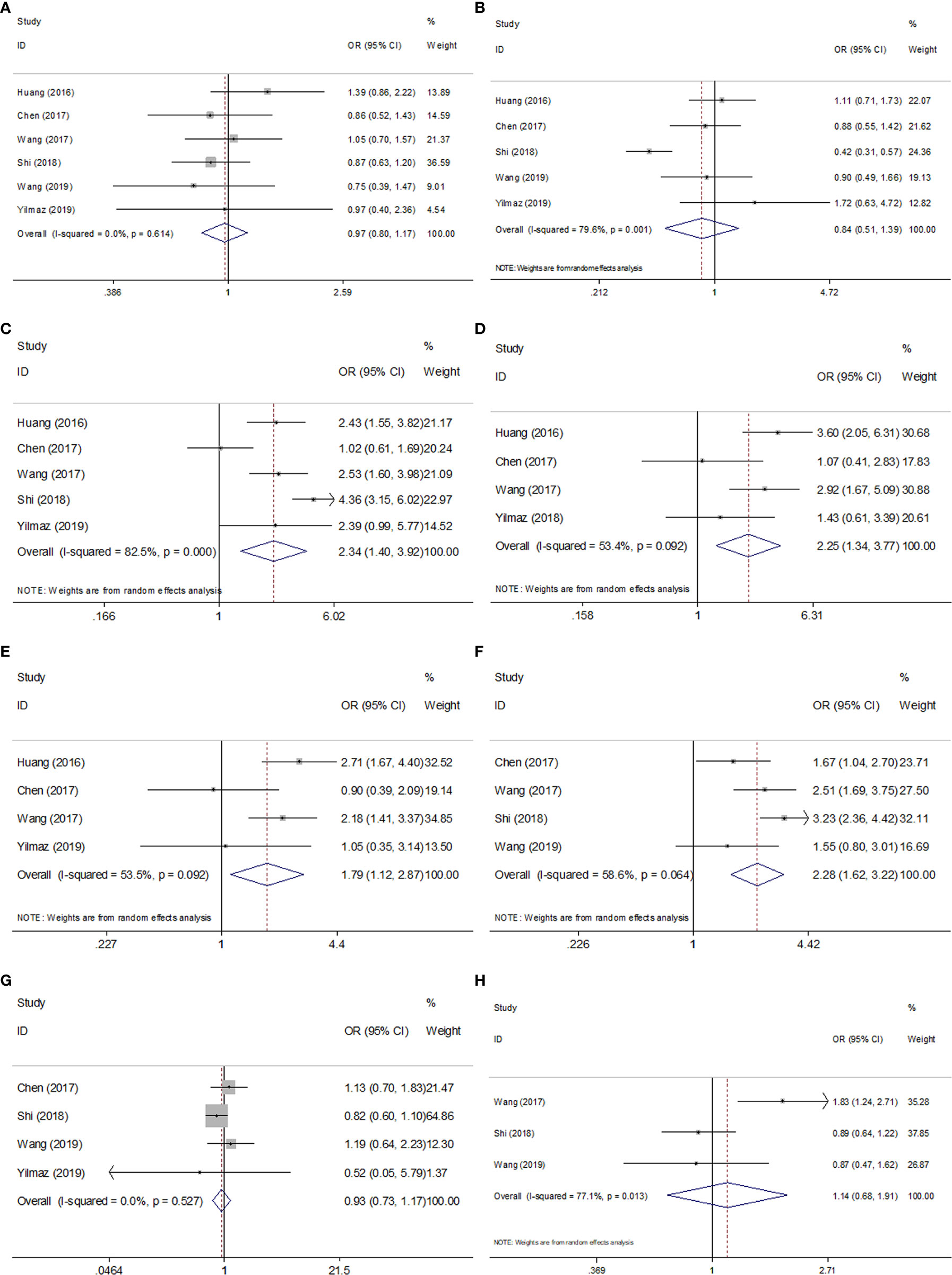
Figure 4 Forest plots of combined analyses between SII and clinical characteristics in GC. (A) Sex (male vs. female); (B) Tumor differentiation (poor vs. well/moderate); (C) TNM stage (III-IV vs. I-II); (D) T stage (T3-T4 vs. T1-T2); (E) N stage (N1-3 vs. N0); (F) Tumor size (≥5cm vs. <5 cm); (G) Lauren type (diffuse/mixed vs. intestinal); (H) Age (≥60 vs. <60).
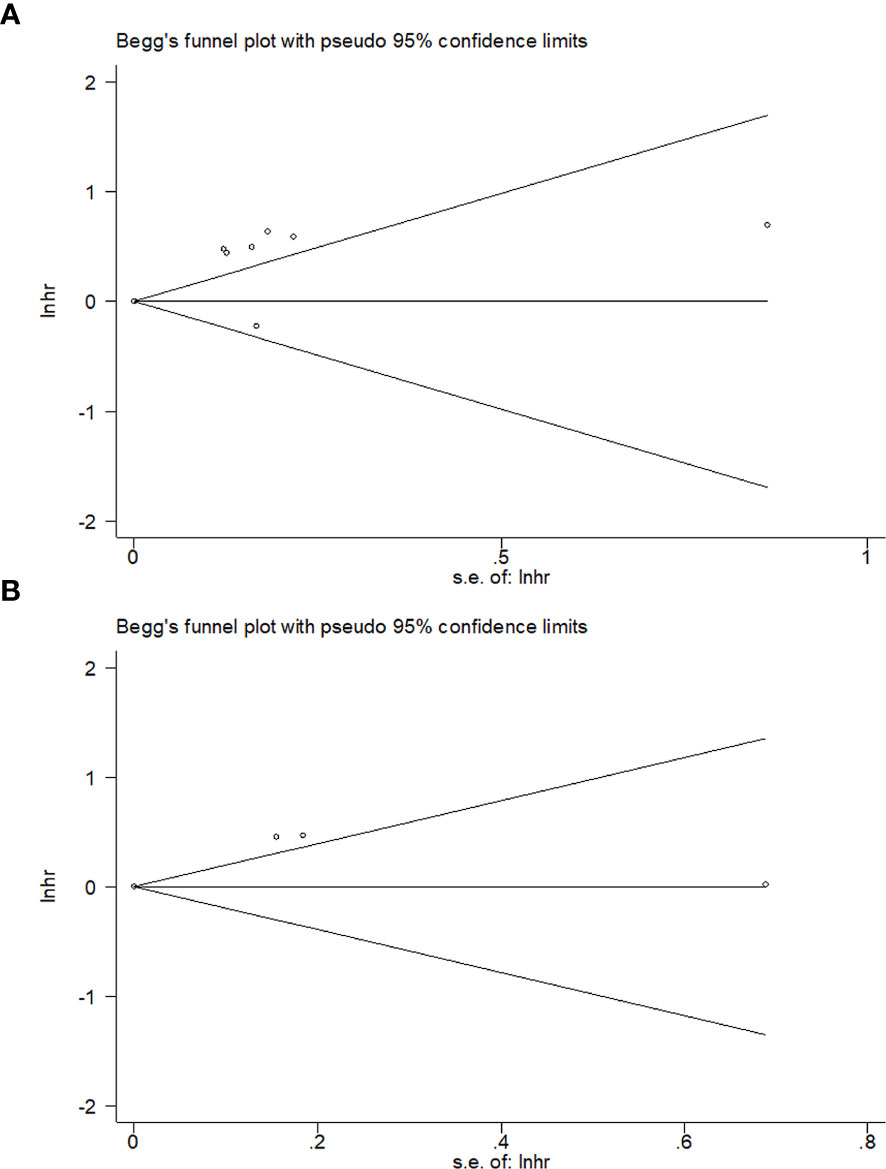
Publication Bias
We evaluated potential publication bias using Begg’s test. As shown in Figure 5, the funnel plot of publication bias assessment was symmetric, and thus the results suggested that there was no significant publication bias in this meta-analysis (Begg’s p= 0.536 for OS and Begg’s p= 1 for DFS, Figure 5).
Discussion
Previous studies have investigated the potential of SII, a parameter which can be estimated from peripheral blood, as a prognostic indicator in patients with GC (12, 13, 18–23), However, the results from these studies have been inconsistent. Thus, we quantitatively assessed the prognostic and clinical role of pretreatment SII in patients with GC. Our meta-analysis, which incorporated data from eight studies, demonstrated that a high pretreatment SII was significantly associated with worse OS but not DFS. Furthermore, pooled results also indicated the existence of a relationship between an elevated SII and advanced TNM stage, a higher T stage, positive N stage, and larger tumor size in patients with GC. Taken together, our results suggest that a high SII could serve as an independent prognostic marker for poor OS in GC. Similarly, owing to the significant association between SII and clinical factors reflecting disease aggressiveness and invasiveness, monitoring of SII may be beneficial for early detection of disease progression. Based on the findings of this meta-analysis, SII has the potential to be a predictive marker with important clinical utility for patients with GC. Although previous meta-analyses explored the prognostic significance of SII in patients with solid tumors (27, 28), the sample size of GC patients in these studies was limited (27, 28). In this study, we focused on patients with GC and searched the updated literature to further investigate the prognostic value of SII specifically in this disease.
Systemic inflammatory responses have been confirmed to facilitate cancer progression and play important roles in each stage of tumor development, including initiation, invasion, angiogenesis, and metastasis (29, 30). The relationships between cancer cells and inflammatory cells or mediators in the tumor microenvironment are complex. As SII is calculated as platelet count × neutrophil count/lymphocyte count, a high SII could be attributed to high platelet counts, high neutrophil counts, and/or low lymphocyte counts. Although the molecular mechanisms underlying the prognostic value of SII in GC have not been fully elucidated, there are several hypotheses. One possible explanation comes from lymphocytes, and especially tumor-infiltrating lymphocytes (TILs), which play a major role in inducing cytotoxic cell death and suppressing cancer cell proliferation (31). TILs are pivotal components of the antitumor activity, and thus a decrease in TILs leads to tumor progression. Another possible hypothesis involves neutrophils, which are known to secrete a variety of cytokines (vascular epithelial growth factor, IL-8, IL-16, etc.) that stimulate tumor cell growth (32). Finally, platelets, which can form a physical shield around cancer cells to protect them from attacks by immune cells, may also be involved (33). In the circulatory system, platelets promote cancer cell arrest at the endothelium and support the establishment of secondary lesions of cancer cells (34). Thus, an elevation in SII implies a dominance of protumor activity in the tumor microenvironment, which ultimately leads to a poor prognosis.
The prognostic effect of SII in various cancers has been investigated in several meta-analyses (27, 28, 35). A comprehensive meta-analysis containing 22 studies with 7657 patients suggested that a high SII correlated with diverse poor survival outcomes in cancer patients (28). Another meta-analysis showed that an elevated pretreatment SII indicated significantly poorer OS, DFS/progression-free survival, and cancer-specific survival in patients with non-small cell lung cancer (NSCLC) (35). A recent meta-analysis also suggested that an elevated SII was a poor prognostic factor for patients with hepatocellular carcinoma (17). In the present meta-analysis, we also found a prognostic role of SII for OS in GC, which is in line with the results of previous meta-analyses. However, we identified a non-significant association between SII and DFS in patients with GC. This could be attributed to the following reasons: the follow-up of DFS was relatively shorter than that of OS; therefore, the prognostic value may be masked owing to the inadequate duration of DFS follow-up. Moreover, the sample size of the DFS analysis was limited; only four studies with 1,591 patients were included for analysis, which may restrict the statistical power of the results.
Our meta-analysis has several limitations. First, all included studies were conducted in Asia, which may compromise the validity of the results in patients with other ethnicities. The prognostic value of SII in other ethnicities of patients with GC needs to be investigated. Second, the cutoff values for SII differed among the studies, which may contribute to heterogeneity. Third, most enrolled studies were retrospective. Therefore, information bias, selection bias, and misclassification bias might exist in this meta-analysis. Considering these limitations, further individual participant data-based meta-analysis, real-world studies, and prospective studies with larger sample sizes are warranted for validation.
Conclusions
This meta-analysis demonstrated that a high pretreatment SII was significantly associated with poorer OS as well as advanced tumor stage, positive node metastasis, higher T stage, and larger tumor size in patients with GC. We suggest that SII should be monitored to guide prognostication and provide reliable information for the risk of disease progression in GC. However, owing to some limitations in this study, large multicenter prospective trials are required to validate the prognostic role of SII in GC.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
YQ collected and analyzed the data, and wrote the paper. ZZ collected and analyzed the data. ZZ and YC revised the whole paper. All authors reviewed the final paper. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol (2019) 5(12):1749–68. doi: 10.1001/jamaoncol.2019.2996
2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer (2015) 136(5):E359–86. doi: 10.1002/ijc.29210
3. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet (2016) 388(10060):2654–64. doi: 10.1016/s0140-6736(16)30354-3
4. Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric Cancer, Version 3.2016. J Natl Compr Cancer Netw (2016) 14(10):1286–312. doi: 10.6004/jnccn.2016.0137
5. Charalampakis N, Economopoulou P, Kotsantis I, Tolia M, Schizas D, Liakakos T, et al. Medical management of gastric cancer: a 2017 update. Cancer Med (2018) 7(1):123–33. doi: 10.1002/cam4.1274
6. Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol (2016) 17(6):717–26. doi: 10.1016/s1470-2045(16)00175-3
7. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care (2009) 12(3):223–6. doi: 10.1097/MCO.0b013e32832a7902
8. Li HC, Zhao Y, Zheng FY. Prognostic significance of elevated preoperative neutrophil-to-lymphocyte ratio for patients with colorectal cancer undergoing curative surgery A meta-analysis. Medicine (2019) 98(3):e14126. doi: 10.1097/md.0000000000014126
9. Zhang J, Feng W, Ye Z, Wei Y, Li L, Yang Y. Prognostic significance of platelet-to-lymphocyte ratio in patients with nasopharyngeal carcinoma: a meta-analysis. Future Oncol (London England) (2019) 16(5):117–27. doi: 10.2217/fon-2019-0520
10. Wang X, Ni X, Tang G. Prognostic Role of Platelet-to-Lymphocyte Ratio in Patients With Bladder Cancer: A Meta-Analysis. Front Oncol (2019) 9:757. doi: 10.3389/fonc.2019.00757
11. Gao XP, Liu YH, Liu ZY, Wang LJ, Jing CX, Zhu S, et al. Pretreatment lymphocyte-to-monocyte ratio as a predictor of survival among patients with ovarian cancer: a meta-analysis. Cancer Manage Res (2019) 11:1907–20. doi: 10.2147/cmar.S184970
12. Huang L, Liu S, Lei Y, Wang K, Xu M, Chen YB, et al. Systemic immune-inflammation index, thymidine phosphorylase and survival of localized gastric cancer patients after curative resection. Oncotarget (2016) 7(28):44185–93. doi: 10.18632/oncotarget.9923
13. Chen L, Yan Y, Zhu LH, Cong XL, Li S, Song SB, et al. Systemic immune-inflammation index as a useful prognostic indicator predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Cancer Manage Res (2017) 9:849–67. doi: 10.2147/cmar.S151026
14. Zhang Y, Chen B, Wang L, Wang R, Yang X. Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer: A meta-analysis. Medicine (Baltimore) (2019) 98(3):e13788. doi: 10.1097/md.0000000000013788
15. Zhang Y, Xiao G, Wang R. Clinical significance of systemic immune-inflammation index (SII) and C-reactive protein-to-albumin ratio (CAR) in patients with esophageal cancer: a meta-analysis. Cancer Manag Res (2019) 11:4185–200. doi: 10.2147/cmar.S190006
16. Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol (2017) 23(34):6261–72. doi: 10.3748/wjg.v23.i34.6261
17. Wang B, Huang Y, Lin T. Prognostic impact of elevated pre-treatment systemic immune-inflammation index (SII) in hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore) (2020) 99(1):e18571. doi: 10.1097/md.0000000000018571
18. Wang K, Diao FY, Ye ZJ, Zhang XH, Zhai ET, Ren H, et al. Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Chin J Cancer (2017) 36(1):75. doi: 10.1186/s40880-017-0243-2
19. Guner A, Kim SY, Yu JE, Min IK, Roh YH, Roh C, et al. Parameters for Predicting Surgical Outcomes for Gastric Cancer Patients: Simple Is Better Than Complex. Ann Surg Oncol (2018) 25(11):3239–47. doi: 10.1245/s10434-018-6684-2
20. Guo J, Chen S, Chen Y, Li S, Xu D. Combination of CRP and NLR: a better predictor of postoperative survival in patients with gastric cancer. Cancer Manag Res (2018) 10:315–21. doi: 10.2147/cmar.S156071
21. Shi H, Jiang Y, Cao H, Zhu H, Chen B, Ji W. Nomogram Based on Systemic Immune-Inflammation Index to Predict Overall Survival in Gastric Cancer Patients. Dis Markers (2018) 2018:1787424. doi: 10.1155/2018/1787424
22. Wang QS, Zhu DY. The prognostic value of systemic immune-inflammation index (SII) in patients after radical operation for carcinoma of stomach in gastric cancer. J Gastrointest Oncol (2019) 10(5):965–78. doi: 10.21037/jgo.2019.05.03
23. Yilmaz A, Mirili C, Tekin SB, Bilici M. The ratio of hemoglobin to red cell distribution width predicts survival in patients with gastric cancer treated by neoadjuvant FLOT: a retrospective study. Ir J Med Sci (2019) 189(1):91–102. doi: 10.1007/s11845-019-02153-x
24. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol (2009) 62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005
25. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
26. Zhang Y, Sun Y, Zhang Q. Prognostic value of the systemic immune-inflammation index in patients with breast cancer: a meta-analysis. Cancer Cell Int (2020) 20:224. doi: 10.1186/s12935-020-01308-6
27. Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget (2017) 8(43):75381–8. doi: 10.18632/oncotarget.18856
28. Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J Cancer (2018) 9(18):3295–302. doi: 10.7150/jca.25691
29. Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell (2010) 140(6):883–99. doi: 10.1016/j.cell.2010.01.025
30. Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
31. Santoiemma PP, Powell DJ. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther (2015) 16(6):807–20. doi: 10.1080/15384047.2015.1040960
32. Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci (2007) 98(11):1652–8. doi: 10.1111/j.1349-7006.2007.00606.x
33. Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M. The platelet-cancer loop. Eur J Internal Med (2013) 24(5):393–400. doi: 10.1016/j.ejim.2013.01.017
34. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer (2011) 11(2):123–34. doi: 10.1038/nrc3004
Keywords: gastric cancer, systemic immune-inflammation index, meta-analysis, prognosis, clinical management
Citation: Qiu Y, Zhang Z and Chen Y (2021) Prognostic Value of Pretreatment Systemic Immune-Inflammation Index in Gastric Cancer: A Meta-Analysis. Front. Oncol. 11:537140. doi: 10.3389/fonc.2021.537140
Received: 22 February 2020; Accepted: 18 January 2021;
Published: 11 March 2021.
Edited by:
Samuel J. Klempner, Massachusetts General Hospital Cancer Center, United StatesReviewed by:
Yongxi Song, The First Affiliated Hospital of China Medical University, ChinaHyoung-Il Kim, Yonsei University Health System, South Korea
Xiaobin Gu, First Affiliated Hospital of Zhengzhou University, China
Copyright © 2021 Qiu, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Chen, Y2hlbnlpbmcxOTMyQDEyNi5jb20=
 Ye Qiu
Ye Qiu Ying Chen
Ying Chen