
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 01 February 2021
Sec. Genitourinary Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.622134
This article is part of the Research Topic Emerging Molecular Signaling Pathways and Therapeutic Targets for Genitourinary Cancer Metastasis View all 18 articles
 Mehmet A. Bilen1,2*†
Mehmet A. Bilen1,2*† James F. Jiang3†
James F. Jiang3† Caroline S. Jansen3
Caroline S. Jansen3 Jacqueline T. Brown1,2
Jacqueline T. Brown1,2 Lara R. Harik4
Lara R. Harik4 Aarti Sekhar5
Aarti Sekhar5 Haydn Kissick3
Haydn Kissick3 Shishir K. Maithel6
Shishir K. Maithel6 Omer Kucuk1,2
Omer Kucuk1,2 Bradley Carthon1,2
Bradley Carthon1,2 Viraj A. Master2,3*
Viraj A. Master2,3*Introduction: Cabozantinib (XL-184) is a small molecule inhibitor of the tyrosine kinases c-Met, AXL, and VEGFR2 that has been shown to reduce tumor growth, metastasis, and angiogenesis. After the promising results from the METEOR and CABOSUN trials, cabozantinib was approved for use in the first- and second-line setting in patients with advanced RCC. Previously, targeted therapies have been used in the neoadjuvant setting for tumor size reduction and facilitating nephrectomies. The increased response rates with cabozantinib in metastatic renal cell carcinoma (mRCC), along with the other neoadjuvant TKI data, strongly support an expanded role for cabozantinib in the neoadjuvant setting.
Case Description: We report on a 59-year-old gentleman presenting with an unresectable 21.7 cm left renal cell carcinoma (RCC) with extension to soft tissue and muscles of the thoracic cage, psoas muscle, posterior abdominal wall, tail of pancreas, splenic flexure of colon, and inferior margin of spleen. Presurgical, neoadjuvant systemic therapy with cabozantinib was initiated for 11 months in total. Initially after 2 months of cabozantinib, magnetic resonance imaging (MRI) revealed a significant reduction (44.2%) in tumor diameter from 21.7 to 12.1 cm with decreased extension into adjacent structures. After 11 months total of cabozantinib, the corresponding MRI showed grossly stable size of the tumor and significant resolution of invasion of adjacent structures. After washout of cabozantinib, radical resection, including nephrectomy, was successfully performed without any major complications, either intra-operative or perioperative. Negative margins were achieved.
Conclusions: This is a report of neoadjuvant cabozantinib downsizing a tumor and enabling surgical resection in this patient with locally advanced RCC. Our findings demonstrate that neoadjuvant cabozantinib to facilitate subsequent surgical resection may be a feasible option for patients presenting with unresectable RCC.
Cabozantinib is a potent multikinase agent that inhibits, in addition to VEGF receptors, MET, and AXL, both of which are associated with resistance to VEGF-directed therapy. The METEOR phase 3 clinical trial results proved that treatment with cabozantinib increased overall survival, delayed disease progression, and improved the objective response compared with everolimus in advanced renal cell carcinoma (RCC) patients (1). These promising results led to initial approval of cabozantinib treatment for advanced renal cell carcinoma. In the CABOSUN phase 2 clinical trial, cabozantinib treatment demonstrated a significant clinical benefit in progression free survival and objective response rate over standard-of-care sunitinib as first-line therapy in patients with intermediate- or poor-risk metastatic RCC (2). Thus, recently, cabozantinib has been approved for use in the first- and second-line setting in patients with advanced RCC (3). Therefore, cabozantinib was selected to be administered for this patient with locally advanced RCC.
Targeted therapies, primarily inhibitors of the VEGF receptor tyrosine kinase and rapamycin pathways, have changed the management of advanced RCC. Over the past 10 years, studies have established efficacy and have led to approval of sorafenib, sunitinib, temsirolimus, everolimus, pazopanib, axitinib, and also cabozantinib. These agents have significantly improved progression-free survival, with certain therapies achieving a median overall survival of >2 years in advanced RCC patients (4, 5). Most recently, trials on novel multikinase inhibitors, such as cabozantinib, and PD-1 inhibitors, such as nivolumab, have demonstrated significantly prolonged progression free survival and increases in overall survival, compared to standard therapy in metastatic RCC patients (2, 3). Using these agents in the neoadjuvant setting has emerged as a treatment option for locally advanced RCC patients. Neoadjuvant therapy can potentially downsize advanced tumors, enabling surgical interventions when they may not otherwise have been feasible or safe due to unresectable locoregional disease. We describe here, a patient presenting with initially unresectable locally advanced RCC treated with neoadjuvant cabozantinib, downsizing the tumor and enabling surgical resection.
A 59-year-old man with an Eastern Cooperative Oncology Group (ECOG) status of 0 presented with a left renal mass in March 2018. Computed tomography (CT) scans of the chest, abdomen, and pelvis revealed a locally invasive 21-cm left renal mass inseparable from the soft tissue of the thorax, psoas muscle, posterior abdominal wall, tail of pancreas, splenic flexure of colon, and inferior margin of spleen with no evidence of nodal involvement or metastatic disease. In April 2018, magnetic resonance imaging (MRI) supported these findings showing a 21.7 cm renal mass invading the renal hilum and adjacent structures described above (Figure 1A). In April 2018, patient underwent a CT-guided renal biopsy that confirmed renal cell carcinoma (RCC). The tumor was deemed unresectable at our multidisciplinary genitourinary tumor board, and systemic treatment was recommended. In April 2018, patient was seen in genitourinary medical oncology clinic, and cabozantinib 60 mg daily was started. In June 2018, after 2 months of treatment, MRI revealed a significant decrease in tumor size from 21.7 to 12.1 cm with marked decrease of extension into the psoas muscle, posterior abdominal wall, tail of the pancreas, splenic flexure of the colon, and inferior margin of the spleen (Figures 1 and 2). After 11 months of therapy, the corresponding MRI showed grossly stable size of the tumor but resolved invasion of adjacent structures (Figures 1C, 2, and 3).
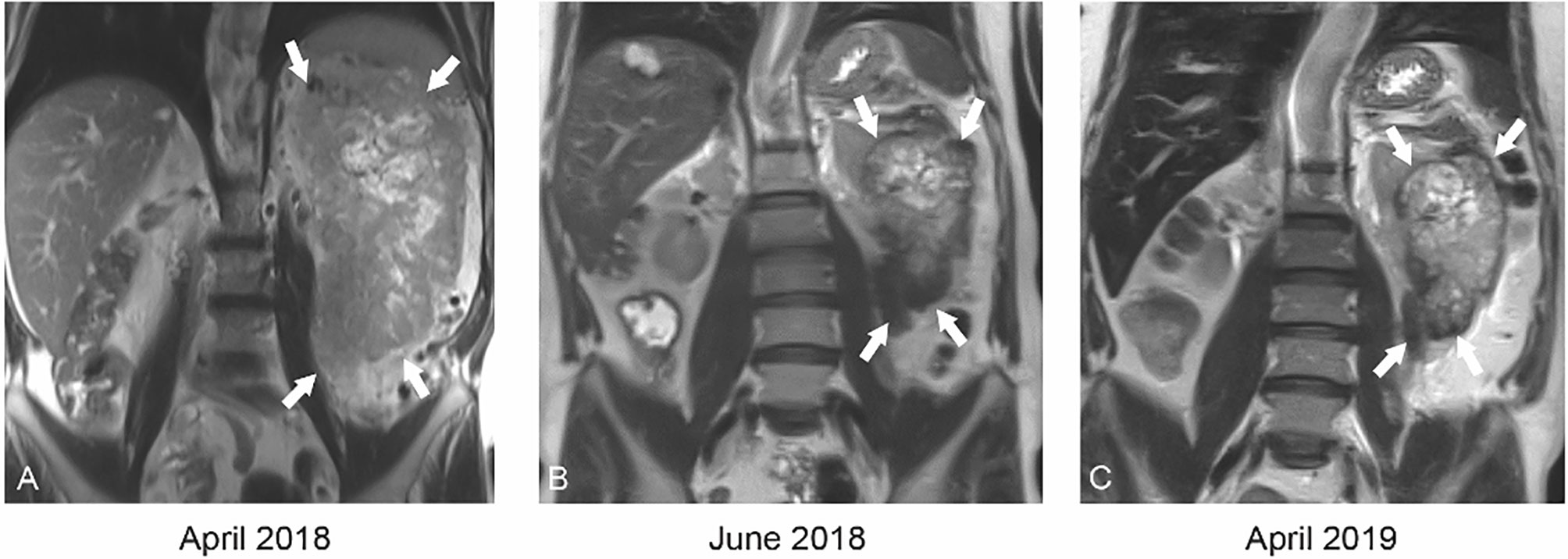
Figure 1 Coronal T2 weighted MRI at baseline (A) demonstrates a large 21.7 cm mass (white arrows) replacing the entire left kidney with central areas of necrosis. After just 2 months of cabozantinib therapy (B), the mass had decreased to 12.1 cm (white arrows). After 12 months of cabozantinib (C), the mass was stable in size.
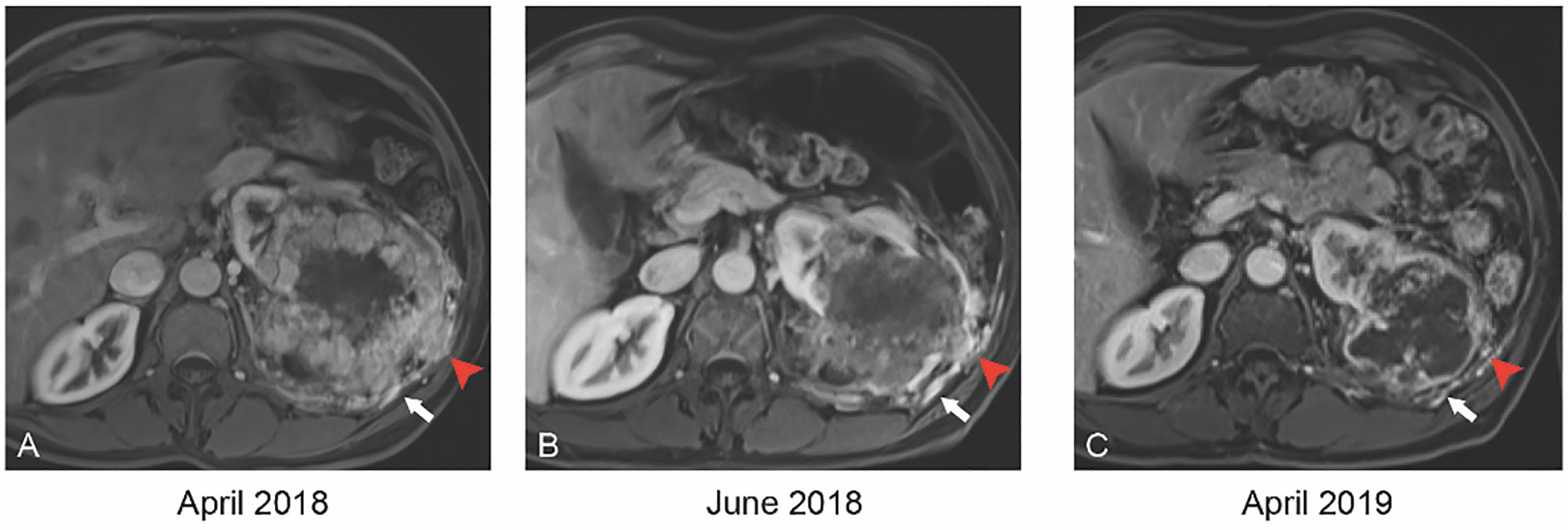
Figure 2 Axial T1 weighted MR with contrast at baseline (A) demonstrates tumor invasion into the intercostal space (red arrowhead) and neovascularity (white arrow). By 2 months of therapy (B), the invasion has retracted (red arrowhead). By 12 months (C), the invasion has resolved and vascularity has decreased significantly.
During cabozantinib therapy, the patient developed hypertension, secondary to cabozantinib, which was well controlled with lisinopril and amlodipine. Otherwise, there were no adverse events during drug therapy, besides mild hand-foot disease, and patient did not require any dose reduction. The patient was therefore scheduled for surgery after a 3-weeks washout from systemic therapy in March 2019.
In April 2019, patient underwent en bloc left radical nephrectomy, left adrenalectomy, retroperitoneal lymph node dissection, omentoplasty, distal pancreatectomy, splenectomy, and resections of quadratus lumborum, left psoas muscle, left crus muscle, and diaphragm with negative margins. Final pathology confirmed a 13.7 cm T4N0M0 grade 3 clear cell renal cell carcinoma invading the renal vein, renal sinus fat, perinephric fat, and psoas/diaphragm muscle and surgical margins were negative (Figure 4). The patient was discharged in a stable clinical status 9 days after surgery. When we wrote this report, the patient was still alive and well, and no evidence of recurrence on imaging.
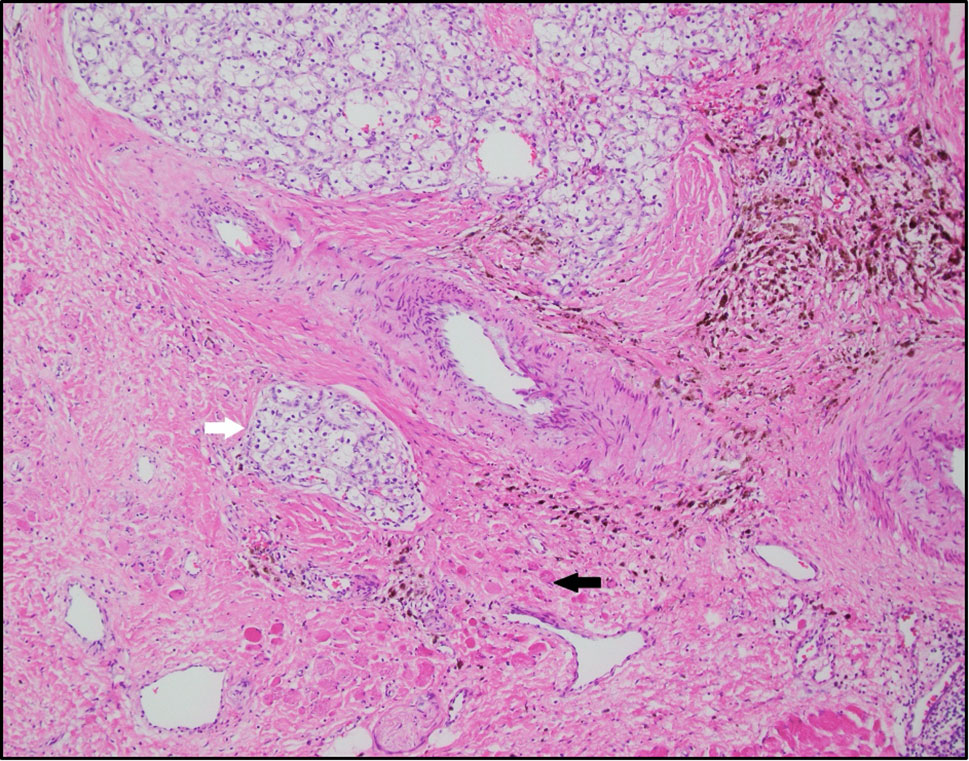
Figure 4 Clear cell renal cell carcinoma (white arrow) infiltrating into skeletal muscle (black arrow), representing psoas muscle and diaphragm. The background is fibrotic with extensive hemosiderin laden macrophages, possibly representing therapy related changes (H&E-10x).
Correlative studies were performed on resected tumor samples. Figure 5 shows this patient’s flow cytometry and pre-operative lab results compared to a cohort of renal cell carcinoma patients. The patient’s intraoperative sample was processed to obtain a single cell suspension, which was analyzed using flow cytometry. This allowed for enumeration of tumor infiltrating T lymphocytes (Figure 5A). The patient’s tumor had extremely few infiltrating CD8 T cells (0.061% CD8 T cells) (Figure 5B), which has been reported to suggest a poor prognosis (6). The patient’s neutrophil to lymphocyte ratio were within the first quartile of the cohort’s results (Figure 5C). His pre-operative C-reactive protein level and albumin level were within the second quartile (Figures 5D, E).
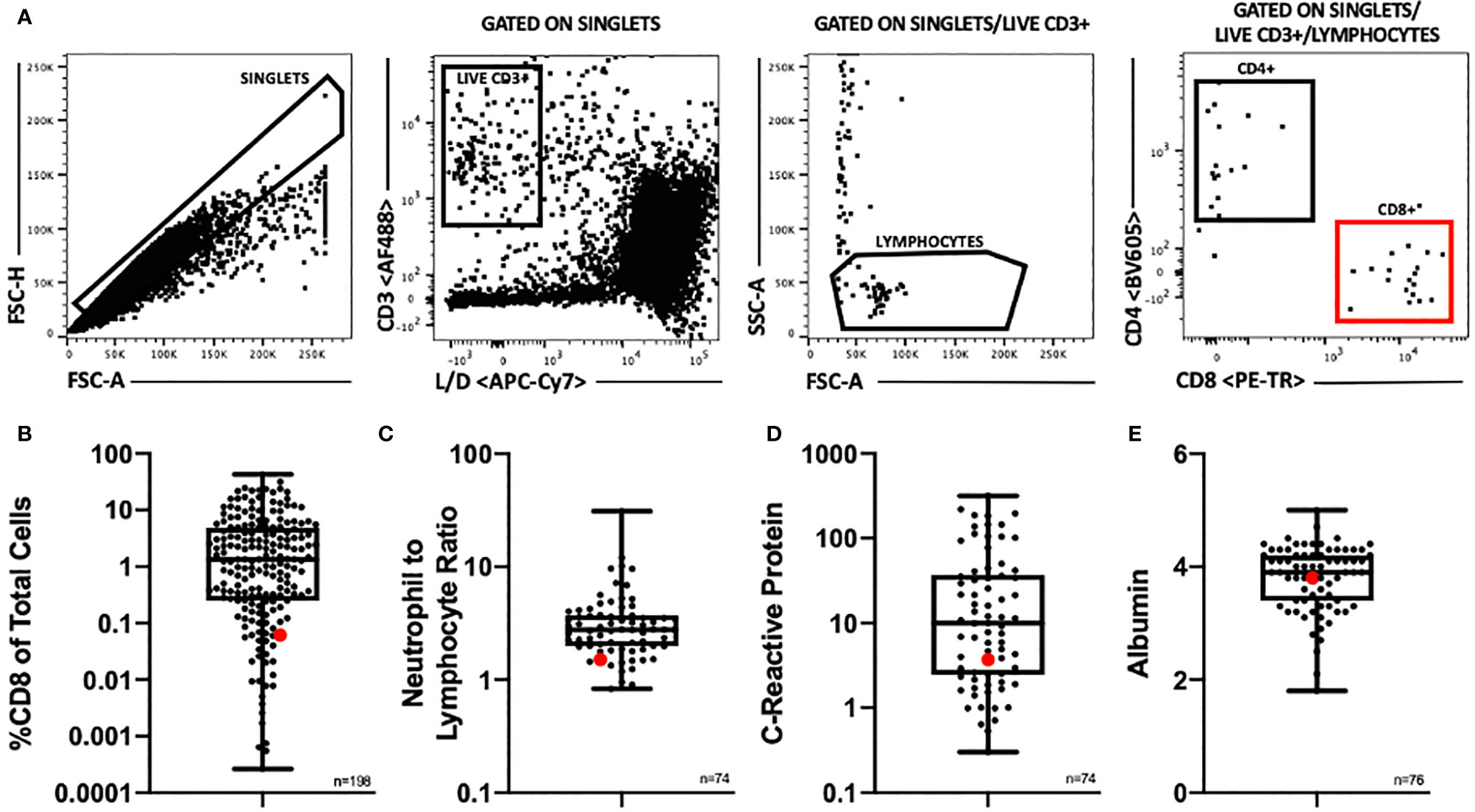
Figure 5 (A) Flow cytometry gating scheme. Samples were gated to exclude doublets and cell aggregates and to include only single cells for further analysis. This single cell population was then gated to include only live, CD3+ cells, then to include only lymphocyte sized cells. These gates insured as pure of a T lymphocyte population as possible for further analysis. T lymphocytes were then divided into CD4+ and CD8+ populations. (B) Distribution of %CD8 of total cells by flow cytometry among a cohort of renal cell carcinoma patients, n = 198. (C) Distribution of pre-operative neutrophil to lymphocyte ratio among a cohort of renal cell carcinoma patients (n=74). (D) Distribution of pre-operative C-reactive protein level (mg/L) among a cohort of renal cell carcinoma patients (n=74). (E) Distribution of pre-operative albumin level (g/dl) among a cohort of renal cell carcinoma patients (n=76). (B–E) Patient of interest highlighted in red. Box plots show middle 50% with the median at the center and the whiskers extending to minimum and maximum values.
Correlative studies were also performed on the formaldehyde fixed paraffin embedded pathology specimens from the tumor resection. Immunofluorescence imaging showed sparse CD8 T cell infiltration in two distinct specimens from the resected tumor lesion (Figures 6A, B), consistent with flow cytometry results (Figures 5A, B). The presence of CD31+ endothelium throughout the tumor specimen was also evident on immunofluorescence imaging (Figures 6A, B). Interestingly, tertiary lymphoid structures (TLS) were identified in both specimens examined (Figures 6C, D), despite the paucity of CD8 T cells identified on flow cytometry and immunofluorescence imaging, which is consistent with a report that there does not appear to be a correlation between CD8 T cell infiltration and the presence of TLS in RCC tumors (6).
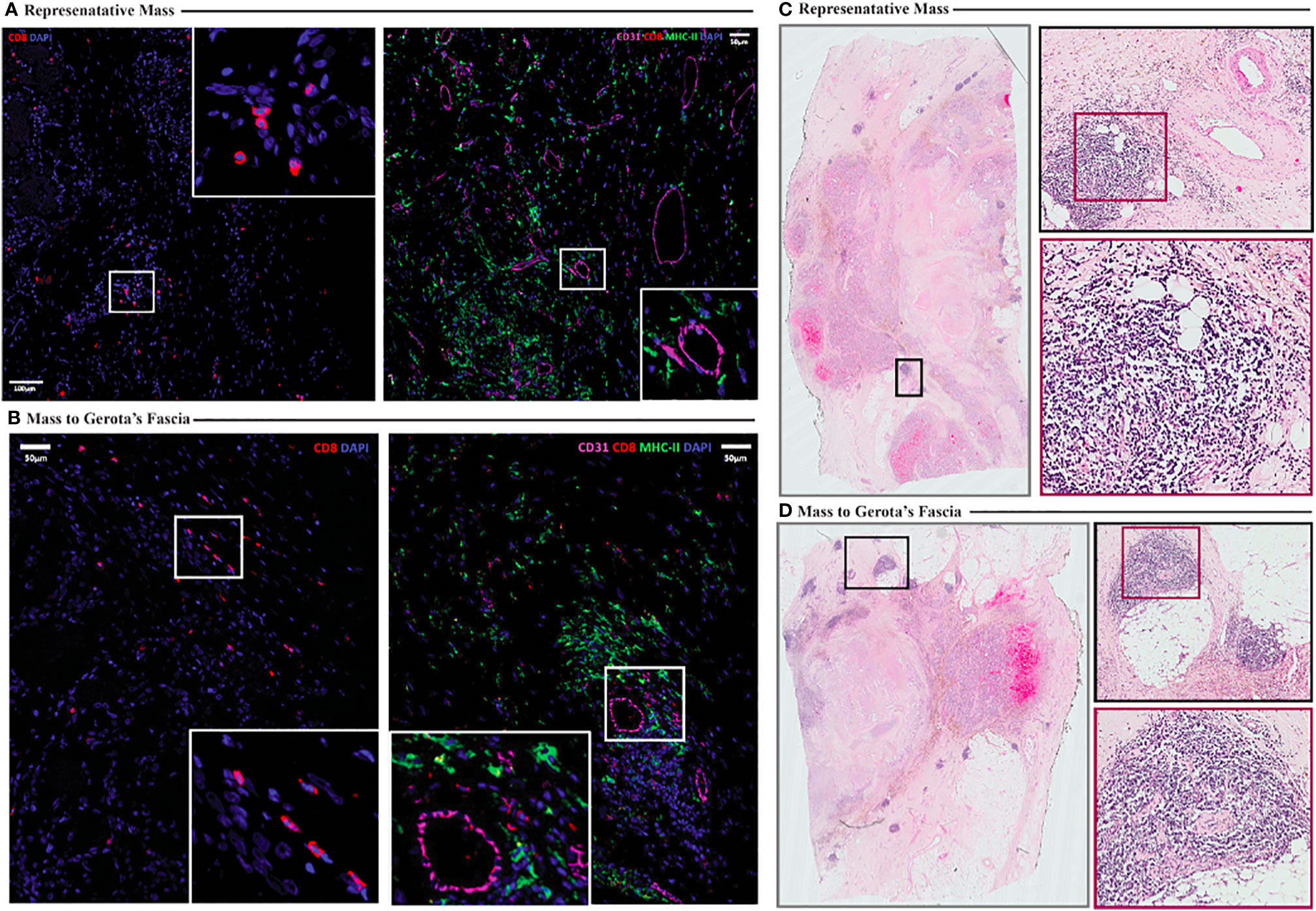
Figure 6 (A, B) Immunofluorescence imaging illustrating sparse CD8 T cell infiltration (A, B, left) and presence of CD31+ endothelium (A, B, right) in resected tumor specimens (A representative. Mass, B mass to Gerota’s fascia). (C, D) Hematoxylin and eosin staining shows presence of tertiary lymphoid structures (highlighted in insets) (C representative mass, D mass to Gerota’s fascia).
Next generation sequencing testing was performed on intra-operative resected tumor samples. No microsatellite instability was detected. No genes with pathogenic or likely pathogenic alternations were detected in the sample. Of note, there were no mutations detected in MET, VHL, PBRM1, BAP1, SETD2, or RET. Immunohistochemistry results were positive for MLH1 (2+, 80%), MSH2 (2+, 90%), MSH6 (1+, 60%), and PMS2 (1+, 80%) and were negative for PD-L1 (SP142).
We reported a patient presenting with initially unresectable locally advanced RCC treated with neoadjuvant cabozantinib, downsizing the tumor, and enabling surgical resection. This case study demonstrates important points about cabozantinib for patients with advanced RCC.
In patients presenting with initially unresectable advanced RCC, neoadjuvant cabozantinib may be of benefit for downsizing tumors to enable surgical resections that otherwise wouldn’t be possible. We also showed that this approach is feasible and safe prior to surgery. Reduction of tumor size was rapid, as the tumor diameter decreased by 44.2% after only 2 months of cabozantinib, a partial response on the Response Evaluation Criteria for Solid Tumors (RECIST). In the following 9 months, of 11 months total, of cabozantinib therapy, tumor size, appearance, and involvement of surrounding tissues remained stable. It has been suggested that one mechanism of cabozantinib’s efficacy is modulation of the immune microenvironment in the tumor (7–9), so it is interesting that this patient had a strong response to cabozantinib, despite a sparsely immune infiltrated tumor.
Although neoadjuvant therapy shows reduction of tumor size, it is yet not clear whether a prolonged survival can be achieved for patients. Several studies of neoadjuvant therapy for RCC have demonstrated consistent primary tumor size reduction to enable surgical resection (10–13). Rini et al. were the first to report response (28% median reduction in primary tumor size) of advanced primary renal tumors to treatment with neoadjuvant sunitinib. Of 28 patients with advanced RCC deemed unsuitable for initial nephrectomy, 13 (45%) were able to undergo nephrectomy following sunitinib (12). Emerging data from recent prospective phase II trials have also reported consistent tumor size reduction facilitating nephrectomies (13–16). In a literature review of neoadjuvant therapy to facilitate nephrectomy for locally advanced disease, Bindayi et al. found that 12 of 14 of the studies investigated neoadjuvant sunitinib or sorafenib (17). In a prospective phase 2 clinical trial, Karam et al. reported that axitinib, when given prior to surgery, resulted in significant shrinking of kidney cancers, facilitating surgical resections (13). Most recently, Roy et al. reported two patients with unresectable RCCs that were treated with cabozantinib, and achieved >50% tumor shrinkage which allowed surgical resection (18).
In summary, we present a patient with locally advanced RCC, treated with neoadjuvant cabozantinib downsizing a tumor and enabling surgical resection in this patient. Our findings demonstrate that neoadjuvant cabozantinib to facilitate subsequent surgical resection may be a feasible option for patients presenting with unresectable RCC. There is still a need for more effective neoadjuvant agents that might improve outcome of kidney cancer patients. Currently, we are conducting a neoadjuvant cabozantinib clinical trial at our institution (NCT04022343), with multiple correlative studies to facilitate identification of the patients most likely to respond.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the relevant individual(s), and/or minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
MB and VM conceived and designed the study. JJ, MB, CJ, and VM wrote the manuscript. MB, VM, JB, LH, AS, SM, OK, and BC provided the study materials or patients. LH, HK, CJ, and VM performed the experiments. MB managed the cabozantinib therapy. VM and SM performed the surgery. All authors contributed to the article and approved the submitted version.
This paper is supported by grants from the John Robinson Family Foundation, the Christopher Churchill Foundation. CJ is supported by a National Cancer Institute grant (1-F30-CA-243250).
MB has acted as a paid consultant for and/or as a member of the advisory boards of Exelixis, Bayer, BMS, Eisai, Pfizer, AstraZeneca, Janssen, Calithera Biosciences, Genomic Health, Nektar, and Sanofi and has received grants to his institution from Xencor, Bayer, Bristol-Myers Squibb, Genentech/Roche, Seattle Genetics, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Genome & Company, AAA, Peloton Therapeutics, and Pfizer for work performed as outside of the current study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank the patients and their families, and all members of the study team.
VEGF, vascular endothelial growth factor; RCC, renal cell carcinoma; CT, computed tomography; MRI, magnetic resonance imaging; TLS, tertiary lymphoid structure.
1. Choueiri TK, Escudier B, Powles T, Tiinar N, Mainwaring P, Rini B, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol (2016) 17(7):917–27. doi: 10.1016/S1470-2045(16)30107-3
2. Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson M, Walsh M, et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol (2017) 35(6):591–7. doi: 10.1200/JCO.2016.70.7398
3. Desai A, Small EJ. Treatment of advanced renal cell carcinoma patients with cabozantinib, an oral multityrosine kinase inhibitor of MET, AXL and VEGF receptors. Future Oncol (2019) 12:3741–9. doi: 10.2217/fon-2019-0021
4. Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med (2007) 356(2):125–34. doi: 10.1056/NEJMoa060655
5. Motzer RJ, Hutson TE, Tomczak P, Michaelson M, Bukowski R, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol (2009) 27(22):3584–90. doi: 10.1200/JCO.2008.20.1293
6. Jansen CS, Prokhnevska N, Master VA, Sanda M, Carlisle J, Bilen M, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature (2019) 576(7787):465–70. doi: 10.1038/s41586-019-1836-5
7. Patnaik A, Swanson KD, Csizmadia E, Solanki A, Landon-Brace N, Gehring M, et al. Cabozantinib Eradicates Advanced Murine Prostate Cancer by Activating Antitumor Innate Immunity. Cancer Discovery (2017) 7(7):750–65. doi: 10.1158/2159-8290.CD-16-0778
8. Lu X, Horner JW, Paul E, Shang X, Troncoso P, Deng P, et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature (2017) 543(7647):728–32. doi: 10.1038/nature21676
9. Kwilas AR, Ardiani A, Donahue RN, Aftab DT, Hodge JW. Dual effects of a targeted small-molecule inhibitor (cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J Transl Med (2014) 12:294. doi: 10.1186/s12967-014-0294-y
10. Amin C, Wallen E, Pruthi RS, Calvo BF, Godley PA, Rathmell WK. Preoperative tyrosine kinase inhibition as an adjunct to debulking nephrectomy. Urology (2008) 72(4):864–8. doi: 10.1016/j.urology.2008.01.088
11. Kondo T, Hashimoto Y, Kobayashi H, Lizuka J, Nishikawa T, Nakano T, et al. Presurgical targeted therapy with tyrosine kinase inhibitors for advanced renal cell carcinoma: clinical results and histopathological therapeutic effects. Jpn J Clin Oncol (2010) 40(12):1173–9. doi: 10.1093/jjco/hyq150
12. Rini BI, Garcia J, Elson P, Wood L, Shah S, Stephenson A, et al. The effect of sunitinib on primary renal cell carcinoma and facilitation of subsequent surgery. J Urol (2012) 187(5):1548–54. doi: 10.1016/j.juro.2011.12.075
13. Karam JA, Devine CE, Urbauer DL, Lozano M, Maity T, Ahrar K, et al. Phase 2 trial of neoadjuvant axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma. Eur Urol (2014) 66(5):874–80. doi: 10.1016/j.eururo.2014.01.035
14. Cowey CL, Amin C, Pruthi RS, Wallen E, Nielson M, Grigson G, et al. Neoadjuvant clinical trial with sorafenib for patients with stage II or higher renal cell carcinoma. J Clin Oncol (2010) 28(9):1502–7. doi: 10.1200/JCO.2009.24.7759
15. Hatiboglu G, Hohenfellner M, Arslan A, Hadaschik B, Teber D, Radtke J, et al. Effective downsizing but enhanced intratumoral heterogeneity following neoadjuvant sorafenib in patients with non-metastatic renal cell carcinoma. Langenbecks Arch Surg (2017) 402(4):637–44. doi: 10.1007/s00423-016-1543-8
16. Hellenthal NJ, Underwood W, Penetrante R, Litwin A, Zhang S, Wilding G, et al. Prospective clinical trial of preoperative sunitinib in patients with renal cell carcinoma. J Urol (2010) 184(3):859–64. doi: 10.1016/j.juro.2010.05.041
17. Bindayi A, Hamilton ZA, McDonald ML, Yim K, Millard F, McKay R, et al. Neoadjuvant therapy for localized and locally advanced renal cell carcinoma. Urol Oncol (2018) 36(1):31–7. doi: 10.1016/j.urolonc.2017.07.015
Keywords: cabozantinib, renal cell carcinoma, neoadjuvant therapy, radical nephrectomy, case report
Citation: Bilen MA, Jiang JF, Jansen CS, Brown JT, Harik LR, Sekhar A, Kissick H, Maithel SK, Kucuk O, Carthon B and Master VA (2021) Neoadjuvant Cabozantinib in an Unresectable Locally Advanced Renal Cell Carcinoma Patient Leads to Downsizing of Tumor Enabling Surgical Resection: A Case Report. Front. Oncol. 10:622134. doi: 10.3389/fonc.2020.622134
Received: 27 October 2020; Accepted: 15 December 2020;
Published: 01 February 2021.
Edited by:
Jinbo Chen, Central South University, ChinaReviewed by:
Brian Rini, Vanderbilt University, United StatesCopyright © 2021 Bilen, Jiang, Jansen, Brown, Harik, Sekhar, Kissick, Maithel, Kucuk, Carthon and Master. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehmet A. Bilen, bWJpbGVuQGVtb3J5LmVkdQ==; Viraj A. Master, dm1hc3RlckBlbW9yeS5lZHU=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.