
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 08 January 2021
Sec. Cancer Molecular Targets and Therapeutics
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.606485
Circular RNAs (circRNAs) are newly discovered intriguing RNAs due to the covalently closed loop structure, high stability, tissue specificity, and functional diversity. In recent years, a large number of circRNAs have been identified through high-throughput sequencing technology and bioinformatics methods, the abnormal expression of circRNAs are closely related to many diseases including bladder cancer (BC). CircRNAs have been proven to have several functions, such as acting as a regulator of parental gene transcription, miRNA sponge and interacting with proteins to regulate its expression. In addition, some circRNAs have been identified to encode proteins. CircRNAs have the characteristics of high abundance, high stability, wide distribution in body fluids, tissue specificity, and developmental stage specificity, which determine that circRNAs has great potential to be utilized as biomarkers for BC. Herein, we briefly summarize the biogenesis, functions and roles, and the current research progress of circRNAs in BC with a focus on the potential application for BC diagnosis, treatment, and prognosis.
Bladder cancer (BC) is one of the most common malignant cancers with an estimated 549,000 new cases and 200,000 deaths per year (1). Tumorigenesis processes of BC are a multicellular, multifactorial, and multistage process. Although the pathogenesis of BC are not well known, BC has been linked to tobacco smoke, parasitic infection, exposure to radiation, or chemicals and other risk factors (2, 3). Malignant transformation of normal cells, the communication of BC cells, BC stem cells, and microenvironment cells determines the initiation and progression of BC. However, the molecular mechanisms and the early diagnosis of BC have not been well elucidated. Therefore, it is urgent to explore new molecular mechanism and effective diagnosis biomarkers for BC.
Circular RNA (circRNA), a novel type of RNAs with covalently closed loop structure and lack of 3′ polyadenylated tails, are becoming a new hotspot in the field of non-coding RNAs in the recent years. CircRNAs were first discovered in the pathogenic plant viruses and considered to be the rare errors in RNA splicing. More recently, with high-throughput RNA sequencing technology, RNase R-treated transcriptomes and novel circRNA-specific bioinformatics, a large amount of circRNAs have identified in mammals, plants, fungi, worms, fish, insects, and other eukaryotes (4, 5). Since then, circRNAs have attracted widespread attention and relevant studies have been conducted on their biological properties, functions, molecular mechanisms, and potential application in clinical diagnosis and treatment.
In recent years, the expansion of knowledge of non-coding RNA biology has revealed critical roles in tumorigenesis processes (6). An increasing number of circRNAs have been found to participate in many pathological processes such as tumorigenesis through regulating genes expression at transcriptional, posttranscriptional, and translational (7–9). It is also reported that a variety of circRNAs are abnormally expressed in BC tissues or cell lines (10–13).
Since circRNAs have tissue and cell specificity, high abundance and stability, evolutionary species conservation and spread in various body fluids, exploring BC-related circRNAs as biomarkers or targets might be create new possibilities for the early diagnosis, effective treatment and prognosis evaluation of BC. In this review, we summarize the biological characteristics, functions, mechanisms of BC-related circRNAs, and finally discuss the potential applications as biomarkers, therapeutic targets.
CircRNAs are generated from precursor mRNA, and different from the canonical splicing of mRNA, circRNAs are produced by back-splicing process, which connects a 5′ splicing donor site with an upstream 3′ splicing receptor site to form a single chain covalent closed loop (9). The currently discovered circRNAs can be divided into three types: exonic circRNAs which contain exons only, intronic circRNAs which synthesized by introns, and exon-intron circRNAs which contain both exons and introns (9, 14).
However, the mechanisms for circRNAs biogenesis are not fully understood. Jeck et al. proposed two hypothetical models of exonic circRNAs formation: lariat-driven circularization and intron-pairing-driven circularization (15). Zhang et al. demonstrated that exon circularization is dependent on the complementary sequence of flanking introns (16). It was reported that microintrons containing splice sites and short reverse repeats can also form circRNAs (17). Subsequently, a new hypothetical model has been reported: RNA binding protein-mediated cyclization. ADAR1, muscle blind protein, and RNA-binding protein QKI are found to be critical in the formation of circRNAs (18–20).
In the current research, various techniques have been used detect and quantify circRNAs, including high-throughput RNA-seq, circRNA microarray, RT-PCR/qPCR, Northern blot, and some other technologies (21). High-throughout RNA-seq using next-generation sequencing, combine with depletion of ribosomal RNA to reveal the existence and quantity of circRNA (22). CircRNA microarray analysis uses circular ligation sequence specific probes combined with external nuclease linear RNA depletion to capture and quantify circRNAs at high sensitivity and specificity (15). Northern blot using the backsplice junction sequence specific probes and qRT‐PCR using divergent primers are used to verify and quantify limited known circRNAs (21).
Nowadays, with the continuous progress of circRNAs field, many circRNAs-associated databases have been built. The establishment of these databases makes it easier for researchers to analyze circRNAs’ information, regulatory networks, and role in diseases. The Circbase, CIRC pedia v2, and Deepbase 2.0 databases contain a large number of circRNAs and related detailed information about different species (23–25). The Circnet, Starbase v2.0, and circInteractome databases predict the circRNA-miRNA-gene network and interaction between circRNA and RNA-binding proteins (26–28). The CircRNADb and CSCD databases offer researchers the analysis of protein-encoding ability (29, 30). The CircRNADb database also contains genomic information, genome sequence, exon splicing, and references about circRNAs (29). The TRCirc database provides the regulatory information of circRNAs transcription (31). The CirclncRNAnet database provides a simple method for researchers to analyze sequencing results (32). The ExoRBase database and circRNA disease provides circRNAs existed in mutiple diseases or the related exosomes (33, 34). In addition to the above, there are some other related databases in constant establishment. However, due to the lack of standardized nomenclature, it is still difficult to search all the different databases for the same circRNA and compare these studies. In addition, database management and updates are still limited.
As a novel type of non-coding RNAs, circRNAs have circular structure, which is produced by unique back-splicing process with different mechanisms. Circular structure and different formation mechanism confer many unique characteristics to circRNAs such as high stability, evolutionary conservation, and tissues/cells specificity. These characteristics make it is possible to apply in the diagnosis, treatment and prognosis of diseases.
CircRNAs have been demonstrated to serve as miRNA sponges, regulate alternative splicing or transcription, bind to RNA binding proteins, perform rolling translation, encode proteins, as well as derive pseudo genes (4, 5, 35). CircRNAs are involved in many pathological processes, such as diabetes and its complications, nervous system disease, cardiovascular diseases as well as various tumors (5, 36–44).
In this review, we focus on the role, function and clinical application of circRNAs in the initiation, development, diagnosis, and prognosis of BC.
Increasing evidences indicated that circRNAs exert critical function in regulating BC occurrence and progression (Figure 1). More and more circRNAs have been discovered to regulate the proliferation, apoptosis, migration and invasion of BC cells. They played an important role in the occurrence and development of BC through various modes such as miRNA sponges and interaction with proteins (Table 1).
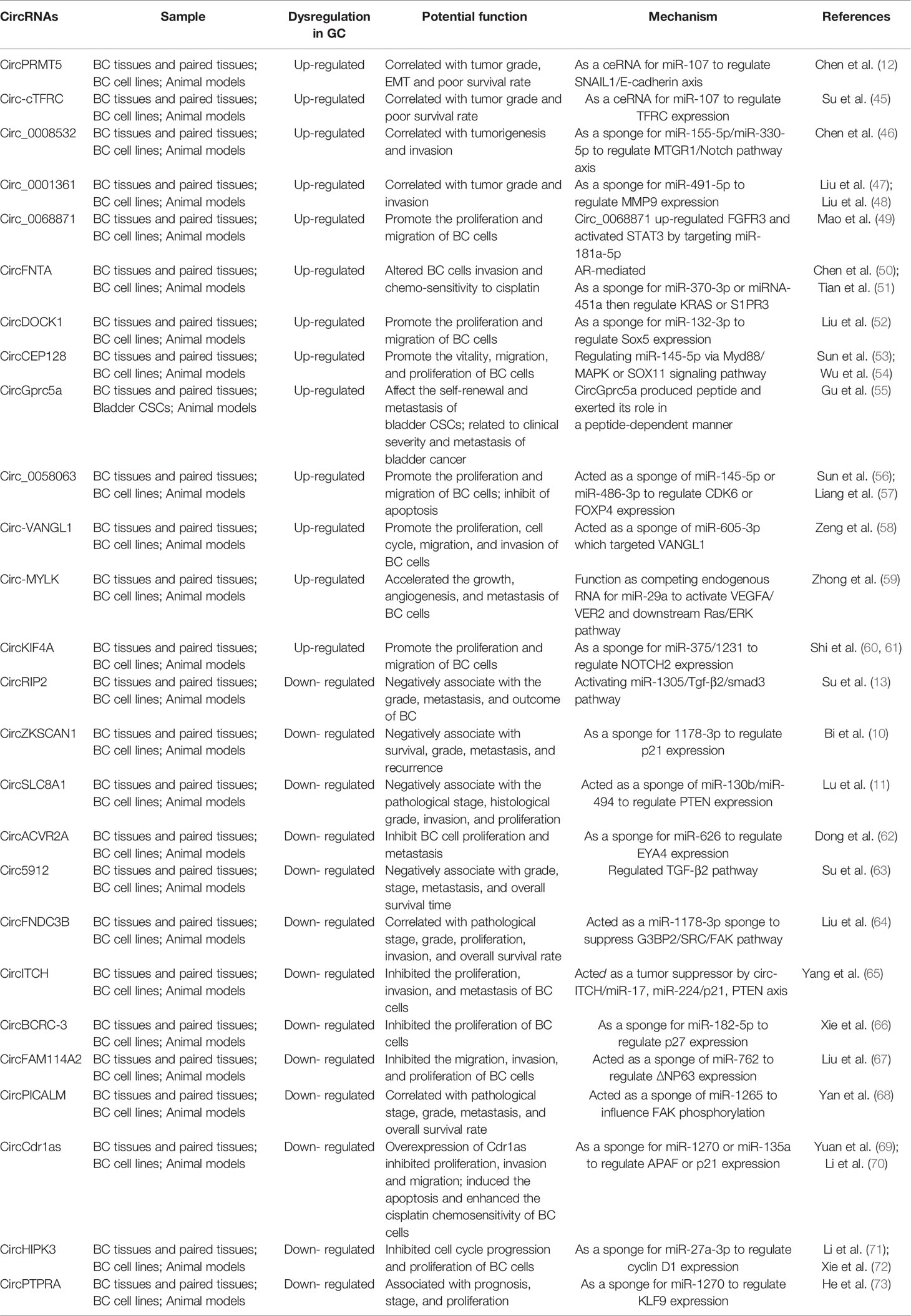
Table 1 Some examples of circRNAs that play an important role in the occurrence and development of BC.
Circ-cTFRC might be correlated with grade and poor survival rate of BC patients through circ-cTFRC/miR-107/TFRC axis. As known a sponge for miR-107, circ-cTFRC are up-regulated in BC tissues and cell lines, which related to grade, invasion, proliferation and poor survival rate. The expression of circ-cTFRC correlated with TFRC and negatively correlated with miR-107 in BC tissues and cell lines (45).
CircPRMT5 promoted metastasis of BC through sponging miR-30c to induce EMT. CircPRMT5 was up-regulated in BC tissues and associated with advanced clinical stage and worse survival of BC patients. Moreover, circPRMT5 is also up-regulated in serum and urine exosomes of BC patients, indicating that it may be significantly related to metastasis (12).
Circ_0008532 was revealed as miR-155-5p and miR-330-5p sponge and could regulate the capacity for invasive in BC cells through MTGR1/Notch pathway. Circ_0008532 is up-regulated in BC cells and tissues, which acted as an oncogene in BC, which may provide potential biomarkers and a therapeutic target for the treatment of BC (50).
A novel circRNA, circ_0001361 was recently found to act as oncogenic circRNA (47, 48). Circ_0001361 was highly expressed in BC tissues and cell lines, and it was positively correlated with grade, invasion, and poor overall survival. Mechanistically, circ_0001361 could directly interact with miR-491-5p to up-regulate MMP9, and MMP9 was verified to mediate circ_0001361-induced migration and invasion of BC cells (47).
Circ_0068871 was a circRNA derived from several exons of FGFR3, plays the role of oncogenes in the occurrence and development of BC, and serves as a potential biomarker (49). Circ_0068871 up-regulated expression of FGFR3 and activated STAT3 through sponging miR-181a-5p to promote cells proliferation and migration.
There are many other circRNAs (such as CircFNTA, circDOCK1, CircGprc5a, CircCEP128, Circ_0058063, Circ-VANGL1, CircRMYLK, and CircKIF4A) that play important roles in promoting BC, which could be used as potential clinical BC diagnosis and prognosis biomarkers (46, 51–60).
Su et al. found that circRIP2 may serve as tumor suppressor in BC through sponge for miR-1305 (13). Higher circRIP2 expression negatively associates with the grade, metastasis, and outcome of BC patients. They found that circRIP2 sponge miR-1305 to accelerate BC progression by activating TGF-β2/smad3 pathway.
Circ ZKSCAN1 was markedly down-regulated in BC tissues and cells and with survival, tumor grade, pathological stage, and tumor recurrence (10). Overexpressed circ-ZKSCAN1 inhibited the proliferation, invasion, and metastasis of BC cells. They also demonstrated that circZKSCAN1 suppressed the aggressive biological behaviors of BC cells through up-regulates the expression of p21 by sponging miR-1178-3p.
CircSLC8A1 is a circRNA transcribed from gene SLC8A1. CircSLC8A1 was identified from RNA-sequencing data, might serve as potential tumor suppressor for BC (11). They found that circSLC8A1 was down-regulated in BC tissues and cells, which was negatively correlated with the stage and grade of BC. CircSLC8A1 acted as miRNA sponge to regulate the expression of the miR-130b/miR-494 target gene PTEN, which suppressed the migration, invasion, and proliferation of BC cells.
CircACVR2A might serve as potential suppressive factor for BC, which selected from RNA-sequence, was found to be decreased in BC tissues and cell lines (62). CircACVR2A acted as a miRNA sponge of miR-626 to regulate EYA4 expression and suppressed the proliferation, migration, and invasion of BC cells.
CircFNDC3B acted as a miR-1178-3p sponge to suppress G3BP2, which served as a novel tumor suppressive factor and potential target for new therapies in human BC. CircFNDC3B was dramatically down-regulated in BC tissues and correlated with grade, proliferation, migration, invasion, and overall survival rate (64).
Circ5912 played the role of tumor suppressor gene in BC by regulating TGF-β2. Circ5912 expression was significantly lower in BC tissues. Higher circ5912 levels associated with better clinical outcomes, including grade, stage, metastasis, and longer overall survival time (63).
There are many other anti-cancer circRNAs that play important roles in the diagnosis, treatment and prognosis of BC and may be used in clinical practice in the future (58, 65–74).
The high incidence, high mortality and poor prognosis of BC make new requirements for early diagnosis and the prognosis. CircRNAs have been shown to have great potential as cancer diagnostic and prognostic biomarkers. Firstly, circRNAs can be easily detected due to the high stability and abundance in various tissue of human. Secondly, many circRNAs expression are tissue specific and development stage specific, which has an important role in diagnosis and prognosis. In addition, circRNAs are also exists in serum, plasma, and other body fluids, which can be used for non-invasive detection (9, 75, 76).
The clinical value of circRNAs as diagnostic and prognostic biomarkers of BC has been explored in many studies (62, 77–79). Subsequently, through the correlation analysis of clinic pathological factors and prognosis and survival analysis, a set of potential circRNAs biomarkers appeared for the early diagnosis of BC and the prediction of recurrence and metastasis (Table 2).
According to the detection of tissue and serum samples from BC patients and the controls, followed with clinic pathologic factors correlation analysis as well as prognostic and survival analysis, a set of potential circRNAs biomarkers are verified for BC diagnosis and prognosis. Circ_0077837 and circ_0004826 was significantly correlated with worse clinicopathological features and poor prognosis of BC patients. The area under the ROC curve of them was 0.775 and 0.790, respectively (80). Circ_0137439 was correlated with tumor stage, grade, lymph node status, and history of muscle-invasive BC. Urinary cell-free circ_0137439 could not only distinguish BC from normal controls, but also distinguish muscle-invasive BC from non-muscle-invasive BC. In addition, circ_0137439 in urine supernatant could be used as an independent prognostic indicator of recurrence-free survival and overall survival of BC patients (81). Circ_0000285 was significantly reduced in BC tissues and serum. Moreover, circ_0000285 was associated with tumor size (p < 0.001), differentiation (p < 0.001), distant metastasis (p = 0.004), TNM stage (p = 0.013), and lymph node metastasis (p = 0.038) (82). Higher circ5912 levels associates with better clinical outcomes, including grade (p = 0.008), metastasis (p = 0.016), and longer overall survival time (p = 0.0157) (63).
In conclusion, circRNAs play an important role in the occurrence and development of BC, which may be served as potential biomarkers for the diagnosis, prognosis, and therapy of BC.
As the roles in BC are gradually being clarified, circRNAs might be developed as potential therapeutic targets. Studies have proposed several strategies based on the function of circRNAs to treat BC. Exogenous up-regulation or down-regulation of related circRNAs to regulate miRNA may be the useful methods, and several of them have been applied. SiRNA or shRNA targeting a specific reverse splicing sequence of circRNAs was used to inhibit its expression (9, 83, 84). Stable circRNAs knockdown was generated using specific lentiviral short hairpin RNA (85–87). Some researchers have also tried to use the CRISPR/Cas9 system to achieve circRNAs knockout (88, 89). It was also reported that plasmids and lentiviral vectors used to increase the expression of circRNAs (88, 90). Overexpression vectors with short intronic repeat sequences also could increase the levels of circRNAs (17). In addition to exogenous regulation of circRNAs expression, endogenous regulation also has broad application prospects. However, there are still no relevant reports on how to control the process of endogenous cyclization.
Artificial circRNAs sponges targeting miRNAs may be a simple, effective, and convenient strategy for BC treatment. A large number of studies have found that circRNAs as miRNA sponges can inhibit the progression of BC (10, 11, 13, 62). These results suggested that BC can be treated by synthesizing related circRNAs sponges. Recently, synthetic circRNA presented a new treatment approach for critical global pathologies, including cardiovascular disease, hepatitis, esophageal carcinoma, and Gastric Carcinoma (91–94). These findings establish that the design and construction of highly efficient artificial circRNAs sponges represent a novel strategy in the treatment of cancer and other diseases.
Artificially controlled endogenous circularization may be another approach to apply circRNAs to the treatment of BC. On one hand, the “mRNA trap” can be used to isolate the translation initiation site of these abnormal mRNA to reduce the production of BC-related functional proteins (9, 95). On the other hand, using circRNAs to regulate the release and activity of BC-related proteins, or targeting coding circRNAs involved in tumorigenesis or development may be potential therapeutic methods (9).
CircRNAs are becoming an emerging field of tumor diagnosis and treatment research, but the experimental and clinical researches in BC still many unresolved. Firstly, the mechanism of cyclization and degradation of BC-related circRNAs is still unclear. Secondly, the number of BC-related circRNAs with clear biological functions and mechanisms of action is limited. Besides, under what conditions circRNAs play a cancer-promoting effect, and what conditions play a cancer-suppressing effect is not clear. In addition, it is not clear whether circRNAs can affect BC microenvironment cells and play a role in BC development.
Exosomes are nano-scale vesicles released by many cells, which can transfer signal molecules including circRNAs to recipient cells and regulate the progression of many diseases, including cancer. Exosomes might extremely extend circRNAs’ studies and applications. It is reported that exosomal circRNAs can be used as emerging tools for cancer diagnosis, prognosis and risk assessment (9, 61, 96, 97). Based on these studies, we believe exosomal circRNAs may be expected to become a promising biomarker and therapeutic tool for BC.
Noncoding RNA methylation modification in cancer is getting more and more attention from researchers. Recent studies have indicated that several circRNAs are closely related to the tumorigenesis of cancers via post-transcriptional methylation modificationsp (98–100). It provides a new research direction for us to explore the role of circRNAs in the occurrence and development of BC.
Nevertheless, the clinical application of circRNAs as drugs or targets in BC needs more detailed and complete experimental data such as safety, efficacy, and potential side effects. The systematic assessment including the cost, accuracy, repeatability, specificity, and sensitivity of circRNAs biomarkers in large samples is not sufficient. Furthermore, standardized methodology for circRNAs detecting in the process of clinical application is lacked. In addition, how to safely and effectively deliver circRNAs in vivo is also a problem to be solved. These challenges and deficiencies provide the direction for the follow-up research and technology development. We believe that with the deepening of research, the continuous progress of technology and the development of various aspects, these problems will eventually be solved.
Through traditional/emerging biological methods and informatics technologies along with these further studies, more and more BC-related circRNAs and their physiological and pathological functions have been identified. In addition, many circRNAs have been found to have great potential to be diagnosis and prognosis biomarkers for BC as well as novel therapeutic targets or approaches. The knowledge of the precise mechanisms of circRNAs cyclization, degradation, localization, and function in BC is still limited in current stage. But, we believe that these limitations will eventually be resolved, these novel diagnosis and treatment strategies based on circRNAs will effectively serve BC clinical early diagnosis and treatment in the future.
ZL, HQ, and WX contributed to the conception and design of this review. ZL wrote the first draft of the manuscript. WG, SF, YZ, and LL wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (no. 81602883), Zhenjiang Key Laboratory of High Technology Research on Exosomes Foundation and Transformation Application (Grant SS2018003), Zhenjiang Social Development Guidance Project (No. FZ2019038), the Foundation for Excellent Young Teachers of Jiangsu University, and Research and Practice innovation program for graduate students of Jiangsu Province (No. KYCX20_3089).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin (2020) 70(5):404–23. doi: 10.3322/caac.21631
3. Liang Z, Lu L, Mao J, Li X, Qian H, Xu W. Curcumin reversed chronic tobacco smoke exposure induced urocystic EMT and acquisition of cancer stem cells properties via Wnt/beta-catenin. Cell Death Dis (2017) 8(10):e3066. doi: 10.1038/cddis.2017.452
4. Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol (2020) 21(8):475–90. doi: 10.1038/s41580-020-0243-y
5. Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet (2019) 20(11):675–91. doi: 10.1038/s41576-019-0158-7
6. Hanniford D, Ulloa-Morales A, Karz A, Berzoti-Coelho MG, Moubarak RS, Sanchez-Sendra B, et al. Epigenetic Silencing of CDR1as Drives IGF2BP3-Mediated Melanoma Invasion and Metastasis. Cancer Cell (2020) 37(1):55–70 e15. doi: 10.1016/j.ccell.2019.12.007
7. Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao L, Zhang Y, et al. The Landscape of Circular RNA in Cancer. Cell (2019) 176(4):869–81 e13. doi: 10.1016/j.cell.2018.12.021
8. Guarnerio J, Bezzi M, Jeong JC, Paffenholz SV, Berry K, Naldini MM, et al. Oncogenic Role of Fusion-circRNAs Derived from Cancer-Associated Chromosomal Translocations. Cell (2016) 165(2):289–302. doi: 10.1016/j.cell.2016.03.020
9. Li R, Jiang J, Shi H, Qian H, Zhang X, Xu W. CircRNA: a rising star in gastric cancer. Cell Mol Life Sci (2020) 77(9):1661–80. doi: 10.1007/s00018-019-03345-5
10. Bi J, Liu H, Dong W, Xie W, He Q, Cai Z, et al. Circular RNA circ-ZKSCAN1 inhibits bladder cancer progression through miR-1178-3p/p21 axis and acts as a prognostic factor of recurrence. Mol Cancer (2019) 18(1):133. doi: 10.1186/s12943-019-1060-9
11. Lu Q, Liu T, Feng H, Yang R, Zhao X, Chen W, et al. Circular RNA circSLC8A1 acts as a sponge of miR-130b/miR-494 in suppressing bladder cancer progression via regulating PTEN. Mol Cancer (2019) 18(1):111. doi: 10.1186/s12943-019-1040-0
12. Chen X, Chen RX, Wei WS, Li YH, Feng ZH, Tan L, et al. PRMT5 Circular RNA Promotes Metastasis of Urothelial Carcinoma of the Bladder through Sponging miR-30c to Induce Epithelial-Mesenchymal Transition. Clin Cancer Res (2018) 24(24):6319–30. doi: 10.1158/1078-0432.CCR-18-1270
13. Su Y, Feng W, Shi J, Chen L, Huang J, Lin T. circRIP2 accelerates bladder cancer progression via miR-1305/Tgf-beta2/smad3 pathway. Mol Cancer (2020) 19(1):23. doi: 10.1186/s12943-019-1129-5
14. Shang Q, Yang Z, Jia R, Ge S. The novel roles of circRNAs in human cancer. Mol Cancer (2019) 18(1):6. doi: 10.1186/s12943-018-0934-6
15. Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. Rna (2013) 19(2):141–57. doi: 10.1261/rna.035667.112
16. Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL, Yang L. Complementary sequence-mediated exon circularization. Cell (2014) 159(1):134–47. doi: 10.1016/j.cell.2014.09.001
17. Liang D, Wilusz JE. Short intronic repeat sequences facilitate circular RNA production. Genes Dev (2014) 28(20):2233–47. doi: 10.1101/gad.251926.114
18. Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell (2014) 56(1):55–66. doi: 10.1016/j.molcel.2014.08.019
19. Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, et al. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep (2015) 10(2):170–7. doi: 10.1016/j.celrep.2014.12.019
20. Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell (2015) 160(6):1125–34. doi: 10.1016/j.cell.2015.02.014
21. Altesha MA, Ni T, Khan A, Liu K, Zheng X. Circular RNA in cardiovascular disease. J Cell Physiol (2019) 234(5):5588–600. doi: 10.1002/jcp.27384
22. Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol (2014) 32(5):453–61. doi: 10.1038/nbt.2890
23. Glazar P, Papavasileiou P, Rajewsky N. circBase: a database for circular RNAs. Rna (2014) 20(11):1666–70. doi: 10.1261/rna.043687.113
24. Dong R, Ma XK, Li GW, Yang L. CIRCpedia v2: An Updated Database for Comprehensive Circular RNA Annotation and Expression Comparison. Genomics Proteomics Bioinformatics (2018) 16(4):226–33. doi: 10.1016/j.gpb.2018.08.001
25. Zheng LL, Li JH, Wu J, Sun WJ, Liu S, Wang ZL, et al. deepBase v2.0: identification, expression, evolution and function of small RNAs, LncRNAs and circular RNAs from deep-sequencing data. Nucleic Acids Res (2016) 44(D1):D196–202. doi: 10.1093/nar/gkv1273
26. Liu YC, Li JR, Sun CH, Andrews E, Chao RF, Lin FM, et al. CircNet: a database of circular RNAs derived from transcriptome sequencing data. Nucleic Acids Res (2016) 44(D1):D209–15. doi: 10.1093/nar/gkv940
27. Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res (2014) 42(Database issue):D92–7. doi: 10.1093/nar/gkt1248
28. Dudekula DB, Panda AC, Grammatikakis I, De S, Abdelmohsen K, Gorospe M. CircInteractome: A web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol (2016) 13(1):34–42. doi: 10.1080/15476286.2015.1128065
29. Chen X, Han P, Zhou T, Guo X, Song X, Li Y. circRNADb: A comprehensive database for human circular RNAs with protein-coding annotations. Sci Rep (2016) 6:34985. doi: 10.1038/srep34985
30. Xia S, Feng J, Chen K, Ma Y, Gong J, Cai F, et al. CSCD: a database for cancer-specific circular RNAs. Nucleic Acids Res (2018) 46(D1):D925–D9. doi: 10.1093/nar/gkx863
31. Tang Z, Li X, Zhao J, Qian F, Feng C, Li Y, et al. TRCirc: a resource for transcriptional regulation information of circRNAs. Briefings Bioinform (2019) 20(6):2327–33. doi: 10.1093/bib/bby083
32. Wu SM, Liu H, Huang PJ, Chang IY, Lee CC, Yang CY, et al. circlncRNAnet: an integrated web-based resource for mapping functional networks of long or circular forms of noncoding RNAs. GigaScience (2018) 7(1):1–10. doi: 10.1093/gigascience/gix118
33. Li S, Li Y, Chen B, Zhao J, Yu S, Tang Y, et al. exoRBase: a database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res (2018) 46(D1):D106–D12. doi: 10.1093/nar/gkx891
34. Zhao Z, Wang K, Wu F, Wang W, Zhang K, Hu H, et al. circRNA disease: a manually curated database of experimentally supported circRNA-disease associations. Cell Death Dis (2018) 9(5):475. doi: 10.1038/s41419-018-0503-3
35. Baumann K. CircRNAs in lifespan. Nat Rev Mol Cell Biol (2020) 21(8):420. doi: 10.1038/s41580-020-0269-1
36. Tian Y, Xu J, Du X, Fu X. The interplay between noncoding RNAs and insulin in diabetes. Cancer Lett (2018) 419:53–63. doi: 10.1016/j.canlet.2018.01.038
37. Shan K, Liu C, Liu BH, Chen X, Dong R, Liu X, et al. Circular Noncoding RNA HIPK3 Mediates Retinal Vascular Dysfunction in Diabetes Mellitus. Circulation (2017) 136(17):1629–42. doi: 10.1161/CIRCULATIONAHA.117.029004
38. Chen B, Li Y, Liu Y, Xu Z. circLRP6 regulates high glucose-induced proliferation, oxidative stress, ECM accumulation, and inflammation in mesangial cells. J Cell Physiol (2019) 234(11):21249–59. doi: 10.1002/jcp.28730
39. Kumar L, Shamsuzzama, Jadiya P, Haque R, Shukla S, Nazir A. Functional Characterization of Novel Circular RNA Molecule, circzip-2 and Its Synthesizing Gene zip-2 in C. elegans Model of Parkinson’s Disease. Mol Neurobiol (2018) 55(8):6914–26. doi: 10.1007/s12035-018-0903-5
40. Lo I, Hill J, Vilhjalmsson BJ, Kjems J. Linking the association between circRNAs and Alzheimer’s disease progression by multi-tissue circular RNA characterization. RNA Biol (2020) 17(12):1789–97. doi: 10.1080/15476286.2020.1783487
41. Huang S, Li X, Zheng H, Si X, Li B, Wei G, et al. Loss of Super-Enhancer-Regulated circRNA Nfix Induces Cardiac Regeneration After Myocardial Infarction in Adult Mice. Circulation (2019) 139(25):2857–76. doi: 10.1161/CIRCULATIONAHA.118.038361
42. Vausort M, Salgado-Somoza A, Zhang L, Leszek P, Scholz M, Teren A, et al. Myocardial Infarction-Associated Circular RNA Predicting Left Ventricular Dysfunction. J Am Coll Cardiol (2016) 68(11):1247–8. doi: 10.1016/j.jacc.2016.06.040
43. Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun (2016) 7:12429. doi: 10.1038/ncomms12429
44. Guarnerio J, Zhang Y, Cheloni G, Panella R, Mae Katon J, Simpson M, et al. Intragenic antagonistic roles of protein and circRNA in tumorigenesis. Cell Res (2019) 29(8):628–40. doi: 10.1038/s41422-019-0192-1
45. Su H, Tao T, Yang Z, Kang X, Zhang X, Kang D, et al. Circular RNA cTFRC acts as the sponge of MicroRNA-107 to promote bladder carcinoma progression. Mol Cancer (2019) 18(1):27. doi: 10.1186/s12943-019-0951-0
46. Chen J, Sun Y, Ou Z, Yeh S, Huang CP, You B, et al. Androgen receptor-regulated circFNTA activates KRAS signaling to promote bladder cancer invasion. EMBO Rep (2020) 21(4):e48467. doi: 10.15252/embr.201948467
47. Liu F, Zhang H, Xie F, Tao D, Xiao X, Huang C, et al. Hsa_circ_0001361 promotes bladder cancer invasion and metastasis through miR-491-5p/MMP9 axis. Oncogene (2020) 39(8):1696–709. doi: 10.1038/s41388-019-1092-z
48. Liu L, Wu SQ, Zhu X, Xu R, Ai K, Zhang L, et al. Analysis of ceRNA network identifies prognostic circRNA biomarkers in bladder cancer. Neoplasma (2019) 66(5):736–45. doi: 10.4149/neo_2019_190107N25
49. Mao W, Huang X, Wang L, Zhang Z, Liu M, Li Y, et al. Circular RNA hsa_circ_0068871 regulates FGFR3 expression and activates STAT3 by targeting miR-181a-5p to promote bladder cancer progression. J Exp Clin Cancer Res (2019) 38(1):169. doi: 10.1186/s13046-019-1136-9
50. Chen L, Yang X, Zhao J, Xiong M, Almaraihah R, Chen Z, et al. Circ_0008532 promotes bladder cancer progression by regulation of the miR-155-5p/miR-330-5p/MTGR1 axis. J Exp Clin Cancer Res (2020) 39(1):94. doi: 10.1186/s13046-020-01592-0
51. Tian J, Fan J, Xu J, Ren T, Guo H, Zhou L. circ-FNTA accelerates proliferation and invasion of bladder cancer. Oncol Lett (2020) 19(1):1017–23. doi: 10.3892/ol.2019.11150
52. Liu P, Li X, Guo X, Chen J, Li C, Chen M, et al. Circular RNA DOCK1 promotes bladder carcinoma progression via modulating circDOCK1/hsa-miR-132-3p/Sox5 signalling pathway. Cell Prolif (2019) 52(4):e12614. doi: 10.1111/cpr.12614
53. Sun M, Zhao W, Chen Z, Li M, Li S, Wu B, et al. Circular RNA CEP128 promotes bladder cancer progression by regulating Mir-145-5p/Myd88 via MAPK signaling pathway. Int J Cancer (2019) 145(8):2170–81. doi: 10.1002/ijc.32311
54. Wu Z, Huang W, Wang X, Wang T, Chen Y, Chen B, et al. Circular RNA CEP128 acts as a sponge of miR-145-5p in promoting the bladder cancer progression via regulating SOX11. Mol Med (2018) 24(1):40. doi: 10.1186/s10020-018-0039-0
55. Gu C, Zhou N, Wang Z, Li G, Kou Y, Yu S, et al. circGprc5a Promoted Bladder Oncogenesis and Metastasis through Gprc5a-Targeting Peptide. Mol Ther Nucleic Acids (2018) 13:633–41. doi: 10.1016/j.omtn.2018.10.008
56. Sun M, Zhao W, Chen Z, Li M, Li S, Wu B, et al. Circ_0058063 regulates CDK6 to promote bladder cancer progression by sponging miR-145-5p. J Cell Physiol (2019) 234(4):4812–24. doi: 10.1002/jcp.27280
57. Liang H, Huang H, Li Y, Lu Y, Ye T. CircRNA_0058063 functions as a ceRNA in bladder cancer progression via targeting miR-486-3p/FOXP4 axis. Biosci Rep (2020) 40(3):BSR20193484. doi: 10.1042/BSR20193484
58. Zeng Z, Zhou W, Duan L, Zhang J, Lu X, Jin L, et al. Circular RNA circ-VANGL1 as a competing endogenous RNA contributes to bladder cancer progression by regulating miR-605-3p/VANGL1 pathway. J Cell Physiol (2019) 234(4):3887–96. doi: 10.1002/jcp.27162
59. Zhong Z, Huang M, Lv M, He Y, Duan C, Zhang L, et al. Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Cancer Lett (2017) 403:305–17. doi: 10.1016/j.canlet.2017.06.027
60. Shi YR, Wu Z, Xiong K, Liao QJ, Ye X, Yang P, et al. Circular RNA circKIF4A Sponges miR-375/1231 to Promote Bladder Cancer Progression by Upregulating NOTCH2 Expression. Front Pharmacol (2020) 11:605:605. doi: 10.3389/fphar.2020.00605
61. Shi X, Wang B, Feng X, Xu Y, Lu K, Sun M. circRNAs and Exosomes: A Mysterious Frontier for Human Cancer. Mol Ther Nucleic Acids (2020) 19:384–92. doi: 10.1016/j.omtn.2019.11.023
62. Dong W, Bi J, Liu H, Yan D, He Q, Zhou Q, et al. Circular RNA ACVR2A suppresses bladder cancer cells proliferation and metastasis through miR-626/EYA4 axis. Mol Cancer (2019) 18(1):95. doi: 10.1186/s12943-019-1025-z
63. Su Y, Du Z, Zhong G, Ya Y, Bi J, Shi J, et al. circ5912 suppresses cancer progression via inducing MET in bladder cancer. Aging (2019) 11(23):10826–38. doi: 10.18632/aging.102464
64. Liu H, Bi J, Dong W, Yang M, Shi J, Jiang N, et al. Invasion-related circular RNA circFNDC3B inhibits bladder cancer progression through the miR-1178-3p/G3BP2/SRC/FAK axis. Mol Cancer (2018) 17(1):161. doi: 10.1186/s12943-018-0908-8
65. Yang C, Yuan W, Yang X, Li P, Wang J, Han J, et al. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol Cancer (2018) 17(1):19. doi: 10.1186/s12943-018-0771-7
66. Xie F, Li Y, Wang M, Huang C, Tao D, Zheng F, et al. Circular RNA BCRC-3 suppresses bladder cancer proliferation through miR-182-5p/p27 axis. Mol Cancer (2018) 17(1):144. doi: 10.1186/s12943-018-0892-z
67. Liu T, Lu Q, Liu J, Xie S, Feng B, Zhu W, et al. Circular RNA FAM114A2 suppresses progression of bladder cancer via regulating NP63 by sponging miR-762. Cell Death Dis (2020) 11(1):47. doi: 10.1038/s41419-020-2226-5
68. Yan D, Dong W, He Q, Yang M, Huang L, Kong J, et al. Circular RNA circPICALM sponges miR-1265 to inhibit bladder cancer metastasis and influence FAK phosphorylation. EBioMedicine (2019) 48:316–31. doi: 10.1016/j.ebiom.2019.08.074
69. Yuan W, Zhou R, Wang J, Han J, Yang X, Yu H, et al. Circular RNA Cdr1as sensitizes bladder cancer to cisplatin by upregulating APAF1 expression through miR-1270 inhibition. Mol Oncol (2019) 13(7):1559–76. doi: 10.1002/1878-0261.12523
70. Li P, Yang X, Yuan W, Yang C, Zhang X, Han J, et al. CircRNA-Cdr1as Exerts Anti-Oncogenic Functions in Bladder Cancer by Sponging MicroRNA-135a. Cell Physiol Biochem (2018) 46(4):1606–16. doi: 10.1159/000489208
71. Li Y, Zheng F, Xiao X, Xie F, Tao D, Huang C, et al. CircHIPK3 sponges miR-558 to suppress heparanase expression in bladder cancer cells. EMBO Rep (2017) 18(9):1646–59. doi: 10.15252/embr.201643581
72. Xie F, Zhao N, Zhang H, Xie D. Circular RNA CircHIPK3 Promotes Gemcitabine Sensitivity in Bladder Cancer. J Cancer (2020) 11(7):1907–12. doi: 10.7150/jca.39722
73. He Q, Huang L, Yan D, Bi J, Yang M, Huang J, et al. CircPTPRA acts as a tumor suppressor in bladder cancer by sponging miR-636 and upregulating KLF9. Aging (2019) 11(23):11314–28. doi: 10.18632/aging.102530
74. Zheng F, Wang M, Li Y, Huang C, Tao D, Xie F, et al. CircNR3C1 inhibits proliferation of bladder cancer cells by sponging miR-27a-3p and downregulating cyclin D1 expression. Cancer Lett (2019) 460:139–51. doi: 10.1016/j.canlet.2019.06.018
75. Memczak S, Papavasileiou P, Peters O, Rajewsky N. Identification and Characterization of Circular RNAs As a New Class of Putative Biomarkers in Human Blood. PloS One (2015) 10(10):e0141214. doi: 10.1371/journal.pone.0141214
76. Lin X, Lo HC, Wong DT, Xiao X. Noncoding RNAs in human saliva as potential disease biomarkers. Front Genet (2015) 6:175:175. doi: 10.3389/fgene.2015.00175
77. Kouhsar M, Azimzadeh Jamalkandi S, Moeini A, Masoudi-Nejad A. Detection of novel biomarkers for early detection of Non-Muscle-Invasive Bladder Cancer using Competing Endogenous RNA network analysis. Sci Rep (2019) 9(1):8434. doi: 10.1038/s41598-019-44944-3
78. Cong L, Yang Q, Hu C, Yu Q, Hao S, Li D. Current Status of Functional Studies on Circular RNAs in Bladder Cancer and their Potential Role as Diagnostic and Prognostic Biomarkers: A Review. Med Sci Monit (2019) 25:3425–34. doi: 10.12659/MSM.916697
79. Li M, Wang Y, Liu Y, Zhang X, Liu J, Wang P. Low Expression of hsa_circ_0018069 in Human Bladder Cancer and Its Clinical Significance. BioMed Res Int (2019) 2019:9681863. doi: 10.1155/2019/9681863
80. Shen C, Wu Z, Wang Y, Gao S, Da L, Xie L, et al. Downregulated hsa_circ_0077837 and hsa_circ_0004826, facilitate bladder cancer progression and predict poor prognosis for bladder cancer patients. Cancer Med (2020) 9(11):3885–903. doi: 10.1002/cam4.3006
81. Song Z, Zhang Q, Zhu J, Yin G, Lin L, Liang C. Identification of urinary hsa_circ _0137439 as potential biomarker and tumor regulator of bladder cancer. Neoplasma (2020) 67(1):137–46. doi: 10.4149/neo_2018_181214N970
82. Chi BJ, Zhao DM, Liu L, Yin XZ, Wang FF, Bi S, et al. Downregulation of hsa_circ_0000285 serves as a prognostic biomarker for bladder cancer and is involved in cisplatin resistance. Neoplasma (2019) 66(2):197–202. doi: 10.4149/neo_2018_180318N185
83. Lu D, Thum T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat Rev Cardiol (2019) 16(11):661–74. doi: 10.1038/s41569-019-0218-x
84. Yang H, Li X, Meng Q, Sun H, Wu S, Hu W, et al. CircPTK2 (hsa_circ_0005273) as a novel therapeutic target for metastatic colorectal cancer. Mol Cancer (2020) 19(1):13. doi: 10.1186/s12943-020-1139-3
85. Li LJ, Zhu ZW, Zhao W, Tao SS, Li BZ, Xu SZ, et al. Circular RNA expression profile and potential function of hsa_circ_0045272 in systemic lupus erythematosus. Immunology (2018) 155(1):137–49. doi: 10.1111/imm.12940
86. Li C, Zhou H. Circular RNA hsa_circRNA_102209 promotes the growth and metastasis of colorectal cancer through miR-761-mediated Ras and Rab interactor 1 signaling. Cancer Med (2020) 9(18):6710–25. doi: 10.1002/cam4.3332
87. Feng F, Chen AP, Wang XL, Wu GL. Circ_0061140 promotes metastasis of bladder cancer through adsorbing microRNA-1236. Eur Rev Med Pharmacol Sci (2020) 24(10):5310–9. doi: 10.26355/eurrev_202005_21313
88. Gupta SK, Garg A, Bar C, Chatterjee S, Foinquinos A, Milting H, et al. Quaking Inhibits Doxorubicin-Mediated Cardiotoxicity Through Regulation of Cardiac Circular RNA Expression. Circ Res (2018) 122(2):246–54. doi: 10.1161/CIRCRESAHA.117.311335
89. Piwecka M, Glazar P, Hernandez-Miranda LR, Memczak S, Wolf SA, Rybak-Wolf A, et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science (2017) 357(6357):eaam8526. doi: 10.1126/science.aam8526
90. Liang J, Shen YC, Zhang XY, Chen C, Zhao H, Hu J. Circular RNA HIPK3 downregulation mediates hydrogen peroxide-induced cytotoxicity in human osteoblasts. Aging (2020) 12(2):1159–70. doi: 10.18632/aging.102674
91. Lavenniah A, Luu TDA, Li YP, Lim TB, Jiang J, Ackers-Johnson M, et al. Engineered Circular RNA Sponges Act as miRNA Inhibitors to Attenuate Pressure Overload-Induced Cardiac Hypertrophy. Mol Ther (2020) 28(6):1506–17. doi: 10.1016/j.ymthe.2020.04.006
92. Jost I, Shalamova LA, Gerresheim GK, Niepmann M, Bindereif A, Rossbach O. Functional sequestration of microRNA-122 from Hepatitis C Virus by circular RNA sponges. RNA Biol (2018) 15(8):1032–9. doi: 10.1080/15476286.2018.1435248
93. Wang Z, Ma K, Cheng Y, Abraham JM, Liu X, Ke X, et al. Synthetic circular multi-miR sponge simultaneously inhibits miR-21 and miR-93 in esophageal carcinoma. Lab Invest J Tech Methods Pathol (2019) 99(10):1442–53. doi: 10.1038/s41374-019-0273-2
94. Liu X, Abraham JM, Cheng Y, Wang Z, Wang Z, Zhang G, et al. Synthetic Circular RNA Functions as a miR-21 Sponge to Suppress Gastric Carcinoma Cell Proliferation. Mol Ther Nucleic Acids (2018) 13:312–21. doi: 10.1016/j.omtn.2018.09.010
95. Chao CW, Chan DC, Kuo A, Leder P. The mouse formin (Fmn) gene: abundant circular RNA transcripts and gene-targeted deletion analysis. Mol Med (1998) 4(9):614–28. doi: 10.1007/BF03401761
96. Zhang H, Zhu L, Bai M, Liu Y, Zhan Y, Deng T, et al. Exosomal circRNA derived from gastric tumor promotes white adipose browning by targeting the miR-133/PRDM16 pathway. Int J Cancer (2019) 144(10):2501–15. doi: 10.1002/ijc.31977
97. Bar C, Thum T, de Gonzalo-Calvo D. Circulating miRNAs as mediators in cell-to-cell communication. Epigenomics (2019) 11(2):111–3. doi: 10.2217/epi-2018-0183
98. Yi YC, Chen XY, Zhang J, Zhu JS. Novel insights into the interplay between m(6)A modification and noncoding RNAs in cancer. Mol Cancer (2020) 19(1):121. doi: 10.1186/s12943-020-01233-2
99. He Y, Zhang Q, Zheng Q, Yu X, Guo W. Distinct 5-methylcytosine profiles of circular RNA in human hepatocellular carcinoma. Am J Trans Res (2020) 12(9):5719–29.
Keywords: CircRNAs, bladder cancer, functions, biomarker, targets
Citation: Liang Z, Guo W, Fang S, Zhang Y, Lu L, Xu W and Qian H (2021) CircRNAs: Emerging Bladder Cancer Biomarkers and Targets. Front. Oncol. 10:606485. doi: 10.3389/fonc.2020.606485
Received: 15 September 2020; Accepted: 24 November 2020;
Published: 08 January 2021.
Edited by:
Kailin Xu, Xuzhou Medical University, ChinaReviewed by:
Shou-Lin Wang, Nanjing Medical University, ChinaCopyright © 2021 Liang, Guo, Fang, Zhang, Lu, Xu and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenrong Xu, aWNsc0B1anMuZWR1LmNu; Hui Qian, bHN0bW1tbHN0QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.