- 1Department of Radiation Medicine, Georgetown University Hospital, Washington, DC, United States
- 2Georgetown University School of Medicine, Washington, DC, United States
- 3George Washington University School of Medicine, Washington, DC, United States
- 4Department of Nuclear Medicine, Georgetown University Hospital, Washington, DC, United States
- 5Department of Radiation Oncology, Beth Israel Deaconess Medical Center, Boston, MA, United States
- 6Biotechnology Research Institute, North Carolina Central University, Durham, NC, United States
- 7Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, United States
- 8Department of Urology, Georgetown University Hospital, Washington, DC, United States
Lymph node recurrent prostate cancer is a common clinical scenario that is likely to increase significantly with the widespread adoption of novel positron emission tomography (PET) agents. Despite increasing evidence that localized therapy is disease modifying, most men with lymph node recurrent prostate cancer receive only systemic therapy with androgen deprivation therapy (ADT). For men who receive localized therapy the intent is often to delay receipt of systemic therapy. Little evidence exists on the optimal combination of local and systemic therapy in this patient population. In this hypothesis generating review, we will outline the rationale and propose a framework for combining involved field SBRT with risk adapted intermittent ADT for hormone sensitive nodal recurrent prostate cancer. In patients with a limited number of nodal metastases, involved field stereotactic body radiation therapy (SBRT) may have a role in eliminating castrate-resistant clones and possibly prolonging the response to intermittent ADT. We hypothesize that in a small percentage of patients, such a treatment approach may lead to long term remission or cure.
Androgen Deprivation Therapy For Lymph Node-Positive Prostate Cancer Following Radical Prostatectomy
The prognosis for men with lymph node-positive prostate cancer is superior to patients with bone metastases (1–4). Survival varies depending on the timing, location, and extent of disease (5). The optimal treatment strategy for these patients remains an area of active investigation. While most patients receive androgen deprivation therapy (ADT) as the standard of care, the ideal timing for the initiation of ADT (immediate versus delayed until time of symptoms) remains controversial. The major prospective randomized trials guiding this debate in post-prostatectomy patients provide conflicting evidence and were conducted prior to the advent of widespread PSA monitoring making it unclear if their findings still apply to today's patient population (6, 7). Results from a large national database, suggest that the prostate-cancer specific survival and overall survival are similar between immediate and delayed ADT in a patient population with PSA monitoring (8). Based on data such as this, the National Comprehensive Cancer Network (NCCN) and the International Society of Geriatric Oncology (SIOG) guidelines currently recommend immediate ADT but observation remains an option for the well informed asymptomatic patient who will be closely monitored (9, 10).
Intermittent Androgen Deprivation Therapy
Side effects of ADT include hot flashes, decreased libido, fatigue, osteoporosis, weight gain, sarcopenia, and increased risk of cardiovascular disease. In general, side effects from ADT increase with the length of treatment duration (11, 12). Intermittent ADT (I-ADT) is a treatment option for men with biochemically recurrent prostate cancer with the aim of decreasing long term side effects from ADT (9, 13). Multiple large randomized trials comparing continuous ADT (C-ADT) to intermittent ADT in the metastatic setting have showed potential small improvements in overall survival with continuous ADT but improved quality of life (QOL) in the I-ADT arm (14–16). Likewise, multiple meta-analyses confirmed an improvement in quality of life for patients receiving intermittent ADT (17–19). Intermittent ADT requires close monitoring with regular PSA and total testosterone tests. Despite the increase in laboratory testing, patients on I-ADT require approximately one-third fewer LHRH injections compared to patients on continuous ADT. This leads to an overall cost savings of approximately 48% for patients receiving intermittent therapy (13, 18). With the advent of additional effective therapeutic agents in the metastatic setting, appropriate patient selection for intermittent ADT is critical (19). Patients with isolated lymph node metastases achieve longer remissions and are therefore most likely to benefit from this treatment strategy (13, 20). Currently, there are many unanswered questions regarding the logistics of administering intermittent ADT. These include the criteria for discontinuing/re-initiating ADT and which patients benefit most from transitioning from intermittent to continuous ADT. Past trials have used hormone responsiveness as a criterion for initiation and discontinuation of ADT without strong rationale for specific PSA cut-offs (21).
Rationale For The Treatment of Nodal Oligo-Recurrent Disease
Prostate cancer has a tropism for lymph nodes, making it the second most common and with advanced PET imaging, frequently the only site of disease recurrence (22). Early in the natural history of the disease, nodal metastases are small with a median size of approximately 1 cm, which limits detection by standard imaging (< 2 cm) (23, 24). However, newer PET agents overcome many of these limitations. Imaging with 68Ga - prostate specific membrane antigen PET/CT (PSMA-PET) allows for the detection of small lymph node metastases at low PSA levels, shortening the time between biochemical and clinical recurrence (21). Using PSMA-PET imaging, greater than 50% of men with early PSA progression (≤ 0.5 ng/ml) have radiographic evidence of recurrence with >50% of those having abdominopelvic nodal involvement (25, 26). The radiographic detection rate of PSMA-PET continues to improve with increasing PSA levels (25–27).
With the advent of new sensitive and specific imaging the group of patients with oligo-recurrent nodal prostate cancer is likely to increase significantly (24). Despite calls for aggressive local therapy in these patients, the significance of treating isolated lymph node metastases remains controversial. Some have hypothesized that this is an intermediary stage prior to diffuse metastases and that treatment might delay or even prevent distant dissemination (28, 29). Others have hypothesized that lymph node metastases represent a distinct metastatic lineage, completely separate from bone or visceral metastases (30, 31). Those who believe that lymph node metastases are an intermediate step prior to widespread disease would argue for metastasis directed therapy while those who subscribe to the hypothesis that lymph nodes metastases are a distinct metastatic lineage believe there is likely little rationale or clinical benefit to treating lymph node metastases. In practice, distinct patterns of spread are likely not mutually exclusive and multiple patterns of metastatic seeding may occur (32). Additionally, both patterns of spread likely harbor castrate resistant clones which when treated may prolong a patient's response to ADT.
Defining Oligo-Recurrent Nodal Disease
Despite a growing number of publications and even consensus statements there is no consistent definition for oligo-recurrent prostate cancer (33). Given that lymph node only prostate cancer may represent a favorable biology distinct from those with bone and visceral involvement, and that it certainly confers an improved prognosis, the authors of this paper argue against proposing a strict numerical cut-off for the number of metastases to qualify for oligo-recurrent nodal disease. Instead we are advocating for any volume of nodal disease that can be safely treated with curative intent. This approach preserves the rationale for treating nodal recurrent disease, which is to halt or delay widespread disease progression, while maximizing the number of patients who may benefit (28, 29). Because the potential benefit for treating nodal disease is diminished if cancer has already spread to the bone or visceral sites, one must be relatively certain no other systemic disease is present (34). For this reason, we recommend oligo-recurrence only be defined with the use of advanced PET imaging, preferably PSMA-PET, if available, which has been shown to outperform other agents in this clinical setting (24, 35).
Overview of Local Therapy Treatment Options
The intent of ADT in this patient population is to delay progression and all patients with metastases not treated with localized therapy will ultimately progress. Successful treatment of nodal oligo-metastatic disease relies on treating all the involved nodes (34). Recently, data is emerging that localized therapy in the form of surgery or radiotherapy may be effective in treating these nodal oligo-recurrences. With evidence accumulating, 75% of panelists at the most recent Advanced Prostate Cancer Consensus Conference (APCCC) recommend systemic therapy and local treatment of all lesions for the majority of men with oligo-recurrent prostate cancer (33). While the optimal local therapy is an area of active clinical investigation, below we will review the most common types: surgery and radiation therapy.
Salvage Lymph Node Dissection
Surgery in the form of salvage pelvic lymphadenectomies (sLND) may be beneficial for patients with hormone sensitive oligo-recurrent nodal disease (36). Studies have shown that sLND can delay clinical progression in up to 40% of patients with prior prostatectomy, with a subset potentially cured (37). Unfortunately, imaging is not yet sensitive enough to detect very small (< 4–5 mm) involved nodes, requiring sLND expansions beyond the PET positive fields (23, 38, 39). Despite these extensive interventions, many patients develop a second biochemical failure 1–2 years after sLND (37, 40). Additionally, sLND is technically challenging with a risk of significant complications requiring surgical management (41). Given the lack of durable response, technical difficulty and risk of morbidity from sLND, it continues to be recommended only on an experimental basis for a highly selective cohort of patients who are most likely to benefit from the procedure and high-level evidence is still missing to draw any clinically meaningful conclusion about the oncological impact of salvage lymph node dissection on long-term outcomes (36, 42).
Radiation Therapy
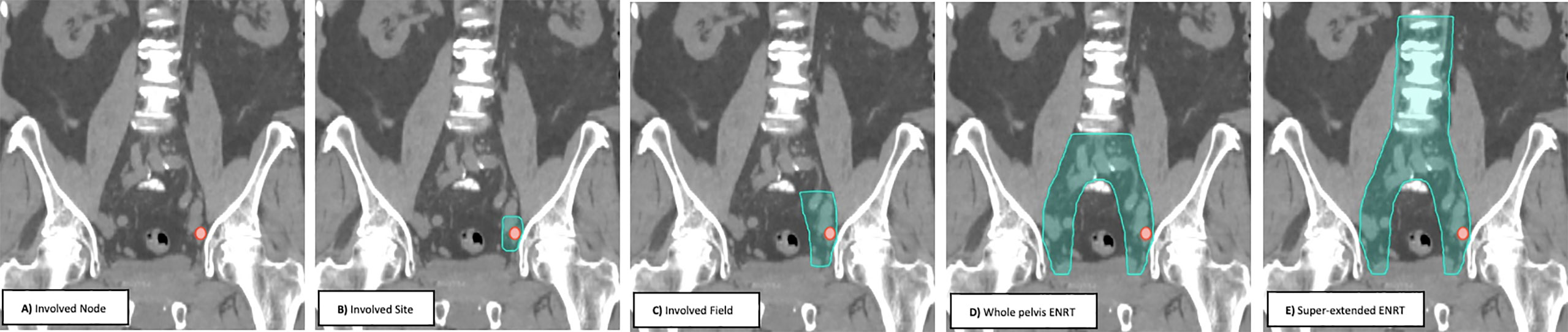
Prospective trials and retrospective multi-institutional reviews have used several radiotherapy treatment approaches (Figure 1) ranging from involved node (focal) stereotactic body radiotherapy (SBRT), involved site SBRT, involved field conventionally fractionated radiotherapy, whole pelvis elective nodal conventionally fractioned radiotherapy +/- a boost to gross disease (ENRT) and super-extended elective nodal radiotherapy (super-extended ENRT) (43). With the available data, no consensus treatment approach has emerged, with each approach having potential benefits and drawbacks (43). Below, we will review each of the treatment approaches with available data.
Whole Pelvis Elective Nodal Radiation Therapy and Super-Extended Elective Nodal Radiation Therapy
Multi-institution reviews have suggested that involved node SBRT is safe and effective in treating nodal oligo-recurrences (44). However, several questions remain given the lack of long-term data compared to more conventional radiation therapy (45). Further, recurrences following more focalized SBRT generally occur in the adjacent, untreated pelvic or retroperitoneal lymph nodes (46). Because of this, some groups have advocated for elective nodal radiotherapy to consensus nodal volumes (ENRT) to reduce the risk of regional recurrences. This treatment strategy has been applied to patients with lymph node positive prostate cancer in the definitive and adjuvant setting and with long term follow-up has been found to be well tolerated (47). Because of the larger volumes being treated, ENRT is generally delivered in 25–30 small fractions (1.8–2 Gy/fx) (45). Retrospective data suggests that ENRT may decrease recurrences in patients with a solitary lymph node recurrence when compared to involved node SBRT, albeit at the cost of increased toxicity (45). A major limitation to this approach includes the increase of atypical patterns of lymphatic spread following primary treatment which consensus contouring atlases may omit in >50% of cases and which may necessitate an increase in field size (super extended-ENRT) to include the para-aortic, pre-sacral and peri-rectal regions, among others (48). Additionally, with a low (α / β) ratio, prostate cancer generally responds best to a relatively higher dose per fraction, which is limited with an ENRT or super-extended ENRT approach.
Focal or Involved Node Stereotactic Body Radiation Therapy
For patients with limited metastatic sites, SBRT to the oligo-metastases may offer long-term local control and increased progression free survival (49). SBRT accurately delivers high doses to the target while sparing adjacent normal tissues (50). SBRT may improve tumor control and reduce treatment-related toxicity through improved target accuracy (51). This allows for high-dose (> 5 Gy per fraction), extremely hypofractionated treatment courses (1–5 fractions) that may be more radiobiologically effective and are certainly more convenient for patients than conventionally fractionated radiotherapy (51). Because of the high dose per fraction, SBRT targeting just the gross recurrent disease (focal or involved node SBRT) has been most extensively studied. Several recent clinical trials have confirmed the benefit of involved node (focal) SBRT to treat oligo-recurrent prostate cancer (34, 52, 53). The STOMP Trial randomized asymptomatic oligo-recurrent (≤ 3 sites) prostate cancer patients to observation or metastasis directed therapy (MDT) (metastasectomy or involved node (focal) SBRT) (52). The primary endpoint was ADT-free survival. ADT was initiated in both arms at time of symptoms, local progression or poly-metastatic disease (> 3 metastatic lesions). The median ADT-free survival was 13 months for the surveillance group and 21 months for the treated group. No grade 2 or higher toxicities were observed. More recently, the ORIOLE Trial was published which randomized asymptomatic oligo-metastatic (≤ 3 sites) prostate cancer patients to observation or involved node (focal) SBRT (19.5 Gy to 48 Gy in 3–5 fractions) (34). Progression at 6 months occurred in 61% of the patients in the observation arm but only 19% in the treated group. With increased follow-up of the STOMP trial, data is emerging that patients treated with MDT may experience increased time to castrate resistance (54). Criticisms of these trials include the lack of control arm of immediate ADT and the use of the surrogate end point ADT-free survival, however, for older men, with limited life expectancy this remains a clinically meaningful endpoint.
Involved Site and Field Stereotactic Body Radiation Therapy
Most studies until this point have focused on involved node (focal) SBRT or ENRT, without significant investigation of alternative treatment volumes or synergy with systemic therapies. Additional treatment volumes include involved site and involved field radiotherapy (please refer to Figure 1 for a graphical representation). These volumes expand on involved node to include an expansion on the gross disease to cover for adjacent microscopic disease. The rationale for these approaches stem from the subsequent regional nodal failures often seen when patients are treated with involved node SBRT.
Involved site radiotherapy includes a pre-specified expansion along the lymphatic nodal basin, while involved field generally involves an expansion to include the involved nodal basin as well as adjacent nodal basins. There is limited data on patterns of recurrence for involved site SBRT. Kneebone et al, noted distant lymph node recurrences as the most frequent site of recurrence without specific mention to their precise locations (55). To the authors' knowledge there has been no published data on involved field SBRT. Soldatov et el. used conventionally fractionated involved field radiotherapy in patients with oligo-recurrent nodal prostate cancer. The authors of this study noted relatively few adjacent or contralateral pelvic nodal failures using this treatment strategy but a relatively high rate of infield recurrences, perhaps owing to the small dose/fraction used in the study (56). Given the radiobiological advantages of SBRT, it is reasonable to believe that when combined with an involved field treatment volume, the rate of local control could improve.
Proposed Treatment Volumes, Dose, and Fractionation Scheme For Involved Field Stereotactic Body Radiation Therapy
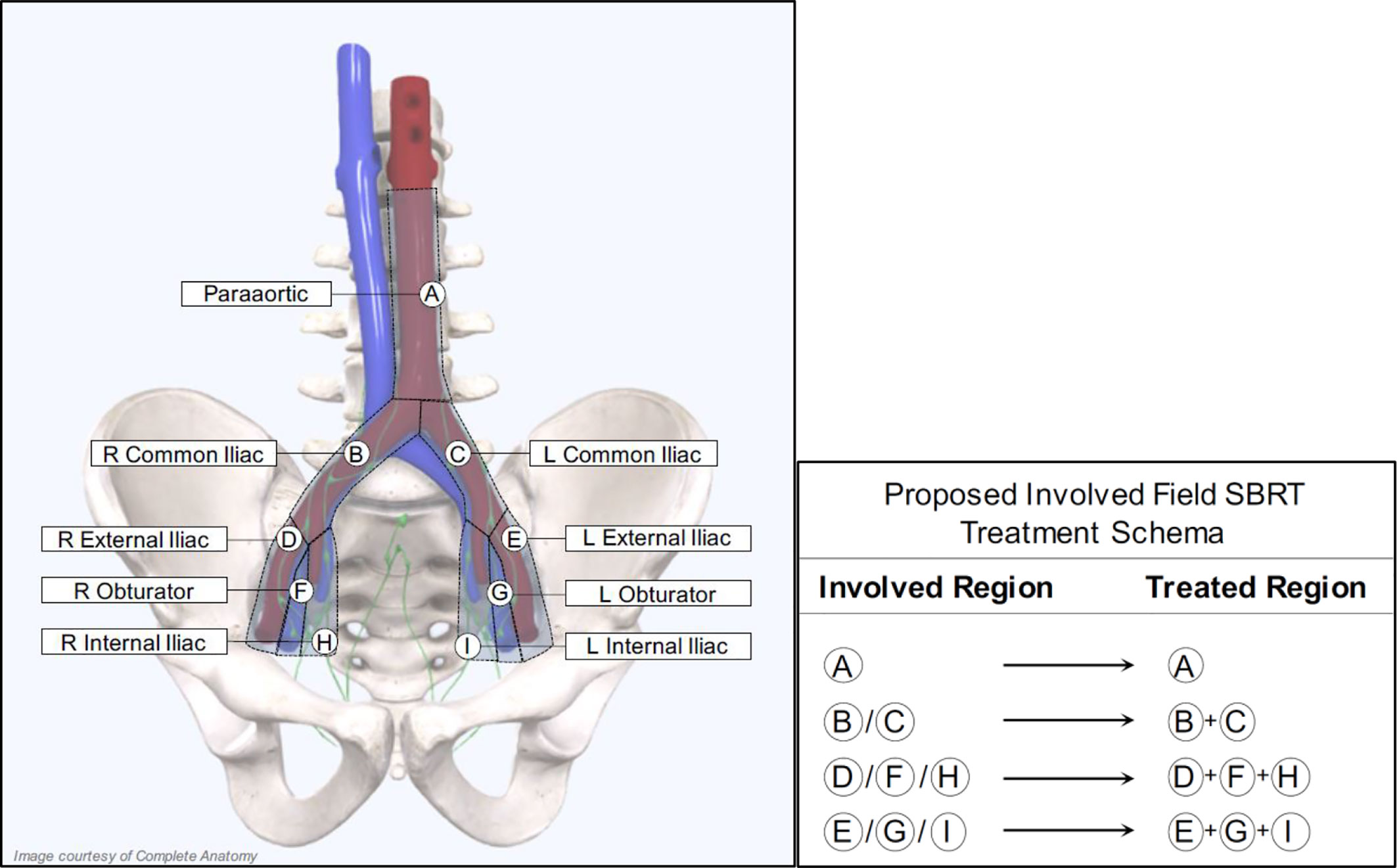
For patients receiving salvage radiation for oligo-recurrent nodal prostate cancer, the optimal systemic therapy, radiation dose, fractionation schedule, and treatment volume remain unanswered questions. Given the potential radiobiological advantages, improved local control, increased patient convenience and the poor coverage of consensus treatment atlases utilizing ENRT the authors of this paper advocate for treatment of nodal metastases with a form of involved field SBRT. This treatment approach has several distinct advantages over the other commonly utilized treatment options and at the very least, requires further exploration. First, it expands on the gross disease to include the adjacent nodes where an involved node SBRT approach is most likely to fail. Further, because this approach utilizes relatively small treatment volumes, it allows for safe dose escalation and hypo-fractionation, improving local control and patient convenience. Additionally, from the available literature, treatment with involved field has a low rate of contralateral pelvic nodal failure, providing a rationale for the omission of these volumes in many situations (56). Further, because of the conformality and dose fall of from SBRT, if a patient were to fail in the contralateral pelvis, these could be salvaged with additional involved field SBRT, without a significantly increased risk of toxicity. Lastly, and perhaps most importantly, many of these patients will eventually progress regardless of the type of local therapy used, so it is critically important to minimize treatment burden in this population. Figure 2 provides an example of involved field utilized for the treatment of a patient with oligo-recurrent prostate cancer to the left external iliac lymph node. Figure 3 shows our proposed treatment volumes for pelvic nodal oligo-recurrences.
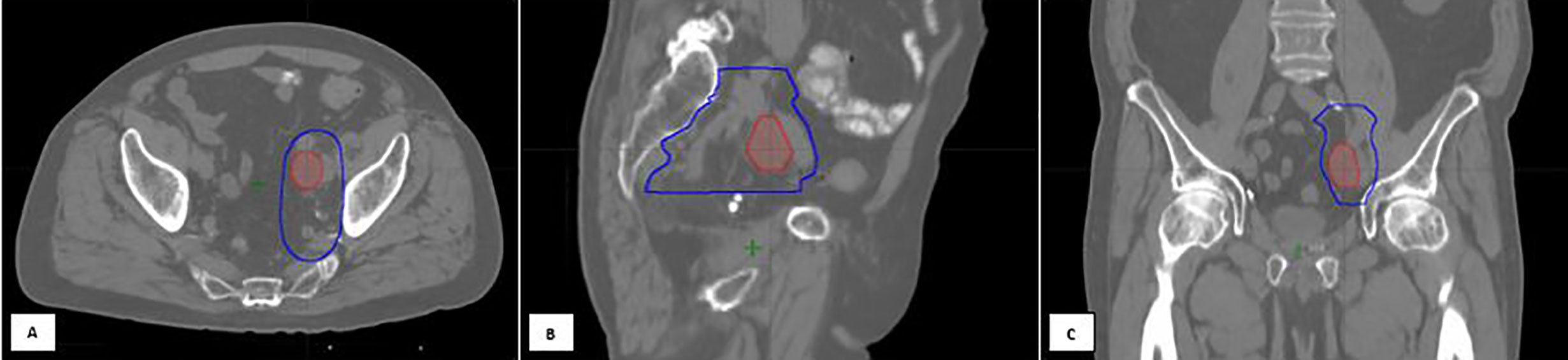
Figure 2 An 80-year-old gentleman with oligo-recurrent prostate cancer with left external iliac adenopathy identified on PET imaging [(A) Axial, (B) Sagittal, (C) Coronal views]. The decision was to proceed with SBRT assisted Intermittent ADT. An involved field SBRT approach was utilized. The left external iliac, left obturator, and left internal iliac nodal regions were treated with 27.5 Gy in 5 fractions (high risk CTV), with a 30 Gy simultaneous integrated boost to the gross disease. Treatment planning computed tomography images showing the isodose lines for the GTV prescription of 30 Gy (red) and the High-risk CTV prescription of 27.5 Gy (dark blue line). Following SBRT, induction ADT was initiated.
No optimal SBRT dose regimen has been established due to the variation in target volume and proximity to normal structures (57). Retrospective data suggests that a biologically effective dose (BED) ≥100Gy (assuming an α / β = 3) is needed for adequate long-term local control following SBRT (39). Because of the need to balance safety with efficacy, we hypothesize that treating the gross nodal disease to 30 Gy/5 fx with a simultaneous high-risk CTV expansion of 27.5 Gy/5 fx to the adjacent nodal basin(s) allows for high rates of local control with minimal toxicity. Because the BED of this radiation scheme is less than 100 Gy (BED = 88 Gy, (α / β = 3), the threshold that retrospective data suggests is needed for favorable long-term local control, it needs to be given concurrently with a radiosensitizer. Here, we plan to utilize ADT, which has been shown to increase the effectiveness of radiation by decreasing prostate cancer specific DNA repair mechanisms, allowing for the use of lower radiation doses without sacrificing local control (58). We hypothesize that this treatment dose and fractionation, when combined with concurrent ADT, will allow for high rates of long term local control with minimal acute and late toxicity. This strategy also allows for the safe and effective treatment of para-aortic nodal metastases, which has been limited due to the sensitivity of the adjacent small bowel to radiation (59). Our early experience with our proposed approach suggests that prostate SBRT will not adversely affect this (49, 60).
Proposed Dose Constraints
We hypothesize that involved field SBRT will decrease tumor burden in lymph nodes and delay transition to bone metastases and castration resistance (54). However, if involved field SBRT to oligo-recurrent nodal disease causes a significant rate of high-grade late toxicity and/or adversely affected patients' long-term quality of life this approach would not be worth pursuing further. Data on the dose tolerance of the bowel to SBRT is limited (61). It is clear that high doses of radiation to small portions of the bowel can cause significant complications (≥ grade 3) (62). Currently, the impact of SBRT to oligometastatic disease on overall survival is unknown. Therefore, we recommend a very conservative constraint of 30 Gy <1 cc to the bowel, a constraint for which the risk of significant toxicity is very low (5%) (63). Moderate doses to larger portions of bowel may also impact the risk of toxicity so we recommend the volume of bowel receiving 20 Gy <15 cc (64). (Please see Table 1 for additional proposed dose constraints).

Table 1 Proposed Dose Constraints for OARs when treating gross nodal disease to 30 Gy/5 fx with a simultaneous high-risk CTV expansion of 27.5 Gy/5 fx.
A Cycle-Based Treatment Approach
PSA response to ADT as a Prognostic Tool
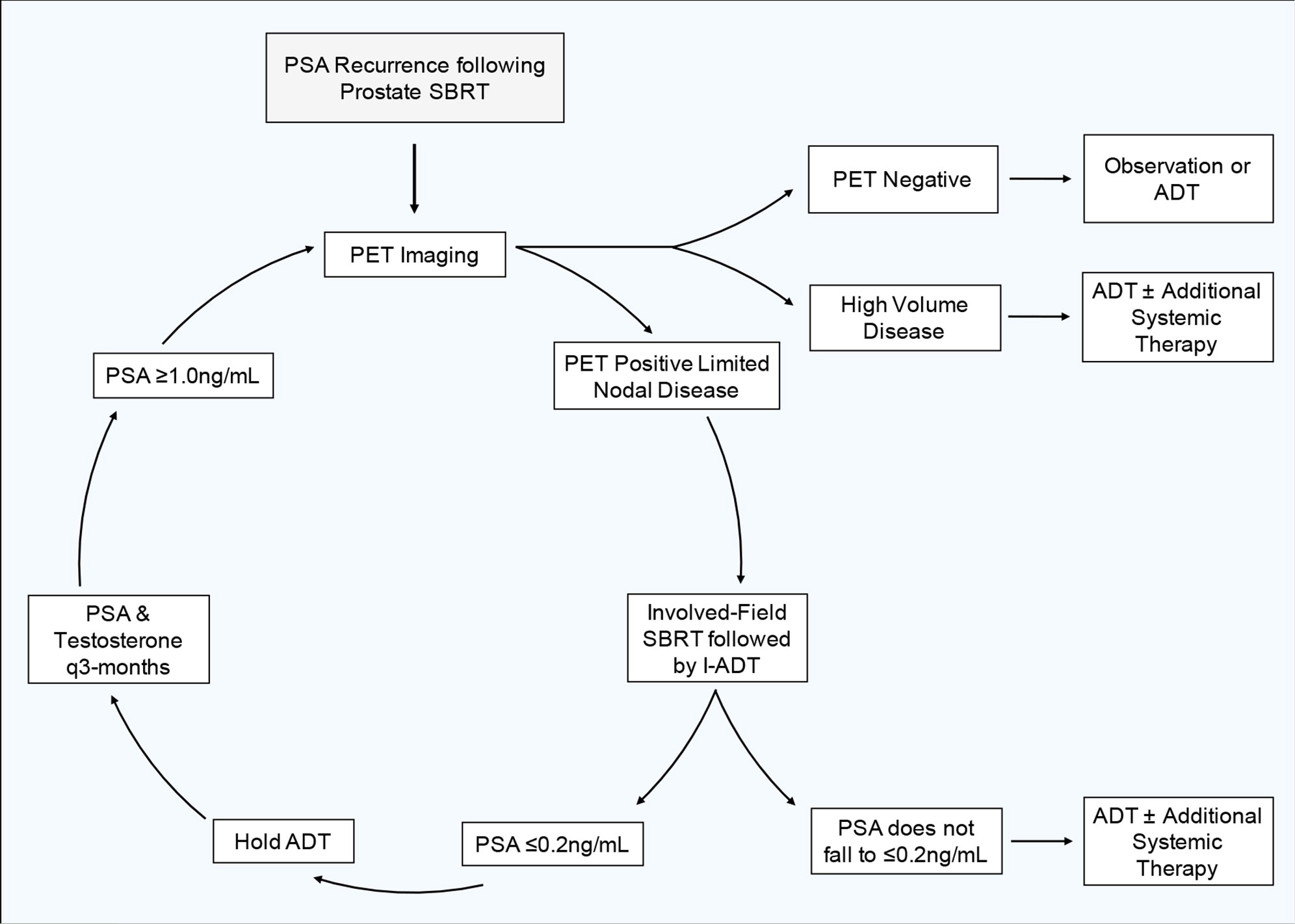
Early PSA response to ADT and other therapies is prognostic (9, 21, 65). In general, optimal PSA suppression takes 6–9 months (66). A PSA ≤0.2 ng/ml after 7 months of ADT suggests a long median survival (21, 65). Here, we plan to utilize induction ADT as a prognostic tool to determine if a patient would benefit from more intensive systemic therapy or if they would be reasonable candidates for an intermittent treatment approach such as this. We therefore recommend a conservative, risk adaptive approach for determining the length of induction ADT and which patients would be candidates for continued I-ADT. Following involved field SBRT, we recommend a 3-month induction period where all patients receive ADT. At 3 months, we recommend checking PSA and testosterone levels, if the PSA is ≤0.2 ng/ml, the PSA level that is associated with improved prognosis, then hold further ADT and continue to follow PSA and testosterone levels every 3 months. However, if at 3 months the PSA fails to fall ≤0.2 ng/ml, we recommend an additional 3-month induction period of ADT. If after an additional 3 months (6 months following the initiation of ADT) the PSA still fails to fall ≤0.2 ng/ml, this is indicative of a less favorable prognosis and we do not recommend the patient proceed further with this intermittent treatment strategy. Instead, these patients would likely benefit from continuous ADT with the possible addition of other systemic agents.
Timing of Repeat Imaging and Re-Initiation of Androgen Deprivation Therapy
Although a subset of patients may have a prolonged clinical response and may never need subsequent treatment, most patients will eventually relapse. From published patterns of recurrence following SBRT, the majority of patients re-present with ≤3 new metastases, allowing for subsequent MDT (46). For this reason, and because intermittent ADT requires frequent total testosterone and PSA checks, we recommend following these values every 3 months. If a patient's PSA starts to rise following a cycle of ADT and SBRT, we recommend repeat PET imaging when the patient's PSA rises to ≥1 ng/ml. At this PSA level, PSMA-PET has been shown to perform well in the detection (> 80%) of recurrent prostate cancer (67). While the performance of PET imaging improves with increasing PSA, this PSA cut off balances a relatively high rate of disease detection without allowing undue progression of disease that may sacrifice clinical outcomes (68). If imaging reveals new oligo-recurrent disease, we would treat the disease with another cycle of involved field SBRT and re-initiate ADT using the parameters previously outlined. Figure 4 shows our proposed treatment cycle. If imaging reveals high burden metastatic disease, we recommend re-initiating ADT with the possible addition of other systemic agents.
Conclusion
Recurrent prostate cancer remains a complex disease. ADT is successful in delaying the progression and improving overall survival. Unfortunately, castrate-resistant clones may be present early in the disease process even prior to the initiation of ADT, creating the need for alternative treatments. SBRT has been demonstrated as a safe and efficacious modality. Implementation of involved field SBRT early in the disease process may reduce overall tumor burden, in turn delaying progression of disease, prolonging response to intermittent ADT and improving both the quality and length of life. Strengths of this approach include: minimizing a patient's exposure to ADT and the side effects related to its receipt, utilizing large fraction sizes to capitalize on the low intrinsic (α / β) of prostate cancer to improve local control and short treatment courses that improve patient convenience and reduce indirect patient costs. Potential limitations of this approach include: adverse patient outcomes in those that may have benefited from more aggressive upfront systemic therapy and a potential sacrifice in long term local control with the delivery of less than a truly ablative dose of radiation in order to prioritize long term patient safety. In conclusion, the current cyclic approach balances the benefits of local treatment and limited ADT versus the potential benefit of long-term ADT in those with unfavorable features. The authors plan to test this treatment strategy in a single institution phase II clinical trial.
Author Contributions
MC is the lead author who participated in manuscript drafting, table/figure creation, and manuscript revision. MLC, AP, EW, MF, and MM aided in the table/figure creation. LB and SL are the dosimetrists who contributed dosimetric data and figures. PL, RH, SC, DK, NA, GE, SS, BC, JL, and ND are senior authors who aided in drafting the manuscript and manuscript revision. SC is the corresponding author who initially developed the concept, and drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by NIH Grant P30CA051008. We gratefully acknowledge the Grant No R01MD012767 from the National Institute on Minority Health and Health Disparities (NIMHD), NIH to DK and SC.
Conflict of Interest
SP Collins and BT Collins serve as clinical consultants to Accuray Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ADT, androgen deprivation therapy; CRPC, Castrate-resistant prostate cancer; DVH, dose-volume histogram; GTV, gross target volume; IGRT, image-guided radiation treatment; LHRH, luteinizing hormone-releasing hormone; PSA, prostate-specific antigen; PTV, planning target volume; SBRT, stereotactic body radiation therapy; TURP, transurethral resection of the prostate.
References
1. Gandaglia G, Karakiewicz PI, Briganti A, Passoni NM, Schiffmann J, Trudeau V, et al. Impact of the Site of Metastases on Survival in Patients with Metastatic Prostate Cancer. Eur Urol (2015) 68(2):325–34. doi: 10.1016/j.eururo.2014.07.020
2. Halabi S, Kelly WK, Ma H, Zhou H, Solomon NC, Fizazi K, et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men With Castration-Resistant Prostate Cancer. J Clin Oncol (2016) 34(14):1652–9. doi: 10.1200/JCO.2015.65.7270
3. James ND, Spears MR, Clarke NW, Dearnaley DP, De Bono JS, Gale J, et al. Survival with Newly Diagnosed Metastatic Prostate Cancer in the “Docetaxel Era”: Data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019). Eur Urol (2015) 67(6):1028–38. doi: 10.1016/j.eururo.2014.09.032
4. Jackson WC, Desai NB, Abugharib AE, Tumati V, Dess RT, Lee JY, et al. Anatomical patterns of recurrence following biochemical relapse after post-prostatectomy salvage radiation therapy: a multi-institutional study. BJU Int (2017) 120(3):351–7. doi: 10.1111/bju.13792
5. Ost P, Decaestecker K, Lambert B, Fonteyne V, Delrue L, Lumen N, et al. Prognostic factors influencing prostate cancer-specific survival in non-castrate patients with metastatic prostate cancer. Prostate (2014) 74(3):297–305. doi: 10.1002/pros.22750
6. Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol (2006) 7(6):472–9. doi: 10.1016/S1470-2045(06)70700-8
7. Schröder FH, Kurth K-H, Fossa SD, Hoekstra W, Karthaus PP, De Prijck L, et al. Early Versus Delayed Endocrine Treatment of T2-T3 pN1-3 M0 Prostate Cancer Without Local Treatment of the Primary Tumour: Final Results of European Organisation for the Research and Treatment of Cancer Protocol 30846 After 13 Years of Follow-up (A Randomised Controlled Trial). Eur Urol (2009) 55(1):14–22. doi: 10.1016/j.eururo.2008.09.008
8. Wong Y-N, Freedland S, Egleston B, Hudes G, Schwartz JS, Armstrong K. Role of androgen deprivation therapy for node-positive prostate cancer. J Clin Oncol (2009) 27(1):100–5. doi: 10.1200/JCO.2007.14.2042
9. Mohler J, Antonarakis E. NCCN Guidelines Updates: Management of Prostate Cancer. J Natl Compr Canc Netw (2020) 17(5.5):583–6. doi: 10.6004/jnccn.2019.5011
10. Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol (2017) 71(4):630–42. doi: 10.1016/j.eururo.2016.08.002
11. Duchesne GM, Woo HH, King M, Bowe SJ, Stockler MR, Ames A, et al. Health-related quality of life for immediate versus delayed androgen-deprivation therapy in patients with asymptomatic, non-curable prostate cancer (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol (2017) 18(9):1192–201. doi: 10.1016/S1470-2045(17)30426-6
12. Tsai H-T, Pfeiffer RM, Philips GK, Barac A, Fu AZ, Penson DF, et al. Risks of Serious Toxicities from Intermittent versus Continuous Androgen Deprivation Therapy for Advanced Prostate Cancer: A Population Based Study. J Urol (2017) 197(5):1251–7. doi: 10.1016/j.juro.2016.12.022
13. Abrahamsson P. Intermittent androgen deprivation therapy in patients with prostate cancer: Connecting the dots. Asian J Urol (2017) 4(4):208–22. doi: 10.1016/j.ajur.2017.04.001
14. Crook JM, O’Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, Horwitz EM, et al. Intermittent Androgen Suppression for Rising PSA Level after Radiotherapy. N Engl J Med (2012) 367(10):895–903. doi: 10.1056/NEJMoa1201546
15. Calais da Silva F, Calais da Silva FM, Gonçalves F, Santos A, Kliment J, Whelan P, et al. Locally advanced and metastatic prostate cancer treated with intermittent androgen monotherapy or maximal androgen blockade: results from a randomised phase 3 study by the South European Uroncological Group. Eur Urol (2014) 66(2):232–9. doi: 10.1016/j.eururo.2013.03.055
16. Hussain M, Tangen CM, Berry DL, Higano CS, Crawford ED, Liu G, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med (2013) 368(14):1314–25. doi: 10.1056/NEJMoa1212299
17. Magnan S, Zarychanski R, Pilote L, Bernier L, Shemilt M, Vigneault E, et al. Intermittent vs Continuous Androgen Deprivation Therapy for Prostate Cancer: A Systematic Review and Meta-analysis. JAMA Oncol (2015) 1(9):1261–9. doi: 10.1001/jamaoncol.2015.2895
18. Niraula S, Le LW, Tannock IF. Treatment of prostate cancer with intermittent versus continuous androgen deprivation: a systematic review of randomized trials. J Clin Oncol (2013) 31(16):2029–36. doi: 10.1200/JCO.2012.46.5492
19. Botrel TEA, Clark O, dos Reis RB, Pompeo ACL, Ferreira U, Sadi MV, et al. Intermittent versus continuous androgen deprivation for locally advanced, recurrent or metastatic prostate cancer: a systematic review and meta-analysis. BMC Urol (2014) 14:9. doi: 10.1186/1471-2490-14-9
20. Perera M, Roberts MJ, Klotz L, Higano CS, Papa N, Sengupta S, et al. Intermittent versus continuous androgen deprivation therapy for advanced prostate cancer. Nat Rev Urol (2020) 17(8):469–81. doi: 10.1038/s41585-020-0335-7
21. Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol (2006) 24(24):3984–90. doi: 10.1200/JCO.2006.06.4246
22. Gandaglia G, Abdollah F, Schiffmann J, Trudeau V, Shariat SF, Kim SP, et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate (2014) 74(2):210–6. doi: 10.1002/pros.22742
23. Budäus L, Leyh-Bannurah S-R, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial Experience of (68)Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur Urol (2016) 69(3):393–6. doi: 10.1016/j.eururo.2015.06.010
24. Barbosa FG, Queiroz MA, Nunes RF, Viana PCC, Marin JFG, Cerri GG, et al. Revisiting Prostate Cancer Recurrence with PSMA PET: Atlas of Typical and Atypical Patterns of Spread. Radiogr Rev Publ Radiol Soc N Am Inc (2019) 39(1):186–212. doi: 10.1148/rg.2019180079
25. Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of Hybrid 68Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J Nucl Med (2015) 56(5):668–74. doi: 10.2967/jnumed.115.154153
26. Calais J, Czernin J, Cao M, Kishan AU, Hegde JV, Shaverdian N, et al. 68Ga-PSMA-11 PET/CT Mapping of Prostate Cancer Biochemical Recurrence After Radical Prostatectomy in 270 Patients with a PSA Level of Less Than 1.0 ng/mL: Impact on Salvage Radiotherapy Planning. J Nucl Med (2018) 59(2):230–7. doi: 10.2967/jnumed.117.201749
27. Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol (2019) 5(6):856–63. doi: 10.1001/jamaoncol.2019.0096
28. Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, et al. The evolutionary history of lethal metastatic prostate cancer. Nature (2015) 520(7547):353–7. doi: 10.1038/nature14347
29. Hong MKH, Macintyre G, Wedge DC, Van Loo P, Patel K, Lunke S, et al. Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nat Commun (2015) 6:6605. doi: 10.1038/ncomms7605
30. Mangiola S, Hong MKH, Cmero M, Kurganovs N, Ryan A, Costello AJ, et al. Comparing nodal versus bony metastatic spread using tumour phylogenies. Sci Rep (2016) 6:33918. doi: 10.1038/srep33918
31. Datta K, Muders M, Zhang H, Tindall DJ. Mechanism of lymph node metastasis in prostate cancer. Future Oncol Lond Engl (2010) 6(5):823–36. doi: 10.2217/fon.10.33
32. Naxerova K, Reiter JG, Brachtel E, Lennerz J, Van de Wetering M, Rowan A, et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science (2017) 357(6346):55–60. doi: 10.1126/science.aai8515
33. Gillessen S, Attard G, Beer TM, Beltran H, Bjartell A, Bossi A, et al. Management of Patients with Advanced Prostate Cancer: Report of the Advanced Prostate Cancer Consensus Conference 2019. Eur Urol (2020) 77(4):508–47. doi: 10.1016/j.eururo.2020.01.012
34. Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol (2020). doi: 10.1001/jamaoncol.2020.0147
35. Calais J, Ceci F, Eiber M, Gartmann J, Nguyen K, Lok V, et al. 68Ga-PSMA-11 PET/CT detects prostate cancer at early biochemical recurrence with superior detection rate and reader agreement when compared to 18F-Fluciclovine PET/CT in a prospective head-to-head comparative phase 3 study. J Nucl Med (2019) 60(supplement 1):587–7. doi: 10.1097/01.JU.0000557513.53829.a8
36. Suardi N, Briganti A, Gandaglia G, Fossati N, Montorsi F. Salvage Lymph Node Dissection for Node-only Recurrence of Prostate Cancer: Ready for Prime Time? Eur Urol (2017) 71(5):693–4. doi: 10.1016/j.eururo.2016.12.001
37. Suardi N, Gandaglia G, Gallina A, Di Trapani E, Scattoni V, Vizziello D, et al. Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: results of a single-institution series with a minimum follow-up of 5 years. Eur Urol (2015) 67(2):299–309. doi: 10.1016/j.eururo.2014.02.011
38. Dundee P, Gross T, Moran D, Ryan A, Ballok Z, Peters J, et al. Ga-labeled Prostate-specific Membrane Antigen Ligand-positron-emission Tomography: Still Just the Tip of the Iceberg. Urology (2018) 120:187–91. doi: 10.1016/j.urology.2018.06.029
39. Mandel P, Tilki D, Chun FK, Pristupa E, Graefen M, Klutmann S, et al. Accuracy of 68Ga-Prostate-specific Membrane Antigen Positron Emission Tomography for the Detection of Lymph Node Metastases Before Salvage Lymphadenectomy. Eur Urol Focus (2020) 6(1):71–3. doi: 10.1016/j.euf.2018.07.025
40. Siriwardana A, Thompson J, van Leeuwen PJ, Doig S, Kalsbeek A, Emmett L, et al. Initial multicentre experience of 68 gallium-PSMA PET/CT guided robot-assisted salvage lymphadenectomy: acceptable safety profile but oncological benefit appears limited. BJU Int (2017) 120(5):673–81. doi: 10.1111/bju.13919
41. Brassetti A, Proietti F, Pansadoro V. Oligometastatic prostate cancer and salvage lymph node dissection: systematic review. Minerva Chir (2019) 74(1):97–106. doi: 10.23736/S0026-4733.18.07796-9
42. Bravi CA, Fossati N, Gandaglia G, Suardi N, Mazzone E, Robesti D, et al. Long-term Outcomes of Salvage Lymph Node Dissection for Nodal Recurrence of Prostate Cancer After Radical Prostatectomy: Not as Good as Previously Thought. Eur Urol (2020). doi: 10.1016/j.eururo.2020.06.043
43. Achard V, Bottero M, Rouzaud M, Lancia A, Scorsetti M, Filippi AR, et al. Radiotherapy treatment volumes for oligorecurrent nodal prostate cancer: a systematic review. Acta Oncol Stockh Swed (2020) 1–11. doi: 10.1080/0284186X.2020.1775291
44. Ost P, Jereczek-Fossa BA, As NV, Zilli T, Muacevic A, Olivier K, et al. Progression-free Survival Following Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Treatment-naive Recurrence: A Multi-institutional Analysis. Eur Urol (2016) 69(1):9–12. doi: 10.1016/j.eururo.2015.07.004
45. De Bleser E, Jereczek-Fossa BA, Pasquier D, Zilli T, Van As N, Siva S, et al. Metastasis-directed Therapy in Treating Nodal Oligorecurrent Prostate Cancer: A Multi-institutional Analysis Comparing the Outcome and Toxicity of Stereotactic Body Radiotherapy and Elective Nodal Radiotherapy. Eur Urol (2019) 76(6):732–9. doi: 10.1016/j.eururo.2019.07.009
46. Ost P, Jereczek-Fossa BA, Van As N, Zilli T, Tree A, Henderson D, et al. Pattern of Progression after Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Nodal Recurrences. Clin Oncol R Coll Radiol G B (2016) 28(9):e115–120. doi: 10.1016/j.clon.2016.04.040
47. Ventimiglia E, Seisen T, Abdollah F, Briganti A, Fonteyne V, James N, et al. A Systematic Review of the Role of Definitive Local Treatment in Patients with Clinically Lymph Node-positive Prostate Cancer. Eur Urol Oncol (2019) 2(3):294–301. doi: 10.1016/j.euo.2019.02.001
48. Schiller K, Stöhrer L, Düsberg M, Borm K, Devecka M, Vogel MME, et al. PSMA-PET/CT–based Lymph Node Atlas for Prostate Cancer Patients Recurring After Primary Treatment: Clinical Implications for Salvage Radiation Therapy. Eur Urol Oncol (2020) 0(0). doi: 10.1016/j.euo.2020.04.004
49. Bhattasali O, Chen LN, Tong M, Lei S, Collins BT, Krishnan P, et al. Rationale for stereotactic body radiation therapy in treating patients with oligometastatic hormone-naïve prostate cancer. Front Oncol (2013) 3:293. doi: 10.3389/fonc.2013.00293
50. Frelinghuysen M, Schillemans W, Hol L, Verhoef C, Hoogeman M, Nuyttens JJ. Acute toxicity of the bowel after stereotactic robotic radiotherapy for abdominopelvic oligometastases. Acta Oncol Stockh Swed (2018) 57(4):480–4. doi: 10.1080/0284186X.2017.1378432
51. Timmerman RD, Kavanagh BD, Cho LC, Papiez L, Xing L. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol (2007) 25(8):947–52. doi: 10.1200/JCO.2006.09.7469
52. Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol (2018) 36(5):446–53. doi: 10.1200/JCO.2017.75.4853
53. Siva S, Bressel M, Murphy DG, Shaw M, Chander S, Violet J, et al. Stereotactic Abative Body Radiotherapy (SABR) for Oligometastatic Prostate Cancer: A Prospective Clinical Trial. Eur Urol (2018) 74(4):455–62. doi: 10.1016/j.eururo.2018.06.004
54. Ost P. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence (STOMP): Five-Year Results of a Randomized Phase II Trial. (2020).
55. Kneebone A, Hruby G, Ainsworth H, Byrne K, Brown C, Guo L, et al. Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Detected via Prostate-specific Membrane Antigen Positron Emission Tomography. Eur Urol Oncol (2018) 1(6):531–7. doi: 10.1016/j.euo.2018.04.017
56. Soldatov A, von Klot CAJ, Walacides D, Derlin T, Bengel FM, Ross TL, et al. Patterns of Progression After 68Ga-PSMA-Ligand PET/CT-Guided Radiation Therapy for Recurrent Prostate Cancer. Int J Radiat Oncol Biol Phys (2019) 103(1):95–104. doi: 10.1016/j.ijrobp.2018.08.066
57. Salama JK, Kirkpatrick JP, Yin F-F. Stereotactic body radiotherapy treatment of extracranial metastases. Nat Rev Clin Oncol (2012) 9(11):654–65. doi: 10.1038/nrclinonc.2012.166
58. Spratt DE, Evans MJ, Davis BJ, Doran MG, Lee MX, Shah N, et al. Androgen Receptor Upregulation Mediates Radioresistance after Ionizing Radiation. Cancer Res (2015) 75(22):4688–96. doi: 10.1158/0008-5472.CAN-15-0892
59. Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys (2010) 37(8):4078–101. doi: 10.1118/1.3438081
60. Chen LN, Suy S, Uhm S, Oermann EK, Ju AW, Chen V, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol Lond Engl (2013) 8:58. doi: 10.1186/1748-717X-8-58
61. LaCouture TA, Xue J, Subedi G, Xu Q, Lee JT, Kubicek G, et al. Small Bowel Dose Tolerance for Stereotactic Body Radiation Therapy. Semin Radiat Oncol (2016) 26(2):157–64. doi: 10.1016/j.semradonc.2015.11.009
62. Hoyer M, Roed H, Traberg Hansen A, Ohlhuis L, Petersen J, Nellemann H, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol Stockh Swed (2006) 45(7):823–30. doi: 10.1080/02841860600904854
63. Chuong MD, Springett GM, Freilich JM, Park CK, Weber JM, Mellon EA, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys (2013) 86(3):516–22. doi: 10.1016/j.ijrobp.2013.02.022
64. Colbert LE, Rebueno N, Moningi S, Beddar S, Sawakuchi GO, Herman JM, et al. Dose escalation for locally advanced pancreatic cancer: How high can we go? Adv Radiat Oncol (2018) 3(4):693–700. doi: 10.1016/j.adro.2018.07.008
65. Harshman LC, Chen Y-H, Liu G, Carducci MA, Jarrard D, Dreicer R, et al. Seven-Month Prostate-Specific Antigen Is Prognostic in Metastatic Hormone-Sensitive Prostate Cancer Treated With Androgen Deprivation With or Without Docetaxel. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(4):376–82. doi: 10.1200/JCO.2017.75.3921
66. Klotz L, Toren P. Androgen deprivation therapy in advanced prostate cancer: is intermittent therapy the new standard of care? Curr Oncol (2012) 19(Suppl 3):S13–21. doi: 10.3747/co.19.1298
67. Fendler WP, Ferdinandus J, Czernin J, Eiber M, Flavell RR, Behr SC, et al. Impact of 68Ga-PSMA-11 PET on the Management of recurrent Prostate Cancer in a Prospective Single-Arm Clinical Trial. J Nucl Med (2020). doi: 10.2967/jnumed.120.242180
68. Duchesne GM, Woo HH, Bassett JK, Bowe SJ, D’Este C, Frydenberg M, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol (2016) 17(6):727–37. doi: 10.1016/S1470-2045(16)00107-8
Keywords: prostate cancer, involved field SBRT, intermittent ADT, Nodal Oligo-recurrence, hormone sensitive, prostate SBRT
Citation: Carrasquilla M, Creswell ML, Pepin AN, Wang E, Forsthoefel M, McGunigal M, Bullock E, Lei S, Collins BT, Lischalk JW, Esposito G, Aghdam N, Kumar D, Suy S, Leger P, Hankins RA, Dawson NA and Collins SP (2021) Rationale for Involved Field Stereotactic Body Radiation Therapy-Enhanced Intermittent Androgen Deprivation Therapy in Hormone-Sensitive Nodal Oligo-Recurrent Prostate Cancer Following Prostate Stereotactic Body Radiation Therapy. Front. Oncol. 10:606260. doi: 10.3389/fonc.2020.606260
Received: 14 September 2020; Accepted: 25 November 2020;
Published: 18 January 2021.
Edited by:
Rosa M. Nadal, National Heart, Lung, and Blood Institute (NHLBI), United StatesReviewed by:
Marijo Bilusic, National Institutes of Health (NIH), United StatesBenjamin A. Teply, University of Nebraska Medical Center, United States
Copyright © 2021 Carrasquilla, Creswell, Pepin, Wang, Forsthoefel, McGunigal, Bullock, Lei, Collins, Lischalk, Esposito, Aghdam, Kumar, Suy, Leger, Hankins, Dawson and Collins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sean P. Collins, U1BDOUBnZW9yZ2V0b3duLmVkdQ==
 Michael Carrasquilla
Michael Carrasquilla Michael L. Creswell
Michael L. Creswell Abigail N. Pepin
Abigail N. Pepin Edina Wang1
Edina Wang1 Matthew Forsthoefel
Matthew Forsthoefel Brian T. Collins
Brian T. Collins Jonathan W. Lischalk
Jonathan W. Lischalk Giuseppe Esposito
Giuseppe Esposito Nima Aghdam
Nima Aghdam Deepak Kumar
Deepak Kumar Ryan A. Hankins
Ryan A. Hankins Sean P. Collins
Sean P. Collins