- 1Department of Thoracic Surgery, Affiliated Provincial Hospital of Anhui Medical University, Hefei, China
- 2Department of Thoracic Surgery, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China
Objective: This study aimed to analyze the relationship between the number of examined lymph nodes (ELNs) at the N1 station and the postoperative clinicopathological features and prognosis of patients with pT1-3N0M0 non-small cell lung cancer (NSCLC).
Methods: The cut-off value of the number of ELNs at the N1 station was obtained using X-tile software analysis. Kaplan-Meier survival curve analysis and the Cox proportional hazard model were used to study the impact of the number of ELNs at the N1 station on the prognosis of postoperative patients with pT1-3N0M0 NSCLC.
Results: The median survival time and 1-, 3- and 5-year survival rates of 0 ELNs at the N1 station were 28.0 months and 74.8%, 45.4%, and 21.2%, respectively. The median survival time and 1-, 3-, and 5-year survival rates of 1–4 ELNs at the N1 station were 45.0 months and 85.5%, 55.4%, and 39.1%, respectively. In the group with ≥ 5 ELNs at the N1 station, the median survival time and the 1-, 3- and 5-year survival rates were 59.0 months and 94.0%, 62.7%, and 48.2%, respectively. Both univariate and multivariate Cox regression analyses showed that the number of ELNs at the N1 station, T stage and operation type were independent factors affecting the prognosis of patients with pT1-3N0M0 NSCLC.
Conclusion: Increasing the number of ELNs at the N1 station is positively correlated with the long-term survival rate of patients with T1-3N0M0 NSCLC. At least 5 LNs at the N1 station should be examined in pathological examination.
Introduction
The morbidity and mortality of lung cancer are ranked first among all malignant tumors in the world (1, 2). The lung cancer histological types small cell lung cancer and non-small cell lung cancer (NSCLC) comprise 85% of all lung cancers, of which NSCLC accounts for 80% (1, 3). Radical resection remains the primary treatment for early resectable NSCLC. However, the postoperative 5-year survival rate is only 50% to 60% (4).
NSCLC patients with positive lymph nodes have a high risk of recurrence after surgery. Lymph node involvement is an important factor that determines the prognosis and treatment decision of postoperative NSCLC patients. Lymph node resection or sampling plays a crucial role in accurate lymph node staging. The results of lymph node pathological examination can determine the postoperative lymph node stage of the patient, determine the postoperative pathological stage of the patient, and guide the next step of treatment. Accurate postoperative staging is the key to determine whether patients should receive adjuvant therapy after surgery. Additionally, according to the International Association for Lung Cancer Research’s lymph node map, the NCCN guidelines recommend that thoracic surgeons sample only one or more lymph nodes from mediastinal lymph node stations (2R, 4R, 7, 8 and 9 on the right; 4L, 5, 6, 7, 8 and 9 on the left) (5, 6).
Because lymph nodes at the N1 station (stations 10, 11, 12, 13 and 14) are located in the lungs, they are often not examined by surgeons and pathologists after surgery. Thus, postoperative pathological N1 stage is evaluated as N0, resulting in the lack of adjuvant treatment after an operation and increasing the risk of postoperative recurrence. Presently, many studies have reported on the number of examined lymph nodes (ELNs) in patients with resectable NSCLC (7–9), but few have focused on the minimum number of ELNs at the N1 station. Therefore, this study aimed to explore whether the number of ELNs at the N1 station affects the long-term survival of patients with pT1-3N0M0 NSCLC.
Methods
Patient Selection
This study evaluated 1,510 NSCLC patients who had undergone radical resection of lung cancer at the affiliated Provincial Hospital of Anhui Medical University between January 2010 and April 2015. All the patients received lobectomy (or pneumonectomy) plus systematic hilar and mediastinal lymph node dissection. The inclusion criteria were as follows: 1) pathological diagnosis of NSCLC; 2) postoperative pathological stage of pT1-3N0M0; and 3) R0 resection. The exclusion criteria were as follows: 1) distant metastasis found during surgery or confirmed to be T4N1-2M1, 2) neoadjuvant therapy before surgery, and 3) incomplete medical records.
The clinical data were obtained from the LinkDoc database, supplemented by electronic medical record review. In this study, TNM staging, T staging, N staging and M staging were performed according to the 8th edition of the Lung Cancer Staging Project of the American Joint Commission on Cancer (AJCC). This study was approved by the Affiliated Provincial Hospital of Anhui Medical University.
Variables and Outcomes
This study collected and analyzed sex, age, smoking history, operation type, tumor location, tumor diameter, histology, tumor differentiation, T stage, preoperative complications and postoperative chemotherapy in patients with T1-3N0M0 NSCLC. The primary endpoint was overall survival (OS), which was defined as the interval between the date of operation and date of death from any cause or the last follow-up. We surveyed the patients at 3-month intervals for the first 2 years and at 6-month intervals thereafter. The follow-up evaluations generally included a physical examination, blood analysis (including pertinent tumor markers), chest radiography, and chest CT. Whenever there were symptoms or signs indicative of recurrence, further evaluations, including CT of the abdomen, brain magnetic resonance imaging, positron emission tomography-CT or bone scintigraphy, were performed.
Statistical Analyses
The categorical and continuous variables between the groups were compared by χ2-test and independent samples t-test as appropriate. The optimal number of ELNs at the N1 station was calculated using the X-tile model based on the maximum chi-squared value when a series of log-rank tests were conducted. Survival curves were depicted by the Kaplan-Meier method and compared among groups using the log-rank test. Multivariate Cox regression analyses were applied to identify the independent predictors for survival. Statistical analyses were conducted using SPSS Statistics (version 24; IBM, NY, USA). Differences with p < 0.05 were considered statistically significant.
Results
Patient Characteristics
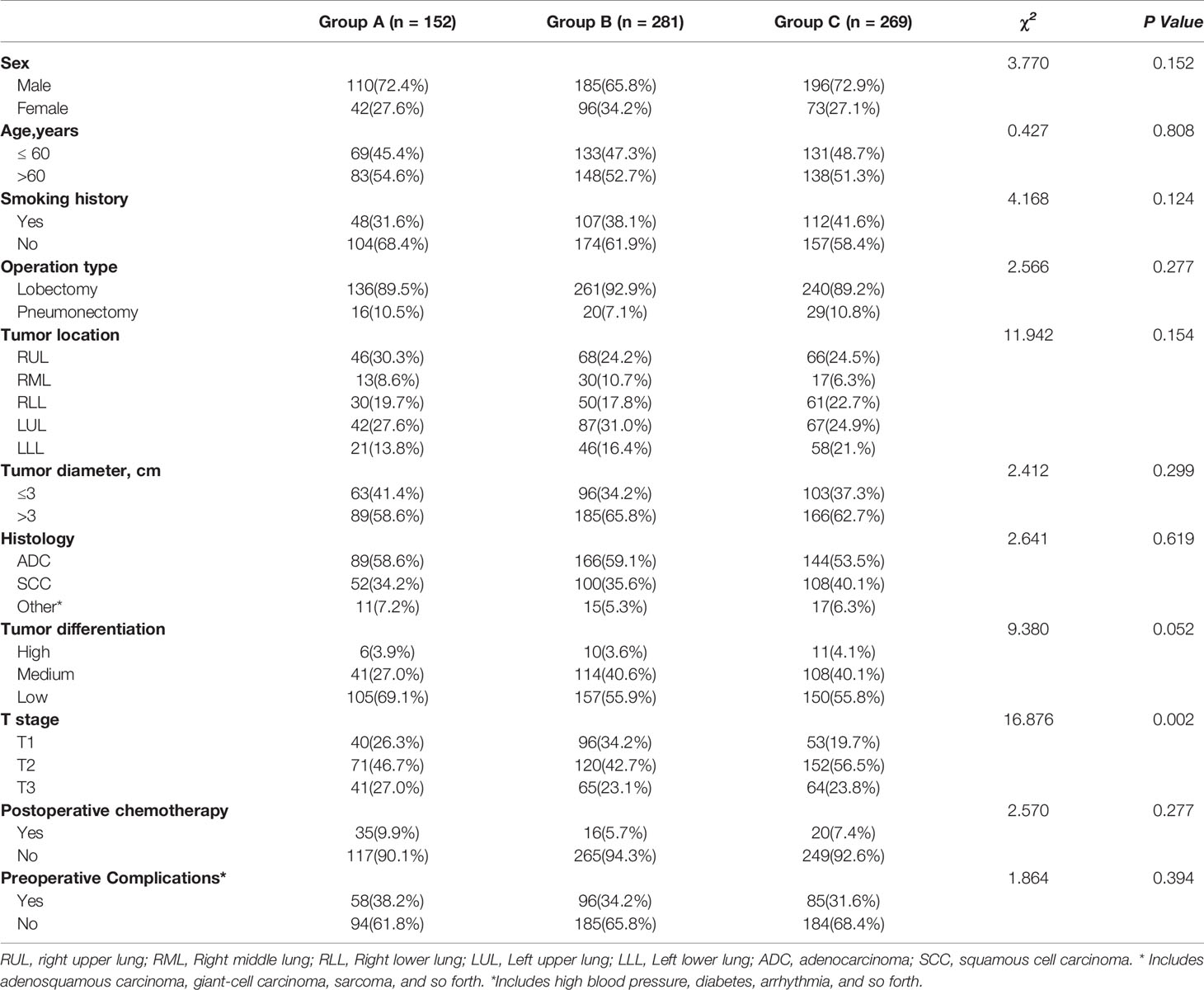
In total, 702 patients were included in this study. According to the relationship between the number of ELNs at the N1 station and OS in patients with pT1-3N0M0 NSCLC, the cohort was divided into three groups based on the X-tile model: 0 ELNs at the N1 station, 1–4 ELNs at the N1 station and ≥ 5 ELNs at the N1 station. 0 ELNs at the N1 station were defined as Group A, 1–4 ELNs at the N1 station were defined as Group B, and ≥ 5 ELNs at the N1 station were defined as Group C. The baseline characteristics are depicted in Table 1. The T stage had a significant influence on the number of ELNs at the N1 station. The results showed that the later was the T stage, the greater was the effect on the number of ELNs at the N1 station. Additionally, no significant difference was found in age, sex, smoking history, operation type, tumor location, tumor diameter, histology, tumor differentiation, preoperative complications or postoperative chemotherapy among the three groups.
In total, 9,608 lymph nodes were examined in the whole group, of which the number of ELNs at the N1 station was 2,849. The median number of ELNs was 13 (range: 0–35), and the median number of stations at which lymph nodes were examined was 3 (range: 0–8). The median number of ELNs at the N1 station was 3 (range: 0–20). The distribution of the number of ELNs at the N1 station in the NSCLC patients is shown in Figure 1.
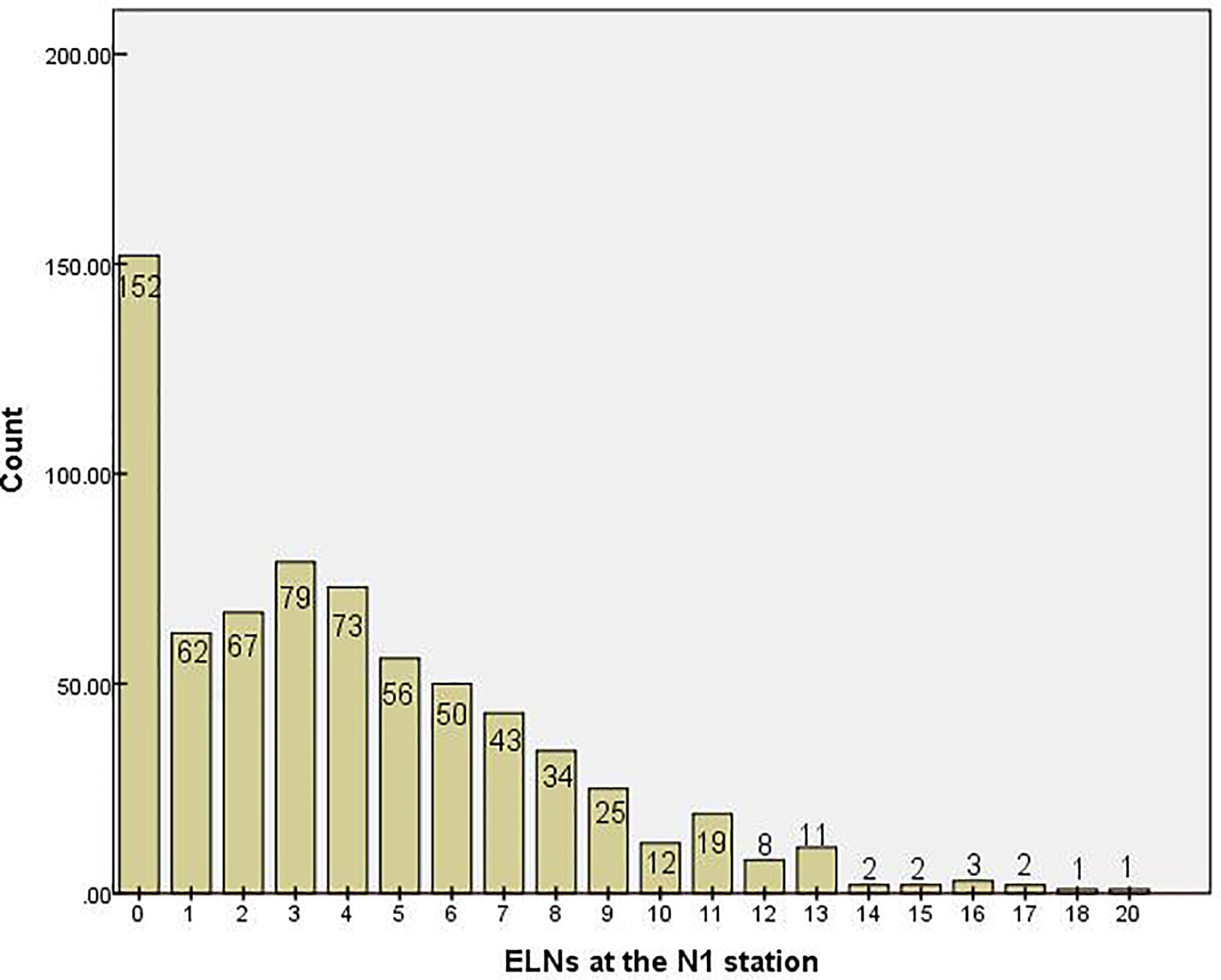
Figure 1 Distribution of the number of examined lymph nodes at the N1 stationin. ELN, examined lymph node.
Impact of the Number of Examined Lymph Nodes on Survival
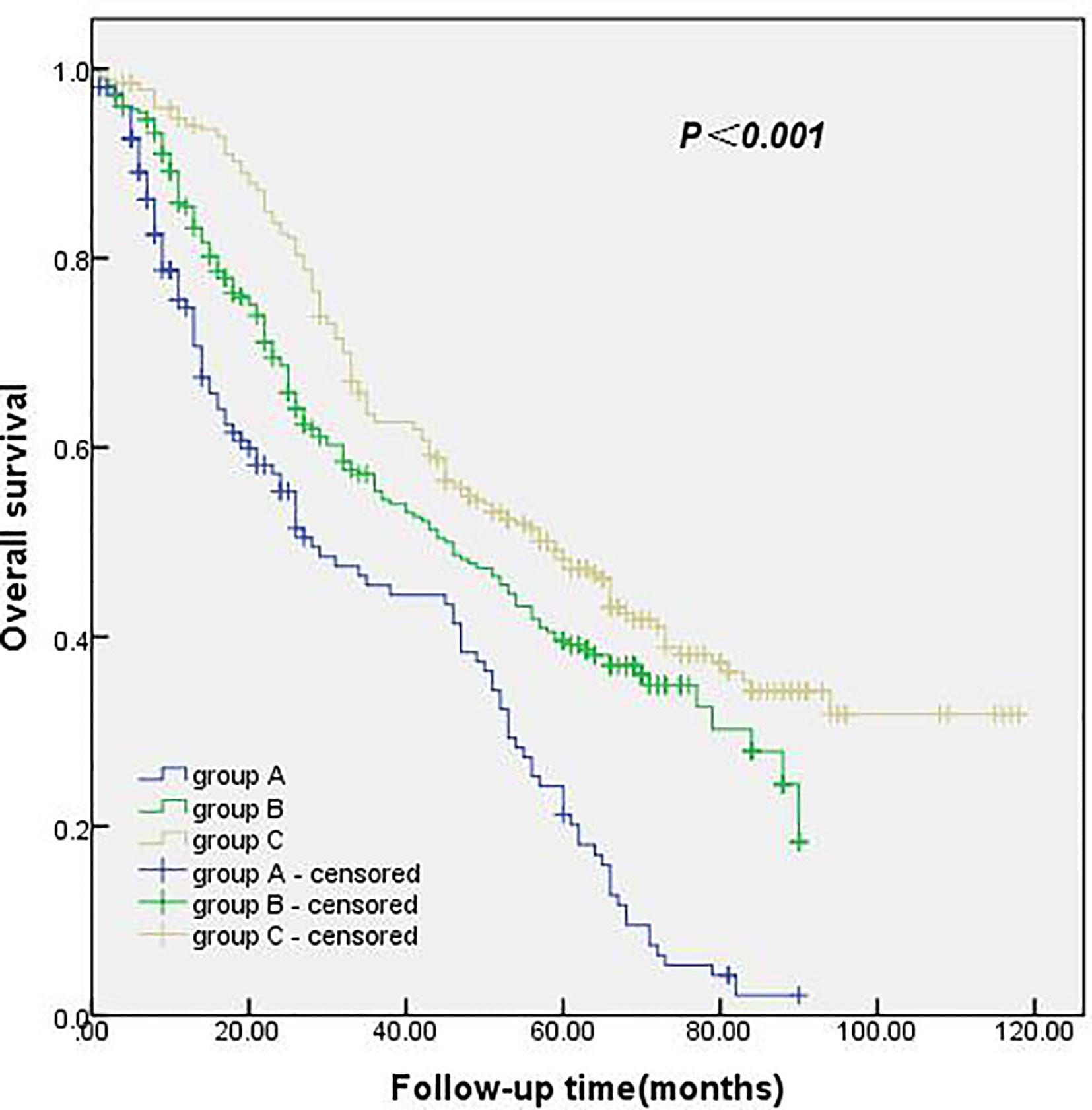
The follow-up period was from January 2010 to April 2020, with a total follow-up period of 112 months. The median survival time and 1-, 3- and 5-year survival rates were 46.0 months and 86.6%, 56.1%, and 39.5%, respectively. The median survival time and 1-, 3- and 5-year survival rates of 0 ELNs at the N1 station were 28.0 months and 74.8%, 45.4%, and 21.2%, respectively. The median survival time and 1-, 3-, and 5-year survival rates of 1–4 ELNs at the N1 station were 45.0 months and 85.5%, 55.4%, and 39.1%, respectively. In the group with ≥ 5 ELNs at the N1 station, the median survival time and 1-, 3- and 5-year survival rates were 59.0 months and 94.0%, 62.7%, and 48.2%, respectively. The survival rate of the group with ≥ 5 ELNs at the N1 station was significantly better than that of the group with 0 ELNs at the N1 station and the group with 1–4 ELNs at the N1 station. These results were statistically significant (p<0.001). The details are shown in Figure 2.
Univariate analysis of the clinicopathological data showed that the operation type, histology, T stage and ELNs at the N1 station were significantly correlated with patient survival (p<0.05). The details are shown in Table 2. The clinicopathological data of the patients were incorporated into the Cox model for multivariate analysis. The results showed that the operation type, T stage and ELNs at the N1 station were independent prognostic factors affecting patient survival (p<0.05). The details are shown in Table 3.
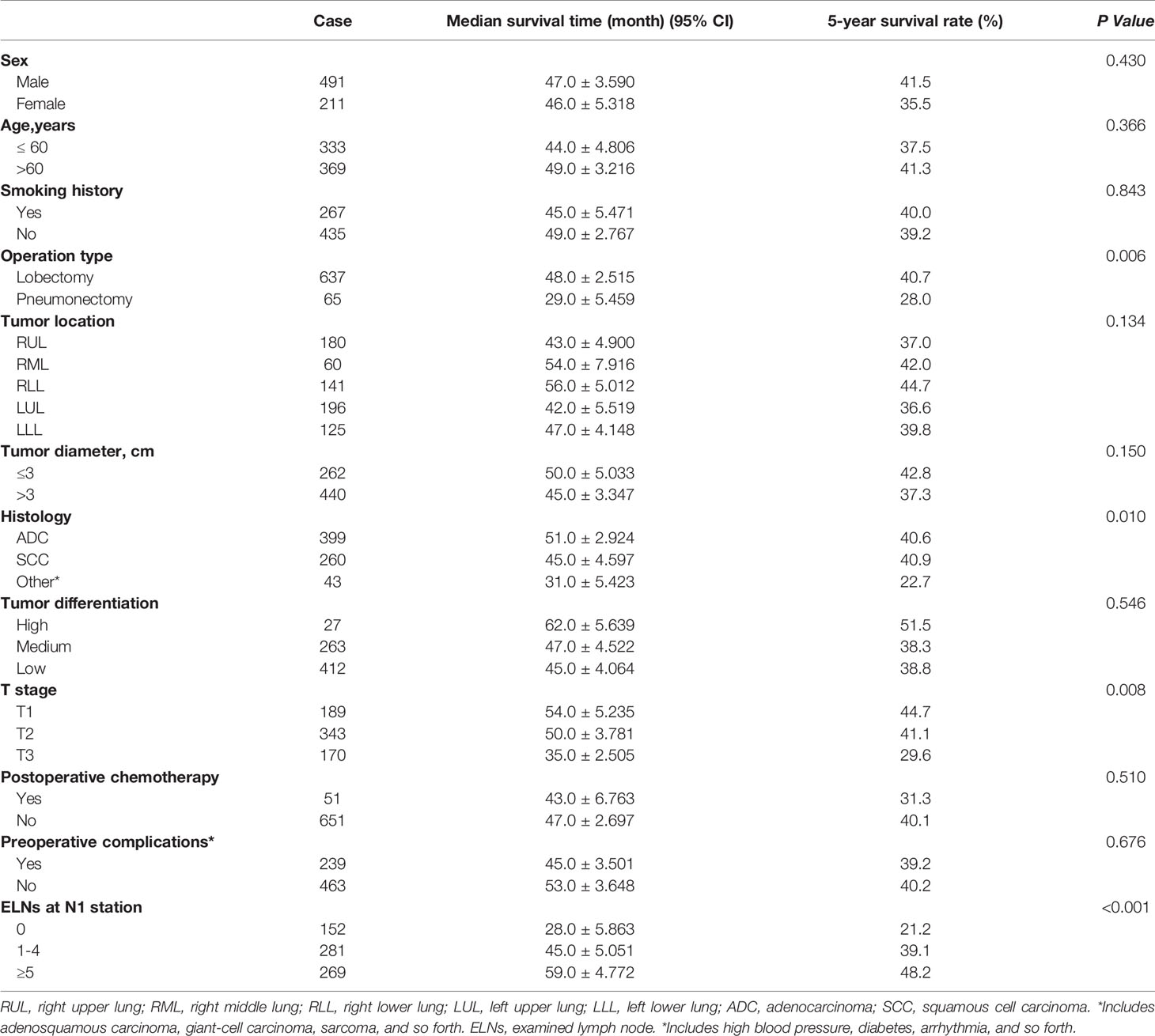
Table 2 The prognostic factors associated with overall survival of patients in groups A, B, and C by univariate analysis.
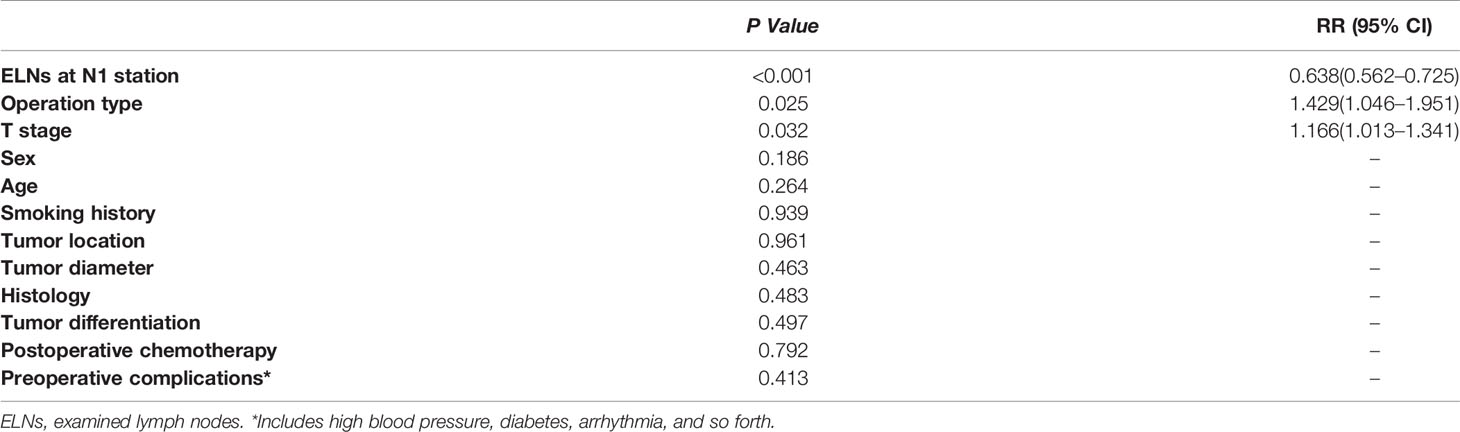
Table 3 The prognostic factors associated with overall survival of patients in groups A, B, and C by multivariate Cox regression.
Impact of the Number of Examined Lymph Nodes on T Stage
In comparing the pathological data, we found a significant difference in T stage among the three groups. Therefore, all the patients were classified according to T stage to test whether the number of ELNs at the N1 station in this study was suitable for patients with different T stages of NSCLC. In patients with stage T1, stage T2 and stage T3 NSCLC, the survival rate of the group with ≥ 5 ELNs at the N1 station was significantly better than that of the group with 0 ELNs at the N1 station and the group with 1–4 ELNs at the N1 station, with a statistically significant difference (Figures 3A–C).
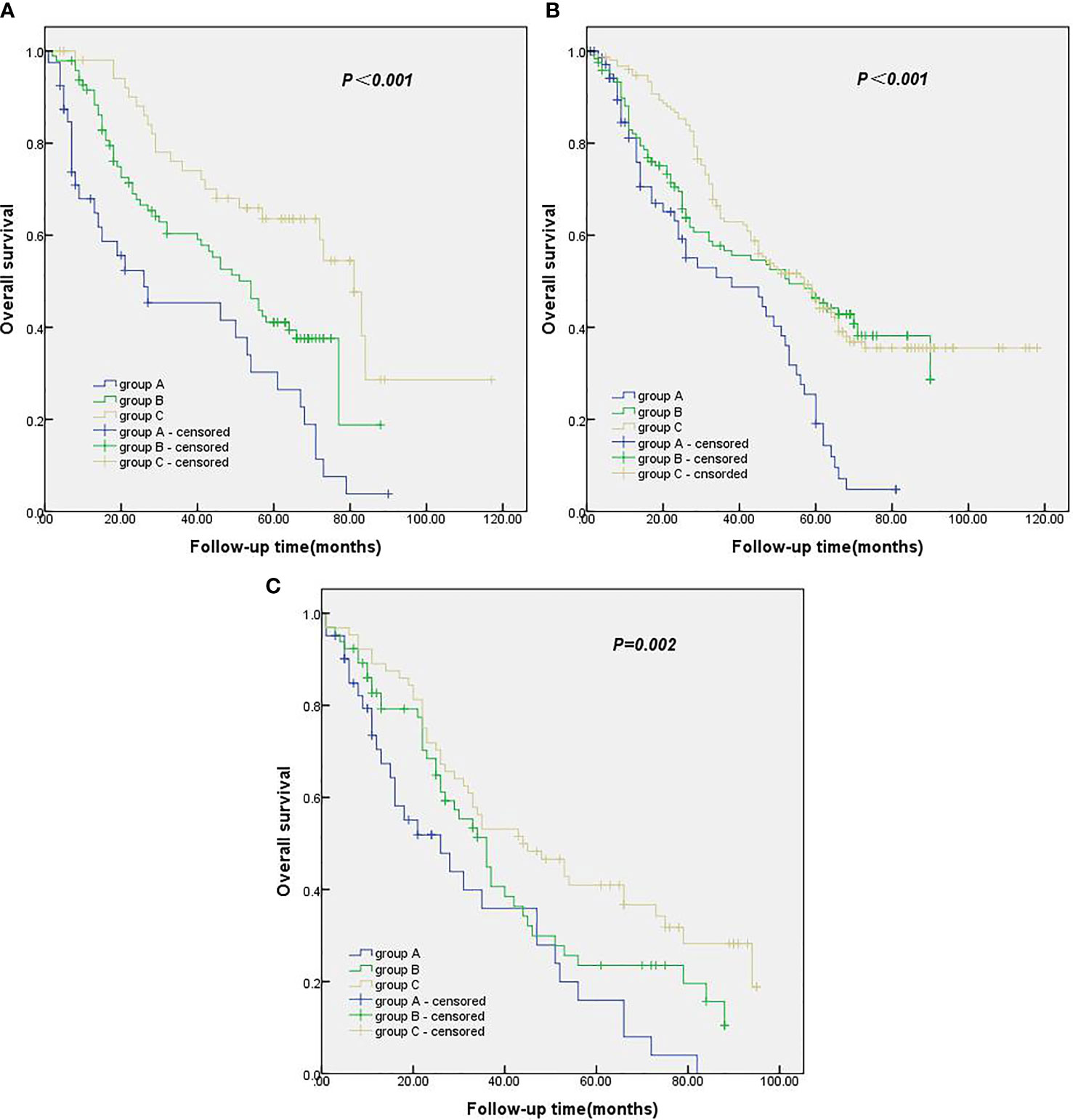
Figure 3 (A) Kaplan-Meier survival curves of groups A, B and C in T1 stage. (B) Kaplan-Meier survival curves of groups A, B and C in T2 stage. (C) Kaplan-Meier survival curves of groups A, B and C in T3 stage.
Discussion
The problem of postoperative recurrence in patients with NSCLC has always been a major issue faced by clinicians. The NCCN guidelines suggest that inadequate examination of lymph nodes reduces survival and recommend a minimum number of ELNs. For many cancers, such as gastric and breast cancer, the relationship between more lymph node detection and improved long-term survival has been well studied (10, 11). Additionally, studies have shown that insufficient detection of lymph nodes will increase the risk of recurrence in patients with NSCLC. However, few studies have investigated whether the number of ELNs at the N1 station affects the prognosis of patients with NSCLC (12–14). An accurate examination of lymph nodes has important reference significance for the postoperative pathological staging and prognosis evaluation of patients with NSCLC. In this study, the number of ELNs at the N1 station was significantly correlated with the long-term survival rate of pT1-3N0M0 NSCLC patients. The survival rate of patients with ≥ 5 ELNs at the N1 station was higher than that of the other two groups. Additionally, the survival rate of patients with 1–4 ELNs at the N1 station was higher than that of patients with 0 ELNs at the N1 station.
In this study, pT1-3N0M0 NSCLC patients who had undergone radical resection of lung cancer were divided into groups according to the number of ELNs at the N1 station. Through survival and multivariate analyses, we found that the long-term survival rate of pT1-3N0M0 NSCLC patients with ≥ 5 ELNs at the N1 station was higher than that of patients with 1–4 ELNs at the N1 station and patients with 0 ELNs at the N1 station, and the number of ELNs at the N1 station was an independent prognostic factor. Through analyses of the Chinese registry and Surveillance, Epidemiology, and End Results (SEER) database, Liang et al. found that a greater number of ELNs was related to more accurate lymph node staging and the long-term prognosis of NSCLC patients after surgery (15). Additionally, Liang et al. suggested that 16 lymph nodes should be examined as the best cut-off point for postoperative lymph node examination or prognosis stratification in N0 patients (15). Saynak M et al., by evaluating stage Ia NSCLC patients who had undergone lung cancer surgery, found that the local recurrence rate of patients with N1 station lymph nodes examined was 14%, while the recurrence rate of patients without N1 station lymph nodes examined was 31%, suggesting that insufficient examination of N1 station lymph nodes cannot accurately determine the patient’s pathological stage, resulting in insufficient postoperative adjuvant therapy (16).
We believe that the most important findings from this study are as follows. First, when the number of ELNs at the N1 station is low, the probability of positive hilar and intrapulmonary lymph nodes will be reduced, resulting in some postoperative NSCLC patients being mistakenly classified as N0 and in lower chances of postoperative adjuvant therapy and a worse prognosis. When more lymph node examinations are performed at the N1 station, the postoperative lymph node staging of NSCLC patients is more accurate, the chance of receiving adjuvant therapy is increased, and the prognosis is better. Such differences could explain the different survival times among the three groups. This phenomenon is considered the main reason for the results of this study. Second, the first site of lymph node metastasis in patients with NSCLC is the lymph nodes at the N1 station, and the examination of additional pathological sections of lymph nodes at the N1 station will increase the chance of lymph node micrometastasis at the N1 station being detected by pathologists. Thus, patients with stage N0 NSCLC can be accurately classified as stage N1, thus receiving adjuvant therapy postoperation to prolong survival.
In this study, NSCLC patients were refined according to T stage. Survival analysis showed that, in stages T1, T2 and T3, the long-term survival rate of patients with more than 5 ELNs at the N1 station was higher than that of patients with 1–4 ELNs at the N1 station and of patients with 0 ELNs at the N1 station. According to the results of this study, for patients with stage T1 to T3 NSCLC, thoracic surgeons should examine 5 or more N1 lymph nodes during the operation so that patients can obtain good and accurate postoperative pathological staging and sufficient postoperative adjuvant therapy to improve their prognosis. Additionally, through univariate and multivariate Cox regression, we found that the operation type is an independent prognostic factor for the prognosis of patients with pT1-3N0M0 NSCLC. We believe there are several reasons for this finding. First, 637 patients had undergone lobectomy, accounting for 90.7% of the whole population, whereas the percentage of patients who had undergone pneumonectomy accounted for 9.3%. Therefore, there was a certain statistical deviation. Second, most of the patients who had undergone pneumonectomy had central lung cancer, although they were pT1-3N0M0 patients, but the pathological stage was “later” than that of peripheral lung cancer, their survival time was generally short, and their prognosis was poor. Finally, patients who undergo pneumonectomy are more likely to develop respiratory failure, heart failure and other multiple organ failure than those who undergo lobectomy, thus affecting the prognosis.
Our study has several limitations. The main limitations are the retrospective nature of the analysis and nonrandomization, which may have resulted in potential selection bias. Another limitation is that it is difficult to separate each lymph node in the dissected tissue, leading to varying numbers of ELNs due to the fragmentation of lymph node tissue in the process of lymph node resection and possibly affecting the best cut-off point of the number of ELNs.
Conclusions
In patients with pT1-3N0M0 NSCLC, the long-term survival rate of those with ≥ 5 ELNs at the N1 station was higher than that of patients with 1–4 ELNs at the N1 station and with 0 ELNs at the N1 station, and the number of ELNs at the N1 station was an independent prognostic factor. T stage and surgical method were identified as independent prognostic factors in patients with pT1-3N0M0 NSCLC. Considering some of the limitations of this study, a large cohort, multicenter and prospective study is needed to confirm our findings.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Affiliated Provincial Hospital of Anhui Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
GXW conceptualized the study, contributed to the methodology, provided the software, contributed to the investigation of the study, wrote the original draft, and wrote, reviewed, and edited the manuscript. XNW wrote the original draft, and wrote, reviewed, and edited the manuscript. XHS and TL contributed to the visualization and investigation of the study. MQX and LDX provided the software Software and contributed to the validation of the study. MRX acquired funding, and wrote, reviewed, and edited the manuscript. All authors contributed to the article andapproved the submitted version.
Funding
This work was supported by the grants from the National Natural Science Foundation of China, The Fundamental Research Funds for the Central Universities and Key research and development projects in Anhui Province (Nos. 81973643, WK9110000021, and 202004j07020017).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Linkdoc for her help with the regulatory management, data collection, and preparation of figures.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
3. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev (2010) 19:1893–907. doi: 10.1158/1055-9965.EPI-10-0437
4. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forth-coming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol (2007) 2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a
5. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Non-small cell lung cancer, V.3. (2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
6. Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P, et al. The IASLC lung cancer staging project: A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol (2009) 4:568–77. doi: 10.1097/JTO.0b013e3181a0d82e
7. Yendamuri S, Dhillon SS, Groman A, Dy G, Dexter E, Picone A, et al. Effect of the number of lymph nodes examined on the survival of patients with stage I non-small cell lung cancer who undergo sublobar resection. J Thorac Cardiovasc Surg (2018) 156:394–402. doi: 10.1016/j.jtcvs.2018.03.113
8. Chen Y, Zhang J, Chen L, Dai J, Hu J, Zhu X, et al. Lymph Node Examination for Stage I Second Primary Lung Cancer Patients Who Received Second Surgical Treatment. Ann Surg Oncol (2020). doi: 10.1245/s10434-020-08975-9
9. Ding H, Wang H, Xu L, Song N, Jiang G. Survival and Resected Lymph Node Number During Sublobar Resection for N0 Non-Small Cell Lung Cancer 2 cm or Less. Ann Thorac Surg (2019) 107:1647–55. doi: 10.1016/j.athoracsur.2018.12.024
10. National Comprehensive Cancer Network:. NCCN clinical practice guidelines in oncology: Gastric cancer, V.2. (2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Breast cancer, V.4. (2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
12. Wang X, Yan S, Lv C, Wang Y, Wang J, Li S, et al. Impact of Omission of Intrapulmonary Lymph Node Retrieval on Outcome Evaluation of Lung Cancer Patients Without Lymph Node Metastasis: A Propensity Score Matching Analysis. Clin Lung Cancer (2017) 18(6):e411–6.4. doi: 10.1016/j.cllc.2017.05.001
13. Ramirez RA, Wang CG, Miller LE, Adair CA, Berry A, Yu X, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol (2012) 30(23):2823–8. doi: 10.1200/JCO.2011.39.2589
14. Eichhorn F, Klotz LV, Muley T, Kobinger S, Winter H, Eichhorn ME, et al. Prognostic relevance of regional lymph-node distribution in patients with N1-positive non-small cell lung cancer: A retrospective single-center analysis. Lung Cancer (2019) 138:95–101. doi: 10.1016/j.lungcan.2019.10.018
15. Liang W, He J, Shen Y, Shen J, He Q, Zhang J, et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol (2017) 35(11):1162–70. doi: 10.1200/JCO.2016.67.5140
Keywords: examined lymph node, non-small cell lung cancer, survival, prognosis, surgery
Citation: Wang G, Wu X, Sun X, Li T, Xu M, Xu L and Xie M (2020) Value of N1 Lymph Node Examination in the Prognosis of Patients With pT1-3N0M0 Non-Small Cell Lung Cancer. Front. Oncol. 10:603378. doi: 10.3389/fonc.2020.603378
Received: 06 September 2020; Accepted: 16 November 2020;
Published: 15 December 2020.
Edited by:
Pasquale Pisapia, University of Naples Federico II, ItalyReviewed by:
Davide Franceschini, University of Milan, ItalyRocco Trisolini, Fondazione Policlinico Universitario A. Gemelli IRCCS, Italy
Copyright © 2020 Wang, Wu, Sun, Li, Xu, Xu and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingran Xie, eG1yMTk4MUB1c3RjLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Gaoxiang Wang
Gaoxiang Wang Xianning Wu
Xianning Wu Xiaohui Sun1,2
Xiaohui Sun1,2 Mingran Xie
Mingran Xie