
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 07 January 2021
Sec. Gastrointestinal Cancers
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.601240
This article is part of the Research Topic Immunotherapy in Hepatocellular Carcinoma View all 12 articles
 Clelia Donisi1
Clelia Donisi1 Marco Puzzoni1
Marco Puzzoni1 Pina Ziranu1
Pina Ziranu1 Eleonora Lai1
Eleonora Lai1 Stefano Mariani1
Stefano Mariani1 Giorgio Saba1
Giorgio Saba1 Valentino Impera1,2
Valentino Impera1,2 Marco Dubois1
Marco Dubois1 Mara Persano1
Mara Persano1 Marco Migliari1
Marco Migliari1 Andrea Pretta1,2
Andrea Pretta1,2 Nicole Liscia1,2
Nicole Liscia1,2 Giorgio Astara1
Giorgio Astara1 Mario Scartozzi1*
Mario Scartozzi1*Hepatocellular carcinoma (HCC) is the typical inflammation-induced neoplasia. It often prospers where a chronic liver disease persists, thus leading a strong rationale for immune therapy. Several immune-based treatments, including immune checkpoint inhibitors (ICI), cytokines, adoptive cell transfer, and vaccines, have been tested in the treatment of HCC. In this review, we summarize the role of the ICI in HCC patients in various sets of treatment. As for advanced HCC, the anti-Programmed cell Death protein 1 (PD1) antibodies and the anti-Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) antibodies have been examined in patients with enthusiastic results in phase I-II-III studies. Overall, this led the Food and Drug Administration (FDA) to approve pembrolizumab, nivolumab, and nivolumab + ipilimumab in the second-line setting. The anti- Programmed Death-Ligand 1 (PDL-1) antibodies have also been evaluated. Thanks to the results obtained from phase III IMbrave study, atezolizumab + bevacizumab is now the standard of care in the first-line advanced setting of HCC. As for localized HCC, the putative immunological effect of locoregional therapies led to evaluate the combination strategy with ICI. This way, chemoembolization, ablation with radiofrequency, and radioembolization combined with ICI are currently under study. Likewise, the study of adjuvant immunotherapy following surgical resection is underway. In addition, the different ICI has been studied in combination with other ICI as well as with multikinase inhibitors and anti-angiogenesis monoclonal antibody. The evidence available suggests that combining systemic therapies and locoregional treatments with ICI may represent an effective strategy in this context.
Hepatocellular Carcinoma (HCC) claims to be 90% of primary liver cancer and represents the second cause of death due to malignancy in males (1). The triggers most likely involved in cancer development are chronic infections by Hepatitis B or C viruses, diabetes, aflatoxin-B1 (AFB1) exposure, obesity, alcohol abuse, nonalcoholic steatohepatitis (NASH), nonalcoholic fatty liver disease (NAFLD), and metabolic syndrome (2–11).
Indeed, chronic inflammation boosts the tumor immunogenicity and induces hepatocellular DNA damage, genetic and epigenetic mutations. Furthermore, chronic inflammation allows to escape the host immune surveillance in cooperation with an immunosuppressive surrounding (2–8).
The impairment of various immune components promotes tumorigenesis. The liver immune milieu consists of an assortment of innate and adaptive immune cells that undergo alterations that promote cancer development and progression. Immune checkpoints are involved in the inhibition of T- or natural killer cell activation as well as in the initiation and preservation of tumor immune tolerance. B and T cells, natural killer cells, dendritic cells, tumor-associated macrophages, monocytes, and myeloid-derived suppressor cells express on their surface immune-checkpoints and their ligands. The most well-known of them are cytotoxic T-lymphocyte protein 4 (CTLA-4), which promotes immunosuppression, and programmed cell death protein 1 (PD-1) that leads to the T-cell exhaustion status, which inhibits T-cell multiplication and release of cytotoxic mediators (2–8).
In a physiological state, antigens are presented to CD4+ T cells that consequently promote the activity of CD8+ T cells. Thus, leading to an upregulation of CTLA-4 and PD-1. Consequently, the immune checkpoints prevent hyperactivation of the immune response. That way, the tolerogenic environment of the liver is preserved. Therefore, HCC is an immunogenic tumor that builds-up in an immune-suppressed microenvironment. In the setting of chronic inflammation, the cancer develops and flourishes thanks to the recruitment of regulatory T cells (Treg), myeloid-derived suppressor cells (MDSCs), and the upregulation of immune checkpoints, CTLA-4 and PD-1. PD-1 binding its ligand PD-L1 prevents TCR signaling, blocks T cell proliferation, and induces the exhaustion of T cells. Tregs constitutively express CTLA-4 and preclude the immune response through it. CTLA-4 binds CD80/CD86, competing with CD28, and blocks activation of the T cells. It appears clear that the inhibition of immune checkpoints avoids immune exhaustion, reduces Treg activity, and leads to the reactivation of the anticancer immune response (2–5). Thus, immune-checkpoint inhibitors (ICI) seem to be promising treatment strategies (Figure 1).
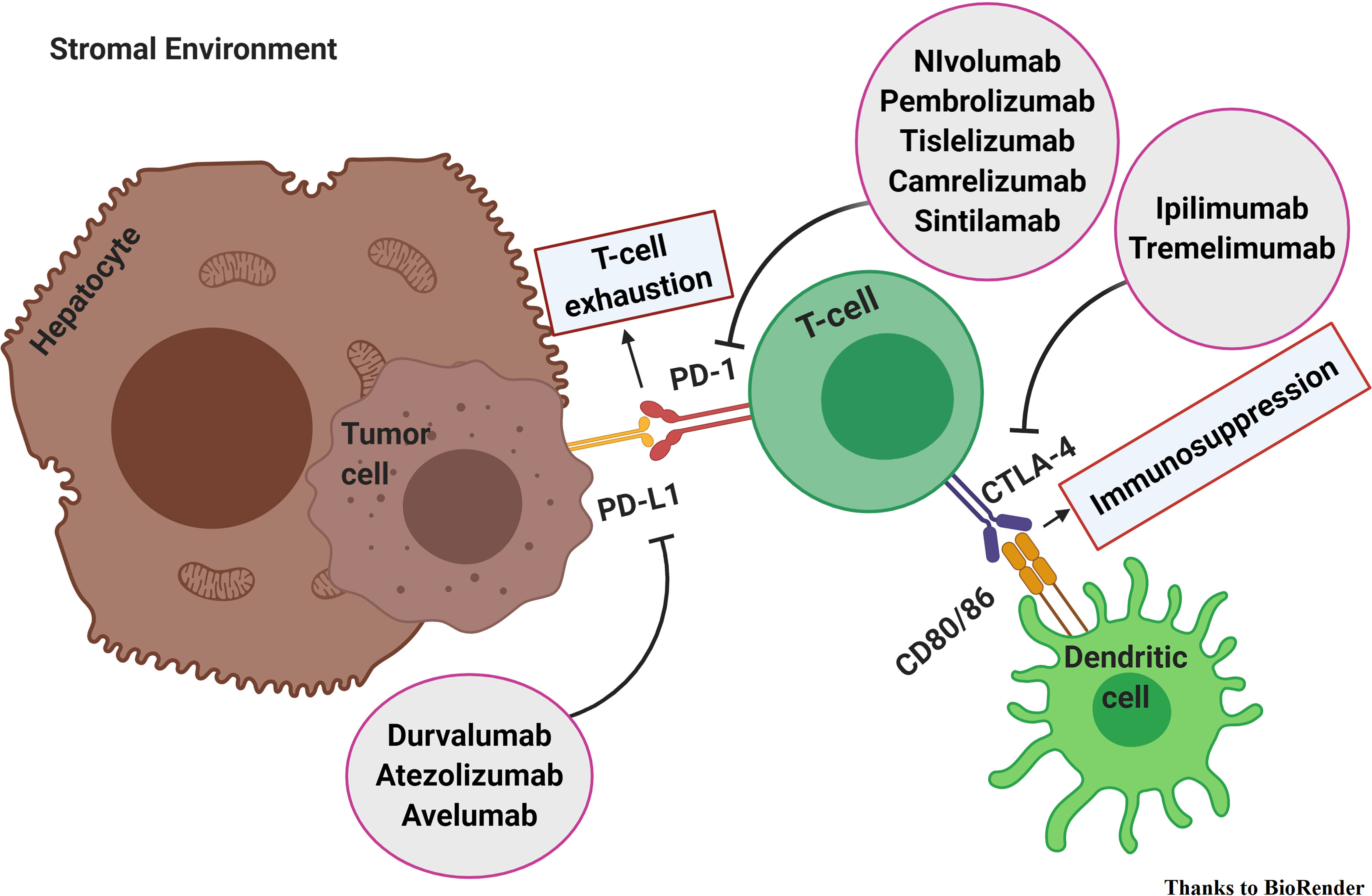
Figure 1 Immune checkpoint inhibitors in hepatocellular carcinoma. PD-1 binding its ligand PD-L1 prevents TCR signaling, blocks T cell proliferation, and induces the exhaustion of T cells. CTLA-4 binds CD80/CD86 and blocks activation of the T cells. The inhibition of immune checkpoints avoids immune exhaustion, reduces Treg activity, and leads to the reactivation of the anticancer immune response.
The systemic therapies for patients with HCC in advanced and intermediate stage, according to Barcelona Clinic Liver Cancer (BCLC), refractory to locoregional therapy was limited to sorafenib for a long time (12). Instead, since 2017, several effective systemic therapies have been recommended by the Food and Drug Administration (FDA), thus turning treatment decision making into a challenge. Several TKIs are now available for first-line [sorafenib (13–22), lenvatinib (23, 24)], second and third-line treatment [regorafenib (25), cabozantinib (26)]. Also, a monoclonal antibody [ramucirumab (27)] is available for second-line treatment. In addition, two anti-PD-1 antibodies [nivolumab (28) and pembrolizumab (29)] and the combination anti-PD1 + anti-CTLA4 [nivolumab + ipilimumab (30)] received FDA’s accelerated approval. On the whole, the anti- angiogenesis remains a cardinal point for treatment, whereas the ICI, including anti-PD-1, anti-PDL-1, and anti-CTLA-4, are becoming increasingly important in the therapeutic scenario.
As for anti-CTLA-4, tremelimumab (31) has been evaluated in a phase II, in a non-controlled, open-label, multicenter clinical trial, in patients with HCC not amenable to locoregional treatment and chronic hepatitis C. Tremelimumab showed a good safety profile along with encouraging outcomes in terms of RR (17.6%), disease control rate (DCR) (76,4%) and time to progression (TTP) (6.48 months).
On this basis, tremelimumab, in combination with durvalumab, has been evaluated. A randomized phase II trial (NCT02519348) has been examined tremelimumab and durvalumab as single-agent as well in combination with two different dosage regimens (tremelimumab 300 + durvalumab vs tremelimumab 75 + durvalumab) in advanced HCC patients. A safety profile along with an antitumor activity were demonstrated in the preliminary results, especially for the tremelimumab 300 + durvalumab regimen. Grade 3/4 adverse events were reported in 28.9% of patients (tremelimumab 300 + durvalumab, 35.1%; tremelimumab 75 + durvalumab, 25.6%; durvalumab, 19.8%; tremelimumab, 42%). The ORR observed were the following: 22.7% for tremelimumab 300 + durvalumab; 9.5% for tremelimumab + durvalumab; 9.6% for durvalumab, and 7.2% for tremelimumab (32).
As a result, the data from the phase III Himalaya trial (33) are expected to assess the efficacy of tremelimumab + durvalumab versus sorafenib in the first-line setting of HCC patients not susceptible to locoregional therapy.
As regards anti-PD-1, nivolumab and pembrolizumab have been investigated in phase II (CheckMate 040 and Keynote 224, respectively) and phase III studies (CheckMate 459 and Keynote 240, respectively).
The CheckMate 040 phase I/II non-comparative study evaluated nivolumab in patients with unresectable HCC with or without previous treatment with sorafenib. The phase II study showed a promising ORR of 20% with a median extent response of 9.9 months along with a manageable safety profile. The 9-month overall survival (OS) rate was 74%. On this basis, the FDA speeded up the acceptance of nivolumab for HCC pretreated with sorafenib (29). Conversely, the CheckMate 459 trial phase III study (34) failed to demonstrate improved OS with nivolumab versus sorafenib in this setting. Although the results obtained are impressive, showing improvements in survival and response rate along with a lack of adverse events, they were not statistically significant. Median overall survival was 16.4 months for nivolumab and 14.7 for sorafenib [Hazard Ratio (HR): 0.85 p 0.0752], the ORR was 15% for nivolumab and 7% for sorafenib. Also, nivolumab has been assessed in combination with ipilimumab in the Cohort 4 of Checkmate 040 (30). The ORR was 31% with a median duration of response (DOR) of 17 months; DCR was 49%, and 24 months OS rate was 40%.
Based on these impressive results, the FDA recommended the combination of nivolumab and ipilimumab for HCC patients previously treated with sorafenib.
As regards of pembrolizumab, it has been evaluated in the phase II Keynote 224, non-randomized, multicentre, open-label, in HCC BCLC B-C patients pre-treated with sorafenib. Pembrolizumab demonstrated a manageable safety profile along with antineoplastic activity with an ORR of 17%. On this basis, pembrolizumab received FDA’s accelerated approval, and it has been evaluated versus placebo in pre-treated advanced HCC patients in phase III randomized, placebo-controlled Keynote 240 (35). Pembrolizumab improved OS (13.9 months vs 10.6 months HR: 0.78 p: 0.0238), progression free survival (PFS) (3.0 months vs 2.8 months HR: 0.77 p: 0.022) and ORR (16.9% vs 2.2%) with durable responses (DoR 13.8 months) vs placebo. The study, however, was negative. The outcome measures OS and PFS, although impressive, did not achieve statistical significance. Regarding anti-PDL-1, atezolizumab has been tested as first-line treatment in combination with bevacizumab in the phase Ib GO30140 Study (NCT02715531). Patients included in arm A received atezolizumab + bevacizumab IV every three weeks, whereas patients included in arm F were randomized 1:1 and took atezolizumab-bevacizumab (F1) or single-agent atezolizumab (F2). In arm A, the ORR (primary endpoint) was 36%, with 76% of responses still ongoing. In arm F, the primary endpoint was PFS. A statistically significant improvement in median PFS was reached with the combination therapy respect to single-agent atezolizumab (F1: 5.6 versus F2: 3.4 months, HR 0.55, 80% confidence interval (CI), 0.40–0.74, P = 0.0108). As for safety, another one primary endpoint for both arms, any-grade treatment-related adverse events (TRAEs) were 68% in arm F1 and 41% in arm F2 (36).
Another crucial study that represents a turning point in the treatment of HCC was the phase III IMbrave 150 Study. In this randomized, open-label trial, advanced HCC patients were randomized 2:1 to receive atezolizumab + bevacizumab or sorafenib until loss of clinical benefit or unacceptable toxicity. Co-primary endpoints were OS and PFS by independent review facility (IRF)-assessed response evaluation criteria in solid tumors (RECIST) 1.1, whereas key secondary endpoints were IRF-ORR per RECIST 1.1 and IRF-ORR per HCC modified RECIST (mRECIST). The primary data analysis showed the achievement of both co-primary endpoints: in the intent-to-treat (ITT) population, at a median follow-up of 8.6 months, OS HR was 0.58 (95% CI, 0.42, 0.79; P = 0.0006) and PFS HR was 0.59 (95% CI, 0.47, 0.76; P < 0.0001) in the atezolizumab plus bevacizumab arm vs the control arm. ORR was 27% in patients receiving atezolizumab and bevacizumab vs 12% in patients receiving sorafenib (P < 0.0001) per IRF RECIST 1.1 and 33 vs 13% (P < 0.0001) per IRF HCC mRECIST for experimental arm vs control arm, respectively. Median treatment duration was of 7.4 months for atezolizumab, 6.9 for bevacizumab, and 2.8 for sorafenib. Moreover, the association of atezolizumab and bevacizumab was well tolerated and procrastinated time to deterioration (TTD) of the quality of life (QoL) of the patients [median TTD, 11.2 vs 3.6 mo; HR, 0.63 (95% CI: 0.46, 0.85)], physical functioning [median TTD, 13.1 vs 4.9 mo; HR, 0.53 (95% CI: 0.39, 0.73)], and role functioning [median TTD, 9.1 vs 3.6 mo; HR, 0.62 (95% CI: 0.46, 0.84)] compared with sorafenib. Furthermore, the combination therapy postponed TTD in patient-reported symptoms (loss of appetite, fatigue, pain, diarrhea) and led to meaningful clinical symptoms deterioration in a lower proportion of patients. Based on this data, atezolizumab + bevacizumab was approved as the first-line standard of care in advanced HCC (37, 38).
Hepatic resection (HR), liver transplantation (LT), and ablation (39) are treatments with curative intent in HCC, according to the EASL clinical practice guidelines (40).
To date, no therapy has proven to be effective in the adjuvant setting (41, 42). Nonetheless, the promising results of immunotherapy in advanced HCC have led to a growing interest in the adjuvant setting too. It is well-known that the liver has an immune suppressive microenvironment to avoid autoimmune phenomena (43, 44). However, in patients with HCC, persistent inflammatory state upregulates the expression of PD-1 (45) and PD-L1 (46), leading to CD8+ T-cells apoptosis and a decrease of their action against tumor cells (47, 48). Moreover, this effect relates to a poor prognosis and a considerable aggressiveness of the tumor and promotes postoperative recurrences in HCC patients (49–52). An increased PD-1 and PD-L1 expression could provide the rationale for the employment of both PD-1 and PD-L1 ICI as adjuvant treatment in HCC.
Adjuvant Immunotherapy with ICI is currently under investigation in HCC patients who underwent loco-regional treatment and are at high risk of recurrence. Unfortunately, no published randomized trials are yet available.
Nivolumab, an anti-PD-1 monoclonal antibody (mAb), is being assessed in a phase III, multicenter, randomized, double-blind CheckMate 9DX trial (NCT03383458). The study has an estimated enrollment of 530 HCC patients who will randomly receive either nivolumab (arm A) or placebo (arm B) (53).
Pembrolizumab, an anti-PD-1 mAb, is now being studied in a phase III, multicenter, randomized, double-blinded, two-arm study Keynote-937 (NCT03867084). Participants (estimated enrollment: 950 patients) will receive intravenous (IV) pembrolizumab if assigned to arm A, and IV placebo if assigned to arm B.
Durvalumab, an anti-PD-L1 mAb, alone or combined with bevacizumab, is under examination in a phase III, randomized, double-blind, placebo-controlled, multicenter study, EMERALD-2 (NCT03847428), in the same HCC high-risk population of the abovementioned studies. Patients randomized to arm A will receive IV durvalumab plus IV bevacizumab; arm B patients will receive durvalumab plus placebo, and arm C subjects will be assigned two placebos. The estimated enrollment is of 888 participants.
Atezolizumab, an anti-PD-L1 mAb, is under evaluation in association with bevacizumab in phase III, multicenter, randomized, open-label IMbrave050 study (NCT04102098). Patients will be randomly allocated to arm A to receive IV atezolizumab plus IV bevacizumab or to arm B to active surveillance. The study estimates to enroll 662 participants.
Toripalimab, an anti-PD-1 mAb, is under study in a phase II/III, randomized, double-blind, placebo- controlled study, the JUPITER 04 trial (NCT03859128). The estimated 530 participants enrolled will be treated with toripalimab if assigned to arm A, whereas they will not receive it if assigned to arm B.
The primary outcome of these trials is the measure of the recurrence-free survival (RFS), except for Keynote-937, which will consider both RFS and OS. However, it is significant to specify that EMERALD-2 will evaluate only the RFS for arm B versus arm C as primary endpoint, while the RFS for arm A versus arm C represented the secondary endpoint.
Loco-regional treatments in HCC are used in patients with early-stage (0-A BSCL staging) who are not eligible for surgical treatment or transplant, or in patients with advanced-stage (B-C BSCL) not amenable to kinase-inhibitor drugs (Sorafenib or Regorafenib). The most used local procedures are transarterial chemoembolization (TACE) (54–59), radiofrequency ablation (RFA) (60), stereotactic body radiotherapy (SBRT) (61, 62), transarterial radioembolization, and embolization via microspheres loaded with yttrium-90 (Y-90) (63–65).
These loco-regional treatments allow to release a high quantity of tumor antigens through the destruction of the tumor cells. For this reason, the effectiveness of their combination with the ICI has been investigated with encouraging results (66).
The results of two studies are currently available. In the study conducted by Duffy et al., 32 patients were started on tremelimumab therapy at two dose levels every four weeks for six administrations total, then followed by 3-monthly infusions until they matched up off-treatment. On the 36th day, subtotal radiofrequency ablation or chemoablation were performed. Of the 19 evaluable patients, 5 (26,3%) reached a firm partial response. Six-week tumor biopsies displayed an increase in CD8+ T cells in patients who presented a clinical benefit alone. For this refractory HCC population, six and twelve- month probabilities of tumor progression-free survival were 57,1 and 33,1%, respectively, with a median time to tumor progression of 7,4 months. The mOS was 12,3 months (67).
Furthermore, the phase II trial by Zao et al. (NCT03939975) assessed the response of 50 HCC patients who progressed to a first-line with sorafenib and started a second-line treatment with anti-PD1 (pembrolizumab or nivolumab). Of these, 33 patients underwent subtotal thermal ablation because the disease did not progress or had an atypical response to anti-PD-1 inhibitor. Additional ablation ameliorated effectiveness with acceptable toxicity, and the RR rose from 10 to 24% (12/50). The median time to progression (MTP), PFS, and OS was 6.1, 5, and 16.9 months, respectively (68).
Currently, there are several trials underway to evaluate which combination is more useful and could allow us to get the best results in terms of ORR.
The combination of ICI with stereotactic radiotherapy (SBRT) is still under study. In particular, the phase II/III trial NCT04167293 (ISBRT01) is evaluating this type of local treatment in association with sintilimab (a monoclonal antibody anti-PD1) in an advanced stage of HCC. Another study is NCT03380130 (NASIR-HCC), a phase II clinical trial that is investigating nivolumab combination in the same patient settings. While phase II study NCT03316872 is studying SBRT combined with pembrolizumab.
The role of TACE in combined therapy is also under study. In phase II trial IMMUTACE (NCT03572582), the procedure is associated with nivolumab administration in patients affected by intermediate-stage hepatocellular carcinoma. Moreover, in the phase II study TRIPLET (NCT04191889), the association of TACE with apatinib plus camrelizumab is under investigation in patients with C staged HCC, in BCLC classification. Even the phase II trial LEAP-012 (NCT04246177) is evaluating TACE combined with the administration of lenvatinib and pembrolizumab. In addition to the classic TACE (c-TACE), a variant is the drug-eluting bead transarterial chemoembolization (DEB-TACE). This type of procedure is also under investigation in combination with ICI, such as durvalumab and tremelimumab (NCT03638141) or nivolumab (NCT03143270).
A recent phase II study, NCT03259867 (TATE-PD1), involves the use of trans-arterial tirapazamine embolization (TATE) in patients with advanced HCC or other malignancies, simultaneously treated with nivolumab or pembrolizumab. The results of this new procedure are particularly interesting.
Considering radioembolization with yttrium 90 (Y90-RE), the results of a phase II, non-randomized trial (NCT03033446), and analyzing the combination with nivolumab in Asian advanced HCC patients, were recently presented. It enrolled 40 patients with a median follow-up of 16.4 months, and 36 patients were assessed. The combination of nivolumab plus Y90-RE resulted in an encouraging ORR of 31% (95%CI 16,4–48,1%), median PFS of 4.6 months (95%CI 2.3–4.8 months), and mOS of 15.1 months (95%CI 7.8–NE) (69). Furthermore, other trials are currently investigating Y90-RE in combination with nivolumab (NCT02837029) or pembrolizumab (NCT03099564).
In addition to the trials involving a single loco-regional procedure, several combination trials compare different methods. Among these, there is the phase III study NCT03949231 that confronts the hepatic artery infusion with the vein infusion of toripalimab (monoclonal anti-PD1 Ab) in patients with (BCLC) C-stage hepatocellular carcinoma. Furthermore, the phase II study NCT02821754 is estimating differences between chemoembolization (TACE), radiofrequency ablation (RFA), and cryoablation (CA) in patients with HCC and biliary tract cancer treated with tremelimumab and durvalumab. Another comparison study is the phase II trial NCT03753659, in which patients with early HCC received pembrolizumab and then underwent RFA versus Microwave Ablation (MWA).
HCC has a less dense vasculature with abnormal leaky and fragile tumor vessels, which lead to interstitial hypertension, tumor hypoxia, and necrosis (70–74). Hypoxia can, in turn, stimulate the angiogenic process, the tumor growth (71, 73, 75, 76), and may recruit immunosuppressive cells (77). Indeed, there is a complex bidirectional relationship between angiogenesis and immunity (78–88).
In particular, vascular endothelial growth factor (VEGF), in association with other pro-angiogenic determinants in the tumor microenvironment (TME), may down-regulate intercellular adhesion molecule 1 (ICAM-1) or vascular cell adhesion protein 1 (VCAM-1), repress T cell trafficking and dendritic cell (DC) maturation (77, 89). Moreover, the VEGF-A and pro-inflammatory cytokines cause Fas ligand (FasL) expression by tumor endothelial cells that gain the capacity to put CD8+ T cells but not T-reg cells to death (90). VEGF also increases PD-1 expression of tumor-infiltrating CD8+ T-cells (79). Also, PD-L1 expression is strongly dependent on transcriptional regulation of hypoxia-inducible factor 1-alpha (79, 91). Therefore, the blockade of the angiogenesis pathway might modify the immune TME, up-regulating CD8+ T-cells, and down-regulating immunosuppressor cells. That way, ICIs may improve the effectiveness of anti-angiogenic drugs inducing antibody-related cytotoxicity on endothelial cells. As a result, the destruction of the malignancy’s vasculature was obtained (92).
A Phase 1b study evaluated the safety and effectiveness of the association of durvalumab with ramucirumab, an anti-VEGF receptor-2 (VEGFR-2) IgG1 mAb, in different cohorts of advanced pre-treated cancer patients, including one cohort of 28 HCC subjects (NCT02572687). In the HCC cohort, ORR was 11%, but in patients that had “high” PD-L1 expression (≥25% of tumor cells or immune cells) achieved 18%. No significant differences in median PFS were observed accordingly to PD-L1 expression (4.4 in overall patients and 5.6 months in patients with high PD-L1 expression) as well as in mOS (10.7 and 16.5 months, respectively). Hypertension (17.9%), anemia (21.4%), and fatigue (10.7%) were the most frequent 3/4 TRAEs reported. Grade 3/4 TRAEs of interest reported in >5% of patients were hypertension, bleeding events (10.7%), and venous thromboembolic events (7.1%) for ramucirumab and lipase (10.6%) and AST increase (17.9%). Globally, the combination of durvalumab and ramucirumab did not show new safety signals and suggested potential anti-tumor activity, especially in the case of high PD-L1 expression. Further results are expected (93).
A multicenter, open-label, phase I/II dose-escalation and expansion study is assessing the harmlessness and benefit of MGD013, an anti-PD-1/anti-LAG-3 Dual-Affinity Re-Targeting (DART) protein in monotherapy and in combination with brivanib, a selective dual inhibitor of VEGFR and fibroblast growth factor receptors (FGFR) in advanced liver cancer patients (phase I- dose escalation also included intrahepatic cholangiocarcinoma) (NCT04212221).
Most TKIs have a remarkable anti-angiogenic effect through the inhibition of the VEGFRs (70) and have an immune-modulatory role as immune effectors involved in the TME and antigen presentation process (82). The association with ICI opens to the exploration of new treatment combinations to improve the anti-tumor immune response (94, 95).
Sorafenib is a multi-target TKI, approved since 2007 for first-line treatment of HCC, which can block the RAS, VEGFR, platelet-derived growth factor receptor (PDGFR), fms related tyrosine kinase 3 (FLT3), and KIT kinases, inducing apoptosis and blocking cell proliferation, migration, and cancer angiogenesis (96). Among the explored mechanisms of resistance to sorafenib in HCC, Liu et al. reported PD-L1 and DNA methyltransferases contribution (97). Currently, TKI and anti- PD-1 mAbs combination therapies were under study as first-line treatment for advanced HCC. In particular, the association with nivolumab is being assessed in a phase II, multicenter pilot trial in advanced HCC patients not eligible for surgery (NCT03439891). This trial will estimate the maximum tolerated dose, the safety, and ORR of the combination of sorafenib and nivolumab, along with the DOR, PFS, OS, peripheral and tumor immune cell profiling, PD-L1 expression, and alpha-fetoprotein (AFP) response (98).
A phase Ib/II study is evaluating sorafenib and pembrolizumab combination therapy in advanced HCC (NCT03211416). The primary endpoint is RR; secondary endpoints are safety, OS, and PFS. Moreover, the study will compare in blood and cancer samples the pre-treatment quantity of immunosuppressive cells and the functional activity of effector T cells post-treatment (99). Another phase Ib of dose-escalation and dose-expansion study is assessing the safety and tolerability of the combination of sorafenib with spartalizumab, an anti-PD-1 mAb, in advanced HCC (NCT02988440).
Lenvatinib is a small multi-TKI which works against VEGFR-1,-2, and -3, FGFR-1,-2,-3, and -4, PDGFRα, KIT, and (RET), approved on August 2018 by FDA for first-line treatment of unresectable HCC (100). Some ongoing clinical trials are studying its association with ICI.
The association between sorafenib and nivolumab is under evaluation in advanced HCC patients in two trials. In particular, a Japanese phase Ib trial aims to assess the tolerability and safety of this combination. Its secondary endpoints include OS, PFS, ORR, DOR, DCR, TTP, clinical Benefit Rate (CBR), and pharmacokinetics (PK) (NCT03418922). On the other hand, an exploratory, open-label, single-arm, multicenter phase II study evaluates the effectiveness and feasibility (as determined by safety and tolerability) of first-line sorafenib combined with nivolumab in patients with multinodular, advanced stage HCC. Primary endpoints are ORR, safety, and tolerability; secondary endpoints are TTP, PFS, OS, and translational research that consists of correlation of biomarkers potentially associated with clinical efficacy (NCT03841201-IMMUNIB).
Regarding the association of lenvatinib with pembrolizumab, preliminary data from a phase Ib study analyzing this combination in first-line setting for advanced HCC (NCT03006926) reported an ORR of 42.3%, and a median PFS of 9.69 months (95% CI 5.55–not evaluable). The most frequent any-grade TRAEs were decreased appetite and hypertension (53.3% each), diarrhea (43.3%), and fatigue (40%). The most common grade ≥3 TRAEs described were hypertension (16.7%), aspartate aminotransferase (AST) increment (16.7%), neutropenia (13.3%), and hyponatremia (10.0%). Eight patients had severe adverse events (SAEs) (26.7%), and 16.7% discontinued lenvatinib and or pembrolizumab due to TRAEs, but side effects were controlled (101).
Based on these results, the phase III multicenter, randomized, double-blinded, active-controlled, LEAP-002 trial (NCT03713593) is testing the effectiveness and safety of lenvatinib and pembrolizumab combination therapy versus lenvatinib combined with placebo as first-line treatment in advanced HCC Child-Pugh class A patients. This trial estimates to randomize 750 patients approximately. The primary endpoints are OS and PFS, whereas secondary endpoints include ORR, DOR, DCR, TTP, adverse events, and PK (102). Also, a single-arm phase IIb study is assessing lenvatinib and pembrolizumab combination therapy as second-line treatment in patients with unresectable hepatobiliary tumors, including the analysis of potential biomarkers of response (NCT03895970).
Regorafenib is a multi-target TKI that actively suppresses VEGFR-1,-2,-3, PDGFR, TIE-2, fibroblast growth factor receptor 1 (FGFR1), KIT (CD117), RET, and B-Raf (103). It is under evaluation in combination with ICI in two ongoing studies.
A multicenter, non-randomized, open-label, dose-escalation, phase Ib study is assessing the harmlessness and tolerability of the association of regorafenib and pembrolizumab as first-line treatment for patients with advanced HCC (NCT03347292). Moreover, the study aims to explore the anti-tumor activity of this combination and to determine blood/tissue biomarkers related to the tumor activity, status or response.
The REGOMUNE trial (NCT03475953) is a multicenter phase I/II trial which is estimating the combination of regorafenib and avelumab in solid tumors, including HCC, after at least one previous line of systemic therapy. Phase I will establish the recommended phase II dose (RP2D), whereas phase II will assess the efficacy and safety of the drugs combination.
Cabozantinib is a TKI targeting VEGFR-2, c-MET, AXL, RET and FLT-3 (100, 104). One cohort of the Checkmate040 phase I/II trial (NCT01658878) is assessing the potential synergistic activity of cabozantinib combined with nivolumab, with or without ipilimumab, in Child-Pugh A advanced HCC patients; primary endpoints are safety and ORR (29, 105, 106).
A phase Ib, open-label trial will explore the safety, tolerability, preliminary efficacy, and PK of cabozantinib combined with atezolizumab in advanced HCC patients (NCT03170960). In the dose-escalation phase (3 + 3 design), a recommended dose for cabozantinib and atezolizumab combination therapy will be determined. In the expansion phase, 18 cohorts will be recruited at the recommended dose of cabozantinib and atezolizumab, comprising one cohort of advanced systemic-treatment naïve HCC. The primary objective is the ORR for each cohort (107).
The phase III COSMIC-312 trial (NCT03755791) is appraising cabozantinib plus atezolizumab versus sorafenib in the first-line setting in advanced HCC patients, Child-Pugh A. Patients will be randomized in a 2:1:1 ratio to take cabozantinib plus atezolizumab, sorafenib, or single-agent cabozantinib. The study has two primary endpoints: compare OS and PFS for cabozantinib + atezolizumab versus sorafenib; the secondary endpoint is PFS for cabozantinib versus sorafenib (108).
The open-label, single-arm, CAMILLA trial is a phase Ib study of cabozantinib and durvalumab combination therapy in pretreated patients with advanced HCC (NCT03539822). The study intends to examine the safety and tolerability and display preliminary data on effectiveness (109).
Axitinib is a TKI selective for VEGFR-1/2/3. VEGF Liver 100 (NCT03289533) is a Phase Ib study assessing the feasibility of the combination of avelumab plus axitinib in treatment-naive patients with HCC in terms of harmlessness and effectiveness. Provisory results of the analysis showed an ORR of 13.6% based on RECIST 1.1 and 31.8% based on mRECIST criteria. mPFS was 5.5 and 3.8 months, according to RECIST and mRECIST, respectively. Tumor shrinkage was reported in 68.2% of patients by RECIST and 72.7% of patients by mRECIST. OS data were still immature. The most common grade 3 TRAEs were hypertension (50.0%) and hand-foot syndrome (22.7%); no grade 4/5 TRAEs were mentioned. Immune-related AEs (irAEs) occurring in ≥10% of patients were hypothyroidism (31.8%) and hyperthyroidism (13.6%). None of irAEs were grade ≥3. No treatment discontinuations due to TRAEs or irAEs were registered. Thus, safety and efficacy results were promising, but further follow-up is required (110).
Apatinib is an impressive TKI inhibitor of VEGFR-2, c-Kit, c-Src, and PDGFR. An open-label, dose-escalation (phase Ia) and expansion study (phase Ib) evaluated the safety and efficacy of the camrelizumab, an anti-PD-1 mAb, and apatinib combination therapy in advanced HCC patients (NCT02942329). The main goals were harmlessness and tolerability and RP2D determination. A grade 3 TRAE was reported in 60.6%. Hypertension (15.2%) and elevated AST (15.2%) were the most common. Results showed that camrelizumab and apatinib combination had a feasible safety profile and activity against cancer cells in HCC patients (111). The phase II, single-arm, RESCUE study (NCT03463876) is preliminary exploring the efficacy and safety of the combination of apatinib and camrelizumab regimen as second-line treatment in advanced HCC; the primary endpoint is ORR.
Currently, is ongoing a randomized, open-label, international, multicenter, phase III trial of camrelizumab plus apatinib versus sorafenib in first-line setting in patients with unresectable HCC that did not receive systemic treatment in the past (NCT03764293). The co-primary endpoints are OS and PFS.
The MET/HGF pathway stimulate cellular proliferation, survival, and invasion and progression in HCC and has been associated with TKI resistance (112–114). A phase Ib/II, open-label, multicenter study is assessing the association of capmatinib (INC280), a selective oral c-MET recently developed in HCC, and spartalizumab versus spartalizumab single-agent in advanced HCC patients, progressing after sorafenib (NCT02795429).
Another phase I/II dose-escalation, and expansion study is testing bozitinib, a c-MET inhibitor, combined with genolimzumab, an anti-PD-1 mAb, after first-line treatment for locally advanced or unresectable HCC not pretreated with a PD-1 inhibitor or a c-MET inhibitor (NCT03655613).
Another promising approach is represented by the association of ICI with inhibitors of the fibroblast growth factor 19 (FGF19)/FGF receptor 4 (FGFR4) pathway (115). The alteration of the FGF19/FGFR4 signaling is a known driver of HCC carcinogenesis (116). It suppresses E-cadherin expression and promotes the expression of epithelial-to-mesenchymal transition (EMT)-related genes, leading to increased HCC cell invasion. FGF19/FGFR4 axis has been associated with poor prognosis. Moreover, FGF19 expression has been related with early relapse and shorter disease-specific recurrence in a cohort of resected HCC patients and appears implicated in sorafenib resistance (117, 118).
A Phase I/II, multicenter, open-label study is assessing the combination of oral FGF401, an FGFR4 inhibitor, with spartalizumab in refractory HCC patients harboring FGFR4 and KLB (an FGF19 co- receptor) expression and FGF401 as single-agent in other advanced solid tumors. The study is investigating the efficacy as the dose-limiting toxicity to detect the maximum tolerated dose and/or RP2D (NCT02325739).
TGF-β contributes to cell invasion, angiogenesis, EMT, and drug resistance in HCC, as demonstrated by several preclinical findings (119–121). Moreover, TGF-β may induce in vitro FGFR4 expression through the extracellular-signal-regulated kinase (ERK) pathway, and its interaction with FGFR4 promotes the metastatic spread of HCC in vivo (122). TGF-β also plays a critical role in HCC immune-tolerance. Indeed, it is secreted by Kupffer cells and liver sinusoidal endothelial cells, and it can up-regulate the Treg, and recently, Mariathasan et al. reported that TGF-β weakened tumor response to PD-L1 inhibition by contributing to exclude T cells (123–127). For these reasons, a combined approach of the TGF-β pathway and PD-1/PD-L1 inhibitors, or a managing bifunctional fusion proteins targeting both TGF-β and PD-L1, might overcome drug resistance and have a synergistic effect (128–130).
Galunisertib (LY2157299 Monohydrate) is an oral TGF-β receptor-1 (TGF-βR1) inhibitor that showed a favorable safety profile as single-agent or in combination with sorafenib (131). Currently, galunisertib is under investigation in combination with nivolumab in a phase Ib/II (dose escalation and cohort expansion) study in advanced solid tumors, including HCC with AFP ≥200 ng/ml, as second-line treatment. The main goal of this study is to estimate the harmlessness, tolerability, and effectiveness of this drug association (NCT02423343).
A phase I/Ib, open-label, multi-center, dose-escalation ongoing trial is assessing the safety and tolerability of NIS793, a novel anti-TGF-β antibody (Ab) alone or in combination with spartalizumab in advanced refractory solid tumors, including HCC (NCT02947165). The study also aims to identify recommended doses and schedules of these drugs (NIS793: every 2 or every 3 weeks; spartalizumab: every 3 or 4 weeks) for future studies.
Another promising approach for the future might be M7824 (MSB0011359C), an innovative first- in-class bifunctional fusion protein that consists of a human IgG1 anti-PD-L1 mAb (avelumab) fused to the extracellular domain of TGFβ receptor II (TGF-βRII) to act as a TGFβ “trap”. Results of a phase I dose-escalation study with M7824 showed an amenable safety profile in heavily pre- treated patients with advanced solid tumors. Multiple expansion cohorts are ongoing in various tumor types (NCT02517398) (132).
The EACH trial, a randomized, multicenter, open-label study of palliative FOLFOX versus doxorubicin in Asian patients with advanced HCC, has led the China FDA to introduce FOLFOX4 in the clinical practice guideline (PR 8.6%, 38.6% SD, median OS 5.7 months) (133).
It has been reported that oxaliplatin can induce an anti-tumor immune response and immunogenic cell death, more specifically by activation of DCs, the enhancement of cross-priming of CD8- positive (CD8+) T cells, the stimulation of the anti-tumor CD4+ T cells phenotype, and down- regulation of MDSC and T-reg cells. Moreover, oxaliplatin promotes tumor cell death through lytic receptors/pathways, boosted serum inflammatory cytokines, and switch to pro-inflammatory status in the TME (133, 134). A Phase II, non-randomized study is assessing the combination of camrelizumab with apatinib or with chemotherapy in patients with advanced HCC (FOLFOX4) who failed or were unbearable to prior systemic therapy (NCT03092895).
It is well-known that in some patients, due to the lack of tumor-infiltrating effector T cells, checkpoint inhibitors were ineffective. However, cancer vaccines seem to be able to increase effector T-cells infiltration into tumors. Therefore, a strategy combining a cancer vaccine with an immune checkpoint inhibitor may be promising. The synergistic action of the two drugs may lead to an effective antitumor immune response: whilst the vaccine raises the number of tumor-infiltrating effector T cells, the anti-PD-1 makes sure that these cells stay active (135). Hence, clinical trials are warranted.
In the last few years, several studies evaluated new drug combinations (134, 136). These new therapeutic approaches could soon make a difference.
As for the adjuvant setting, there are no available data up to now, but there are several phase III trials ongoing on various immunocheckpoint inhibitors. We will look forward to the results of these studies, which would seem to prospect the best disease control rate. If data will be statistically significant, we will make a relevant step forward. Anyhow, for now, in the localized HCC, surgery represents the standard of care (Table 1).
Regarding the combination of locoregional treatments and immunocheckpoint inhibitors, several phase II trials are underway. There is only a phase III trial on Toriliplimab, but no data is available yet. The unique existing data are related to a small cohort. Thus, the results are not reliable (Table 2).
Nonetheless, the available evidence suggests that combining systemic therapies and locoregional treatments with immune checkpoint inhibitors may represent a useful strategy in this context.
In the advanced HCC, thanks to the improvement of OS, PFS, and QoL achieved by the phase III IMbrave150 trial, the FDA approved atezolizumab + bevacizumab as first-line therapy in this setting (26).
Another drug that seems to be promising is tremelimumab, but we are looking forward to the phase III Himalaya trial results. This trial is assessing the combination of tremelimumab + durvalumab.
As for anti-PD-1, nivolumab and pembrolizumab, there are controversial results. Based on the results of phase II trials (CheckMate 040 and Keynote 224), the FDA approved nivolumab and pembrolizumab for advanced HCC. However, the phase III trials (CheckMate 459 and Keynote 240) did not match up to their primary endpoints of OS and PFS. Nonetheless, there are some aspects to take into consideration. CheckMate 040 was a non-comparative study on advanced HCC patients not all pre-treated with Sorafenib. On the other hand, CheckMate 459 compared Nivolumab with Sorafenib in the first-line setting. Although the design of the studies was different, phase III data were interesting thanks to the best tolerability of the drug in the patients, along with a positive trend in terms of response rate and overall survival. Likewise, the Keynote 224 examined the use of Pembrolizumab in 104 advanced HCC patients pre-treated with Sorafenib, whereas the Keynote 240 analyzed pembrolizumab vs placebo in 413 patients as second line treatment. Maybe a first-line setting could have different outcomes or maybe an enlarged sample of patients might have led to different results. Even so, the patients did not suffer the side effects as well as an improvement in survival and response rate. Therefore, taking in consideration the QoL of the patients the approval of these drugs was considerate.
Also, due to the promising results of the combination of nivolumab + ipilimumab, analyzed in cohort 4 in phase II CheckMate 040 trial, the FDA approved them for usage in clinical practice.
No phase III trials are ongoing, so they are warranted (Tables 3 and 4).
Many studies are analyzing the combination of ICI + TKI in the first-line in the metastatic setting. A few of them are phase III trials such as the LEAP-002 trial that is evaluating lenvatinib + pembrolizumab versus placebo, whereas the COSMIC-312 trial is assessing cabozantinib + atezolizumab versus sorafenib as the NCT03764293 trial camrelizumab + apatinib versus sorafenib. Their results were awaited. Other combinations of ICI with target therapies as C-Met, FGFR, and TGF-β, are understudy for the second-line in advanced HCC. However, they are still phase I or II trials. For sure, these emerging combinations represent the most promising therapies so far, on which we could rely more in the future.
Also, a combination of chemotherapy, oxaliplatin, and ICI is evaluating in phase II trials based on the role that oxaliplatin plays in promoting the action of immunotherapy.
However, it appears clear that we should opt for combining therapies over a single-agent treatment to overcome the drug-resistance. Nevertheless, in order to tailor a therapy that fits the single patient perfectly, we need to determine some specified biomarkers.
In conclusion, given the encouraging results emerging from the preliminary data of some phase I-II trials, and waiting for the results of the ongoing studies, it is possible to hope that some agents can be successfully combined in the second-line as well as in the first-line. Indeed, these new promising therapeutic options may soon change the clinical practice. Nonetheless, other clinical trials are needed to define a better treatment sequence.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Iñarrairaegui M, Melero I, Sangro B. Immunotherapy of Hepatocellular Carcinoma: Facts and Hopes. Clin Cancer Res (2018) 24(7):1518–24. doi: 10.1158/1078-0432
3. Heinrich B, Czauderna C, Marguardt JU. Immunotherapy of Hepatocellular Carcinoma. Oncol Res Treat (2018) 41(5):292–7. doi: 10.1159/000488916
4. Brown ZJ, Greten TF. Immune Therapies. In: Hoshida Y, editor. Hepatocellular carcinoma: Translational Precision Medicine Approaches (2019). doi: 10.1007/978-3-030-21540-8_12
5. Cariani E, Missale G. Immune landscape of hepatocellular carcinoma microenvironment: Implications for prognosis and therapeutic applications. Liver Int (2019) 39(9):1608–21. doi: 10.1111/liv.14192
6. Lee HW, Cho KJ, Park JY. Current status and future direction of Immunotherapy in Hepatocellualr Carcinoma: What do the data suggest? Immune Netw (2020) 20(1):e11. doi: 10.4110//in.2020.20.e11
7. Prieto J, Melero I, Sangro B. Immunological landscape and Immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol (2015) 12(12):681–700. doi: 10.1038/nrgastro.2015.173
8. Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol (2018) 15(10):599–616. doi: 10.138/s41571-018-0073-4
9. D’Anzeio M, Faloppi L, Scartozzi M, Giampieri R, Bianconi M, Del Prete M, et al. The role of micro-RNAs in hepatocellular carcinoma: from molecular biology to treatment. Molecules (2014) 19(5):6393–406. doi: 10.3390/molecules19056393
10. De Matteis S, Granato AM, Napolitano R, Molinari C, Valgiusti M, Santini D, et al. Interplay Between SIRT-3, Metabolism and Its Tumor Suppressor Role in Hepatocellular Carcinoma. Dig Dis Sci (2017) 62(8):1872–80. doi: 10.1007/s10620-017-4615-x
11. Casadei Gardini A, Marisi G, Scarpi E, Scartozzi M, Faloppi L, Silvestris N, et al. Effects of metformin on clinical outcome in diabetic patients with advanced HCC receiving sorafenib. Expert Opin Pharmacother (2015) 16(18):2719–25. doi: 10.1517/14656566.2015.1102887
12. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
13. Marisi G, Cucchetti A, Ulivi P, Canale M, Cabibbo G, Solaini L, et al. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J Gastroenterol (2018) 24(36):4152–63. doi: 10.3748/wjg.v24.i36.4152
14. Marisi G, Petracci E, Raimondi F, Faloppi L, Foschi FG, Lauletta G, et al. ANGPT2 and NOS3 Polymorphisms and Clinical Outcome in Advanced Hepatocellular Carcinoma Patients Receiving Sorafenib. Cancers (Basel) (2019) 11(7):1023. doi: 10.3390/cancers11071023
15. Rovesti G, Orsi G, Kalliopi A, Vivaldi C, Marisi G, Faloppi L, et al. Impact of Baseline Characteristics on the Overall Survival of HCC Patients Treated with Sorafenib:Ten Years of Experience. Gastrointest Tumors (2019) 6(3-4):92–107. doi: 10.1159/000502714
16. Casadei Gardini A, Faloppi L, De Matteis S, Foschi FG, Silvestris N, Tovoli F, et al. Metformin and insulin impact on clinical outcome in patients with advanced hepatocellular carcinoma receiving sorafenib: Validation study and biological rationale. Eur J Cancer (2017) 86:106–14. doi: 10.1016/j.ejca.2017.09.003
17. Casadei Gardini A, Solaini L, Riggi L, Molinaro E, Dadduzio V, Rizzato MD, et al. Prognostic Role of a New Index (RAPID Index) in Advanced Hepatocellular Carcinoma Patients Receiving Sorafenib: Training and Validation Cohort. Gastrointest Tumors (2019) 6(3-4):71–80. doi: 10.1159/000501593
18. Faloppi L, Scartozzi M, Maccaroin E, Di Pietro Paolo M, Berardi R, Del Prete M, et al. Evolving strategies for the treatment of hepatocellular carcinoma: from clinical-guided to molecularly-tailored therapeutic options. Cancer Treat Rev (2011) 37(3):169–77. doi: 10.1016/j.ctrv.2010.08.001
19. Caputo F, Dadduzio V, Tovoli F, Bertolini G, Cabibbo G, Crema K, et al. The role of PNI to predict survival in advanced hepatocellular carcinoma treated with Sorafenib. PloS One (2020) 15(5):e0232449. doi: 10.1371/journal.pone.0232449
20. Di Costanzo GG, Casadei Gardini A, Marisi G, Foschi FG, Scartozzi M, Granata R, et al. Validation of a Simple Scoring System to Predict Sorafenib Effectiveness in Patients with Hepatocellular Carcinoma. Target Oncol (2017) 12(6):795–803. doi: 10.1007/s11523-017-0522-5
21. Casadei Gardini A, Scarpi E, Marisi G, Foschi FG, Donati G, Giampalma E, et al. Early onset of hypertension and serum electrolyte changes as potential predictive factors of activity in advanced HCC patients treated with sorafenib: results from a retrospective analysis of the HCC-AVR group. Oncotarget (2016) 7(12):15243–51. doi: 10.18632/oncotarget.7444
22. Casadei Gardini A, Dadduzio V, Rovesti G, Cabibbo G, Vukotic R, Rizzato MD, et al. Utility of neutrophil-to-lymphocyte ratio to identify long-term survivors among HCC patients treated with sorafenib. Med (Baltimore) (2020) 99(22):e19958. doi: 10.1097/MD.0000000000019958
23. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. A Randomised Phase 3 trial of lenvatinib vs. sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma. Lancet (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
24. Casadei Gardini A, Puzzoni M, Montagnani F, Marisi G, Tamburini E, Cucchetti A, et al. Profile of lenvatinib in the treatment of hepatocellular carcinoma: design, development, potential place in therapy and network meta-analysis of hepatitis B and hepatitis C in all Phase III trials. Onco Targets Ther (2019) 12:2981–8. doi: 10.2147/OTT.S192572
25. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2017) 389:56–66. doi: 10.1016/S0140-6736(16)32453-9
26. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med (2018) 379:54–63. doi: 10.1056/NEJMoa1717002
27. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. REACH-2: A randomized, double-blind, placebo-controlled phase 3 study of ramucirumab versus placebo as second- line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated baseline alpha-fetoprotein (AFP) following first-line sorafeib. J Clin Oncol (2018) 36(15_suppl):4003–3. doi: 10.1200/JCO.2018.36.15_suppl.4003
28. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol (2019) 30(SUPPLEMENT 5):v874-5 doi: 10.1093/annonc/mdz394.029
29. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non- comparative, phase 1/2 dose escalation and expansion trial. Lancet (2017) 389(10088):2492–502. doi: 10.1016/S0140-6736(17)31046-2
30. He AR, Yau T, Hsu C, Kang YK, Kim TY, Santoro A, et al. Nivolumab + ipilimumab combination therapy in patients with advanced hepatocellular carcinoma: subgroup analysis from CheckMate 040. J Clin Oncol (2020) 38(4_suppl):512–2. doi: 10.1200/JCO.2020.38.4_suppl.512
31. Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol (2013) 59:81–8. doi: 10.1016/j.jhep.2013.02.022
32. Kelley RK, Kudo M, Harris W, Ikeda M, Okusaka T, Kang Y, et al. (2020). The novel regimen of tremelimumab in combination with durvalumab provides a favorable safety profile and clinical activity for patients with advanced hepatocellular carcinoma (aHCC). Ann Onc (2020) 31(Supplement 3):233–4. doi: 10.1016/j.annonc.2020.04.059
33. Abou-Alfa GK, Chan SL, Furuse J, Galle PR, Kelley RK, Qin S, et al. A randomized, multicenter phase 3 study of durvalumab (D) and tremelimumab (T) as first-line treatment in patients with unresectable hepatocellular carcinoma (HCC): HIMALAYA study. J Clin Oncol (2018) 36:TPS4144–TPS4144. doi: 10.1200/JCO.2018.36
34. LBA3 -, Sangro B, Park J, Finn R, Cheng A, Mathurin P, et al. CheckMate 459: Long-term (minimum follow-up months) survival outcomes with nivolumab versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. Ann Onc (2020) 31:S241–2. doi: 10.1016/j.annonc.2020.04.078
35. Finn RS, Ryoo B-Y, Merle P, Kudo M, Bouattour M, Lim H-Y, et al. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol (2019) 37:4004. doi: 10.1200/JCO.2019.37.15_suppl.4004
36. Lee M, Ryoo B, Hsu C, Numata K, Stein S, Verret W, et al. Randomised Efficacy And Safety Results For Atezolizumab (Atezo) + Bevacizumab (Bev) In Patients (Pts) With Previously Untreated, Unresectable Hepatocellular Carcinoma (Hcc). Ann OF Oncol (2019) 30(SUPPL_5):V851–934. doi: 10.1093/ANNONC/MDZ394
37. Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, et al. Patient-reported outcomes from the phase III IMbrave150 trial of atezolizumab plus bevacizumab vs sorafenib as first- line treatment for patients with unresectable hepatocellular carcinoma. Gastrointest Cancer Symposium (2020) 38:476. doi: 10.1200/JCO.2020.38.4_suppl.476
38. LBA3-, Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, et al. Efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol (2019) 30(suppl_9):ix183–ix202. doi: 10.1093/annonc/mdz446
39. Canale M, Ulivi P, Foschi FG, Scarpi E, De Matteis S, Donati G, et al. Clinical and circulating biomarkers of survival and recurrence after radiofrequency ablation in patients with hepatocellular carcinoma. Crit Rev Oncol Hematol (2018) 129:44–53. doi: 10.1016/j.critrevonc.2018.06.017
40. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol (2018) 69:182–236. doi: 10.1016/j.jhep.2018.03.019
41. Wang J, He XD, Yao N, Liang WJ, Zhang YC. A meta-analysis of adjuvant therapy after potentially curative treatment for hepatocellular carcinoma. Can J Gastroenterol (2013) 27(6):351–63. doi: 10.1155/2013/417894
42. Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol (2015) 16(13):1344–54. doi: 10.1016/S1470-2045(15)00198-9
43. Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol (2003) 3:51–62. doi: 10.1038/nri981
44. Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology (2008) 47(1):296–305. doi: 10.1002/hep.21965
45. Gehring AJ, Ho ZZ, Tan AT, Aung MO, Lee KH, Tan KC, et al. Profile of tumor antigen- specific CD8 T-cells in patients with hepatitis B virus-related hepatocellular carcinoma. Gastroenterology (2009) 137:682–90. doi: 10.1053/j.gastro.2009.04.045
46. Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology (2016) 64(6):2038–46. doi: 10.1002/hep.28710
47. Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer (2011) 128(4):887–96. doi: 10.1002/ijc.25397
48. Chang H, Jung W, Kim A, Kim HK, Kim WB, Kim JH, et al. Expression and prognostic significance of programmed death protein 1 and programmed death ligand-1, and cytotoxic T lymphocyte-associated molecule-4 in hepatocellular carcinoma. APMIS (2017) 125(8):690–8. doi: 10.1111/apm.12703
49. Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res (2009) 15(3):971–9. doi: 10.1158/1078-0432.CCR-08-1608
50. Gu X, Gao XS, Xiong W, Guo W, Han L, Bai Y, et al. Increased programmed death ligand-1 expression predicts poor prognosis in hepatocellular carcinoma patients. OncoTargets Ther (2016) 9:4805–13. doi: 10.2147/OTT.S110713
51. Dai X, Xue J, Hu J, Yang SL, Chen GG, Lai PBS, et al. Positive expression of programmed death ligand 1 in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. Transl Oncol (2017) 10:511–7. doi: 10.1016/j.tranon.2017.03.009
52. Finkelmeier F, Canli Ö, Tal A, Pleli T, Trojan J, Schmidt M, et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer (2016) 59:152–9. doi: 10.1016/j.ejca.2016.03.002
53. Jimenez Exposito MJ, Akce M, Montero Alvarez JL, Assenat E, Balart LA, Baron AD, et al. 783TiP CA209-9DX: phase III, randomized, double-blind study of adjuvant nivolumab vs placebo for patients with hepatocellular carcinoma (HCC) at high risk of recurrence after curative resection or ablation. Ann Oncol (2018) 29(suppl_8):viii205–viii270. doi: 10.1093/annonc/mdy282
54. Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology (2002) 224(1):47–54. doi: 10.1148/radiol.2241011262
55. Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet (2002) 359(9319):1734–9. doi: 10.1016/S0140-6736(02)08649-X
56. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology (2003) 37(2):429–42. doi: 10.1053/jhep.2003.50047
57. Faloppi L, Bianconi M, Memeo R, Casadei Gardini A, Giampieri R, Bittoni A, et al. Lactate Dehydrogenase in Hepatocellular Carcinoma: Something Old, Something New. BioMed Res Int (2016) 2016:7196280. doi: 10.1155/2016/7196280
58. Scartozzi M, Faloppi L, Bianconi M, Giampieri R, Maccaroni E, Bittoni A, et al. The role of LDH serum levels in predicting global outcome in HCC patients undergoing TACE: implications for clinical management. PloS One (2012) 7(3):e32653. doi: 10.1371/journal.pone.0032653
59. Scartozzi M, Svegliati Baroni G, Faloppi L, Di Pietro Paolo M, Pierantoni C, Candelari R, et al. Trans-arterial chemo-embolization (TACE), with either lipiodol (traditional TACE) or drug-eluting microspheres (precision TACE, pTACE) in the treatment of hepatocellular carcinoma: efficacy and safety results from a large mono-institutional analysis. J Exp Clin Cancer Res (2010) 29(1):164. doi: 10.1186/1756-9966-29-164
60. Gerum S, Jensen AD, Roeder F. Stereotactic body radiation therapy in patients with hepatocellular carcinoma: A mini-review. World J Gastrointest Oncol (2019) 11(5):367–76. doi: 10.4251/wjgo.v11.i5.367
61. Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg (2010) 252(6):903–12. doi: 10.1097/SLA.0b013e3181efc656
62. Shen A, Zhang H, Tang C, Chen Y, Wang Y, Zhang C, et al. Systematic review of radiofrequency ablation versus percutaneous ethanol injection for small hepatocellular carcinoma up to 3 cm. J Gastroenterol Hepatol (2013) 28(5):793–800. doi: 10.1111/jgh.12162
63. Morgan B, Kennedy AS, Lewington V, Jones B, Sharma RA. Intra-arterial brachytherapy of hepatic malignancies: watch the flow. Nat Rev Clin Oncol (2011) 8(2):115–20. doi: 10.1038/nrclinonc.2010.153
64. Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Nemcek AA Jr, Kulik L, et al. Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Intervent Radiol (2007) 30(4):571–92. doi: 10.1007/s00270-007-9064-z
65. Salem R, Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Ibrahim S, et al. Technical aspects of radioembolization with 90Y microspheres. Tech Vasc Interv Radiol (2007) 10(1):12–29. doi: 10.1053/j.tvir.2007.08.001
66. Slovak R, Ludwig JM, Gettinger SN, Herbst RS, Kim HS. Immuno-thermal ablations- boosting the anticancer immune response. J Immunother Cancer (2017) 5(1):78. doi: 10.1186/s40425-017-0284-8
67. Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol (2017) 66(3):545–51. doi: 10.1016/j.jhep.2016.10.029
68. Zhao M, Huang J, Lyu N, Kong Y, Mu L, Lin Y. Local thermal ablation reboots the response in advanced hepatocellular carcinoma with stable or atypical progressive diseases during anti-PD-1 therapy. Ann Oncol (2019) 30(suppl_11):xi33–47. doi: 10.1093/annonc/mdz451
69. Tai D, Loke K, Gogna A, Tan SH, Ng DCE, Hennedige TP, et al. A phase II open-label, single-center, nonrandomized trial of Y90-radioembolization in combination with nivolumab in Asian patients with advanced hepatocellular carcinoma: CA 209-678. J Clin Oncol (2020) 38(15_suppl):4590–0. doi: 10.1200/JCO.2020.38.15_suppl.4590
70. Liu K, Zhang X, Xu W, Chen J, Yu J, Gamble JR, et al. Targeting the vasculature in hepatocellular carcinoma treatment: Starving versus normalizing blood supply. Clin Transl Gastroenterol (2017) 8:e98. doi: 10.1038/ctg.2017.28
71. Yang ZF, Poon RTP. Vascular changes in hepatocellular carcinoma. Anat Rec (2008) 291:721–34. doi: 10.1002/ar.20668
72. De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer (2017) 17:457–74. doi: 10.1038/nrc.2017.51
73. Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, et al. Control of the immune response by pro-angiogenic factors. Front Oncol (2014) 4:70. doi: 10.3389/fonc.2014.00070
74. Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med (2001) 7:987–9. doi: 10.1038/nm0901-987
75. Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, et al. Hypoxia- inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA (1997) 94:8104–9. doi: 10.1073/pnas.94.15.8104
76. Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, et al. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin Cancer Res (2019) 25:912–20. doi: 10.1158/1078-0432.CCR-18-1254
77. Kwilas AR, Donahue RN, Tsang KY, Hodge JW. Immune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapy. Cancer Cell Microenviron (2015) 2(01):e677. doi: 10.14800/ccm.677
78. Schaaf MB, Garg AD, Agostinis P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis (2018) 9:115. doi: 10.1038/s41419-017-0061-0
79. Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis (2017) 20(02):185–204. doi: 10.1007/s10456-017-9552-y
80. De Matteis S, Scarpi E, Granato AM, Vespasiani-Gentilucci U, La Barba G, Foschi FG, et al. Role of SIRT-3, p-mTOR and HIF-1α in Hepatocellular Carcinoma Patients Affected by Metabolic Dysfunctions and in Chronic Treatment with Metformin. Int J Mol Sci (2019) 20(6):1503. doi: 10.3390/ijms20061503
81. Gnoni A, Santini D, Scartozzi M, Russo A, Licchetta A, Palmieri V, et al. Hepatocellular carcinoma treatment over sorafenib: epigenetics, microRNAs and microenvironment. Is there a light at the end of the tunnel? Expert Opin Ther Targets (2015) 19(12):1623–35. doi: 10.1517/14728222.2015.1071354
82. Casadei Gardini A, Santini D, Aprile G, Silvestris N, Felli E, Foschi FG, et al. Antiangiogenic agents after first line and sorafenib plus chemoembolization: a systematic review. Oncotarget (2017) 8(39):66699–708. doi: 10.18632/oncotarget.19449
83. Casadei Gardini A, Faloppi L, Aprile G, Brunetti O, Caparello C, Corbelli J, et al. Multicenter prospective study of angiogenesis polymorphism validation in HCC patients treated with sorafenib. An INNOVATE study protocol. Tumori (2018) 104(6):476–9. doi: 10.5301/tj.5000704
84. Casadei Gardini A, Marisi G, Faloppi L, Scarpi E, Foschi FG, Iavarone M, et al. eNOS polymorphisms and clinical outcome in advanced HCC patients receiving sorafenib: final results of the ePHAS study. Oncotarget (2016) 7(19):27988–99. doi: 10.18632/oncotarget.8569
85. Faloppi L, Puzzoni M, Casadei Gardini A, Silvestris N, Masi G, Marisi G, et al. Angiogenesis Genotyping and Clinical Outcomes in Patients with Advanced Hepatocellular Carcinoma Receiving Sorafenib: The ALICE-2 Study. Target Oncol (2020) 15(1):115–26. doi: 10.1007/s11523-020-00698-x
86. Scartozzi M, Faloppi L, Svegliati Baroni G, Loretelli C, Piscaglia F, Iavarone M, et al. VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving sorafenib: the ALICE-1 study. Int J Cancer (2014) 135(5):1247–56. doi: 10.1002/ijc.28772
87. Faloppi L, Scartozzi M, Bianconi M, Svegliati Baroni G, Toniutto P, Giampieri R, et al. The role of LDH serum levels in predicting global outcome in HCC patients treated with sorafenib: implications for clinical management. BMC Cancer (2014) 14:110. doi: 10.1186/1471-2407-14-110
88. Casadei Gardini A, Marisi G, Dadduzio V, Gramantieri L, Faloppi L, Ulivi P, et al. Association of NOS3 and ANGPT2 Gene Polymorphisms with Survival in Patients with Hepatocellular Carcinoma Receiving Sorafenib: Results of the Multicenter Prospective INNOVATE Study. Clin Cancer Res (2020) 26:4485–93. doi: 10.1158/1078-0432.CCR-19-3897
89. Suzuki H, Onishi H, Wada J, Yamasaki A, Tanaka H, Nakano K, et al. VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur J Immunol (2010) 40(1):197–203. doi: 10.1002/eji.200939887
90. Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med (2014) 20(6):607–15. doi: 10.1038/nm.3541
91. Chen J, Jiang C, Jin L, Zhang X. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol (2015) 27:409–16. doi: 10.1093/annonc/mdv615
92. Khan KA, Kerbel RS. Improving immunotherapy outcomes with antiangiogenic treatments and vice versa. Nat Rev Clin Oncol (2018) 15(5):310–24. doi: 10.1038/nrclinonc.2018.9
93. Bang YJ, Golan T, Lin CC, Dahan L, Fu S, Moreno V, et al. Ramucirumab (Ram) and durvalumab (Durva) treatment of metastatic non-small cell lung cancer (NSCLC), gastric/gastroesophageal junction (G/GEJ) adenocarcinoma, and hepatocellular carcinoma (HCC) following progression on systemic treatment(s). J Clin Oncol (2019) 37(15_suppl):2528–8. doi: 10.1200/JCO.2019.37.15_suppl.2528
94. Lin YY, Tan CT, Chen CW, Ou DL, Cheng AL, Hsu C. Immunomodulatory Effects of Current Targeted Therapies on Hepatocellular Carcinoma: Implication for the Future of immunotherapy. Semin Liver Dis (2018) 38(4):379–88. doi: 10.1055/s-0038-1673621
95. Harding JJ, El Dika I, Abou-Alfa GK. Immunotherapy in hepatocellular carcinoma: Primed to make a difference? Cancer (2016) 122:367–77. doi: 10.1002/cncr.29769
96. Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, et al. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat Rev Drug Discovery (2006) 5:835–44. doi: 10.1038/nrd2130
97. Liu J, Liu Y, Meng L, Liu K, Ji B. Targeting the PDL1/DNMT1 axis in acquired resistance to sorafenib in human hepatocellular carcinoma. Oncol Rep (2017) 38:899–907. doi: 10.3892/or.2017.5722
98. Keenan B, Griffith MJ, Bauer K, Bracci PM, Behr S, Umetsu SE, et al. Phase II multicenter pilot study of safety, efficacy, and immune cell profiling in advanced hepatocellular carcinoma (HCC) on combination of sorafenib (SOR) plus nivolumab (NIVO). J Clin Oncol (2019) 37(4_suppl):464. doi: 10.1200/JCO.2019.37.4_suppl.TPS464
99. Gosain R, Mukherjee S, Lee SS, Miller A, Minderman H, Maguire O, et al. Phase Ib/II study of sorafenib (SOR) and pembrolizumab (PEM) in advanced hepatocellular cancer (HCC). J Clin Oncol (2020) 38(4_suppl):596. doi: 10.1200/JCO.2020.38.4_suppl.TPS596
100. Mossenta M, Busato D, Baboci L, Di Cintio F, Tooli G, Dal Bo M. New Insight into Therapies Targeting Angiogenesis in Hepatocellular Carcinoma. Cancers (Basel) (2019) 11(8):1086. doi: 10.3390/cancers11081086
101. Ikeda M, Sung MW, Kudo M, Kobayashi M, Baron AD, Finn RS, et al. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients (pts) with unresectable hepatocellular carcinoma (uHCC). J Clin Oncol (2018) 36(15_suppl):4076–6. doi: 10.1200/JCO.2018.36.15_suppl.4076
102. Llovet JM, Kudo M, Cheng AL, Finn RS, Galle PR, Kaneko S, et al. Lenvatinib (len) plus pembrolizumab (pembro) for the first-line treatment of patients (pts) with advanced hepatocellular carcinoma (HCC): Phase 3 LEAP-002 study. J Clin Oncol (2019) 37(15_suppl):4152. doi: 10.1200/JCO.2019.37.15_suppl.TPS4152
103. Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, et al. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer (2011) 129:245–55. doi: 10.1002/ijc.25864
104. Zhu XD, Sun HC. Emerging agents and regimens for hepatocellular carcinoma. Zhu Sun J Hematol Oncol (2019) 12:110. doi: 10.1186/s13045-019-0794-6
105. Kudo M, Matilla A, Santoro A, Melero I, Gracian AC, Acosta-Rivera M, et al. Checkmate- 040: Nivolumab (NIVO) in patients (pts) with advanced hepatocellular carcinoma (aHCC) and Child-Pugh B (CPB) status. J Clin Oncol (2019) 37(4_suppl):327–7. doi: 10.1200/JCO.2019.37.4_suppl.327
106. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. J Clin Oncol (2019) 37(15_suppl):4012–2. doi: 10.1200/JCO.2019.37.15_suppl.4012
107. Spencer KR, Ramsingh G, Mohamed N, Pal SK, Rimassa L. Phase Ib trial of cabozantinib (C) in combination with atezolizumab (A) in patients (pts) with advanced hepatocellular carcinoma (HCC), gastric or gastroesophageal junction cancer (GC/GEJC), or colorectal cancer (CRC). J Clin Oncol (2019) 37:478. doi: 10.1200/JCO.2019.37.4_suppl.TPS478
108. Kelley RK, Cheng AL, Braiteh FS, Park JW, Benzaghou F, Milwee S, et al. Phase 3 (COSMIC-312) study of cabozantinib (C) in combination with atezolizumab (A) versus sorafenib (S) in patients (pts) with advanced hepatocellular carcinoma (aHCC) who have not received previous systemic anticancer therapy. J Clin Oncol (2019) 37:4157. doi: 10.1200/JCO.2019.37.15_suppl.TPS4157
109. Saeed A, Koestler D, Williamson SK, Baranda JC, Sun W, Al-Rajabi RMT, et al. A phase Ib trial of cabozantinib in combination with durvalumab (MEDI4736) in previously treated patients with advanced gastroesophageal cancer and other gastrointestinal (GI) malignancies (CAMILLA). J Clin Oncol (2019) 37(8_suppl):56. doi: 10.1200/JCO.2019.37.8_suppl.TPS56
110. Kudo M, Motomura K, Wada Y, Inaba Y, Sakamoto Y, Kurosaki M, et al. First-line avelumab+ axitinib in patients with advanced hepatocellular carcinoma: Results from a phase 1b trial (VEGF Liver 100). J Clin Oncol (2019) 37(15_suppl):4072–2. doi: 10.1200/JCO.2019.37.15_suppl.4072
111. Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, et al. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin Cancer Res (2019) 25(2):515–23. doi: 10.1158/1078-0432.CCR-18-2484
112. Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer (2006) 6:637–45. doi: 10.1038/nrc1912
113. Vejchapipat P, Tangkijvanich P, Theamboonlers A, Chongsrisawat V, Chittmittrapap S, Poovorawan Y. Association between serum hepatocyte growth factor and survival in untreated hepatocellular carcinoma. J Gastroenterol (2004) 39:1182–8. doi: 10.1007/s00535-004-1469-8
114. Chen J, Jin R, Zhao J, Liu J, Ying H, Yan H, et al. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett (2015) 367:1–11. doi: 10.1016/j.canlet.2015.06.019
115. Raja A, Park I, Haq F, Ahn SM. FGF19-FGFR4 signaling in hepatocellular carcinoma. Cells (2019) 8:E536. doi: 10.3390/cells8060536
116. Sawey ET, Chanrion M, Cai C, Wu G, Zhang J, Zender L, et al. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell (2011) 19(3):347–58. doi: 10.1016/j.ccr.2011.01.040
117. Kang HJ, Haq F, Sung CO, Choi J, Hong SM, Eo SH, et al. Characterization of hepatocellular carcinoma patients with FGF19 amplification assessed by fluorescence in situ hybridization: a large cohort study. Liver Cancer (2019) 8(1):12–23. doi: 10.1159/000488541
118. Hyeon J, Ahn S, Lee JJ, Song DH, Park CK. Expression of fibroblast growth factor 19 is associated with recurrence and poor prognosis of hepatocellular carcinoma. Dig Dis Sci (2013) 58(7):1916–22. doi: 10.1007/s10620-013-2609-x
119. Gao L, Shay C, Lv F, Wang X, Teng Y. Implications of FGF19 on sorafenib-mediated nitric oxide production in hepatocellular carcinoma cells – a short report. Cell Oncol (Dordr) (2018) 41(1):85–91. doi: 10.1007/s13402-017-0354-4
120. Pickup M, Novitskiy S, Moses HL. The roles of TGFb in the tumour microenvironment. Nat Rev Cancer (2013) 13:788–99. doi: 10.1038/nrc3603
121. Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S, Raymond E, et al. Targeting the TGFb pathway for cancer therapy. Pharmacol Ther (2015) 147:22–31. doi: 10.1016/j.pharmthera.2014.11.001
122. Koudelkova P, Costina V, Weber G, Dooley S, Findeisen P, Winter P, et al. Transforming growth factor-b drives the transendothelial migration of hepatocellular carcinoma cells. Int J Mol Sci (2017) 18:E2119. doi: 10.3390/ijms18102119
123. Huang J, Qiu M, Wan L, Wang G, Huang T, Chen Z, et al. TGF-b1 promotes hepatocellular carcinoma invasion and metastasis via ERK pathwaymediated FGFR4 expression. Cell Physiol Biochem (2018) 45:1690–9. doi: 10.1159/000487737
124. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcrip¬tion factor Foxp3. Science (2003) 299:1057–61. doi: 10.1126/science.1079490
125. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol (2012) 30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623
126. Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell (2006) 10:99–111. doi: 10.1016/j.ccr.2006.06.016
127. Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFb attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature (2018) 554:544–8. doi: 10.1038/nature25501
128. Lan Y, Zhang D, Xu C, Hance KW, Marelli B, Qi J, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-b. Sci Transl Med (2018) 10:eaan5488. doi: 10.1126/scitranslmed.aan5488
129. Strauss J, Heery CR, Schlom J, Madan RA, Cao L, Kang Z, et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFb, in advanced solid tumors. Clin Cancer Res (2018) 24(6):1287–95. doi: 10.1158/1078-0432.CCR-17-2653
130. De Gramont A, Faivre S, Raymond E. Novel TGF-b inhibitors ready for prime time in onco-immunology. Oncoimmunology (2016) 6:e1257453. doi: 10.1080/2162402X.2016.1257453
131. Yingling JM, McMillen WT, Yan L, Huang H, Sawyer JS, Graff J, et al. Preclinical assessment of galunisertib (LY2157299 monohydrate), a first-in-class transforming growth factor-b receptor type I inhibitor. Oncotarget (2017) 9:6659–77. doi: 10.18632/oncotarget.23795
132. Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol (2013) 31:3501–8. doi: 10.1200/JCO.2012.44.5643
133. Garg AD, Dudek-Peric AM, Romano E, Agostinis P. Immunogenic cell death. Int J Dev Biol (2015) 59:131–40. doi: 10.1387/ijdb.150061pa
134. Liu X, Qin S. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: Opportunities and Challenges. Oncologist (2019) 24(Suppl 1):S3–S10. doi: 10.1634/theoncologist.2019-IO-S1-s01
135. Lai X, Friedman A. Combination therapy of cancer with cancer vaccine and immune checkpoint inhibitors: A mathematical model. PloS One (2017) 12(5):e0178479. doi: 10.1371/journal.pone.0178479
Keywords: Hepatocellular carcinoma, immune checkpoint inhibitors, atezolizumab, pembrolizumab, nivolumab
Citation: Donisi C, Puzzoni M, Ziranu P, Lai E, Mariani S, Saba G, Impera V, Dubois M, Persano M, Migliari M, Pretta A, Liscia N, Astara G and Scartozzi M (2021) Immune Checkpoint Inhibitors in the Treatment of HCC. Front. Oncol. 10:601240. doi: 10.3389/fonc.2020.601240
Received: 31 August 2020; Accepted: 11 November 2020;
Published: 07 January 2021.
Edited by:
Ka On Lam, The University of Hong Kong, Hong KongReviewed by:
Sri Harsha Tella, University of South Carolina, United StatesCopyright © 2021 Donisi, Puzzoni, Ziranu, Lai, Mariani, Saba, Impera, Dubois, Persano, Migliari, Pretta, Liscia, Astara and Scartozzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mario Scartozzi, bWFyaW9zY2FydG96emlAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.