- 1Department of Medical Oncology, Xuzhou first People’s Hospital, The Affiliated Xuzhou Municipal Hospital of Xuzhou Medical University, Xuzhou, China
- 2Department of General Surgery, Jiangsu Taizhou People’s Hospital, Taizhou, China
- 3Department of Oncology, Jiangsu Taizhou People’s Hospital, Taizhou, China
Esophageal cancer is one of the most common cancers with a low overall 5-year relative survival rate of approximately 20%. Trastuzumab (Herceptin®) targets HER2 and is an effective therapeutic strategy in HER2-positive breast cancer. However, few reports have described targeted therapy for treating esophageal squamous cell carcinoma (ESCC). A patient with advanced ESCC who had received chemotherapy, radiotherapy, and had undergone a clinical study is described here. The tumor had not been controlled. Herceptin and chemotherapy were used as salvage therapy in this patient because of high HER2 expression. Good therapeutic results were observed in this patient. Therefore, Herceptin is a potential target therapy for patients with HER2-positive advanced ESCC. A study with a large population and a prospective random study are necessary to validate these results.
Highlights
Herceptin is a potential target therapy for patients with advanced HER2-positive esophageal squamous cell carcinoma.
HER2 gene testing should not be ignored in squamous cell carcinoma.
Introduction
Esophageal cancer is one of the most common cancers worldwide. The low overall 5-year relative survival rate of patients with esophageal cancer of approximately 20% is one of the lowest of all cancers (1). Therefore, it is urgent and necessary to identify novel treatment strategies in esophageal cancer. The two major esophageal cancer subtypes are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) (2). With the development of precision medicine, targeted therapy is becoming one of the most promising approaches for treating all cancers. Human epidermal growth factor receptor 2 (HER2) is overexpressed in 20–30% of breast cancers (3, 4). HER2 over-expression leads to abnormal cell proliferation and results in poor prognosis in HER2-positive breast cancer (5). HER2 overexpression is also observed in primary squamous cell carcinoma of the breast (6). Apart from breast, gastric, and gastroesophageal cancers, HER2 amplification of about 2% is observed in multiple tumors (7). Trastuzumab (Herceptin®), targets HER2 and is an effective therapeutic strategy in HER2-positive breast cancer (4). However, there have been few reports exploring targeted therapy in esophageal squamous cell carcinoma (ESCC). We reported a case of HER2-positive advanced ESCC that was treated using Herceptin and chemotherapy with good therapeutic results in our department.
Case Report
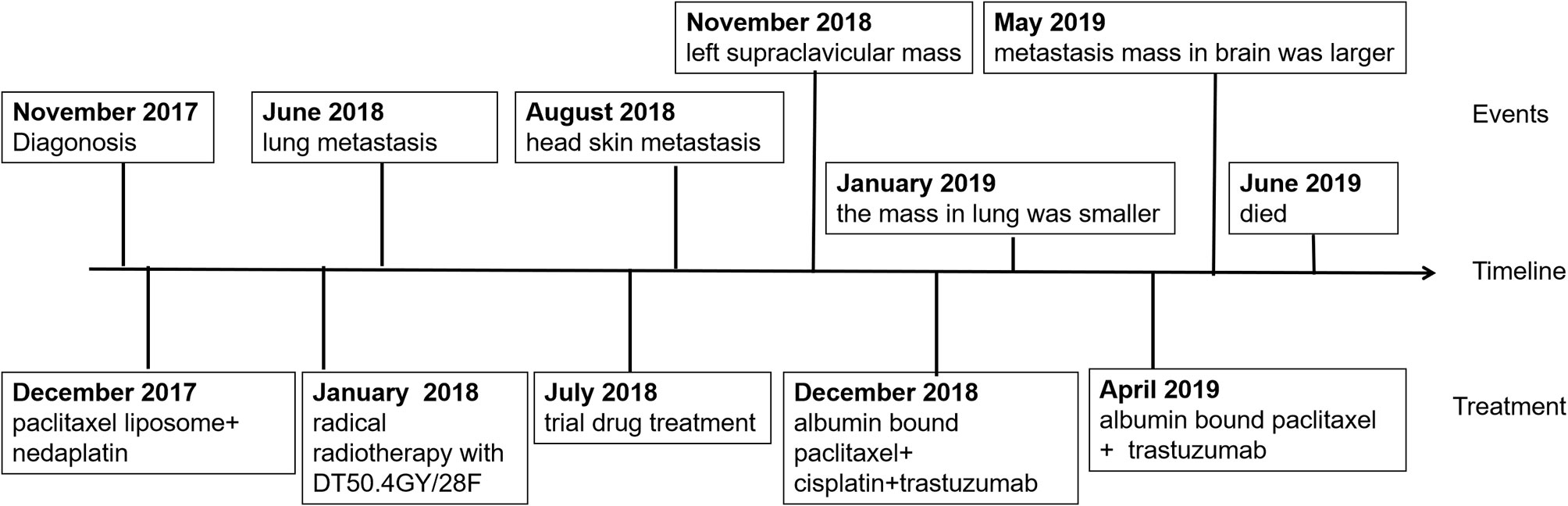
A 61-year-old man was referred to our hospital with dysphagia in November 2017. Gastroscopy showed that two new growths were observed in his esophageal mucosa. One was an 18–22 cm growth originating from his incisor, and the other was a 25–40 cm ring wall growth. Pathology results showed that the new tumors were squamous cell carcinoma and the diagnosis was cervical and lower thoracic esophageal carcinoma. Surgery was not an option for this patient because of the two long lesions in esophagus. Therefore, two cycles of chemotherapy were administered to the patient using “paclitaxel liposome 240 mg (135 mg/m2) + nedaplatin 100 mg (55 mg/m2)” in December 2017. The curative effect was evaluated by partial remission (PR). From January 2 to March 1, 2018, the patient received radical radiotherapy with DT50.4GY/28F. The patient also received concurrent chemotherapy with “paclitaxel liposome 240 mg + nedaplatin 100 mg” on January 22 2018. However, lung computed tomography (CT) on June 27, 2018 showed stage IV lung metastasis. Meanwhile, dysphagia was not relieved. Subsequently, the patient was enrolled in the “BGB-A317-302” clinical study in July 2018. “BGB-A317-302” is a randomized, controlled, open, global phase three study, comparing the efficacy of the BGB-A317 anti-PD-1 antibody with chemotherapy as a second-line treatment in patients with advanced esophageal cancer. Two cycles of trial drug treatment were performed on July 23, 2018 and August 13, 2018. Unfortunately, head skin metastasis was identified on August 22, 2018. The curative effect was evaluated as progressive disease (PD), and the patient was excluded from the study.
Two months later, the patient had increasing lymphadenopathy in the left supraclavicular mass enlargement with skin ulceration. On November 14 2018, he came to our hospital for further treatment of the left supraclavicular mass. Laboratory tests showed carbohydrate antigen 125 (CA125): 106.1 µ/ml, neuron-specific enolase (NSE): 32.59 µ/ml, CYFR-211: 89.00 ng/ml. The masses were visualized by chest and head CT (Figure 1). Resection of the left supraclavicular mass was performed on November 15 2018. Postoperative pathology showed that squamous cell carcinoma was moderately differentiated, with ulcer formation and invasion of striated muscle tissue (Figures 2A–C). It was considered as metastasis based on medical history (IV stage).
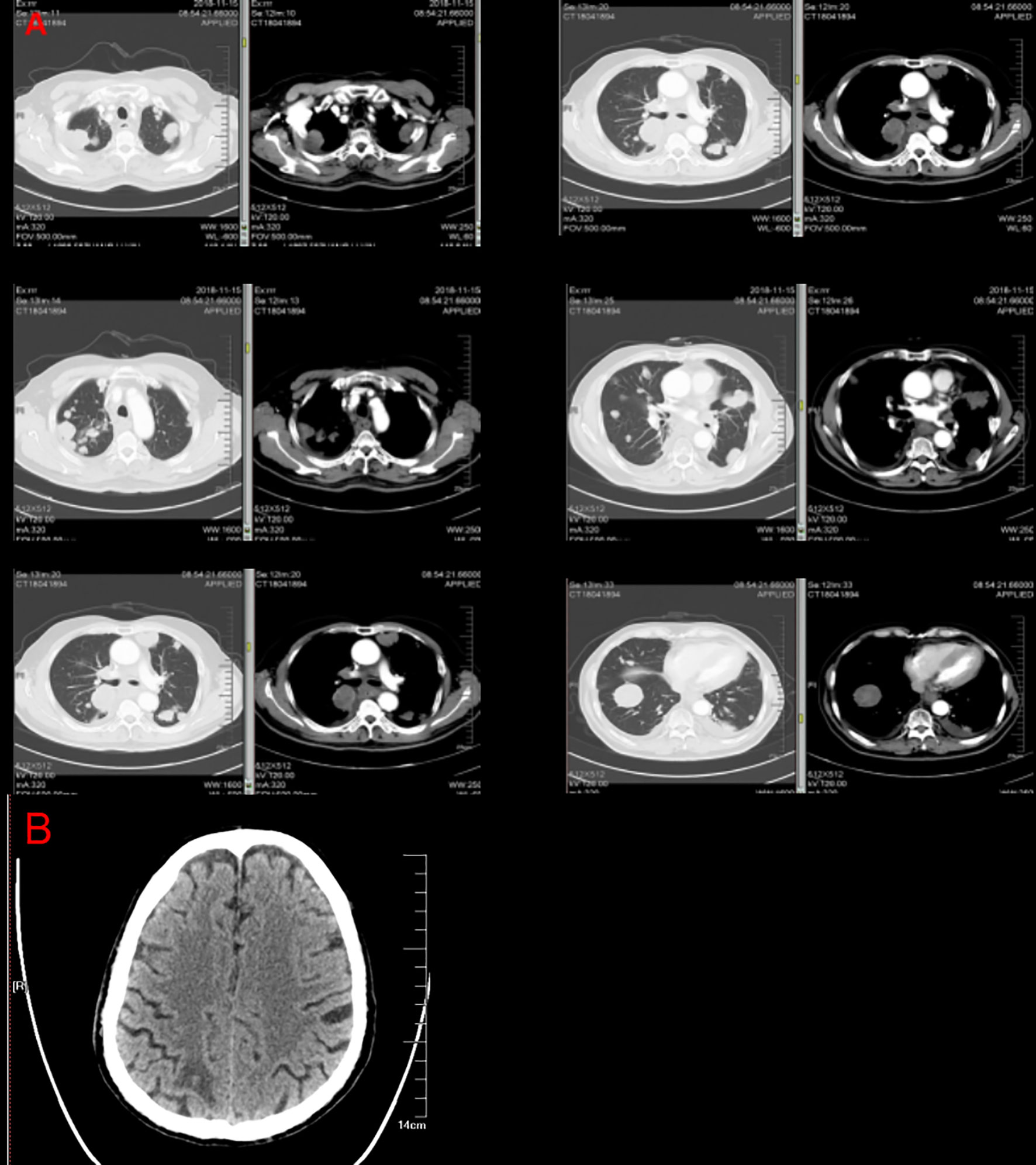
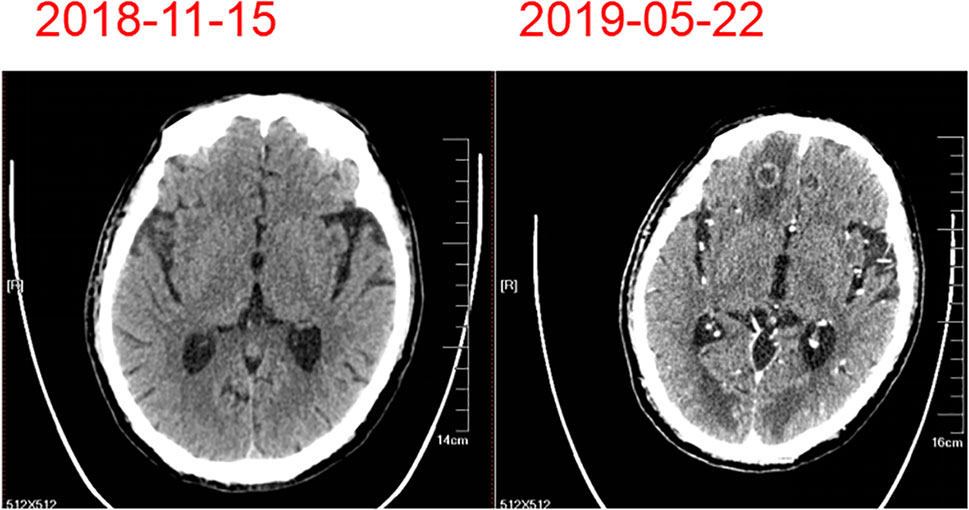
Figure 1 Computed tomography images from the patient with esophageal squamous cell carcinoma. Computed tomography imaging revealed an expansile intraluminal esophageal mass and lung masses (A). The mass observed in brain computed tomography (B).
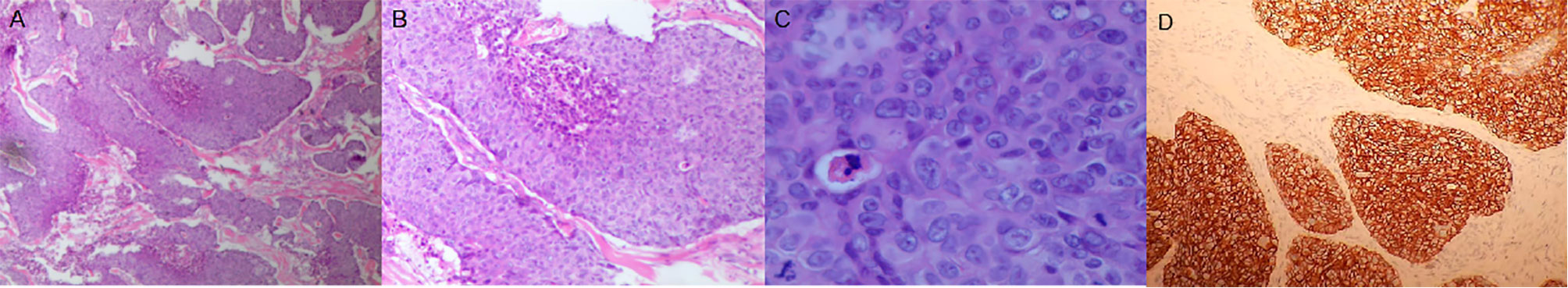
Figure 2 The mass on the chest wall was composed of poorly differentiated mucinous adenocarcinoma of the solid type. (A) Hematoxylin and eosin (H&E) staining, ×40. (B) H&E staining, ×200. (C) H&E staining, ×400. (D) Her2 staining, ×200. H&E, hematoxylin and eosin.
The patient had a strong desire for survival and his intervention adherence was high. Larotrecinib was approved by the Food and Drug Administration for the treatment of NTRK (Neuro Trophin Receptor Kinase) gene carriers on November 26 2018. Moreover, larotrecinib is safe, well tolerated, and a highly effective treatment for all NTRK positive cancer patients (8). Therefore, to assess future treatment options for esophageal carcinoma, tissue from the left supraclavicular mass of this patient was subjected to gene testing (Beijin Geneis Diag). This analysis revealed that the tumor sample was EGFR (epidermal growth factor receptor)-, HER2+ (>1%), NTRK-, and had MSS (Microsatellite stabilization). The immunohistological staining result showed HER2 (3+) (Figure 2D). It showed that larotrecinib was not a suitable treatment option. However, trastuzumab, the anti-HER2 directed therapy, can produce therapeutic benefits for HER2-positive patients. Laboratory tests results showed: carbohydrate antigen 125 (CA125), 239 µ/ml; neuron-specific enolase (NSE), 58.59 µ/ml; CYFR-211, 194.8 ng/ml (Figure 3).

Figure 3 The tumor markers after treatment with Herceptin combined with chemotherapy. CA125 (A), NSA (B), and CYFR-211 (C) decreased after treatment with Herceptin combined with chemotherapy.
Therefore, 400 mg of (230 mg/m2) albumin bound paclitaxel, 90 mg of (50 mg/m2) cisplatin, and 420 mg of (6 mg/kg) trastuzumab (Herceptin) were administered to the patient on December 10, 2018 and January 04, 2019 as the first-line anti-HER-2 drug treatment. Chest CT re-examination on January 23, 2019 showed that the mass was significantly reduced in size (Figure 4). Tumor biomarkers gradually decreased (Figure 3). PR was evaluated according using RECIST1.1 (9) and the esophageal obstruction was relieved. Treatment was well tolerated by the patient without any discomfort. The patient was again treated with the above regimen on January 25 and February 16. The effect of this treatment was reviewed and evaluated every two cycles and the patient was still evaluated by PR status.
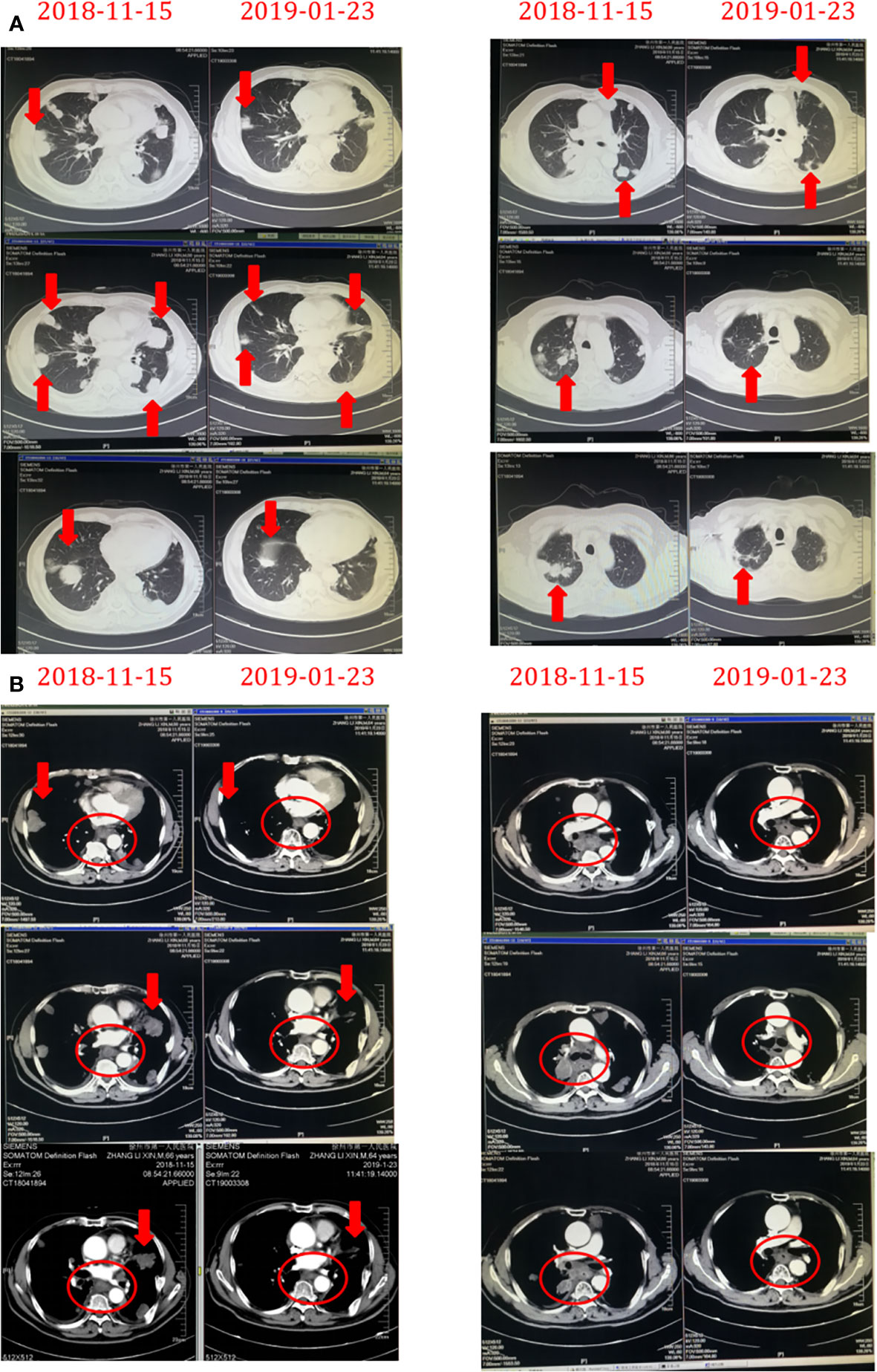
Figure 4 The chest masses before and after treatment with Herceptin combined with chemotherapy. The masses rapidly decreased in size on aortopulmonary window images (A) and mediastinum window images (B) derived by chest computed tomography after treatment with Herceptin combined with chemotherapy.
On February 23, 2019, the patient developed grade IV myelosuppression. Peripheral blood count results were: white blood cell count, 1.2 × 109/liter; red blood cell count, 2.11 × 109/liter; hemoglobin 66 g/liter; and platelet count, 64 × 109/liter. After supportive transfusions of two units of red blood cells over two days, 200 µg granulocyte colony stimulating factor, and 3 mg of interleukin-6 for four days, the patient’s condition quickly improved. Subsequently, following the insistence of the patient, one more cycle of the anti-HER-2 drug treatment was administered on March 21. However, the patient developed numbness in both lower limbs which was considered a side effect caused by cisplatin. Therefore, cisplatin was discontinued and only 400 mg of albumin bound paclitaxel and 420 mg of trastuzumab (Herceptin) were administered on April 26, 2019. The patient was in good clinical status with complete remission of dysphagia until May 19, 2019. At this time, the patient experienced dizziness and unstable walking. The metastatic mass was larger than before and new multiple lesions were found in brain CT (Figure 5). Whole brain radiotherapy was recommended to mitigate neurological symptoms. However, the patient refused further treatment and died of brain metastases in June 2019. This patient had over 6 months progression-free survival (PFS) and an overall survival (OS) of 19 months. This patient’s diagnosis and treatment are summarized in Figure 6.
Discussion
Esophageal cancer is one of the most common malignant cancers in China (10). Drinking hot tea may be an important risk for esophageal cancer (11). We reported a patient with advanced ESCC with brain, lung, and skin metastases. The optimal treatment course for patients with advanced ESCC remains controversial (12). Comprehensive treatment (chemotherapy and radiotherapy) is the only treatment with potentially curative intent in patients with advanced ESCC (13). Even so, advanced ESCC still has poor prognosis and a low survival rate. Therefore, there remains a need for the development of effective treatment. Herceptin, a recombinant monoclonal antibody to HER2, is as an effective drug for improving outcomes in patients with HER2-positive breast cancer (14). Other adenocarcinomas also have high HER2 expression. For example, HER2 is also overexpressed in approximately 20% of gastric and esophagogastric junction adenocarcinoma (15). HER2 expression and amplification is common in esophageal adenocarcinoma (2). HER2 amplification is observed in 17% of esophageal adenocarcinoma (16).
Trastuzumab combined with chemotherapy is recommended as a new therapy for patients with HER2-positive advanced gastric and esophagogastric junction adenocarcinoma (17). This treatment improved the median OS by 2.7 months in patients with HER2-positive gastric/gastroesophageal junction cancer compared with chemotherapy alone (18). Additionally, HER2-targeted therapies were recently updated in the NCCN Guidelines to a category 2A recommendation in rectal cancer (19). To date, there are no reports of HER2-targeted therapies in esophageal cancer. This is the first report of a patient with HER2-positive advanced ESCC being treated with Herceptin and achieving clinical remission. It is a pity that neurological symptoms appeared following the progression of brain metastases 6 months after successful treatment. We postulate that this may be caused by many different reasons. Firstly, discontinuation of cisplatin in the sixth chemotherapy course because of myelosuppression could be a major element. Previous research has indicated clinical synergy between cisplatin and trastuzumab (20). Cisplatin discontinuation may reduce Herceptin efficacy. Secondly, resistance to Herceptin may contribute to cancer progression. The mechanism of HER2-targeted resistance remains unknown. At present, many potential pathways are involved in HER2-targeted resistance, including cross-talk with estrogen receptors, immune response, and cell cycle control mechanisms (21). MyPathway, a phase IIa multiple basket study, indicated that 38% of patients with colorectal cancer with HER2 amplification achieved PR after treatment with patuzumab combined with the HER2 inhibitor trastuzumab (22). Mengjun et al. found the GB235 anti-HER2 antibody can be considered a complementary therapy for HER2-positive breast cancer (23). Therefore, it is necessary to formulate complementary therapy for HER2-positive patients. Lastly, the blood brain barrier may contribute to brain metastasis. Indeed, the concentration of Herceptin in cerebrospinal fluid is only 0.5% that measured in blood (24). While the mechanism of this is not clear, it may be related to the difficulty of macromolecules passing through the blood-brain barrier.
We reported a patient with HER2-positive ESCC who received trastuzumab combined with chemotherapy and who experienced over 6 months PFS and 19 months OS. In clinical practice observation, patients with gastroesophageal or gastric metastatic adenocarcinoma treated with trastuzumab had 7.9 months PFS and 14.1 months OS (25). This suggests that the effect of Herceptin treatment is likely similar in HER2-positive patients with ESCC and those with adenocarcinoma. However, the response rate of Herceptin treatment in all patients with HER2-positive ESCC remains unknown and needs to be determined in a large population.
Conclusion
Herceptin is a potential therapy for patients with advanced HER2-positive ESCC. Therefore, HER2-positive gene tests should not be ignored in squamous cell carcinoma. This conclusion needs validation through prospective random and large population studies.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials; further inquiries can be directed to the corresponding author.
Ethics Statement
The study was approved by the Human Ethics Review Committee of Xuzhou first People’s Hospital. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
LH and TY provided this case. LH, QN, and CP wrote, reviewed, and/or modified the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, et al. Oesophageal cancer. Nat Rev Dis Primers (2017) 3:17048. doi: 10.1038/nrdp.2017.48
3. Nami B, Maadi H, Wang Z. Mechanisms Underlying the Action and Synergism of Trastuzumab and Pertuzumab in Targeting HER2-Positive Breast Cancer. Cancers (Basel) (2018) 10(10):342. doi: 10.3390/cancers10100342
4. Xu L, Liu S, Yang T, Shen Y, Zhang Y, Huang L, et al. DNAzyme Catalyzed Tyramide Depositing Reaction for In Situ Imaging of Protein Status on the Cell Surface. Theranostics (2019) 9(7):1993–2002. doi: 10.7150/thno.31943
5. Budhraja RH, Shah MA, Suthar M, Yadav A, Shah SP, Kale P, et al. LC-MS/MS Validation Analysis of Trastuzumab Using dSIL Approach for Evaluating Pharmacokinetics. Molecules (2016) 21(11):1464. doi: 10.3390/molecules21111464
6. Lei R, Miao L. Primary squamous cell carcinoma of the breast: report of two cases with HER2 overexpression. Cancer Biol Ther (2020) 21(12):1081–6. doi: 10.1080/15384047.2020.1838033
7. Jhaveri KL, Wang XV, Makker V, Luoh SW, Mitchell EP, Zwiebel JA, et al. Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: results from the NCI-MATCH trial (EAY131) subprotocol Q. Ann Oncol (2019) 30(11):1821–30. doi: 10.1093/annonc/mdz291
8. Hong DS, Bauer TM, Lee JJ, Dowlati A, Brose MS, Farago AF, et al. Larotrectinib in adult patients with solid tumours: a multi-centre, open-label, phase I dose-escalation study. Ann Oncol (2019) 30(2):325–31. doi: 10.1093/annonc/mdy539
9. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
10. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
11. Yu C, Tang H, Guo Y, Bian Z, Yang L, Chen Y, et al. Hot Tea Consumption and Its Interactions With Alcohol and Tobacco Use on the Risk for Esophageal Cancer: A Population-Based Cohort Study. Ann Intern Med (2018) 168(7):489–97. doi: 10.7326/M17-2000
12. Monjazeb AM, Riedlinger G, Aklilu M, Geisinger KR, Mishra G, Isom S, et al. Outcomes of patients with esophageal cancer staged with [(1)(8)F]fluorodeoxyglucose positron emission tomography (FDG-PET): can postchemoradiotherapy FDG-PET predict the utility of resection? J Clin Oncol (2010) 28(31):4714–21. doi: 10.1200/JCO.2010.30.7702
13. Liu S, Ren SN, Ding WX, Ge XL, Cao YD, Zhang S, et al. Concurrent liposomal paclitaxel and cisplatin chemotherapy improved outcomes for locally advanced esophageal squamous cell carcinoma treated with intensity-modulated radiotherapy. Ann Transl Med (2019) 7(14):331. doi: 10.21037/atm.2019.06.45
14. Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet (2017) 389(10075):1195–205. doi: 10.1016/S0140-6736(16)32616-2
15. Mondaca S, Margolis M, Sanchez-Vega F, Jonsson P, Riches JC, Ku GY, et al. Phase II study of trastuzumab with modified docetaxel, cisplatin, and 5 fluorouracil in metastatic HER2-positive gastric cancer. Gastric Cancer (2019) 22(2):355–62. doi: 10.1007/s10120-018-0861-7
16. Yoon HH, Shi Q, Sukov WR, Lewis MA, Sattler CA, Wiktor AE, et al. Adverse prognostic impact of intratumor heterogeneous HER2 gene amplification in patients with esophageal adenocarcinoma. J Clin Oncol (2012) 30(32):3932–8. doi: 10.1200/JCO.2012.43.1890
17. Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2016) 14(10):1286–312. doi: 10.6004/jnccn.2016.0137
18. Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer (2015) 18(3):476–84. doi: 10.1007/s10120-014-0402-y
19. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J Natl Compr Canc Netw (2020) 18(7):806–15. doi: 10.6004/jnccn.2020.0032
20. Slamon D, Pegram M. Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials. Semin Oncol (2001) 28(1 Suppl 3):13–9. doi: 10.1016/s0093-7754(01)90188-5
21. Luque-Cabal M, Garcia-Teijido P, Fernandez-Perez Y, Sanchez-Lorenzo L, Palacio-Vazquez I. Mechanisms Behind the Resistance to Trastuzumab in HER2-Amplified Breast Cancer and Strategies to Overcome It. Clin Med Insights Oncol (2016) 10(Suppl 1):21–30. doi: 10.4137/CMO.S34537
22. Meric-Bernstam F, Hurwitz H, Raghav K, McWilliams RR, Fakih M, VanderWalde A, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol (2019) 20(4):518–30. doi: 10.1016/S1470-2045(18)30904-5
23. Shu M, Yan H, Xu C, Wu Y, Chi Z, Nian W, et al. A novel anti-HER2 antibody GB235 reverses Trastuzumab resistance in HER2-expressing tumor cells in vitro and in vivo. Sci Rep (2020) 10(1):2986. doi: 10.1038/s41598-020-59818-2
24. Chang HY, Morrow K, Bonacquisti E, Zhang W, Shah DK. Antibody pharmacokinetics in rat brain determined using microdialysis. Mabs-Austin (2018) 10(6):843–53. doi: 10.1080/19420862.2018.1473910
25. Al-Batran SE, Moorahrend E, Maintz C, Goetze TO, Hempel D, Thuss-Patience P, et al. Clinical Practice Observation of Trastuzumab in Patients with Human Epidermal Growth Receptor 2-Positive Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. Oncologist (2020) 25(8):e1181–7. doi: 10.1634/theoncologist.2020-0109
Keywords: esophageal squamous cell carcinoma, herceptin, chemotherapy, targeted therapy, human epidermal growth factor receptor 2 (Her2) positive
Citation: Han L, Pan C, Ni Q and Yu T (2021) Case Report: Herceptin as a Potentially Valuable Adjuvant Therapy for a Patient With Human Epidermal Growth Factor Receptor 2-Positive Advanced Esophageal Squamous Cell Carcinoma. Front. Oncol. 10:600459. doi: 10.3389/fonc.2020.600459
Received: 07 September 2020; Accepted: 11 December 2020;
Published: 01 February 2021.
Edited by:
Cyril Corbet, Fonds National de la Recherche Scientifique (FNRS), BelgiumReviewed by:
Yuqin Shang, Arizona State University, United StatesWencheng Zhang, Tianjin Medical University Cancer Institute and Hospital, China
Copyright © 2021 Han, Pan, Ni and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Yu, Z2xhY2llcnlvb0BvdXRsb29rLmNvbQ==
†These authors have contributed equally to this study and share first authorship
 Li Han1†
Li Han1† Qingtao Ni
Qingtao Ni