
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 02 November 2020
Sec. Hematologic Malignancies
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.596134
This article is part of the Research Topic Advances and Challenges of Allogeneic Stem Cell Transplantation View all 19 articles
Relapse is the main cause of mortality in patients with acute myeloid leukemia (AML) after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Adverse cytogenetic or molecular risk factors, as well as refractory disease or persistent measurable residual disease (MRD) at the time of transplantation are associated with an increased risk of recurrence. Salvage therapy for AML relapse after allo-HSCT is often limited to chemotherapy, donor lymphocyte infusions and/or second transplants and is rarely successful. Effective post-transplant preventive intervention in high risk AML may be crucial. The most frequent and promising approach is the use of post-transplant maintenance with hypomethylating agents or with FLT3 tyrosine kinase inhibitors when the target is present. Moreover, IDH1/IDH2 inhibitors and BCL-2 inhibitors in combination with other strategies are promising approaches in the maintenance setting. Here we summarize the current knowledge about the preemptive and prophylactic use of pharmacologic agents after allo-HSCT to prevent relapse of AML.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is currently considered the optimal curative treatment option for patients with unfavorable risk acute myeloid leukemia (AML) (1–3). The implementation of non-myeloablative conditioning regimens and the improvement in supportive care has led to decrease in the transplant-related mortality (TRM) and to significant increase in the number of transplant candidates, including older patients and/or those with comorbidities (4, 5). However, reduced-intensity conditioning (RIC) is associated with higher rate of relapse (6). Allo-HSCT is generally recommended when the benefit of relapse reduction outweighs the risk of non-relapse mortality (NRM)/morbidity and this is based on the assessment of cytogenetic and molecular genetic features as well as donor, patient, and transplant-related factors (7–10). This includes intermediate or high-risk cytogenetic/molecular disease groups defined by the 2017 European Leukemia Net (ELN) guidelines, achievement of complete remission (CR) after more than one induction chemotherapy, refractory disease and the presence of pre-transplant measurable residual disease (MRD) positivity (7, 11, 12).
Disease relapse in transplanted patients in first CR (CR1) occurs in 30%–40% of cases and harbors a particular poor prognosis if it occurs in the first 6 months post-transplant (13). Relapse rates are even higher among patients who undergo allo-HSCT beyond CR1 or those with refractory disease (14, 15).
The treatment options for AML patients who relapse after transplant are very limited and highly depend on the patient performance status at the time of relapse (16). Commonly used treatment options for patients who are candidates for intensive therapy are salvage chemotherapy, often associated with donor lymphocyte infusion (DLI), allogeneic stem cell boost, or even second allo-HSCT from the same or different donor (17–24). In contrast, patients who are not eligible for intensive therapy are usually offered low intensity chemotherapy, hypomethylating agents (HMA), targeted therapies, participation in clinical trials, and withdrawal of immunosuppression or supportive care, all aiming at controlling the disease rather than achieving remission (25–27).
Salvage treatments post-allo-HSCT can induce remissions only in a minority of patients (20%) and the 2-year overall survival (OS) rates are usually below 20% (28–30). Alternatively, preventive strategies have been studied to reduce the incidence of relapse including the use of myeloablative conditioning (MAC), prophylactic DLI, graft manipulation, early withdrawal of immunosuppression or intensive surveillance. Intensification of conditioning regimen by using MAC is associated with a lower relapse rate but with higher TRM. Thus, there is no difference in OS when MAC or RIC are used in allo-HSCT for AML (31). Prophylactic DLI is associated with a decrease in the relapse rate at the expense of more graft-versus-host disease (GVHD) and therefore an increased morbidity and mortality (32).
The low efficacy of these strategies to prevent post-transplant relapse led to the introduction of alternative approaches such as prophylactic pharmacological interventions for patients with unfavorable risk, or preemptive strategies for patients with risk of imminent recurrence indicated by MRD positivity by flow cytometry, cytogenetic testing, molecular analysis or loss of donor chimerism. The ideal maintenance agent should target an active driver pathway, such as tyrosine kinase inhibitors (TKIs) targeting FLT3 (such as sorafenib and midostaurin) or HMA (i.e., azacitidine and decitabine). These agents have an acceptable non-hematologic toxicity with manageable drug–drug interactions. Moreover, they enhance the graft-versus-leukemia effect (GVL) with non-significant effect on GVHD. For instance, in vitro and murine studies showed that HMAs has an important immunologic effect after transplantation in expanding circulating T regulatory (Tregs)/natural killer (NK) cells and up-regulating the expression of tumor antigens on leukemic blasts leading to increased GVL effect without increasing the risk of GVHD (33–35). Moreover, the use of FLT3 inhibitors as maintenance post-transplant is supported by the observation of an anti-leukemic synergism between sorafenib and alloreactive donor cells (36, 37). One recent study demonstrated that sorafenib promotes GVL activity in mice and humans through interleukin-15 production in FLT3-ITD leukemia cells (38).
Here, we summarize the clinical data on a number of agents being studied as maintenance/preemptive therapies after allo-HSCT in AML focusing mainly on TKIs (FLT3 inhibitors) and HMAs (azacitidine and decitabine).
There are two approaches to reduce the risk of frank AML relapse following allo-HSCT, prophylactic and preemptive strategies. Prophylactic strategies are defined as the initiation of treatment in the absence of any measurable disease after transplant. Prophylactic therapy is given to patients with high risk of relapse in the aim to eradicate residual malignant cells which are undetectable by currently available monitoring techniques. In contrast, preemptive strategies are initiated for patients with risk of imminent relapse presenting as any evidence of disease activity at MRD level to prevent frank hematological relapse.
MRD persistence at transplant has been identified as an independent and strong risk factor for post-transplant relapse that can be at least partially overcome by additional intervention such as augmented conditioning (7, 39, 40). Similarly, growing evidence strongly suggests that MRD detection by multi-parametric flow cytometry (MFC), molecular techniques, or chimerism analyses after allo-HSCT may be used as a predictor of imminent relapse (41). These should be part of routine post-transplant follow-up since MRD detection can improve outcomes by guiding subsequent therapy aiming to unleash or enhance the GVL effect (39).
Dynamic MRD monitoring after allo-HSCT may improve outcomes; however, there is a relative paucity of data and lack of clear recommendation on how we should test MRD (frequency, qualitative and/or quantitative, on peripheral blood or bone marrow), when we should react and what could be the best available MRD-directed intervention post-allo HSCT (42).
The main methods for detection of MRD in patients with AML after allo-HSCT are MFC, molecular genetics and chimerism analyses (43). MFC is the standard and most commonly used MRD method to identify residual leukemic cells reaching a sensitivity of 10−3 to 10−5 (39–44). Several studies have demonstrated a higher risk of relapse in AML patients with positive MRD detected by MFC after transplant compared to those without evidence of MRD (≤ 0.1% leukemia cells) by the same detection method (45, 46). MRD by flow cytometry has many drawbacks including the lack of standardization, its lower sensitivity, the need for high technical expertise to differentiate between leukemic from regenerating bone marrow cells, biological heterogeneity of the leukemic population and the possibility of false negative results related to sample processing, hemodilution, number of events analyzed and immunophenotypic switch (45–47).
Another method of MRD assessment is donor/recipient chimerism analysis that can detect host-derived hematopoiesis based on genomic differences between the recipient and the donor. Decrease in donor chimerism in AML is often associated with disease relapse (48). Sensitivity of chimerism is dependent on the method applied, ranging from only 10−2–10−3 in the conventional method using fragment analysis of short tandem repeats (STR) or in XY-FISH analysis method in sex-mismatched donor/recipient, to a high sensitivity of 10−4–10−5 if variant-allele-specific quantitative PCR that can detect small DNA insertions or deletion or evaluation of CD34+ cell subset in AML were used (48–50). In consequence, chimerism analysis should be routinely performed after allo-HSCT on days +30, +100, +270, and +365 in conjunction with other MRD markers and clinical parameters to wisely decide on preemptive intervention (51).
The last method of MRD assessment is molecular analysis. Currently, the most widely applied strategy for molecular MRD monitoring is real-time quantitative PCR (RQ-PCR) which can detect mutated genes, fusion gene transcripts or overexpressed genes and can detect leukemic cells at 10−6 sensitivity (42, 43). PCR based methods are characterized by high specificity and sensitivity for leukemic cells detection and low risk of contamination; however, their use depends on identifying pretreatment AML-associated mutation at diagnosis and these molecular targets must be stable while on therapy (52, 53). For instance, some mutations like NPM1 mutation, RUNX1-RUNX1T1 and CBF-MYH11 in core binding factor (CBF) AML are relatively stable during disease course hereby are suitable for PCR MRD monitoring (12). It was recently shown that NPM1 MRD-positivity at levels >0.1% to >10% beyond Day +60 post allo-HSCT are associated with increased relapse rates and reduced survival. Hence, preemptive interventions are considered for patients with persistent NPM1 MRD levels at >0.1%–1% and more intervention should be considered if MRD is >10% (54, 55). Persistent CBF-fusion transcripts after allo-HSCT are translated into higher cumulative relapse incidence (RI) and shorter leukemia-free survival (LFS). Thus, preemptive interventions should be considered in case of persistent MRD positivity (>1%) of RUNX1–RUNX1T1 or CBFB-MYH11 in two consecutive measurements or if there is >0.5 log increase in the transcripts in repeated analysis (56, 57).
Other mutations such as FLT3 (ITD and TKD), RAS, IDH1, IDH2, and MLL-PTD may theoretically be measurable by MRD detection but are poor MRD markers and have not been integrated into routine care yet, since these mutations are relatively unstable throughout treatment. Moreover, some of these mutations are lost during disease course and treatment due to leukemia clonal evolution (58). As a result, ELN guidelines recommend against using them as single markers (39).
In contrast to the limited frequency (50%) of mutations mentioned above, over-expression of Wilms Tumor 1 (WT1) gene is present in almost 90% of patients with AML and can be measured in peripheral blood with better sensitivity and specificity than in bone marrow. WT1 expression analysis in MRD assessment is recommended by ELN using a standardized and certified ELN assay (59). Several reports showed that persistent high bone marrow or continuous increase in peripheral blood WT1 transcripts at 3 months post-transplant are associated with higher risk of relapse (60, 61). Conversely, patients with sustained low WT1 levels after transplant have excellent outcomes (62).
Other emerging technologies like digital-droplet based PCR and next-generation sequencing (NGS) assays are expected to be particularly useful in AML (63–65).
Table 1 summaries the studies that use HMA for relapse prevention after allo-HSCT in AML. HMAs are clinically active in AML and myelodysplastic syndromes (MDS) and represent an important new treatment modality, particularly in elderly and/or unfit patients, due to their favorable toxicity profile (77). HMA have significant antitumor activity in relapsed AML patients after allo-HSCT with a 20%–40% CR rate (78, 79). Azacitidine (AZA) which is the first reported DNMT inhibitor, appears to be well tolerated after transplantation. In vitro and murine studies showed that AZA has an important immunologic effect after transplantation in expanding circulating Treg cells and up-regulating the expression of tumor antigens on leukemic blasts leading to increased GVL effect without increasing the risk of GVHD (33).
AZA and decitabine have been tested in several prospective and retrospective studies as maintenance therapy to avoid relapse post-allo-HSCT. These early-phase studies generally demonstrated tolerability, feasibility and established the optimal dosage and schedule for future trials (66, 68–72, 74–76). de Lima et al. (66) reported the results of the first phase 1 dose-finding study of maintenance AZA post-transplant in 45 patients with high-risk AML (n = 37) or MDS (n = 8). The investigators examined subcutaneous AZA at different dosing schedule (8, 16, 24, 32, and 40 mg/m2). The optimal dose was 32 mg/m2 given for 5 consecutive days every 28 days. After a median follow up of 20.5 months, the NRM was 9%. One-year event-free survival (EFS) and 1-year OS were 58% and 77%, respectively. The rates of grade II-III acute GVHD and chronic GVHD were 27% and 37%, respectively. The authors concluded that low dose azacitidine is safe and may prolong OS and EFS in heavily pretreated AML and MDS patients as post-transplant maintenance (66).
In another report by Oshikawa and colleagues (68), AZA plus gemtuzumab ozogamicin (GO) were used in 10 patients with high-risk AML after allo-HSCT. After a median follow-up of 474 days from allo-HSCT, the NRM rate was 10% and the 1-year disease-free survival (DFS) and OS were 60% and 70%, respectively (68).
Furthermore, in a prospective trial by Craddock et al. (71), 37 AML patients received AZA at a median time of 54 days post-transplant and at a dose of 36 mg/m2/day for 5 days every 28 days up to 12 months. AZA was well tolerated in the majority of patients. Only 17 patients had grade I–II acute GVHD. Day 100 and 1-year NRM were 0% and 8%, respectively. The 1-year and 2-year OS were 81% and 49%, respectively (71).
Moreover, El-Cheikh and colleagues (72) reported their results of an observational study on AML (n = 13) and MDS (n = 5) patients who received post-transplant reduced dose AZA of 32 mg/m2/day for 5 days monthly, for up to five years. At the time of last follow up, 13 patients were still alive in CR, and had full donor chimerism. The 1-year DFS and OS were 63% and 70%, respectively (72).
More recently, MD Anderson Cancer Center group reported the results of first randomized controlled trial (74). In this study, 187 patients with high-risk AML or MDS who were in CR after allo-HSCT received AZA (n = 93) or placebo (n = 94) at a dose of 32 mg/m2/day for 5 days for 12 months. However, most of the patients in the AZA arm (74.6%) did not receive the planned 12 cycles of treatment due to relapse, death, toxicity or upon patient’s request. The investigators closed the study early due to slow accrual. Relapse-free survival (RFS) was comparable between both groups; however, stratification by number of AZA cycles administered showed a trend toward improved RFS in patients receiving more AZA therapy cycles (74).
In addition to injectable AZA, an oral formulation of AZA (CC-486) has been recently tested in a phase 1/2 dose-finding study on 30 patients with AML (n = 26) and MDS (n = 4) in CR as maintenance therapy after allo-HSCT (75). The study included 4 dosing schedules of 150-300 mg per day for 7 or 14 days every 28 days for up to 12 cycles. Oral AZA (CC-486) seemed safe and generally well tolerated with only 3 patients (10%) developing grade III acute GVHD. Median OS was not reached after 19 months follow-up and the 1-year OS were 86% and 81% in the 7-day and 14-day dosing cohorts, respectively (75).
Decitabine is another HMA that has been evaluated in the maintenance setting post allo-HSCT. Pusic et al. (69) tested the safety and efficacy of decitabine maintenance after allo-HSCT in 22 patients with AML (n = 17) and MDS (n = 5). Decitabine was given at a dose of 5, 7.5, 10, and 15 mg/m2/day for 5 consecutive days every 6 weeks. The toxicity profile was acceptable. Acute GVHD grade I-II and grade III–IV occurred in 27% and 9%, respectively. The 2-year DFS and OS were 48% and 56%, respectively. The investigators concluded that the dose of 10 mg/m2 for 5 days every 6 weeks appeared safe and optimal rather than the 15 mg/m2 and could be administered after transplant in high-risk patients (69).
In another study, decitabine was evaluated in a phase 1 dose-finding study as maintenance therapy post allo-HSCT in 16 patients with MDS (n = 11) or secondary AML (n = 5) (70). No aggravation of preexisting acute GVHD was observed and mild/moderate chronic GVHD occurred in only 2 patients (12.5%). In conclusion, the investigators considered 5 mg/m2/day to be the most appropriate starting dose for decitabine maintenance (70).
MRD-triggered preemptive therapy with HMA is another strategy to avoid relapse of AML after transplant. The German group has tested this concept in 2 prospective studies (67, 73). The first trial was a single-center phase II study of 20 patients with MDS/AML evaluating the administration of AZA preemptively post allo-HSCT after a decrease of CD34+ donor chimerism to <80%, while still in complete hematologic remission (67). All patients received AZA for 4 cycles at a dose of 75 mg/m2/day for 7 days. Sixteen-patients (80%) had response with either increasing CD34+ donor chimerism to >80% (n = 10; 50%) or stabilization (n = 6; 30%) with no evidence of relapse. Furthermore, 11 patients (55%) with stable disease or with subsequent drop in donor chimerism to <80% after initial response received a median of 4 (range: 1–11) additional cycles of AZA. Most patients (65%) ultimately developed hematologic relapse but their relapse was delayed by a median of 231 days after the decrease in donor chimerism.
In the second prospective trial (RELAZA-2) (73), 53 AML/MDS patients who developed MRD positivity after transplant (n = 24) or after conventional chemotherapy (n = 29) received AZA at a dose of 75 mg/m2/day for 7 days monthly for up to 24 cycles. MRD positivity were defined by a drop of 80% or less in CD34+ donor chimerism or an increase in NPM1 mutation, RUNX1-RUNX1T1 and CBFb–MYH11 >1% in the bone marrow or peripheral blood without evidence of hematological relapse. One-year RFS was 46%, and 26 (49%) patients eventually relapsed. The authors concluded that AZA could be effectively used to prevent or delay hematologic relapse in MRD-positive patients with AML/MDS (73).
Overall, these data clearly show that AML patients can tolerate maintenance therapy after allo-HSCT with HMA (azacitidine or decitabine) albeit at lower doses, with a favorable safety profile and apparently a reduction in the risk of disease relapse after transplant. Moreover, the results of preemptive studies could serve as the basis to design future studies of MRD-guided therapy using HMAs with other targeted therapies, including immuno-modulating agents.
Table 2 summaries the studies that use FLT3 inhibitors for relapse prevention after allo-HSCT in AML. FLT3-internal tandem duplication (ITD) mutation is found in approximately 30% of patients with AML (91, 92). These patients have a high risk of relapse and low cure rates (93, 94). Patients with FLT3-ITD mutation also have a higher risk of early relapse after allo-HSCT compared to patients with wild type FLT3 (38% vs. 28% in Center for International Blood and Marrow Transplant Research (CIBMTR) analysis) (94, 95). Treatment options for patients with FLT3-mutated AML who relapse after transplant are limited to chemotherapy, second allo-HSCT, and FLT3 inhibitors alone or combined with DLI, all of which are rarely effective in the long term, even though, a small fraction of those patients can achieve long-standing responses with sorafenib (22, 96–99). The use of FLT3 inhibitors as maintenance treatment after allo-HSCT is supported by the observation of an anti-leukemic synergism between sorafenib and allo-reactive donor cells (36, 37). Moreover, marrow aplasia induced by chemotherapy leads to elevated FLT3-ligand levels that may increase on-target activity of FLT3 inhibitors (100–103).
Sorafenib was the first TKI studied in the setting of post-transplant maintenance therapy in AML with FLT3-ITD mutation. It showed benefit in survival and improvement of outcomes in a phase I study, several retrospective studies and two randomized studies (80–86, 104). Chen and colleagues (80) reported the results of the first phase I trial on sorafenib after transplant in 22 patients with FLT3 mutated AML. They found that sorafenib could be safely used after allo-HSCT with a maximum tolerated dose (MTD) of 400 mg twice daily. The 2-year progression-free survival (PFS) was 72% with a corresponding 2-year OS of 78% after allo-HSCT. Our group has reported the results of a pilot study in 6 patients with FLT3-ITD AML who received sorafenib (n = 5 maintenance, n = 1 salvage) after transplant. Grade II skin GVHD was observed in 5 of 6 patients shortly after sorafenib initiation, suggesting a possible immunomodulatory effect. Remarkably, all patients were alive after a median follow-up of 16 months and had sustained molecular remission (81). In a single institution observational study, sorafenib maintenance was evaluated in patients with FLT3-ITD AML who underwent allo-HSCT in CR1. Patients on sorafenib maintenance (n = 26) had an improved 2-year OS (81% vs. 62%, p = 0.029) and improved PFS (82% vs. 53%, p = 0.008) compared to historical controls (n = 54) (82).
In a multicenter study, single agent sorafenib was used as post-transplant maintenance in 28 adults with FLT3 positive AML (83, 84). Twenty-five patients were given sorafenib as primary prophylaxis and three patients received it after relapse post allo-HSCT in combination with salvage chemotherapy and were then continued as maintenance after achievement of CR. At a median follow-up of 18 months, 25 patients were in CR with full donor chimerism with 1-year DFS and OS of 91% and 89%, respectively. A recent update of this study after a median follow-up of 40 months further demonstrated promising long-term outcomes with sorafenib maintenance with 2-year PFS and OS of 73% and 80%, respectively.
Recently Bazarbachi and colleagues (85) reported the results of European Society for Blood and Marrow Transplantation (EBMT) registry-based study on 462 allo-grafted FLT3-mutated AML patients (FLT3-ITD-95%) over a median follow-up of 39 months for surviving patients. Among these patients, 28 received post-transplant sorafenib maintenance as prophylactic (n = 19) or preemptive therapy (n = 9), started at a median of 55 days post-transplant (range 1–173 days) and a median dose of 800 mg/day (range 200–800 mg/day). Multivariate analysis showed that maintenance sorafenib significantly decreased RI [hazard ratio (HR) = 0.39; p = 0.05] with improvement in LFS (HR = 0.35; p = 0.01) and OS (HR = 0.36; p = 0.03). A matched-pair analysis was then performed on 52 patients (26 patients in the sorafenib group and 26 in the control group). The 2-year LFS and OS were 79% and 83%, respectively, in the sorafenib group (p = 0.02) vs. 54% and 62%, respectively, in the control group (p = 0.007).
In a recent double-blind prospective trial (SORMAIN) (86), 83 transplanted FLT3-ITD adult AML patients were randomized to receive either maintenance sorafenib (n = 43, up to 400 mg twice daily) or placebo (n = 40) started between days 60 and 100 after transplant for up to 24 months. The 2-year RFS was significantly improved in the sorafenib group (85%) compared to the placebo group (53%) (HR = 0.39, 95% CI, 0.18 to 0.85 p = 0.01). Sorafenib was generally well tolerated and the most common grade III–IV adverse events was acute GVHD (20%) in sorafenib group compared to (17%) in the placebo group.
More recently the Chinese group reported the results of a phase III randomized open-label multi-centers trial on 202 FLT3-ITD AML adult patients who underwent allo-HSCT (87). The patients received either sorafenib maintenance (n = 100; 400 mg BID) or placebo (n = 102) within 30–60 days post-transplant and for 6 months. After median follow up of 22 months, eleven and 30 patients relapsed in the sorafenib and control groups. The 2-year OS were 83% and 71%, (P = 0.025) and LFS were 81% and 54% (P < 0.001) in the sorafenib and control groups, respectively.
Acute Leukemia Working Party of the EBMT published a very recent clinical practice recommendation on allo-HSCT in AML patients with FLT3-ITD (105). The group recommends post-transplant maintenance with sorafenib in all cases except in patients with active acute GVHD. Sorafenib should be started as soon as possible after disease evaluation and MRD assessment at a dose of 400 mg daily in two divided doses and the dose may be increased to 800 mg daily in case of positive MRD and for a minimum of 2 years, depending on tolerance.
Midostaurin is another FLT3 inhibitor that has activity as single agent in AML harboring FLT3-ITD or FLT3 tyrosine kinase domain (TKD) mutation. It was also evaluated in the maintenance setting. Based on the RATIFY trial (106), midostaurin received FDA approval in combination with 3 + 7 induction chemotherapy for newly diagnosed FLT3-mutated AML. However, in this trial midostaurin maintenance was not offered for patients who underwent allo-HSCT.
The RADIUS phase II prospective trial randomized 60 patients with FLT3-ITD AML to standard of care (n = 30) or midostaurin (n = 30) starting 28–60 days post-transplant (88). The estimated RFS at 18-month was 76% in the standard of care arm compared to 89% in the midostaurin arm (HR = 0.46; 95% CI 0.12–1.86, P = 0.26), corresponding to relapse rates of 24% and 11%, respectively (P = 0.27).
In another phase II prospective study by Schlenk et al. (89) on 284 newly diagnosed FLT3-ITD AML patients, midostaurin maintenance treatment was also offered for patients receiving allo-HSCT in CR1 (56%). In a landmark analysis in patients who were event-free at day +100 after transplant (n = 116), those who received maintenance therapy within 100 days post-transplant (n = 72) had better EFS and OS (p = 0.004 and p = 0.01, respectively) than patients who did not.
Gilteritinib is another potent inhibitor of FLT3 with activity against FLT3-ITD and FLT3-TKD. In the phase 3 ADMIRAL trial, 371 adult patients with relapsed or refractory FLT3-mutated AML were randomly assigned in a 2:1 ratio to receive either gilteritinib or salvage chemotherapy. Patients who had a response and proceeded to allo-HSCT continued in the trial and could resume gilteritinib as maintenance therapy. Median OS in gilteritinib arm was 9.3 months compared to 5.6 months in the chemotherapy arm (107). A follow up on long-term survivors was recently presented in ASCO meeting 2020 (108). After 18 months of follow-up, gilteritinib continued to show better OS rates compared to salvage chemotherapy (27% vs. 15%). A total of 63 gilteritinib-treated patients had OS more than 18 months. A higher proportion of patients on gilteritinib achieved remission and underwent allo-HSCT. After a median of 3.5 months, 35 of 63 (56%) patients underwent allo-HSCT; 25 of these 35 patients (71%) received post-transplant gilteritinib maintenance. The authors concluded that the long-term survival in patients receiving gilteritinib is related to ongoing remission, subsequent allo-HSCT, or post-transplant gilteritinib maintenance therapy. Gilteritinib is currently being prospectively tested as maintenance therapy after allo-HSCT in FLT3-ITD AML patients in an ongoing randomized, double-blind, placebo-controlled phase III trial (NCT02997202) (109). This study aims to enroll and randomize 346 adult patients with AML in CR1 to receive maintenance therapy with either 120 mg gilteritinib per day or placebo for 24 months.
Quizartinib, another selective and highly potent FLT3 inhibitor, was also evaluated in a phase I dose-finding and safety study (90). Thirteen adult patients with FLT3-ITD mutated AML in morphological remission following allo-HSCT received one of two quizartinib dose levels at 40 mg/day (n = 7) and 60 mg/day (n = 6), administered orally for up to 24 months. Around 77% of patients received quizartinib for at least 1 year and preliminary data indicated an acceptable tolerability and a reduced relapse rate compared with historical cohorts with only one (1/13) relapse.
Based on the previously discussed trials, introducing single agent AZA as maintenance therapy can generally delay but mostly not prevent relapse after allo-HSCT. Combining AZA with DLI is a promising concept of MRD-guided post-transplant interventions since it reduces disease burden by cytotoxic therapy and reinforce an allo-immune reaction by cellular approach. This concept was evaluated in a phase II study of 30 patients with high-risk AML (n = 20) and MDS (n = 10) who were treated with prophylactic post-transplant AZA followed by escalated doses of DLI. Two-year OS and DFS were both 65.5%. Acute and chronic GVHD were reported in 31.5% and 53% of patients, respectively (110).
Many targeted agents such as isocitrate dehydrogenase (IDH) Inhibitors (IDH1, ivosidenib; IDH2, enasidenib), hedgehog (Hh) inhibitor (glasdegib), and BCL2 inhibitor (venetoclax) in combination therapy have been evaluated and showed encouraging results in relapsed/refractory (R/R) AML or in AML/MDS patients ineligible for intensive chemotherapy (111–117). Both IDH inhibitors were approved by the FDA for the treatment of R/R AML. These drugs induce cellular differentiation and may promote an allo-immunologic reaction by antigen upregulation on leukemic cells. This mode of action implies that these agents may have an interesting activity in IDH-mutated AML patients as salvage or even as maintenance therapy after transplant (111, 112). Currently, there are several ongoing prospective trials evaluating the role of IDH inhibitors in the maintenance setting after transplant in IDH-mutated AML (NCT03515512 and NCT03564821). The safety and efficacy of combination venetoclax plus AZA in R/R AML after allo-HSCT has been proven only in case series (113–116). The same combination is being tested in post-transplant AML patients as maintenance therapy (NCT04128501).
Although combination HMA and FLT3 inhibitors was not investigated in the setting of maintenance therapy after allo-HSCT in AML, this combination has shown efficacy in AML. DiNardo and colleagues reported the results of the combination of venetoclax with low dose AZA in 81 elderly patients; analysis of primary and adaptive resistance was caused by an enrichment of clones harboring activated signaling pathways such as FLT3 or RAS or biallelically perturbing TP53 which helped in determining the predictors of outcome using this combination therapy (117). And we know from previous studies that combination of AZA plus sorafenib is effective and well tolerated in relapsed/refractory FLT3-ITD AML (118). Thus, the combination of FLT3 inhibitor and HMA seems to be a potential strategy to prevent relapse post-transplant in high risk AML patients and it is worth being investigated.
Hedgehog inhibitor (glasdegib) has recently shown promising results in a randomized phase II study when combined with low-dose cytarabine (LDAC) as compared to LDAC alone in AML/MDS frail patients (119). A single agent glasdegib is being investigated in a phase II study as maintenance therapy following allo-HSCT for high-risk patients (NCT01841333).
Finally, despite maintenance treatment, most of the patients still relapse. Different mechanisms of resistance may emerge. For example, in patients with FLT3-ITD mutation, acquisition of point mutations in the FLT3 drug binding site, or activation of alternative pathways such as mutations of the NRAS gene are the most described mechanism of resistance.91 Many combinatorial strategies have evolved and probably overcome this resistance such as combination of FLT3-TKIs with epigenetic therapy including histone deacetylase inhibitors and HMA, which revealed promising and synergistic antileukemic in vitro efficacy mainly by downregulation of the JAK/STAT pathway (120).
Figure 1 summarizes the treatment guidelines to prevent relapse of AML after allo-HSCT.
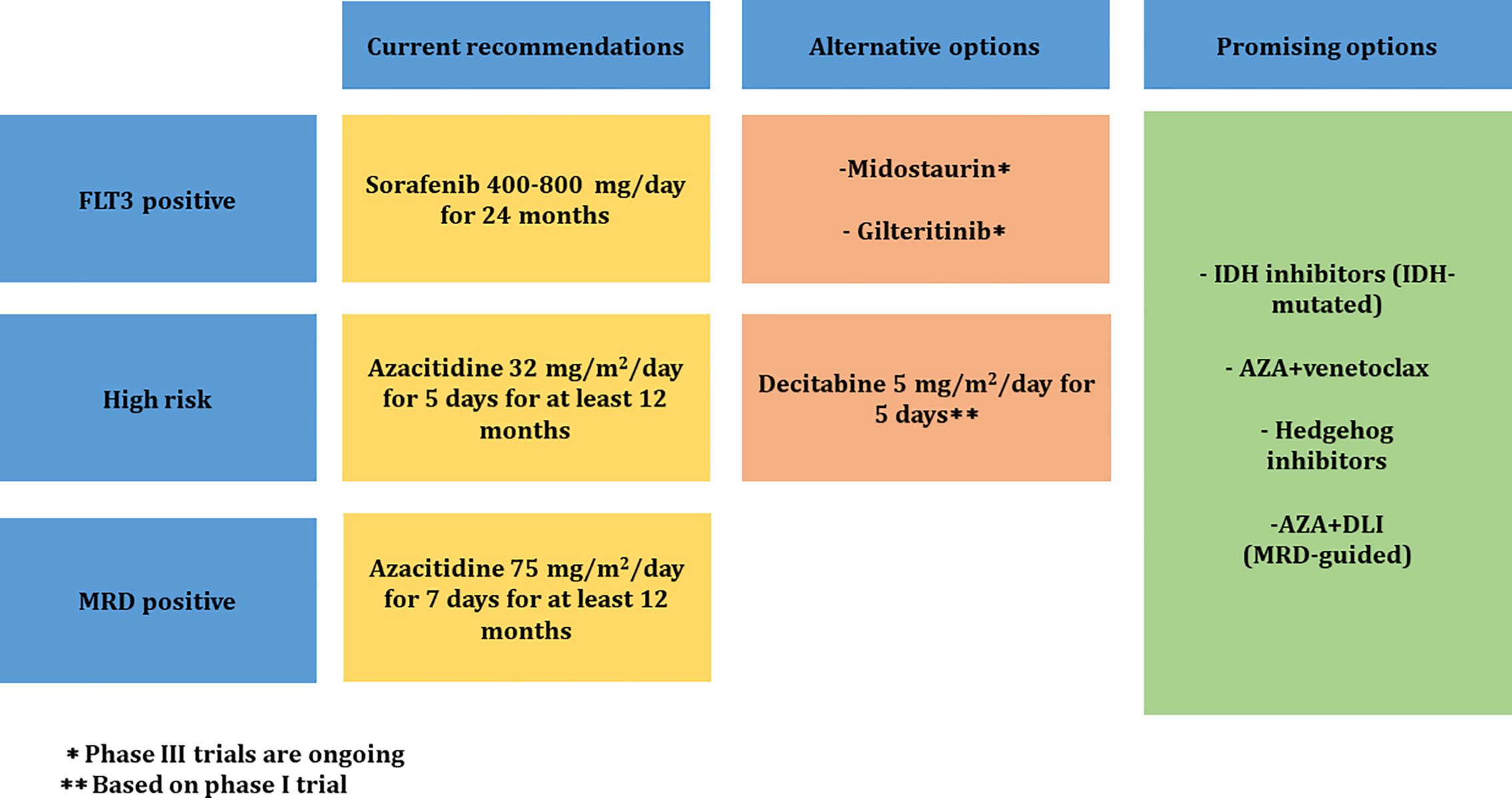
Figure 1 Proposed treatment guidelines to prevent relapse of AML after allo-HSCT. MRD, measurable residual disease; IDH, isocitrate dehydrogenase; AZA, azacitidine; DLI, donor lymphocyte infusion.
● MRD measurement using MFC and RQ-PCR methods should be incorporated in the treatment decision process for adult AML patients after transplant.
● MRD will enable to identify high-risk patients to define patients at risk of relapse who would benefit from preemptive approaches with HMA and targeted therapies.
● Azacitidine use as maintenance therapy in high-risk AML and as preemptive MRD-triggered therapy could be considered after transplant for at least 12 months at a dose of 32 mg/m2 for 5 days and 75 mg/m2 for 7 days, respectively.
● In FLT3-ITD AML patients, post-transplant maintenance therapy with sorafenib at a dose 400–800 mg/day in two divided doses should be strongly considered for 24 months.
● Other FLT3 inhibitors such as midostaurin and gilteritinib are attractive in the maintenance setting and warrant further investigation in larger prospective studies.
● The use of other agents (IDH inhibitors, BCL-2 inhibitors, Hedgehog inhibitors) and combination therapy with DLI are being evaluated and could have a promising result in the post-transplant maintenance setting.
AA reviewed the literature and wrote the manuscript. ZO reviewed the literature and wrote the manuscript. IA reviewed the literature and wrote the manuscript. JE-C reviewed the literature and wrote the manuscript. AB reviewed the literature and wrote the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Liu H, Stock W, Bishop MR. Expanded indications for allogeneic stem cell transplantation in patients with myeloid malignancies. Curr Opin Hematol (2013) 20:115–22. doi: 10.1097/MOH.0b013e32835dd84a
2. Cornelissen JJ, Breems D, van Putten WL, Gratwohl AA, Passweg JR, Pabst T, et al. Comparative analysis of the value of allogeneic hematopoietic stem-cell transplantation in acute myeloid leukemia with monosomal karyotype versus other cytogenetic risk categories. J Clin Oncol (2012) 30:2140–6. doi: 10.1200/JCO.2011.39.6499
3. DeZern AE, Sung A, Kim S, Smith BD, Karp JE, Gore SD, et al. Role of allogeneic transplantation for FLT3/ITD acute myeloid leukemia: outcomes from 133 consecutive newly diagnosed patients from a single institution. Biol Blood Marrow Transplant (2011) 17:1404–9. doi: 10.1016/j.bbmt.2011.02.003
4. Storb RF, Champlin R, Riddell SR, Murata M, Bryant S, Warren EH. Non- myeloablative transplants for malignant disease. Hematol Am Soci Hematol Educ Prog (2001), 375–91. doi: 10.1182/asheducation-2001.1.375
5. Bornhauser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomized phase 3 trial. Lancet Oncol (2012) 13:1035–44. doi: 10.1016/S1470-2045(12)70349-2
6. Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J Clin Oncol (2017) 35:1154–61. doi: 10.1200/JCO.2016.70.7091
7. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood (2017) 129:424–47. doi: 10.1182/blood-2016-08-733196
8. Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, et al. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant (2015) 21:1863–9. doi: 10.1016/j.bbmt.2015.07.032
9. Sureda A, Bader P, Cesaro S, Dreger P, Duarte RF, Dufour C, et al. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant (2015) 50:1037–56. doi: 10.1038/bmt.2015.6
10. Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood (2005) 106:2912–9. doi: 10.1182/blood-2005-05-2004
11. Araki D, Wood BLOM, Radich JP, Halpern AB, Zhou Y, Mielcarek M, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: time to move toward a minimal residual disease-based definition of complete remission? J Clin Oncol (2016) 34:329–36. doi: 10.1200/JCO.2015.63.3826
12. Ivey AHR, Simpson MA, Jovanovic JV, Gilkes A, Grech A, Patel Y, et al. Assessment of minimal residual disease in standard-risk AML. N Engl J Med (2016) 374:422–33. doi: 10.1056/NEJMoa1507471
13. Cornelissen JJ, Bidier B. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood (2016) 127:62–70. doi: 10.1182/blood-2015-07-604546
14. Meijer E, Cornelissen JJ. Allogeneic stem cell transplantation in acute myeloid leukemia in first or subsequent remission: weighing prognostic markers predicting relapse and risk factors for non-relapse mortality. Semin Oncol (2008) 35:449e57. doi: 10.1053/j.seminoncol.2008.04.015
15. Bosi A, Laszlo D, Labopin M, Reffeirs J, Michallet M, Gluckman E, et al. Second allogeneic bone marrow transplantation in acute leukemia: results of a survey by the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol (2001) 19:3675–84. doi: 10.1200/JCO.2001.19.16.3675
16. Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant (2015) 21:454–9. doi: 10.1016/j.bbmt.2014.11.007
17. Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol (2007) 25:4938–45. doi: 10.1200/JCO.2007.11.6053
18. Eapen M, Giralt SA, Horowitz MM, Klein JP, Wagner JE, Zhang MJ, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant (2004) 34:721–7. doi: 10.1038/sj.bmt.1704645
19. Loren AW, Porter DL. Donor leukocyte infusions for the treatment of relapsed acute leukemia after allogeneic stem cell transplantation. Bone Marrow Transplant (2008) 41:483–93. doi: 10.1038/sj.bmt.1705898
20. Lee JH, Lee KH, Kim S, Seol M, Kim SH, Kim WK, et al. Combination chemotherapy of intermediate-dose cytarabine, idarubicin, plus etoposide and subsequent mobilized donor leukocyte infusion for relapsed acute leukemia after allogeneic bone marrow transplantation. Leuk Res (2001) 25:305–12. doi: 10.1016/S0145-2126(00)00142-9
21. Christopeit M, Kuss O, Finke J, Bacher U, Beelen DW, Bornhauser M, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol (2013) 31:3259–71. doi: 10.1200/JCO.2012.44.7961
22. Kharfan-Dabaja MA, Labopin M, Polge E, Nishihori T, Bazarbachi A, Finke J, et al. Association of Second Allogeneic Hematopoietic Cell Transplant vs Donor Lymphocyte Infusion With Overall Survival in Patients With Acute Myeloid Leukemia Relapse. JAMA Oncol (2018) 4:1245–53. doi: 10.1001/jamaoncol.2018.2091
23. Yafour N, Beckerich F, Bulabois CE, Chevallier P, Daguindau É, Dumesnil C, et al. Preventative and therapeutic relapse strategies after allogeneic hematopoietic stem cell transplantation: Guidelines from the Francophone society of bone marrow transplantation and cellular therapy (SFGM-TC). Bull Cancer (2017) 104:S84–98. doi: 10.1016/j.bulcan.2017.05.009
24. El Cheikh J, Otrock ZK, Qannus AA, Kharfan-Dabaja MA, Bazarbachi A. Risk-Adapted Approach to HLA-Matched Sibling Hematopoietic Cell Allografting: Impact of Adjusting Conditioning Intensity and Integrating Post-Transplant Therapeutic Interventions. Clin Lymph Myeloma Leuk (2016) 16:304–10. doi: 10.1016/j.clml.2016.01.005
25. Tessoulin B, Delaunay J, Chevallier P, Loirat M, Ayari S, Peterlin P, et al. Azacitidine salvage therapy for relapse of myeloid malignancies following allogeneic hematopoietic SCT. Bone Marrow Transplant (2014) 49:567–1. doi: 10.1038/bmt.2013.233
26. Ganguly S, Amin M, Divine C, Aljitawi OS, Abhyankar S, McGuirk JP. Decitabine in patients with relapsed acute myeloid leukemia (AML) after allogeneic stem cell transplantation (allo-SCT). Ann Hematol (2013) 92:549–50. doi: 10.1007/s00277-012-1607-y
27. Bazarbachi A, Labopin M, Battipaglia G, Djabali A, Passweg J, Socié G, et al. Sorafenib improves survival of FLT3-mutated acute myeloid leukemia in relapse after allogeneic stem cell transplantation: a report of EBMT acute leukemia Working Party. Haematologica (2019) 104:e398–401. doi: 10.3324/haematol.2018.211615
28. Devillier R, Crocchiolo R, Etienne A, Prebet T, Charbonnier A, Fürst S, et al. Outcome of relapse after allogeneic stem cell transplant in patients with acute myeloid leukemia. Leuk Lymph (2013) 54:1228–34. doi: 10.3109/10428194.2012.741230
29. Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood (2012) 119:1599–606. doi: 10.1182/blood-2011-08-375840
30. Schmid C, de Wreede LC, van Biezen A, Finke J, Ehninger G, Ganser A. et al, Outcome after relapse of myelodysplastic syndrome and secondary acute myeloid leukemia following allogeneic stem cell transplantation: A retrospective registry analysis on 698 patients by the Chronic Malignancies Working Party of the European Society of Blood and Marrow Transplantation. Haematologica (2018) 103:237–45. doi: 10.3324/haematol.2017.168716
31. Abdul Wahid SF, Ismail NA, Mohd-Idris MR, Jamaluddin FW, Tumian N, Sze-Wei EY, et al. Comparison of Reduced-Intensity and Myeloablative Conditioning Regimens for Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Acute Myeloid Leukemia and Acute Lymphoblastic Leukemia: A Meta-Analysis. Stem Cells Dev (2014) 23:2535–52. doi: 10.1089/scd.2014.0123
32. de Lima M, Bonamino M, Vasconcelos Z, Colares M, Diamond H, Zalcberg I, et al. Prophylactic donor lymphocyte infusions after moderately ablative chemotherapy and stem cell transplantation for hematological malignancies: high remission rate among poor prognosis patients at the expense of graft-versus-host disease. Bone Marrow Transplant (2001) 27:73–8. doi: 10.1038/sj.bmt.1702726
33. Goodyear OC, Dennis M, Jilani NY, Loke J, Siddique S, Ryan G, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood. (2012) 119:3361–9. doi: 10.1182/blood-2011-09-377044
34. Santourlidis S, Trompeter HI, Weinhold S, Eisermann B, Meyer KL, Wernet P, et al. Crucial role of DNA methylation in determination of clonally distributed killer cell Ig-like receptor expression patterns in NK cells. J Immunol (2002) 169:4253–61. doi: 10.4049/jimmunol.169.8.4253
35. Schroeder T, Fröbel J, Cadeddu RP, Czibere A, Dienst A, Platzbecker U, et al. Salvage therapy with azacitidine increases regulatory T cells in peripheral blood of patients with AML or MDS and early relapse after allogeneic blood stem cell transplantation. Leukemia (2013) 27:1910–3. doi: 10.1038/leu.2013.64
36. Metzelder SK, Schroeder T, Finck A, Scholl S, Fey M, Gotze K, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia (2012) 26:2353–9. doi: 10.1038/leu.2012.105
37. Tschan-Plessl A, Halter JP, Heim D, Medinger M, Passweg JR, Gerull S. Synergistic effect of sorafenib and cGvHD in patients with high-risk FLT3-ITD+AML allows long-term disease control after allogeneic transplantation. Ann Hematol (2015) 94:1899–905. doi: 10.1007/s00277-015-2461-5
38. Mathew NR, Baumgartner F, Braun L, O’Sullivan D, Thomas S, Waterhouse M, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med (2018) 24:282–91. doi: 10.1038/nm.4484
39. Schuurhuis GJ, Heuser M, Freeman S, Béné MC, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European Leukemia Net MRD Working Party. Blood (2018) 131:1275–91. doi: 10.1182/blood-2017-09-801498
40. Hourigan CS, Dillon LW, Gui G, Logan BR, Fei M, Ghannam J, et al. Impact of Conditioning Intensity of Allogeneic Transplantation for Acute Myeloid Leukemia With Genomic Evidence of Residual Disease. J Clin Oncol (2020) 38:1273–83. doi: 10.1200/JCO.19.03011
41. Shah MV, Jorgensen JL, Saliba RM, Wang SA, Alousi AM, Andersson BS, et al. Early Post-Transplant Minimal Residual Disease Assessment Improves Risk Stratification in Acute Myeloid Leukemia. Biol Blood Marrow Transplant (2018) 24:1514–20. doi: 10.1016/j.bbmt.2018.02.003
42. Spyridonidis A. How I treat measurable (minimal) residual disease in acute leukemia after allogeneic hematopoietic cell transplantation. Blood (2020) 135:1639–49. doi: 10.1182/blood.2019003566
43. Ravandi F, Walter RB, Freeman SD. Evaluating measurable residual disease in acute myeloid leukemia. Blood Adv (2018) 2:1356–66. doi: 10.1182/bloodadvances.2018016378
44. Tsirigotis P, Byrne M, Schmid C, Baron F, Ciceri F, Esteve J, et al. Relapse of AML after hematopoietic stem cell transplantation: Methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant (2016) 51:1431–8. doi: 10.1038/bmt.2016.167
45. Bastos-Oreiro M, Perez-Corral A, Martínez-Laperche C, Bento L, Pascual C, Kwon M, et al. Prognostic impact of minimal residual disease analysis by flow cytometry in patients with acute myeloid leukemia before and after allogeneic hemopoietic stem cell transplantation. Eur J Haematol (2014) 93:239–46. doi: 10.1111/ejh.12336
46. Rossi G, Carella AM, Minervini MM, Savino L, Fontana A, Pellegrini F, et al. Minimal residual disease after allogeneic stem cell transplant: A comparison among multiparametric flow cytometry, Wilms tumor 1 expression and chimerism status (Complete chimerism versus Low Level Mixed Chimerism) in acute leukemia. Leuk Lymph (2013) 54:2660–6. doi: 10.3109/10428194.2013.789508
47. Rossi G, Nomdedeu Guinot JF, Fontana A, Minervini MM, Garcia-Dabrio MC, Cascavilla N. CD117-CD15 in acute myeloid leukemia: no role as LAIP in the study of minimal residual disease. Eur J Haematol (2013) 90:171–4. doi: 10.1111/ejh.12042
48. Bornhauser M, Thiede C. Chimerism analysis after allogeneic stem cell transplantation. Haematologica (2005) 90:1301A.
49. Thiede C, Bornhäuser M, Ehninger G. Evaluation of STR informativity for chimerism testing–comparative analysis of 27 STR systems in 203 matched related donor recipient pairs. Leukemia (2004) 18:248–54. doi: 10.1038/sj.leu.2403212
50. Stahl T, Rothe C, Böhme MU, Kohl A, Kröger N, Fehse B. Digital PCR Panel for Sensitive Hematopoietic Chimerism Quantification after Allogeneic Stem Cell Transplantation. Int J Mol Sci (2016) 17:1515. doi: 10.3390/ijms17091515
51. Bornhäuser M, Oelschlaegel U, Platzbecker U, Bug G, Lutterbeck K, Kiehl MG. Monitoring of donor chimerism in sorted CD34+ peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica (2009) 94:1613–7. doi: 10.3324/haematol.2009.007765
52. Buccisano F, Maurillo L, Schuurhuis GJ, Del Principe MI, Di Veroli A, Gurnari C, et al. The emerging role of measurable residual disease detection in AML in morphologic remission. Semin Hematol (2019) 56:125–30. doi: 10.1053/j.seminhematol.2018.09.001
53. Voso MT, Ottone T, Lavorgna S, Venditti A, Maurillo L, Lo-Coco F, et al. MRD in AML: The Role of New Techniques. Front Oncol (2019) 9:655. doi: 10.3389/fonc.2019.00655
54. Schnittger S, Kern W, Tschulik C, Weiss T, Dicker F, Falini B, et al. Minimal residual disease levels assessed by NPM1 mutation-specific RQ-PCR provide important prognostic information in AML. Blood (2009) 114:2220–31. doi: 10.1182/blood-2009-03-213389
55. Shayegi N, Kramer M, Bornhauser M, Schaich M, Schetelig J, Platzbecker U, et al. The level of residual disease based on mutant NPM1 is an independent prognostic factor for relapse and survival in AML. Blood (2013) 122:83–92. doi: 10.1182/blood-2012-10-461749
56. Wang Y, Wu DP, Liu QF, Qin YZ, Wang JB, Xu LP, et al. In adults with t(8;21)AML, posttransplant RUNX1/RUNX1T1-based MRD monitoring, rather than c-KIT mutations, allows further risk stratification. Blood (2014) 124:1880–6. doi: 10.1182/blood-2014-03-563403
57. Qin YZ, Wang Y, Xu LP, Zhang XH, Chen H, Han W, et al. The dynamics of RUNX1-RUNX1T1 transcript levels after allogeneic hematopoietic stem cell transplantation predict relapse in patients with t(8;21) acute myeloid leukemia. J Hematol Oncol (2017) 10:44. doi: 10.1186/s13045-017-0414-2
58. Cloos J, Goemans BF, Hess CJ, van Oostveen JW, Waisfisz Q, Corthals S, et al. Stability and prognostic influence of FLT3 mutations in paired initial and relapsed AML samples. Leukemia (2006) 20:1217–20. doi: 10.1038/sj.leu.2404246
59. Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, et al. Real-Time Quantitative Polymerase Chain Reaction Detection of Minimal Residual Disease by StandardizedWT1Assay to Enhance Risk Stratification in Acute Myeloid Leukemia: A European LeukemiaNet Study. J Clin Oncol (2009) 27:5195–201. doi: 10.1200/JCO.2009.22.4865
60. Cho BS, Min GJ, Park SS, Shin SH, Yahng SA, Jeon YW, et al. WT1 Measurable Residual Disease Assay in Patients With Acute Myeloid Leukemia Who Underwent Allogeneic Hematopoietic Stem Cell Transplantation: Optimal Time Points, Thresholds, and Candidates. Biol Blood Marrow Transplant (2019) 25:1925–32. doi: 10.1016/j.bbmt.2019.05.033
61. Rautenberg C, Pechtel S, Hildebrandt B, Betz B, Dienst A, Nachtkamp K, et al. Wilms’ Tumor 1 Gene Expression Using a Standardized European LeukemiaNet-Certified Assay Compared to Other Methods for Detection of Minimal Residual Disease in Myelodysplastic Syndrome and Acute Myelogenous Leukemia after Allogeneic Blood Stem Cell Transplantation. Biol Blood Marrow Transplant (2018) 24:2337–43. doi: 10.1016/j.bbmt.2018.05.011
62. Nomdedéu JF, Esquirol A, Carricondo M, Pratcorona M, Hoyos M, Garrido A, et al. Bone Marrow WT1 Levels in Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplasia: Clinically Relevant Time Points and 100 Copies Threshold Value. Biol Blood Marrow Transplant (2018) 24:55–63. doi: 10.1016/j.bbmt.2017.09.001
63. Schmitt MW, Kennedy SR, Salk JJ, Fox EJ, Hiatt JB, Loeb LA. Detection of ultra-rare mutations by next-generation sequencing. Proc Natl Acad Sci USA (2012) 109:14508–13. doi: 10.1073/pnas.1208715109
64. Kim T, Moon JH, Ahn JS, Kim YK, Lee SS, Ahn SY, et al. Next-generation sequencing-based posttransplant monitoring of acute myeloid leukemia identifies patients at high risk of relapse. Blood. (2018) 132:1604–13. doi: 10.1182/blood-2018-04-848028
65. Getta BM, Devlin SM, Levine RL, Arcila ME, Mohanty AS, Zehir A, et al. Multicolor Flow Cytometry and Multigene Next-Generation Sequencing Are Complementary and Highly Predictive for Relapse in Acute Myeloid Leukemia after Allogeneic Transplantation. Biol Blood Marrow Transplant (2017) 23:1064–71. doi: 10.1016/j.bbmt.2017.03.017
66. de Lima MG, Thall S, de Padua Silva PF, Jones L, Komanduri RB, Braun K, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoi- etic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. (2010) 116:5420–31. doi: 10.1002/cncr.25500
67. Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia (2012) 26:381–9. doi: 10.1038/leu.2011.234
68. Oshikawa G, Kakihana K, Saito M, Aoki J, Najima Y, Kobayashi T, et al. Post-transplant maintenance therapy with azacitidine and gemtuzumab ozogamicin for high-risk acute myeloid leukaemia. Br J Haematol (2015) 169:756–9. doi: 10.1111/bjh.13248
69. Pusic I, Choi J, Fiala MA, Gao F, Holt M, Cashen AF, et al. Maintenance therapy with decitabine after allogeneic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant (2015) 21:1761–9. doi: 10.1016/j.bbmt.2015.05.026
70. Han S, Kim YJ, Lee J, Jeon S, Hong T, Park GJ, et al. Model-based adaptive phase I trial design of post-transplant decitabine maintenance in myelodysplastic syndrome. J Hematol Oncol (2015) 8:118. doi: 10.1186/s13045-015-0208-3
71. Craddock C, Jilani N, Siddique S, Yap C, Khan J, Nagra S, et al. Tolerability and clinical activity of post-transplantation azacitidine in patients allografted for acute myeloid leukemia treated on the RICAZA trial. Biol Blood Marrow Transplant (2016) 22:385–90. doi: 10.1016/j.bbmt.2015.09.004
72. El-Cheikh J, Massoud R, Fares E, Kreidieh N, Mahfouz R, Charafeddine M, et al. Low-dose 5-azacytidine as preventive therapy for relapse of AML and MDS following allogeneic HCT. Bone Marrow Transplant (2017) 52:918–21. doi: 10.1038/bmt.2017.31
73. Platzbecker U, Middeke JM, Sockel K, Herbst R, Wolf D, Baldus CD, et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): An open-label, multicentre, phase 2 trial. Lancet Oncol (2018) 19:1668–79. doi: 10.1016/S1470-2045(18)30580-1
74. Oran B, deLima M, Garcia-Manero G, Thall PF, Lin R, Alousi AM, et al. Maintenance with 5-azacytidine for acute myeloid leukemia and myelodysplastic syndrome patients. Blood (2018) 132:971. doi: 10.1182/blood-2018-99-111582
75. de Lima M, Oran B, Champlin RE, Papadopoulos EB, Giralt SA, Scott BL, et al. CC-486 maintenance after stem cell transplantation in patients with acute myeloid leukemia or myelodysplastic syndromes. Biol Blood Marrow Transplant (2018) 24:2017–24. doi: 10.1016/j.bbmt.2018.06.016
76. Marini C, Brissot E, Bazarbachi A, Duléry R, Sestili S, Pattipaglia G, et al. Tolerability and efficacy of treatment with azacytidine as prophylactic or pre-emptive therapy for myeloid neoplasms after allogeneic stem cell transplantation. Clin Lymph Myeloma Leuk (2020) 20:377–82. doi: 10.1016/j.clml.2019.10.011
77. Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol (2010) 28:562–9. doi: 10.1200/JCO.2009.23.8329
78. Czibere A, Bruns I, Kroger N, Platzbecker U, Lind J, Zohren F, et al. 5-Azacytidine for the treatment of patients with acute myeloid leukemia or myelodysplastic syndrome who relapse after allo-SCT: a retrospective analysis. Bone Marrow Transplant (2010) 45:872–6. doi: 10.1038/bmt.2009.266
79. Jabbour E, Giralt S, Kantarjian H, Garcia-Manero G, Jagasia M, Kebriaei P, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer. (2009) 115:1899–905. doi: 10.1002/cncr.24198
80. Chen YB, Li S, Lane AA, Connolly C, Del Rio C, Valles B, et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol Blood Marrow Transplant (2014) 20:2042–8. doi: 10.1016/j.bbmt.2014.09.007
81. Antar A, Kharfan-Dabaja MA, Mahfouz R, Bazarbachi A. Sorafenib Maintenance Appears Safe and Improves Clinical Outcomes in FLT3-ITD Acute Myeloid Leukemia After Allogeneic Hematopoietic Cell Transplantation. Clin Lymph Myeloma Leuk (2015) 15:298–302. doi: 10.1016/j.clml.2014.12.005
82. Brunner AM, Li S, Fathi AT, Wadleigh M, Ho VT, Collier K, et al. Haematopoietic cell transplantation with and without sorafenib maintenance for patients with FLT3-ITD acute myeloid leukaemia in first complete remission. Br J Haematol (2016) 175:496–504. doi: 10.1111/bjh.14260
83. Battipaglia G, Ruggeri A, Massoud R, El Cheikh J, Jestin M, Antar A, et al. Efficacy and feasibility of sorafenib as a maintenance agent after allogeneic hematopoietic stem cell transplantation for Fms-like tyrosine kinase 3-mutated acute myeloid leukemia. Cancer (2017) 123:2867–74. doi: 10.1002/cncr.30680
84. Battipaglia G, Massoud R, Ahmed SO, Legrand O, El Cheikh J, Youniss R, et al. Efficacy and Feasibility of Sorafenib as a Maintenance Agent After Allogeneic Hematopoietic Stem Cell Transplantation for Fms-like Tyrosine Kinase 3 Mutated Acute Myeloid Leukemia: An Update. Clin Lymph Myeloma Leuk (2019) 19:506–8. doi: 10.1016/j.clml.2019.04.004
85. Bazarbachi A, Labopin M, Battipaglia G, Azedine Djabali A, Forcade E, Arcese W, et al. Allogeneic Stem Cell Transplantation for FLT3-Mutated Acute Myeloid Leukemia: In vivo T-Cell Depletion and Posttransplant Sorafenib Maintenance Improve Survival. A Retrospective Acute Leukemia Working Party-European Society for Blood and Marrow Transplant Study. Clin Hematol Int (2019) 1:58–74. doi: 10.2991/chi.d.190310.001
86. Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Röllig C, et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN). J Clin Oncol (2020) 38:2993–3002. doi: 10.1200/JCO.19.03345
87. Xuan L, Wang Y, Huang F, Fan Z, Xu Y, Sun J, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol (2020) 21:1201–12. doi: 10.1016/S1470-2045(20)30455-1
88. Maziarz RTT, Patnaik MM, Scott BL, Mohan SR, Deol A, Rowley SD, et al. Radius: A Phase 2 Randomized Trial Investigating Standard of Care ± Midostaurin after Allogeneic Stem Cell Transplant in FLT3-ITD-Mutated AML. Blood (2018) 132(Suppl 1):662. doi: 10.1182/blood-2018-99-113582
89. Schlenk RF, Weber D, Fiedler W, Salih HR, Wulf G, Salwender H, et al. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood (2019) 133:840–51. doi: 10.1182/blood-2018-08-869453
90. Sandmaier BM, Khaled S, Oran B, Gammon G, Trone D, Frankfurt O. Results of a phase 1 study of quizartinib as maintenance therapy in subjects with acute myeloid leukemia in remission following allogeneic hematopoietic stem cell transplant. Am J Hematol (2018) 93:222–31. doi: 10.1002/ajh.24959
91. Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. (2002) 100:1532–42. doi: 10.1182/blood-2002-02-0492
92. Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med (2016) 374:2209–21. doi: 10.1056/NEJMoa1516192
93. Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood (2002) 99:4326–35. doi: 10.1182/blood.V99.12.4326
94. Deol A, Sengsayadeth S, Ahn KW, Wang HL, Aljurf M, Antin JH, et al. Does FLT3 mutation impact survival after hematopoietic stem cell transplantation for acute myeloid leukemia? A Center for International Blood and Marrow Transplant Research (CIBMTR) analysis. Cancer (2016) 122:3005–14. doi: 10.1002/cncr.30140
95. Schiller GJ, Tuttle P, Desai P. Allogeneic Hematopoietic Stem Cell Transplantation in FLT3-ITD-Positive Acute Myelogenous Leukemia: The Role for FLT3 Tyrosine Kinase Inhibitors Post-Transplantation. Biol Blood Marrow Transplant (2016) 22:982–90. doi: 10.1016/j.bbmt.2016.01.013
96. Salem R, Massoud R, Haffar B, Mahfouz R, Bazarbachi A, El-Cheikh J. Dynamics of molecular response in AML patients with NPM1 and FLT3 mutations undergoing allogeneic stem cell transplant. Bone Marrow Transplant (2017) 52:1187–90. doi: 10.1038/bmt.2017.82
97. Brunet S, Labopin M, Esteve J, Cornelissen J, Socié G, Iori AP, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol (2012) 30:735–41. doi: 10.1200/JCO.2011.36.9868
98. Sharma M, Ravandi F, Bayraktar UD, Chiattone A, Bashir Q, Giralt S, et al. Treatment of FLT3-ITD-positive acute myeloid leukemia relapsing after allogeneic stem cell transplantation with sorafenib. Biol Blood Marrow Transplant (2011) 17:1874–7. doi: 10.1016/j.bbmt.2011.07.011
99. Sid S, Rey J, Charbonnier A, D’Incan E, Mohty B, Blaise D, et al. Treatment of Post-transplant Relapse of FLT3-ITD Mutated AML Using 5-Azacytidine and Sorafenib Bitherapy. Clin Lymph Myeloma Leuk (2017) 17:241–2. doi: 10.1016/j.clml.2016.10.002
100. Sato T, Yang X, Knapper S, White P, Smith BD, Galkin S, et al. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood (2011) 117:3286–93. doi: 10.1182/blood-2010-01-266742
101. Antar AI, Otrock ZK, Jabbour E, Mohty M, Bazarbachi A. FLT3 inhibitors in acute myeloid leukemia: ten frequently asked questions. Leukemia (2020) 34:682–96. doi: 10.1038/s41375-019-0694-3
102. Bazarbachi AH, Al Hamed R, Malard F, Mohty M, Bazarbachi A. Allogeneic transplant for FLT3-ITD mutated AML: a focus on FLT3 inhibitors before, during, and after transplant. Ther Adv Hematol (2019) 10:2040620719882666. doi: 10.1177/2040620719882666
103. Yafour N, Beckerich F, Bulabois CE, Chevallier P, Daguindau É, Dumesnil C, et al. How to prevent relapse after allogeneic hematopoietic stem cell transplantation in patients with acute leukemia and myelodysplastic syndrome. Curr Res Transl Med (2017) 65:65–9. doi: 10.1016/j.retram.2017.06.001
104. Antar A, Otrock ZK, El-Cheikh J, Kharfan-Dabaja MA, Battipaglia G, Mahfouz R, et al. Inhibition of FLT3 in AML: a focus on sorafenib. Bone Marrow Transplant (2017) 52:344–51. doi: 10.1038/bmt.2016.251
105. Bazarbachi A, Bug G, Baron F, Brissot E, Ciceri F, Dalle IA, et al. Clinical practice recommendation on hematopoietic stem cell transplantation for acute myeloid leukemia patients with FLT3-internal tandem duplication: a position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica (2020) 105:1507–16. doi: 10.3324/haematol.2019.243410
106. Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med (2017) 377:454–64. doi: 10.1056/NEJMoa1614359
107. Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N Engl J Med (2019) 381:1728–40. doi: 10.1056/NEJMoa1902688
108. Perl AE, Martinelli G, Neubauer A, Berman E, Baer MR, Larson RA, et al. Long-term survivors and gilteritinib safety beyond one year in FLT3-mutated R/R AML: ADMIRAL trial follow-up. J Clin Oncol (2020) 38:7514. doi: 10.1200/JCO.2020.38.15_suppl.7514
109. Levis MJ, Hamadani M, Logan B, Rosales M, Perl AE, Devine SM, et al. A phase 3, trial of gilteritinib, as maintenance therapy after allogeneic hematopoietic stem cell transplantation in patients with FLT3-ITD+ AML. J Clin Oncol (2018) 36(15_Suppl):TPS7075. doi: 10.1200/JCO.2018.36.15_suppl.TPS7075
110. Guillaume T, Malard F, Magro L, Labopin M, Tabrizi R, Borel C, et al. Prospective phase II study of prophylactic low-dose azacitidine and donor lymphocyte infusions following allogeneic hematopoietic stem cell transplantation for high-risk acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant (2019) 54:1815–26. doi: 10.1038/s41409-019-0536-y
111. DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N Engl J Med (2018) 378:2386–98. doi: 10.1056/NEJMoa1716984
112. Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. (2017) 130:722–31. doi: 10.1182/blood-2017-04-779405
113. DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol (2018) 93:401–7. doi: 10.1002/ajh.25000
114. Aldoss I, Yang D, Aribi A, Ali H, Sandhu K, Al Malki MM, et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica (2018) 103:e404–7. doi: 10.3324/haematol.2018.188094
115. Moukalled N, El Darsa H, Haibe Y, Massoud R, Kanj SS, Mahfouz R, et al. Feasibility of Venetoclax-based combinations for adult patients with acute myeloid leukemia relapsing after allogenic stem cell transplantation. Bone Marrow Transplant (2019) 54:620–4. doi: 10.1038/s41409-018-0347-6
116. Vigil C, Silverman M, Carter T. Hypomethylating Agents and Low-Dose Venetoclax for Relapse Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant (2020) 26:S104. doi: 10.1016/j.bbmt.2019.12.608
117. DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood (2020) 135:791–803. doi: 10.1182/blood.2019003988
118. Ravandi F, Alattar ML, Grunwald MR, Rudek MA, Rajkhowa T, Richie MA, et al. Phase II study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood (2013) 121:4655–62. doi: 10.1182/blood-2013-01-480228
119. Cortes JE, Heidel FH, Heuser M, Fiedler W, Smith BD, Robak T. A phase 2 randomized study of low dose Ara-C with or without Glasdegib (PF-04449913) in untreated patients with acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood (2016) 128:99. doi: 10.1182/blood.V128.22.99.99
Keywords: acute myeloid leukemia, stem cell transplantation, allogeneic, relapse, prevention, hypomethylating agents
Citation: Antar AI, Otrock ZK, Abou Dalle I, El-Cheikh J and Bazarbachi A (2020) Pharmacologic Therapies to Prevent Relapse of Acute Myeloid Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation. Front. Oncol. 10:596134. doi: 10.3389/fonc.2020.596134
Received: 18 August 2020; Accepted: 09 October 2020;
Published: 02 November 2020.
Edited by:
Jacopo Peccatori, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Matthew D. Dun, University of Newcastle, AustraliaCopyright © 2020 Antar, Otrock, Abou Dalle, El-Cheikh and Bazarbachi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Bazarbachi, YmF6YXJiYWNAYXViLmVkdS5sYg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.