- 1Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, TX, United States
- 2Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, TX, United States
Cancer/Testis (C/T) antigens are a group of antigens, expressed in almost all types of cancers, which can elicit an immune response in patients whose cancers express these antigens. They are currently of great interest as targets for the development of cancer biomarkers and the creation of immunotherapies that directly target tumors in patients. Currently there are 280 C/T antigens and their variants listed on the C/T antigen data base. All known C/T antigens are encoded for by genes which are normally only expressed in the male testis; specifically during the process of spermatogenesis. They are therefore only expressed in germ cells that are in the process of differentiating into sperm. Expression of C/T antigens in tumors is thus a biological anomaly as, with the exception of germ cell tumors, cancers arise from somatic tissues which are not known to express any of the genes specifically involved in spermatogenesis. How and why C/T antigens are expressed in tumors remains an enigma. In this paper we present a hypothesis which proposes a mechanism for the activation of C/T antigen encoding genes in tumors. We propose that aberrant activation of the human autosomal retrogene, EIF2S3B, which regulates initiation and maintenance of spermatogenesis in males, is responsible for C/T expression. Because both male and females have tumors that express C/T antigens activation of spermatogenesis genes in tumors must involve a non-sex specific pathway. This can be explained by the copy number of EIF2S3 genes uniquely present within the human genome.
Introduction
One of the characteristics of cancers is their ability to express genes beyond the pattern that is normally found in the cells or tissues from which they originate. The universal nature of this ability is due to the selective advantage that may be conferred to cells during oncogenesis, tumor growth, tumor maintenance and metastasis. These advantages are manifested as dysregulation of cell division, changes in cell metabolism (such as a shift to glycolysis and expression of the testis specific retrogene PGK2), increased protein synthesis, avoidance of the immune system, recruitment of blood vessels and an ability to spread beyond the site of origin. Of particular interest is the aberrant expression of genes that are normally associated with the process of spermatogenesis that typically only occurs in the male testis. The expression of spermatogenesis associated genes in a cancer was first noted in 1991 (1) and by 1997 strategies had been developed to identify cancer specific antigens that elicit an immune response so that the number of recognized C/T antigens was significantly increased across a variety of cancers (2, 3). Currently 280 C/T antigens and their variants are listed on the CTDatabase (http://www.cta.lncc.br/). There has been an explosion of interest in the use of these antigens as cancer biomarkers and therapeutic targets (4–8). In spite of this, there has been little effort expended to understand the relationship between C/T antigen expression and cancer biology.
A direct link between gametogenesis and cancer was first proposed by Old in 2001 (9). Several groups have explored the relationship between expression of some of the C/T antigens during the process of spermatogenesis and their potential contribution to cancer development (10–13). It is now well established that C/T antigens are encoded for by a variety of genes that are activated during all the various stages of spermatogenesis (10). One possible explanation for the activation C/T antigens in cancers is that both spermatogenesis and oncogenesis may share common pathways of gene activation. An understanding of the spermatogenic pathways that lead to sperm production in the human testis could thus provide an explanation for the aberrant expression of spermatogenesis associated genes in tissues outside of the testis.
Testis display a phenomenon known as “immune privilege” whereby spermatogenesis is protected from the immune system. This means that antigens produced by meiotic and haploid germ cells, that are present in the testis of males post puberty, are protected from autoimmune attack even though they are produced long after the formation of systemic self-tolerance. In contrast any spermatogenesis associated proteins that are produced by tumors are not protected from the immune system and, as such, are immunogenic and can be recognized as being outside of systemic self-tolerance which is established during fetal development. Thus cancerous cells, which are derived from somatic tissues, can illicit an immune response to spermatogenesis associated proteins which may be produced via expression of spermatogenesis specific genes. This is particularly the case if these antigens are released into the blood stream by the tumor and give rise to the C/T antigens.
Recently it has been demonstrated that activation of the gene EIF2S3Y is primarily responsible for the initiation of spermatogenesis in the testis of juvenile male mammals and its subsequent maintenance in the adult. Most mammals carry two versions of EIF2S3 within their genomes, one on the X chromosome (EIF2S3X) and the other on the Y chromosome (EIF2S3Y). Mouse models have firmly established that the initiation of spermatogenesis in the juvenile testis, and its subsequent maintenance in the adult, is regulated by the Y chromosome linked gene EIF2S3Y (14–17). This pattern of gene expression is currently accepted for most mammalian species. However, humans are an exception to this, having lost EIF2S3Y from the human Y chromosome (18). Its function has been replaced by a retrotransposed copy of EIF2S3X that is located on human chromosome 12 and known as EIF2S3B (19). This is depicted in Figure 1. This results in human females carrying four copies of the EIF2S3 gene (two on the X chromosome and two on chromosome 12) versus males that carry three copies (one on the X chromosome and two on chromosome 12). This contrasts with the ancestral situation in most mammals where both males and females carry two copies of the EIF2S3 gene, with females having two copies (one on each X chromosome) and males having two copies (one on the X chromosome and another on the Y chromosome). Thus, unlike most other mammals, initiation and maintenance of spermatogenesis in humans is regulated by the activation of an autosomal retrogene (EIF2S3B) that is shared by both males and females. It is this uniquely human situation that could lead to activation of EIF2S3B during oncogenesis and cancer development and provide an explanation for the expression of C/T antigens in the tumors of both human sexes.
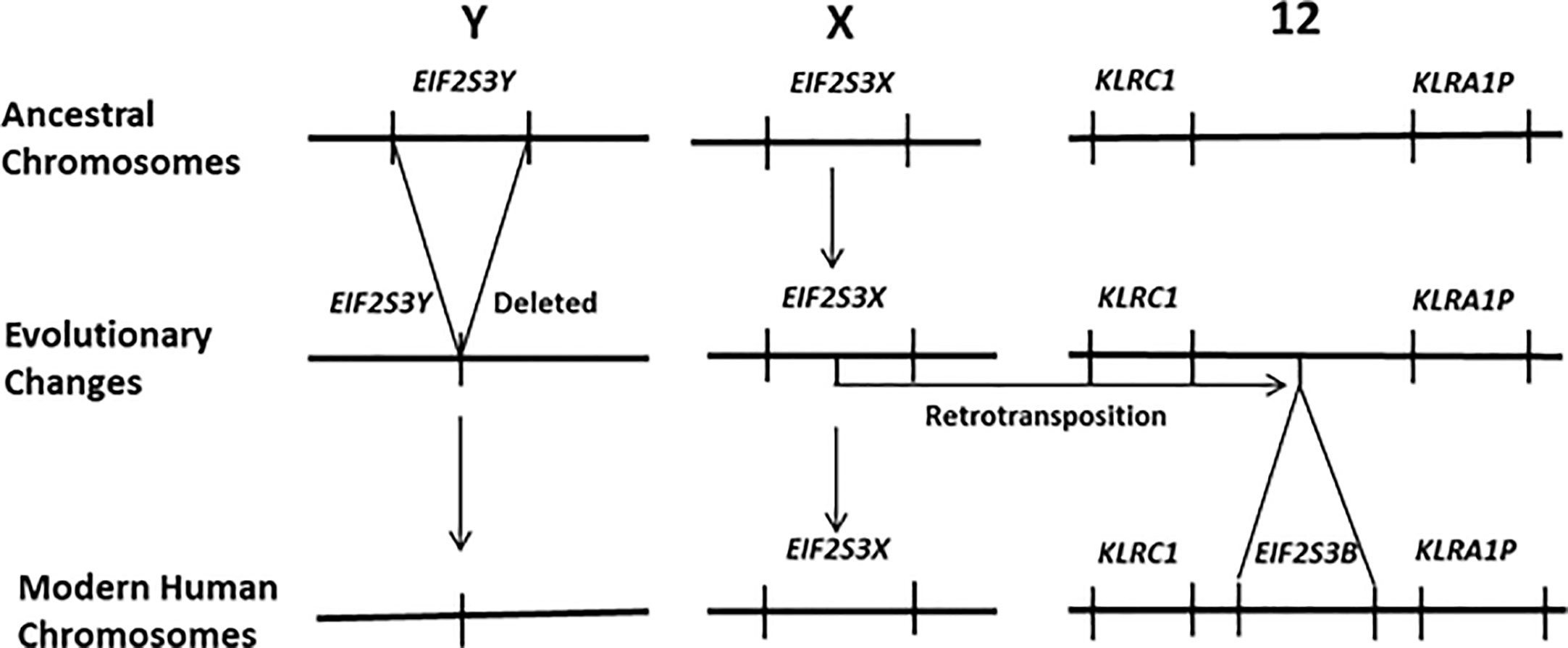
Figure 1 In humans the EIF2S3Y gene has been deleted from the Y chromosome. It has been functionally replaced by a new gene that arose via a retrotransposition event that moved an intronless copy of EIF2S3X on to human chromosome 12 giving rise to the retrogene EIF2S3B. It is EIF2S3B that now regulates the initiation and maintenance of spermatogenesis in the human testis. Females carry four copies of the EIF2S3 gene. Each of the two X chromosomes contains a copy of EIF2S3X and two copies EIF2S3B located on the chromosome 12 pair within their genome. In contrast males possess only three copies of EIF2S3 within their genome. A single copy of EIF2S3X on the X chromosome and two copies EIF2S3B located on the chromosome 12 pair.
It is our hypothesis that activation of the autosomal retrogene, EIF2S3B, in somatic cells, pre-cancerous cells, or tumors results in the expression of genes normally involved only in spermatogenesis. This in turn results in the production of the C/T antigens by human cancers.
Supporting Evidence
EIF2S3B Expression and Regulation of Spermatogenesis
EIF2S3X is an essential housekeeping gene that is ubiquitously expressed in all male and female mammals. EIF2S3 is a subunit of the eukaryotic initiation factor 2 (eIF2) that is involved in the initiation of protein synthesis. When bound to GTP, eIF2 forms a tertiary complex with tRNA Met-tRNAi which subsequently binds to the 40S ribosomal complex. These interactions are rate limiting in eukaryotic protein synthesis. The 40S ribosomal complex binds to the start codon of the protein encoding mRNA to form the 43S pre-initiation complex. Cleavage of the GTP precedes junction with the 60S ribosomal subunit and formation of the 80S ribosome that translates the bound mRNA. Hydrolysis of the GTP results in the release of the eIF2-GDP binary complex, which is recycled. Expression of EIF2S3X in mammalian cells is therefore essential for protein synthesis. EIF2S3Y presumably has a similar function in spermatogonial stem cells. In this case it is responsible for the initiation and maintenance of mRNA translation that is associated with the expression of spermatogenesis-specific genes during male gametogenesis.
The essential role of EIF2S3Y in the initiation of spermatogenesis was first identified, and subsequently explored, in mouse models. In 2001 Mazeyrat et al. (14) reported that in male mice carrying the Spy deletion on the Y chromosome, which phenotypically displays a failure to initiate spermatogenesis in the testis, could have spermatogenesis restored by expression of EIF2S3Y transgenes. This demonstrated that in mice only two genes were absolutely necessary for spermatogenesis; the gene SRY that drives testis development in the embryonic male and EIF2S3Y that is required for completion of the first meiotic division of spermatogenesis. This work was later confirmed and expanded upon by Yamauchi et al. (17) who used female mice containing a single X chromosome (XO) supplemented with SRY transgenes and an X chromosome linked transgenically derived copy of EIF2S3Y. the presence of the SRY transgene in these mice (XOSry mice) results in the development of testis populated with spermatogonia. In mice carrying the X linked EIF2S3Y transgene (XEO mice), addition of the SRY transgene (XEOSry mice) results in testis development, initiation of spermatogenesis and development of sperm up until the round spermatid stage. In 2016 Yamauchi et al. (17) were able to create XO mice which were completely devoid of any Y chromosome genes that were able to produce sperm. This was achieved by developing a line of mice containing a SOX9 transgene (XOSox9 mice) that develop testis. When these mice were made transgenic with overexpressing EIF2S3X transgenes (XOSox9, Eif2s3x mice) spermatogenesis preceded up to the round spermatid stage. This clearly demonstrated that regulated over expression of the X chromosome linked gene, EIF2S3X, can substitute of EIF2S3Y in the process of initiating spermatogenesis in testis. It also shows that EIF2S3X and EIF2S3Y share functional equivalence with regard to the initiation and regulation of protein synthesis. In addition it has now been definitively established that a high level of EIF2S3 expression is required for the initiation of spermatogenesis in the juvenile testis and its maintenance in the adult male. Depending on the developmental stage EIF2S3Y expression during spermatogenesis is 4–39 times higher than of EIF2S3X in the testis (Table 1). EIF2S3X expression decreases during sperm development so that the process is eventually dominated by EIF2S3Y. This would suggest that EIF2S3Y expression is regulated by a testis specific mechanism that does not co-regulate EIF2S3X. Thus a testis specific pathway regulates the levels of EIF2S3Y expression that facilitate the high rates of protein synthesis required to maintain healthy levels of spermatogenesis in the testis.
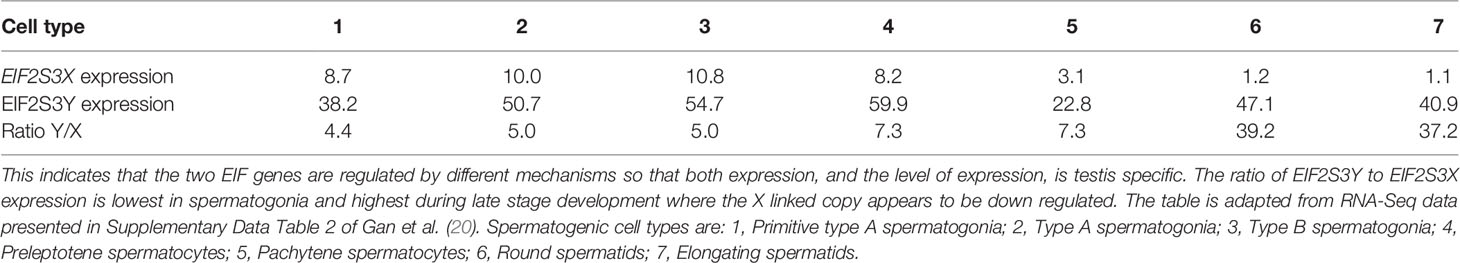
Table 1 During spermatogenesis in mice levels of EIF2S3Y expression significantly exceed those of EIF2S3X during both early and late stages of sperm development.
In humans two autosomal copies of the EIF2S3B retrogene functionally substitute for the loss of a single copy of EIF2S3Y that has been deleted from the human Y chromosome (18). Human females carrying four copies of the EIF2S3 gene within their genome; a copy of EIF2S3X on each of the two X chromosomes and a copy of EIF2S3B on the autosomal chromosome 12 pair. In contrast human males have only three copies of EIF2S3 within their genomes; one copy of EIF2S3X on the single X chromosome within the male genome and two copies of EIF2S3B on the chromosome 12 pair. The X linked copy of EIF2S3 is expressed in all cells within the human body but the retrogene EIF2S3B is only expressed in the male testis at puberty and there is no evidence on its expression in any other male or female tissue.
Because of its critical function in protein synthesis it would be expected that there would be an advantage in maximizing EIF2S3 expression in somatic cells. This is indeed the case in human females where EIF2S3X is one of the few X chromosome genes that escape X inactivation during embryonic development, so that both copies are transcriptionally active in females (19, 21). In males there is only one active copy of EIF2S3X and expression of EIF2S3B is tightly regulated so that outside of the testis any expressional leakage is biologically insignificant. This is evidenced by the fact that several X linked human disorders associated with mutations in the EIF2S3X gene that predominantly affect males have been identified. The most commonly encountered is X linked intellectual disability syndrome (MEHMO) which in the male is characterized by profound intellectual disability, epilepsy, hypogonadism, hypogenitalism, microcephaly, obesity and growth retardation that may be lethal. Female carriers are unaffected and several different family specific mutations in EIF2S3X have been linked to this disorder (22–24). A mild variation of EIF2S3X linked disease has also been identified and results in hypopituitarism and dysregulation of glucose metabolism (25). The fact that mutations within the EIF2S3X gene predominantly affect males, but not females who are obligate carriers, indicates that any incidental expression of EIF2S3B in male tissues outside of the testis is insufficient to make up for the absence of a second functional copy of EIF2S3X. It can thus be concluded that functional expression of EIF2S3B is tightly regulated in humans and its expression is primarily limited to the testis of males where it regulates the activation of spermatogenesis genes.
Link Between Spermatogenesis and Tumor Development
The presence of two autosomal copies of EIF2S3B in humans leads to the hypothesis that aberrant activation of EIF2S3B during cancer development could result in the activation of spermatogenic pathways within cancer stem cells in both males and females. Because levels of EIF2S3 expression represent a “choke point” during protein synthesis, it is logical to propose that supplementation of EIF2S3X by activation of EIF2S3B in tumors would result in enhanced protein synthesis. This, in turn, would increase the rate at which proteins are produced, accumulated or replaced, thus promoting tumor cell division, growth and survival. Since activation of spermatogenic pathways is dependent on the level of EIF2S3 expression as outlined above, aberrant activation of EIF2S3B during tumor-genesis and differentiation could regulate the expression of C/T antigen genes. In turn this leads to the production of C/T antigens in tumors.
Discussion
Activation of EIF2S3B has been documented in thirty five different types of human cancers (https://cansarblack.icr.ac.uk/target/Q2VIR3/expression, https://www.proteinatlas.org/ENSG00000180574-EIF2S3B/pathology). However, activation of EIF2S3B appears to be sample specific so that this cannot be considered a universal characteristic of all tumors. This observation is consistent with the fact that C/T antigens have been documented in many different types of cancers, but not all cancer samples display C/T antigen production. To test our hypothesis there is a need to determine if there is any correlation between EIF2S3B activation and C/T antigen production in individual tumor samples. A systematic approach to this is needed rather that the present random sampling currently available in the literature. It is possible that even transient activation of EIF2S3B could result in the expression of C/T antigens. In addition transfection of somatic cells, such as human fibroblasts could provide insight into how aberrant expression of EIF2S3B in cells modifies their phenotype and directly demonstrate if spermatogenic pathways are activated. Similar experiments involving transfection of fibroblasts with the testis specific human PIWIL2 gene have previously demonstrated transformation into a cancer stem cell like phenotype (26).
Identifying the pathways that regulate C/T antigen activation can lead to a better understanding of tumor initiation and development. It could also determine if C/T antigen activation, in the many different tumor types it has been documented in to date, is the result of a shared pathway. This may yield new insights into how subsets of human tumors which express C/T antigens arise and develop.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://asia.ensembl.org/Homo_sapiens/Info/Index.
Author Contributions
JR and CE contributed equally to the conception of the article. JR wrote and submitted the article. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by internal funds.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. van der Bruggen P, Traversari C, Chomez P, Lurquin C, De PE, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science (1991) 254:1643–7. doi: 10.1126/science.1840703
2. Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA (1995) 92:11810–3. doi: 10.1073/pnas.92.25.11810
3. Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA (1997) 94:1914–8. doi: 10.1073/pnas.94.5.1914
4. Fratta E, Coral S, Covre A, Parisi G, Colizzi F, Danielli R, et al. The biology of cancer testis antigens: putative function, regulation and therapeutic potential. Mol Oncol (2011) 5:164–82. doi: 10.1016/j.molonc.2011.02.001
5. Salmaninejad A, Zamani MR, Pourvahedi M, Golchehre Z, Hosseini BA, Rezaei N. Cancer/Testis Antigens: Expression, Regulation, Tumor Invasion, and Use in Immunotherapy of Cancers. Immunol Invest (2016) 45:619–40. doi: 10.1080/08820139.2016.1197241
6. Wei X, Chen F, Xin K, Wang Q, Yu L, Liu B, et al. Cancer-Testis Antigen Peptide Vaccine for Cancer Immunotherapy: Progress and Prospects. Transl Oncol (2019) 12:733–8. doi: 10.1016/j.tranon.2019.02.008
7. Xie K, Fu C, Wang S, Xu H, Liu S, Shao Y, et al. Cancer-testis antigens in ovarian cancer: implication for biomarkers and therapeutic targets. J Ovarian Res (2019) 12:1. doi: 10.1186/s13048-018-0475-z
8. Yao J, Caballero OL, Yung WK, Weinstein JN, Riggins GJ, Strausberg RL, et al. Tumor subtype-specific cancer-testis antigens as potential biomarkers and immunotherapeutic targets for cancers. Cancer Immunol Res (2014) 2:371–9. doi: 10.1158/2326-6066.CIR-13-0088
9. Old LJ. Cancer/testis (CT) antigens - a new link between gametogenesis and cancer. Cancer Immun (2001) 1:1.
10. Babatunde KA, Najafi A, Salehipour P, Modarressi MH, Mobasheri MB. Cancer/Testis genes in relation to sperm biology and function. Iran J Basic Med Sci (2017) 20:967–74. doi: 10.22038/ijbms.2017.9259
11. Chen YT, Chiu R, Lee P, Beneck D, Jin B, Old LJ. Chromosome X-encoded cancer/testis antigens show distinctive expression patterns in developing gonads and in testicular seminoma. Hum Reprod (2011) 26:3232–43. doi: 10.1093/humrep/der330
12. Cheng YH, Wong EW, Cheng CY. Cancer/testis (CT) antigens, carcinogenesis and spermatogenesis. Spermatogenesis (2011) 1:209–20. doi: 10.4161/spmg.1.3.17990
13. Whitehurst AW. Cause and consequence of cancer/testis antigen activation in cancer. Annu Rev Pharmacol Toxicol (2014) 54:251–72. doi: 10.1146/annurev-pharmtox-011112-140326
14. Mazeyrat S, Saut N, Grigoriev V, Mahadevaiah SK, Ojarikre OA, Rattigan A, et al. A Y-encoded subunit of the translation initiation factor Eif2 is essential for mouse spermatogenesis. Nat Genet (2001) 29:49–53. doi: 10.1038/ng717
15. Vernet N, Mahadevaiah SK, Ellis PJ, de Rooij DG, Burgoyne PS. Spermatid development in XO male mice with varying Y chromosome short-arm gene content: evidence for a Y gene controlling the initiation of sperm morphogenesis. Reproduction (2012) 144:433–45. doi: 10.1530/REP-12-0158
16. Yamauchi Y, Riel JM, Stoytcheva Z, Ward MA. Two Y genes can replace the entire Y chromosome for assisted reproduction in the mouse. Science (2014) 343:69–72. doi: 10.1126/science.1242544
17. Yamauchi Y, Riel JM, Ruthig VA, Ortega EA, Mitchell MJ, Ward MA. Two genes substitute for the mouse Y chromosome for spermatogenesis and reproduction. Science (2016) 351:514–6. doi: 10.1126/science.aad1795
18. Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature (2003) 423:825–37. doi: 10.1038/nature01722
19. Ehrmann IE, Ellis PS, Mazeyrat S, Duthie S, Brockdorff N, Mattei MG, et al. Characterization of genes encoding translation initiation factor eIF-2gamma in mouse and human: sex chromosome localization, escape from X-inactivation and evolution. Hum Mol Genet (1998) 7:1725–37. doi: 10.1093/hmg/7.11.1725
20. Gan H, Wen L, Liao S, Lin X, Ma T, Liu J, et al. Dynamics of 5-hydroxymethylcytosine during mouse spermatogenesis. Nat Commun (2013) 4:1995. doi: 10.1038/ncomms2995
21. Wainer KK, Linial M. Human genes escaping X-inactivation revealed by single cell expression data. BMC Genomics (2019) 20:201. doi: 10.1186/s12864-019-5507-6
22. Borck G, Shin BS, Stiller B, Mimouni-Bloch A, Thiele H, Kim JR, et al. eIF2gamma mutation that disrupts eIF2 complex integrity links intellectual disability to impaired translation initiation. Mol Cell (2012) 48:641–6. doi: 10.1016/j.molcel.2012.09.005
23. Moortgat S, Desir J, Benoit V, Boulanger S, Pendeville H, Nassogne MC, et al. Two novel EIF2S3 mutations associated with syndromic intellectual disability with severe microcephaly, growth retardation, and epilepsy. Am J Med Genet A (2016) 170:2927–33. doi: 10.1002/ajmg.a.37792
24. Skopkova M, Hennig F, Shin BS, Turner CE, Stanikova D, Brennerova K, et al. EIF2S3 Mutations Associated with Severe X-Linked Intellectual Disability Syndrome MEHMO. Hum Mutat (2017) 38:409–25. doi: 10.1002/humu.23170
25. Gregory LC, Ferreira CB, Young-Baird SK, Williams HJ, Harakalova M, van HG, et al. Impaired EIF2S3 function associated with a novel phenotype of X-linked hypopituitarism with glucose dysregulation. EBioMedicine (2019) 42:470–80. doi: 10.1016/j.ebiom.2019.03.013
Keywords: EiF2S3 retrogene, spermatogenesis, therapy, Cancer/Testis-antigen, oncogenesis
Citation: Rohozinski J and Edwards CL (2020) Does EIF2S3 Retrogene Activation Regulate Cancer/Testis Antigen Expression in Human Cancers? Front. Oncol. 10:590408. doi: 10.3389/fonc.2020.590408
Received: 01 August 2020; Accepted: 20 October 2020;
Published: 30 November 2020.
Edited by:
Giuseppe Giaccone, Cornell University, United StatesReviewed by:
Chang Li, University of Washington, United StatesRobert Getzenberg, Veru Inc., United States
Copyright © 2020 Rohozinski and Edwards. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Rohozinski, amFuckBiY20uZWR1
 Jan Rohozinski
Jan Rohozinski Creighton L. Edwards1,2
Creighton L. Edwards1,2