- 1Affiliated Zhongda Hospital of Southeast University, Southeast University, Nanjing, China
- 2Department of Urology, Jinhu People’s Hospital, Jinghua, China
- 3Department of Urology, JinTan People’s Hospital, Changzhou, China
- 4Department of Urology, Affiliated Zhongda Hospital of Southeast University, Nanjing, China
- 5Department of Nosocomial Infection, Affiliated Zhongda Hospital of Southeast University, Nanjing, China
Background: Contrast-enhanced ultrasound (CEUS) is an examination mode for detecting blood vessels in tissues, and it has been gradually used in the diagnosis of kidney cancer in recent years. This study explores the value of contrast-enhanced ultrasound in the clinical diagnosis of renal cancer, and provides an accurate and effective method for clinical diagnosis of renal cancer.
Methods: CEUS and RCC were selected as the keywords. Searching the PubMed and Embase from 2007 to 2020, the original data were abstracted and performed heterogeneity test with the Meta-Disc software. The weighted sensitivity, specificity, positive likelihood ratio and negative likelihood ratio were calculated, as well as the summary receiver operating characteristic (SROC) curve. Further estimated the diagnostic value of CEUS in the research of renal cancer by calculating the area under the curve (AUC). The quality of evidence in researches was evaluated by QUADAS items. Meta-disc, Review Manager 5.3, and STATA 13 were used.
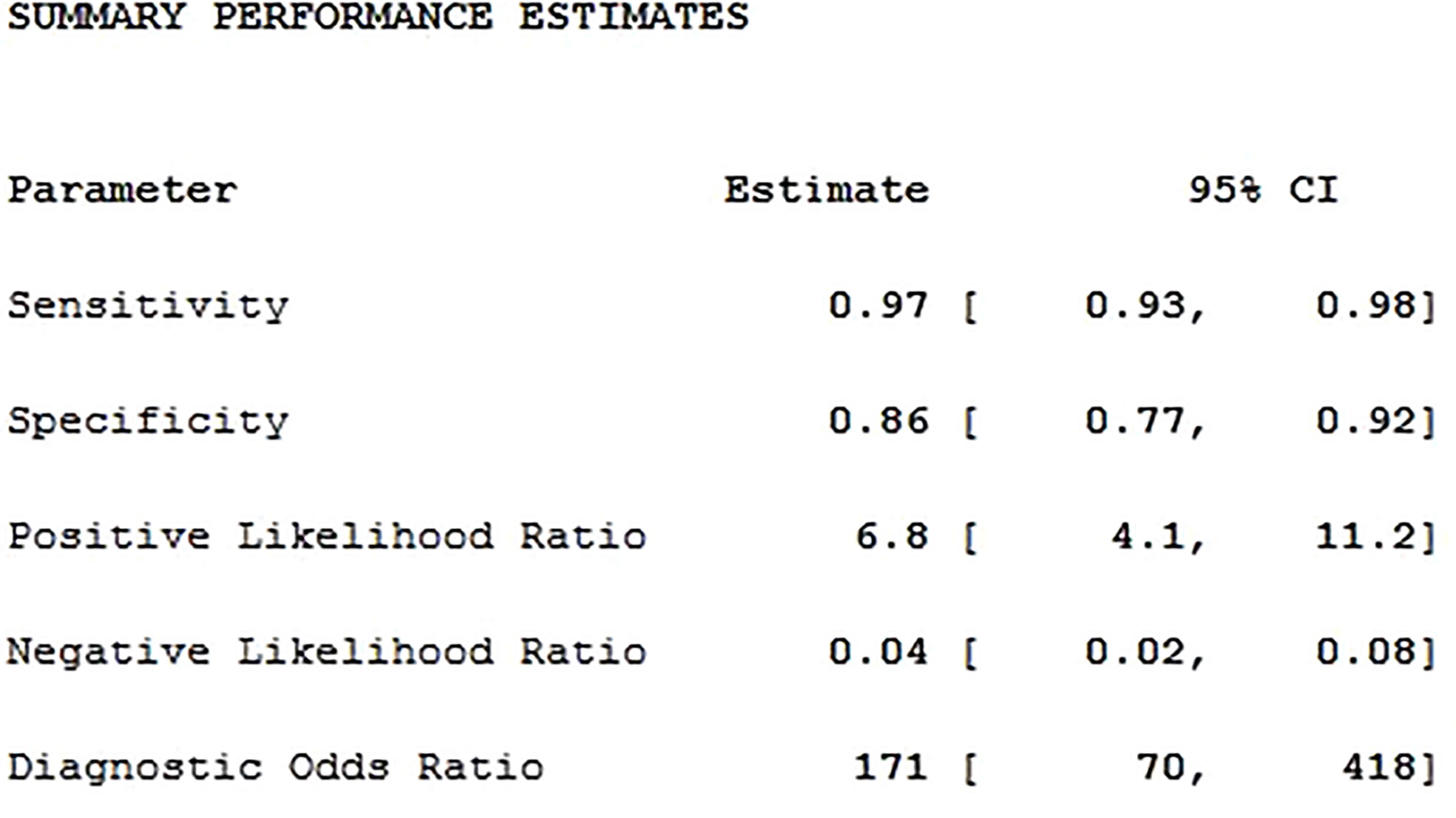
Results: A total of 20 studies were adopted for Meta-analysis. The weighted sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio were 0.97, 0.86, 6.8, 0.04 and 171, respectively; and AUC was 0.97. The results showed that there was high heterogeneity.
Conclusion: CEUS technology has a good diagnostic value for RCC.
Introduction
Renal cancer (RCC) is the most common primary malignant tumor of the kidney, accounting for 80% to 90% of primary malignant tumors of the kidney (1). In recent years, the incidence of kidney cancer and the number of deaths has increased significantly (2). Most patients with kidney cancer lack typical clinical symptoms and signs at an early stage (3). One third of RCC cases were reported with metastasis by the time of diagnosis (4). Therefore, there is still a need for an effective kidney cancer imaging diagnosis method.
RCC usually presents as a large mass on CT, mostly with soft tissue density; papillary RCC is less malignant than RCC and has less blood supply than its blood supply. Therefore, enhanced CT scan show either uneven or relatively uniform mild to moderate enhancement. CT examination is considered to be a gold standard for the diagnosis of kidney tumors, but CT can easily confuse cystic kidney cancer with renal abscess and hydronephrosis. MRI is usually used as a diagnostic tool for kidney tumors that cannot be characterized by CT, and is mainly used for typical lesions in CT. MRI is also often used in patients who cannot undergo CT enhancement due to impaired renal function. The limitation of MRI is that the acquisition time is long and people with metal implants such as pacemakers cannot be examined. Its availability and timeliness are not as good as CT.
The current clinical diagnosis methods for RCC are mainly imaging examinations such as ultrasound, contrast-enhanced CT, contrast-enhanced MR, non-contrast CT, non-contrast MR among which ultrasound has become the main method due to its simplicity and non-invasiveness, but the accuracy of conventional ultrasound for qualitative diagnosis of tumors is limited. Non-contrast CT/MR can only observe a specific section at a specific time, and the display rate of necrotic lesions is not as good as that of contrast enhanced ultrasound (CEUS), and it may be misdiagnosed due to missing the peak period of tumor enhancement and making the contrast enhancement characteristics unclear.
Contrast-enhanced CT and MR contrast agents can cause certain damage to the physiological functions of the liver and kidneys, and can also cause allergic reactions.
Contrast-enhanced ultrasound is a new detection method developed in ultrasound contrast agent and contrast imaging technology. It can observe blood perfusion in tumor in real time, continuously and dynamically, which further improves the accuracy of clinical diagnosis (5–7). CEUS can effectively display the low blood perfusion state and ischemic necrosis of tumor lesions with a diameter of less than 1 cm, thereby providing more information for the diagnosis of renal cancer, and at the same time eliminates the disadvantages of enhanced CT and MRI examinations (8, 9). CT/MR can only observe a specific section at a specific time. In addition, CEUS can display small blood vessels more sensitively than CT/MR, so as to more accurately observe blood perfusion of new tumors, which can evaluate the angiogenesis of renal cancer before surgery. CEUS cannot observe the surrounding and distant metastasis of the tumor, and cannot provide information on the clinical staging of renal cancer.
This study explores the value of contrast-enhanced ultrasound in the clinical diagnosis of renal cancer. The contrast-enhanced ultrasound examination method has high efficiency in diagnosing kidney cancer, nonradioactive and has very few contrast agents to cause allergic reactions. Compared with MRI, the examination time is short, therefore, it has higher clinical promotion value. With improvement of functions and performance of Doppler ultrasound equipment, the development of safer, cheaper, and better imaging performance contrast agents, contrast-enhanced ultrasound may become the first choice for renal cancer in the near future.
Methods
Search Strategy
Computer searches include PubMed, Embase to collect relevant literature on the diagnosis of kidney cancer by contrast-enhanced ultrasound. Search period: 2007 to 2020. Subject terms include contrast-enhanced ultrasound, kidney tumor, kidney cancer, renal cancer and renal tumor, and the search method is adjusted according to the specific database, and the search strategy is determined after multiple pre-searches. Using a combination of database retrieval and manual retrieval, two evaluators independently retrieve and re-search the references of the included literature. Another reviewer Xu Bin and Ke-Hao Pan are both medically-trained urologists in China with certain clinical and imaging experience. Xu Bin is the deputy chief physician of Chinese Urology. The deviation between the two reviewers is relatively small. The language is limited to Chinese or English.
Study Selection
The exclusion criteria for the systematic review were: (a) articles not within the field of interest; (b) editorials or letters, review articles, comments, conference proceedings; and (c) case reports.
Literature Screening
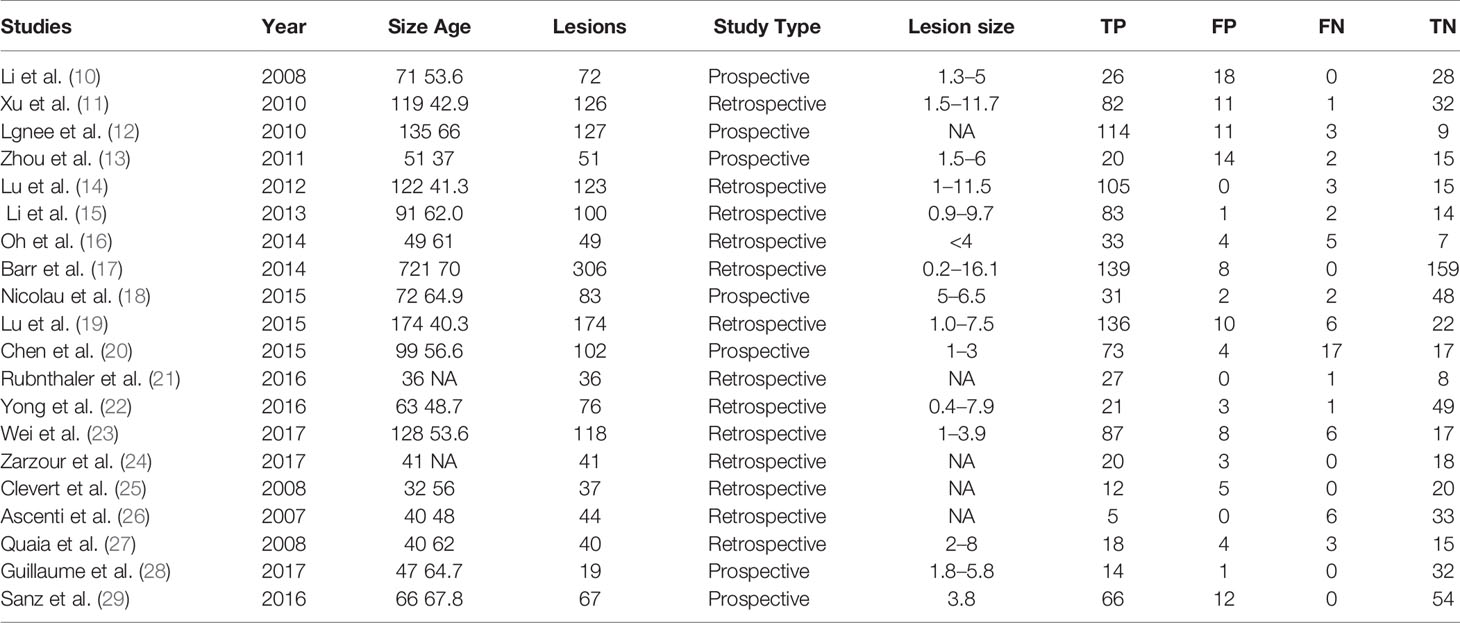
Literature was independently screened by 2 reviewers based on the inclusion criteria, first reading the title and abstract. Then read the full text of the documents that may meet the inclusion criteria. After cross-checking the results, data were extracted from cohort studies. The basic characteristics of the included literature are shown in Table 1.
Quality Assessment
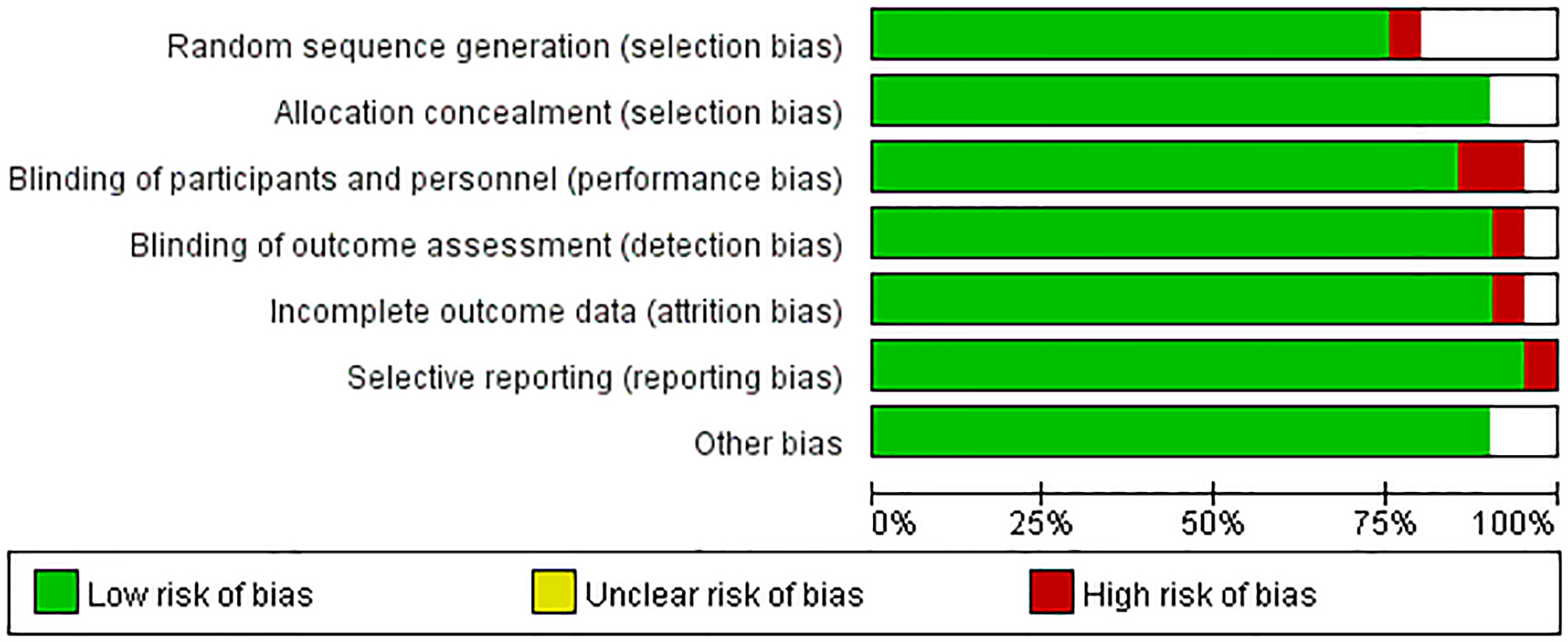
Two reviewers individually evaluated the quality of the included literature, and discussed when they disagree. This meta-analysis was carried out according to the QUADAS (Quality Assessment of Diagnostic Accuracy Studies) standard, which can be divided into three situations: “yes,” “no,” and “unclear.” “Yes” means that the criteria for this item are met, “no” means that the criteria are not met, and “unclear” means that the standards are partially met or sufficient information cannot be obtained from the document (30).
Statistical Analysis
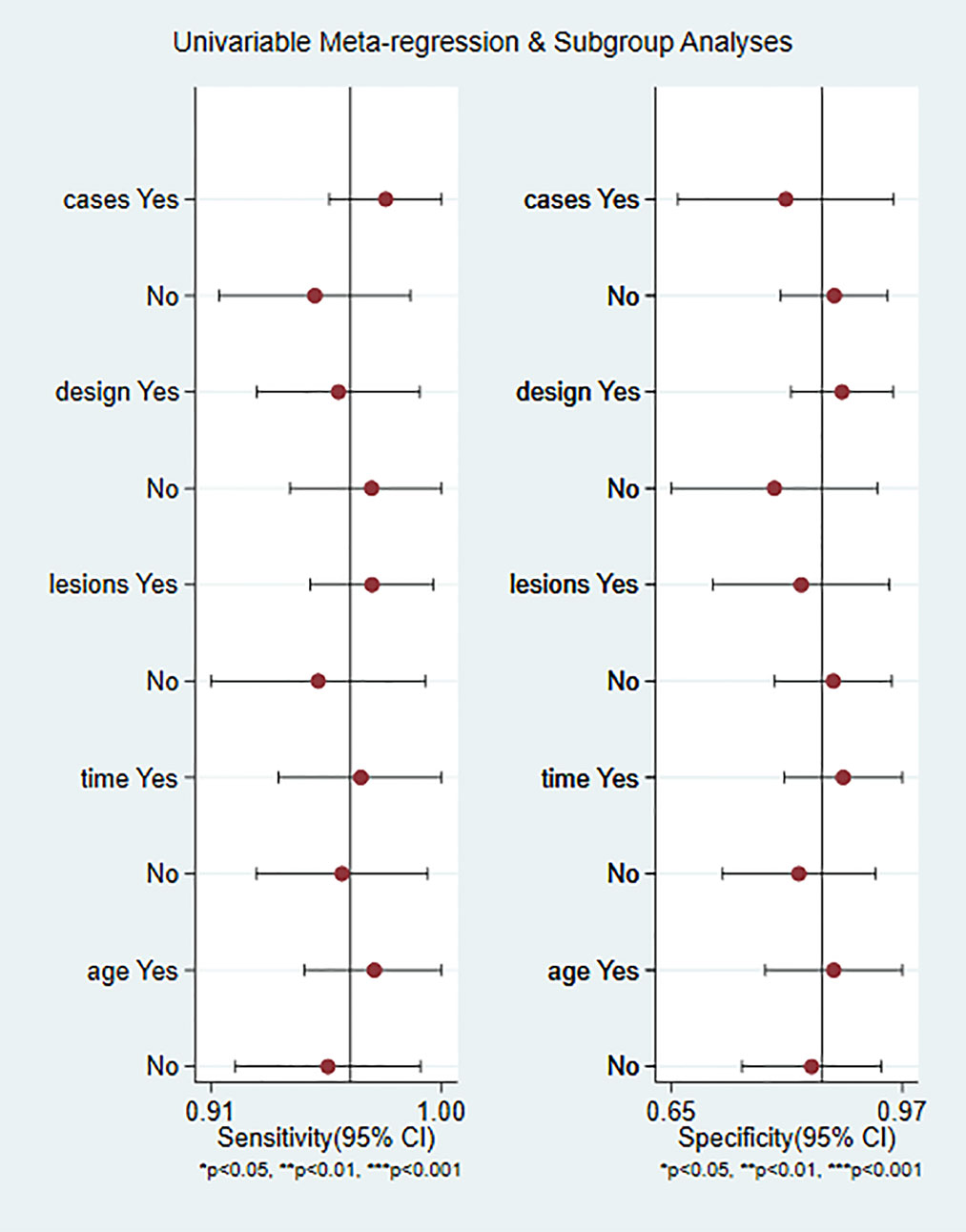
The weighted sensitivity, specificity, positive likelihood ratio and negative likelihood ratio were calculated, as well as the summary receiver operating characteristic (SROC) curve. The larger the area under the curve and the closer the SROC curve is to the upper left corner, the higher the value of the diagnostic test. Between-study statistical heterogeneity was assessed using I2 and the Cochrane Q test. The meta-regression and subgroup analysis of CEUS are shown in Figure 1, divided into five sub-groups according to whether the article publication year is beyond 2015, whether the case was greater than 100, whether lesion was greater than 100, whether the study was retrospective or prospective, and whether the age of patients above 60. The number of articles published before and after 2015 is close.
Results
Literature Search
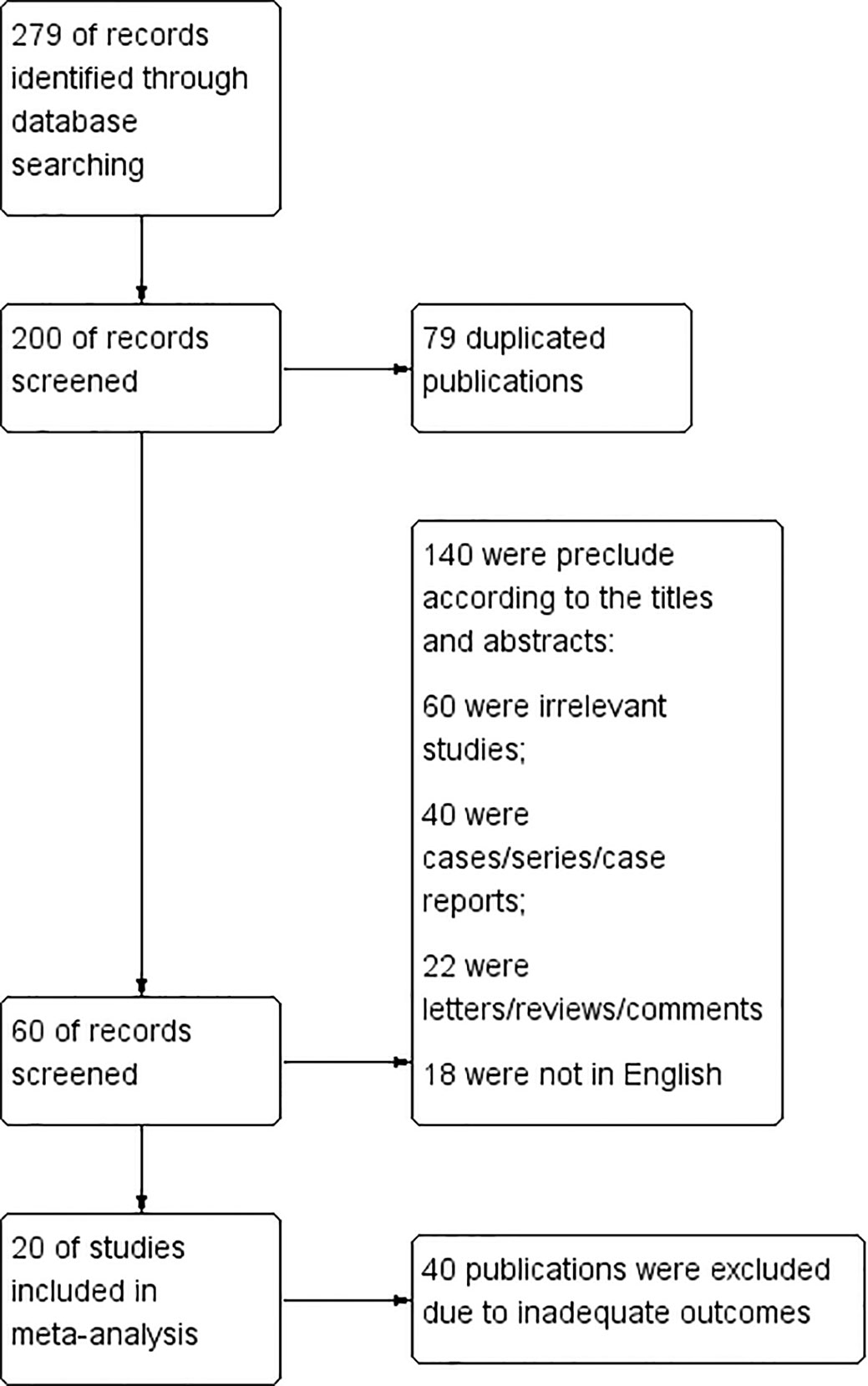
279 documents were first detected. 79 duplicated publications were excluded through literature manager software. And after the abstracts were screened, 140 records were excluded. 40 publications were excluded due to inadequate outcome because they lack information about the true positive rate, true negative rate, false negative rate and false positive rate of CEUS diagnosis. Finally, a total of 20 articles were included (10–29). The flow chart is shown in Figure 2.
Twenty studies including 2197 patients and 1791 lesions were selected for the meta-analysis. The basic characteristics of the included literature are shown in Table 1.
Inclusion criteria: 1 Pathological diagnosis should be adopted as “gold standard” for all adopted literature; 2 The research object is the literature using contrast-enhanced ultrasound to diagnose RCC; 3 The interval between ultrasound examination and pathological examination should not exceed 1 month; 4 Each study can be successfully extracted to TP, FP, TN, and FN.
Exclusion criteria: 1 Excluded documents that did not use contrast enhancement technology. 2 Excluded secondary literature and conference papers such as experience exchange, abstracts, lectures and reviews.
Histopathological Results
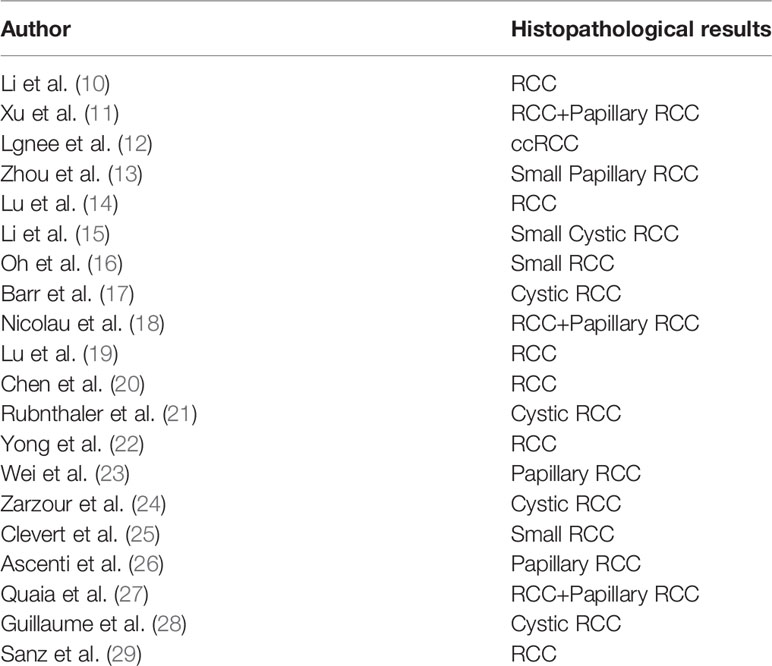
The histopathological results of included studies are shown in Table 2. Most of the included articles are RCC, Papillary RCC and so on. CEUS is effective in diagnosing these kidney cancers.
Qualitative Analysis
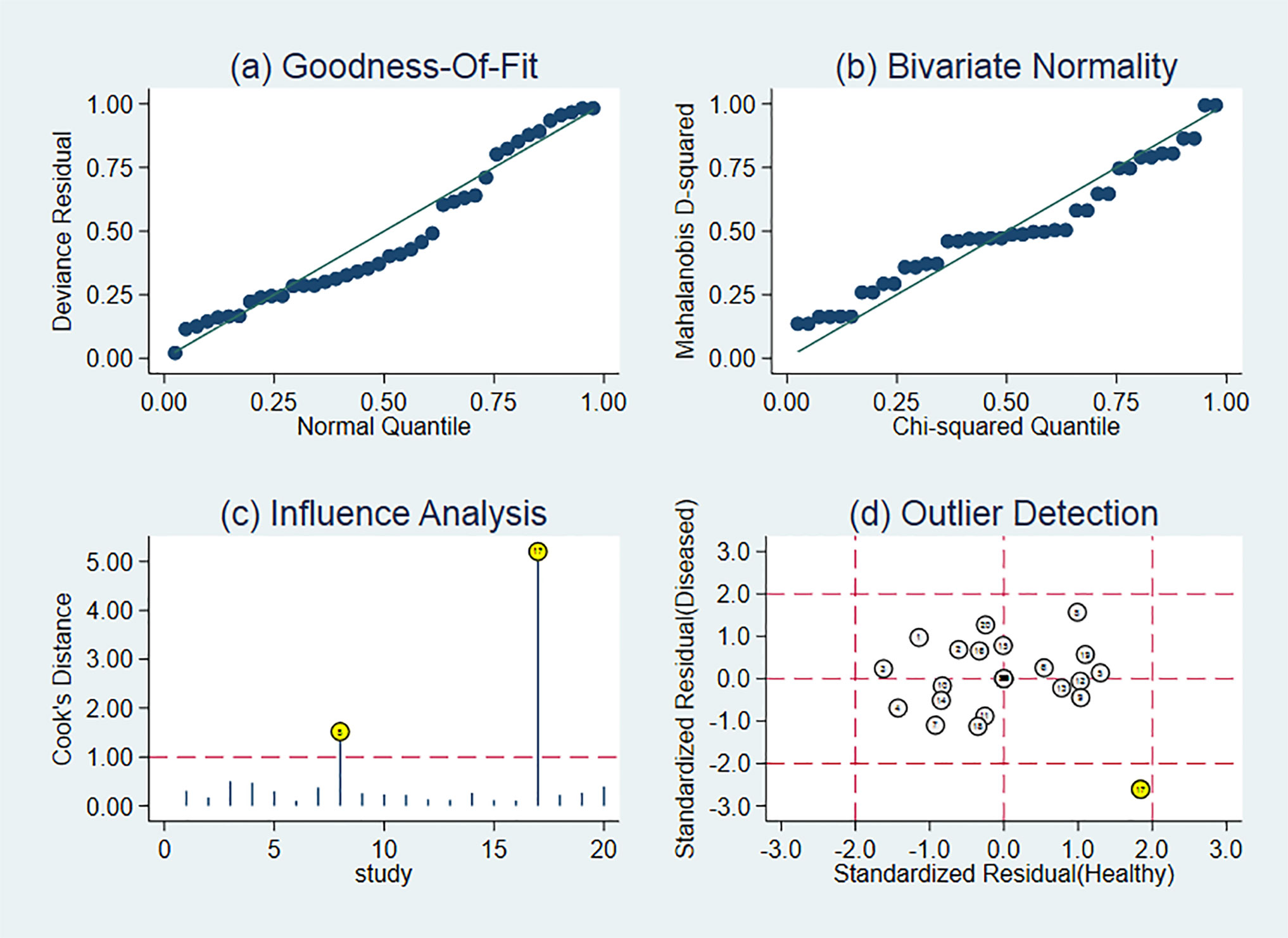
The quality of the articles included was satisfactory. The research quality evaluation is shown in Figure 3. In patient selection, one article is high risk. On Index Test, there is no high risk, however, one article on Reference Standard is high risk. The overall quality of the article is high.
Meta Analysis
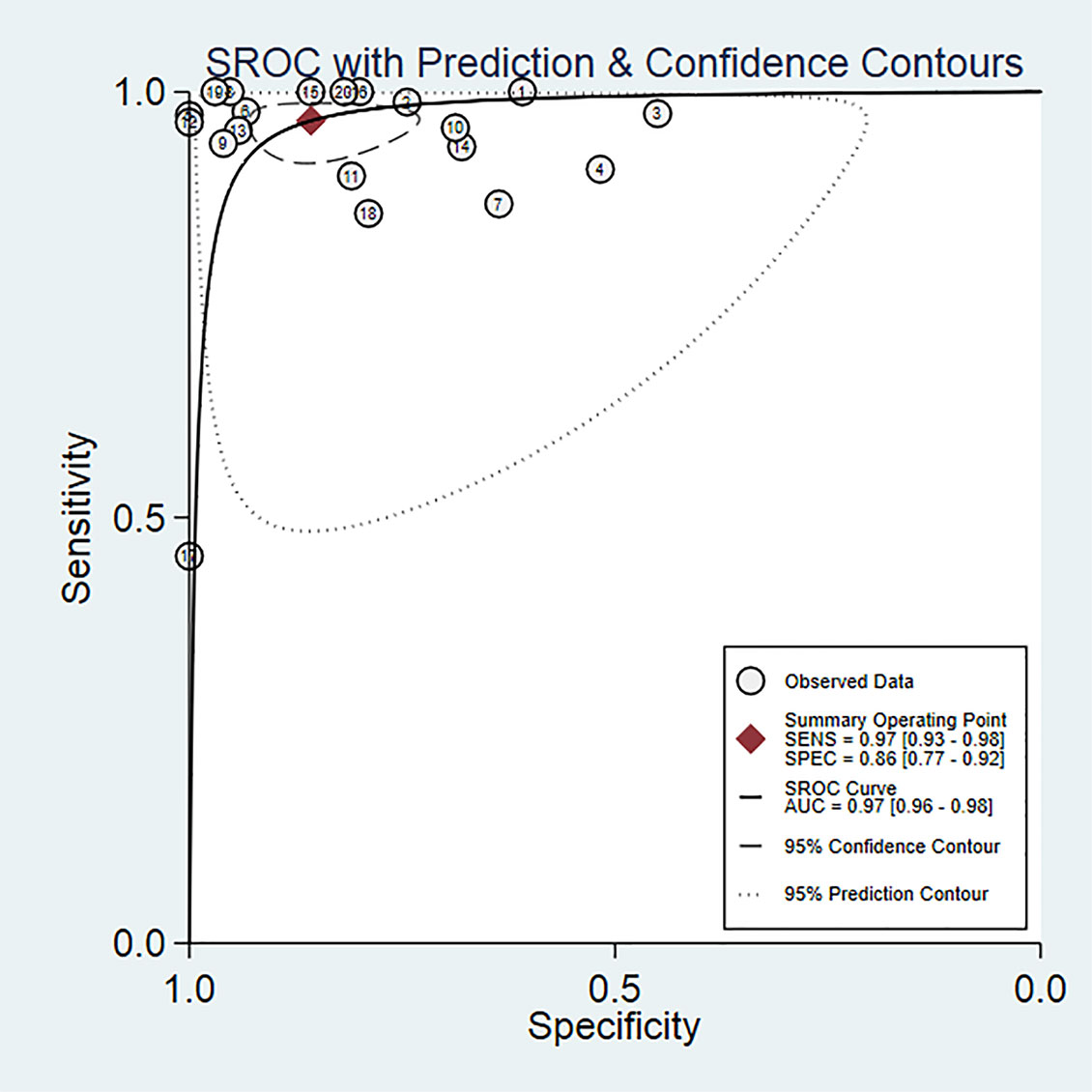
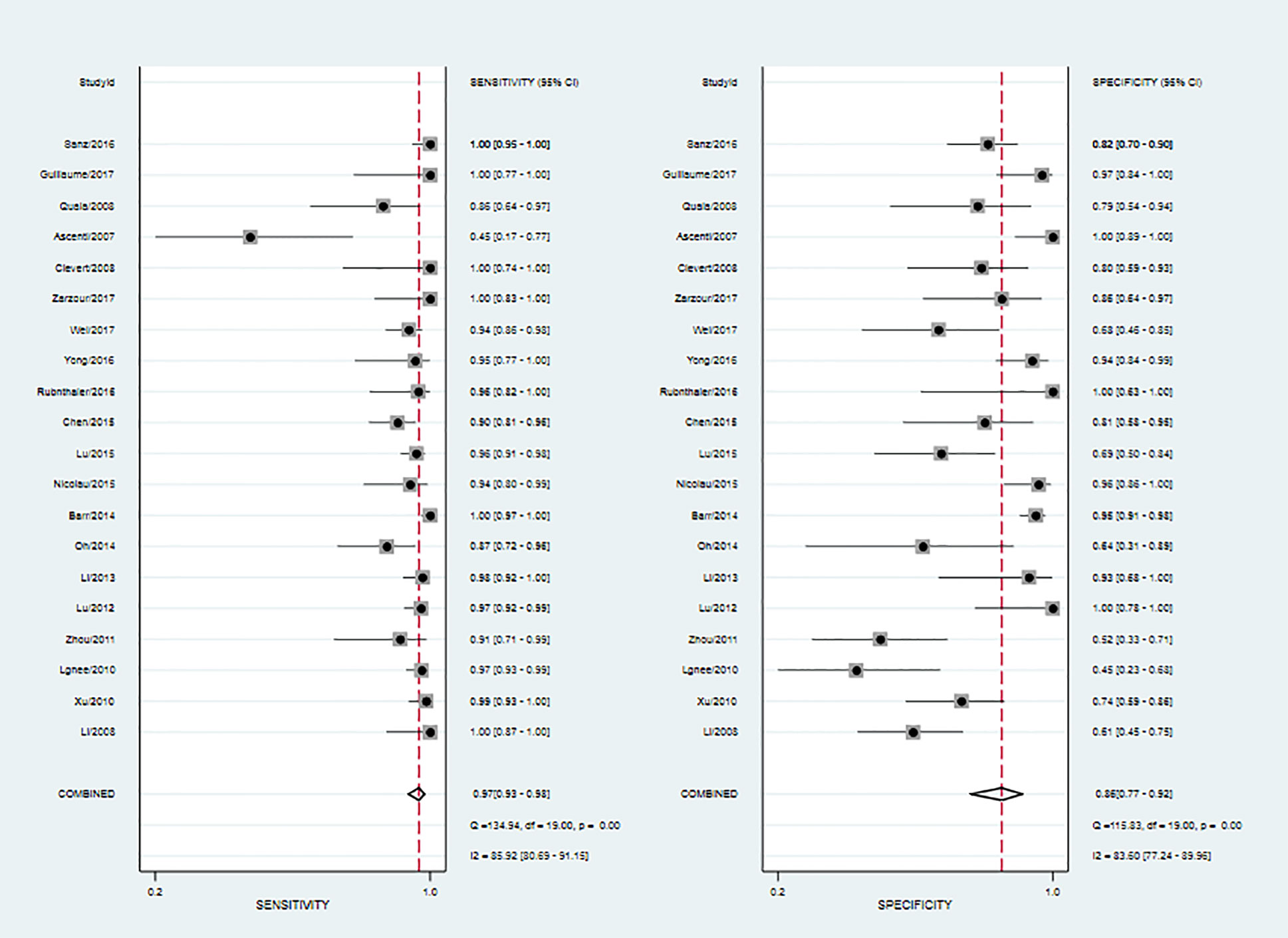
Twenty studies including 2197 patients and 1791 lesions were selected for the meta-analysis. Results of the meta-analysis are presented in Figure 4. The SROC curve and the forest map of CEUS are shown in Figures 5 and 6, respectively. Pooled Sen, Spe, LR+, LR-, DOR were 0.97, 0.86, 6.8, 0.04, and 171, respectively. In Figure 5, the areas under the SROC curve are 0.97 (95% CI, 0.96–0.98).
Heterogeneity Analysis
As shown in Figure 6, CEUS has heterogeneity in the sensitivity and specificity of the diagnosis of kidney cancer (Q value, P value, I2 value are 134.94, < 0.01, 85.92% and 115.84, <0.01, 83.60%, respectively). A randomed-effects model was used. The Spearman correlation coefficients of the sensitivity logarithm and (1-specificity) logarithm of CEUS diagnosis of renal cancer were −0.190 (P>0.05), indicating that there is no threshold effect. To further explore the potential sources of heterogeneity, a subgroup analysis and meta-regression was performed. It showed that no definite variable was the source of heterogeneity in the current meta-analysis (Figure 1).
Sensitivity Analysis
Sensitivity analysis is shown in Figure 7. The results showed that the meta-analysis results are stable.
Clinical Application Analysis
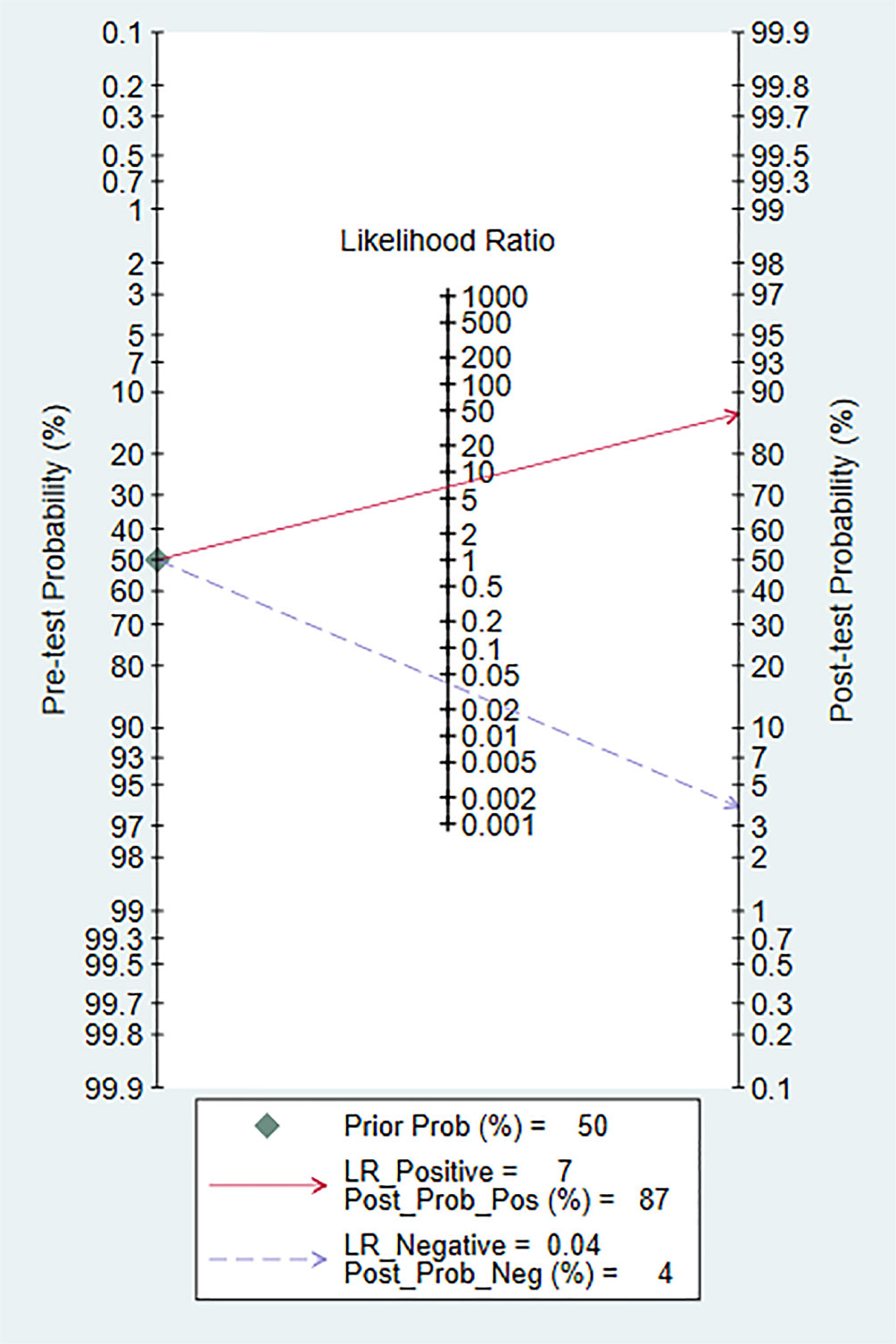
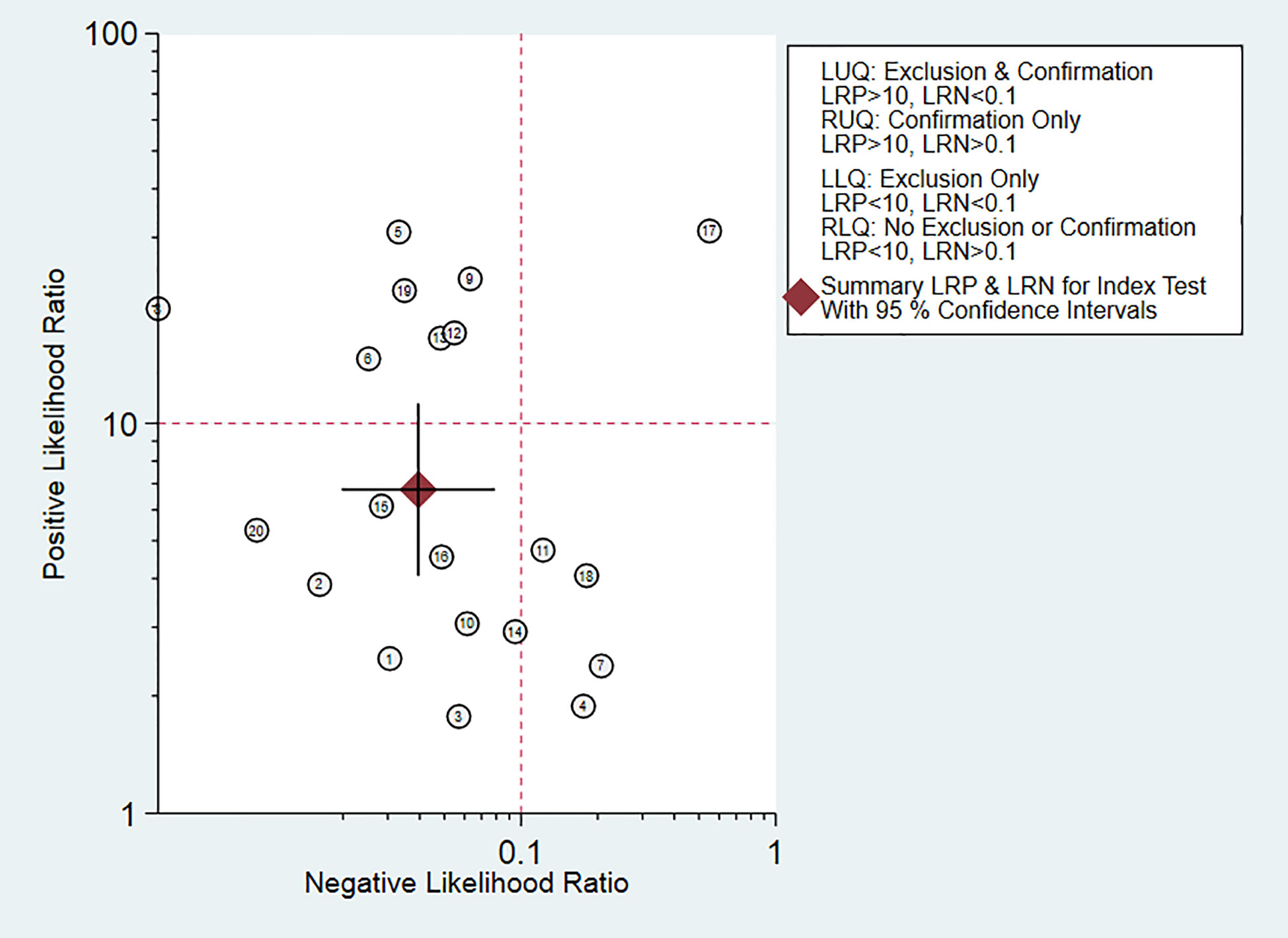
Fagan diagram was constructed for clinical application analysis as shown in Figure 8. The post-test probability of CEUS was 87% and is higher than the pre-test probability (50%), indicating that CEUS is effective in the diagnosis of renal cancer. As can be seen from Figure 9, the combined negative likelihood ratios for the diagnosis of renal cancer were >0.1 and the positive likelihood ratio was <10.
Publication Bias
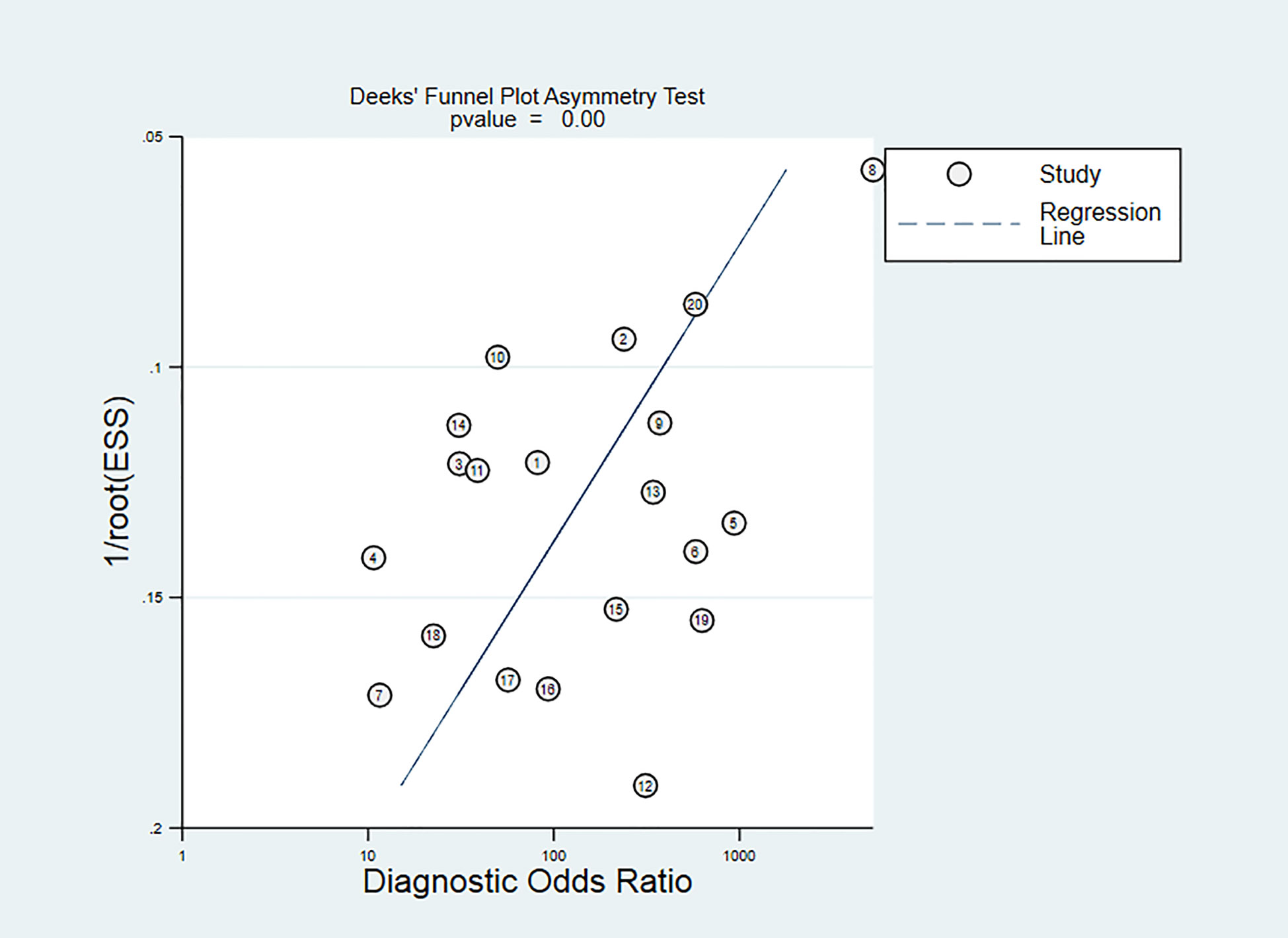
The Deeks’ funnel chart shows asymmetry in scattered points, suggesting that there is publication bias (P<0.05). It is shown in Figure 10. However, the sensitivity analysis showed that our results are stable. Despite there is publication bias, our sensitivity test found that the article is stable, indicating that our results are reliable.
Discussion
CEUS is a new type of ultrasound diagnosis technology that uses contrast enhancers and corresponding analysis software to display the state of tissue blood perfusion on the basis of conventional ultrasound. CEUS uses high-intensity nonlinear harmonic signals generated by contrast agents to increase the contrast between normal tissues and lesions. Kidney cancer has the characteristics of infinite growth of microvessels in malignant tumors. Most malignant tumors have a large number of aggressive capillary formations around and inside the tumor. CEUS can enhance the display of blood perfusion of kidney and tumor microvessels. During the examination, the contrast agent is injected into the blood circulation through the peripheral vein, and the microbubbles are in full contact with the red blood cells in the capillaries, forming many blood bubble interfaces, thereby changing the basic function between the ultrasound and the basic tissues, enhancing the ultrasound signal of the whole body blood pool, and improving the signal-to-noise ratio of echo, thereby improving the display of tumors. In addition, CEUS can also improve the sensitivity of detecting small tumors or slow blood vessels. The results of this study show that the weighted sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio were 0.97, 0.86, 6.8, 0.04 and 171, respectively; and AUC is 0.97. This suggests that CEUS could be used as a diagnostic tool for RCC.
CEUS features of renal cancers are that the cortical phase contrast agent can quickly fill the lesion tissue, and the enhancement degree of CEUS is equal to or significantly higher than that of the renal parenchyma, and in the late medulla and delayed phase, the contrast agent quickly exits the lesion organization, mainly manifested as low enhanced performance. CEUS shows the enhancement feature of fast forward and fast out.
CEUS provides a new method for diagnosing kidney tumors. The application of TIC can make the diagnosis of RCC more objective by analyzing the AT, TP, and DPI values of the tumor and surrounding renal cortex. Most renal cancers have pseudo-capsules. The display rate of pseudo-capsules after contrast is higher than that of conventional ultrasound, and the enhancement time is long and obvious. In CEUS, the hemorrhagic and necrotic foci in the tumor are in sharp contrast with the enhanced foci, and the display rate of the necrotic foci is higher than that of conventional ultrasound. Although CT and MRI have high diagnostic rates, contrast agents can cause certain damage to the physiological functions of the liver and kidneys. At the same time, the patient’s body can also be damaged by radiation. CT/MR can only observe a specific section at a specific time, and the display rate of necrotic lesions is not as good as that of CEUS, and it may be misdiagnosed by missing the peak period of tumor enhancement and making the contrast enhancement characteristics unclear. In addition, CEUS can display small blood vessels more sensitively than CT/MR, so more accurate to observe the blood perfusion of new tumors, which can used to evaluate the angiogenesis of renal cancer before surgery. CEUS cannot observe the surrounding and distant metastasis of the tumor, and it has no guidance on the clinical staging of renal cancer.
The heterogeneity of this study is high. According to the results of the subgroup and meta-regression analysis, the five subgroups are not sources of heterogeneity and a comprehensive analysis requires more subgroup data. First, the characteristics of ultrasonography determine that factors such as the experience and skills of the diagnostician, as well as the subjective evaluation of the imaging results, have a great influence on the diagnosis. In Rubnthaler (21) article, all CEUS examinations were performed and interpreted by a single radiologist with more than 15 years of experience in CEUS. In Lu (19) article, a sonologist with 10 years’ experience with CEUS did diagnosis. Obviously, different experiences of the two sonologists will lead to different diagnosis of kidney cancer. Then, although each study uses the same contrast agent, the ultrasound equipment and probe models used are different, and even the same study uses different ultrasound equipment and probes. In Wei (23) article, CEUS examinations were performed using a Sequoia 512ultrasound system (Siemens, Mountain View, CA, USA). In Quaia (27) article, CEUS examinations were performed using Sequoia, Acuson-Siemens. These factors may affect the diagnosis rate and cause heterogeneity. These may be the reason for the heterogeneity.
This research still has some limitations. First, the number of documents is limited and retrospective studies account for a large amount, so can cause selection bias; second, due to the different publication time of the literature, the CEUS diagnostic standards in some literatures have certain differences, which may affect the results; third, There are poor quality research in these documents, which leads to the bias of the publication of this article.
Conclusion
Contrast enhanced ultrasound technology has a good diagnostic clinical value for RCC.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
AN and W-JC revised the manuscript. K-HP, LJ and WJ-C contributed equally to this work and should be considered co-first authors. AN and FK contributed equally to this work and should be considered second authors. XB, Y-LW and MC contributed equally to this work and should be considered corresponding authors. All authors contributed to the article and approved the submitted version.
Funding
Foundations: This study was funded by The National Natural Science Foundation of China (No. 81872089, 81370849, 81672551, 81300472, 81070592, 81202268, 81202034), Natural Science Foundation of Jiangsu Province (BK20161434, BL2013032, BK20150642 and BK2012336), Six talent peaks project in Jiangsu Province, Jiangsu Provincial Medical Innovation Team (CXTDA2017025), The National Key Research and Development Program of China (SQ2017YFSF090096), Jiangsu Provincial Key Research and Development Program (BE2019751), Innovative Team of Jiangsu Provincial (2017ZXKJQWO7), Jiangsu Provincial Medical Talent (ZDRCA2016080).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
RCC, renal cell carcioma; CEUS, contrast-enhanced ultrasound; AUC, area under the curve.
References
1. Udea N, Nagira H, Sannomiya N, Ikunishi S, Hattori Y, Kamida A, et al. Contrast-enhanced ultrasonography in evaluation of therapeutic effect of chemotherapy for patients with liver metastases. Yonago Acts Med (2016) 59(4):255–61.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin (2017) 67:177–93. doi: 10.3322/caac.21395
3. Skinner DG, Vermillion CD, Pfister RC, Leadbetter WF. Renal cell carcinoma. Am Fam Physician (1971) 4:89–94.
4. Gore ME, Larkin JM. Challenges and opportunities for converting renal cell carcinoma into a chronic disease with targeted therapies. Br J Cancer (2011) 104:399–406. doi: 10.1038/sj.bjc.6606084
5. Zarzour JG, Porter KK, Tchelepi H, Robbin ML. Contrast-enhanced ultrasound of benign liver lesions. Abdom Radiol (NY) (2018) 43:848–60. doi: 10.1007/s00261-017-1402-2
6. Nakata N, Ohta T, Nishioka M, Takeyama H, Toriumi Y, Kato K, et al. Optimization of region of interest drawing for quantitative analysis: differentiation between benign and malignant breast lesions on contrast-enhanced sonography. J Ultrasound Med (2015) 34:1969–76. doi: 10.7863/ultra.14.10042
7. Kasoji SK, Chang EH, Mullin LB, Chong WK, Rathmell WK. Dayton PA. A pilot clinical study in characterization of malignant renal-cell carcinoma subtype with contrast-enhanced ultrasound. Ultrason Imaging (2017) 39:126–36. doi: 10.1177/0161734616666383
8. Kabakci N, Igci E, Secil M, Yorukoglu K, Mungan U, Celebi I, et al. Echo contrast-enhanced power Doppler ultrasonography for assessment of angiogenesis in renal cell carcinoma. J Ultrasound Med (2005) 24:747–53. doi: 10.7863/jum.2005.24.6.747
9. Matsumoto S, Minami T, Yamamoto Y, Nishioka T, Akiyama T, Onoue A. Efficacy of contrast-enhanced color Doppler ultrasonography for the diagnosis of renal mass lesions. Hinyokika Kiyo (2001) 47:299–302.
10. Li F, Du LF, Xing JF, Su YJ, Wu Y. Diagnostic efficacy of contrast-enhanced ultrasonography in solid renal parenchymal lesions with maximum diameters of 5 cm. J Ultrasound Med (2008) 27:875–85. doi: 10.7863/jum.2008.27.6.875
11. Xu ZF, Xu HX, Xie XY, Liu GL, Zheng YL, Lu MD. Renal cell carcinoma and renal angiomyolipoma: differential diagnosis with real-time contrast-enhanced ultrasonography. J Ultrasound Med (2010) 29:709–17. doi: 10.7863/jum.2010.29.5.709
12. Ignee A, Straub B, Brix D, Schuessler G, Ott M, Dietrich CF. The value of contrast enhanced ultrasound (CEUS) in the characterisation of patients with renal masses. Clin Hemorheol Microcirc (2010) 46:275–90. doi: 10.3233/CH-2010-1352
13. Zhou X, Yan F, Luo Y, Peng Y-L, Parajuly SS, Wen X-R, et al. Characterization and diagnostic confidence of contrast-enhanced ultrasound for solid renal tumors. Ultrasound Med Biol (2011) 37:845–53. doi: 10.1016/j.ultrasmedbio.2011.02.015
14. Lu Q, Wang W, Huang B, Li C, Li C. Minimal fat renal angiomyolipoma: the initial study with contrast-enhanced ultrasonography. Ultrasound Med Biol (2012) 38:1896–901. doi: 10.1016/j.ultrasmedbio.2012.07.014
15. Li X, Liang P, Guo M, Yu J, Yu X, Cheng Z, et al. Real-time contrast-enhanced ultrasound in diagnosis of solid renal lesions. Discovery Med (2013) 16:15–25.
16. Oh TH, Lee YH, Seo IY. Diagnostic efficacy of contrast-enhanced ultrasound for small renal masses. Korean J Urol (2014) 55:587–92. doi: 10.4111/kju.2014.55.9.587
17. Barr RG, Peterson C, Hindi A. Evaluation of indeterminate renal masses with contrast-enhanced US: a diagnostic performance study. Radiology (2014) 271:133–42. doi: 10.1148/radiol.13130161
18. Nicolau C, Buñesch L, Paño B, Salvador R, Ribal MJ, Mallofré C, et al. Prospective evaluation of CT indeterminate renal masses using US and contrast-enhanced ultrasound. Abdom Imaging (2015) 40:542–51. doi: 10.1007/s00261-014-0237-3
19. Lu Q, Huang BJ, Wang WP, Li CX, Xue LY. Qualitative and quantitative analysis with contrast-enhanced ultrasonography: diagnosis value in hypoechoic renal angiomyolipoma. Korean J Urol (2015) 16:334–41. doi: 10.3348/kjr.2015.16.2.334
20. Chen L, Wang L, Diao X, Qian W, Fang L, Pang Y, et al. The diagnostic value of contrast-enhanced ultrasound in differentiating small renal carcinoma and angiomyolipoma. Biosci Trends (2015) 9:252–8. doi: 10.5582/bst.2015.01080
21. Rubenthaler J, Paprottka K, Marcon J, Hameister E, Hoffmann K, Joiko N, et al. Comparison of magnetic resonance imaging (MRI) and contrast-enhanced ultrasound(CEUS) in the evaluation of unclear solid renal lesions. ClinHemorheol Microcirc (2016) 64:757–63. doi: 10.3233/CH-168034
22. Yong C, Teo YM, Jeevesh K. Diagnostic performance of contrast-enhanced ultrasound in the evaluation of renal masses inpatients with renal impairment. Med J Malaysia (2016) 71:193–8.
23. Wei SP, Xu CL, Zhang QR, Zhang Q-R, Zhao Y-E, Huang P-F, et al. Contrast-enhanced ultrasound for differentiating benign from malignant solid small renal masses: comparison with contrast-enhanced CT. Abdom Radiol (NY) (2017) 42:2135–45. doi: 10.1007/s00261-017-1111-x
24. Zarzour JG, Lockhart ME, West J, Turner E, Jackson BE, Thomas JV, et al. Contrast-enhanced ultrasound classification of previously indeterminate renal lesions. J Ultrasound Med (2017) 36:1819–27. doi: 10.1002/jum.14208
25. Clevert DA, Minaifar N, Weckbach S, Jung EM, Stock K, Reiser M, et al. Multislice computed tomography versus contrast-enhanced ultrasound in evaluation of complex cystic renal masses using the Bosniak classification system. Clin Hemorheol Microcirc (2008) 39(1-4):171–8. doi: 10.3233/CH-2008-1083
26. Ascenti G, Mazziotti S, Zimbaro G, Settineri N, Magno C, Melloni D, et al. Complex cystic renal masses: characterization with contrast-enhanced US. Radiology (2007) 243(1):158–65. doi: 10.1148/radiol.2431051924
27. Quaia E, Bertolotto M, Cioffi V, Rossi A, Baratella E, Pizzolato R, et al. Comparison of contrast -enhanced sonography with unenhanced sonography and contrast -enhanced CT in the diagnosis of malignancy in complex cystic renal masses. AJR (2008) 191(4):1239–49. doi: 10.2214/AJR.07.3546
28. Defortescu G, Cornu J-N, Béjar S, Giwerc A, Gobet F, Werquin C, et al. Diagnostic performance of contrast-enhanced ultrasonography and magnetic resonance imaging for the assessment of complex renal cysts: A prospective study. Int J Urol (2017) 24:184–9. doi: 10.1111/iju.13289
29. Sanz E, Hevia V, Gómez V, Álvarez S, Fabuel JJ, Martínez L, et al. Renal Complex Cystic Masses: Usefulness of Contrast-Enhanced Ultrasound (CEUS) in Their Assessment and Its Agreement with Computed Tomography. Curr Urol Rep (2016) 17:89. doi: 10.1007/s11934-016-0646-7
Keywords: renal cancer, contrast-enhanced ultrasound, diagnosis, meta-analysis, tumor imaging
Citation: Pan K-H, Jian L, Chen W-J, Nikzad AA, Kong FQ, Bin X, Wang Y-L and Chen M (2020) Diagnostic Performance of Contrast-Enhanced Ultrasound in Renal Cancer: A Meta-Analysis. Front. Oncol. 10:586949. doi: 10.3389/fonc.2020.586949
Received: 24 July 2020; Accepted: 20 October 2020;
Published: 18 November 2020.
Edited by:
Po-Hsiang Tsui, Chang Gung University, TaiwanReviewed by:
Christoph Dietrich, Hirslanden Private Hospital Group, SwitzerlandKun Zheng, Peking Union Medical College Hospital (CAMS), China
Copyright © 2020 Pan, Jian, Chen, Nikzad, Kong, Bin, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Bin, eGIxNTg5NjQ1MDgxMEAxMjYuY29t; Ya-Li Wang, MTUxNTA2NjYyNjBAMTYzLmNvbQ==; Ming Chen, bWluZ2NoZW5zZXVAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Ke-Hao Pan1†
Ke-Hao Pan1† Ming Chen
Ming Chen