- 1Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
- 2Department of Radiation Physics, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
Objectives: To investigate the tumor volume and its change on short-term outcome in esophageal squamous cell carcinoma (ESCC) patients who underwent definitive radiotherapy or chemoradiotherapy.
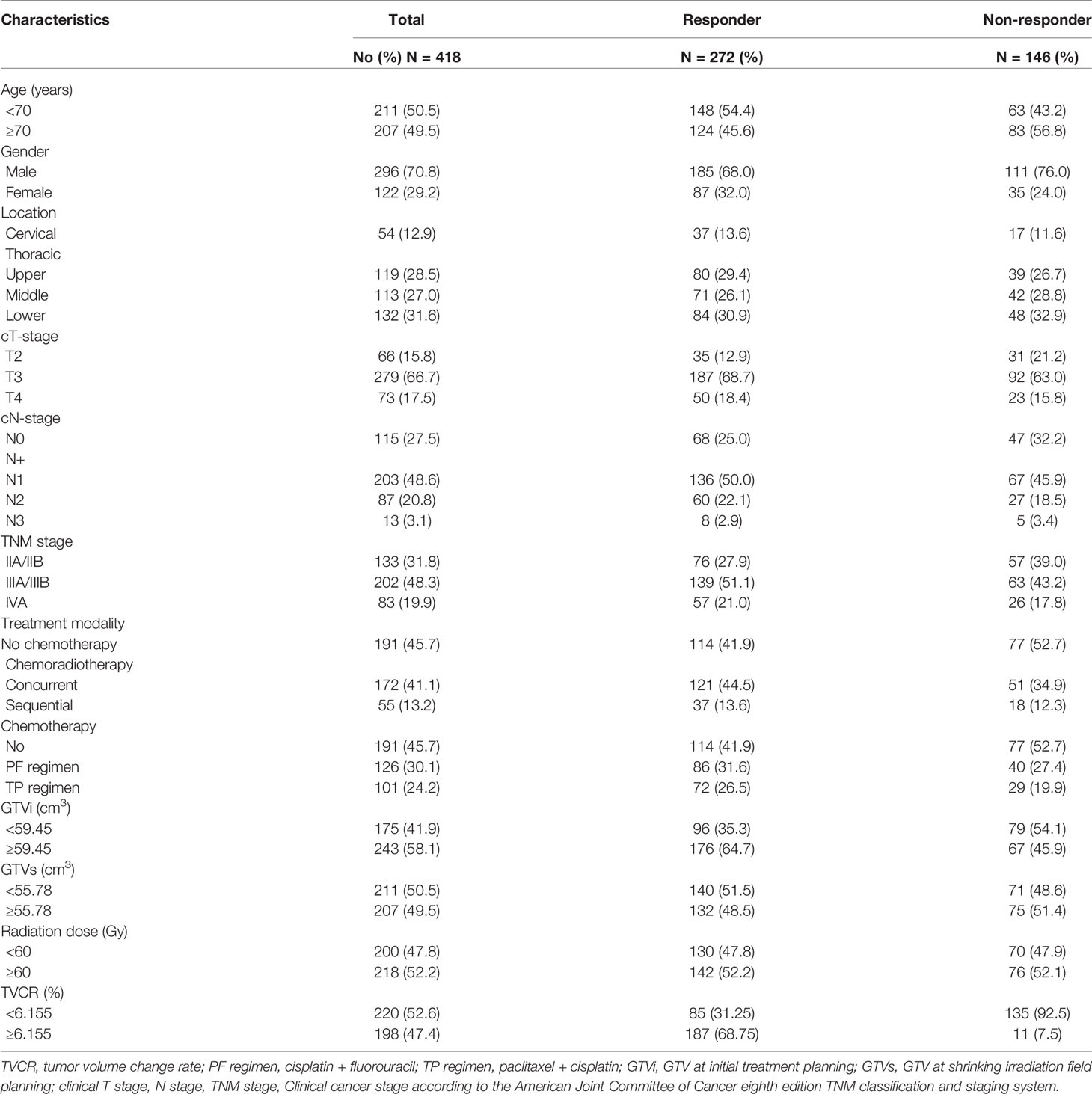
Methods and Materials: All data were retrospectively collected from 418 ESCC patients who received radiotherapy or chemoradiotherapy at our institution between 2015 and 2019. Short-term outcome using the treatment response evaluation was assessed according to the RECIST 1.1. The tumor volume change rate (TVCR) was defined as follows: TVCR = {1 − [gross tumor volume (GTV) at shrinking irradiation field planning)]/(GTV at the initial treatment planning)} ×100%. Chi square test was used to compare the clinic characteristics in different TVCR groups, and the difference between initial GTV (GTVi) and shrinking GTV (GTVs) was compared using Wilcoxon’s sign rank test. Logistic regression analysis and Spearman correlation was performed.
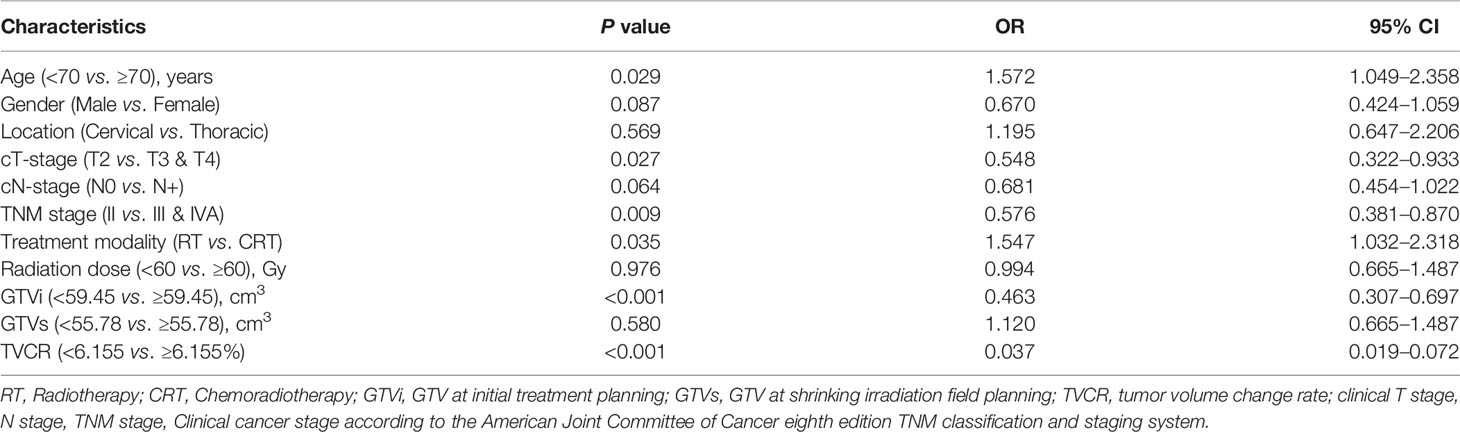
Results: There was a significant decrease in GTVi compared to GTVs (P < 0.001). In univariate analysis, age, cT-stage, TNM stage, treatment modality, GTVi, and TVCR were associated with short-term outcome (all P < 0.05). In multivariate analysis, gender and TVCR were statistically significant (P = 0.010, <0.001) with short-term outcome, and the combined predictive value of gender and TVCR exceeded that of TVCR (AUC, 0.876 vs 0.855).
Conclusions: TVCR could serve to forecast short-term outcome of radiotherapy or chemoradiotherapy in ESCC. It was of great significance to guide the individualized treatment of ESCC.
Introduction
Esophageal cancer (EC) has become the seventh most common tumor and the sixth leading cause of cancer death in 2018 worldwide (1). About 90% of its pathological type is esophageal squamous cell carcinoma (ESCC) in China (2). Generally speaking, surgical treatment is the best choice for early EC patients, but because of the incidence of EC is relatively not known, the early symptoms are not obvious, and it is often detected at the advanced clinical stage once patient with the obvious symptom of dysphagia. So, about 40 to 60% of the patients lose the opportunity of operation, and these patients can be only treated with radiotherapy and chemotherapy (3). Radiation sensitive patients can achieve the same survival time as surgery, especially due to the continuous improvement of the radiotherapy technology in recent years, maximize the appropriate target area, improve the target dose, and effectively protect organs at risk (OARs) and normal tissues, and significantly improve the local control rate (4).
However, because of the tumor heterogeneity, differences in tumor gene expression and tumor microenvironment, it is clinically observed that even if the EC patients have the same clinical stage, pathological type and degree of differentiation, they are also given the same radiotherapy method and dose, but the curative effect may be very different (5). Even receiving standard treatment, about 27–50% of the patients would have local recurrence and distant metastasis or both (6). Furthermore, the survival time of patients who were resistant to radiotherapy could not benefit or even be significantly shortened, and they had to bear many side effects of radiotherapy, including ulcer, esophageal fistula, pulmonary fibrosis, radiation cardiotoxicity and so on (7). Therefore, it is very necessary to predict the short-term outcome (STO) of radiotherapy in EC patients. This can help us to find potential sensitive or insensitive patients and carry out personalized and accurate treatment according to individual sensitivity in order to improve the curative effect, the local control rate and prolong the survival time.
The gross tumor volume (GTV) defined on radiotherapy planning refers to the range of tumor lesions with certain shape and size displayed by existing auxiliary examination methods including computed tomography (CT) and esophagography, and its stereoscopic imaging reflects the actual shape of the tumor. With the improvement of radiotherapy technology, we have ability to routinely evaluate GTV during radiotherapy, and it has been confirmed as a predictor in non-small cell lung cancer (8, 9) and nasopharyngeal carcinoma (10, 11). Although GTV as a prognostic factor has been studied in EC treated by surgery alone, no studies investigated the tumor volume change rate (TVCR) during radiotherapy (12, 13). In clinical work, we found that STO after treatment was the important guiding significance for indicating tumor recurrence, metastasis. So, in this study, we conducted GTV and TVCR in ESCC patients during radiotherapy and investigate whether it is a predictor of STO with the aim of making individualized treatment as soon as possible.
Methods and Materials
Characteristics of Patients
This retrospective study included 418 cases of ESCC patients who received radiotherapy or chemoradiotherapy (CRT) in Shandong Cancer Hospital between October 2015 and May 2019. Inclusion criteria include the following: (1) patients were aged 18 years or older; (2) new diagnosed ESCC and confirmed by histopathology; (3) receipt of ≥50Gy radiotherapy with or without chemotherapy, and a repeated computed tomography (CT) scan was performed before and follow 40Gy for shrinking irradiation field; (4) availability of dosimetry, radiotherapy planning evaluation system and imaging data; (5) the local focus could not be resected; (6) Karnofsky performance status ≥70; (7) no past history of malignant tumors, no intolerable serious medical diseases. The exclusion criteria included: (1) distant metastasis; (2) esophagography showed signs of esophageal perforation; (3) active esophageal bleeding; (4) complete esophageal obstruction. This was a retrospective study, and it was approved by the Ethics Committee of Shandong Cancer Hospital, and informed consents were obtained from all included individuals.
Radiotherapy
The GTV was identified by diagnostic and radiotherapy planning CT images and esophagography. All patients were treated with 3-dimensional conformal radiotherapy or intensity-modulated radiotherapy. Radiotherapy alone, radical simultaneous or sequential CRT was performed according to the clinical stage. The delineation of target volumes and OARs referred to the Radiotherapy and Oncology Group (RTOG) guidelines. All radiotherapy plans were generated in the Eclipse system (Varian Medical Systems, Palo Alto, CA, Version 13.5.35), and delivered with 6 MV photons beams. The prescribed doses of radiotherapy were 45–66 Gy at 1.8–2.0 Gy per fraction once daily and five fractions per week. Plans were normalized to 95% of the plan tumor volume received 100% of the prescribed dose. The dose of all OARs was controlled below the safe range.
Chemotherapy
Some patients underwent concurrent or sequential CRT based on individualized treatment strategy. The regimens mainly consisted of two kinds of platinum-based chemotherapy. One was cisplatin with fluorouracil, one was cisplatin with paclitaxel. The doses of chemotherapy regimens followed the guidelines of Chinese Society of Clinical Oncology and National Comprehensive Cancer Network for EC.
Response Evaluation
All patients performed esophagography and enhanced CT of chest, abdomen and neck before and at 1 month after radiotherapy or CRT. The imaging data were analyzed and the STO of all patients were assessed by an imaging deputy chief physician and a radiotherapy deputy chief physician according to Response Evaluation Criteria in Solid Tumor 1.1 (RECIST 1.1) without knowledge of the results of TVCR studies. Patients with an outcome of complete response (CR) or partial response (PR) were subsequently classified as responders according to RECIST 1.1. Meanwhile, those who had an outcome of stable disease (SD) or progressive disease (PD) were defined as non-responders.
TVCR Calculation
We defined GTV including the primary tumor and involved lymph nodes at initial treatment planning as GTVi, at shrinking irradiation field planning as GTVs. All GTVi and GTVs were extracted from the Varian treatment planning system. The TVCR were calculated as follows:
Statistical Analysis
Using receiver operating characteristic (ROC) curve to convert continuous variables into binary variables. Chi square test was used to compare the clinic characteristics in different TVCR, and the difference between GTVi and GTVs was compared using Wilcoxon’s sign rank test. Univariate logistic regressions analysis was performed to estimate the odds ratio (OR) and confidence interval (CI) to evaluate the effect of independent variables on STO. In order to avoid omitting indicators that might be of clinical significance, factors that had P <0.1 in univariate analyses were subjected to multivariate analysis. Spearman’s rank correlation coefficient (r) values were calculated to analyze how other independent variables related to TVCR. A significant difference was considered if a P value was <0.05 in two-sided. And all statistical analyses were conducted with IBM SPSS Statistics 25.0 software (SPSS Inc., Chicago, IL).
Results
Clinical Parameters of the Patients
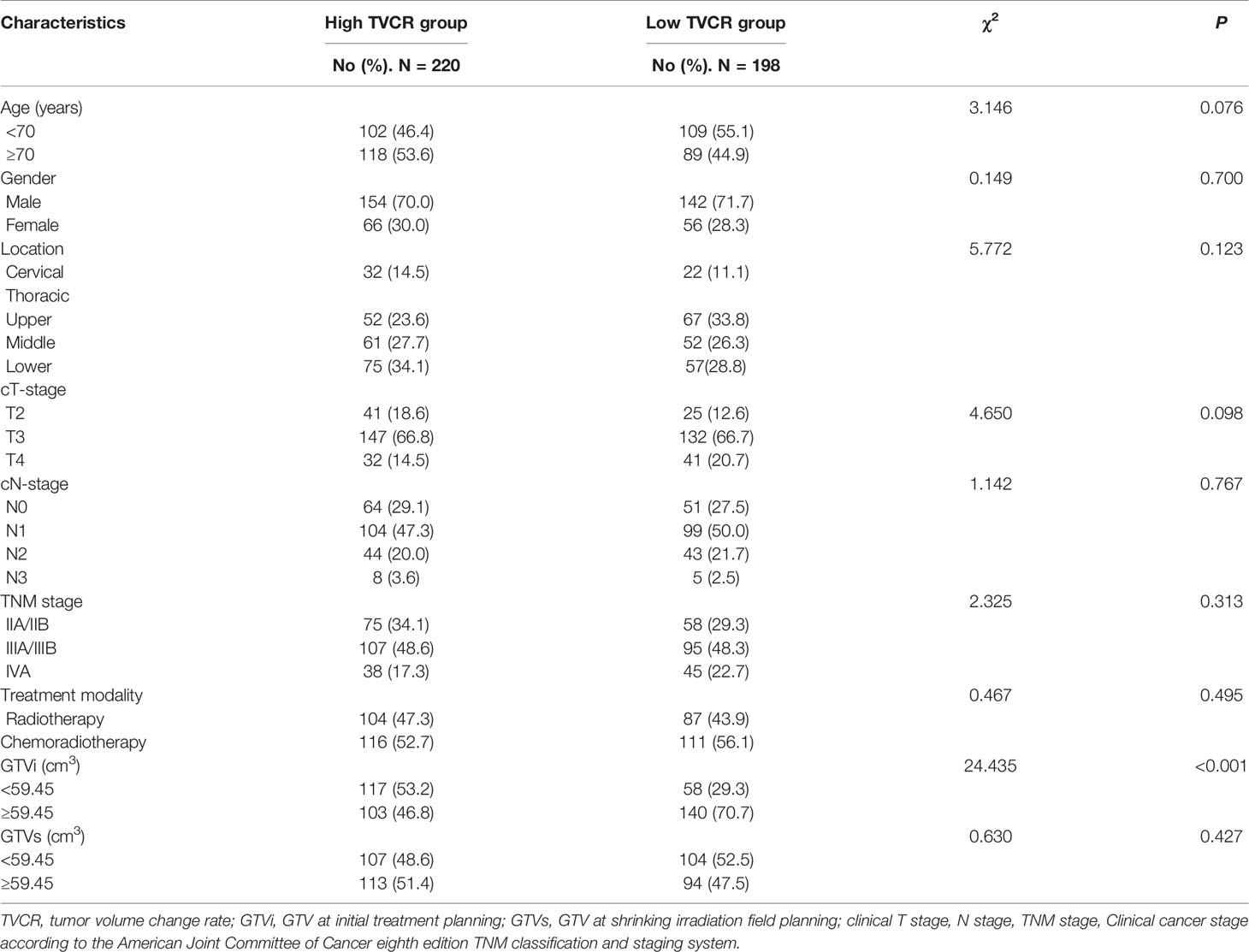
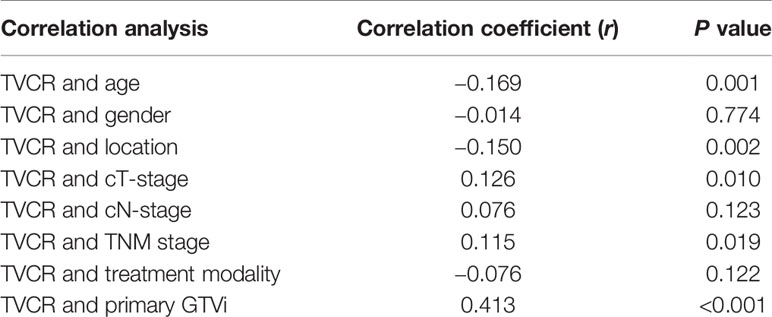
The median age and radiation dose were 70 years (range, 45–92 years) and 60 Gy (range, 50–66 Gy), respectively. The optimal cut-off values of GTVi, GTVs, and TVCR were 59.45 (range, 5.4–312.0 cm3; sensitivity 51.4%; specificity 51.4%; 95%CI, 0.526–0.644), 55.78 (range, 5.7–338.0 cm3; sensitivity 64.7%; specificity 54.1%; 95%CI, 0.526–0.644) and 6.155% (range, −29.3 to 90.0%; sensitivity 68.8%; specificity 92.5%; 95%CI, 0.819–0.891), respectively. 227 patients (54.3%) underwent concurrent or sequential CRT, and 191 patients (45.7%) only received radiotherapy. All patient characteristics were listed in Table 1. The tumor volume changes in all patients at shrinking irradiation field planning were as follows: 217 patients (51.9%) had a decrease (mean ± standard deviation, 50.91 ± 56.82); 139 patients (33.3%) showed no change; and 62 patients (14.8%) demonstrated an increase (mean ± standard deviation, 6.635 ± 7.116). There was a significant decrease in GTVi (mean ± standard deviation, 100.5 ± 79.54) compared to GTVs (mean ± standard deviation, 75.03 ± 63.52), with GTVi decreasing by a median of 1.67% (range −29.3 to 90.4%; P<0.001) (Figure 1). At 1 month after radiotherapy or CRT, 272 patients (65.1%) were assessable for responders, 146 patients (34.9%) were assessable for non-responders. And individual change rate in tumor volume at shrinking irradiation field planning were shown in Figure 2. According to cut-off values, we also divided all patients into high TVCR group (≥6.155%) and low TVCR group (<6.155%). There was significant correlation between the GTVi with high and low TVCR groups (χ2 = 24.435, P < 0.001), which was shown in the Table 2.
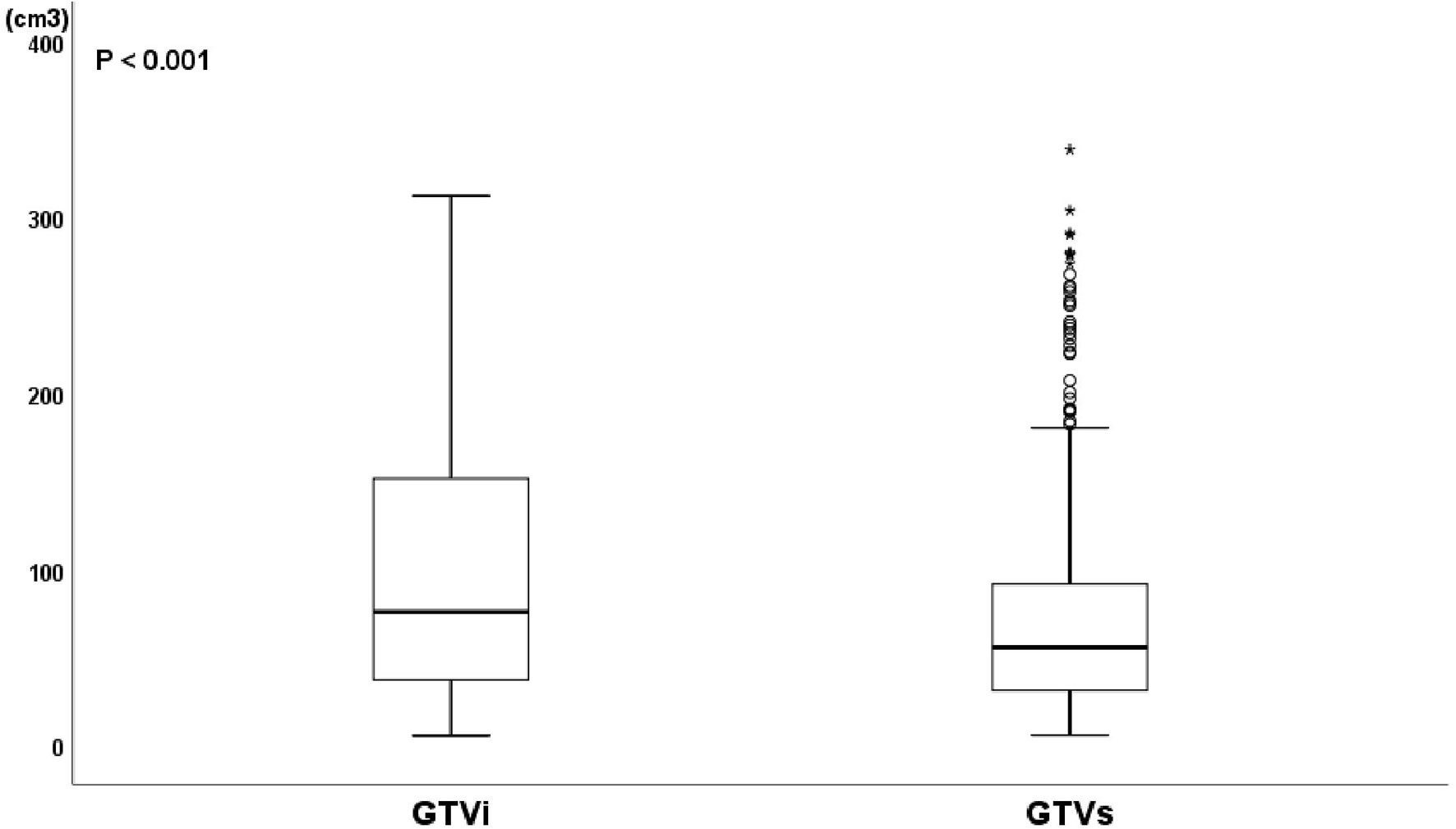
Figure 1 Boxplot of GTV at initial treatment planning (GTVi) and GTV at shrinking irradiation field planning (GTVs). Wilcoxon’s signed rank test, P < 0.001.
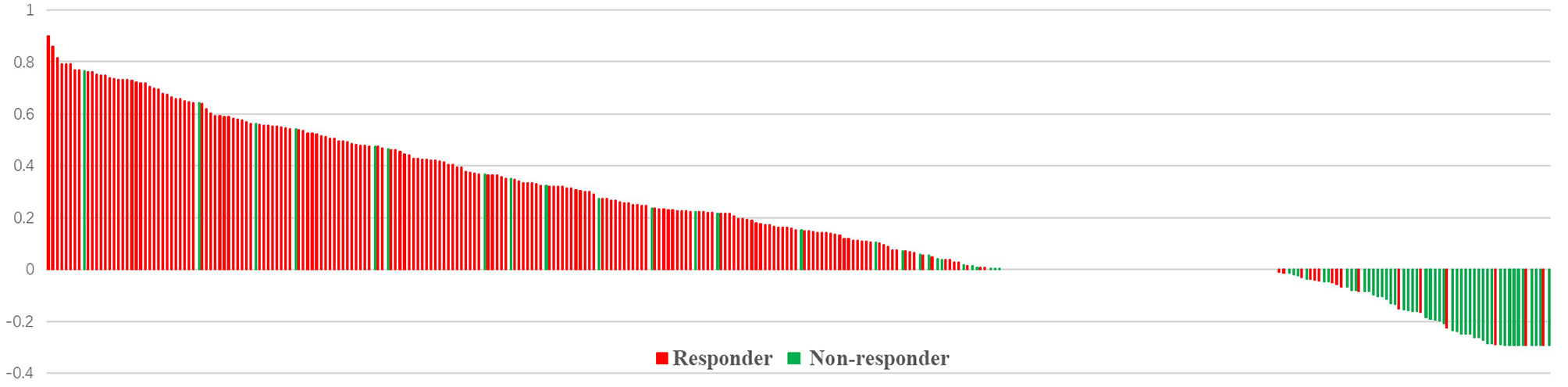
Figure 2 Individual changes in tumor volume at shrinking irradiation field planning. Bar length indicates the tumor volume change rate. Patients with responder (red) or non-responder (green).
Univariate and Multivariate Analyses for Short-Term Outcome
In univariate analysis, age [OR, 1.572; 95% CI, 1.049–2.358; P = 0.029], cT-stage [OR, 0.548; 95% CI, 0.322–0.933; P = 0.027], TNM stage [OR, 0.576; 95% CI, 0.381–0.870; P = 0.009], treatment modality [OR, 1.547; 95% CI, 1.032–2.318; P = 0.035], GTVi [OR, 0.463; 95% CI, 0.307–0.697; P < 0.001], and TVCR [OR, 0.037; 95% CI, 0.019–0.072; P < 0.001] were significantly associated with STO (Table 3). The variables with P <0.1 in univariate analysis were subjected to multivariate analysis. In addition to TVCR [OR, 0.036; 95% CI, 0.018–0.071; P < 0.001], gender [OR, 0.469; 95% CI, 0.264–0.835; P = 0.010] was also a potential factor which could predict STO (Figure 3). And the combined predictive value (AUC, 0.876; 95%CI, 0.843–0.910; P < 0.001) of gender and TVCR is exceeded of TVCR (AUC, 0.855; 95%CI, 0.819–0.891; P < 0.001) (Figure 4).
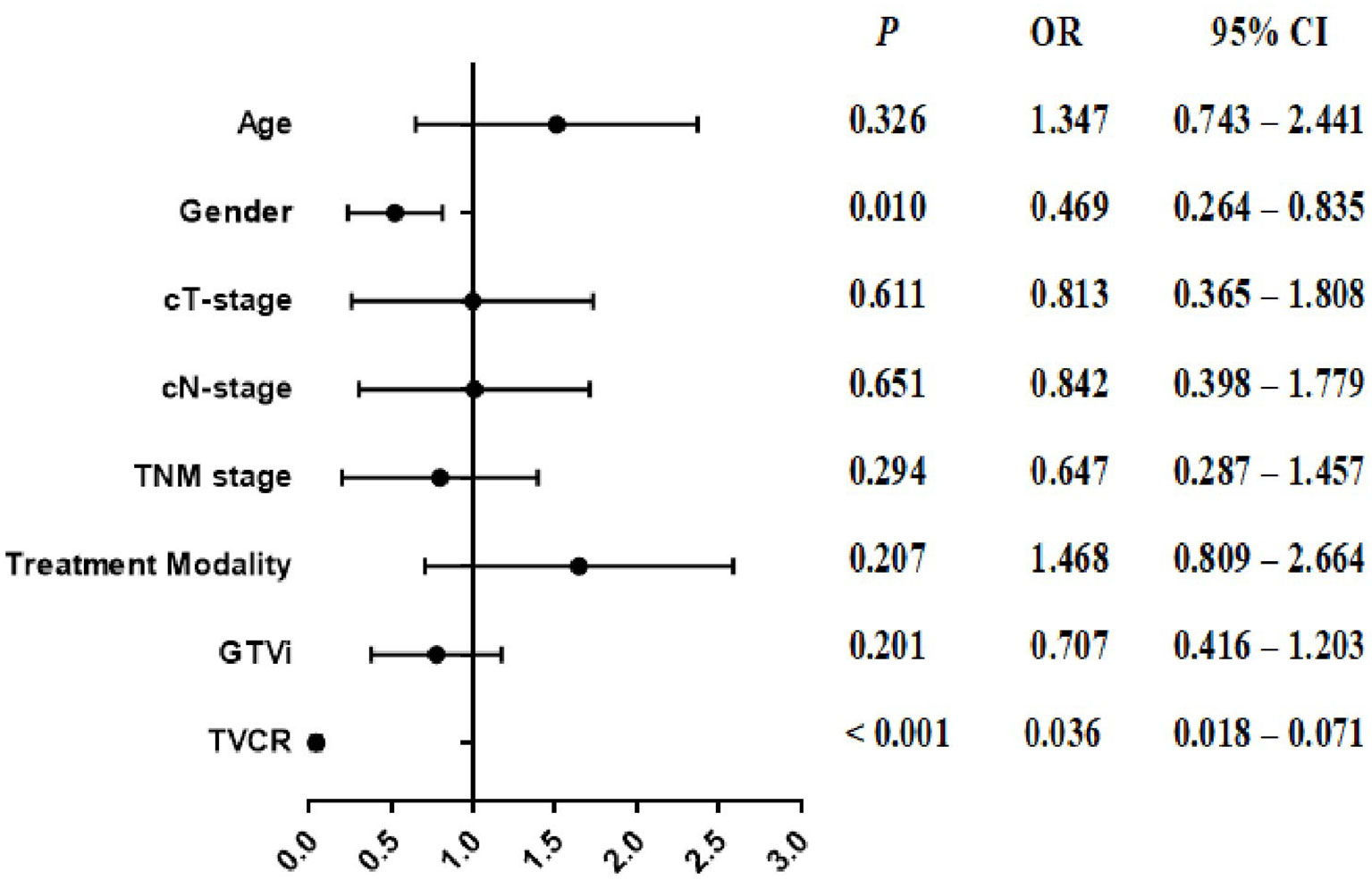
Figure 3 The forest plot of the multivariate analysis of clinical, and tumor volume in predicting the short-term outcome.
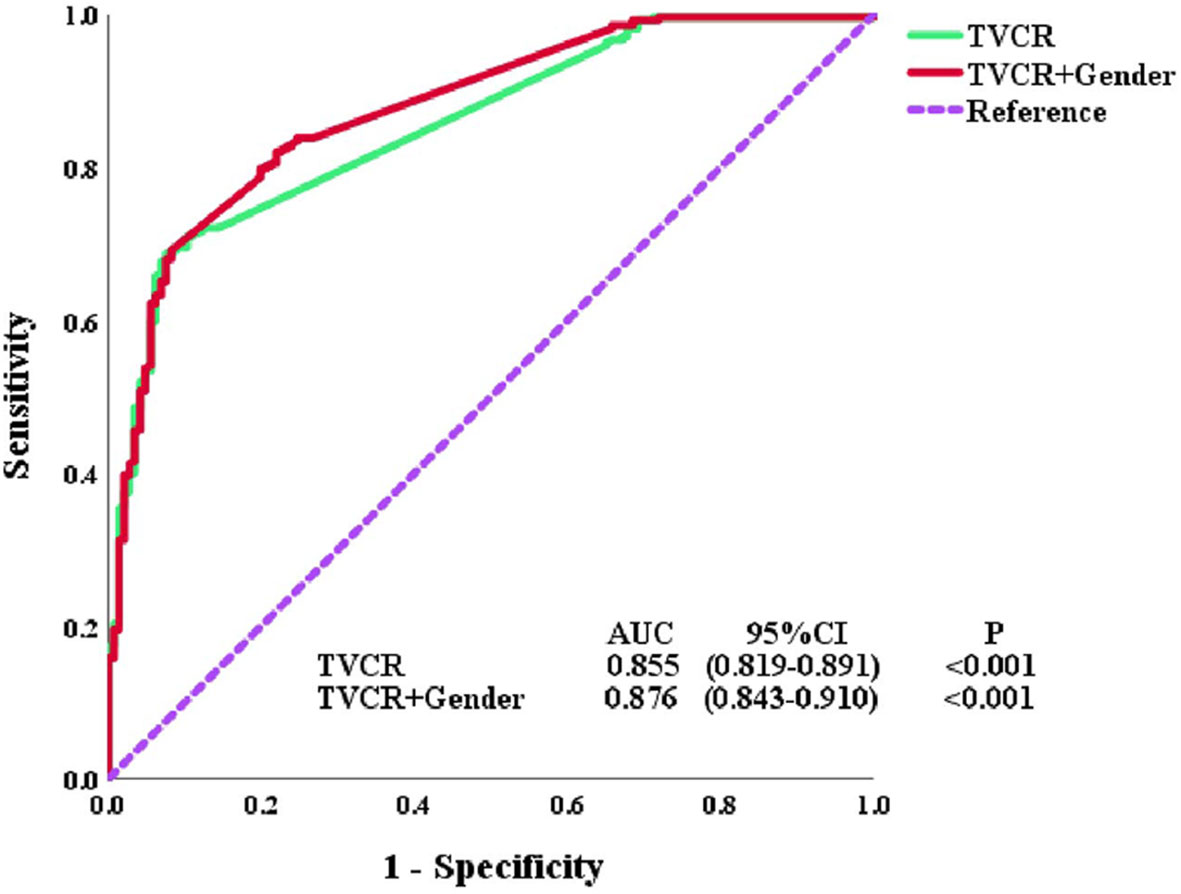
Figure 4 Receiver operator characteristic (ROC) curves for tumor volume change rate (TVCR) and multivariate.
Spearman Correlation Coefficient in Tumor Volume Change Rate
Further correlation studies indicated that TVCR was positively correlated with GTVi (r = 0.413, P < 0.001). The P value of TVCR with age, location, cT-stage, and TNM stage were all <0.05, but r value of them were <0.2 (Table 4).
Subgroup Analysis
Gender [OR, 0.287; 95% CI, 0.107–0.768; P = 0.013] and TVCR [OR, 0.042; 95% CI, 0.015–0.116; P < 0.001] were independent predictors of STO in multivariate analysis for patients with big GTVi (≥59.45 cm3) (Table 5).
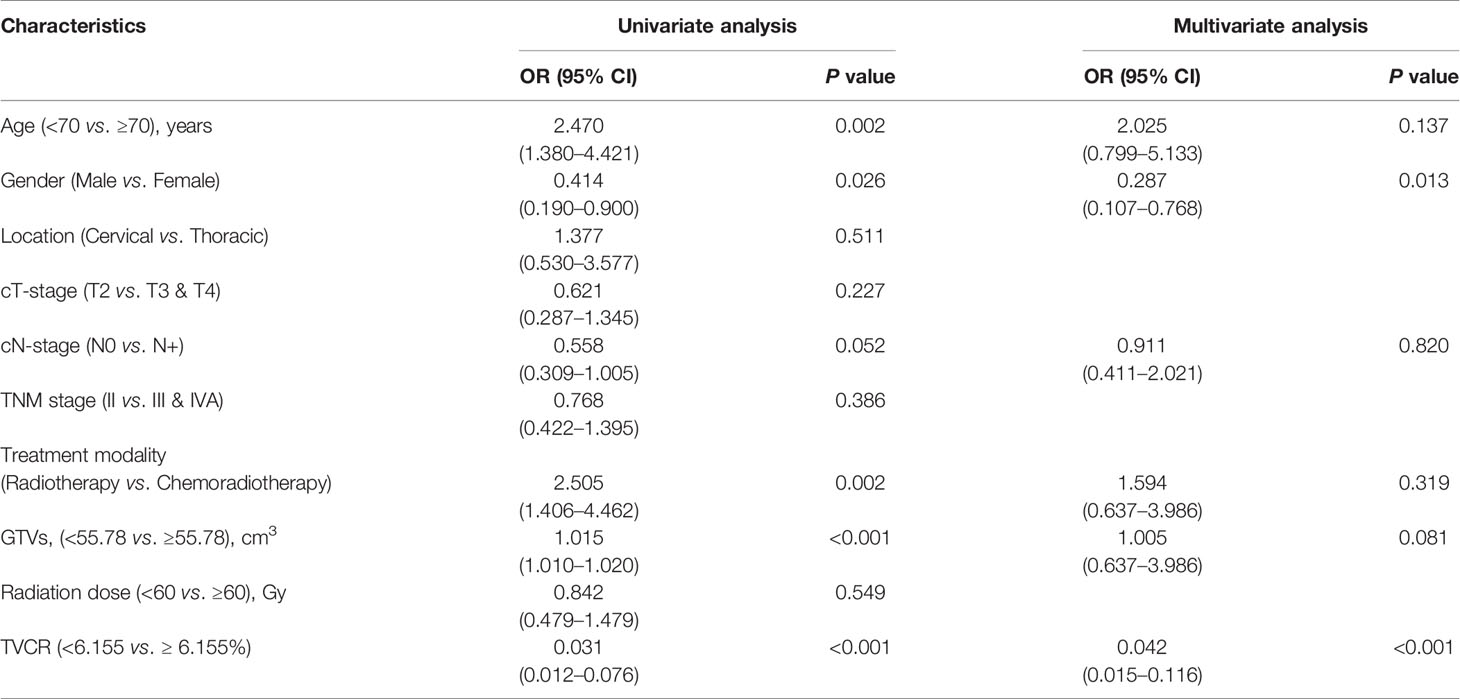
Table 5 Univariate and multivariate logistic regressions analysis for short-term outcome (STO) in big GTVi group (≥59.45 cm3).
Discussion
At present, the standard treatment scheme recommended by the guidelines for inoperable patients with locally advanced EC is concurrent or sequential CRT. But it is not clear whether further consolidation therapy is needed after CRT. How to select patients who need further consolidation therapy after CRT has become an urgent problem to be solved in clinical work. Furthermore, the main evaluation criteria of RECIST 1.1 is to measure changes in the longest diameter of assessable lesions, while ignoring short diameter and tumor volume. So, we assessed the relationship with GTVi, TVCR and STO, demonstrating that TVCR was a strong prognostic factor for unresectable ESCC patients who underwent radiotherapy or CRT. Moreover, patients with TVCR ≥6.155% during radiotherapy or CRT could have better STO. This was the first time to report the significance of TVCR during radiotherapy or CRT for ESCC. And TVCR would be a feasible predictive factor to find patients who are not sensitive to radiotherapy and develop individualized treatment as soon as possible.
GTV changes during radiotherapy or CRT differ widely in different studies. In our study, there were 62 patients (14.8%) whose GTVs were bigger than GTVi. Similar to our study, Wang et al. have reported that some patients (four of 11, 36.4%) with EC demonstrated an increase in GTV at week 2 during radiotherapy, and there were still some patients (three of 11, 27.3%) demonstrated an increase in GTV at week 4 (14). Christina et al. have also found that some patients (three3 of 19, 15.8%) with high grade glioma demonstrated an increase in GTV at week 3 during radiotherapy (15). One reason was the surrounding of the tumor might be edema after radiotherapy that made it difficult to identify whether it is the tumor invasion or the acute radiation reaction of the normal esophageal mucosa during radiotherapy, or be further enlarged without obvious retraction at the initial stage of radiotherapy. Barker et al. have also reported that tumor growth would accelerate at the initial stage of radiotherapy (16). Another reason might be the error by CT image parameters, which was compared with enhanced CT for localization, the image of tumor tissue on plain scan CT, was similar to that of surrounding normal tissue, which lead to the increase of GTV.
GTVi as a predictive factor has been confirmed in many studies. Bradley et al. reported that GTV had a high prognostic value in patients with unresectable non-small cell lung cancer (8). Chua et al. also reported that larger GTV are also associated with inferior local control rates in malignancies of the nasopharynx, larynx, and hypopharynx (10). In general, larger GTV means a heavier tumor load, more radiation-resistant hypoxic tumor cells and clonal cells, and greater restrictions on related organs that could lead to poor survival. Meanwhile, our study showed that GTVi was significant with STO in univariate analyses. Similar to our results, Chen et al. found that GTV could serve as a good prognostic factor for ESCC patients underwent radiotherapy, and Créhange et al. reported that tumor volume affected outcomes of EC (17, 18). But it was not an independent factor in our study. This might be that 54.3% of the patients in our study received CRT. So, the predictive value of GTVi still needs to be further explored.
Tumor is constantly changing during treatment and it seems that the rate of change between GTVs and GTVi which we defined as TVCR is more reasonable than GTVi in predicting effect. From disease regression, one potential advantage of quantifying TVCR during radiotherapy was to redefine the tumor volume to assess curative effect. Previous studies in other primary sites found that the larger reduction in tumor volume resulted in better local control, better disease-free survival, and better OS in cervical, non-small-cell lung and head-and-neck cancers, but some studies believed that tumor regression rate was not a predictor of survival (19–22). In our study, TVCR was a powerful predictor for STO. Similar with our result, Yang et al. have found TVCR during radiotherapy could be used as an independent factor affecting survival rate in head-and-neck cancer (21). As expected, our data demonstrated a significant correlation of GTVi with tumor volume (r = 0.413). However, GTVi was not independent predictors of STO in our study, suggesting that TVCR may be a more sensitive indicator. The TVCR showed a better predictive value (AUC, 0.855; 95% CI, 0.819–0.891; P < 0.001) on STO in this study. All GTVi and GTVs parameters came from the evaluation system after the target area was sketched. But, at present, the target and OARs are still sketched manually by doctors in clinical radiotherapy, while many factors such as doctors’ clinical knowledge, experience, energy and status determine that there are great differences in drawing quality between different doctors and different patients (23). With the development of artificial intelligence (AI) in the field of precision radiotherapy, it is possible to solve these problems. Pinnacle et al. initially realized the automatic delineation of regions of interest using atlas template library; Google developed a set of AI target delineation system based on atlas, which automatically delineated head and neck tumor lesions through machine learning (24); Sims et al. used the atlas tool to automatically draw the brainstem, parotid gland, and mandible of patients, and compared the results with the results of manual sketching (25); Lin et al. used AI technology to automatically draw nasopharyngeal tumors on magnetic resonance images, which provided a solution for accurate and efficient delineation of radiotherapy targets for nasopharyngeal carcinoma (26). All these show that AI plus radiotherapy can improve the accuracy radiotherapy and promote the automation and intelligence of radiotherapy.
Many prognostic factors achieved significance in our study, but TVCR still maintained significance for STO in multivariate analysis. Meanwhile, gender was also significant for STO in multivariate analysis. Similar with our results, Pierre et al. have found that gender is an independent prognostic factor for patients with ESCC, and female gender was a positive prognostic factor (27). In addition, studies have shown that tumor location was also related to prognosis, but in this study, tumor location was very weakly negative correlated with TVCR (r = −0.150). This was might be that thoracic EC spread unnoticed before the appearance of the first symptom. Créhange et al. have reported that tumor volume was correlated with tumor location, and tumors below the carina had a worse prognosis (18). They all indicated that the individualized treatment was becoming more and more important. And the combined predictive value of gender and TVCR exceeded that of TVCR (AUC 0.876 vs 0.855).
Several limitations would be addressed here. First, this was a retrospective, single-center study that was inevitably affected by a number of confounding factors. Second, although esophagography, CT and other auxiliary examinations were used, compared with pathological TNM staging, clinical TNM staging was still not accurate. Last, the combined of gender and TVCR had a good predictive value, but it was not tested in clinic, and its clinical application value remained to be determined.
In conclusion, our study confirmed that STO could be predicted by the changes of GTV before and during radiotherapy or chemoradiotherapy for ESCC, which had an important clinical significance for adjusting the treatment strategy and guiding individualized treatment.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of Shandong Cancer Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
XM and JY contributed to the conception and design of the study. SL and CL organized the database. SL, ZG, and DS performed the statistical analysis. SL and XM wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81972864), Academic Promotion Program of Shandong First Medical University (2019RC002), Science and Technology Support Plan for Youth Innovation Teams of Universities in Shandong Province (2019KJL001) and Science and Technology Plan of Jinan (201907113).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank patients and their kin for supporting our work and thank editors as well as reviewers for reading the manuscript.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338
3. Tachibana M, Kinugasa S, Hirahara N, Yoshimura H. Lymph node classification of esophageal squamous cell carcinoma and adenocarcinoma. Eur J Cardiothorac Surg (2008) 34:427–31. doi: 10.1016/j.ejcts.2008.04.022
4. Teoh AY, Chiu PW, Yeung WK, Liu SY, Wong SK, Ng EK. Long-term survival outcomes after definitive chemoradiation versus surgery in patients with resectable squamous carcinoma of the esophagus: results from a randomized controlled trial. Ann Oncol (2013) 24:165–71. doi: 10.1093/annonc/mds206
5. Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med (2008) 358:36–46. doi: 10.1056/NEJMoa073149
6. Lou F, Sima CS, Adusumilli PS, Bains MS, Sarkaria IS, Rusch VW, et al. Esophageal cancer recurrence patterns and implications for surveillance. J Thorac Oncol (2013) 8:1558–62. doi: 10.1097/01.JTO.0000437420.38972.fb
7. Hamai Y, Hihara J, Emi M, Taomoto J, Aoki Y, Kishimoto I, et al. Treatment outcomes and prognostic factors for thoracic esophageal cancer with clinical evidence of adjacent organ invasion. Anticancer Res (2013) 33:3495–502. doi: 10.3109/0284186X.2013.806820
8. Bradley JD, Ieumwananonthachai N, Purdy JA, Wasserman TH, Lockett MA, Graham MV, et al. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys (2002) 52:49–57. doi: 10.1016/s0360-3016(01)01772-2
9. Werner-Wasik M, Swann RS, Bradley J, Graham M, Emami B, Purdy J, et al. Increasing tumor volume is predictive of poor overall and progression-free survival: secondary analysis of the Radiation Therapy Oncology Group 93-11 phase I-II radiation dose-escalation study in patients with inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys (2008) 70:385–90. doi: 10.1016/j.ijrobp.2007.06.034
10. Chua DT, Sham JS, Kwong DL, Tai JS, Wu PM, Lo M, et al. Volumetric analysis of tumor extent in nasopharyngeal carcinoma and correlation with treatment outcome. Int J Radiat Oncol Biol Phys (1997) 39:711–9. doi: 10.1016/s0360-3016(97)00374-x
11. Guo R, Sun Y, Yu XL, Yin WJ, Li WF, Chen YY, et al. Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiother Oncol (2012) 104:294–9. doi: 10.1016/j.radonc.2012.09.001
12. Li H, Chen TW, Li ZL, Zhang XM, Chen XL, Wang LY, et al. Tumour size of resectable oesophageal squamous cell carcinoma measured with multidetector computed tomography for predicting regional lymph node metastasis and N stage. Eur Radiol (2012) 22:2487–93. doi: 10.1007/s00330-012-2512-4
13. Li R, Chen TW, Hu J, Guo D, Zhang X, Deng D, et al. Tumor volume of resectable adenocarcinoma of the esophagogastric junction at multidetector CT: association with regional lymph node metastasis and N stage. Radiology (2013) 269:130–8. doi: 10.1148/radiol.13122269
14. Wang Q, Zhang W, Chen X, Zhang Z, Han W, Yang AL, et al. Study on relationship between the beginning plan and the anatomy and dosimetry change during intensity modulated radiotherapy for esophageal carcinoma. Chin J Radiat Oncology (2010) 19:512–6. doi: 10.3760/cma.j.issn.1004-4221.2010.06.008
15. Tsien C, Gomez-Hassan D, Ten Haken RK, Tatro D, Junck L, Chenevert TL, et al. Evaluating changes in tumor volume using magnetic resonance imaging during the course of radiotherapy treatment of high-grade gliomas: Implications for conformal dose-escalation studies. Int J Radiat Oncol Biol Phys (2005) 62:328–32. doi: 10.1016/j.ijrobp.2004.10.010
16. Barker JL Jr, Garden AS, Ang KK, O’Daniel JC, Wang H, Court LE, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys (2004) 59:960–70. doi: 10.1016/j.ijrobp.2003.12.024
17. Chen Y, Zhang Z, Jiang G, Zhao K. Gross tumor volume is the prognostic factor for squamous cell esophageal cancer patients treated with definitive radiotherapy. J Thorac Dis (2016) 8:1155–61. doi: 10.21037/jtd.2016.04.08
18. Créhange G, Bosset M, Lorchel F, Buffet-Miny J, Dumas JL, Mercier M, et al. Tumor Volume as Outcome Determinant in Patients Treated With Chemoradiation for Locally Advanced Esophageal Cancer. Am J Clin Oncol (2006) 29:583–7. doi: 10.1097/01.coc.0000242346.25229.48
19. Jabbour SK, Kim S, Haider SA, Xu X, Wu A, Surakanti S, et al. Reduction in tumor volume by cone beam computed tomography predicts overall survival in non-small cell lung cancer treated with chemoradiation therapy. Int J Radiat Oncol Biol Phys (2015) 92:627–33. doi: 10.1016/j.ijrobp.2015.02.017
20. Brink C, Bernchou U, Bertelsen A, Hansen O, Schytte T, Bentzen SM. Locoregional control of non-small cell lung cancer in relation to automated early assessment of tumor regression on cone beam computed tomography. Int J Radiat Oncol Biol Phys (2014) 89:916–23. doi: 10.1016/j.ijrobp.2014.03.038
21. Yang SN, Liao CY, Chen SW, Liang JA, Tsai MH, Hua CH, et al. Clinical implications of the tumor volume reduction rate in head-and-neck cancer during definitive intensity-modulated radiotherapy for organ preservation. Int J Radiat Oncol Biol Phys (2011) 79:1096–103. doi: 10.1016/j.ijrobp.2009.12.055
22. Mayr NA, Wang JZ, Lo SS, Zhang D, Grecula JC, Lu L, et al. Translating response during therapy into ultimate treatment outcome: a personalized 4-dimensional MRI tumor volumetric regression approach in cervical cancer. Int J Radiat Oncol Biol Phys (2010) 76:719–27. doi: 10.1016/j.ijrobp.2009.02.036
23. Pham DL, Xu C, Prince JL. Current methods in medical image segmentation. Annu Rev BioMed Eng (2000) 2:315–37. doi: 10.1146/annurev.bioeng.2.1.315
24. Powles J1, Hodson H. Google DeepMind and healthcare in an age of algorithms. Health Technol (Berl) (2017) 7:351–67. doi: 10.1007/s12553-017-0179-1
25. Sims R, Isambert A, Grégoire V, Bidault F, Fresco L, Sage J, et al. A pre-clinical assessment of an atlas-based automatic segmentation tool for the head and neck. Radiother Oncol (2009) 93:474–8. doi: 10.1016/j.radonc.2009.08.013
26. Lin L, Dou Q, Jin YM, Zhou GQ, Tang YQ, Chen WL, et al. Deep Learning for Automated Contouring of Primary Tumor Volumes by MRI for Nasopharyngeal Carcinoma. Radiology (2019) 291:677–86. doi: 10.1148/radiol.2019182012
Keywords: esophageal squamous cell carcinoma, tumor volume change, radiotherapy, gross tumor volume, short-term outcome
Citation: Liang S, Li C, Gao Z, Shang D, Yu J and Meng X (2021) The Predictive Value of Tumor Volume and Its Change on Short-Term Outcome for Esophageal Squamous Cell Carcinoma Treated With Radiotherapy or Chemoradiotherapy. Front. Oncol. 10:586145. doi: 10.3389/fonc.2020.586145
Received: 22 July 2020; Accepted: 14 December 2020;
Published: 01 February 2021.
Edited by:
Gene A. Cardarelli, Brown University, United StatesReviewed by:
Mary Feng, University of California, San Francisco, United StatesVinay Sharma, University of the Witwatersrand, South Africa
Copyright © 2021 Liang, Li, Gao, Shang, Yu and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue Meng, c2Rzemx5eW1lbmd4dWVAeWVhaC5uZXQ=
 Shuai Liang1
Shuai Liang1 Chengming Li
Chengming Li Xue Meng
Xue Meng