- 1The First Central Clinical College of Tianjin Medical University, Tianjin, China
- 2Nankai University School of Medicine, Tianjin, China
- 3Department of Hematology, Tianjin First Central Hospital, Tianjin, China
B cell lymphoma 2 (BCL-2) family proteins play an important role in intrinsic apoptosis. Overexpression of BCL-2 proteins in acute myeloid leukemia can circumvent resistance to apoptosis and chemotherapy. Considering this effect, the exploration of anti-apoptotic BCL-2 inhibitors is considered to have tremendous potential for the discovery of novel pharmacological modulators in cancer. This review outlines the impact of BCL-2 family proteins on intrinsic apoptosis and the development of acute myeloid leukemia (AML). Furthermore, we will also review the new combination therapy with venetoclax that overcomes resistance to venetoclax and discuss biomarkers of treatment response identified in early-phase clinical trials.
Introduction
Acute myeloid leukemia (AML) is one of the most common hematological malignancies in adults, with a median age at diagnosis of 68 (1). For the past 30 years, the main treatment has been chemotherapy alone or combined with hematopoietic stem cell transplantation (HSCT). In 2017, there was a breakthrough, and several new drugs, including venetoclax and selective B cell lymphoma-2 (BCL-2) inhibitors, seemed to reshape the therapeutic landscape of AML (2). The BCL-2 family of proteins plays a significant role in the intrinsic apoptotic pathway. They are also critical for cell survival and are overexpressed in many tumors, including AML. In addition, aberrant overexpression of BCL-2 family proteins causes resistance to chemotherapy and is associated with a poor prognosis (3–7). Recently, BCL-2 inhibitors, such as venetoclax and novel MCL-1 inhibitors, have shown anti-leukemic activity in preclinical AML models. In 2018, venetoclax in combination with azacitidine, decitabine, or low-dose cytarabine (LDAC) was approved for use in untreated patients with AML aged 75 years or older or patients with comorbidities that preclude the use of intensive induction chemotherapy (8). Here, we review how they regulate apoptosis and how the targeting of BCL-2 has developed in AML. Furthermore, we summarize the mechanism of resistance to venetoclax and provide some potential solutions.
Apoptosis and the BCL-2 Family
There are two main pathways for apoptosis: the intrinsic pathway and the extrinsic pathway (9). The extrinsic apoptotic pathway is initiated by the interaction of an extracellular ligand and tumor necrosis factor (TNF) family death receptors. Then, procaspase-8 is activated by the binding of the death-inducing signaling complex (DISC) to a series of adaptors, and activated caspase-8 further initiates the caspase-3 cascade, which eventually leads to cell death via apoptosis (10, 11) (Figure 1). The intrinsic pathway is initiated by internal cellular stress, such as DNA damage, growth factor deprivation, and oxidative stress. These alterations then lead to mitochondrial depolarization, which allows the release of cytochrome c, which is the hallmark of the intrinsic apoptotic pathway. Cytochrome c binds to apoptosis protease-activating factor 1 (APAF1) and procaspase-9, forming an intracellular “apoptosome” that can activate caspase-9. Then, active caspase-9 leads to executioner caspase-3 activation (11) (Figure 1). The BCL-2 family of proteins regulates the intrinsic apoptotic pathway by controlling mitochondrial outer membrane permeabilization (MOMP) (12).
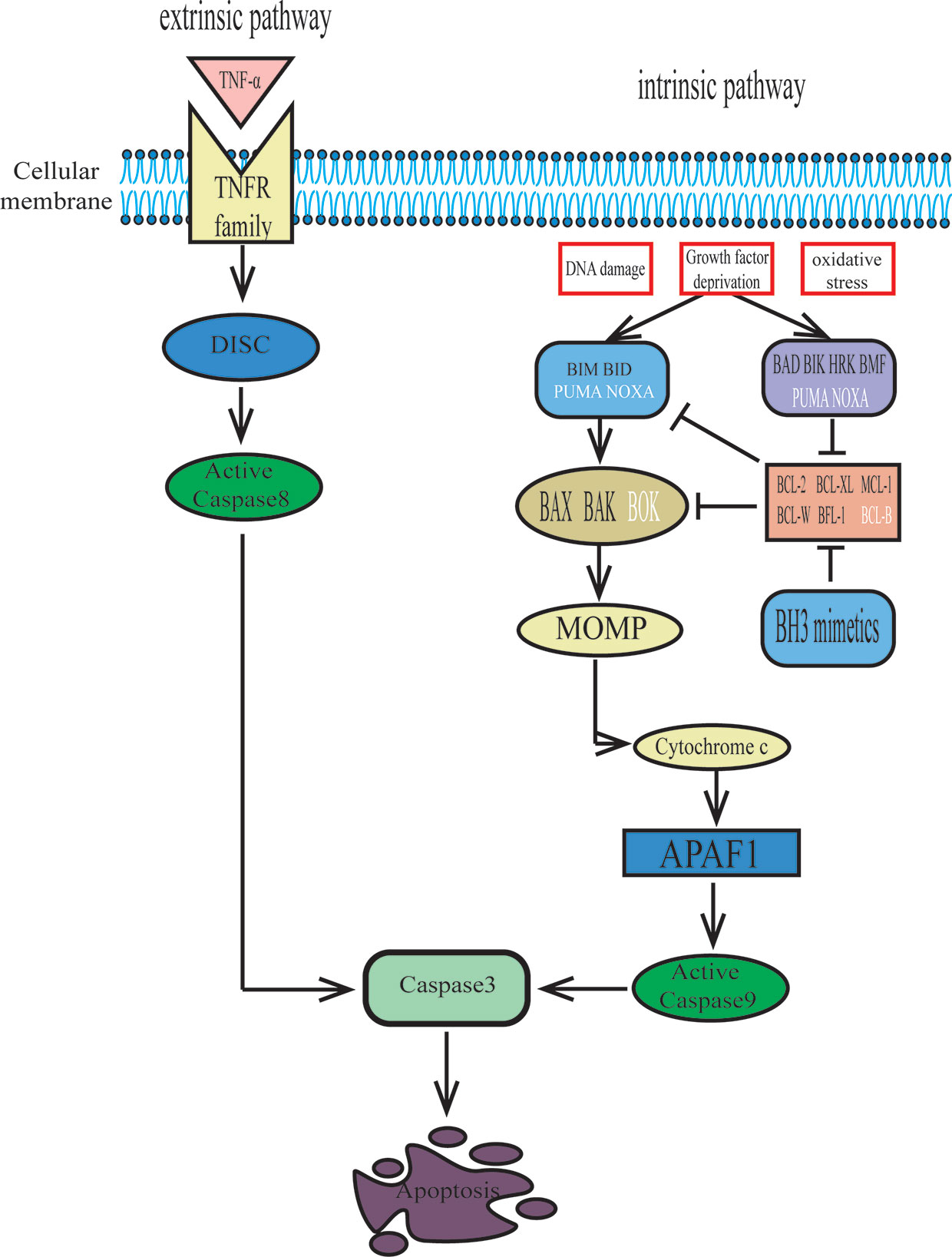
Figure 1 The extrinsic and intrinsic pathways to apoptosis. The extrinsic pathway of apoptosis is activated when certain death receptor ligands of the tumour necrosis factor (TNF) family (such as TNF) engage their cognate death receptors on the plasma membrane, leading to caspase-8 activation via the death-inducing signalling complex (DISC), which results in apoptosis. The intrinsic pathway of apoptosis is activated by cellular stresses (such as DNA damage, growth factor deprivation or oxidative stress) and is regulated by BCL-2 family proteins. BH3 proteins bind to and activate pro-apoptotic proteins BAX, BAK and possibly BOK, which oligomerize in the mitochondrial membranes and release cytochrome c, then interact with apoptosis protease-activating factor 1(APAF1) and initiate caspase activation that results in apoptosis. Futhermore, BH3-only activator proteins (BID, BIM, Puma and NOXA) directly bind to interact with BAX and BAK to promote mitochondrial outer membrane permeabilization (MOMP). While the BH3-only sensitizer proteins (BAD, BIK, HRK, BMF, PUMA, NOXA) bind to anti-apoptotic proteins with higher affinity, freeing the activator proteins from the BH3 binding pockets in the anti-apoptotic proteins and executing cell death by binding to BAX or BAK. The two pathways converge at activation of the effector caspase-3.
The BCL-2 gene, whose transcription can be upregulated, was first discovered as a part of t(14;18) chromosomal translocation in follicular lymphoma and diffuse large B cell lymphoma (13, 14). It was first recognized as a classical growth-driving oncogene, but later proved that BCL-2 promotes malignant cell survival by attenuating apoptosis (15, 16). The BCL-2 family consists of more than 20 proteins, which may be functionally classified as either anti-apoptotic or pro-apoptotic proteins (17). The anti-apoptotic BCL-2 proteins, which include four BCL-2 homology domains (BH1–4), include BCL-2, BCL-XL (BCL-2-like protein 1), MCL-1 (induced myeloid leukemia cell differentiation protein MCL-1), BCL-W (Bcl-2-like protein 2), BFL-1/A1 (BCL-2-related protein A1), and possibly BCL-B (Bcl-2-like protein 10). The pro-apoptotic proteins can be divided into effector proteins and BH3-only proteins. The former group contains three BH domains and includes BAX (BCL-2 associated X protein), BAK (BCL-2 antagonist killer), and possibly BOK (BCL-2-related ovarian killer). The latter group possesses only a single BH3 domain and includes BID (BH3-interacting domain death antagonist), BIK (BCL2 interacting killer), BIM (BCL-22L11; BCL2-interacting mediator of cell death), BAD (BCL2 antagonist of cell death), BMF (BCL-2 modifying factor), HRK (activator of apoptosis hara-kiri), PUMA (p53 upregulated modulator of apoptosis; also called BBC3 [BCL2 binding component 3]), and NOXA (PMA induced protein 1; also called PMAIP1 [phorbol-12-myristate-13-acetate-induced protein 1]) (11, 17, 18). Once BAX and BAK are activated, they oligomerize and form pores to induce MOMP, releasing cytochrome c from mitochondria and finally inducing cell apoptosis (19). The anti-apoptotic BCL-2 proteins can protect cells from apoptosis by directly binding to BAX/BAK or antagonizing pro-apoptotic BH3-only proteins. Furthermore, the BH3-only proteins can be subdivided into activator and sensitizer proteins. The activator proteins, which include BID, BIM, Puma, and NOXA, directly bind to and interact with BAX and BAK to promote MOMP. The sensitizer proteins bind to anti-apoptotic proteins with higher affinity, freeing the activator proteins from the BH3 binding pockets in the anti-apoptotic proteins and executing cell death by binding to BAX or BAK (20, 21) (Figure 1).
Owing to subtle differences in their BH3 domains and in the grooves of the anti-apoptotic proteins, the various BCL-2 family proteins have differential specificity of binding to one another. For example, BAX and BAK have high affinities for BCL-XL, and BAK has higher affinities for MCL-1, as do BAX and BCL-2 (22). Interestingly, some BH3-only proteins, such as BAD and NOXA, are selective for subsets of their anti-apoptotic relatives, whereas other BH3-only proteins, particularly BIM, BID, and PUMA, probably neutralize all of the anti-apoptotic proteins (23) (Figure 2). In healthy cells, anti-apoptotic proteins and pro-apoptotic proteins maintain a delicate balance, while cancer cells overexpress different anti-apoptotic proteins to facilitates prolonged tumor cell survival (24). A good knowledge of the roles of BCL-2 family proteins in promoting tumorigenesis has contributed to the development of numerous novel drugs targeting aberrant apoptotic pathways in cancer. Thus, targeting BCL-2 family proteins may be a prominent strategy for cancer therapy.
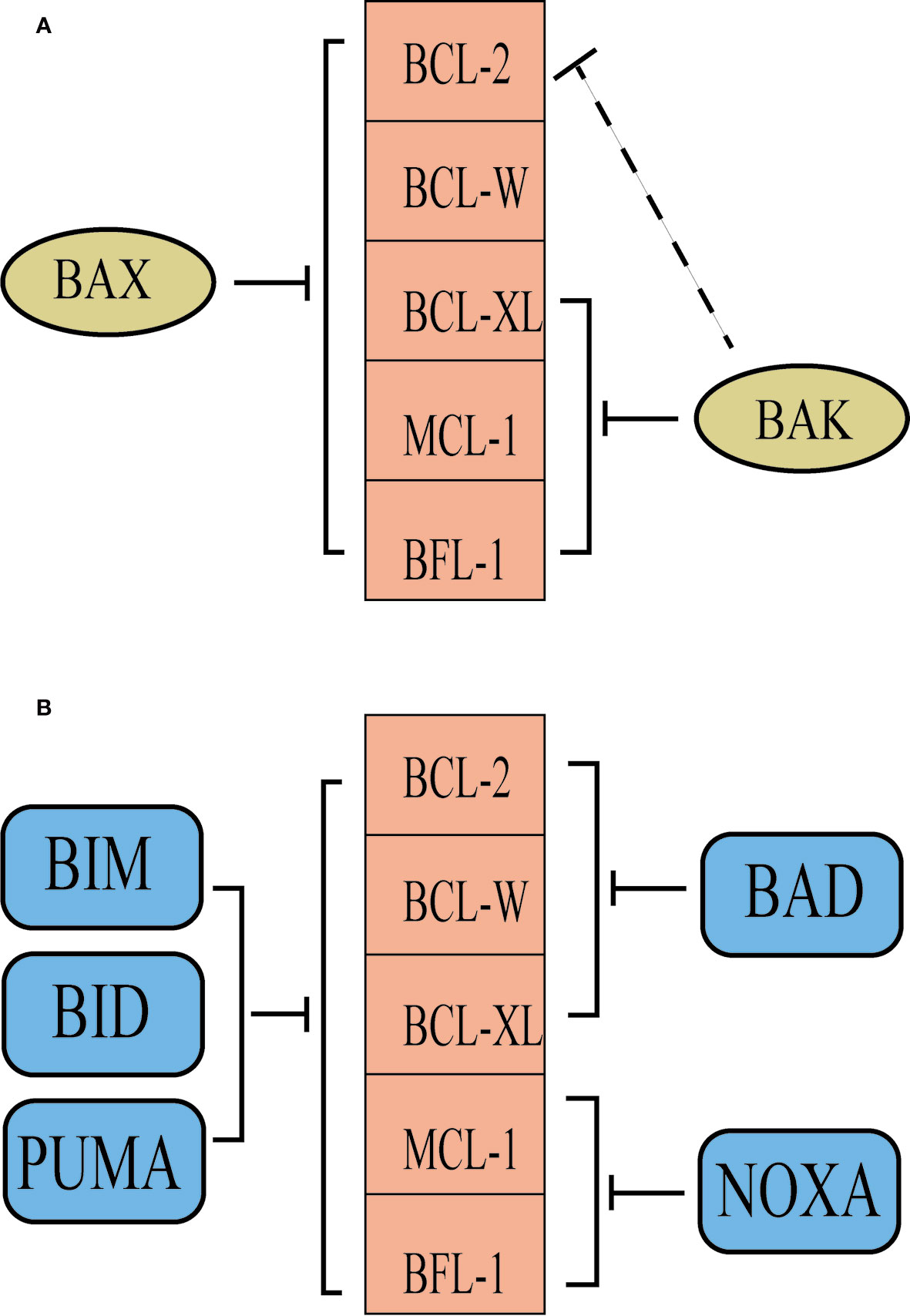
Figure 2 The selectivity of BCL-2 family proteins. (A) BAK is inhibited predominantly by BCL-XL, MCL-1 and BFL-1, while BCL-2 contributes in some situations. However, BAX is probably inhibited by all of the pro-survival proteins. (B) Some BH3-only proteins, such as BAD and NOXA, are selective for subsets of their anti-apoptotic relatives, whereas other BH3-only proteins, particularly BIM, BID and PUMA, probably neutralize all of the anti-apoptotic proteins.
BCL-2 in AML
A number of early studies showed that BCL-2 was overexpressed in CD34+ AML cells and was associated with poor prognosis and resistance to chemotherapy (25–27). Overexpression of MCL-1 and BCL-XL also confers chemotherapy resistance in AML (4, 28, 29). Thus, antisense oligonucleotides targeting BCL-2 were explored and decreased the number of leukemia cells in vitro (30). A better understanding of the intrinsic apoptosis pathway has contributed to the focus on the development of novel small molecules that mimic the BH3 domain found in all pro-apoptotic BCL-2 family proteins. These drugs mimic the action of certain pro-apoptotic BH3-only proteins by binding directly to the BH3-binding domains of anti-apoptotic molecules, thereby displacing native BH3-only proteins and thus inducing apoptosis (Figure 1). These protein-protein interaction inhibitors that directly activate apoptosis represented a milestone event in medicinal chemistry, as well as in the cell death field. With the inspired success in B cell lymphoid malignancies, these new BH3 mimetics have also been used in AML.
Early Attempts to Target BCL-2 in AML
Oblimersen
Oblimersen, which can target the first six codons of the human BCL-2 mRNA, is an 18-mer phosphorothioate Bcl-2 antisense oligonucleotide. It can increase cancer cell apoptosis and overcome chemotherapy resistance by binding to BCL-2 mRNA, thus downregulating BCL-2 protein expression (30, 31). The first use in a clinical trial of AML was a phase I study in relapsed or refractory acute leukemia in which patients received a combination with fludarabine, cytarabine, and granulocyte colony-stimulating factor (FLAG) salvage chemotherapy (32). Seventeen patients with relapsed and refractory AML and 3 patients with acute lymphoblastic leukemia (ALL) were recruited. Five of 17 (29%) achieved complete response (CR), and 2 patients achieved complete response with incomplete blood count recovery (CRi) (11%), yielding an objective response of 41%. Furthermore, no dose-limiting toxicity was observed in any of the cohorts examined. Another phase 1 trial conducted by the same group combined two different doses of oblimersen with chemotherapy in untreated older patients with AML (33). In this trial, 14 of 29 (48%) patients achieved CR, with no significant increase compared with the previously reported remission rates of 40% in the absence of oblimersen. The side effects were similar to those expected with chemotherapy alone and were not dose limiting at either dose level, which proved that the drug was safe and tolerable. The preclinical and early clinical data led the Cancer and Leukemia Group B (CALGB) to conduct a large phase III trial in which older adults with newly diagnosed AML were randomized to standard induction chemotherapy with or without oblimersen (34). Regrettably, there were no differences observed in CR rates (48% vs 52%; p=0.75) or overall survival rates (OS at 1 year 36% vs 40%; p=0.83), which may lead to no further trials of this drug in AML. However, the failure of this trial did not dispel the exploration of BCL-2 inhibitors in AML.
Obatoclax (GX15-070)
Obatoclax is a pan-Bcl-2 antagonist and was the first BH3 mimetic used in clinical trials of AML. It can bind to the BH3 domain of BCL-2 (as well as those of BCL-XL, MCL-1, BCL-w, A1, and BCL-b) (35) and then prevent the anti-apoptotic proteins from sequestering pro-apoptotic BH3-only proteins. Obatoclax potently induced apoptosis and decreased leukemia cell proliferation in AML cell lines and primary AML samples (36). In a phase I trial of 44 patients with advanced hematological malignancies, which included 25 AML, 14 myelodysplasia (MDS), 4 chronic lymphocytic leukemia (CLL), and 1 ALL, patients showed good tolerance but modest efficacy in the clinic (37). Only one patient with AML with mixed lineage leukemia t(9;11) rearrangement achieved complete remission. In addition, obatoclax had neurological side effects, which further limited its clinical development. In addition, another phase I/II study of obatoclax in older patients with previously untreated AML showed similar results in that obatoclax did not seem to be associated with an objective response (38). The failure of this drug may encourage a future study on its combination with other new drugs, such as histone deacetylase inhibitors or sorafenib (39, 40). Furthermore, the insolubility of this compound also limits its function, so new BH3 mimetics should be explored.
Dual BCL-2/BCL-XL Inhibitors
ABT-737
ABT-737 is a BH3 mimetic with high potency activity against BCL-2, BCL-XL, BCL-W, and, to a lesser extent, MCL-1. It was discovered as the first high-affinity inhibitor of BCL-2 family proteins by using nuclear magnetic resonance (NMR)-based screening (41). In a preclinical study, ABT-737, which can effectively trigger Bax/Bak-mediated apoptosis, induced AML cell apoptosis in vitro, and the same activity was demonstrated in a murine xenograft model in vivo (42–44). High expression of MCL-1 and phosphorylation of BCL-2 are associated with resistance to ABT-737, as they lead to a reduction in MCL-1. Thus, targeting these changes may be a potential way to enhance ATB-737 response. For example, ABT-737 could be combined with pan-Bcl-2 family inhibitors, MEK inhibitors, or PI3K/mTOR inhibitors (45–47). However, owing to its poor oral bioavailability and solubility in water, further studies on this drug are likely to be limited.
Navitoclax (ABT-263)
Navitoclax, which is a derivative of ABT-737, is an improved orally bioavailable BH3 mimetic with high affinity for BCL-2, BCL-XL, and BCL-w and substantially lower affinity for MCL-1 than ABT-737 (48). Navitoclax also showed efficacy in AML preclinical studies (49–51). In early clinical trials, it has shown activity in CLL and small-cell lung cancer (52, 53). However, it can cause thrombocytopenia due to its effect on BCL-XL (54–56). This on-target toxicity of navitoclax has undoubtedly limited its further development, which prompted AbbVie’s development of venetoclax (ABT-199).
Selective BCL-2 Inhibitors
Venetoclax
Venetoclax is a highly selective small molecule BH3 mimetic with subnanomolar affinity (Ki<0.01 nM) for BCL-2 and nanomolar affinity (Ki<245 nM) for BCL-XL. Based on this feature, it circumvents significant thrombocytopenia due to concomitant inhibition of BCL-XL, making it clinically available for the treatment of AML, a disease typically associated with thrombocytopenia (57, 58). In preclinical studies, venetoclax induced rapid cell death in AML cell lines and primary patient samples in vitro and in a mouse xenograft model in vivo (58). In the study of this drug, mitochondrial BH3 profiling could be used as a biomarker for predicting the response of AML primary cells to venetoclax in vitro and in a patient-derived xenograft model in vivo. Considering the preclinical data for ABT-767 and navitoclax and the efficiency and safety of venetoclax in CLL, venetoclax was immediately moved into clinical trials.
Selective BCL-XL Inhibitors
To date, many studies on BCL-XL inhibitors have focused on solid tumors (such as breast cancer, non-SCLC, ovarian cancer, colorectal cancer, and multiple myeloma) because solid tumors rely on BCL-XL for their survival. Selective BCL-XL inhibitors include WEHI-539, A-1155463, and A-1331852 (59–63), and further study may focus on the use of these inhibitors in AML.
Selective MCL-1 Inhibitors
MCL-1 has been shown to play a significant role in promoting cell survival in AML cell lines (64), and its overexpression in tumor cells may be associated with resistance to radiotherapy, chemotherapy, and BH3-mimetics targeting BCL-2/BCL-XL (65–67). Overexpression of MCL-1 has been identified in many primary AML cells and as a major factor in the development of resistance to venetoclax (68, 69). Thus, it led to the development of MCL-1 inhibitors. There are a number of MCL-1 inhibitors, and we reviewed several MCL-1 inhibitors that have entered clinical trials.
To date, S63845 has been identified as the most promising selective MCL-1 inhibitor. It showed low nanomolar cytotoxic activity in multiple hematological cancer-derived cell lines in vitro and potent efficacy in vivo in preclinical mouse models of diverse hematological malignancies (70). A side-by-side comparison of BH3 mimetics showed that MCL-1 may be a more potent therapeutic target than BCL-2 in AML (71). Therefore, the utilization of S63845 should be the first line of choice in future clinical trials.
Very little information on S64315 (MIK665) has been disclosed, with two clinical trials under investigation in patients with AML or myelodysplastic syndrome (NCT02979366, NCT03672695) (72).
AZD5991, which has high selectivity and affinity for MCL-1, has been shown to cause an effective apoptotic response in AML cell lines at a low nanomolar range. Furthermore, AZD5991 binds directly to the MCL-1 and BAK interaction and was shown to have potent antitumor activity in vivo, as demonstrated by high tumor regression in an AML xenograft model. Based on these promising data, a phase I clinical trial is undergoing relapsed or refractory hematological malignancies (NCT03218683) (73, 74).
AMG176 is a first-in-class selective MCL-1 inhibitor that is being studied in humans. It targets the BH3-binding groove of MCL-1 and thus frees BAX, resulting in activation of the intrinsic apoptotic pathway. In preclinical studies, it showed potent effects on AML cell lines, xenograft models, and primary patient samples. Based on this background, AMG176 is under several clinical investigations for AML (NTC02675452, NCT03797261) (75, 76).
Clinical Uses of Venetoclax
Single-Agent Venetoclax in Patients With Relapsed/Refractory AML
The first clinical trial of venetoclax in AML was a phase 2 single-agent study in patients with relapsed/refractory disease or untreated patients ineligible for intensive chemotherapy (77). In this study, they recruited 32 patients, with a median age of 71 years, which consisted of 30 relapsed/refractory patients and 2 treatment-naïve patients that were ineligible for intensive chemotherapy. This study showed a modest overall response rate of 19% (6/32), with 6% of patients (2/32) achieving CR and 13% of patients (4/32) achieving CRi; another 19% of patients had anti-leukemic activity that did not meet the criteria for response. The median duration of remission was only 48 days, and the median time spent on the study was only 63.5 days. Most patients had high-risk features, including pre-existing myelodysplastic syndrome or myeloproliferative neoplasm (MDS or MPN, 41%), FLT3-ITD mutations (13%), and older age (median 71 years).
Interestingly, patients with IDH1/2 mutations had a stronger response, with 4 of 12 (33%) patients achieving CR/CRi. Moreover, another 2 patients with IDH mutations demonstrated anti-leukemic activity that did not meet formal criteria for response due to a lack of hematological recovery. This is consistent with preclinical studies, in which IDH1/2 mutant AML suppresses the activity of cytochrome c oxidase and lowers the mitochondrial threshold to trigger apoptosis upon BCL-2 inhibition (78). Another impressive discovery was that all 6 patients who achieved CR/CRi received prior hypomethylating agents, which may lead to the exploration of its combination with hypomethylating agents. The most common grade 3/4 adverse events (AEs) were febrile neutropenia (31%), hypokalemia (22%), and pneumonia (19%), and no tumor lysis syndrome (TLS) events were reported. Overall, this study showed that single-agent venetoclax demonstrated antitumor activity in AML with good tolerability.
Venetoclax-Based Combinations in Treatment-Naïve Patients With AML
Preclinical models have proven a synergistic effect upon combining venetoclax and hypomethylating agents (HMAs) in AML cell lines and primary AML patient samples (79, 80). Moreover, studies have shown that azacitidine can reduce the protein levels of MCL-1, an anti-apoptotic protein that is associated with potential mechanism of resistance to venetoclax (81, 82). Based on these data, the combination of venetoclax with HMAs for the treatment of AML may be a promising therapeutic approach.
AbbVie launched a phase 1b clinical study (NCT02203773) that recruited 212 patients over the age of 60 years with treatment-naïve AML. They conducted a dose escalation and expansion study of venetoclax (83, 84). At first, a total of 45 patients were enrolled in the dose escalation, a dose of 400 mg of venetoclax was administered to 10 patients (4 in the azacitidine group, and 6 in the decitabine group), a dose of 800 mg of venetoclax was administered to 24 patients (12 each in the azacitidine and decitabine groups), and a dose of 1200 mg of venetoclax was administered to 11 patients (6 in the azacitidine group, and 5 in the decitabine group). Although the maximum tolerated dose was not reached in any group, the 1200 mg dose led to a high frequency of gastrointestinal AEs (nausea in 82% and diarrhea in 64% of patients) (83). As a result, the 400 mg and 800 mg dose cohorts for both azacitidine and decitabine were expanded, and 50 patients were added to each group. This study demonstrated a remarkable overall response rate of 68% and included CR and CRi rates of 37% and 30%, respectively. The median follow-up was 15.1 months, and the median OS for all groups was 17.5 months. In addition, the patients who received 400 mg venetoclax plus HMA achieved a remarkable CR/CRi rate of 73% (76% for azacitidine and 74% for decitabine), which revealed that the combinations with azacitidine or decitabine were not significantly different and that 400 mg of venetoclax might be a favorable dose (84, 85). Furthermore, patients with IDH1/2 mutations, FLT3 mutations, or NPM1 mutations had CR/CRi rates of 71%, 72%, and 91.5%, respectively. Even patients with poor risk features showed impressive responses, including those with high-risk cytogenetics (60% CR/CRi) and TP53 mutant patients (47% CR/CRi). The most common grade 3/4 adverse effects for all groups were thrombocytopenia (47%), febrile neutropenia (42%), and neutropenia (40%). No laboratory or clinical TLS was observed. Further studies explained how this treatment executed anti-leukemia activity. Leukemic stem cells (LSCs) rely on amino acid metabolism for oxidative phosphorylation and survival. Venetoclax with azacitidine was able to induce LSC toxicity in vitro by decreasing amino acid uptake, as confirmed by decreased α-ketoglutarate and increased succinate levels, suggesting inhibition of electron transport chain complex II. These metabolic perturbations suppress oxidative phosphorylation, which in turn efficiently and selectively targets LSCs (86, 87).
Similarly, preclinical studies demonstrated that cytarabine can boost venetoclax activity in AML by reducing MCL-1 levels (88, 89). An open-label, multicenter phase trial phase 1b/2 study of venetoclax in combination with LDAC recruited 82 patients over the age of 60 years (median age 74 years) with treatment-naïve AML (90). In the dose escalation phase, most patients needed dose interruption to permit blood count recovery and had a higher rate of hematological toxicity. Therefore, a 600 mg dose was recommended for the phase 2 study of the trial. The CR/CRi rate was 54% (CR, 26%; CRi, 28%). The median OS was 10.1 months, and the median duration of response was 8.1 months. Fifty-eight patients were treatment naïve; the CR/CRi rate for this group was 62%, which is similar to the 67% observed with venetoclax+ HMAs, whereas the group of patients who had prior HMA exposure only obtained a CR/CRi rate of 33%. Patients with mutations in NPM1 or IDH1/2 mutations had CR/CRi rates of 89% and 72%, respectively, which are higher than the average CR/CRi rate. However, patients with TP53 or FLT3 mutations had worse CR/CRi rates (30% and 44%, respectively). The most common grade 3/4 AEs were febrile neutropenia (42%), thrombocytopenia (38%), and neutropenia (27%). There were 2 cases of laboratory TLS and no evidence of clinical TLS.
In addition, a “real-world” report of venetoclax combined with azacitidine for the same patient populations at the same institution has been published (91). Thirty-three patients who received venetoclax + azacitidine off-trial were retrospectively analyzed and compared with 33 patients who received the same therapy on trial. The CR/CRi rate was 63.3% for off-trial patients who received treatment and 84.9% for trial patients, with a median OS periods of 381 days and 880 days, respectively. Although the responses and survival with venetoclax in real-world AML were inferior to those treated in a clinical trial, it still remained effective and tolerable compared with induction chemotherapy.
In summary, venetoclax in combination with HMAs or LDAC seemed to achieve a positive outcome in similar patient populations. On the basis of the above-described clinical data, two phase 3 trials comparing venetoclax + azacitidine/LDAC with azacitidine/LDAC alone are ongoing (NCT02993523, NCT03069352). The promising responses observed in these trials led to the FDA approval of venetoclax in combination with HMAs or LDAC for treatment-naïve AML patients who were at least 75 years old or ineligible for intensive induction chemotherapy because of comorbidities.
Venetoclax-Based Combinations in Relapsed/Refractory Patients With AML
Less than 10% of patients with relapsed/refractory AML are cured with current standard chemotherapy. Allogeneic HSCT is the only realistic hope of a cure for these patients, but only a minority of patients consider this option (92). There is an urgent need to explore new strategies for these patients. Several retrospective studies of relapsed or refractory patients receiving venetoclax-based therapies have been reported. A single institution retrospectively analyzed 33 consecutive adults with relapsed/refractory AML in the real world, and the combination with HMAs achieved a CR/CRi rate of 33%, which included patients with no prior HMAs or allogeneic stem cell transplants (93). However, another series of 39 patients with relapsed/refractory AML achieved a dismal CR/CRi rate of 12% (8/39) (94). Moreover, in a series of 90 patients analyzed after either HMA treatment (51%) or allogeneic stem cell transplant (29%), the combination of venetoclax and HMAs achieved a CR/CRi rate of 46% in patients at a younger median age than the former study (95). Hence, the data revealed that venetoclax-based combinations may be a potential treatment strategy for patients with relapsed/refractory AML.
Venetoclax Resistance and Combination Strategies to Overcome
Although numerous reports have shown that venetoclax-based therapies exert a promising effect on AML, they still remain partly resistant to venetoclax. It is necessary to explore how resistance evolves and how this can be overcome. The upregulation of MCL-1 protein in AML cells is one of the most well-known reasons for resistance to treatment with venetoclax (69). Therefore, targeting MCL-1 directly or indirectly is a new way to solve the resistance. Moreover, mitochondrial cristae structure may be another reason for resistance. Targeting mitochondrial architecture may provide a promising approach to circumvent it (96). Directly targeting MCL-1 has been introduced previously.
Here, we introduce several combinations that can reduce the MCL-1 level indirectly to circumvent the resistance. Indirect MCL-1 inhibitors include the following compounds: bromodomain extra-terminal protein inhibitors (BETis), which reduce MCL-1 and BCL-XL levels while increasing BIM levels and enhance the lethal effects of venetoclax on AML (97); cyclin-dependent kinase 9 (CDK9) inhibitors, which inhibit the transcription of MCL-1 (98); midostaurin or gilteritinib, FLT3 inhibitors that induce downregulation of MCL-1 to increase venetoclax activity (99); CUDC-907, a dual PI3K and histone deacetylase inhibitor that downregulates MCL-1, upregulates BIM, and induces DNA damage (100); MEK inhibitors (101); MDM2 inhibitors (102); PI3K inhibitors (103); and selinexor, an XPO1-selective inhibitor (104). In addition, an inhibitor of the Nedd8-activating enzyme (MLN4924) can upregulate Noxa, and 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) inhibitors (statins) can upregulate PUMA. These two pro-apoptotic proteins, NOXA and PUMA, can neutralize Mcl-1 to boost the activity of venetoclax (105, 106). Ibrutinib, a Burton’s tyrosine kinase inhibitor, and ArQule 531, a multi-kinase inhibitor of Src family kinases and Burton’s tyrosine kinase, can also synergize with venetoclax (107, 108). Pharmacologic inhibition of mitochondrial protein synthesis with antibiotics that target the ribosome, including tedizolid and doxycycline, can potently reverse venetoclax resistance. Thus, inhibition of mitochondrial translation may be a new approach to overcoming venetoclax resistance (109). Owing to these preclinical results, an increasing number of clinical trials are ongoing.
Predictors of Response to Venetoclax
The specific population that is most suitable for venetoclax remains unknown. However, recent studies have shown that mutations in NPM1, RAD21, MLL, or IDH1/IDH2 may predict venetoclax sensitivity (110, 111). In addition, a FLT3 internal tandem duplication gain or TP53 loss confers cross-resistance to both venetoclax and cytotoxicity-based therapies (112). According to a retrospective analysis, HMA plus venetoclax produces better results than traditional standard-of-care regimens in older patients with NPM1+ AML (113). To date, an increasing number of studies have shown that tumor cells with an NPM1 or IDH1/IDH2 mutation may more sensitive to venetoclax. Regarding other mutations, including FLT3 and TP53, VEN-based therapy may be a more effective therapy than traditional chemotherapy. Furthermore, blast cells of FAB M0/1 AML show higher sensitivity to venetoclax, while differentiated monocytic cells abundantly present in M4/5 subtypes show resistance to BCL-2 inhibition (114, 115). This may be associated with decreased expression of BCL-2 and a reliance on MCL-1 to mediate oxidative phosphorylation and survival. Therefore, as the number of patients treated with venetoclax increases, precise predictors of the response will be revealed.
Conclusions and Prospects
Targeting the BCL-2 protein has shown compelling clinical promise in AML. Venetoclax, a selective BCL-2 inhibitor that was approved by the FDA for treatment-naïve elderly AML patients, has shown potential to be further explored. According to a recent study, previously untreated patients aged 75 years or older who were ineligible for intensive chemotherapy experienced a longer overall survival and a higher incidence of remission after treatment with azacitidine plus venetoclax than patients who received azacitidine alone (116). In addition, venetoclax+HMA has also been confirmed to be safe and effective in younger patients (117). Based on the encouraging results achieved with venetoclax, the scope of application of venetoclax is expected to be expanded in the near future for patients who are 75 years of age or older who are not suitable for standard chemotherapy, and potentially even for younger patients. In our center, we administer this treatment to elderly patients and even considered administering it to some young patients who are sensitive to venetoclax in a previous study. Of course, more standardized and larger clinical trials are needed to confirm the safety and effectiveness of VEN in younger patients.
Chimeric antigen receptor T cells, which have shown exciting results in B cell lymphocytic leukemia, are another a novel strategy for AML treatment. What outcomes would occur if this or other immune therapies was combined with venetoclax? Moreover, venetoclax-based therapies have shown effects on some high-risk AML subtypes. Could these therapies be combined with allogeneic HSCT or be used as a consolidation therapy after HSCT? Will these therapies affect graft versus-host disease or graft anti-leukemia? All of these questions remain unanswered. Furthermore, preclinical studies with MCL-1 inhibitors have demonstrated effective antitumor activity in AML. Will MCL-1 inhibitors be another promising strategy to cure AML? In summary, continued efforts to explore BCL-2 family proteins are necessary.
Author Contributions
MZ designed the research. YW, YC, RS, LC, XX, XJ, XH and WL performed the research and analyzed the data. YW wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Sciences Foundation of China (81970180; to MZ), the National Natural Sciences Foundation of China (81800105; to WL), and Tianjin First Central Hospital.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. National Cancer Institute Surveillance. Epidemiology, and End Results Program. Cancer Stat Facts: Acute Myeloid Leukemia (AML). Available at: https://seer.cancer.gov/statfacts/html/amyl.html.
2. Wei AH, Tiong IS. Midostaurin, enasidenib, CPX-351, gemtuzumab ozogamicin, and venetoclax bring new hope to AML. Blood (2017) 130(23):2469–74. doi: 10.1182/blood-2017-08-784066
3. Tothova E, Fricova M, Stecova N, Kafkova A, Elbertova A. High expression of Bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Neoplasma (2002) 49(3):141–4.
4. Kaufmann SH, Karp JE, Svingen PA, Krajewski S, Burke PJ, Gore SD, et al. Elevated expression of the apoptotic regulator Mcl-1 at the time of leukemic relapse. Blood (1998) 91(3):991–1000.
5. Fennell DA, Corbo MV, Dean NM, Monia BP, Cotter FE. In vivo suppression of Bcl-XL expression facilitates chemotherapy-induced leukaemia cell death in a SCID/NOD-Hu model. Br J Haematol (2001) 112(3):706–13. doi: 10.1046/j.1365-2141.2001.02603.x
6. Del Poeta G, Venditti A, Del Principe MI, Maurillo L, Buccisano F, Tamburini A, et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood (2003) 101(6):2125–31. doi: 10.1182/blood-2002-06-1714
7. Venditti A, Del Poeta G, Maurillo L, Buccisano F, Del Principe MI, Mazzone C, et al. Combined analysis of bcl-2 and MDR1 proteins in 256 cases of acute myeloid leukemia. Haematologica (2004) 89(8):934–9.
8. VENCLEXTA®. (venetoclax tablets) for oral use U.S. Food and Drug Administration website. (2018). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208573s009lbl.pdf.
9. Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood (2005) 106(2):408–18. doi: 10.1182/blood-2004-07-2761
10. Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol (1999) 17:331–67. doi: 10.1146/annurev.immunol.17.1.331
11. Jin ZY, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther (2005) 4(2):139–63. doi: 10.4161/cbt.4.2.1508
12. Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science (2004) 305(5684):626–9. doi: 10.1126/science.1099320
13. Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science (1985) 228(4706):1440–3. doi: 10.1126/science.3874430
14. Tsujimoto Y, Yunis J, Onorato-Showe L, Erikson J, Nowell PC, Croce CM. Molecular cloning of the chromosomal breakpoint of B-cell lymphomas and leukemias with the t(11;14) chromosome translocation. Science (1984) 224(4656):1403–6. doi: 10.1126/science.6610211
15. Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature (1988) 335(6189):440–2. doi: 10.1038/335440a0
16. Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature (1990) 348(6299):334–6. doi: 10.1038/348334a0
17. Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell (2010) 37(3):299–310. doi: 10.1016/j.molcel.2010.01.025
18. Merino D, Kelly GL, Lessene G, Wei AH, Roberts AW, Strasser A. BH3-Mimetic Drugs: Blazing the Trail for New Cancer Medicines. Cancer Cell (2018) 34(6):879–91. doi: 10.1016/j.ccell.2018.11.004
19. Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science (2001) 292(5517):727–30. doi: 10.1126/science.1059108
20. Shamas-Din A, Brahmbhatt H, Leber B, Andrews DW. BH3-only proteins: Orchestrators of apoptosis. Biochim Biophys Acta (2011) 1813(4):508–20. doi: 10.1016/j.bbamcr.2010.11.024
21. Chen HC, Kanai M, Inoue-Yamauchi A, Tu HC, Huang Y, Ren D, et al. An interconnected hierarchical model of cell death regulation by the BCL-2 family. Nat Cell Biol (2015) 17(10):1270–81. doi: 10.1038/ncb3236
22. Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell (2011) 44(4):517–31. doi: 10.1016/j.molcel.2011.10.001
23. Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol (2014) 15(1):49–63. doi: 10.1038/nrm3722
24. Timucin AC, Basaga H, Kutuk O. Selective targeting of antiapoptotic BCL-2 proteins in cancer. Med Res Rev (2019) 39(1):146–75. doi: 10.1002/med.21516
25. Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood (1993) 81(11):3091–6.
26. Delia D, Aiello A, Soligo D, Fontanella E, Melani C, Pezzella F, et al. bcl-2 proto-oncogene expression in normal and neoplastic human myeloid cells. Blood (1992) 79(5):1291–8.
27. Karakas T, Maurer U, Weidmann E, Miething CC, Hoelzer D, Bergmann L. High expression of bcl-2 mRNA as a determinant of poor prognosis in acute myeloid leukemia. Ann Oncol (1998) 9(2):159–65. doi: 10.1023/a:1008255511404
28. Pallis M, Zhu YM, Russell NH. Bcl-x(L) is heterogenously expressed by acute myeloblastic leukaemia cells and is associated with autonomous growth in vitro and with P-glycoprotein expression. Leukemia (1997) 11(7):945–9. doi: 10.1038/sj.leu.2400705
29. Konopleva M, Zhao S, Hu W, Jiang S, Snell V, Weidner D, et al. The anti-apoptotic genes Bcl-X(L) and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br J Haematol (2002) 118(2):521–34. doi: 10.1046/j.1365-2141.2002.03637.x
30. Campos L, Sabido O, Rouault JP, Guyotat D. Effects of BCL-2 antisense oligodeoxynucleotides on in vitro proliferation and survival of normal marrow progenitors and leukemic cells. Blood (1994) 84(2):595–600.
31. Cotter FE, Johnson P, Hall P, Pocock C, al Mahdi N, Cowell JK, et al. Antisense oligonucleotides suppress B-cell lymphoma growth in a SCID-hu mouse model. Oncogene (1994) 9(10):3049–55.
32. Marcucci G, Byrd JC, Dai G, Klisovic MI, Kourlas PJ, Young DC, et al. Phase 1 and pharmacodynamic studies of G3139, a Bcl-2 antisense oligonucleotide, in combination with chemotherapy in refractory or relapsed acute leukemia. Blood (2003) 101(2):425–32. doi: 10.1182/blood-2002-06-1899
33. Marcucci G, Stock W, Dai G, Klisovic RB, Liu S, Klisovic MI, et al. Phase I study of oblimersen sodium, an antisense to Bcl-2, in untreated older patients with acute myeloid leukemia: pharmacokinetics, pharmacodynamics, and clinical activity. J Clin Oncol (2005) 23(15):3404–11. doi: 10.1200/JCO.2005.09.118
34. Marcucci G, Moser B, Blum W, Stock W, Wetzler M, Kolitz JE, et al. A phase III randomized trial of intensive induction and consolidation chemotherapy +/- oblimersen, a pro-apoptotic Bcl-2 antisense oligonucleotide in untreated acute myeloid leukemia patients > 60 years old. J Clin Oncol (2007) 25(18):1. doi: 10.1200/jco.2007.10.8720
35. Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci USA (2007) 104(49):19512–7. doi: 10.1073/pnas.0709443104
36. Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax). Cancer Res (2008) 68(9):3413–20. doi: 10.1158/0008-5472.CAN-07-1919
37. Schimmer AD, O’Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res (2008) 14(24):8295–301. doi: 10.1158/1078-0432.CCR-08-0999
38. Schimmer AD, Raza A, Carter TH, Claxton D, Erba H, DeAngelo DJ, et al. A multicenter phase I/II study of obatoclax mesylate administered as a 3- or 24-hour infusion in older patients with previously untreated acute myeloid leukemia. PloS One (2014) 9(10):e108694. doi: 10.1371/journal.pone.0108694
39. Wei Y, Kadia T, Tong W, Zhang M, Jia Y, Yang H, et al. The combination of a histone deacetylase inhibitor with the BH3-mimetic GX15-070 has synergistic antileukemia activity by activating both apoptosis and autophagy. Autophagy (2010) 6(7):976–8. doi: 10.4161/auto.6.7.13117
40. Rahmani M, Aust MM, Attkisson E, Williams DC Jr., Ferreira-Gonzalez A, Grant S. Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood (2012) 119(25):6089–98. doi: 10.1182/blood-2011-09-378141
41. Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature (2005) 435(7042):677–81. doi: 10.1038/nature03579
42. van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell (2006) 10(5):389–99. doi: 10.1016/j.ccr.2006.08.027
43. Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell (2006) 10(5):375–88. doi: 10.1016/j.ccr.2006.10.006
44. Beurlet S, Omidvar N, Gorombei P, Krief P, Le Pogam C, Setterblad N, et al. BCL-2 inhibition with ABT-737 prolongs survival in an NRAS/BCL-2 mouse model of AML by targeting primitive LSK and progenitor cells. Blood (2013) 122(16):2864–76. doi: 10.1182/blood-2012-07-445635
45. Pan R, Ruvolo VR, Wei J, Konopleva M, Reed JC, Pellecchia M, et al. Inhibition of Mcl-1 with the pan-Bcl-2 family inhibitor (-)BI97D6 overcomes ABT-737 resistance in acute myeloid leukemia. Blood (2015) 126(3):363–72. doi: 10.1182/blood-2014-10-604975
46. Rahmani M, Aust MM, Attkisson E, Williams DC Jr., Ferreira-Gonzalez A, Grant S. Dual inhibition of Bcl-2 and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in human myeloid leukemia cells through a GSK3- and Bim-dependent mechanism. Cancer Res (2013) 73(4):1340–51. doi: 10.1158/0008-5472.CAN-12-1365
47. Konopleva M, Milella M, Ruvolo P, Watts JC, Ricciardi MR, Korchin B, et al. MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex. Leukemia (2012) 26(4):778–87. doi: 10.1038/leu.2011.287
48. Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res (2008) 68(9):3421–8. doi: 10.1158/0008-5472.CAN-07-5836
49. Polier G, Giaisi M, Kohler R, Muller WW, Lutz C, Buss EC, et al. Targeting CDK9 by wogonin and related natural flavones potentiates the anti-cancer efficacy of the Bcl-2 family inhibitor ABT-263. Int J Cancer (2015) 136(3):688–98. doi: 10.1002/ijc.29009
50. Kivioja JL, Thanasopoulou A, Kumar A, Kontro M, Yadav B, Majumder MM, et al. Dasatinib and navitoclax act synergistically to target NUP98-NSD1(+)/FLT3-ITD(+) acute myeloid leukemia. Leukemia (2019) 33(6):1360–72. doi: 10.1038/s41375-018-0327-2
51. Kontro M, Kumar A, Majumder MM, Eldfors S, Parsons A, Pemovska T, et al. HOX gene expression predicts response to BCL-2 inhibition in acute myeloid leukemia. Leukemia (2017) 31(2):301–9. doi: 10.1038/leu.2016.222
52. Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol (2012) 30(5):488–96. doi: 10.1200/JCO.2011.34.7898
53. Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol (2011) 29(7):909–16. doi: 10.1200/JCO.2010.31.6208
54. Schoenwaelder SM, Jarman KE, Gardiner EE, Hua M, Qiao J, White MJ, et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood (2011) 118(6):1663–74. doi: 10.1182/blood-2011-04-347849
55. Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ (2007) 14(5):943–51. doi: 10.1038/sj.cdd.4402081
56. Carrington EM, Zhan Y, Brady JL, Zhang JG, Sutherland RM, Anstee NS, et al. Anti-apoptotic proteins BCL-2, MCL-1 and A1 summate collectively to maintain survival of immune cell populations both in vitro and in vivo. Cell Death Differ (2017) 24(5):878–88. doi: 10.1038/cdd.2017.30
57. Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med (2013) 19(2):202–8. doi: 10.1038/nm.3048
58. Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discovery (2014) 4(3):362–75. doi: 10.1158/2159-8290.CD-13-0609
59. Tao ZF, Hasvold L, Wang L, Wang X, Petros AM, Park CH, et al. Discovery of a Potent and Selective BCL-XL Inhibitor with in Vivo Activity. ACS Med Chem Lett (2014) 5(10):1088–93. doi: 10.1021/ml5001867
60. Leverson JD, Phillips DC, Mitten MJ, Boghaert ER, Diaz D, Tahir SK, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med (2015) 7(279):279ra40. doi: 10.1126/scitranslmed.aaa4642
61. Zhang H, Xue J, Hessler P, Tahir SK, Chen J, Jin S, et al. Genomic analysis and selective small molecule inhibition identifies BCL-X(L) as a critical survival factor in a subset of colorectal cancer. Mol Cancer (2015) 14:126. doi: 10.1186/s12943-015-0397-y
62. Punnoose EA, Leverson JD, Peale F, Boghaert ER, Belmont LD, Tan N, et al. Expression Profile of BCL-2, BCL-XL, and MCL-1 Predicts Pharmacological Response to the BCL-2 Selective Antagonist Venetoclax in Multiple Myeloma Models. Mol Cancer Ther (2016) 15(5):1132–44. doi: 10.1158/1535-7163.MCT-15-0730
63. Bessou M, Lopez J, Gadet R, Deygas M, Popgeorgiev N, Poncet D, et al. The apoptosis inhibitor Bcl-xL controls breast cancer cell migration through mitochondria-dependent reactive oxygen species production. Oncogene (2020) 39(15):3056–74. doi: 10.1038/s41388-020-1212-9
64. Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev (2012) 26(2):120–5. doi: 10.1101/gad.182980.111
65. Trivigno D, Essmann F, Huber SM, Rudner J. Deubiquitinase USP9x confers radioresistance through stabilization of Mcl-1. Neoplasia (2012) 14(10):893–904. doi: 10.1593/neo.12598
66. Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature (2011) 471(7336):110–4. doi: 10.1038/nature09779
67. Williams MM, Lee L, Hicks DJ, Joly MM, Elion D, Rahman B, et al. Key Survival Factor, Mcl-1, Correlates with Sensitivity to Combined Bcl-2/Bcl-xL Blockade. Mol Cancer Res (2017) 15(3):259–68. doi: 10.1158/1541-7786.MCR-16-0280-T
68. Xiang Z, Luo H, Payton JE, Cain J, Ley TJ, Opferman JT, et al. Mcl1 haploinsufficiency protects mice from Myc-induced acute myeloid leukemia. J Clin Invest (2010) 120(6):2109–18. doi: 10.1172/JCI39964
69. Ramsey HE, Fischer MA, Lee T, Gorska AE, Arrate MP, Fuller L, et al. A Novel MCL1 Inhibitor Combined with Venetoclax Rescues Venetoclax-Resistant Acute Myelogenous Leukemia. Cancer Discovery (2018) 8(12):1566–81. doi: 10.1158/2159-8290.CD-18-0140
70. Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature (2016) 538(7626):477–82. doi: 10.1038/nature19830
71. Ewald L, Dittmann J, Vogler M, Fulda S. Side-by-side comparison of BH3-mimetics identifies MCL-1 as a key therapeutic target in AML. Cell Death Dis (2019) 10(12):917. doi: 10.1038/s41419-019-2156-2
72. Wei AH, Roberts AW, Spencer A, Rosenberg AS, Siegel D, Walter RB, et al. Targeting MCL-1 in hematologic malignancies: Rationale and progress. Blood Rev (2020) 100672. doi: 10.1016/j.blre.2020.100672
73. Tron AE, Belmonte MA, Adam A, Aquila BM, Boise LH, Chiarparin E, et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat Commun (2018) 9(1):5341. doi: 10.1038/s41467-018-07551-w
74. Hird AW, Secrist JP, Adam A, Belmonte MA, Gangl E, Gibbons F, et al. AZD5991: A potent and selective macrocyclic inhibitor of Mcl-1 for treatment of hematologic cancers. Cancer Res (2017) 77:2. doi: 10.1158/1538-7445.am2017-ddt01-02
75. Caenepeel S, Brown SP, Belmontes B, Moody G, Keegan KS, Chui D. AMG 176, a Selective MCL1 Inhibitor, Is Effective in Hematologic Cancer Models Alone and in Combination with Established Therapies. Cancer Discovery (2018) 8(12):1582–97. doi: 10.1158/2159-8290.CD-18-0387
76. Caenepeel SR, Belmontes B, Sun J, Coxon A, Moody G, Hughes PE. Preclinical evaluation of AMG 176, a novel, potent and selective Mcl-1 inhibitor with robust anti-tumor activity in Mcl-1 dependent cancer models. Cancer Res (2017) 77:2. doi: 10.1158/1538-7445.am2017-2027
77. Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discovery (2016) 6(10):1106–17. doi: 10.1158/2159-8290.CD-16-0313
78. Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong WJ, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med (2015) 21(2):178–84. doi: 10.1038/nm.3788
79. Bogenberger JM, Kornblau SM, Pierceall WE, Lena R, Chow D, Shi CX, et al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia (2014) 28(8):1657–65. doi: 10.1038/leu.2014.44
80. Bogenberger JM, Delman D, Hansen N, Valdez R, Fauble V, Mesa RA, et al. Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk Lymphoma (2015) 56(1):226–9. doi: 10.3109/10428194.2014.910657
81. Bose P, Gandhi V, Konopleva M. Pathways and mechanisms of venetoclax resistance. Leuk Lymphoma (2017) 58(9):1–17. doi: 10.1080/10428194.2017.1283032
82. Tsao T, Shi Y, Kornblau S, Lu H, Konoplev S, Antony A, et al. Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann Hematol (2012) 91(12):1861–70. doi: 10.1007/s00277-012-1537-8
83. DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol (2018) 19(2):216–28. doi: 10.1016/S1470-2045(18)30010-X
84. DiNardo CD, Pratz KW, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood (2019) 133(1):7–17. doi: 10.1182/blood-2018-08-868752
85. Jonas BA, Pollyea DA. How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia. Leukemia (2019) 33(12):2795–804. doi: 10.1038/s41375-019-0612-8
86. Jones CL, Stevens BM, D’Alessandro A, Reisz JA, Culp-Hill R, Nemkov T, et al. Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells. Cancer Cell (2018) 34(5):724–40 e4. doi: 10.1016/j.ccell.2018.10.005
87. Pollyea DA, Stevens BM, Jones CL, Winters A, Pei S, Minhajuddin M, et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med (2018) 24(12):1859–66. doi: 10.1038/s41591-018-0233-1
88. Niu X, Zhao J, Ma J, Xie C, Edwards H, Wang G, et al. Binding of Released Bim to Mcl-1 is a Mechanism of Intrinsic Resistance to ABT-199 which can be Overcome by Combination with Daunorubicin or Cytarabine in AML Cells. Clin Cancer Res (2016) 22(17):4440–51. doi: 10.1158/1078-0432.CCR-15-3057
89. Teh TC, Nguyen NY, Moujalled DM, Segal D, Pomilio G, Rijal S, et al. Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia (2018) 32(2):303–12. doi: 10.1038/leu.2017.243
90. Wei AH, Strickland SA Jr., Hou JZ, Fiedler W, Lin TL, Walter RB, et al. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J Clin Oncol (2019) 37(15):1277–84. doi: 10.1200/JCO.18.01600
91. Winters AC, Gutman JA, Purev E, Nakic M, Tobin J, Chase S, et al. Real-world experience of venetoclax with azacitidine for untreated patients with acute myeloid leukemia. Blood Adv (2019) 3(20):2911–9. doi: 10.1182/bloodadvances.2019000243
92. Bose P, Vachhani P, Cortes JE. Treatment of Relapsed/Refractory Acute Myeloid Leukemia. Curr Treat Options Oncol (2017) 18(3):17. doi: 10.1007/s11864-017-0456-2
93. Aldoss I, Yang D, Aribi A, Ali H, Sandhu K, Al Malki MM, et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica (2018) 103(9):e404–7. doi: 10.3324/haematol.2018.188094
94. DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol (2018) 93(3):401–7. doi: 10.1002/ajh.25000
95. Aldoss I, Yang D, Pillai R, Sanchez JF, Mei M, Aribi A, et al. Association of leukemia genetics with response to venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Am J Hematol (2019) 94(10):E253–5. doi: 10.1002/ajh.25567
96. Chen X, Glytsou C, Zhou H, Narang S, Reyna DE, Lopez A, et al. Targeting Mitochondrial Structure Sensitizes Acute Myeloid Leukemia to Venetoclax Treatment. Cancer Discovery (2019) 9(7):890–909. doi: 10.1158/2159-8290.CD-19-0117
97. Fiskus W, Cai T, DiNardo CD, Kornblau SM, Borthakur G, Kadia TM, et al. Superior efficacy of cotreatment with BET protein inhibitor and BCL2 or MCL1 inhibitor against AML blast progenitor cells. Blood Cancer J (2019) 9(2):4. doi: 10.1038/s41408-018-0165-5
98. Cidado J, Boiko S, Proia T, Ferguson D, Criscione SW, San Martin M, et al. AZD4573 Is a Highly Selective CDK9 Inhibitor That Suppresses MCL-1 and Induces Apoptosis in Hematologic Cancer Cells. Clin Cancer Res (2020) 26(4):922–34. doi: 10.1158/1078-0432.CCR-19-1853
99. Ma J, Zhao S, Qiao X, Knight T, Edwards H, Polin L, et al. Inhibition of Bcl-2 Synergistically Enhances the Antileukemic Activity of Midostaurin and Gilteritinib in Preclinical Models of FLT3-Mutated Acute Myeloid Leukemia. Clin Cancer Res (2019) 25(22):6815–26. doi: 10.1158/1078-0432.CCR-19-0832
100. Li X, Su Y, Hege K, Madlambayan G, Edwards H, Knight T, et al. The HDAC and PI3K dual inhibitor CUDC-907 synergistically enhances the antileukemic activity of venetoclax in preclinical models of acute myeloid leukemia. Haematologica (2020). doi: 10.3324/haematol.2019.233445
101. Han L, Zhang Q, Dail M, Shi C, Cavazos A, Ruvolo VR, et al. Concomitant targeting of BCL2 with venetoclax and MAPK signaling with cobimetinib in acute myeloid leukemia models. Haematologica (2020) 105(3):697–707. doi: 10.3324/haematol.2018.205534
102. Pan R, Ruvolo V, Mu H, Leverson JD, Nichols G, Reed JC, et al. Synthetic Lethality of Combined Bcl-2 Inhibition and p53 Activation in AML: Mechanisms and Superior Antileukemic Efficacy. Cancer Cell (2017) 32(6):748–760 e6. doi: 10.1016/j.ccell.2017.11.003
103. Rahmani M, Nkwocha J, Hawkins E, Pei X, Parker RE, Kmieciak M, et al. Cotargeting BCL-2 and PI3K Induces BAX-Dependent Mitochondrial Apoptosis in AML Cells. Cancer Res (2018) 78(11):3075–86. doi: 10.1158/0008-5472.CAN-17-3024
104. Luedtke DA, Su Y, Liu S, Edwards H, Wang Y, Lin H, et al. Inhibition of XPO1 enhances cell death induced by ABT-199 in acute myeloid leukaemia via Mcl-1. J Cell Mol Med (2018) 22(12):6099–111. doi: 10.1111/jcmm.13886
105. Knorr KL, Schneider PA, Meng XW, Dai H, Smith BD, Hess AD, et al. MLN4924 induces Noxa upregulation in acute myelogenous leukemia and synergizes with Bcl-2 inhibitors. Cell Death Differ (2015) 22(12):2133–42. doi: 10.1038/cdd.2015.74
106. Lee JS, Roberts A, Juarez D, Vo TT, Bhatt S, Herzog LO, et al. Statins enhance efficacy of venetoclax in blood cancers. Sci Transl Med (2018) 10(445). doi: 10.1126/scitranslmed.aaq1240
107. Eide CA, Kurtz SE, Kaempf A, Long N, Agarwal A, Tognon CE, et al. Simultaneous kinase inhibition with ibrutinib and BCL2 inhibition with venetoclax offers a therapeutic strategy for acute myeloid leukemia. Leukemia (2020) 34:2342–53. doi: 10.1038/s41375-020-0764-6
108. Elgamal OA, Mehmood A, Jeon JY, Carmichael B, Lehman A, Orwick SJ, et al. Preclinical efficacy for a novel tyrosine kinase inhibitor, ArQule 531 against acute myeloid leukemia. J Hematol Oncol (2020) 13(1):8. doi: 10.1186/s13045-019-0821-7
109. Sharon D, Cathelin S, Mirali S, Di Trani JM, Yanofsky DJ, Keon KA, et al. Inhibition of mitochondrial translation overcomes venetoclax resistance in AML through activation of the integrated stress response. Sci Transl Med (2019) 11(516). doi: 10.1126/scitranslmed.aax2863
110. Bisaillon R, Moison C, Thiollier C, Krosl J, Bordeleau ME, Lehnertz B, et al. Genetic characterization of ABT-199 sensitivity in human AML. Leukemia (2020) 34(1):63–74. doi: 10.1038/s41375-019-0485-x
111. Benito JM, Godfrey L, Kojima K, Hogdal L, Wunderlich M, Geng H, et al. MLL-Rearranged Acute Lymphoblastic Leukemias Activate BCL-2 through H3K79 Methylation and Are Sensitive to the BCL-2-Specific Antagonist ABT-199. Cell Rep (2015) 13(12):2715–27. doi: 10.1016/j.celrep.2015.12.003
112. DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood (2020) 135(11):791–803. doi: 10.1182/blood.2019003988
113. Lachowiez CA, Loghavi S, Kadia TM, Daver N, Borthakur G, Pemmaraju N, et al. Outcomes of older patients with NPM1-mutated AML: current treatments and the promise of venetoclax-based regimens. Blood Adv (2020) 4(7):1311–20. doi: 10.1182/bloodadvances.2019001267
114. Pei S, Pollyea DA, Gustafson A, Stevens BM, Minhajuddin M, Fu R, et al. Monocytic Subclones Confer Resistance to Venetoclax-Based Therapy in Patients with Acute Myeloid Leukemia. Cancer Discovery (2020) 10(4):536–51. doi: 10.1158/2159-8290.CD-19-0710
115. Kuusanmaki H, Leppa AM, Polonen P, Kontro M, Dufva O, Deb D, et al. Phenotype-based drug screening reveals association between venetoclax response and differentiation stage in acute myeloid leukemia. Haematologica (2020) 105(3):708–20. doi: 10.3324/haematol.2018.214882
116. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med (2020) 383(7):617–29. doi: 10.1056/NEJMoa2012971
Keywords: B cell lymphoma 2, AML—acute myeloid leukemia, venetoclax (ABT-199), intrinsic apoptosis, Bcl-2 protein
Citation: Wei Y, Cao Y, Sun R, Cheng L, Xiong X, Jin X, He X, Lu W and Zhao M (2020) Targeting Bcl-2 Proteins in Acute Myeloid Leukemia. Front. Oncol. 10:584974. doi: 10.3389/fonc.2020.584974
Received: 19 July 2020; Accepted: 14 September 2020;
Published: 05 November 2020.
Edited by:
Naval Daver, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Luca Maurillo, University of Rome Tor Vergata, ItalyMichael Diamantidis, University Hospital of Larissa, Greece
Copyright © 2020 Wei, Cao, Sun, Cheng, Xiong, Jin, He, Lu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingfeng Zhao, bWluZ2Zlbmd6aGFvQHNpbmEuY29t
 Yunxiong Wei
Yunxiong Wei Yaqing Cao
Yaqing Cao Rui Sun1
Rui Sun1 Wenyi Lu
Wenyi Lu Mingfeng Zhao
Mingfeng Zhao