
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 16 September 2020
Sec. Surgical Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.575316
 Ziyun Shen1,2,3,4†
Ziyun Shen1,2,3,4† Jun Zhang1,2,3,4†
Jun Zhang1,2,3,4† Haoda Chen1,2,3,4†
Haoda Chen1,2,3,4† Weishen Wang1,2,3,4
Weishen Wang1,2,3,4 Wei Xu1,2,3,4
Wei Xu1,2,3,4 Xiongxiong Lu1,2,3,4
Xiongxiong Lu1,2,3,4 Yiran Zhou1,2,3,4
Yiran Zhou1,2,3,4 Shiwei Zhao1,2,3,4
Shiwei Zhao1,2,3,4 Zhiwei Xu1,2,3,4
Zhiwei Xu1,2,3,4 Xiaxing Deng1,2,3,4
Xiaxing Deng1,2,3,4 Jiancheng Wang1,2,3,4*
Jiancheng Wang1,2,3,4* Yuanchi Weng1,2,3,4*
Yuanchi Weng1,2,3,4* Baiyong Shen1,2,3,4*
Baiyong Shen1,2,3,4*Objectives: Whether pre-operative biliary drainage (PBD) affects long-term survival of patients with obstructive jaundice with pancreatic ductal adenocarcinoma (PDAC) who underwent pancreaticoduodenectomy is still controversial. Most of the previous research did not include the important total serum bilirubin (TB) level before intervention as well as before surgery. The aim of this study is to evaluate the impact of PBD on long-term survival after considering the TB level.
Methods: Data were collected retrospectively from patients with obstructive jaundice who underwent resection of pancreatic head cancer in a high-volume center. X-Tile software and Kaplan-Meier survival analysis were applied to determine the optimal cut-off levels for TB and age based on the minimal probability (P)-value and the largest χ2-value. Multivariate Cox regression analyses were performed after univariate analysis to assess independent prognostic factors for overall survival (OS) and disease-free survival (DFS).
Results: Of 426 patients with obstructive jaundice who underwent pancreaticoduodenectomy for resectable pancreatic head cancer during a 7 year period, 242 (56.8%) received PBD and 184 (43.2%) underwent surgery directly. The OS of patients who received PBD was significantly worse than that of patients who did not receive PBD by univariate analysis (median of 16.6 vs. 22.2 months, P = 0.048). After including liver function parameters in the multivariate Cox regression, we found that the use of PBD was not associated with OS or DFS, while TB before intervention >150 μmol/L was an independent adverse prognostic factor for both OS [hazard ratio (HR), 1.42; 95% CI, 1.05–1.91] and DFS (HR, 1.38; 95% CI, 1.08–1.77).
Conclusions: In patients with obstructive jaundice with resectable pancreatic head cancer, undergoing PBD before pancreaticoduodenectomy did not impair or benefit survival rates compared with surgery alone. However, TB before intervention >150 μmol/L predicted an unfavorable prognosis, irrespective of the PBD procedure.
The prevalence of pancreatic cancer has increased in the last decade to become the fourth leading cause of cancer death in the United States (1). As its incidence continues to increase, with a 5 year survival rate of <10%, pancreatic cancer remains a fatal disease for most patients. Although pancreaticoduodenectomy is one of the most complex surgeries and is associated with considerable morbidity and mortality, it is still the only potential therapy to treat patients with resectable pancreatic ductal adenocarcinoma (PDAC).
The most common symptom in patients with pancreatic head cancer is obstructive jaundice. Accordingly, pre-operative biliary drainage (PBD) has been developed to relieve biliary obstruction and aims to potentially reduce post-operative complications by improving liver function pre-operatively. Although routine PBD could not reduce morbidity compared to surgery alone according to previous studies, a considerable proportion of patients could benefit from PBD under strict clinical indications (2–5).
The influence of PBD on short-term outcomes of patients with obstructive jaundice has received increased attention in recent years. In contrast, there has been little agreement about whether PBD has an adverse effect on the long-term survival of patients with obstructive jaundice who underwent resection of pancreatic head cancer. Several studies have reported that the use of PBD had an unfavorable impact on the long-term survival of patients with resected PDAC, while other studies have failed to find a connection between PBD and long-term survival (6–10). However, several studies have found that obstructive jaundice is a negative risk factor in patients with pancreatic head cancer (11–13). Thus, previous studies of the impact of PBD on long-term survival in all patients have suffered from shortcomings in the methods used to select cases. Furthermore, the duration of PBD, which was rarely included in the previous research, may also influence the long-term outcome in patients with obstructive jaundice based on the recent studies (14, 15).
The purpose of this study was to explore the relationship between the severity of obstructive jaundice, PBD, duration of PBD, and long-term survival in patients with obstructive jaundice with resectable pancreatic head cancer.
Clinical data were collected retrospectively in the Pancreatic Surgery Department of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine for consecutive patients who underwent pancreaticoduodenectomy for PDAC between January 2012 and December 2018. Included patients were 18–85 years of age and presented with obstructive jaundice, which was defined as a total serum bilirubin (TB) level exceeding 34.2 μmol/L (2 mg/dL) before intervention (16). All patients with locally advanced pancreatic cancer or metastatic disease by computed tomography or during surgery were excluded. Patients who received neoadjuvant chemotherapy were also excluded.
PBD was performed by using endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiodrainage (PTCD) in patients with acute cholangitis, severe malnutrition, contraindications to surgery or in whom surgical resection was significantly delayed. The PBD methods used on patients were determined by surgeons and interventional radiologists. Additionally, in case of biliary infection related to PBD, patients received antibiotic therapy that was monitored by surgeons. The intervals between PBD and surgery were recorded using the medical records of patients.
The pre-operative data available closest to the time of surgery were collected and used for analysis. For patients who underwent PBD, TB and serum albumin were also collected before PBD when available. What needs illustration is that the TB before intervention was defined as the TB before PBD in patients who underwent PBD as well as the TB before surgery in patients who did not undergo PBD. Neutrophil to lymphocyte ratio (NLR) was defined as the absolute neutrophil count divided by the absolute lymphocyte count.
The intraoperative information included surgical method [i.e., open pancreaticoduodenectomy (OPD) or minimally invasive pancreaticoduodenectomy (MIPD)], venous resection, and resection margins.
Post-operative pathologic stage was classified based on the 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging system. The grade of tumor differentiation was also collected. Post-operative information also included post-operative complications and adjuvant chemotherapy.
All the patients with resected pancreatic cancer in our department were followed up at regular intervals. A telephone follow-up call was made every 3 months with informed consent of the patient, and the follow-up ended with the patient's death. Patients were evaluated for recurrence based on computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography-computed tomography (PET/CT). Survival end points included overall survival (OS) and disease-free survival (DFS). OS of the patients was defined as the time elapsed from pancreaticoduodenectomy to death from any cause. DFS was defined as the surgery to recurrence or death from any cause, whichever occurred first. Survival data were censored at the date of last follow-up.
Data were expressed as medians (ranges) when they showed normal distribution or expressed as medians (interquartile ranges) when they did not. In order to determine the optimal cut-off point of TB level for maximum OS difference, X-tile software version 3.6.1 was used (17). The same method was used to determine the optimal cut-off point for age. The transformation of the albumin level into categories was made based on the lower normal reference value (35 g/L).
Categorical data were compared using Pearson's chi-square test or Fisher's exact test where appropriate. Paired t-tests were also used to analyse paired data. Survival curves were constructed using the Kaplan-Meier method with the log-rank test. Only factors found to be significant in the univariate analysis were entered into the multivariate Cox regression analysis. The Harrell concordance index (C-index) was calculated to measure the performance of the Cox model.
All statistical analyses were performed using X-tile software version 3.6.1 and R version 3.6.3. All tests were two-tailed, and P < 0.05 was considered statistically significant.
During the 7 year study period, a total of 426 patients with obstructive jaundice due to PDAC were included. Baseline clinicopathological characteristics for the study population are listed in Table 1. There were 287 males (67.4%), and the mean age was 63.4 ± 9.6 years. Among them, 242 (56.8%) patients received PBD, 107 (25.1%) via ERCP, and 135 (31.7%) via PTCD, respectively. The median elapsed time from PBD to surgery was 2 weeks. The median TB before surgery was significantly lower than the median TB before intervention in all cohorts (132 μmol/L vs. 216 μmol/L, P < 0.001). Meanwhile, the albumin before surgery was significantly higher than the albumin before intervention (P < 0.001).
At a median follow-up time of 16.7 months (range, 0.2–80.8 months), the median OS time, and the median DFS time were 18.5 and 12.3 months, respectively. At the last follow-up, 112 (26.3%) patients were still alive and 314 (73.7%) patients had died. Five patients were lost from the study population.
According to the X-tile software, the optimal cut-off point of TB before intervention was 151 μmol/L (≤ 151 μmol/L vs. >151 μmol/L, P = 0.002) (Figure 1). Note the slight difference of cut-off point between 150 μmol/L and 151 μmol/L (P = 0.003 and P = 0.002, respectively) (Figure 2). With regard to the feasibility for clinical application, we set 150 μmol/L as the best cut-off point for TB before intervention. The optimal cut-off point of age was 65 years (≤ 65 vs. >65 years, P < 0.001) (Supplementary Figure 1). The optimal cut-off point of TB before surgery was 136 μmol/L (≤ 136 μmol/L vs. >136 μmol/L, P = 0.060) (Supplementary Figure 2).
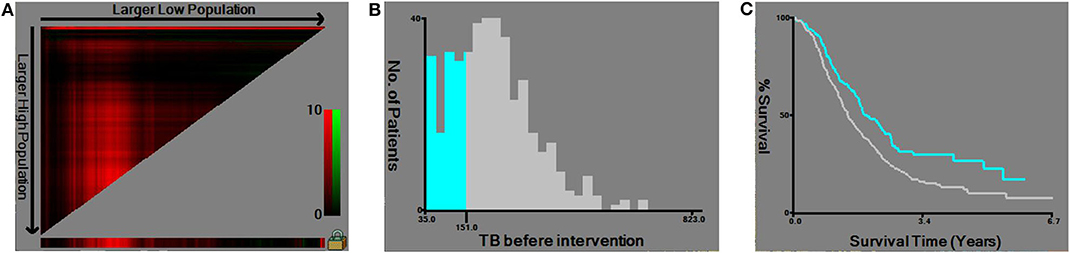
Figure 1. Utilizing X-tile analysis to determine the optimal cut-off level of the total serum bilirubin (TB) before intervention. (A) The graph shows that the optimal cut-off point has been determined by X-tile software. (B) Histogram and (C) Kaplan-Meier analysis were conducted using the optimal cut-off value.
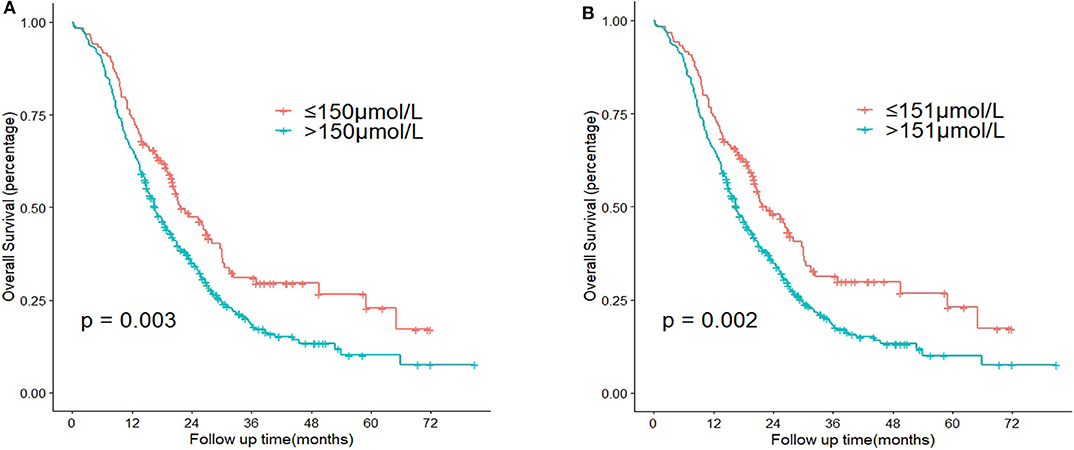
Figure 2. Comparison of P-value according to the cut-off point of total serum bilirubin (TB) before intervention using Kaplan-Meier analysis. (A) Cut-off point = 150 μmol/L. (B) Cut-off point = 151 μmol/L.
Patient characteristics were compared between the two groups based on their PBD status after X-tile analysis (Table 2). Patients who received PBD had higher levels of TB before intervention (P < 0.001). Furthermore, patients who received PBD had lower levels of albumin before intervention (P < 0.001), meaning these patients may have worse liver function. After PBD, patients had lower levels of TB before surgery compared to patients who did not have the PBD procedure (P = 0.001).
Figure 3 shows the Kaplan-Meier curves for OS and DFS among the patients with PDAC stratified by PBD status and type of PBD. Patients who received the PBD procedure (green) had significantly poorer OS than patients undergoing pancreaticoduodenectomy directly (red) (median of 16.6 vs. 22.2 months, P = 0.048). None of the other comparisons were statistically significant.
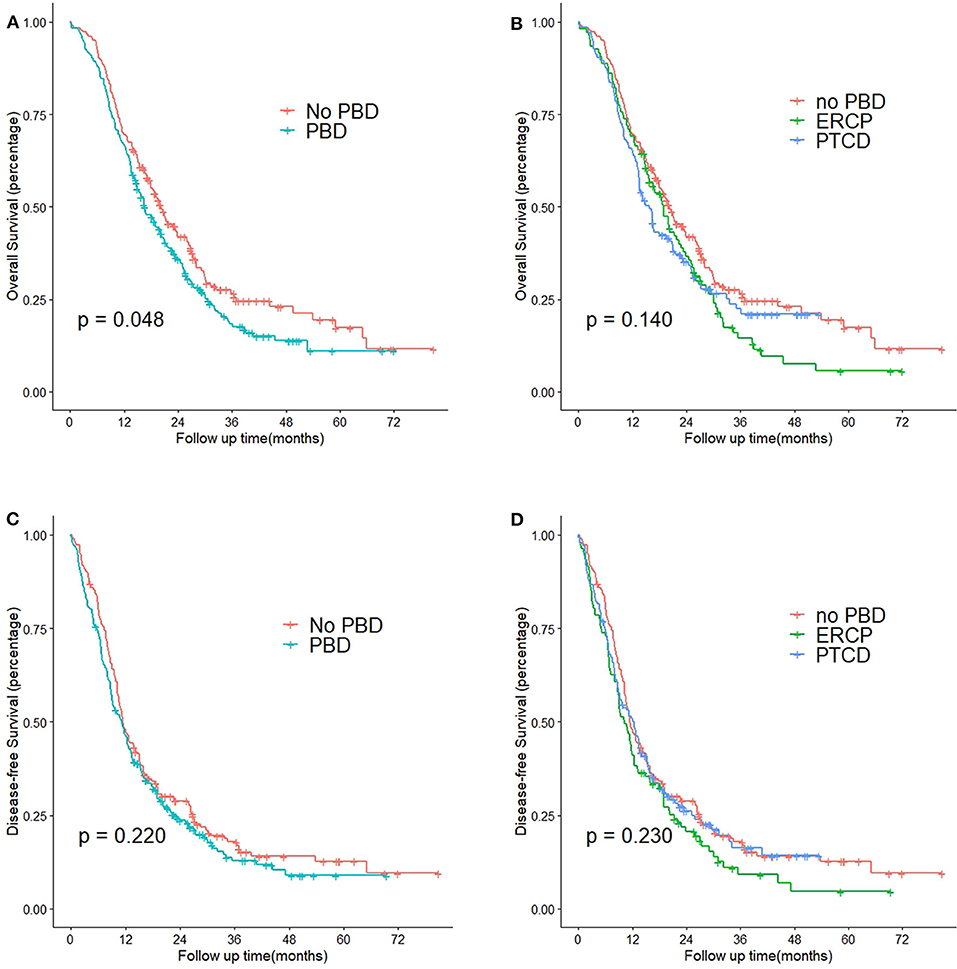
Figure 3. Kaplan-Meier curves of patients with obstructive jaundice with resectable pancreatic head cancer who did or did not undergo PBD. (A) OS curves of patients who did or did not undergo PBD (P = 0.048). (B) OS curves of patients who did not undergo PBD, patients who underwent ERCP, and patients who underwent PTCD (P = 0.140). (C) DFS curves of patients who did or did not undergo PBD (P = 0.220). (D) DFS curves of patients who did not undergo PBD, patients who underwent ERCP, and patients who underwent PTCD (P = 0.230). PBD, pre-operative biliary drainage; ERCP, endoscopic retrograde cholangiopancreatography; PTCD, percutaneous transhepatic cholangiodrainage.
The results of the uni- and multivariate analyses of prognostic factors for OS in patients with obstructive jaundice are shown in Table 3. Displayed are the hazard ratios (HRs) and their 95% confidence intervals (CIs). In the univariate analysis, age, PBD status, TB before intervention, albumin before intervention, NLR, venous resection, T stage, N stage, differentiation, resection margins, and adjuvant chemotherapy were significantly associated with prognosis. To control for potential confounding factors in the patients with obstructive jaundice while investigating the potential relationship between liver function parameters and patient prognosis, we compared two Cox regression models: model 1 did not contain any liver function parameters, and model 2 contained liver function parameters that were significant in the univariate analysis. Therefore, in model 1, the multivariate analysis showed that age >65 years (P = 0.003), PBD (P = 0.026), NLR >2 (P = 0.011), venous resection (P = 0.010), positive lymph node metastasis (P = 0.024), poor or moderate differentiation (P = 0.049), positive surgical margin status (P = 0.002), and no completion of adjuvant chemotherapy (P = 0.001) remained independently associated with worse OS. When liver function parameters were enrolled, model 2 showed that PBD did not affect OS (P = 0.428). Meanwhile, both TB before intervention >150 μmol/L and albumin before intervention <35 g/L were independent risk factors for OS (P = 0.022 and P = 0.022, respectively). We also used the C-index as the evaluation criteria. The C-index for model 2 was 0.681, which was higher compared to model 1 (C-index = 0.676).
Table 4 shows the results of the uni- and multivariate analyses of risk factors for DFS in patients with obstructive jaundice. TB before intervention was an independent risk factor, but PBD status was not an independent risk factor. Two models were constructed in the same way as noted above. In model 1, the multivariate analysis showed that age >65 years (P = 0.027), NLR >2 (P = 0.006), venous resection (P = 0.010), positive lymph node metastasis (P = 0.016), poor or moderate differentiation (P = 0.023), and positive surgical margin status (P = 0.020) affected DFS. We then analyzed the DFS when TB before intervention was added to the model. In model 2, TB before intervention >150 μmol/L was identified as an independent adverse prognostic factor, while the other parameters were still significant, as in model 1. The C-index for model 2 was 0.631, which was higher compared to model 1 (C-index = 0.622).
The initial objective was to assess the impact of PBD on long-term survival in patients with obstructive jaundice. With respect to the first research question, it was found that PBD may have a negative impact on prognosis after pancreaticoduodenectomy in model 1 (Table 3), as mentioned above. Contrary to expectations, our study did not find a significant influence of PBD on OS after considering liver function parameters, including TB and serum albumin. What is surprising is that TB before intervention >150 μmol/L is an independent adverse prognostic factor both for DFS and OS in patients with obstructive jaundice.
Many previous studies have described PBD as an independent predictor for worse prognosis after pancreaticoduodenectomy for PDAC. In the study by Macias et al. (7), histologic grade (P = 0.019) and PBD (P = 0.016) were the sole independent variables predicting worse OS in patients with PDAC. In another study by Furukawa et al. (6), ERCP was associated with poor OS. Two other Japanese studies demonstrated that PTCD was an independent prognostic factor both for peritoneal recurrence and poor OS (8, 9). However, recent research based on the Surveillance, Epidemiology, and End Results (SEER) database found that ERCP before pancreaticoduodenectomy was not associated with long-term survival in patients >65 years old with pancreatic head cancer after using propensity score matching analysis (10).
Nevertheless, the previous studies, as mentioned above, may have selection bias and confounding bias. Selection of the patient population who received PBD to compare with the patient population who did not receive PBD was probably a major bias. A substantial proportion the patient population who did not receive PBD wasn't associated with obstructive jaundice. Not only did these patients not have a plan for PBD in any case but also the presence of jaundice was found to be a negative risk factor in patients with PDAC (11, 12). Thus, patients who did not receive PBD including those without obstructive jaundice, may lead to an apparent selection bias. Therefore, our study selected patients with obstructive jaundice as a specific population to eliminate the potential selection bias.
On the other hand, most of the previous studies did not include liver function parameters in their statistical analyses due to missing data or incomplete data, which may relate to confounding bias. Our study found that TB before intervention >150 μmol/L is an independent adverse prognostic factor in patients with obstructive jaundice with PDAC. In accordance with the present results, a high-quality randomized controlled trial (RCT) has demonstrated that high TB at randomization was found to be a worse prognostic factor for OS after pancreaticoduodenectomy in patients with PDAC, while PBD had no impact on OS (13). The patients with severely obstructive jaundice were more likely to have acute cholangitis and poor nutrition status (18). Hence, high TB could eventually lead to more episodes of PBD before surgery in clinical practice, aiming to reduce post-operative complications. Our data analyses also corroborate this conclusion, the PBD group had a higher rate of TB before intervention >150 μmol/L compared with the no PBD group (87.6 vs. 51.6%, P < 0.001) (Table 2).
After we adjusted for potential confounding factors by including liver function parameters in model 2 of the multivariate analyses for OS, PBD was no longer significant compared with model 1 (Table 3). Additionally, TB before intervention not only was associated with OS but also was associated with DFS. This result may be explained by the fact that patients with severely obstructive jaundice (TB before intervention >150 μmol/L) was the root cause of poor survival, instead of the PBD procedure. These results are in accordance with several previous studies indicating that high TB negatively influences long-term survival in patients with PDAC or periampullary carcinomas (18–20). This result may be explained by the fact that tumor aggressiveness in patients with high TB levels was stronger than patients with low TB levels. Another possible explanation for this is that severe obstructive jaundice may relate to immunosuppression, causing micrometastasis formation of tumors before intervention (21–23). Further experimental studies are needed to identify the relationship between the TB level and nature of pancreatic cancer.
Elderly patients, high NLR, surgery with venous resection and positive resection margins were found to be associated with both OS and DFS after resection. The elapsed time between PBD and surgery did not influence OS or DFS. These results are consistent with those of previous studies (13, 24–27). On the other hand, CA19-9, which is regarded as the most important tumor marker in patients with PDAC, was not included in our study. Although several studies concluded that elevated CA19-9 levels could predict early recurrence and OS after pancreatic resection in patients with PDAC (28–30), a high degree of collinearity is likely present between CA19-9 and TB before intervention based on the past research (31).
There are also several limitations to our study. First, as in any retrospective analysis, there may have been selection bias as well as incomplete information due to the retrospective design. A prospective validation study to confirm the possible risks of TB before intervention is necessary. Second, this study included patients with stage T4 due to the study design. Nevertheless, adjuvant chemotherapy is recommended for all patients with borderline resectable PDAC according to the recent NCCN guideline (4). Third, with the development of the endoscopic ultrasound (EUS) technique, EUS can be used as a preferable alternative drainage technique. However, we didn't perform EUS-guided biliary drainage before 2019, although EUS was widely used in our center.
In conclusion, PBD did not influence long-term OS or DFS in patients with obstructive jaundice with resectable PDAC who underwent pancreaticoduodenectomy, as compared with surgery alone. However, TB before intervention >150 μmol/L is an independent adverse prognostic factor both for DFS and OS in patients with obstructive jaundice. PBD remains the treatment of choice if there is a reasonable indication, considering the risks of the procedure and surgery.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ruijin Hospital Ethics Committee Affiliated to Shanghai JiaoTong University School of Medicine, Shanghai, China. The patients/participants provided their written informed consent to participate in this study.
ZS, JZ, and HC: study conception, design, and drafting of the manuscript. ZS, HC, WW, WX, YZ, XL, SZ, ZX, and XD: acquisition of data. ZS, JZ, and YW: analysis and interpretation of data. JW, YW, and BS: critical revision. All authors contributed to the article and approved the submitted version.
Ruijin Youth NSFC Cultivation Fund (2019QNPY02022).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.575316/full#supplementary-material
Supplementary Figure 1. Utilizing X-tile analysis to determine the optimal cut-off level of age. (A) The graph shows that the optimal cut-off point has been determined by X-tile software. (B) Histogram and (C) Kaplan-Meier analysis were conducted using the optimal cut-off value.
Supplementary Figure 2. Utilizing X-tile analysis to determine the optimal cut-off level of the total serum bilirubin (TB) before surgery. (A) The graph shows that the optimal cut-off point has been determined by X-tile software. (B) Histogram and (C) Kaplan-Meier analysis were conducted using the optimal cut-off value.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
2. van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. (2010) 362:129–37. doi: 10.1056/NEJMoa0903230
3. Scheufele F, Schorn S, Demir IE, Sargut M, Tieftrunk E, Calavrezos L, et al. Preoperative biliary stenting versus operation first in jaundiced patients due to malignant lesions in the pancreatic head: a meta-analysis of current literature. Surgery. (2017) 161:939–50. doi: 10.1016/j.surg.2016.11.001
4. Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2017) 15:1028–61. doi: 10.6004/jnccn.2017.0131
5. Shen Z, Zhang J, Zhao S, Zhou Y, Wang W, Shen B. Preoperative biliary drainage of severely obstructive jaundiced patients decreases overall postoperative complications after pancreaticoduodenectomy: a retrospective and propensity score-matched analysis. Pancreatology. (2020) 20:529–36. doi: 10.1016/j.pan.2020.02.002
6. Furukawa K, Shiba H, Shirai Y, Horiuchi T, Iwase R, Haruki K, et al. Negative impact of preoperative endoscopic biliary drainage on prognosis of pancreatic ductal adenocarcinoma after pancreaticoduodenectomy. Anticancer Res. (2015) 35:5079–83.
7. Macias N, Sayagues JM, Esteban C, Iglesias M, Gonzalez LM, Quinones-Sampedro J, et al. Histologic tumor grade and preoperative bilary drainage are the unique independent prognostic factors of survival in pancreatic ductal adenocarcinoma patients after pancreaticoduodenectomy. J Clin Gastroenterol. (2018) 52:11–7. doi: 10.1097/MCG.0000000000000793
8. Uemura K, Murakami Y, Satoi S, Sho M, Motoi F, Kawai M, et al. Impact of preoperative biliary drainage on long-term survival in resected pancreatic ductal adenocarcinoma: a multicenter observational study. Ann Surg Oncol. (2015) 22:1238–46. doi: 10.1245/s10434-015-4618-9
9. Murakami Y, Uemura K, Hashimoto Y, Kondo N, Nakagawa N, Sasaki H, et al. Does preoperative biliary drainage compromise the long-term survival of patients with pancreatic head carcinoma? J Surg Oncol. (2015) 111:270–6. doi: 10.1002/jso.23797
10. Rustgi SD, Amin S, Yang A, Kim MK, Nagula S, Kumta NA, et al. Preoperative endoscopic retrograde cholangiopancreatography is not associated with increased pancreatic cancer mortality. Clin Gastroenterol Hepatol. (2019) 17:1580–6. doi: 10.1016/j.cgh.2018.11.056
11. Strasberg SM, Gao F, Sanford D, Linehan DC, Hawkins WG, Fields R, et al. Jaundice: an important, poorly recognized risk factor for diminished survival in patients with adenocarcinoma of the head of the pancreas. HPB. (2014) 16:150–6. doi: 10.1111/hpb.12094
12. Nakata B, Amano R, Kimura K, Hirakawa K. Comparison of prognosis between patients of pancreatic head cancer with and without obstructive jaundice at diagnosis. Int J Surg. (2013) 11:344–9. doi: 10.1016/j.ijsu.2013.02.023
13. Eshuis WJ, van er Gaag dNA, Rauws EA, van Eijck CH, Bruno MJ, Kuipers EJ, et al. Therapeutic delay and survival after surgery for cancer of the pancreatic head with or without preoperative biliary drainage. Ann Surg. (2010) 252:840–9. doi: 10.1097/SLA.0b013e3181fd36a2
14. Matsumoto M, Nakabayashi Y, Fujiwara Y, Funamizu N, Noaki R, Eto S, et al. Duration of preoperative biliary drainage as a prognostic factor after pancreaticoduodenectomy for pancreatic head cancer. Anticancer Res. (2017) 37:3215–19. doi: 10.21873/anticanres.11683
15. Shin SH, Han IW, Ryu Y, Kim N, Choi DW, Heo JS. Optimal timing of pancreaticoduodenectomy following preoperative biliary drainage considering major morbidity and postoperative survival. J Hepatobiliary Pancreat Sci. (2019) 26:449–58. doi: 10.1002/jhbp.652
16. Ferri MJ, Saez M, Figueras J, Fort E, Sabat M, Lopez-Ben S, et al. Improved pancreatic adenocarcinoma diagnosis in jaundiced and non-jaundiced pancreatic adenocarcinoma patients through the combination of routine clinical markers associated to pancreatic adenocarcinoma pathophysiology. PLoS ONE. (2016) 11:e0147214. doi: 10.1371/journal.pone.0147214
17. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. (2004) 10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713
18. Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, et al. Tokyo guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. (2018) 25:17–30. doi: 10.1002/jhbp.512
19. Bettschart V, Rahman MQ, Engelken FJ, Madhavan KK, Parks RW, Garden OJ. Presentation, treatment and outcome in patients with ampullary tumours. Br J Surg. (2004) 91:1600–7. doi: 10.1002/bjs.4787
20. Berberat PO, Kunzli BM, Gulbinas A, Ramanauskas T, Kleeff J, Muller MW, et al. An audit of outcomes of a series of periampullary carcinomas. Eur J Surg Oncol. (2009) 35:187–91. doi: 10.1016/j.ejso.2008.01.030
21. Kimmings AN, van Deventer SJ, Obertop H, Rauws EA, Gouma DJ. Inflammatory and immunologic effects of obstructive jaundice: pathogenesis and treatment. J Am Coll Surg. (1995) 181:567–81.
22. Kawarabayashi N, Seki S, Hatsuse K, Kinoshita M, Takigawa T, Tsujimoto H, et al. Immunosuppression in the livers of mice with obstructive jaundice participates in their susceptibility to bacterial infection and tumor metastasis. Shock. (2010) 33:500–6. doi: 10.1097/SHK.0b013e3181c4e44a
23. Chowdhury AH, Camara M, Martinez-Pomares L, Zaitoun AM, Eremin O, Aithal GP, et al. Immune dysfunction in patients with obstructive jaundice before and after endoscopic retrograde cholangiopancreatography. Clin Sci. (2016) 130:1535–44. doi: 10.1042/CS20160326
24. Wu LX, Wang XY, Xu KQ, Lin YL, Zhu WY, Han L, et al. A systematic inflammation-based model in advanced pancreatic ductal adenocarcinoma. J Cancer. (2019) 10:6673–80. doi: 10.7150/jca.30561
25. Pine JK, Haugk B, Robinson SM, Darne A, Wilson C, Sen G, et al. Prospective assessment of resection margin status following pancreaticoduodenectomy for pancreatic ductal adenocarcinoma after standardisation of margin definitions. Pancreatology. (2020) 20:537–44. doi: 10.1016/j.pan.2020.01.004
26. Anger F, Döring A, Schützler J, Germer C-T, Kunzmann V, Schlegel N, et al. Prognostic impact of simultaneous venous resections during surgery for resectable pancreatic cancer. HPB. (2020). doi: 10.1016/j.hpb.2019.12.017. [Epub ahead of print].
27. Stornello C, Archibugi L, Stigliano S, Vanella G, Graglia B, Capalbo C, et al. Diagnostic delay does not influence survival of pancreatic cancer patients. United European Gastroenterol J. (2020) 8:81–90. doi: 10.1177/2050640619879004
28. Distler M, Pilarsky E, Kersting S, Grützmann R. Preoperative CEA and CA 19–9 are prognostic markers for survival after curative resection for ductal adenocarcinoma of the pancreas - a retrospective tumor marker prognostic study. Int J Surg. (2013) 11:1067–72. doi: 10.1016/j.ijsu.2013.10.005
29. Kondo N, Murakami Y, Uemura K, Nakagawa N, Takahashi S, Ohge H, et al. Comparison of the prognostic impact of pre- and post-operative CA19–9, SPan-1, and DUPAN-II levels in patients with pancreatic carcinoma. Pancreatology. (2017) 17:95–102. doi: 10.1016/j.pan.2016.10.004
30. Suzuki S, Shimoda M, Shimazaki J, Maruyama T, Oshiro Y, Nishida K, et al. Predictive early recurrence factors of preoperative clinicophysiological findings in pancreatic cancer. Eur Surg Res. (2018) 59:329–38. doi: 10.1159/000494382
Keywords: obstructive jaundice, pre-operative biliary drainage, pancreaticoduodenectomy, pancreatic cancer, long-term survival
Citation: Shen Z, Zhang J, Chen H, Wang W, Xu W, Lu X, Zhou Y, Zhao S, Xu Z, Deng X, Wang J, Weng Y and Shen B (2020) Does Pre-operative Biliary Drainage Influence Long-Term Survival in Patients With Obstructive Jaundice With Resectable Pancreatic Head Cancer? Front. Oncol. 10:575316. doi: 10.3389/fonc.2020.575316
Received: 23 June 2020; Accepted: 18 August 2020;
Published: 16 September 2020.
Edited by:
Mark Girgis, University of California, Los Angeles, United StatesReviewed by:
Paul Willemsen, Ziekenhuisnetwerk Antwerpen Middelheim, BelgiumCopyright © 2020 Shen, Zhang, Chen, Wang, Xu, Lu, Zhou, Zhao, Xu, Deng, Wang, Weng and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiancheng Wang, amlhbmNoZW5nX3dhbmdAaG90bWFpbC5jb20=; Yuanchi Weng, d3l1YW5jaGlAMTI2LmNvbQ==; Baiyong Shen, c2hlbmJ5QHNoc211LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.