- 1Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN, United States
- 2School of Medicine, Vanderbilt University, Nashville, TN, United States
- 3Department of Radiology, Vanderbilt University Medical Center, Nashville, TN, United States
- 4Department of Cell and Developmental Biology, Vanderbilt University, Nashville, TN, United States
Objective: To determine the relationship between survival and glioblastoma distance from the ventricular-subventricular neural stem cell niche (VSVZ).
Methods: 502 pre-operative gadolinium-enhanced, T1-weighted MRIs with glioblastoma retrieved from an institutional dataset (n = 252) and The Cancer Imaging Atlas (n=250) were independently reviewed. The shortest distance from the tumor contrast enhancement to the nearest lateral ventricular wall, the location of the VSVZ, was measured (GBM-VSVZDist). The relationship of GBM-VSVZDist with the proportion of glioblastomas at each distance point and overall survival was explored with a Pearson’s correlation and Cox regression model, respectively, adjusting for the well-established glioblastoma prognosticators.
Results: 244/502 glioblastomas had VSVZ contact. The proportion of non-VSVZ-contacting glioblastomas correlated inversely with GBM-VSVZDist (partial Pearson’s correlation adjusted for tumor volume R=-0.79, p=7.11x10-7). A fit of the Cox regression model adjusted for age at diagnosis, Karnofsky performance status score, post-operative treatment with temozolomide and/or radiotherapy, IDH1/2 mutation status, MGMT promoter methylation status, tumor volume, and extent of resection demonstrated a significantly decreased overall survival only when glioblastoma contacted the VSVZ. Overall survival did not correlate with GBM-VSVZDist.
Conclusions: In the two independent cohorts analyzed, glioblastomas at diagnosis were found in close proximity or in contact with the VSVZ with a proportion that decreased linearly with GBM-VSVZDist. Patient survival was only influenced by the presence or absence of a gadolinium-enhanced glioblastoma contact with the VSVZ. These results may guide analyses to test differential effectiveness of VSVZ radiation in VSVZ-contacting and non-contacting glioblastomas and/or inform patient selection criteria in clinical trials of glioblastoma radiation.
Introduction
The median survival of patients with glioblastoma is 16 months (1, 2). Survival is even lower in those patients in whom the glioblastoma has invaded or contacted the ventricular-subventricular zone (VSVZ) at diagnosis (3, 4). The VSVZ is a neural stem cell niche in the lateral walls of the lateral ventricles in the brain (5–7). Recent results have supported the hypothesis that VSVZ houses the cellular origins of some human glioblastomas (7, 8).
Glioblastomas with and without VSVZ invasion or contact are not genomically distinct in bulk profiling (9, 10). Therefore, the more severe clinical phenotype of those patients is likely due to the microenvironment of the VSVZ (9). The microenvironment is especially attractive to glioblastoma cells (11, 12), and factors released by the VSVZ can mediate resistance to radiation therapy in glioblastoma cells (13). Consistent with these reports, recurrence is earlier in those patients in whom the initial glioblastoma invaded or contacted the VSVZ (3, 4).
Research efforts in this area have been conducted by dichotomizing glioblastomas as having VSVZ contact or not, demonstrating survival is lower with contact. Whether clinical outcome is related continuously with glioblastoma distance from the VSVZ (GBM-VSVZDist) is unknown. Therefore, in this brief report, we conducted survival analyses to uncover the relationship of patient survival with glioblastoma distance from the VSVZ that yielded consistent findings in two distinct datasets.
Methods
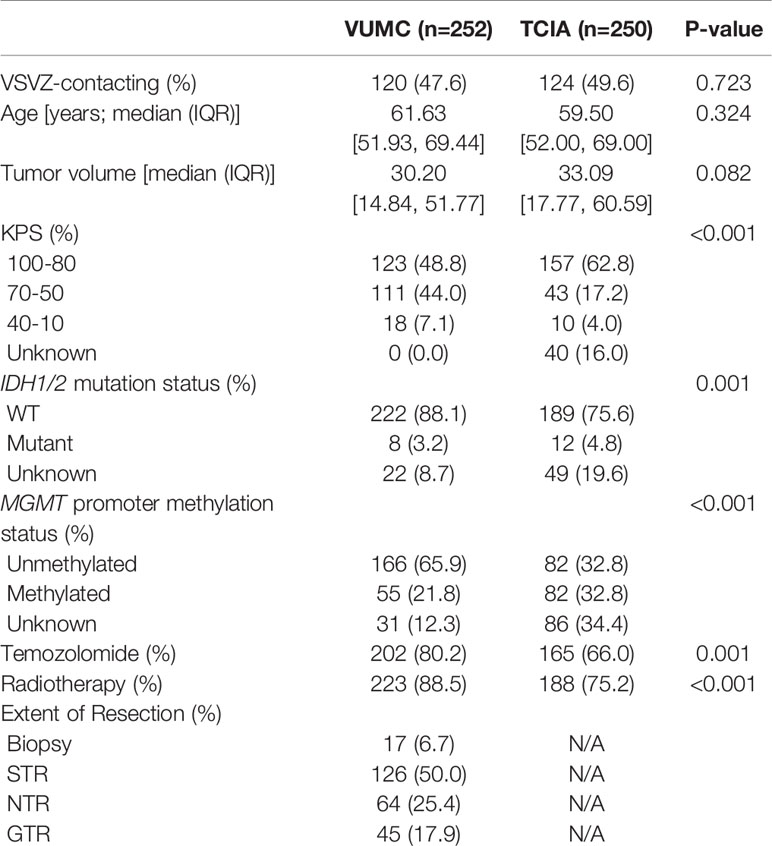
A total of 502 patients with histologically confirmed glioblastoma were analyzed: 252 from a prospectively maintained single-institution (VUMC) registry and 250 from The Cancer Imaging Atlas (TCIA) (14). In the institutional dataset, the median year of diagnosis was 2012 [interquartile range: 2009-2015] and the patients were followed to 2017. Institutional review board approval was obtained; patient consent was waived. Two independent, outcome-blinded reviews of pre-operative gadolinium-enhanced, T1-weighted MRIs were conducted. One of these assessments was made by a board-certified neuro-radiologist. All institutional MRIs were obtained using a 1.5 or 3-tesla magnetic field, and details of the MRIs in the TCIA are published (14). As per standard assessment, all MRIs were first dichotomized as demonstrating contact of the tumor contrast enhancement with the lateral ventricular ependyma—the location of the VSVZ—or not. GBM-VSVZDist was then measured for all non-VSVZ-contacting glioblastomas. Specifically, the shortest distance from the tumor contrast enhancement to the nearest lateral ventricular wall visible on any one of three MRI dimensions (axial, coronal, or sagittal) was measured to the nearest millimeter (mm) (Figure 1). All disagreements were resolved by group consensus. Glioblastoma volume contained within contrast enhancement was also calculated. All of these radiological assessments were performed using OsiriX Lite software (version 9.4, Pixmeo, Geneva, Switzerland).
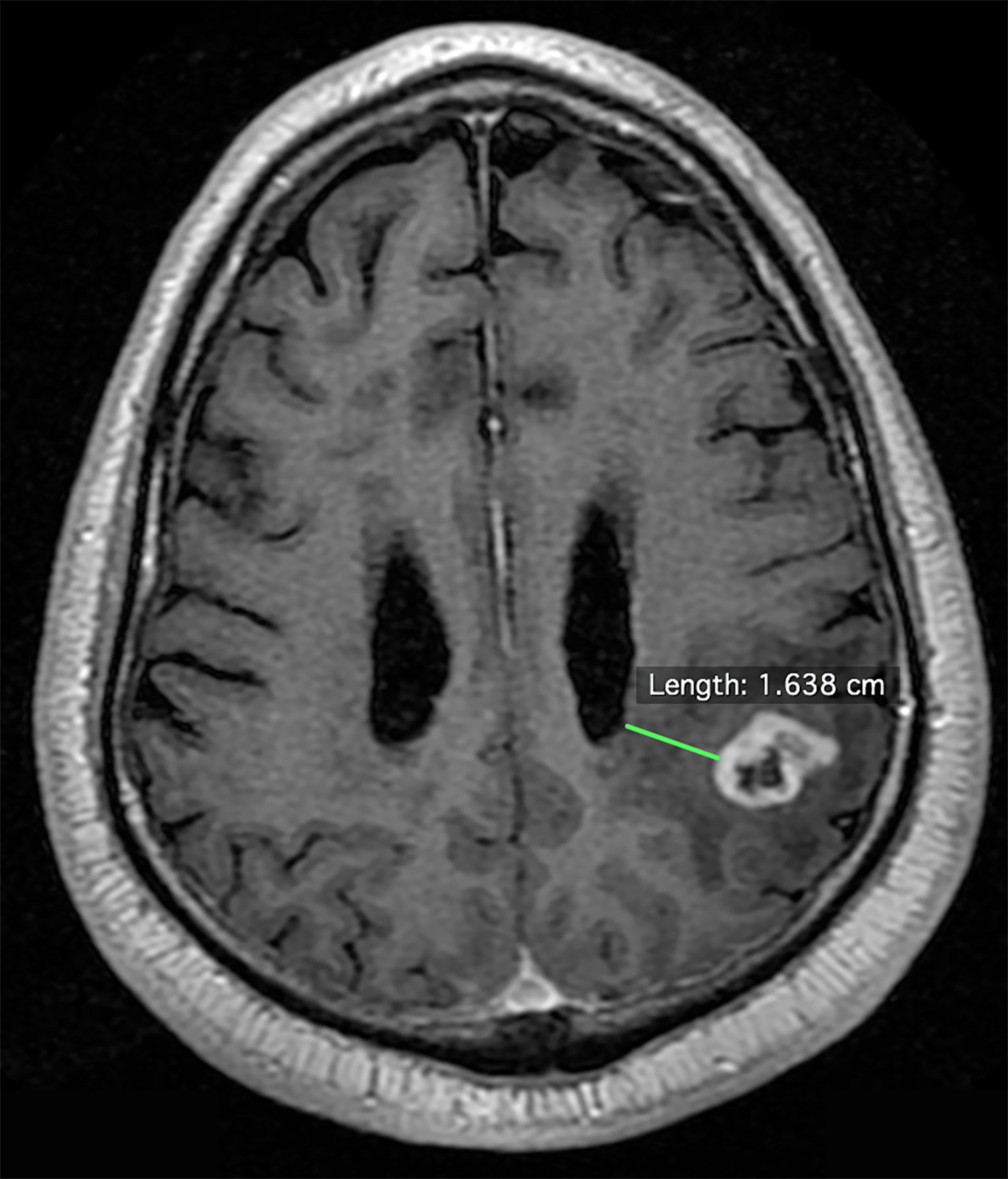
Figure 1 Example measurement of the shortest distance between the enhancing edge of a glioblastoma to the lateral wall of the lateral ventricle on an axial pre-operative gadolinium-enhanced, T1-weighted magnetic resonance image.
Corresponding clinical data were obtained from the institution registry and The Cancer Genome Atlas (9). Patient survival was recorded as the time from initial diagnosis to death or to the last time known to be alive (censored survival). The relationship between GBM-VSVZDist and survival was assessed using the Cox regression model, adjusting for well-established glioblastoma prognosticators. These included age at diagnosis (treated as a continuous variable), pre-operative Karnofsky performance status score (KPS; categorized into 100-80, 70-50, 40-0), postoperative treatment with temozolomide and/or radiotherapy (yes or no), IDH1/2 mutation status (wildtype, mutant, or unassessed), MGMT promoter methylation status (methylated, unmethylated, or unassessed), tumor volume (continuous variable), and extent of resection of the enhancing portion of the glioblastoma. The extent of resection was categorized as biopsy (<50%), subtotal (50%–95%), near-total (95%–99%) and gross total (100%) based on independent assessments by a neurosurgeon and neuroradiologist of the postoperative, post-contrast MRI obtained within 24 h after the operation. The adjusted Cox models were confirmed to meet the proportional hazards assumption, which was tested by assessing the significance of the relationship between Schoenfeld residuals and time for the overall model. The results of the Cox models are reported as hazard ratios with 95% confidence intervals. Finally, the relationship between GBM-VSVZDist and adjusted hazard ratios were depicted using a quadratic spline fit.
Standard descriptive statistical methods were used to report variables and compare distributions of continuous and proportions of categorical variables. Correlation between two variables was assessed using Pearson’s correlation treating both variables continuously. Statistical significance was claimed with a two-sided p-value of ≤ 0.05 or 95% confidence intervals that did not span 1. All analyses were conducted using R version 3.4 (R Foundation for Statistical Computing, Vienna, Austria) and the Survminer package (Version 0.4.0).
Results
Patient and glioblastoma characteristics are listed in Table 1. Out of 502 glioblastomas, 244 (48.6%) had VSVZ contact (GBM-VSVZDist=0). Interestingly, the number of non-VSVZ-contacting glioblastomas correlated inversely with GBM-VSVZDist (R=-0.85, p=3.8x10-9; Figure 2A). This correlation was partly confounded by the inverse correlation of tumor volume with GBM-VSVZDist (R=-0.44, p=2.2x10-16; Figure 2B); therefore, it was adjusted for the median tumor volume at each GBM-VSVZDist value and remained significant (partial Pearson’s correlation R=-0.79, p=7.11x10-7). KPS and extent of resection significantly correlated with GBM-VSVZDist in the institutional VUMC dataset (R=0.19, p=0.002; R=0.28, p=5.7x10-6, respectively), but not in the TCIA dataset (in which extent of resection data are not available; Figure 2C).
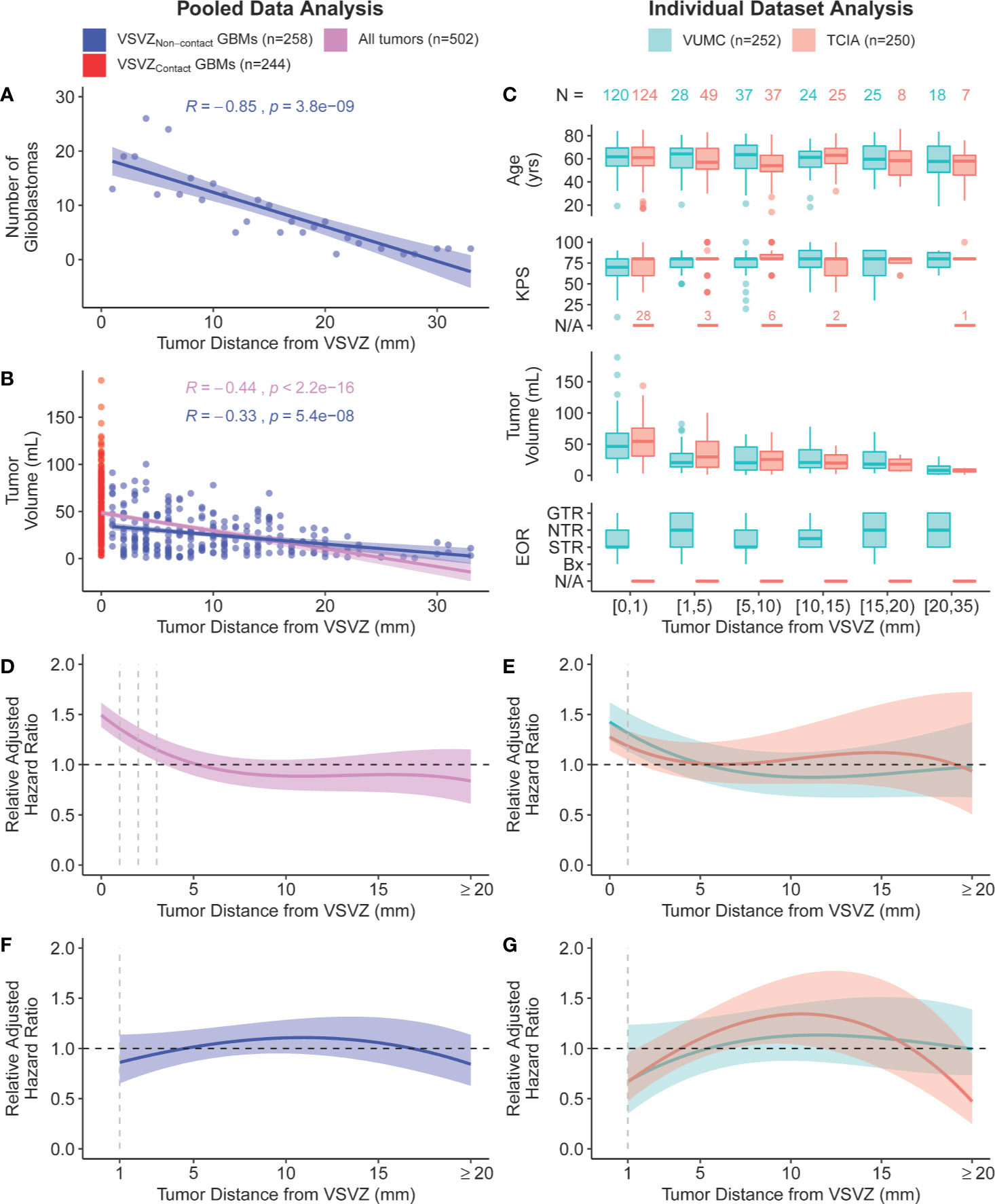
Figure 2 Scatter plot of (A) the number of non-VSVZ-contacting glioblastomas (VSVZNon-contact GBMs) and (B) tumor volume plotted in relation to their distance to the VSVZ (GBM-VSVZDist). The relationships are quantified with a Pearson’s correlation. (C) Boxplot distributions of age, Karnofsky performance status score (KPS), glioblastoma volume, and extent of resection of enhancing glioblastoma [EOR; <50% (biopsy, bx); 50%–95% (subtotal, STR); 95%–99% (near total, NTR); 100% (gross total, GTR)] plotted against GBM-VSVZDist grouped into select ranges in the single-institution (orange) and The Cancer Imaging Archive (TCIA; teal) datasets. The relationship between GBM-VSVZDist and adjusted hazard ratio relative to the hazard at GBM-VSVZDist of 5mm in (D) pooled data and (E) within each dataset is depicted using a quadratic spline fit. The size of the colored ribbon represents the 95% confidence interval. The hazard ratio is adjusted for the following: age at diagnosis (continuous), Karnofsky performance status score (KPS: 100-80, 70-50, 40-0, or not available, as in the case of few TCIA patients denoted in (C), post-operative treatment with temozolomide and/or radiotherapy (yes or no), IDH1/2 mutation status (wildtype, mutant, or unassessed), MGMT promoter methylation status (methylated, unmethylated, or unassessed), tumor volume, and EOR (not available in TCIA hence unadjusted in the TCIA and pooled analyses). (F, G) Prior analyses repeated with only non-VSVZ-contacting glioblastomas.
Next, we conducted survival analyses by fitting a Cox regression model, adjusting for the available co-variables (Table 1). The adjusted fit demonstrated a significantly decreased survival, or increased hazard, when glioblastoma contacted the VSVZ relative to the hazard at GBM-VSVZDist of 5mm (Figures 2D, E). An increased hazard was also noted within the immediate proximity (≤1–3 mm) of VSVZ. However, it was concluded to be an artifact of the fit manifested by the greatly increased hazard associated with VSVZ contact, because in an adjusted Cox regression analysis of only the non-VSVZ-contacting glioblastomas, the hazard ratio remained non-significantly altered along the range of GBM-VSVZDist values (Figures 2F, G).
Discussion
Recent studies in mouse models and human intraoperative glioblastoma samples suggest that genetically altered neural stem cells can migrate out of the VSVZ and ultimately generate glioblastomas (8). Select glioblastoma cells can, in turn, be attracted to the VSVZ (12, 13). Complimenting these discoveries, our results revealed that about half of glioblastomas radiographically contact the VSVZ at diagnosis. The remaining tumors were found near the VSVZ with a frequency that decreased linearly with GBM-VSVZDist.
The decreasing number of patients with greater GBM-VSVZDist added a limitation for the analysis, as it resulted in increasing confidence intervals around the hazard ratio when examining individual datasets (Figure 2E). Hence, we pooled the datasets to address this limitation, yielding a more constant and precise range of confidence interval around the hazard ratio, which remained steady around 1 (Figures 2D, F). Our results are in accordance with MRI probabilistic maps of glioblastoma that highlight areas associated with lower survival (15, 16).
Patient survival was only influenced by the presence or absence of a gadolinium-enhanced glioblastoma contact with the VSVZ (3). Glioblastoma volume, depth, and any potential influence of these variables on the extent of resection may not solely explain the lower survival associated with VSVZ-contacting glioblastomas.
Several studies have sought to understand the molecular basis for the increased malignancy of glioblastomas with VSVZ contact (17–19). Two of them demonstrate a correlation with increased glioblastoma expression of CD133, a glioma stem cell marker, with proximity to the VSVZ (20, 21). However, large bulk tissue analyses have not revealed a consistent molecular signature of VSVZ-contacting glioblastomas (9, 10). Therefore, the role of the microenvironment of the VSVZ is also being probed to understand the increased malignancy of glioblastomas with VSVZ contact. Changes in the disease course that can occur once glioblastoma cells invade the VSVZ, whereupon they are theorized to become therapy-resistant (13), drive recurrence, and disseminate further (22), are hypothesized to explain the lower survival associated with VSVZ-contacting glioblastomas.
This work has some limitations. First, we solely used uniaxial T1-weighted MRIs that did not uniformly include 3D MRI-based sequences, which could lead to biased conclusions. Second, we acknowledge that the enhancing edge of glioblastoma may not represent the true edge of glioblastoma. Therefore, it is critical to rely on a proper combination of MRI studies, and additional histological validation studies are required.
There is a heightened interest in assessing the effectiveness of VSVZ radiation in addition to the standard of care treatment for glioblastoma. For example, one randomized trial is underway (ClinicalTrials.gov Identifier: NCT02177578). Our results may guide analyses of such trials. For example, in existing trials, it may be beneficial to test the differential effectiveness of VSVZ radiation in niche-contacting and non-contacting glioblastomas. Our results may also be used to inform patient selection criteria of future trials in this area; for example, a trial of VSVZ radiation focused only on patients with niche-contacting glioblastoma.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Vanderbilt University Medical Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
AM conceptualized and designed the study, acquired the data, analyzed the data, drafted the manuscript, and revised the manuscript for intellectual content. NM acquired and analyzed the data, and drafted the manuscript. SS acquired the data. LD acquired and interpreted the data. RI conceptualized the study, interpreted the data, and revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Funding
National Cancer Institute, National Institutes of Health (F32 CA224962 to A.M.M.), 2018 Burroughs Wellcome Fund Physician-Scientist Institutional Award (1018894 to Vanderbilt University and A.M.M.), National Institute of Neurological Disorders and Stroke (R01 NS 096238 to R.A.I.), Michael David Greene Brain Cancer Fund (R.A.I.), and Vanderbilt-Ingram Cancer Center Ambassadors Award (R.A.I.).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med (2014) 370(8):709–22. doi: 10.1056/NEJMoa1308345
2. Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med (2014) 370(8):699–708. doi: 10.1056/NEJMoa1308573
3. Mistry AM, Dewan MC, White-Dzuro GA, Brinson PR, Weaver KD, Thompson RC, et al. Decreased survival in glioblastomas is specific to contact with the ventricular-subventricular zone, not subgranular zone or corpus callosum. J Neurooncol (2017) 132(2):341–9. doi: 10.1007/s11060-017-2374-3
4. Mistry AM, Hale AT, Chambless LB, Weaver KD, Thompson RC, Ihrie RA. Influence of glioblastoma contact with the lateral ventricle on survival: a meta-analysis. J Neurooncol (2017) 131(1):125–33. doi: 10.1007/s11060-016-2278-7
5. Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature (2004) 427(6976):740–4. doi: 10.1038/nature02301
6. Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer (2006) 6(6):425–36. doi: 10.1038/nrc1889
7. Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med (2005) 353(8):811–22. doi: 10.1056/NEJMra043666
8. Lee JH, Lee JE, Kahng JY, Kim SH, Park JS, Yoon SJ, et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature (2018) 560(7717):243–7. doi: 10.1038/s41586-018-0389-3
9. Mistry AM, Wooten DJ, Davis LT, Mobley BC, Quaranta V, Ihrie RA. Ventricular-Subventricular Zone Contact by Glioblastoma is Not Associated with Molecular Signatures in Bulk Tumor Data. Sci Rep (2019) 9(1):1842. doi: 10.1038/s41598-018-37734-w
10. Berendsen S, van Bodegraven E, Seute T, Spliet WGM, Geurts M, Hendrikse J, et al. Adverse prognosis of glioblastoma contacting the subventricular zone: Biological correlates. PLoS One (2019) 14(10):e0222717. doi: 10.1371/journal.pone.0222717
11. Goffart N, Kroonen J, Di Valentin E, Dedobbeleer M, Denne A, Martinive P, et al. Adult mouse subventricular zones stimulate glioblastoma stem cells specific invasion through CXCL12/CXCR4 signaling. Neuro Oncol (2015) 17(1):81–94. doi: 10.1093/neuonc/nou144
12. Qin EY, Cooper DD, Abbott KL, Lennon J, Nagaraja S, Mackay A, et al. Neural Precursor-Derived Pleiotrophin Mediates Subventricular Zone Invasion by Glioma. Cell (2017) 170(5):845–59.e819. doi: 10.1016/j.cell.2017.07.016
13. Goffart N, Lombard A, Lallemand F, Kroonen J, Nassen J, Di Valentin E, et al. CXCL12 mediates glioblastoma resistance to radiotherapy in the subventricular zone. Neuro Oncol (2017) 19(1):66–77. doi: 10.1093/neuonc/now136
14. Clark K, Vendt B, Smith K, Freymann J, Kirby J, Koppel P, et al. The Cancer Imaging Archive (TCIA): maintaining and operating a public information repository. J Digit Imaging (2013) 26(6):1045–57. doi: 10.1007/s10278-013-9622-7
15. Roux A, Roca P, Edjlali M, Sato K, Zanello M, Dezamis E, et al. MRI Atlas of IDH Wild-Type Supratentorial Glioblastoma: Probabilistic Maps of Phenotype, Management, and Outcomes. Radiology (2019) 293(3):633–43. doi: 10.1148/radiol.2019190491
16. Liu TT, Achrol AS, Mitchell LA, Du WA, Loya JJ, Rodriguez SA, et al. Computational Identification of Tumor Anatomic Location Associated with Survival in 2 Large Cohorts of Human Primary Glioblastomas. AJNR Am J Neuroradiol (2016) 37(4):621–8. doi: 10.3174/ajnr.A4631
17. Gollapalli K, Ghantasala S, Kumar S, Srivastava R, Rapole S, Moiyadi A, et al. Subventricular zone involvement in Glioblastoma - A proteomic evaluation and clinicoradiological correlation. Sci Rep (2017) 7(1):1449. doi: 10.1038/s41598-017-01202-8
18. Batista K, Costa B, Pablo I, Vega IF, Morales J, Alvarez AV, et al. Analysis of Olig2 and YKL-40 expression: a clinicopathological/immunohistochemical study for the distinction between subventricular zone II and III glioblastomas. Folia Neuropathol (2016) 54(1):31–9. doi: 10.5114/fn.2016.58913
19. Lin CA, Rhodes CT, Lin C, Phillips JJ, Berger MS. Comparative analyses identify molecular signature of MRI-classified SVZ-associated glioblastoma. Cell Cycle (2017) 16(8):765–75. doi: 10.1080/15384101.2017.1295186
20. Steed TC, Treiber JM, Taha B, Engin HB, Carter H, Patel KS, et al. Glioblastomas located in proximity to the subventricular zone (SVZ) exhibited enrichment of gene expression profiles associated with the cancer stem cell state. J Neurooncol (2020) 148(3):455–62. doi: 10.1007/s11060-020-03550-4
21. Yamaki T, Shibahra I, Matsuda KI, Kanemura Y, Konta T, Kanamori M, et al. Relationships between recurrence patterns and subventricular zone involvement or CD133 expression in glioblastoma. J Neurooncol (2020) 146(3):489–99. doi: 10.1007/s11060-019-03381-y
22. Mistry AM, Kelly PD, Gallant JN, Mummareddy N, Mobley BC, Thompson RC, et al. Comparative Analysis of Subventricular Zone Glioblastoma Contact and Ventricular Entry During Resection in Predicting Dissemination, Hydrocephalus, and Survival. Neurosurgery (2019) 85(5):E924–32. doi: 10.1093/neuros/nyz144
Keywords: glioblastoma, subventricular zone, survival, stem cell, glioma
Citation: Mistry AM, Mummareddy N, Salwi S, Davis LT and Ihrie RA (2020) Glioblastoma Distance From the Subventricular Neural Stem Cell Niche Does Not Correlate With Survival. Front. Oncol. 10:564889. doi: 10.3389/fonc.2020.564889
Received: 22 May 2020; Accepted: 13 November 2020;
Published: 11 December 2020.
Edited by:
Esperanza R. Matarredona, Sevilla University, SpainReviewed by:
Analiz Rodriguez, University of Arkansas for Medical Sciences, United StatesManuel Sarmiento Soto, University of Oxford, United Kingdom
Copyright © 2020 Mistry, Mummareddy, Salwi, Davis and Ihrie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akshitkumar M. Mistry, YXhpdGFtbUBnbWFpbC5jb20=; Rebecca A. Ihrie, cmViZWNjYS5paHJpZUB2YW5kZXJiaWx0LmVkdQ==
 Akshitkumar M. Mistry
Akshitkumar M. Mistry Nishit Mummareddy1
Nishit Mummareddy1