- 1Department of Thoracic Surgery, Tangdu Hospital, Air Force Medical University (Fourth Military Medical University), Xi’an, China
- 2Department of Thoracic Surgery, Sun Yat-sen University Cancer Center, Guangzhou, China
- 3Department of Thoracic Surgery, Shaanxi Provincial People’s Hospital, Xi’an, China
- 4Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Xi’an Medical University, Xi’an, China
- 5Department of Radiation Oncology, Sun Yat-Sen University Cancer Center, Guangzhou, China
Background: Grade prognostic assessment (GPA) is widely used to evaluate the prognosis of non-small cell lung cancer (NSCLC) patients with brain metastases (BMs). This study aimed to investigate whether lymph node status (LNS) could be included as one of the GPA variables for NSCLC with BMs.
Methods: Overall, 586 patients with NSCLC and BMs were retrospectively analyzed. Overall survival stratified by LNS was analyzed using the Kaplan-Meier method. Multivariate analysis was also performed to identify independent prognostic factors using the Cox proportional hazards progression model. In the updated GPA index, prognostic factors and criteria of GPA score were weighted by effect magnitude relative risk (RR) and statistical significance.
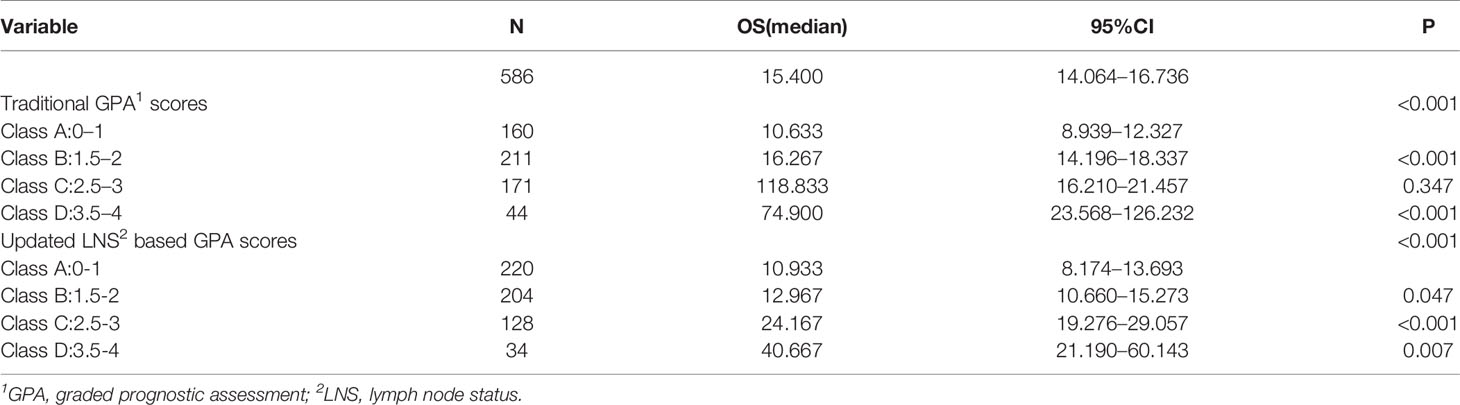
Results: In NSCLC patients with BMs, those with lymph node involvement had worse overall survival (mOS, 13.4 months vs. 25.9 months, P <0.001) than those without lymph node involvement. Multivariate analysis showed that LNS might be an independent prognostic factor (RR: 1.702, CI: 1.340–2.162, P <0.001). Finally, five prognostic factors including LNS, the age of the patient, Karnofsky performance status (KPS), the number of BMs, and extracranial metastases were enrolled in our novel GPA index. With the updated GPA index involving the N stage, survival analysis was also performed. Prognostic results were significantly different among these four subgroups (Class A vs. Class B, P=0.047; Class B vs. Class C, P<0.001; Class C vs. Class D, P=0.007).
Conclusions: These results indicate that LNS might be an indispensable prognostic factor in NSCLC with BM. The novel GPA model involving the N stage could provide more reliable evidence to estimate the survival of NSCLC patients with BMs.
Introduction
About 25%–40% of non-small cell lung cancer (NSCLC) patients experienced brain metastases (BMs) during their disease course (1, 2). Many traditional therapeutic modalities, including surgical resection, stereotactic radiosurgery (SRS), whole-brain radiotherapy (WBRT), and chemotherapy, are available to oncology clinicians for treating NSCLCs with BMs. Unfortunately, the median overall survival remains poor, at only eight months (3, 4). Recently, the advent of targeted therapy and immunotherapy revolutionarily improve the survival of these patients depending on their molecular type and clinical characteristics (5–7). Therefore, it is vital to identify the factors affecting the prognosis of NSCLC. In clinical practice, grade prognostic assessment (GPA) has been widely used to evaluate the prognosis of NSCLC patients with BM and has provided evidence for clinicians to make treatment decisions. The original GPA index was composed of four prognostic factors: age of patients, Karnofsky performance status (KPS), number of BM, and extracranial metastases. Patients with BM were divided into four classes based on this index, with median survival ranging from 3.0 months to 14.8 months (8, 9). However, some studies have indicated that the number of BM might not be an independent prognostic variable in patients with BM (10). Moreover, NSCLC with BM is a systemic disease, and many factors affect its prognosis (11); therefore, it is necessary to update the original GPA index.
Lymph node status (LNS), both in clinical and pathological views, has been recognized as an indispensable prognostic factor for NSCLC. LNS also affects the choice of treatment modality for early stage NSCLC patients (12, 13). In the TNM classification, patients with distant metastasis were categorized as stage IV, regardless of N status (14, 15). One study indicated that LNS cannot be ignored even in stage IV patients. In that study, the author investigated whether LNS affected the prognosis of patients with M1a stage NSCLC (16). Survival analysis was performed in 39,731 patients with M1a disease from the Surveillance, Epidemiology, and End Results (SEER) database. Interestingly, the authors found that LNS was an independent prognostic factor for M1a patients, and similar results were obtained in all subgroups.
Patients with BM are also classified as M stage according to TNM classification, and some scattered data suggests that LNS might be related to BM. A study showed that there was no BM detected by MRI in N0 stage patients, but it was detected in 5.2% of N1 patients and 4.7% of N2 patients (17). Similarly, another study showed that patients with advanced N stage cancer (P=0.009) and diameter of lymph node >2.0 cm (P=0.027) might have a higher risk of experiencing BM during their disease course (18). However, no studies have reported the influence of LNS on the prognosis of NSCLC patients with BM.
Thus, our study was designed to identify whether LNS affects the prognosis of NSCLC patients with BM, and to determine whether the N stage involving GPA index was an important supplement to the original GPA in predicting the survival of NSCLC patients with BM.
Materials and Methods
Patient Selection
All patients who were first diagnosed with BM in NSCLC who were treated at Tangdu Hospital from 2003 to 2013 were eligible for enrollment in our study. The study was approved by the Review Board of the Air Force Medical University. The inclusion criteria were as follows: (1) histologically or cytologically diagnosed as NSCLC; (2) MRI or CT demonstrated the presence of BM; (3) able to be assessed for LNS by thoracic and cervical CT or PET/CT; and (4) able to supply adequate clinical information and available for follow-up.
Evaluation of LNS
The N stage was defined according to the 8th TNM classification (15): The N0 stage was defined as the absence of regional lymph node metastasis; N1 as metastasis in the ipsilateral peribronchial, perihilar, or intrapulmonary lymph nodes; N2 as metastasis in the ipsilateral mediastinal and/or subcarinal lymph nodes; and N3 as metastasis in the contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph nodes. Non-regional lymph node metastasis was defined as M stage. Lymph node involvement was defined as the shortest dimension of any lymph node ≥1.0 cm, according to CT scan or as diagnosed by PET/CT (19, 20). The LNS of the patients was independently reviewed by two radiologists.
Statistical Analysis
The association between LNS and clinicopathologic factors was evaluated using the Chi-square test. Overall survival was defined as the time from diagnosis of BM to cancer-related death. We performed univariate and multivariate analyses using the Kaplan-Meier method and Cox proportional hazards progression model, respectively. A two-sided P-value <0.05 was considered statistically significant. In the updated GPA index, prognostic factors were weighted by effect magnitude relative risk (RR) and statistical significance. The scoring criteria for GPA were based on a previous study and the effect magnitude RR (9, 11). All analyses were performed using SPSS 22.0 software (IBM, Inc., Armonk, NY, USA).
Results
Patient Characteristics
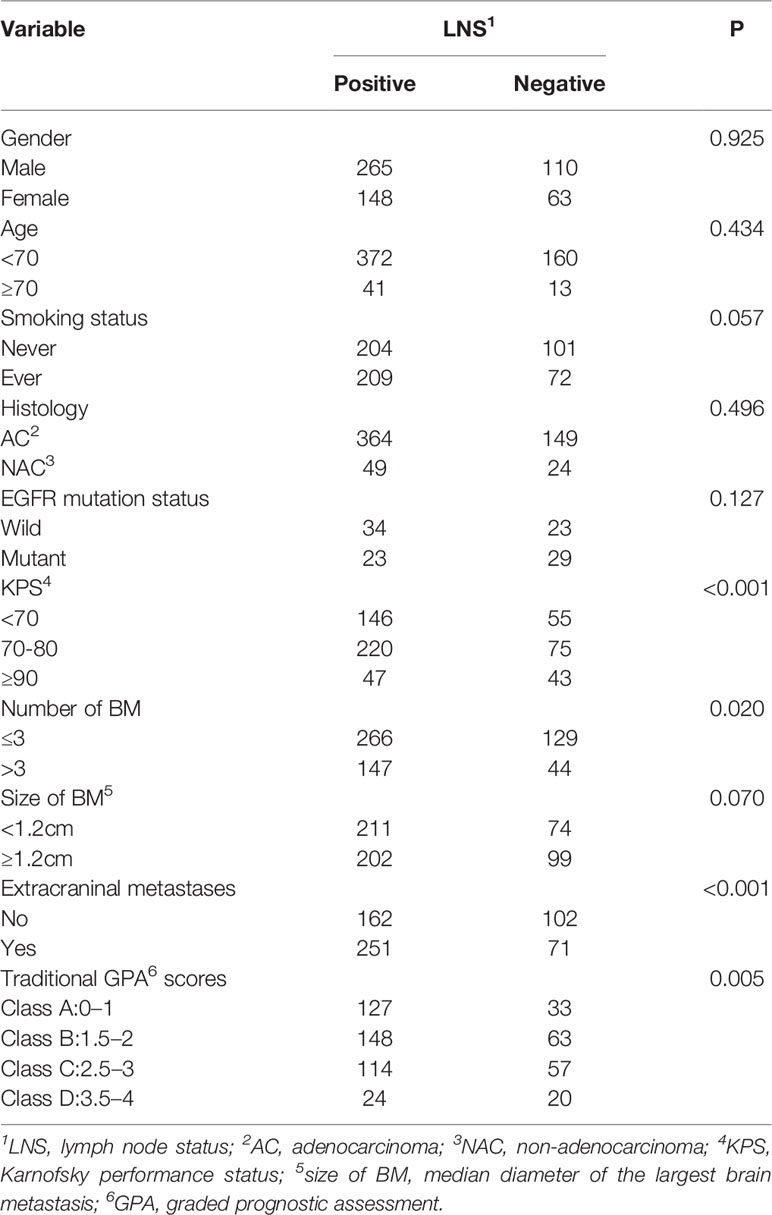
We included 586 patients treated between 2003 and 2013 in our study. Among these patients, 156 patients underwent CT scanning, and three patients were confirmed by MRI to have BM after they received controversial negative CT results. The median age was 55 years (range, 23–80 years), prevalent histology was adenocarcinoma (87.5%), and epidermal growth factor receptor (EGFR) mutation status was detected in 109 patients (18.6%), including 57 patients of wild-type and 52 patients of mutant type. The remaining 477 patients did not undergo EGFR status testing. Among these patients, 201 received first-generation tyrosine kinase inhibitors (TKIs) therapy, such as gefitinib and erlotinib, administered orally. Other clinical-pathologic characteristics of the patients are listed in Table 1.
Correlation Between LNS and Patient Baseline Characteristics
Among the 586 patients, 413 (70.5%) had lymph node involvement, including 136 patients diagnosed with N1 stage, 160 patients diagnosed with N2 stage, and 117 patients diagnosed with N3 stage. Patients with extracranial metastasis and higher GPA scores had a significantly higher rate of lymph node involvement (P <0.001 and P=0.005, respectively) than patients with no extracranial metastasis and lower GPA scores (Table 1).
Comparison of Overall Survival Among Different Clinical N Stages
Patients with lymph node involvement had worse overall survival (mOS, 13.4 months vs. 25.9 months, P <0.001) than those without, as shown in Figure 1A. Further analysis revealed that patients in the N1 stage had better survival than those in the N2 stage, whereas there was no difference between N0 and N1 patients. A similar result was observed in N2 and N3 stage patients (N0 vs. N1, P=0.146; N1 vs. N2, P<0.001; and N2 vs. N3, P=0.256) (Figure 1B).
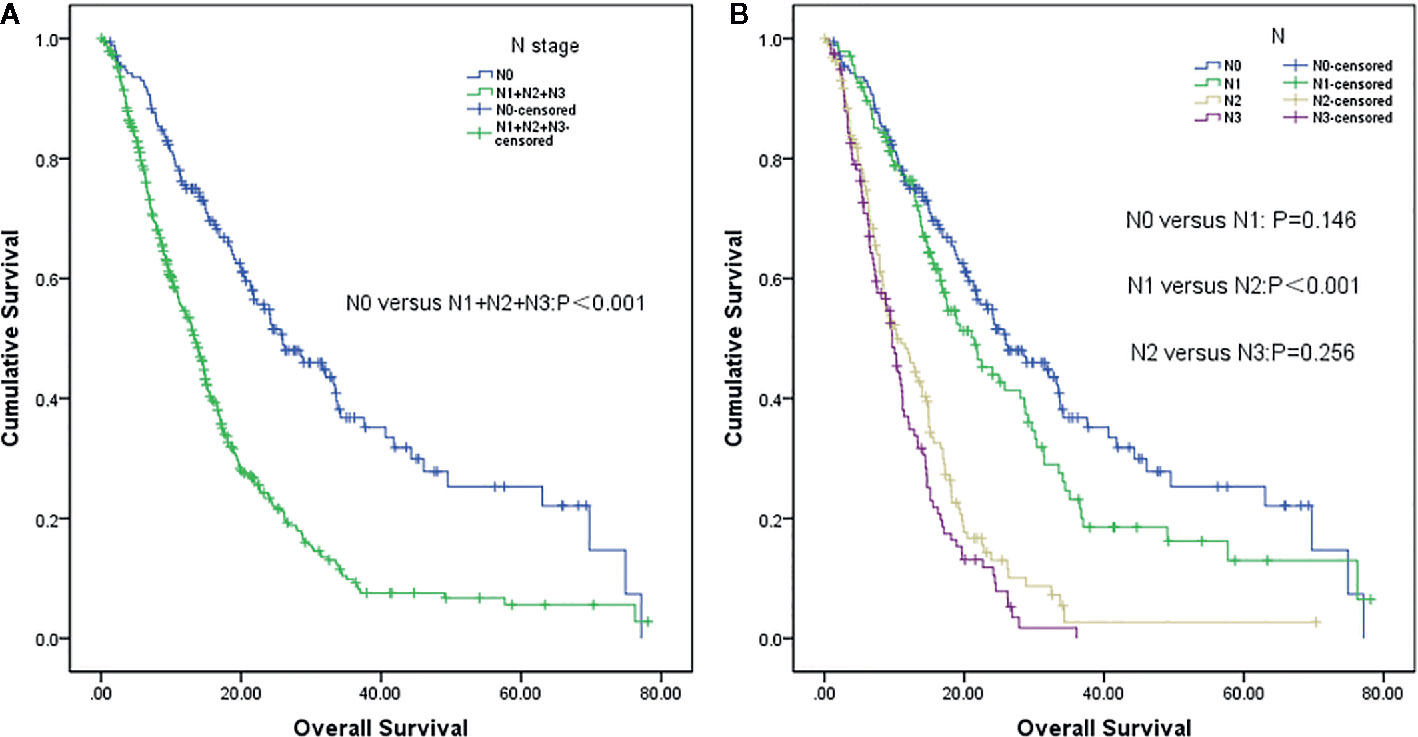
Figure 1 Survival analysis of non-small cell lung cancer (NSCLC) patients with brain metastases (BM) based on lymph node status (LNS). (A) Lymph node negative status (N0) vs. lymph node positive status (N1+N2+N3) (P<0.001). (B) N0 vs N1 (P=0,146), N1 vs. N2 (P<0.001) and N2 vs. N3 (P=0.256), respectively.
Univariate and Multivariate Survival Analyses for the Updated GPA Model
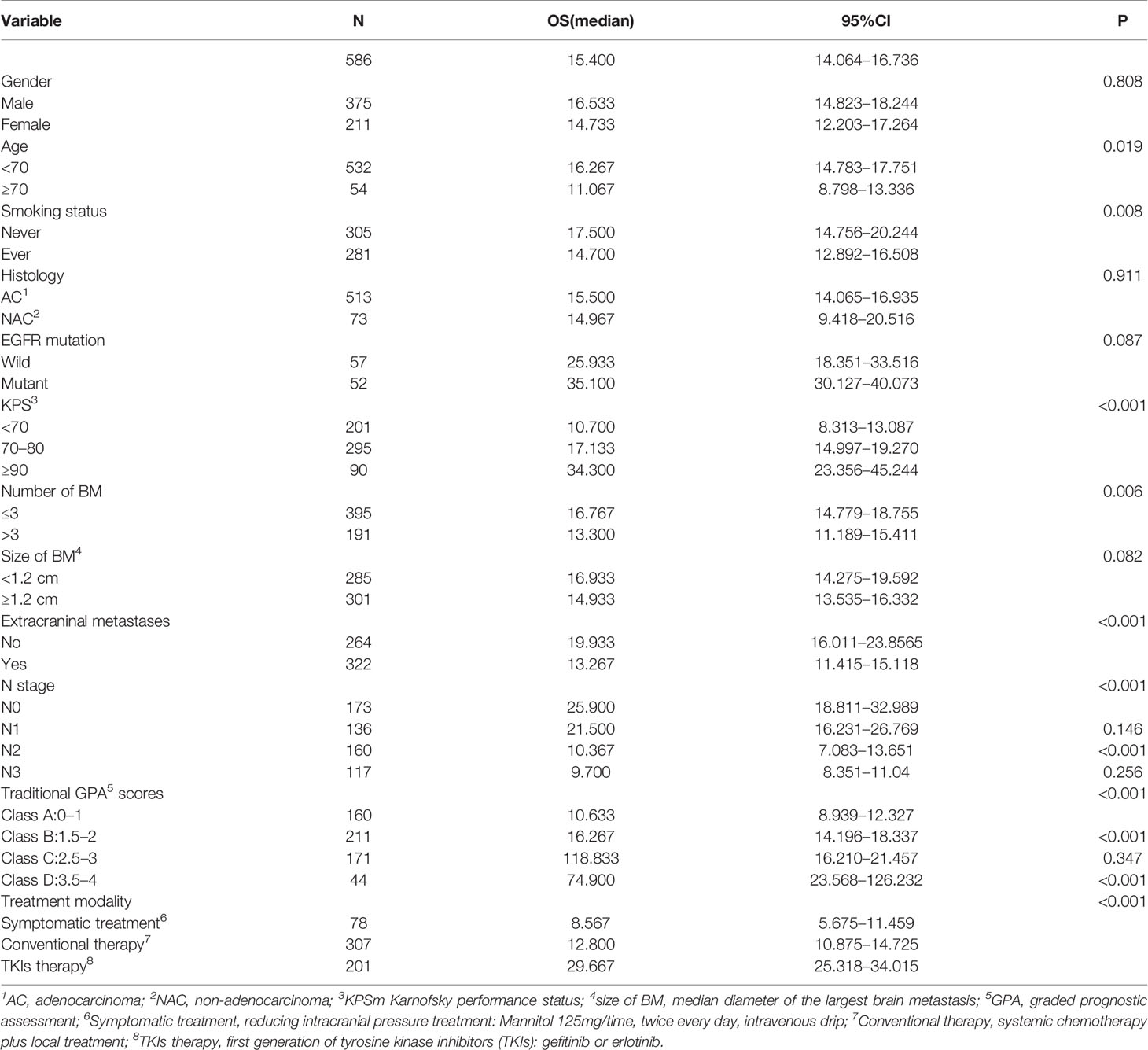
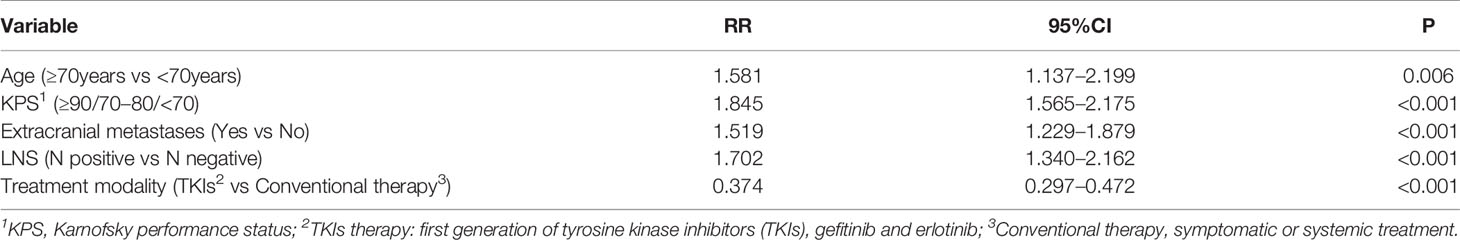
The median overall survival was 15.4 months. Univariate analysis showed that the following factors were significantly correlated with overall survival: LNS (P <0.001), age (P=0.019), KPS (P <0.001), smoking status (P=0.008), number of BM (P=0.006), GPA (P <0.001), extracranial metastases (P <0.001), and therapeutic modality (P <0.001) (Figure 2), which are shown in Table 2. When the above significant variables were selected for inclusion in the multivariate analysis, LNS was found to be an independent prognostic factor in NSCLC patients with BM (RR: 1.702, CI: 1.340–2.162, P <0.001). The age of patients (RR: 1.581), KPS (RR: 1.845), extracranial metastases (RR: 1.519), and treatment modality (RR: 0.374) were shown to be prognostic factors by multivariate analysis (Table 3).
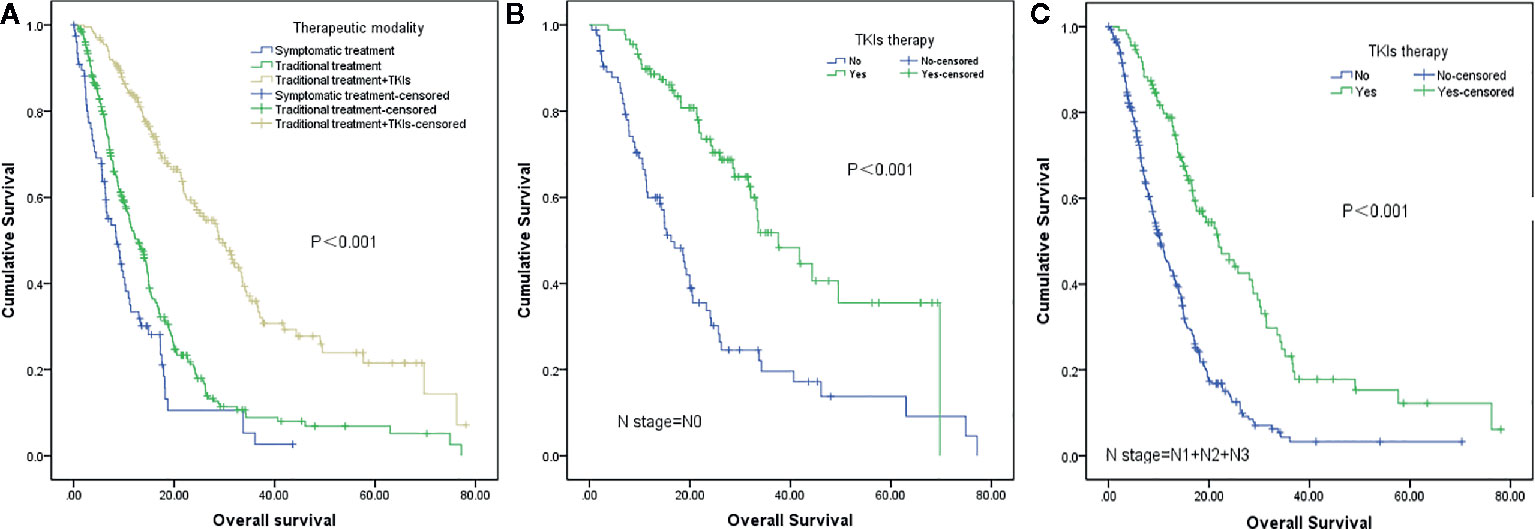
Figure 2 Survival analysis of non-small cell lung cancer (NSCLC) patients with brain metastases (BM) based on different therapeutic strategies. (A) Symptomatic treatment vs traditional treatment vs traditional treatment + TKIs (P<0.001). (B) TKIs therapy vs no TKIs therapy in patients with lymph node-negative (N0) status (P<0.001). (C) TKIs therapy vs no TKIs therapy in patients with lymph node-positive (N1+N2+N3) status (P<0.001).
The original GPA index consisted of four variables, including age, KPS, number of BM, and extracranial metastases (Table 4). We demonstrated that traditional GPA could be used to evaluate the prognosis of BM patients, especially when selecting the patients with the best or worse prognoses (Class A vs. Class B, P<0.001; Class C vs. Class D, P<0.001), but there was no significant difference between Class B and Class C (P=0.347) (Figure 3, Table 2). From our data, age, KPS, and extracranial metastases, were proven to be independent prognostic factors (Table 3).
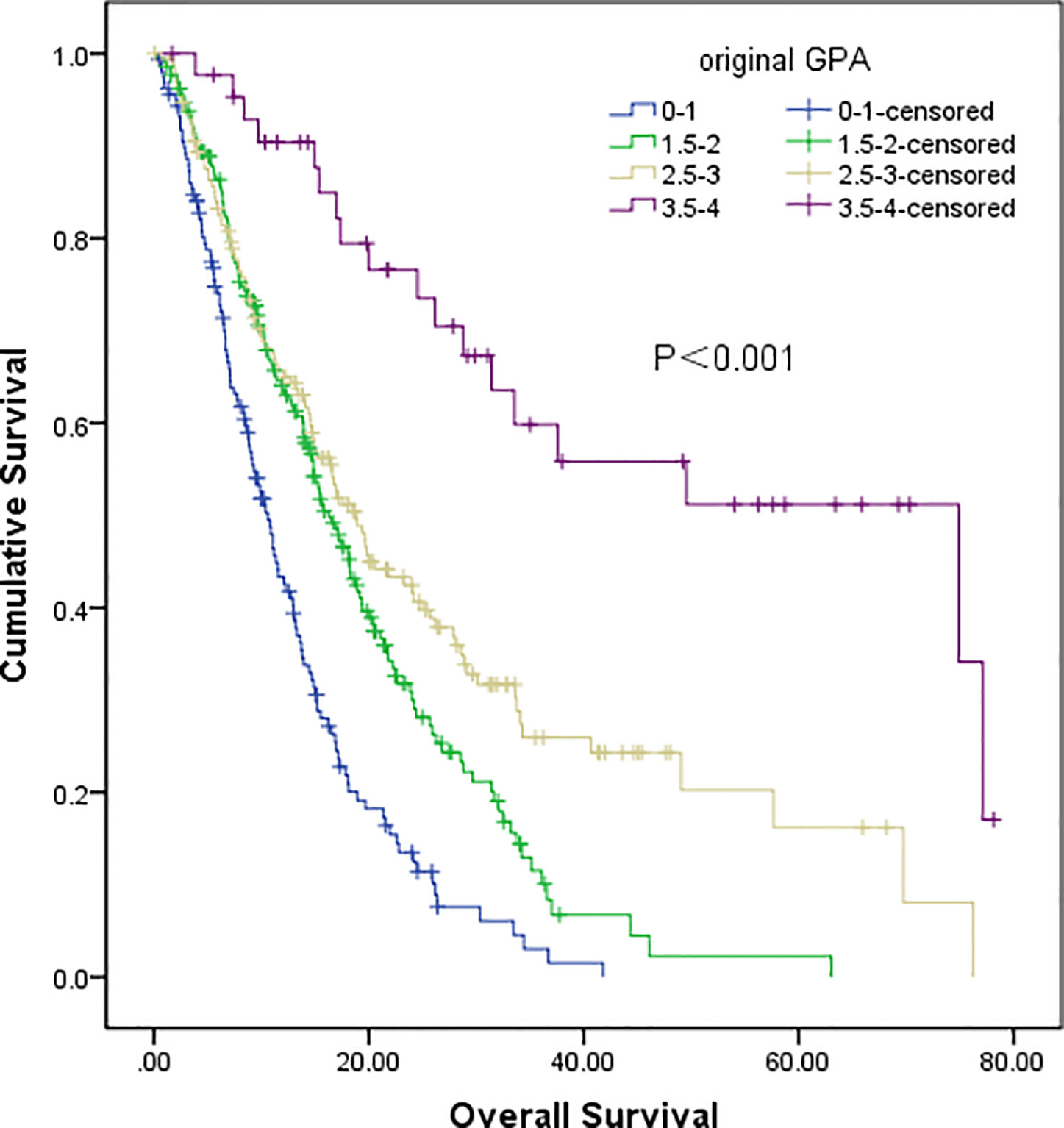
Figure 3 Survival analysis of non-small cell lung cancer (NSCLC) patients with brain metastases (BM) based on original GPA (Class A vs Class B, P<0.001; Class B vs Class C, P=0.347; Class C vs Class D, P<0.001).
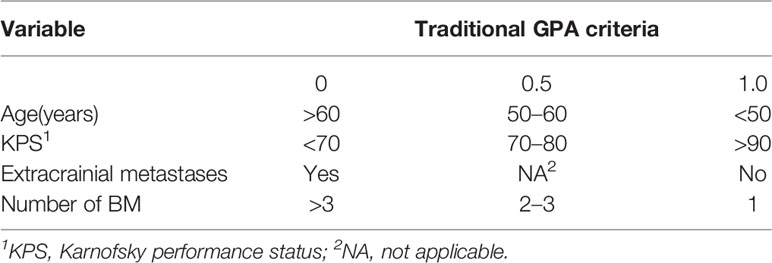
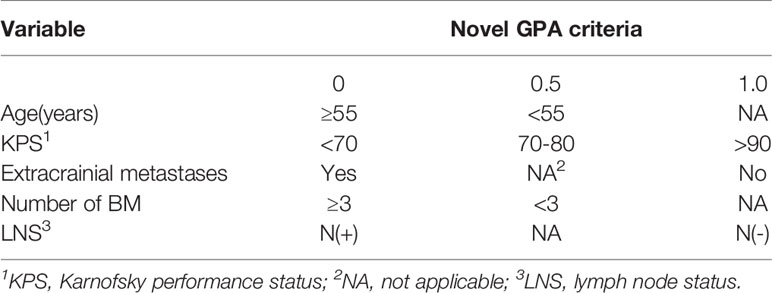
In our updated GPA index, prognostic factors were selected on the basis of the effect magnitude relative risk (RR) and statistical significance. The number of BMs in our novel GPA model was still selected based on previous studies and the univariate analysis results in our study. Finally, five prognostic factors, including LNS, age of patient, KPS, number of BM, and extracranial metastases, were enrolled in our novel GPA index. The scoring criteria were based on a previous study and RR, so extracranial metastases, KPS, and LNS were given a maximum score of 1.0, and the remaining two variables were given a maximum score of 0.5, for a total score of 4.0 (Table 5). With the updated GPA index, survival analysis was also performed. The prognostic results were significantly different among these four subgroups (Class A vs. Class B, P=0.047; Class B vs. Class C, P<0.001; Class C vs. Class D, P=0.007), as shown in Figure 4 and Table 6.
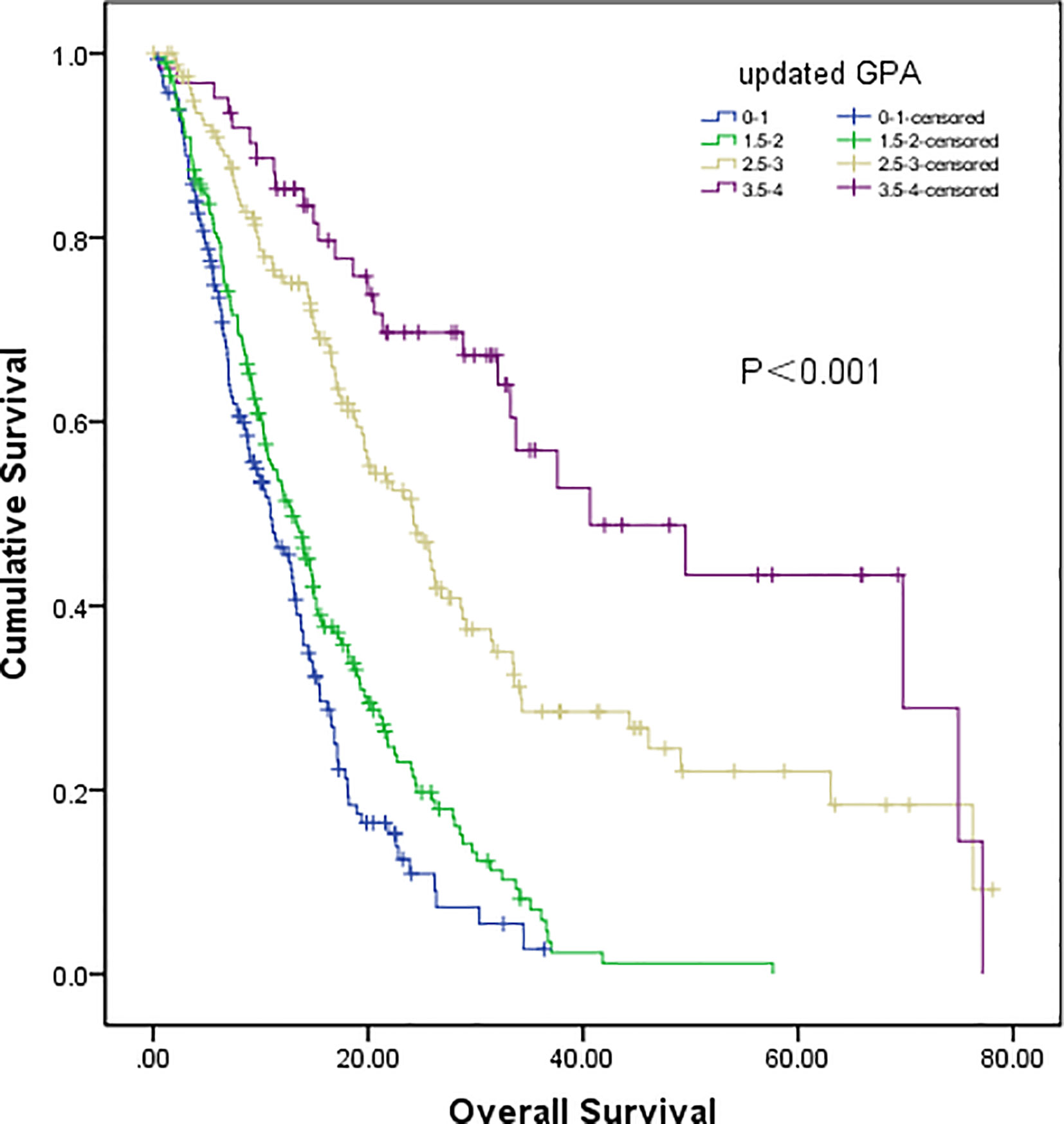
Figure 4 Survival analysis of non-small cell lung cancer (NSCLC) patients with brain metastases (BM) based on updated GPA (Class A vs Class B, P=0.047; Class B vs Class C, P<0.001; Class C vs Class D, P=0.007).
Discussion
We classified NSCLC patients with distant metastasis as stage IV. The 8th edition of the TNM stage classification divided patients into M1a, M1b, and M1c based on the heterogeneity of treatment and prognosis. Patients with NSCLC and BM should be defined as in stage M1b or M1c, according to the new staging version (14). A previous study demonstrated that LNS could provide prognostic information for patients with NSCLC with intrathoracic metastasis (M1a stage) (16). However, there are few related studies on NSCLC with BM. This study strongly suggests that LNS is an indispensable prognostic factor for patients with BM.
The prognostic value of LNS for patients with stage IV NSCLC has not been extensively studied. Iida et al. (21) reported that LNS was an independent prognostic factor for patients with pleural dissemination (M1a stage). Another study included 39,731 patients with M1a disease, and subsequent survival analysis showed that M1a patients without lymph node metastasis had the best survival, followed by patients in the N1 stage; however, there was no significant difference between N2 and N3 stage patients (N0 vs N1, P<0.001; N1 vs. N2, P<0.001; and N2 vs. N3, P=0.478) (16). Similar to the above two studies, our results strongly suggest that the N0 stage was an independent predictor of better survival for NSCLC patients with BM.
The original GPA index, including four variables (age, KPS, number of BM, and extracranial metastases), was widely used to predict the prognosis of NSCLC patients with BM. The four factors of the GPA index could predict the prognosis of these patients (8); however, these results have not been consistently observed in other studies, especially for the number of BM (10, 22). Indeed, although the number of BM (n≤ 3) was not an independent prognostic factor in our study, we still included it in both the original and updated GPA scoring systems. Moreover, we attempted to evaluate the effect of the updated GPA index involving the N stage; hence, the original GPA index was verified equally, which showed that the original GPA could significantly identify the prognosis of NSCLC patients with BM, especially for selecting patients with the best or worst prognoses (Class A and Class D). However, it failed to stratify the difference between the patients of Class B and Class C. According to the updated GPA index involving the N stage, the prognostic results were significantly different among these four subgroups.
Moreover, with extensive research on the mechanism of driver genes in NSCLC, the GPA index has continually been updated to precisely distinguish the prognosis of NSCLC patients with BM. Balasubramanian et al. reported that in NSCLC patients with BM, the median overall survival for patients with EGFR/ALK mutations was longer than that of wild-type patients (P=0.028) (10). Similarly, a meta-analysis that included 4,373 patients from 18 studies was designed to evaluate the relationship between EGFR mutation status and overall survival of patients with NSCLC with BM. The results also confirmed that EGFR mutation status is an important prognostic factor for NSCLC with BM (23). Therefore, a new GPA index was built using the driver genes and molecular alterations of NSCLC patients with BM (11). In Sperduto’s research, significant prognostic factors included the classical four factors (age, KPS, extracranial metastases, and number of BM) and two molecular factors: EGFR and ALK mutant status, which frequently occurred in lung adenocarcinoma. The median OS for the whole cohort in this study setted in US was 12.0 months, and those adenocarcinomas with Lung-molGPA scores of 3.5 to 4.0 had a median survival of nearly 4 years, despite the lower prevalence of drive gene mutation in Caucasian patients compared with East Asian patients. It indicate that driver gene alteration involving GPA scores is a meaningful tool that may facilitate clinical decision-making of NSCLC patient with BM. Similar data were also found in our study (EGFR mutant vs EGFR Wild), although only 109 patients underwent EGFR mutant testing and the other drive genes detections were absent in our study. So a large sample and molecular typing based GPA model is going to be designed in Chinese patients in our future study.
The usability and popularization of a GPA model mostly depends on whether it can widely cover all patients with NSCLC with BM; therefore, the enrolled patients should include patients receiving traditional intervention as well as targeted treatment. In the present study, 201 patients received targeted therapy. The IPASS study (the Iressa Pan-Asia study) is a landmark study on NSCLC, demonstrating the superiority of TKI as a first-line treatment compared to chemotherapy for EGFR mutant patients with respect to progression-free survival (PFS) (24). Moreover, data from the CTONG-0803 trial (25), a phase II, open-label prospective study that evaluated the efficiency of erlotinib in NSCLC with BM, showed that EGFR-positive patients had longer PFS than those with EGFR-negative patients (15.2 months vs. 4.4 months, P = 0.02). In our previous study, we found that TKI treatment was beneficial for BM patients in terms of both PFS and overall survival (OS), independent of the EGFR mutation status (26). Our data confirmed again that TKI therapy could prolong the overall survival of all BM patients, regardless of the N stage.
Finally, it is controversial whether NSCLC patients with BM could benefit from thoracic therapy. Aggressive thoracic therapy (ATT) was defined as resection of the primary disease or chemoradiotherapy in which the total radiation dose exceeded 45 Gy. Gray et al. (27) reported that patients with synchronous and brain-only oligometastatic disease who received ATT had a longer overall survival (P < 0.001), and this survival benefit was also found in patients with stage III disease (P = 0.004). This suggests that thoracic therapy might prolong the survival of BM patients, especially for oligometastatic patients.
Some limitations of this study should be mentioned. The first is that LNS was primarily evaluated by imaging technology (CT or PET/CT), and only a small percentage of patients underwent mediastinoscopy examination. Dales et al. (19) reported that the sensitivity and specificity of CT for mediastinal lymph node diagnosis were 78% and 79%, respectively. Even with PET/CT, the sensitivity and specificity were 85% and 90%, respectively (20). Moreover, the therapeutic schedule of patients was not uniform; only 201 patients received TKI therapy for different lines, and none had been treated with osimertinib, a third-generation TKI, which is widely used in the treatment of BM patients harboring EGFR mutations (28). Finally, data on mediastinal radiotherapy for patients with mediastinal lymph node involvement were limited (29). A study on WBRT/SRS combined with intrathoracic radiotherapy for BM patients positive for lymph node involvement should be designed in the future.
In conclusion, our study provides preliminary evidence that LNS might be an indispensable prognostic factor in NSCLC with BM, and the novel grade prognostic assessment (GPA) model involved in the N stage could provide more information to predict survival.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Tangdu Hospital of Air Force Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
TJ, LZ, and LC participated in study design and study conception. JZ, WPW, SX, HW, and ZZ performed data analysis. JZ, WPW, SX, CJ, YX, WCW, MW, and XW recruited patients. JZ, WPW, SX, and LC drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the Wu Jieping Medical Foundation (320. 6750. 17527) and the Provincial Key R&D Program of Shaanxi (2017ZDCXL-SF-01-04-01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Stuschke M, Eberhardt W, Pottgen C, Stamatis G, Wilke H, Stuben G, et al. Prophylactic cranial irradiation in locally advanced non-small-cell lung cancer after multimodality treatment: long-term follow-up and investigations of late neuropsychologic effects. J Clin Oncol (1999) 17:2700–9. doi: 10.1200/JCO.1999.17.9.2700
2. Ceresoli GL, Reni M, Chiesa G, Carretta A, Schipani S, Passoni P, et al. Brain metastases in locally advanced nonsmall cell lung carcinoma after multimodality treatment: risk factors analysis. Cancer-Am Cancer Soc (2002) 95:605–12. doi: 10.1002/cncr.10687
3. Diener-West M, Dobbins TW, Phillips TL, Nelson DF. Identification of an optimal subgroup for treatment evaluation of patients with brain metastases using RTOG study 7916. Int J Radiat Oncol Biol Phys (1989) 16:669–73. doi: 10.1016/0360-3016(89)90483-5
4. Moscetti L, Nelli F, Felici A, Rinaldi M, De Santis S, D’Auria G, et al. Up-front chemotherapy and radiation treatment in newly diagnosed nonsmall cell lung cancer with brain metastases: survey by Outcome Research Network for Evaluation of Treatment Results in Oncology. Cancer-Am Cancer Soc (2007) 109:274–81. doi: 10.1002/cncr.22399
5. Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol (2018) 2018783118. doi: 10.1200/JCO.2018.78.3118
6. Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol (2016) 17(7):976–83. doi: 10.1016/S1470-2045(16)30053-5
7. Bauer TM, Shaw AT, Johnson ML, Navarro A, Gainor JF, Thurm H, et al. Brain Penetration of Lorlatinib: Cumulative Incidences of CNS and Non-CNS Progression with Lorlatinib in Patients with Previously Treated ALK-Positive Non-Small-Cell Lung Cancer. Target Oncol (2020) 15(1):55–65. doi: 10.1007/s11523-020-00702-4
8. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys (2008) 70:510–4. doi: 10.1016/j.ijrobp.2007.06.074
9. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol (2012) 30:419–25. doi: 10.1200/JCO.2011.38.0527
10. Balasubramanian SK, Sharma M, Venur VA, Schmitt P, Kotecha R, Chao ST, et al. Impact of EGFR mutation and ALK rearrangement on the outcomes of non-small cell lung cancer patients with brain metastasis. Neuro Oncol (2019) 22:267–77. doi: 10.1093/neuonc/noz155
11. Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, et al. Estimating survival in patients with lung cancer and brain metastases: an Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol (2017) 3:827–31. doi: 10.1001/jamaoncol.2016.3834
12. Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest (1997) 111:1710–7. doi: 10.1378/chest.111.6.1710
13. Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest (2009) 136:260–71. doi: 10.1378/chest.08-0978
14. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM Stage Groupings in the forthcoming (eighth) edition of the TNM Classification for Lung Cancer. J Thorac Oncol (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
15. Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin (2017) 67:138–55. doi: 10.3322/caac.21390
16. Dai C, Ren Y, Xie D, Zheng H, She Y, Fei K, et al. Does lymph node metastasis have a negative prognostic impact in patients with NSCLC and M1a disease? J Thorac Oncol (2016) 11:1745–54. doi: 10.1016/j.jtho.2016.06.030
17. Yohena T, Yoshino I, Kitajima M, Uehara T, Kanematsu T, Teruya T, et al. Necessity of preoperative screening for brain metastasis in non-small cell lung cancer patients without lymph node metastasis. Ann Thorac Cardiovasc Surg (2004) 10:347–9.
18. Rice SR, Molitoris JK, Vyfhuis M, Edelman MJ, Burrows WM, Feliciano J, et al. Lymph Node Size Predicts for Asymptomatic Brain Metastases in Patients With Non-small-cell Lung Cancer at Diagnosis. Clin Lung Cancer (2019) 20:e107–14. doi: 10.1016/j.cllc.2018.09.014
19. Dales RE, Stark RM, Raman S. Computed tomography to stage lung cancer. Approaching a controversy using meta-analysis. Am Rev Respir Dis (1990) 141:1096–101. doi: 10.1164/ajrccm/141.5_Pt_1.1096
20. Gould MK, Kuschner WG, Rydzak CE, Maclean CC, Demas AN, Shigemitsu H, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med (2003) 139:879–92. doi: 10.7326/0003-4819-139-11-200311180-00013
21. Iida T, Shiba M, Yoshino I, Miyaoka E, Asamura H, Date H, et al. Surgical intervention for non-small-cell lung cancer patients with pleural carcinomatosis: results from the Japanese Lung Cancer Registry in 2004. J Thorac Oncol (2015) 10:1076–82. doi: 10.1097/JTO.0000000000000554
22. Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol (2014) 15:387–95. doi: 10.1016/S1470-2045(14)70061-0
23. Li WY, Zhao TT, Xu HM, Wang ZN, Xu YY, Han Y, et al. The role of EGFR mutation as a prognostic factor in survival after diagnosis of brain metastasis in non-small cell lung cancer: a systematic review and meta-analysis. BMC Cancer (2019) 19:145. doi: 10.1186/s12885-019-5331-z
24. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med (2009) 361:947–57. doi: 10.1056/NEJMoa0810699
25. Wu YL, Zhou C, Cheng Y, Lu S, Chen GY, Huang C, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann Oncol (2013) 24:993–9. doi: 10.1093/annonc/mds529
26. Cai L, Zhu JF, Zhang XW, Lin SX, Su XD, Lin P, et al. A comparative analysis of EGFR mutation status in association with the efficacy of TKI in combination with WBRT/SRS/surgery plus chemotherapy in brain metastasis from non-small cell lung cancer. J Neurooncol (2014) 120:423–30. doi: 10.1007/s11060-014-1570-7
27. Gray PJ, Mak RH, Yeap BY, Cryer SK, Pinnell NE, Christianson LW, et al. Aggressive therapy for patients with non-small cell lung carcinoma and synchronous brain-only oligometastatic disease is associated with long-term survival. Lung Cancer (2014) 85:239–44. doi: 10.1016/j.lungcan.2014.06.001
28. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med (2018) 378:113–25. doi: 10.1056/NEJMoa1713137
Keywords: non-small cell lung cancer, brain metastases, N stage, prognostic factor, grade prognostic assessment
Citation: Zhu J, Wang W, Xu S, Jia C, Zhang Q, Xia Y, Wang W, Wen M, Wang X, Wang H, Zhang Z, Cai L, Zhang L and Jiang T (2020) Evaluation of the Effect of Lymph Node Status on the Survival of Non-Small Cell Lung Cancer Patients With Brain Metastases: Applications of a Novel Grade Prognostic Assessment Score Model Involving N Stage. Front. Oncol. 10:563700. doi: 10.3389/fonc.2020.563700
Received: 19 May 2020; Accepted: 25 September 2020;
Published: 19 October 2020.
Edited by:
Sara Pilotto, University of Verona, ItalyReviewed by:
Pranshu Mohindra, University of Maryland, Baltimore, United StatesAlessandro Russo, A.O. Papardo, Italy
Copyright © 2020 Zhu, Wang, Xu, Jia, Zhang, Xia, Wang, Wen, Wang, Wang, Zhang, Cai, Zhang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Jiang, amlhbmd0YW90ZEAxNjMuY29t; Lanjun Zhang, emhhbmdsakBzeXN1Y2Mub3JnLmNu; Ling Cai, Y2FpbGluZ0BzeXN1Y2Mub3JnLmNu
†These authors have contributed equally to this work and share first authorship
 Jianfei Zhu
Jianfei Zhu Wuping Wang
Wuping Wang Shuonan Xu
Shuonan Xu Chenghui Jia1
Chenghui Jia1 Ling Cai
Ling Cai Tao Jiang
Tao Jiang